New Advanced Materials and Sorbent-Based Microextraction Techniques as Strategies in Sample Preparation to Improve the Determination of Natural Toxins in Food Samples
Abstract
:1. Introduction
2. Sorbent-Based Microextraction of Natural Toxins from Food Samples
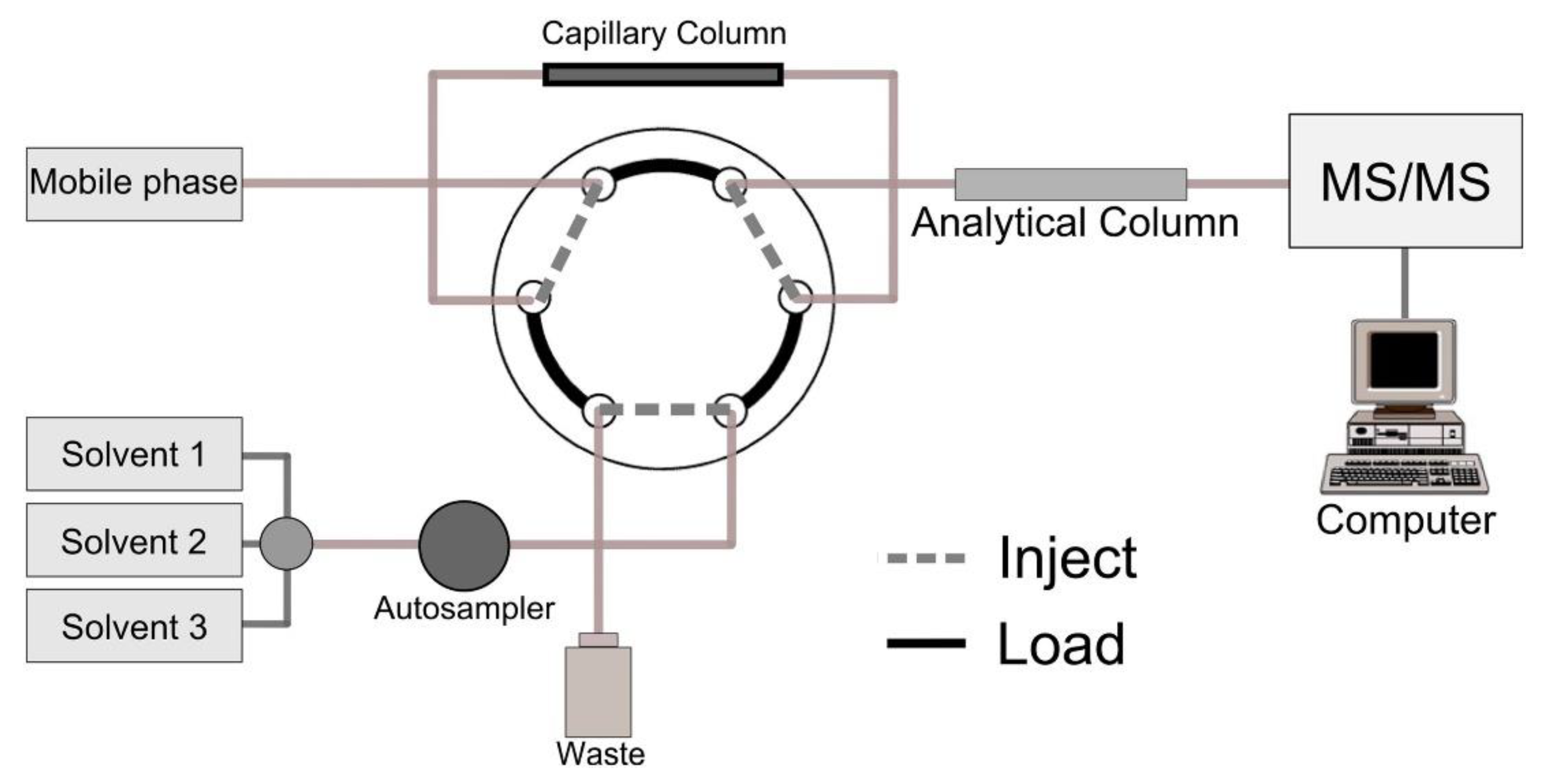
2.1. Solid-Phase Microextraction (SPME)
2.2. Micro-Dispersive Solid-Phase Extraction (µ-dSPE)
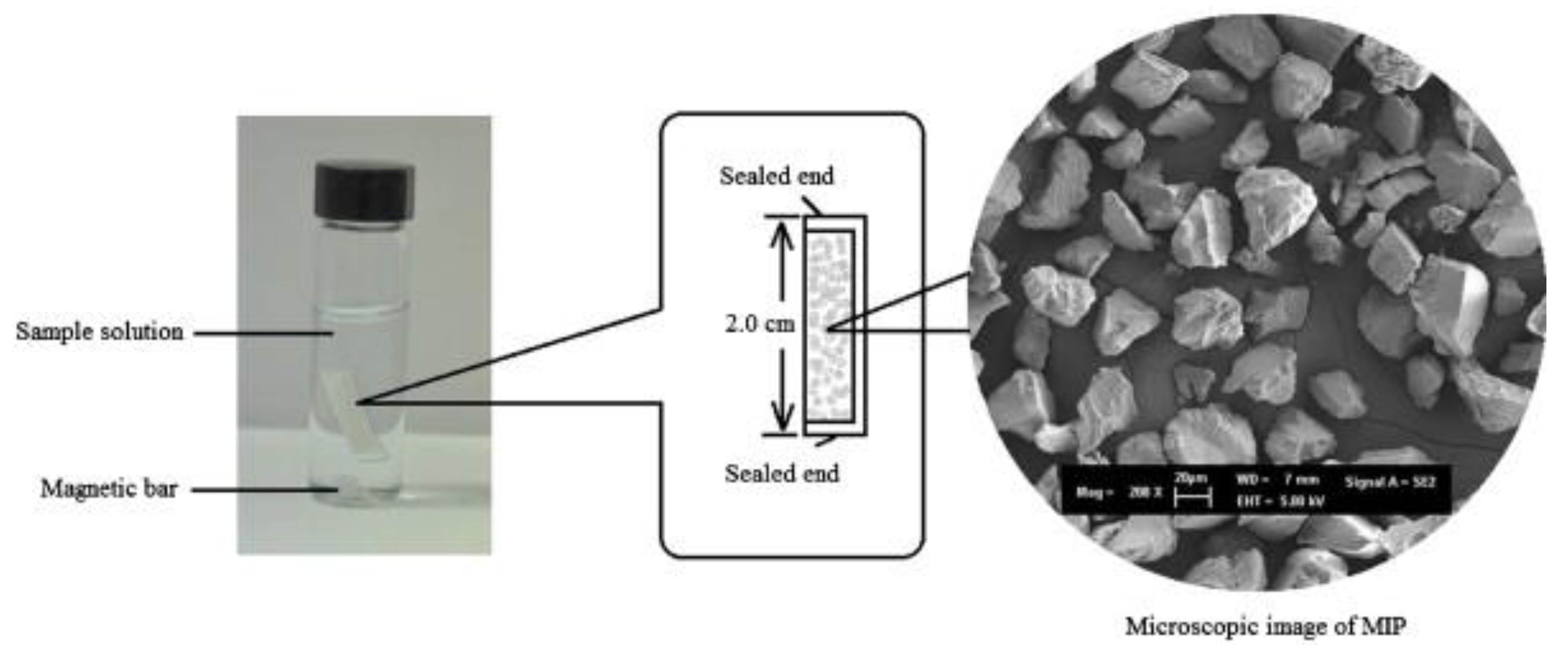
2.3. Micro-Solid-Phase Extraction (µ-SPE)
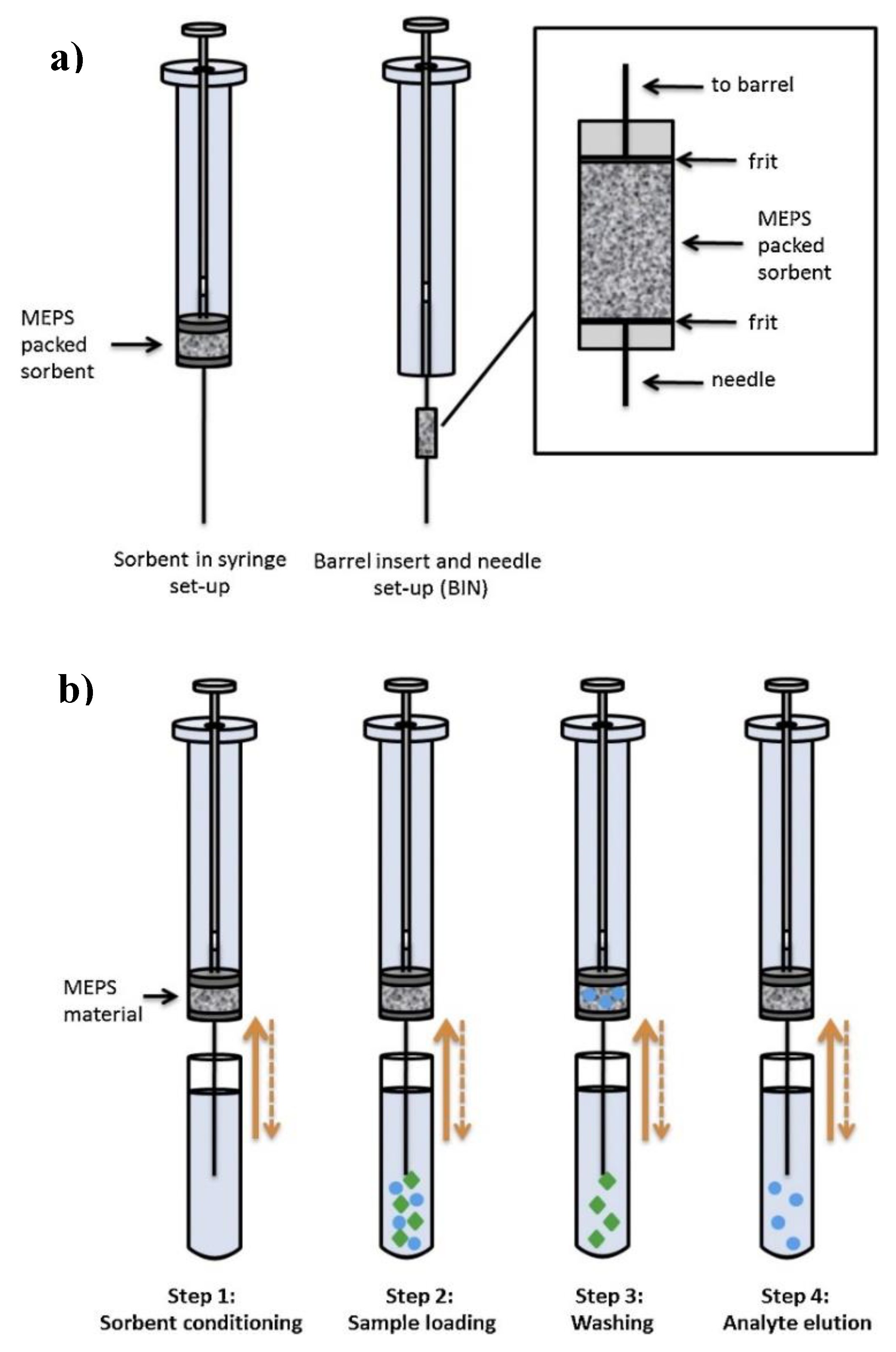
2.4. Microextraction by Packed Sorbent (MEPS)
3. Integration of New Advanced Materials as Sorbents on Microextraction Techniques to Isolate Natural Toxins from Food Samples
3.1. Miniaturized Solid-Phase Extraction (m-SPE)
3.2. In-Syringe Solid-Phase Extraction
3.3. Pipette-Tip Solid-Phase Extraction (PT-SPE)
3.4. Micro-Dispersive Solid-Phase Extraction (µ-dSPE)
3.5. Micro-Magnetic Solid-Phase Extraction (µ-MSPE)
3.6. Micro-Solid-Phase Extraction (µ-SPE)
3.7. Solid-Phase Microextraction (SPME)
3.8. Stir-Bar Sorptive Extraction (SBSE)
4. Future Challenges in the Integration of New Advanced Materials on Microextraction Techniques for the Analysis of Natural Toxins in Food Samples
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization: Natural Toxins in Food. Available online: https://www.who.int/news-room/fact-sheets/detail/natural-toxins-in-food (accessed on 11 December 2019).
- López de Cerain, A.; Gil, A.; Bello, J. Alimentos con sustancias tóxicas de origen natural: Plantas superiores alimenticias. In Toxicología Alimentaria, 1st ed.; Cameán, A., Repetto, M., Eds.; Ediciones Díaz de Santos: Madrid, Spain, 2006; pp. 191–210. [Google Scholar]
- Contaminants in the Food Chain. Available online: http://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/contaminants_in_the_food_chain.pdf (accessed on 31 January 2020).
- EFSA: Plant Toxins. Available online: https://ec.europa.eu/food/safety/chemical_safety/contaminants/catalogue/plant_toxins_en (accessed on 11 December 2019).
- World Health Organization: Mycotoxins. Available online: https://www.who.int/news-room/fact-sheets/detail/mycotoxins (accessed on 11 December 2019).
- Park, D.L.; Guzman-Perez, S.E.; Lopez-Garcia, R. Aquatic Biotoxins: Design and Implementation of Seafood Safety Monitoring Programs. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: New York, NY, USA, 1999; Volume 161, pp. 157–200. [Google Scholar] [CrossRef]
- EFSA: Occurrence of Pyrrolizidine Alkaloids in Food. Available online: http://www.efsa.europa.eu/en/supporting/pub/en-859 (accessed on 11 December 2019).
