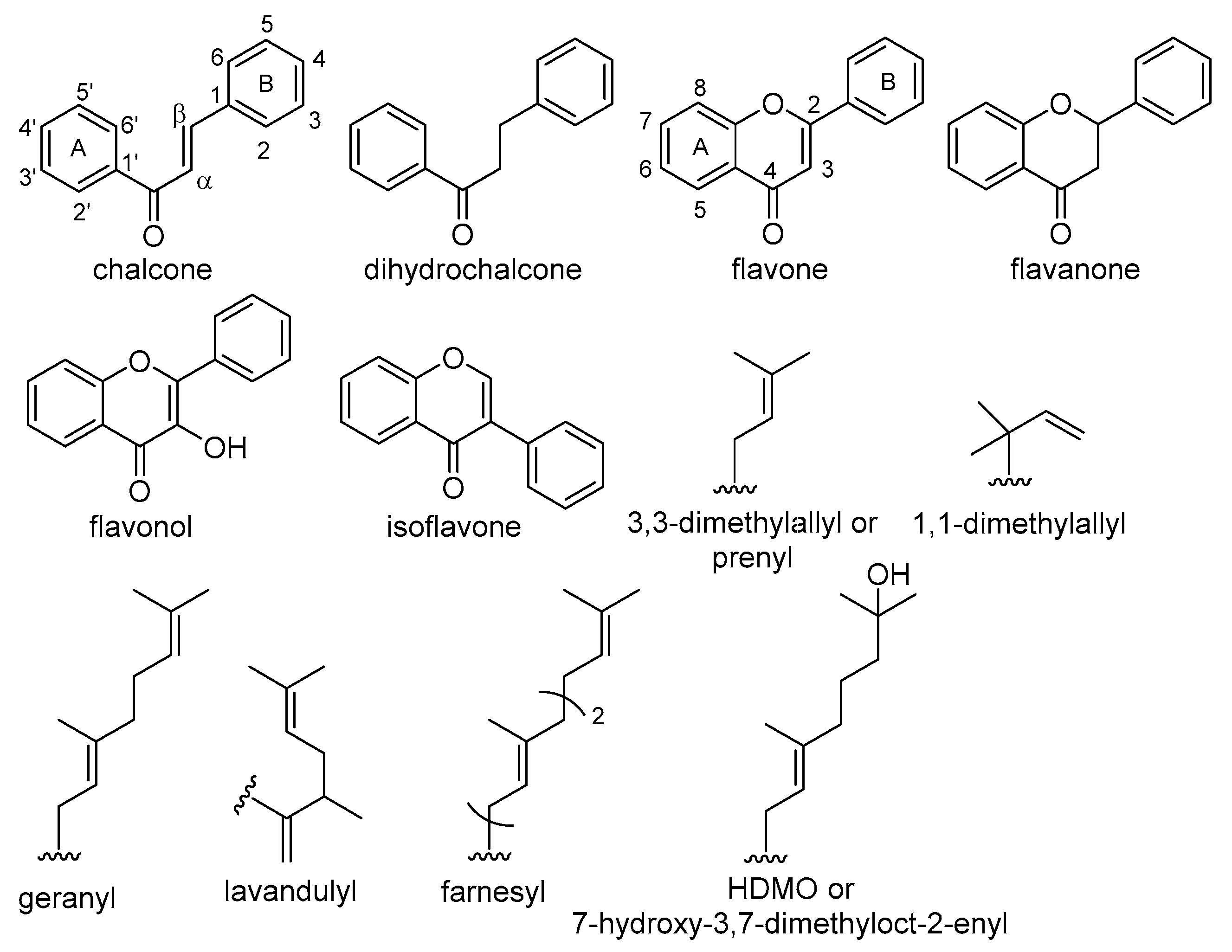
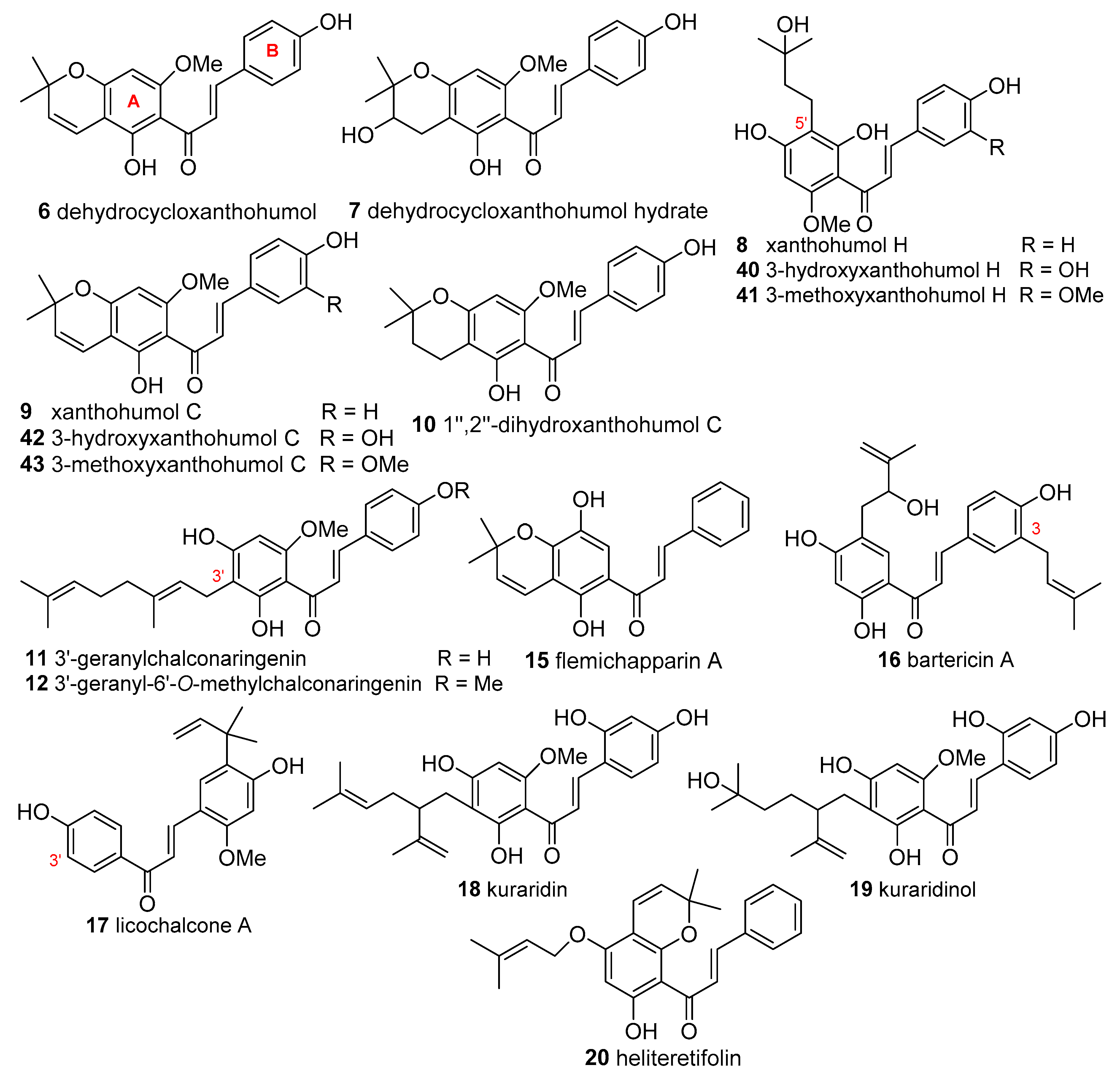
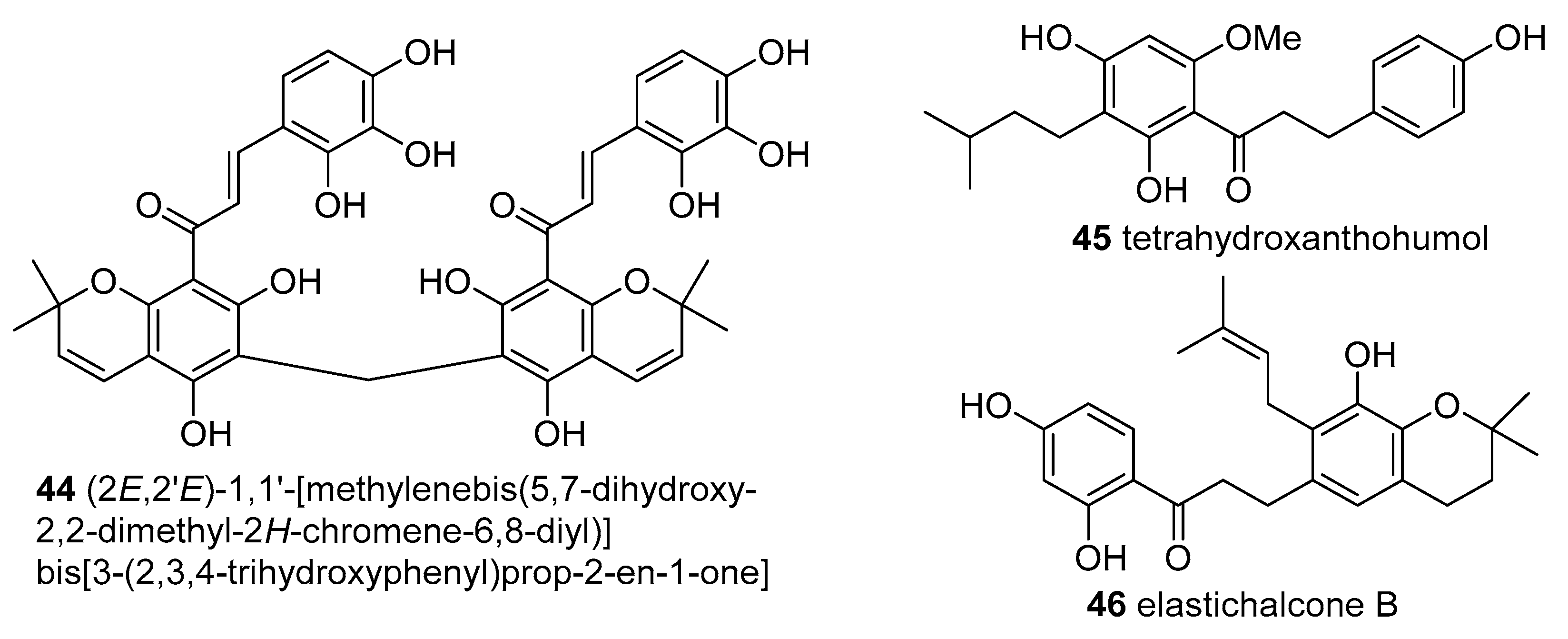
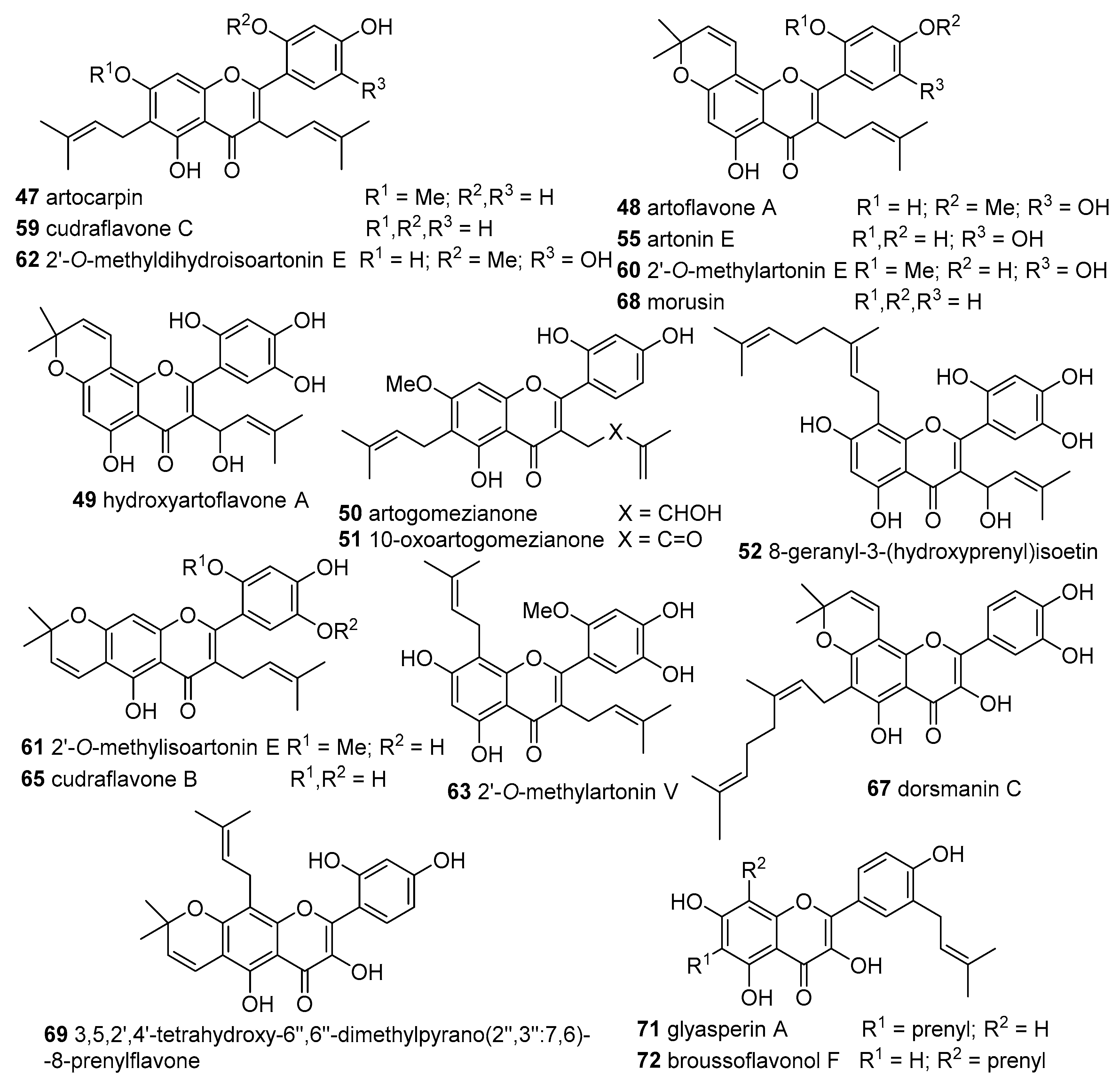
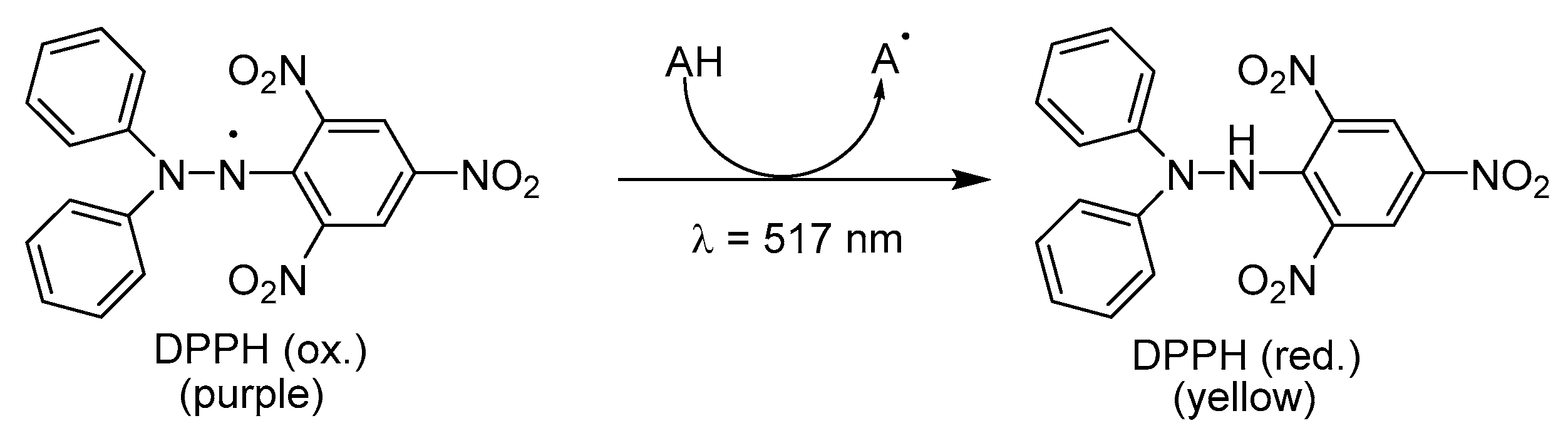
DPPH Radical Scavenging Activity
A great number of prenylated flavonoid derivatives were evaluated for their ability to scavenge DPPH
●. Ko et al. evaluated DPPH
● decolorization induced by xanthone-type cycloheterophyllin (
143), artonin A (
144) and artonin B (
145), isolated from the plant
A. heterophyllus Lam. The scavenging activity was expressed as the concentration (IC
0.20) of the test compounds that induced a decrease of 0.20 in absorbance in a 30 min period of time. The results pointed out that