- EFSA: Scientific Opinion on Tropane Alkaloids in Food and Feed. Available online: http://www.efsa.europa.eu/en/efsajournal/pub/3386 (accessed on 11 December 2019).
- EFSA: Scientific Opinion on the Risks for Public Health Related to the Presence of Opium Alkaloids in Poppy Seeds. Available online: http://www.efsa.europa.eu/en/efsajournal/pub/2405 (accessed on 11 December 2019).
- EFSA: Scientific Opinion on Pyrrolizidine Alkaloids in Food and Feed. Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/j.efsa.2011.2406 (accessed on 11 December 2019).
- EFSA: Dietary Exposure Assessment to Pyrrolizidine Alkaloids in the European Population. Available online: http://www.efsa.europa.eu/en/efsajournal/pub/4572 (accessed on 11 December 2019).
- EFSA: Risks for Human Health Related to the Presence of Pyrrolizidine Alkaloids in Honey, Tea, Herbal Infusions and Food Supplements. Available online: http://www.efsa.europa.eu/en/efsajournal/pub/4908 (accessed on 11 December 2019).
- Commission Regulation (EC) No 1881/2006 of 19 December 2006, Setting Maximum Levels for Certain Contaminants in Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1881&from=ES (accessed on 11 December 2019).
- Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004, Laying Down Specific Hygiene Rules for on the Hygiene of Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R0853&from=ES (accessed on 11 December 2019).
- Commission Recommendation (EU) 2015/976 of 19 June 2015 on the Monitoring of the Presence of Tropane Alkaloids in Food. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015H0976&from=ES (accessed on 11 December 2019).
- Commission Recommendation of 10 September 2014 on Good Practices to Prevent and to Reduce the Presence of Opium Alkaloids in Poppy Seeds and Poppy Seed Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014H0662&from=ES (accessed on 11 December 2019).
- Codex Alimentarius: Code of Practice for Weed Control to Prevent and Reduce Pyrrolizidine Alkaloid Contamination in food and feed (CAC/RCP 74-2014). Available online: http://www.fao.org/input/download/standards/13794/CXP_074e_2014.pdf (accessed on 11 December 2019).
- European Food Safety Authority: Chemical Contaminants. Available online: https://www.efsa.europa.eu/en/topics/topic/chemical-contaminants (accessed on 11 December 2019).
- Commission Regulation (EC) No 401/2006 of 23 February 2006, Laying Down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R0401&from=ES (accessed on 11 December 2019).
- Casado, N.; Pérez-Quintanilla, D.; Morante-Zarcero, S.; Sierra, I. Current development and applications of ordered mesoporous silicas and other solegel silica-based materials in food sample preparation for xenobiotics analysis. TrAC 2017, 88, 167–184. [Google Scholar] [CrossRef]
- Morante-Zarcero, S.; Sierra, I. New advances in food sample preparation with nanomaterials for organic contaminants analysis by liquid chromatography. In Nanomaterials in Chromatography; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 118–154. [Google Scholar] [CrossRef]
- Filippou, O.; Bitas, D.; Samanidou, V. Green approaches in sample preparation of bioanalytical samples prior to chromatographic analysis. J. Chromatogr. B 2017, 1043, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Casado, N.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Câmara, J.S.; Sierra, I. Two novel strategies in food sample preparation for the analysis of dietary polyphenols: Micro-extraction techniques and new silica-based sorbent materials. Trends Food Sci. Technol. 2018, in press. [Google Scholar] [CrossRef]
- Abdel-Rehim, M.; Pedersen-Bjergaard, S.; Abdel-Rehim, A.; Lucena, R.; Moein, M.M.; Cárdenas, S.; Miró, M. Microextraction approaches for bioanalytical applications: An overview. J. Chromatogr. A 2019, in press. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; Owczarek, K.; Namieśnik, J. Miniaturized Solid Phase Extraction. In Green Extraction Techniques: Principles, Advances and Applications; Ibañez, E., Cifuentes, A., Eds.; Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 76, pp. 279–318. [Google Scholar]
- González-Sálamo, J.; Socas-Rodríguez, B.; Hernandez-Borges, J.; Rodríguez-Delgado, M.Á. Nanomaterials as sorbents for food sample analysis. TrAC 2016, 85, 203–220. [Google Scholar] [CrossRef]
- Socas-Rodriguez, B.; Gonzalez-Salamo, J.; Hernandez-Borges, J.; Rodríguez-Delgado, M.Á. Recent applications of nanomaterials in food safety. TrAC 2017, 96, 172–200. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Chen, J.; Jiang, T.; Ye, H.; Ji, H.; Tsang, S.; Zhao, Z.; Yi, T.; Chen, H. Recent progress in nanomaterial-based assay for the detection of phytotoxins in foods. Food Chem. 2019, 277, 162–178. [Google Scholar] [CrossRef]
- Da Silva, M.R.; Fumes, B.H.; Nazario, C.E.D.; Lancas, F.M. New materials for green sample preparation: Recent advances and future trends. In Green Extraction Techniques: Principles, Advances and Applications; Ibañez, E., Cifuentes, A., Eds.; Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 76, pp. 575–599. [Google Scholar]
- Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymers-based microextraction techniques. TrAC 2019, 118, 574–586. [Google Scholar] [CrossRef]
- Turner, N.W.; Bramhmbhatt, H.; Szabo-Vezse, M.; Poma, A.; Coker, R.; Piletsky, S.A. Analytical methods for determination of mycotoxins: An update (2009–2014). Anal. Chim. Acta 2015, 901, 12–33. [Google Scholar] [CrossRef]
- Speltini, A.; Scalabrini, A.; Maraschi, F.; Sturini, M.; Profumo, A. Newest applications of molecularly imprinted polymers for extraction of contaminants from environmental and food matrices: A review. Anal. Chim. Acta 2017, 974, 1–26. [Google Scholar] [CrossRef]
- Hou, X.; Tang, S.; Wang, J. Recent advances and applications of graphene-based extraction materials in food safety. TrAC 2019, 119, 115603. [Google Scholar] [CrossRef]
- Quinto, M.; Spadaccino, G.; Palermo, C.; Centonze, D. Determination of aflatoxins in cereal flours by solid-phase microextraction coupled with liquid chromatography and post-column photochemical derivatization-fluorescence detection. J. Chromatogr. A 2009, 1216, 8636–8641. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, Y.; Saito, K.; Hanioka, N.; Narimatsu, S.; Kataoka, H. Determination of aflatoxins in food samples by automated on-line in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. J. Chromatogr. A 2009, 1216, 4416–4422. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Itano, M.; Ishizaki, A.; Saito, K. Determination of patulin in fruit juice and dried fruit samples by in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. J. Chromatogr. A 2009, 1216, 3746–3750. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Ikeuchi, R.; Kataoka, H. Determination of ochratoxins in nuts and grain samples by in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. J. Chromatogr. A 2012, 1220, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; Lanças, F.M. Determination of Ochratoxin A in wine by packed in-tube solid phase microextraction followed by high performance liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2017, 1493, 41–48. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; González-Sálamo, J.; Hernández-Borges, J.; Rodríguez Delgado, M.Á. Application of multiwalled carbon nanotubes as sorbents for the extraction of mycotoxins in water samples and infant milk formula prior to high performance liquid chromatography mass spectrometry analysis. Electrophoresis 2016, 37, 1359–1366. [Google Scholar] [CrossRef]
- Du, L.J.; Chu, C.; Warner, E.; Wang, Q.Y.; Hu, Y.H.; Chai, K.J.; Cao, J.; Peng, L.Q.; Chen, Y.B.; Yang, J.; et al. Rapid microwave-assisted dispersive micro-solid phase extraction of mycotoxins in food using zirconia nanoparticles. J. Chromatogr. A 2018, 1561, 1–12. [Google Scholar] [CrossRef]
- Lee, T.P.; Saad, B.; Khayoon, W.S.; Salleh, B. Molecularly imprinted polymer as sorbent in micro-solid phase extraction of ochratoxin A in coffee, grape juice and urine. Talanta 2012, 88, 129–135. [Google Scholar] [CrossRef]