143–
145 increased DPPH
● decolorization in a concentration-dependent manner with IC
0.20 of 9.6 ± 0.7, 8.4 ± 0.3, and 12.2 ± 0.6 μM, respectively. The positive control α -tocopherol scavenged DPPH
● with an IC
0.20 of 11.9 ± 0.2 μM [
31].
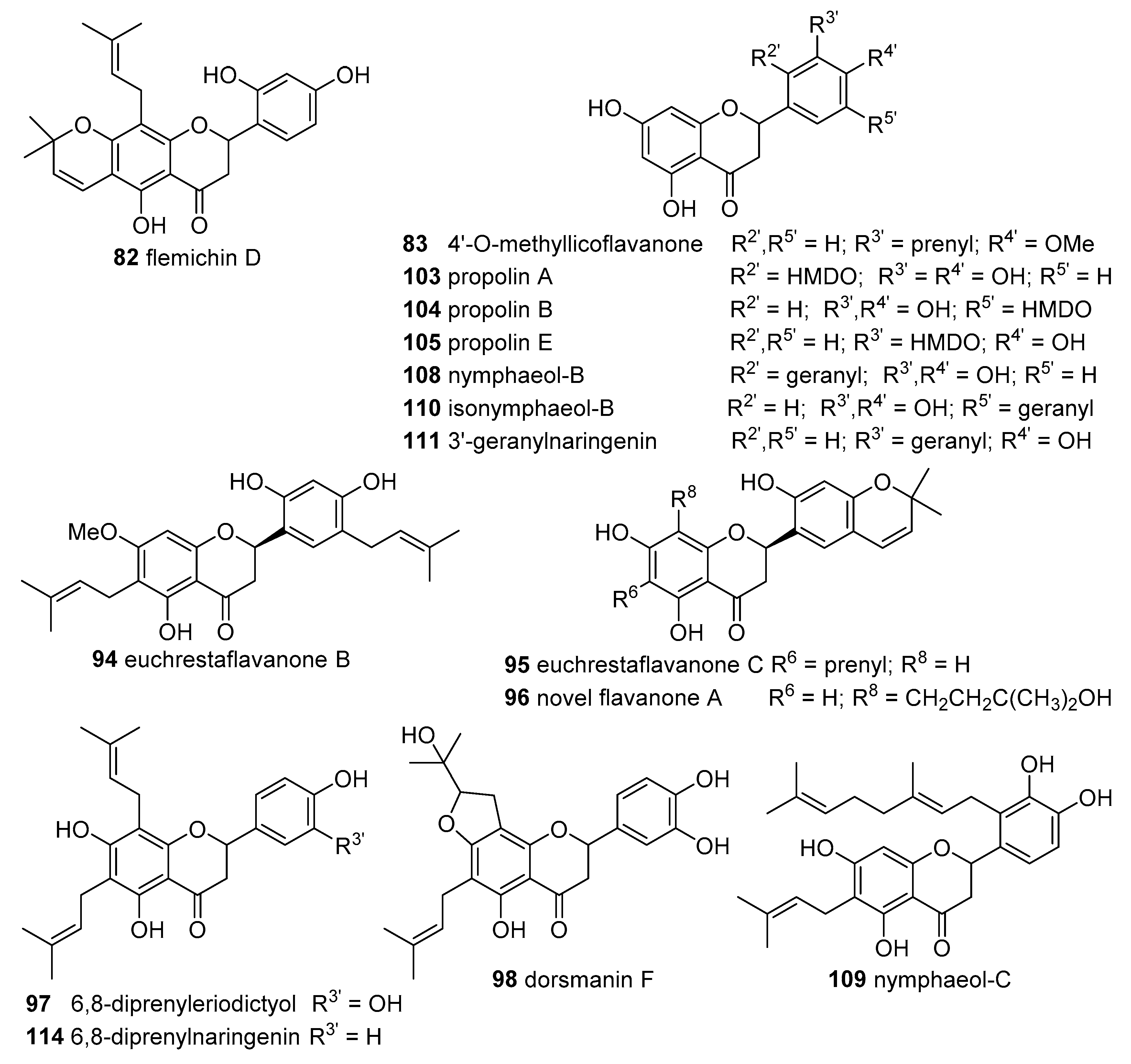
The flavanones 6,8-diprenyleriodictyol (
97) and dorsmanin F (
98) and the flavonol dorsmanin C (
67) were isolated from the twig and leaf of
D. mannii samples collected in Central Province of Cameroon. The DPPH
● scavenging activity assay was performed in experiments of only 20 min since a plateau at 15 min was reached. The prenylated flavonoids were tested in concentrations of 1, 10 and 100 μM, causing a rapid decrease in the absorbance, dependent of the concentration, and compared with butylated hydroxytoluene (BHT), a common antioxidant used as a food additive. The potency of DPPH
● scavenging activity followed the order:
67 >
97 >
98 >> BHT. These results seem to indicate that the C2=C3 double bond and the 3-OH group of the flavonol are important features for the high scavenging potency, when compared with the flavanones [
37].
Two flavanones, propolin A (
103) and propolin B (
104), were isolated and characterized from Taiwanese propolis glue collected from hives located in the area of Bagwa Shan, Taiwan. Both compounds were tested in concentrations ranging from 3.125 to 25 μg/mL and exhibited strong scavenging effects against DPPH
● with IC
50 values of 5.0 and 9.0 μg/mL, respectively [
48].
Omisore et al. tested the DPPH
● scavenging effects of five flavonoids from
Dorstenia species: the chalcones isobavachalcone (
14) and bartericin A (
16) isolated from
D. barteri, the flavone 6-prenyl-apigenin (
66) from
D. kameruniana, and the flavanones 6,8-diprenyleriodictyol (
97) and dorsmanin F (
98) isolated from
D. mannii. The concentration needed to decrease the remaining DPPH
● by 50% (the initial substrate concentration EC
50) was the parameter used to measure the antioxidant capacity. Thus bartericin A (
16, EC
50 47.85 ± 2.15 μg/mL) and 6,8-diprenyleriodictyol (
97, EC
50 32.12 ± 1.10 μg/mL) as well as the positive controls quercitrin (EC
50 28.16 ± 0.84 μg/mL) and ascorbic acid (EC
50 19.33 ± 0.3 μg/mL) showed high antioxidant capacity (EC
50 < 50 μg/mL), while isobavachalcone (
14, EC
50 84.33 ± 0.27 μg/mL), 6-prenylapigenin (
66, EC
50 86.43 ± 0.26 μg/mL) and dorsmanin F (
98, EC
50 53.89 μg/mL) presented moderate antioxidant capacity (EC
50 > 50 μg/mL). The scavenging effects followed the order: ascorbic acid > quercitrin > 6,8-diprenyleriodictyol (
97) > bartericin A (
16) > dorsmanin F (
98) > isobavachalcone (
14) > 6-prenylapigenin (
66) [
20]. A detailed overview on the natural occurrence, synthesis, biosynthesis and pharmacological properties of isobavachalcone (
14) was published by Kuete and Sandjo in 2012 [
57].