- Savastano, M.L.; Losito, I.; Pati, S. Rapid and automatable determination of ochratoxin A in wine based on microextraction by packed sorbent followed by HPLC-FLD. Food Control 2016, 68, 391–398. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Serra-Mora, P.; Moliner-Martínez, Y.; Molins-Legua, C.; Herráez-Hernández, R.; Verdú-Andrés, J.; Campíns-Falcó, P. Trends in Online Intube Solid Phase Microextraction. In Green Extraction Techniques: Principles, Advances and Applications; Ibañez, E., Cifuentes, A., Eds.; Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 76, pp. 575–599. [Google Scholar]
- Abdel-Rehim, M.; Altun, Z.; Blomberg, L. Microextraction in packed syringe (MEPS) for liquid and gas chromatographic applications. Part II—Determination of ropivacaine and its metabolites in human plasma samples using MEPS with liquid chromatography/tandem mass spectrometry. J. Mass Spectrom. 2004, 39, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Silva, C.L.; Castilho, P.C.; Câmara, J.S. An attractive, sensitive and high-throughput strategy based on microextraction by packed sorbent followed by UHPLC-PDA analysis for quantification of hydroxybenzoic and hydroxycinnamic acids in wines. Microchem. J. 2013, 106, 129–138. [Google Scholar] [CrossRef]
- Casado, N.; Perestrelo, R.; Silva, C.L.; Sierra, I.; Câmara, J.S. Comparison of high-throughput microextraction techniques, MEPS and μ-SPEed, for the determination of polyphenols in baby food by ultrahigh pressure liquid chromatography. Food Chem. 2019, 292, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, H.; Xu, Z.; Peng, J.; Zhu, S.; Zhang, H. Development of hyperbranched polymers with non-covalent interactions for extraction and determination of aflatoxins in cereal samples. Anal. Chim. Acta 2013, 797, 40–49. [Google Scholar] [CrossRef]
- Appell, M.; Jackson, M.A. Synthesis and evaluation of cyclodextrin-based polymers for patulin extraction from aqueous solutions. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 117–122. [Google Scholar] [CrossRef]
- Anene, A.; Hosni, K.; Chevalier, Y.; Kalfat, R.; Hbaieb, S. Molecularly imprinted polymer for extraction of patulin in apple juice samples. Food Control 2016, 70, 90–95. [Google Scholar] [CrossRef]
- Zhao, M.; Shao, H.; He, Y.; Li, H.; Yan, M.; Jiang, Z.; Wang, J.; Abd El-Aty, A.M.; Hacimüftüoglu, A.; Yan, F.; et al. The determination of patulin from food samples using dual-dummy molecularly imprinted solid-phase extraction coupled with LC-MS/MS. J. Chromatogr. B 2019, 1125, 121714. [Google Scholar] [CrossRef]
- De Smet, D.; Dubruel, P.; Van Peteghem, C.; Schacht, E.; De Saeger, S. Molecularly imprinted solid-phase extraction of fumonisin B analogues in bell pepper, rice and corn flakes. Food Addit. Contam. 2009, 26, 874–884. [Google Scholar] [CrossRef]
- De Smet, D.; Monbaliu, S.; Dubruel, P.; Van Peteghem, C.; Schacht, E.; De Saeger, S. Synthesis and application of a T-2 toxin imprinted polymer. J. Chromatogr. A 2010, 1217, 2879–2886. [Google Scholar] [CrossRef]
- Jiang, K.; Huang, Q.; Fan, K.; Wu, L.; Nie, D.; Guo, W.; Wu, Y.; Han, Z. Reduced graphene oxide and gold nanoparticle composite-based solid-phase extraction coupled with ultra-high-performance liquid chromatography-tandem mass spectrometry for the determination of 9 mycotoxins in milk. Food Chem. 2018, 264, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Nouri, N.; Sereshti, H.; Farahani, A. Graphene-coated magnetic-sheet solid-phase extraction followed by high-performance liquid chromatography with fluorescence detection for the determination of aflatoxins B1, B2, G1, and G2 in soy-based samples. J. Sep. Sci. 2018, 41, 3258–3266. [Google Scholar] [CrossRef] [PubMed]
- Nouri, N.; Sereshti, H. Electrospun polymer composite nanofiber-based in-syringe solid phase extraction in tandem with dispersive liquid-liquid microextraction coupled with HPLC-FD for determination of aflatoxins in soybean. Food Chem. 2019, 289, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Tezerji, N.S.; Foroughi, M.M.; Bezenjani, R.R.; Jandaghi, N.; Rezaeipour, E.; Rezvani, F. A facile one-pot green synthesis of β-cyclodextrin decorated porous graphene nanohybrid as a highly efficient adsorbent for extracting aflatoxins from maize and animal feeds. Food Chem. 2019, 125747, in press. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Gong, L.; Baibado, J.T.; Dong, W.; Wang, Y.; Dai, Z.; Cheung, H.Y. Graphene based pipette tip solid phase extraction of marine toxins in shellfish muscle followed by UPLC–MS/MS analysis. Talanta 2013, 116, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, P.; Zhang, Q.; Zhang, W.; Ding, X.; Wang, X. Graphene oxide: An adsorbent for the extraction and quantification of aflatoxins in peanuts by high-performance liquid chromatography. J. Chromatogr. A 2013, 1318, 27–34. [Google Scholar] [CrossRef]
- González-Sálamo, J.; Socas-Rodríguez, B.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Core-shell poly (dopamine) magnetic nanoparticles for the extraction of estrogenic mycotoxins from milk and yogurt prior to LC–MS analysis. Food Chem. 2017, 215, 362–368. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Hernández-Borges, J.; Salazar, P.; Martín, M.; Rodríguez-Delgado, M.Á. Core–shell polydopamine magnetic nanoparticles as sorbent in micro-dispersive solid-phase extraction for the determination of estrogenic compounds in water samples prior to high-performance liquid chromatography–mass spectrometry analysis. J. Chromatogr. A 2015, 1397, 1–10. [Google Scholar] [CrossRef]
- McCullum, C.; Tchounwou, P.; Ding, L.S.; Liao, X.; Liu, Y.M. Extraction of aflatoxins from liquid foodstuff samples with polydopamine-coated superparamagnetic nanoparticles for HPLC-MS/MS analysis. J. Agric. Food Chem. 2014, 62, 4261–4267. [Google Scholar] [CrossRef]
- Khodadadi, M.; Malekpour, A.; Mehrgardi, M.A. Aptamer functionalized magnetic nanoparticles for effective extraction of ultratrace amounts of aflatoxin M1 prior its determination by HPLC. J. Chromatogr. A 2018, 1564, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, F.; Wang, C.; Wei, Y. Reversed-phase/weak anion exchange magnetic mesoporous microspheres for removal of matrix effects in lipophilic marine biotoxins analysis by ultrahigh-performance liquid chromatography coupled to tandem mass spectrometry. Food Chem. 2019, 294, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Moreno, V.; Zougagh, M.; Ríos, Á. Hybrid nanoparticles based on magnetic multiwalled carbon nanotube-nano C18 SiO2 composites for solid phase extraction of mycotoxins prior to their determination by LC-MS. Microchim. Acta 2016, 183, 871–880. [Google Scholar] [CrossRef]
- Es’haghi, Z.; Reza Beheshti, H.; Feizy, J. Extraction of aflatoxins from food samples using graphene-based magnetic nanosorbents followed by high-performance liquid chromatography: A simple solution to overcome the problems of immunoaffinity columns. J. Sep. Sci. 2014, 37, 2566–2573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, Y.; Ling, Y.C. Graphene oxide-based magnetic solid phase extraction combined with high performance liquid chromatography for determination of patulin in apple juice. Food Anal. Methods 2017, 10, 210–218. [Google Scholar] [CrossRef]
- Gao, S.; Wu, Y.; Xie, S.; Shao, Z.; Bao, X.; Yan, Y.; Wu, Y.; Wang, J.; Zhang, Z. Determination of aflatoxins in milk sample with ionic liquid modified magnetic zeolitic imidazolate frameworks. J. Chromatogr. B 2019, 1128, 121778. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qiao, X.; Sun, W.; Chen, H.; Chen, X.; Zhang, L.; Wang, T. Effective Extraction of Domoic Acid from Seafood Based on Postsynthetic-Modified Magnetic Zeolite Imidazolate Framework-8 Particles. Anal. Chem. 2019, 91, 2418–2424. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, Z.; Gao, J.; Tong, P.; Liu, W.; Zhang, L. Metal–organic framework UiO-66 modified magnetite@ silica core–shell magnetic microspheres for magnetic solid-phase extraction of domoic acid from shellfish samples. J. Chromatogr. A 2015, 1400, 10–18. [Google Scholar] [CrossRef]
- González-Jartín, J.M.; de Castro Alves, L.; Alfonso, A.; Piñeiro, Y.; Vilar, S.Y.; Gomez, M.G.; Osorio, Z.V.; Sainz, M.J.; Vieytes, M.R.; Rivas, J.; et al. Detoxification agents based on magnetic nanostructured particles as a novel strategy for mycotoxin mitigation in food. Food Chem. 2019, 294, 60–66. [Google Scholar] [CrossRef]
- Wu, C.; He, J.; Li, Y.; Chen, N.; Huang, Z.; You, L.; He, L.; Zhang, S. Solid-phase extraction of aflatoxins using a nanosorbent consisting of a magnetized nanoporous carbon core coated with a molecularly imprinted polymer. Microchim. Acta 2019, 185, 515. [Google Scholar] [CrossRef]
- Tan, L.; He, R.; Chen, K.; Peng, R.; Huang, C.; Yang, R.; Tang, Y. Ultra-high performance liquid chromatography combined with mass spectrometry for determination of aflatoxins using dummy molecularly imprinted polymers deposited on silica-coated magnetic nanoparticles. Microchim. Acta 2016, 183, 1469–1477. [Google Scholar] [CrossRef]
- Hu, M.; Huang, P.; Suo, L.; Wu, F. Polydopamine-based molecularly imprinting polymers on magnetic nanoparticles for recognition and enrichment of ochratoxins prior to their determination by HPLC. Microchim. Acta 2018, 185, 300. [Google Scholar] [CrossRef] [PubMed]
- Turan, E.; Şahin, F. Molecularly imprinted biocompatible magnetic nanoparticles for specific recognition of Ochratoxin A. Sens. Actuators B Chem. 2016, 227, 668–676. [Google Scholar] [CrossRef]
- Lee, T.P.; Saad, B.; Ng, E.P.; Salleh, B. Zeolite Linde Type L as micro-solid phase extraction sorbent for the high performance liquid chromatography determination of ochratoxin A in coffee and cereal. J. Chromatogr. A 2012, 1237, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cudjoe, E.; Vuckovic, D.; Pawliszyn, J. Direct monitoring of ochratoxin A in cheese with solid-phase microextraction coupled to liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 7505–7509. [Google Scholar] [CrossRef]
- Es’haghi, Z.; Sorayaei, H.; Samadi, F.; Masrournia, M.; Bakherad, Z. Fabrication of a novel nanocomposite based on sol–gel process for hollow fiber-solid phase microextraction of aflatoxins: B1 and B2, in cereals combined with high performane liquid chromatography–diode array detection. J. Chromatogr. B 2011, 879, 3034–3040. [Google Scholar] [CrossRef]
- Wu, F.; Xu, C.; Jiang, N.; Wang, J.; Ding, C.F. Poly (methacrylic acid-co-diethenyl-benzene) monolithic microextraction column and its application to simultaneous enrichment and analysis of mycotoxins. Talanta 2018, 178, 1–8. [Google Scholar] [CrossRef]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep. 1999, 11, 737–747. [Google Scholar] [CrossRef]
- Díaz-Bao, M.; Regal, P.; Barreiro, R.; Fente, C.A.; Cepeda, A. A facile method for the fabrication of magnetic molecularly imprinted stir-bars: A practical example with aflatoxins in baby foods. J. Chromatogr. A 2016, 1471, 51–59. [Google Scholar] [CrossRef]
- Romera-Torres, A.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. Analytical methods, occurrence and trends of tropane alkaloids and calystegines: An update. J. Chromatogr. A 2018, 1564, 1–15. [Google Scholar] [CrossRef]