From the root bark of
Cudrania tricuspidata (Carr.) Bureau collected in Hyoupchun, Korea it was possible to isolate two flavones—cycloartocarpesin B (
56) and cudraflavone B (
65)—and three flavanones—euchrestaflavanone B (
94), euchrestaflavanone C (
95) and a novel flavanone A (
96). None of the isolated flavonoids were effective against DPPH
●, presenting only 10% scavenging activity at 300 μM concentration [
38].
Kumazawa et al. isolated nine flavanones (propolin A (
103), propolin B (
104), prokinawan (
106), propolin E (
105), nymphaeol A (
107), nymphaeol B (
108), nymphaeol C (
109), isonymphaeol B (
110) and 3′-geranylnaringenin (
111)) from propolis collected in Okinawa, Japan. The antioxidant properties against DPPH
● at concentrations ranging from 3.125 to 100 μM, after 1 h of incubation were examined. BHT, α-tocopherol, and eriodictyol were tested as positive controls. A strong DPPH
● scavenging activity was recorded for compounds
103,
104 and
106–
110 with IC
50 values between 5.2 and 10.9 μM, similar to those obtained for the positive controls BHT (IC
50 16.8 ± 2.7 μM), α-tocopherol (IC
50 11.4 ± 0.9 μM) and eriodictyol (IC
50 4.7 ± 0.7 μM). Propolin E (
105) and 3′-geranylnaringenin (
111) showed IC
50 values of 62.6 ± 2.2 and 64.2 ± 3.5 μM, respectively. The high scavenging effects of these compounds may be related to the catechol unit present in such structures, an important characteristic for the antioxidant activity of flavonoids [
47].
Five flavones—artonin E (
55), 2′-
O-methylartonin E (
60), 2′-
O-methylisoartonin E (
61), 2′-
O-methyldihydroisoartonin E (
62) and 2′-
O-methylartonin V (
63)—and two xanthone-type compounds—artobiloxanthone (
150) and cycloartobiloxanthone (
140)—were obtained from the root bark of
A. nobilis collected in Central Province of Sri Lanka. The authors claimed to have evaluated the DPPH
● scavenging potential by a TLC bio-autography method and that all the compounds were strong scavengers, but in fact no data was published in the manuscript [
33].
Jung et al. isolated from
Sophora flavescens collected in Kyeong Buk Province, Korea eight flavonoids: the chalcones kuraridin (
18) and kuraridinol (
19), the flavonol kushenol C (
70) and the flavanones leachianone (
89), kushenol E (
90,), sophoraflavanone G (
91), kurarinone (
92) and kurarinol (
93). The DPPH
● decolorization was investigated at 520 nm after 30 min of reaction. The results pointed out that flavanones
89–
93 were ineffective scavengers, with percentages of inhibition less than 50 at a concentration of 200 μg/mL. On the other hand, flavonol
70 showed the highest scavenging activity with an IC
50 value of 10.67 ± 0.23 μM, similar to that of the positive control, L-ascorbic acid (IC
50 8.70 ± 0.22 μM) followed by chalcone
19 and chalcone
18 with IC
50 values of 86.23 ± 2.44 and 111.77 ± 0.72 μM, respectively [
22]. Once again, the high scavenging activity of flavonol compared to flavanones can to be related to the presence the C2=C3 double bond and the 3-OH group in the flavonol skeleton. In another study, sophoraflavanone G (
91) and kurarinone (
92) exhibited IC
50 values of 5.26 and 7.73 μg/mL, respectively, in DPPH
● scavenging assay, with no mention to positive control data [
23].