- Ma, C.; Liu, Y.; Zhu, L.; Ji, H.; Song, X.; Guo, H.; Yi, T. Determination and regulation of hepatotoxic pyrrolizidine alkaloids in food: A critical review of recent research. Food Chem. Toxicol. 2018, 119, 50–60. [Google Scholar] [CrossRef]
- López, P.; Pereboom-de Fauw, D.P.; Mulder, P.P.; Spanjer, M.; de Stoppelaar, J.; Mol, H.G.; de Nijs, M. Straightforward analytical method to determine opium alkaloids in poppy seeds and bakery products. Food Chem. 2018, 242, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Prata, M.; Ribeiro, A.; Figueirinha, D.; Rosado, T.; Oppolzer, D.; Restolho, J.; Araújo, A.R.T.S.; Costa, S.; Barroso, M.; Gallardo, E. Determination of opiates in whole blood using microextraction by packed sorbent and gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2019, 1602, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Boojaria, A.; Masrournia, M.; Ghorbani, H.; Ebrahimitalab, A.; Miandarhoie, M. Silane modified magnetic nanoparticles as a novel adsorbent for determination of morphine at trace levels in human hair samples by high-performance liquid chromatography with diode array detection. Forensic Sci. Med. Pathol. 2015, 11, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Ono, M.; Nakajima, T.; Ito, Y.; Aketo, T.; Haginaka, J. Uniformly sized molecularly imprinted polymer for atropine and its application to the determination of atropine and scopolamine in pharmaceutical preparations containing Scopolia extract. J. Pharm. Biomed. Anal. 2005, 37, 231–237. [Google Scholar] [CrossRef]
- Abbasifar, J.; Samadi-Maybodi, A. Selective determination of atropine using poly dopamine-coated molecularly imprinted Mn-doped ZnS quantum dots. J. Fluoresc. 2016, 26, 1645–1652. [Google Scholar] [CrossRef]
- Khataee, A.; Hassanzadeh, J.; Kohan, E. Specific quantification of atropine using molecularly imprinted polymer on graphene quantum dots. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 205, 614–621. [Google Scholar] [CrossRef]
- Zeng, S.; She, Y.; Jiao, B.; Liu, G.; Wang, J.; Su, X.; Ma, X.; Jin, M.; Jin, F.; Wang, S. Molecularly imprinted polymer for selective extraction and simultaneous determination of four tropane alkaloids from Przewalskia tangutica Maxim. fruit extracts using LC-MS/MS. RSC Adv. 2015, 5, 94997–95006. [Google Scholar] [CrossRef]


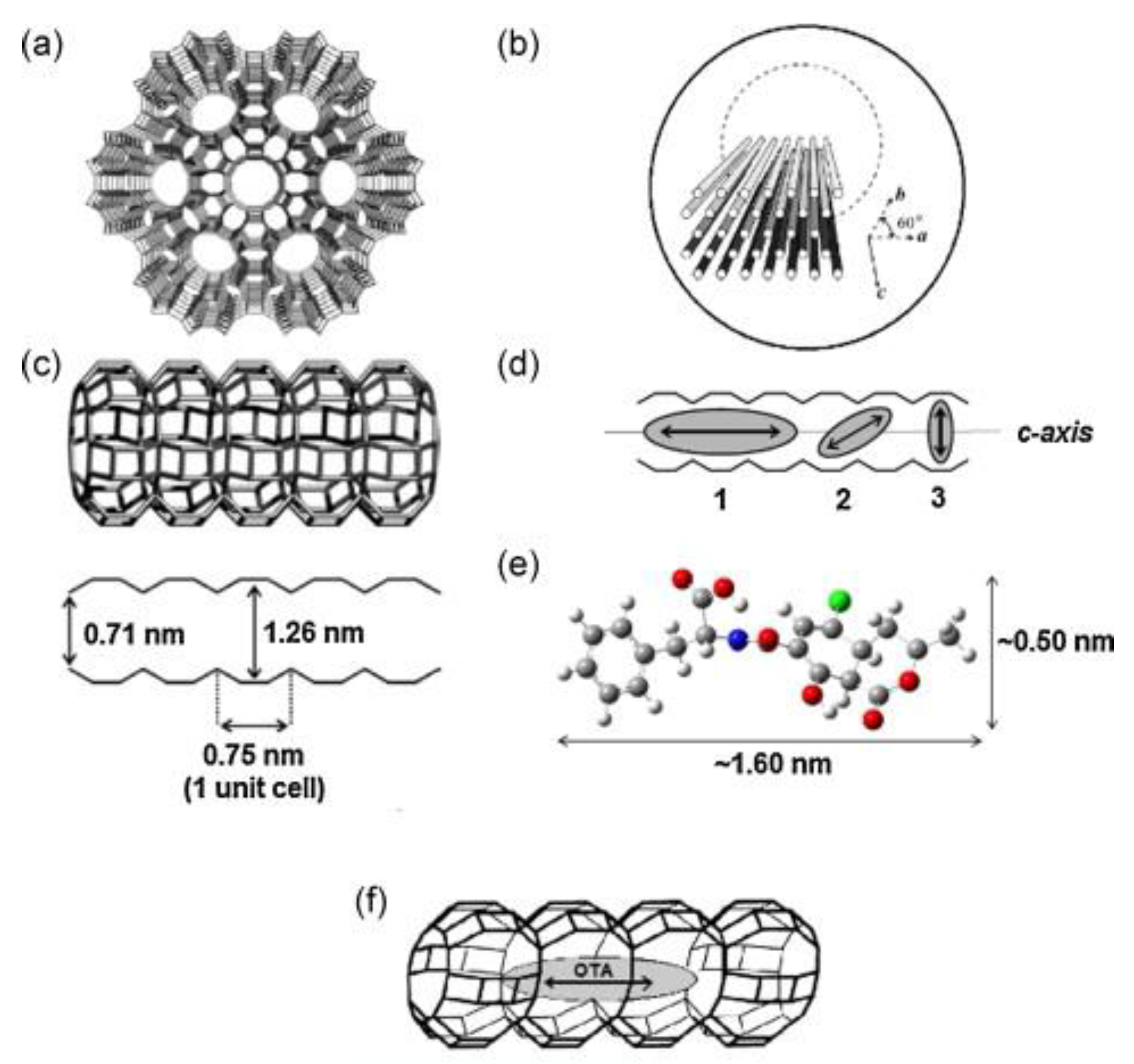
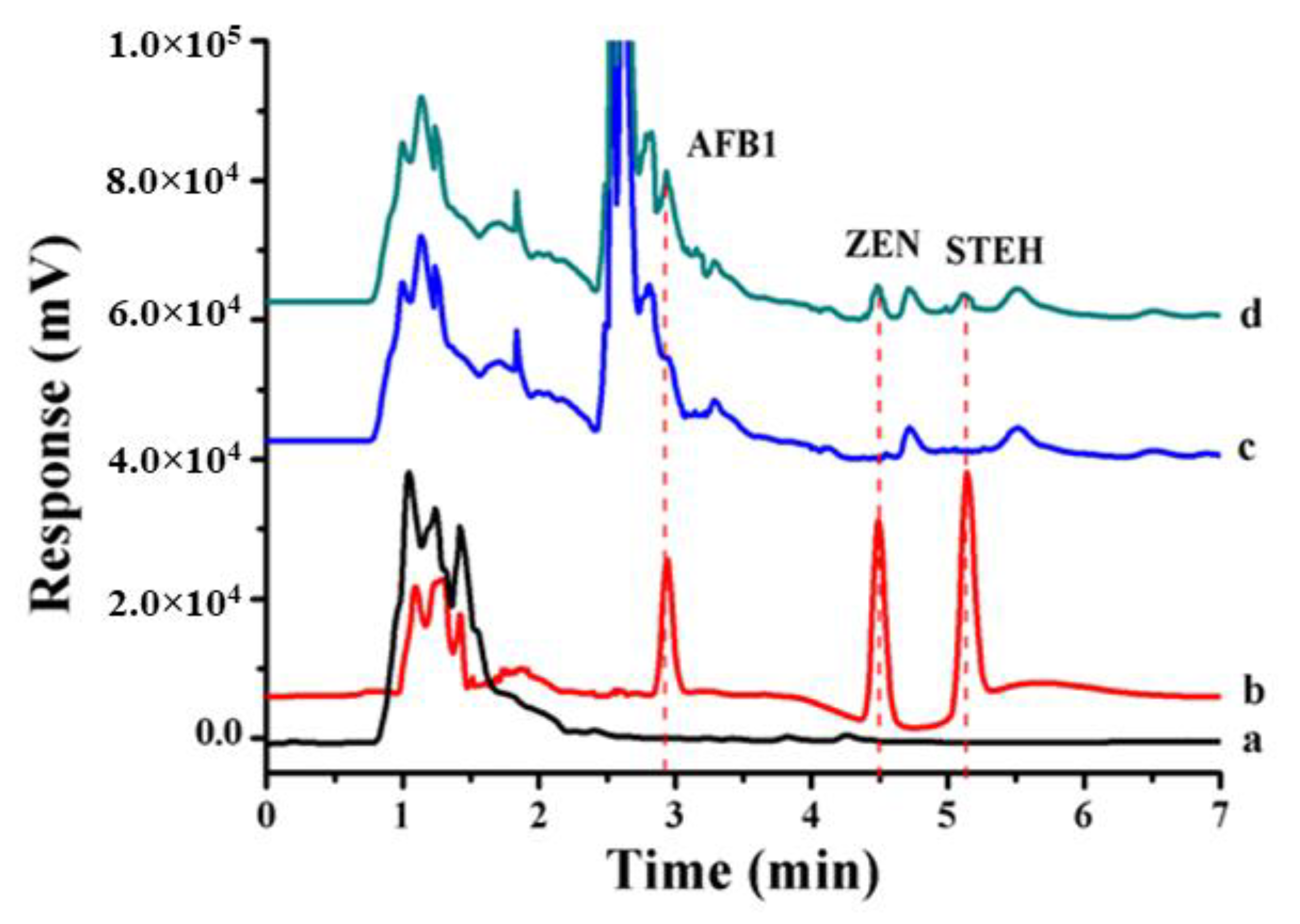
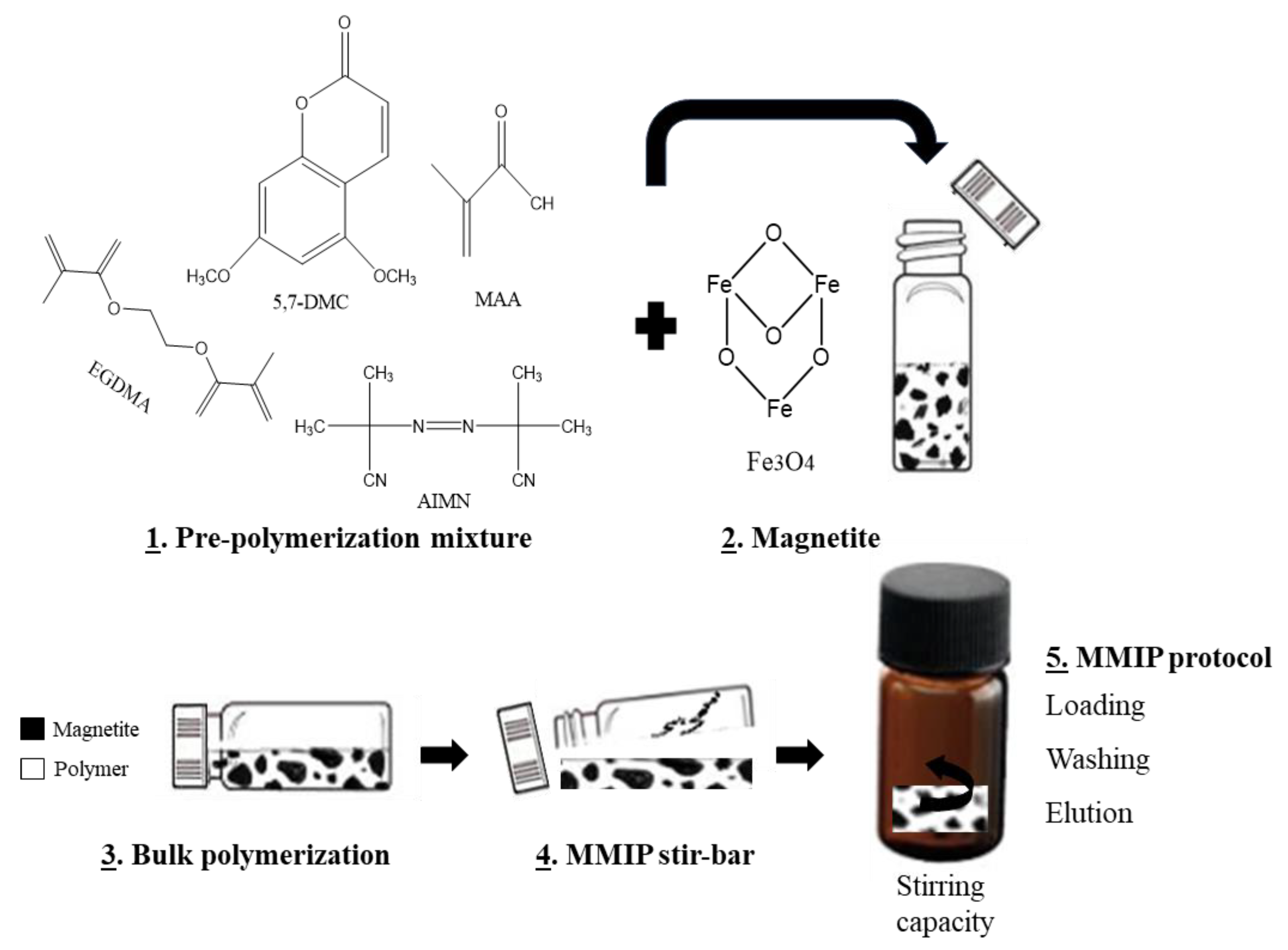
| Food Matrix (Amount) | Analytes | Sample Pretreatment | Microextraction Technique | Analysis | Recovery (%) | LOD | Ref. |
|---|---|---|---|---|---|---|---|
| Cereal flours (2 g) | AF (B1, B2, G1, G2) | Extraction with 10 mL of MeOH/phosphate buffer (80/20, v/v, pH 5.8). Evaporation to dryness and reconstitution with 4 mL of phosphate buffer. An aliquot of the extract (2 mL) subject to microextraction. | SPME Sorbent: Commercial fibers Elution: 0.1 mL MeOH | HPLC-FLD | 49–59 | 0.035-0.2 μg/Kg | [34] |
| Nuts, cereals, dried fruits and spices (0.5 g) | AF (B1, B2, G1, G2) | Extraction with 1 mL of MeOH/H2O (80/20, v/v). An aliquot of the extract (0.1 mL) mixed with 0.1 mL of 50 mM Tris buffer and brought to a total volume of 1 mL with H2O before microextraction. | In-tube SPME * Sorbent: SUPEL-Q PLOT capillary | HPLC-MS | 81–109 | 0.0021-0.0028 μg /L | [35] |
| Fruit juice and dried fruit (1 mL) | PAT | - | In-tube SPME * Sorbent: Carboxen-1006 PLOT capillary | HPLC-MS | > 92 | 0.023 μg /L | [36] |
| Nut and grain samples (0.5 g) | OTA, OTB | Extraction with 1 mL of MeOH/H2O (80/20, v/v). Defatted with 3 mL hexane, supernatant discarded. An aliquot of the clean extract (0.1 mL) brought to a total volume of 1 mL with H2O before microextraction. | In-tube SPME * Sorbent: Carboxen-1006 PLOT capillary | HPLC-MS | 88 | 0.089-0.092 μg /L | [37] |
| Wine (0.05 mL) | OTA | - | In-tube SPME * Sorbent: Luna C18 particles | HPLC-MS/MS | 61–73 | 0.02 μg/L | [38] |
| Powdered infant milk (3 mL) and mineral waters (50 mL) | ZEN, α-ZAL, β-ZAL, α-ZEL, β-ZEL, ZAN | Extraction of milk samples with 0.15 mL acetic acid and 6 mL ACN. Evaporation up to 2.5 mL and reconstitution with H2O to 25 mL, pH adjusted to 3.0 before microextraction. | µ-dSPE Sorbent: 80 mg of MWCNTs Elution: 30 mL MeOH/Acetone (1/1, v/v) | HPLC-MS/MS | 77–120 | 0.05–2.02 µg/L | [39] |
| Peach seed, milk powder, corn flour (0.2 g) and beer (0.2 mL) | AF (B1), OTB, T-2, OTA, ZEN | Microwave assisted extraction of solid samples with 0.2 g NaCl and 5 mL MeOH/H2O (70/30, v/v). An aliquot of the extract (0.2 mL) brought to a total volume of 5 mL with H2O before microextraction. Liquid samples diluted with H2O up to 5 mL before microextraction. | µ-dSPE Sorbent: 12.5 µg zirconia nanoparticles Elution: 0.1 mL MeOH | UHPLC-MS/MS | 84–105 | 0.0022–0.017 µg/L 0.0036–0.033 μg/Kg | [40] |
| Coffee (10 g) and grape juice (10 mL) | OTA | Extraction of coffee samples with 100 mL of carbonate. An aliquot of the extract (10 mL) adjusted to pH 1.5 before microextraction. Grape juice samples adjusted to pH 1.5 before microextraction. | µ-SPE Sorbent: 15 mg AFFINIMIPTM OTA Elution: 0.25 mL MeOH/Acetic acid (98:2, v/v) | HPLC-FLD | 91–101 | 0.02–0.06 μg/Kg | [41] |
| Wine (0.35 mL) | OTA | - | MEPS Sorbent: 4 mg C18 sorbent Elution: 0.05 mL ACN/2% Acetic Acid (90/10, v/v) | HPLC-FLD | 76–108 | 0.08 μg/L | [42] |
| Food Matrix (Amount) | Analytes | Sample Pretreatment | Microextraction Technique | Analysis | Recovery (%) | LOD | Ref. |
|---|---|---|---|---|---|---|---|
| Cereals (5 g) | AF (B1, B2, G1, G2) | Extraction with 25 mL of MeOH/H2O (80/20, v/v). Evaporation of the methanolic fraction of an aliquot of the extract (15 mL). Addition of Britton-Robinson buffer (pH 5.2) up to 3 mL. An aliquot of the extract (2 mL) subject to microextraction. | m-SPE Sorbent: 50 mg hyperbranched polymer Elution: 0.2 mL ACN | HPLC-FLD | 83–103 | 0.012–0.120 μg/Kg | [48] |
| Apple juice (1 mL) | PAT | - | m-SPE Sorbent: 30 mg CD-based polymers Elution: 1 mL Diethyl ether/ACN (4/1, v/v) | HPLC-DAD | n.p. | n.p. | [49] |
| Apple juice (1 mL) | PAT | Dilution with 1 mL of H2O before microextraction. | m-SPE Sorbent: 50 mg SiO2maleicpolymer@MIP Elution: 5 mL de acidified ACN | HPLC-DAD | 82–98 | 8.6 µg/L | [50] |
| Apple, apple juice, hawthorn, hawthorn juice, mixed juice, wines and tomato (10 g) | PAT | Extraction with 10 mL of ACN, 4 mg MgSO4 and 1 g NaCl. An aliquot of the extract (1 mL) evaporated to dryness and reconstituted with 1 mL H2O before microextraction. | m-SPE Sorbent: 30 mg dual dummy-MIP Elution: 3 mL MeOH | HPLC-MS/MS | 81–106 | 0.05–0.2 μg/Kg | [51] |
| Bell pepper, rice and corn flakes (1 g) | F (B1, B2, B3) | Extraction with 6 mL ACN/H2O (84/16, v/v). An aliquot of the extract (1 mL) evaporated to dryness and reconstituted with 1 mL ACN/H2O (90/10, v/v) before microextraction. | m-SPE Sorbent: 20 mg MIP Elution: 1 mL MeOH/Acetic acid (95/5, v/v) | HPLC-MS/MS | 62–86 | 4.5–44 µg/Kg | [52] |
| Maize, barley and oat (5 g) | T-2 | Extraction with 25 mL of ACN/H2O (84/16, v/v). For oat samples, after the solid-liquid extraction, the extract was additionally defatted with 10 mL of hexane. An aliquot of the sample extracts (1 mL) evaporated to dryness and reconstituted with 1 mL MeOH/H2O (20/80, v/v) before microextraction. | m-SPE Sorbent: 50 mg MIP Elution: 3 mL MeOH/Acetic acid (95/5, v/v) | HPLC-MS/MS | 60–73 | 0.4–0.6 µg/Kg | [53] |
| Milk (1 mL) | AF (B1, M1), OTA, ZEN, α-ZEL, β-ZEL, ZAN, α-ZAL, β-ZAL | Extraction with 5 mL ACN with 0.1% formic acid. Supernatant of the extract evaporated to dryness and reconstituted with 0.5 mL ACN/H2O (20/80, v/v) and diluted up to 5 mL with 5 mL of H2O before microextraction. | m-SPE Sorbent: 10 mg rGO/Au Elution: 5 mL MeOH/ACN/Formic acid (50/49/1, v/v/v) | UHPLC-MS/MS | 70–111 | 0.01–0.07 ng/mL | [54] |
| Soy-based foods (2 g) | AF (B1, B2, G1, G2) | Extraction with 10 mL ACN/H2O (75/25, v/v). Diluted up to 50 mL with 10% NaCl aqueous solution before microextraction. | In syringe SPE Sorbent: 30 mg 3DG@Fe3O4 Elution: 0.7 mL MeOH | HPLC-FLD | 83–103 | 0.09–0.15 µg/Kg | [55] |
| Soy-based foods (2 g) | AF (B1, B2, G1, G2) | Extraction with 10 mL ACN/H2O (75/25, v/v). Diluted up to 50 mL with 7% NaCl aqueous solution before microextraction. | In syringe SPE Sorbent: PU/GO nanofibers Elution: 0.75 mL MeOH | HPLC-FLD | 76–101 | 0.09–0.15 µg/Kg | [56] |
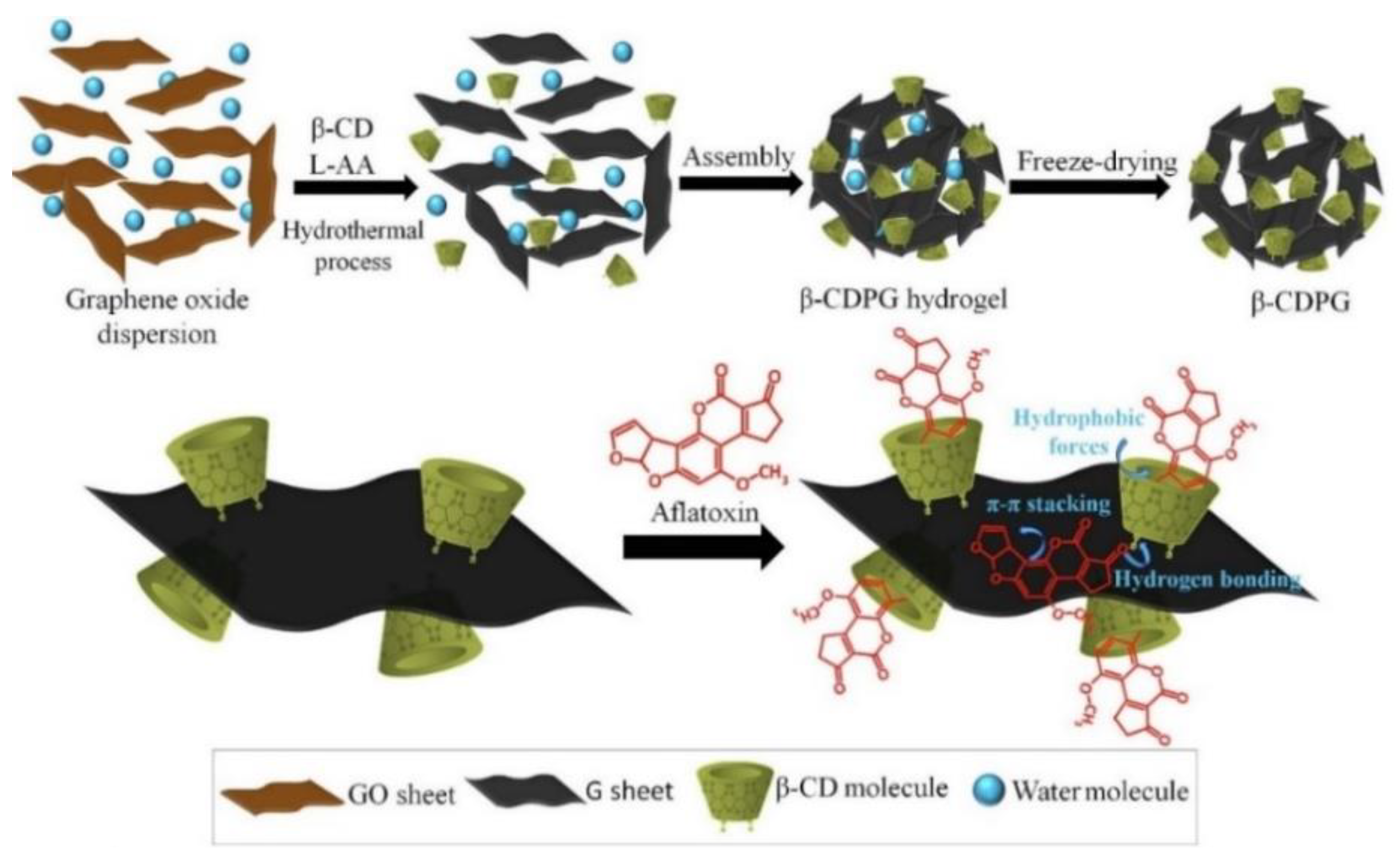
| Maize (5 g) | AF (B1, B2, G1, G2) | Extraction with 20 mL ACN/H2O (80/20, v/v). Evaporation to dryness and reconstituted with 0.1 mL MeOH. Diluted up to 10 mL with H2O before microextraction. | In syringe SPE Sorbent: 15 mg β-CDPG Elution: 2 mL MeOH/DCM (2/1, v/v) | HPLC-FLD | 91–105 | 0.0075–0.030 μg/Kg | [57] |
| Shellfish (0.2 g) | YTX, OA, DTX (1), GYM, SPX (1), PTX (2), AZA (1) | Extraction with 9 mL MeOH. An aliquot of the extract (0.1 mL) evaporated to dryness and reconstituted with 0.2 mL H2O before microextraction. | PT-SPE Sorbent: 2 mg graphene Elution: 2 mL ACN with 0.5% ammonium hydroxide (for basic conditions) or with 0.5% formic acid (for acid conditions) | HPLC-MS/MS | 78–90 | 0.1–1.5 μg/Kg | [58] |
| Peanut (50 g) | AF (B1, B2, G1, G2) | Extraction with MeOH/H2O (80/20, v/v). An aliquot of the extract (8 mL) diluted with H2O before microextraction. | µ-dSPE Sorbent: 5 mg GO Elution: 2 mL MeOH | HPLC-FLD | 85–101 | 0.08–0.65 μg/Kg | [59] |
| Milk and yogurt (1.5 mL) | ZEN, α-ZEL, β-ZEL, ZAN, α-ZAL, β-ZAL | Extraction of milk samples with 3 mL ACN and 0.075 mL acetic acid. Evaporation of the supernatant until 1.5 mL and diluted with H2O up to 25 mL, pH adjusted to 7 before microextraction. Extraction of yogurt samples with 4.5 mL and 0.075 mL acetic acid. The rest of the procedure the same as for milk samples. | µ-MSPE Sorbent: 80 mg Fe3O4@pDA Elution: 8 mL MeOH | HPLC-MS/MS | 70–120 | 0.21–4.77 µg/L | [60] |
| Mineral and tap water (25 mL) | ZEN, α-ZEL, β-ZEL, ZAN, α-ZAL, β-ZAL | Adjustment of pH to 7 before microextraction. | µ-MSPE Sorbent: 60 mg Fe3O4@pDA NPs Elution: 6 mL MeOH | HPLC-MS/MS | 70–119 | 0.02–1.1 µg/L | [61] |
| Red wine (50 mL) | AF (B1, B2, G1, G2) | - | µ-MSPE Sorbent: 4.4 mg PD-MNPs Elution: 0.25 ACN/MeOH (1/1, v/v) | HPLC-MS/MS | 97–108 | 0.0012–0.0031 µg/L | [62] |
| Milk and dairy products (5 mL) | AF (M1) | Extraction with 5 mL hexane and 5 mL MeOH/2 mM NaCl aqueous solution (8/2, v/v) before microextraction. | µ-MSPE Sorbent: 8 mg AMNPs Elution: 2 mL DCM/MeOH/Acetic acid (80/19/1, v/v/v) | HPLC-FLD | 97–116 | 0.2 ng/L | [63] |
| Shellfish (2 g) | AZA (1, 2, 3), OA, DTX (1, 2) | Extraction with 10 mL MeOH/H2O (4/1, v/v). The supernatant mixed with 2 mL hexane, evaporated until 1 mL and addition of 4 mL of H2O before microextraction. | µ-MSPE Sorbent: 50 mg MMM Elution: 2 mL Formic acid/MeOH (5/95, v/v) | UHPLC-MS/MS | 83–119 | 0.4–1.0 μg/Kg | [64] |
| Maize (6 g) | ZEN, α-ZEL, β-ZEL, ZAN, α-ZAL, β-ZAL | Extraction with 24 mL of ACN/H2O (75/25, v/v). The extract diluted up to 25 mL with H2O before microextraction. | µ-MSPE Sorbent: 5 mg MNPs-MWCNT-nanoC18 Elution: 1 mL ACN | HPLC-MS | 92–98 | 0.6–1.0 μg/mL | [65] |