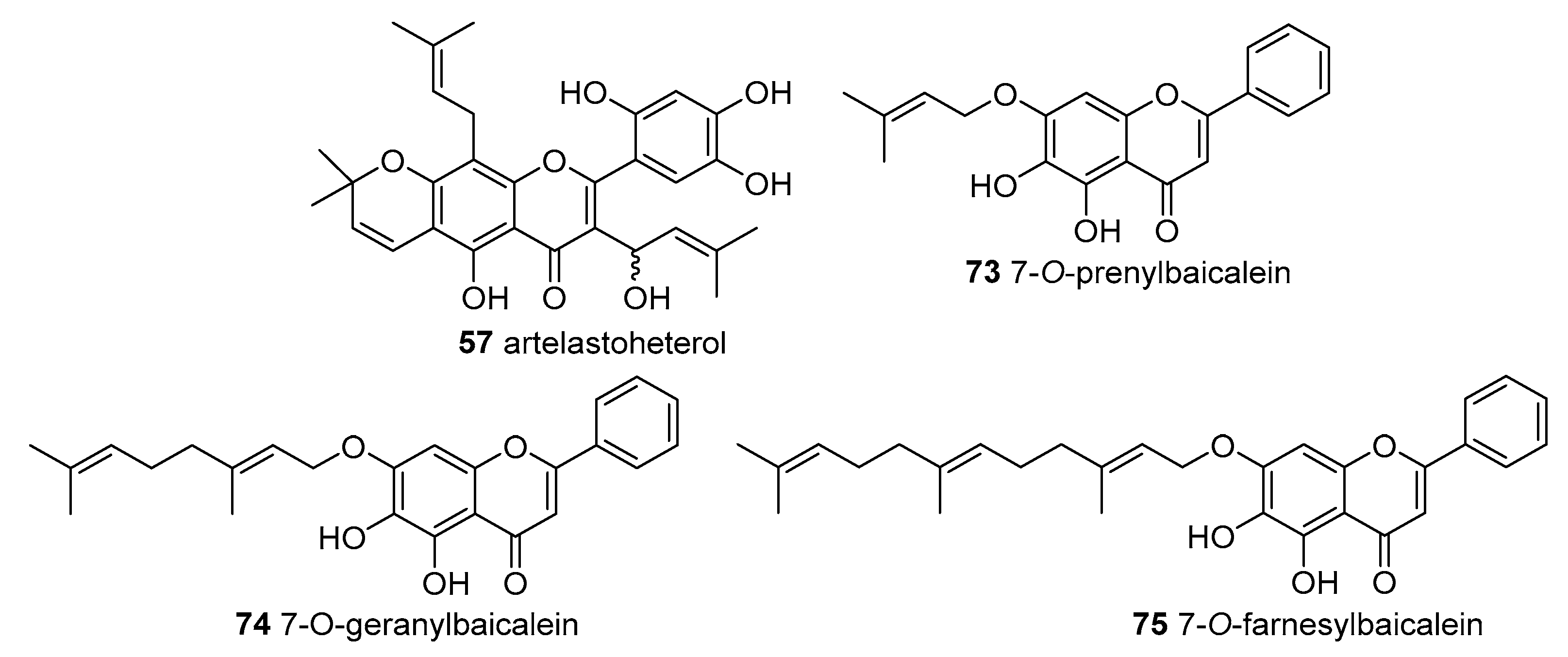
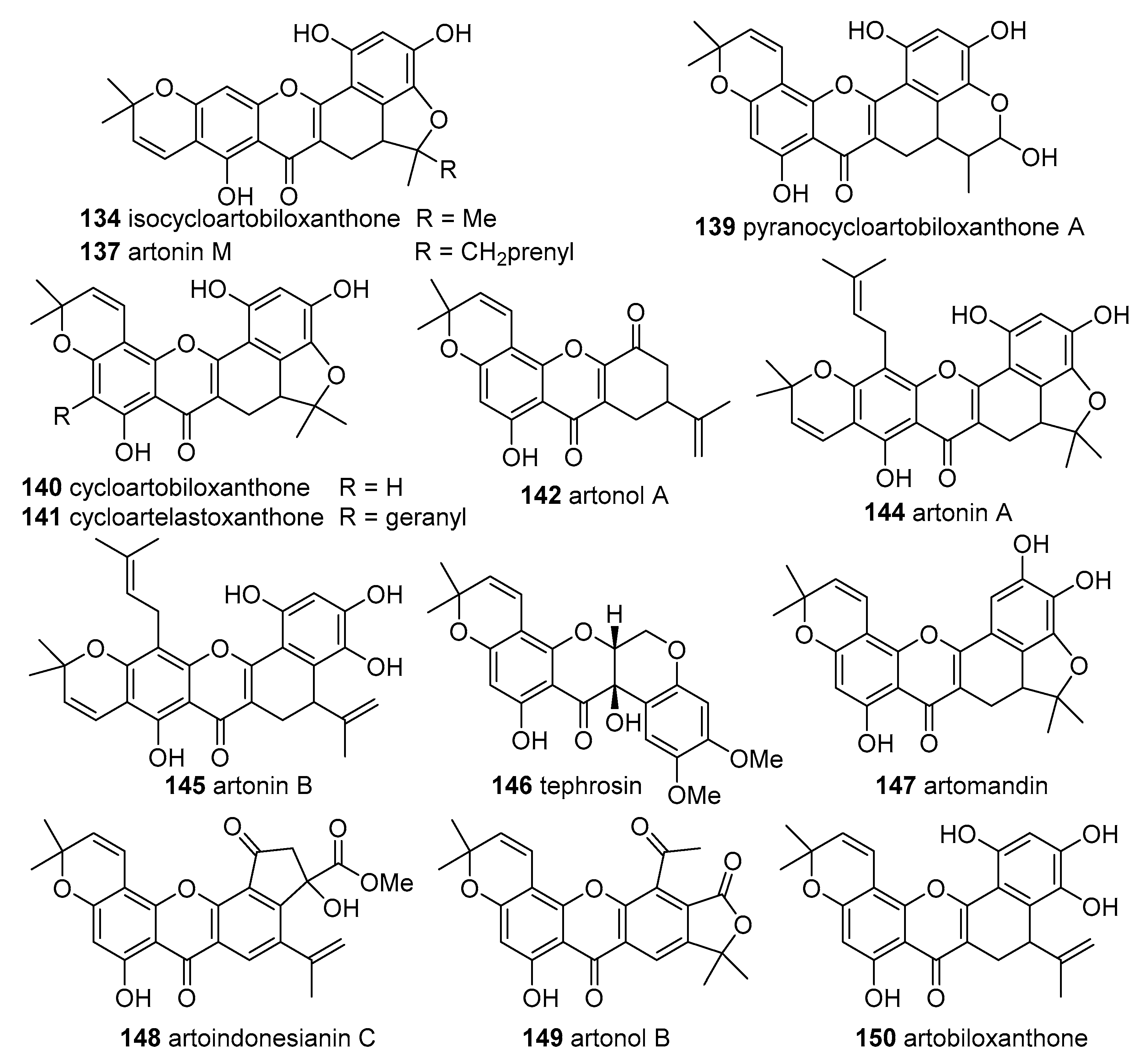
Six derivatives were isolated from
Artocapus species and their DPPH
● scavenging activity investigated. The flavone artoflavone A (
48) and the xanthone-type compound cyclogeracommunin (
133) were isolated from the cortex of roots of
A. communis collected at Kaohsiung Hsien, Taiwan [
34]. The flavone artelastoheterol (
57) and the xanthone-type compounds cycloartobiloxanthone (
140), cycloartelastoxanthone (
141) and artonol A (
142) were isolated from root bark of
A. elasticus collected at Ping-Tung Hsien, Taiwan [
58]. Cyclogeracommunin (
133) and artonol A (
142) were not able to scavenge DPPH
●. Artoflavone A (
48), artelastoheterol (
57), cycloartobiloxanthone (
140) and cycloartelastoxanthone (
141) exhibited scavenging activity in a concentration-dependent manner with IC
50 values of 24.2 ± 0.8, 42.2 ± 2.8, 26.8 ± 1.2 and 18.7 ± 2.2 μM, respectively. In addition, the IC
50 values of the positive controls BHT and α-tocopherol were 80.0 ± 10.9 and 18.1 ± 1.5 μM, respectively. From the results we can state that compounds with a 2,2-dimethylpyran ring substituted at C-7 and C-8 of the flavonoid, such as derivatives
48,
140 and
141, enhanced the DPPH
● scavenging activity of the prenylated flavonoids [
34].
Pyranocycloartobiloxanthone A (
139) was isolated from the stem bark of the endemic and rare
A. obtusus collected from Sarawak, Malaysia and showed strong DPPH
● scavenging activity with an IC
50 value of 2.0 μg/mL. No information was given for the positive control [
55].
Rahman et al. isolated two prenylated isoflavones from the stem bark of