| Rice, wheat and sesame (50 g) | AF (B1, B2, G1, G2) | Extraction of rice and wheat samples with 200 mL Acetone/H2O (50/50, v/v). Elimination of the acetone fraction before microextraction. Extraction of sesame samples with 100 mL hexane and 200 mL Acetone/H2O (50/50, v/v). The rest of the procedure the same as for rice and wheat samples. | µ-MSPE Sorbent: 10 mg MGNP Elution: 2 mL Acetone/H2O (1/1, v/v) | HPLC-FLD | 64–122 | 0.025–0.075 µg/Kg | [66] |
| Apple juice (5 g) | PAT | Extraction with 5 mL ethyl acetate/hexane (96/4, v/v), 1 g NaH2PO4 and 5 g Na2SO4. An aliquot of the organic phase (3 mL) mixed with 0.02 mL acetic acid, evaporated to dryness and reconstituted with 2 mL H2O at pH 6.2 before microextraction. | µ-MSPE Sorbent: 30 mg MGO Elution: 1 mL ACN | HPLC-UV | 69–83 | 2.3 μg/Kg | [67] |
| Milk (20 mL) | AF (B1, B2, G1, G2) | - | µ-MSPE Sorbent: 90 mg M/ZIF-8 Elution: 1 mL ACN/DCM (1/1, v/v) | UHPLC-MS/MS | 79–102 | 2.3–8.1 ng/L | [68] |
| Seafood (5 g) | DA | Extraction with 20 mL MeOH/H2O (1/1, v/v). The resultant sample extract subjected to microextraction. | µ-MSPE Sorbent: 1 mg Fe3O4 SPs@ZIF8/Zn2+ Elution: 0.4 mL 3 mM histidine solution | HPLC-MS/MS | 93−102 | 0.2 ng/L | [69] |
| Shellfish samples (5 g) | DA | Extraction with 20 mL MeOH/H2O (1/1, v/v). The resultant sample extract brought to a total volume of 25 mL with MeOH/H2O (1/1, v/v) before microextraction. | µ-MSPE Sorbent: 1 mg Fe3O4@SiO2@UiO-6 Elution: 1.5 mL ACN with 20% acetic acid | HPLC-MS/MS | 91–107 | 1.45 µg/L | [70] |
| Beer (6 mL) | DON, ZEN, AF (B1, B2, G1, G2), F (B1) | Clean-up with a C18 sorbent. An aliquot of the clean sample (0.1 mL) evaporated to dryness and reconstituted with 0.48 mL ACN/H2O/acetic acid (49/50/1, v/v/v) before microextraction. | µ-MSPE Sorbent: 25 mg MNM Elution: 0.5 mL ACN/H2O/acetic acid (79/20/1, v/v/v) | UHPLC-MS/MS | 87 | n.p. | [71] |
| Corn (25 g) | AF (B1, B2, G1) | Extraction with 5 g NaCl and 125 mL MeOH/H2O (7/3, v/v). An aliquot of the extract (15 mL) mixed with 45 mL of PBS before microextraction. | µ-MSPE Sorbent: 80 mg MNPC Elution: 1.2 mL ACN/H2O (6/4, v/v). | HPLC-FLD HPLC-MS/MS | 75–99 | 0.05–0.07 µg/L | [72] |
| Tea leaves and corn (5 g) | AF (B1, B2, G1, G2) | Extraction with 10 mL ACN/H2O (60/40, v/v). 5 mL of the extract subjected to microextraction. | µ-MSPE Sorbent: 10 mg MMIP Elution: 1 mL ACN/formic acid (95/5, v/v). | UHPLC-MS/MS | 76–95 | 0.05–0.1 μg/Kg | [73] |
| Rice (25 g) and wine (20 mL) | OTA, OTB, OTC | Extraction of rice samples with 100 mL ACN/H2O (60/40, v/v) before microextraction. Wine samples diluted up to 25 mL with a solution of 2.5 M NaCl and 0.24 M NaHCO3 before microextraction. | µ-MSPE Sorbent: 15 mg Fe3O4@PDA MIPs Elution: 1 mL ACN | HPLC-FLD | 71–88 | 0.0018–0.018 µg/Kg | [74] |
| Grape juice | OTA | - | µ-MSPE Sorbent: 5 mg MMIP Elution: - | UV–vis | 97 | 0.374 mg/L | [75] |
| Coffee (10 g) and cereals (5 g) | OTA | Extraction with 10 mL 1% carbonate aqueous solution. Sample extract adjusted to pH 1.5 before microextraction. | µ-SPE Sorbent: 10 mg LTL Elution: 0.4 mL MeOH | HPLC-FLD | 92–101 | 0.09–0.3 μg/Kg | [76] |
| Cheese (0.05 g) | OTA | - | SPME Sorbent: Carbon-tape fiber Elution: 0.15 mL MeOH | HPLC-MS/MS | 93 | 1.5 μg/L | [77] |
| Rice and wheat (10 g) | AF (B1, B2) | Extraction with 1 g NaCl and 100 mL MeOH/H2O (80/20, v/v). Evaporation of the methanolic fraction of the extract and diluted with 40 mL H2O. An aliquot of the extract (25 mL) subject to microextraction. | SPME Sorbent: 50 mg CNT Elution: 2 mL MeOH | HPLC-DAD | 47–103 | 0.061–0.074 μg/L | [78] |
| Rice (2 g) | AF (B1), ZAN, STEH | Extraction with 10 mL ACN/MeOH/H2O (51/9/40, v/v/v), 1.5 g MgSO4 and 0.5 g NaCl. Evaporation to dryness and reconstituted with 3 mL 0.1% TFA/ACN (99/1, v/v) before microextraction. | SPME in-tube * Sorbent: MAA-co-DVB Elution:- | HPLC-PDA | 78–103 | 0.69–2.03 μg/Kg | [79] |
| Milk (1 g) and baby foods (3 g) | AF (B1, B2, G1, G2, M1) | Extraction of milk samples with 3 mL 1% formic acid solution. Supernatant discarded and solid residue extracted with 6 mL chloroform. Evaporation to dryness and reconstitution with 4 mL H2O before microextraction. Baby food samples dissolved with 1% formic acid solution. Supernatant discarded and solid residue extracted with 18 mL chloroform. Evaporation to dryness and reconstitution with 6 mL H2O before microextraction. | SBSE Sorbent: 0.5 g MMIP-SB Elution: 3 mL MeOH/acetic acid (75/25, v/v) | HPLC-MS/MS | 39–60 | 0.3–1.0 ng/Kg | [80] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casado, N.; Gañán, J.; Morante-Zarcero, S.; Sierra, I. New Advanced Materials and Sorbent-Based Microextraction Techniques as Strategies in Sample Preparation to Improve the Determination of Natural Toxins in Food Samples. Molecules 2020, 25, 702. https://doi.org/10.3390/molecules25030702
Casado N, Gañán J, Morante-Zarcero S, Sierra I. New Advanced Materials and Sorbent-Based Microextraction Techniques as Strategies in Sample Preparation to Improve the Determination of Natural Toxins in Food Samples. Molecules. 2020; 25(3):702. https://doi.org/10.3390/molecules25030702
Chicago/Turabian StyleCasado, Natalia, Judith Gañán, Sonia Morante-Zarcero, and Isabel Sierra. 2020. "New Advanced Materials and Sorbent-Based Microextraction Techniques as Strategies in Sample Preparation to Improve the Determination of Natural Toxins in Food Samples" Molecules 25, no. 3: 702. https://doi.org/10.3390/molecules25030702
APA StyleCasado, N., Gañán, J., Morante-Zarcero, S., & Sierra, I. (2020). New Advanced Materials and Sorbent-Based Microextraction Techniques as Strategies in Sample Preparation to Improve the Determination of Natural Toxins in Food Samples. Molecules, 25(3), 702. https://doi.org/10.3390/molecules25030702