E. variegata collected at Dhaka, Bangladesh and evaluated their DPPH
● scavenging potential. 4′,5,7-Trihydroxy-8-prenyl isoflavone (
124) and alpinum isoflavone (
119) demonstrated high antioxidant activity, having IC
50 values of 6.42 ± 1.36 and 8.30 ± 1.41 μg/mL, respectively, similar to that obtained by the positive control,
tert-butyl-1-hydroxytoluene (BHT, IC
50 5.88 μg/mL) [
51]. Very recently, a pharmacological overview on alpinum isoflavone (
119) has been published by Ateba et al. [
59].
Three xanthone-type derivatives were isolated from the stem bark of
A. kemando: artomandin (
147), artoindonesianin C (
148) and artonol B (
149). Although
147 scavenged DPPH
● with an IC
50 of 38.0 μg 6.4 μg/mL, it was considerably lower effect than the positive control vitamin C (IC
50 12.2 μg/mL). In addition, artoindonesianin C (
148) and artonol B (
149) were weak scavengers, with IC
50 values above 120 μg/mL [
54].
5,7-Dihydroxy-6-methyl-8-prenylflavanone (
84) 5,7-dihydroxy-4′-methoxy-6-methyl-8-prenyl-flavanone (
85), 5,7-dihydroxy-6-prenylflavanone (
86), 5-dihydroxy-7-methoxy-6-prenylflavanone (
87), and 5,7-dihydroxy-4′-methoxy-8-prenylflavanone (
88) were isolated from leaves of
E. platycarpa. The scavenging effects were determined using concentrations of 10, 100 and 1000 μM of each compound and the results showed that the scavenging potential is directly proportional to the concentration of the prenylated flavanones. Moreover, flavanones did not show a remarkable reduction of DPPH
● being flavanone
88 the most active, with a 43.1 ± 3.9% of reduction for a 1000 μM concentration (the positive control quercetin presented 92.9% of reduction) [
45].
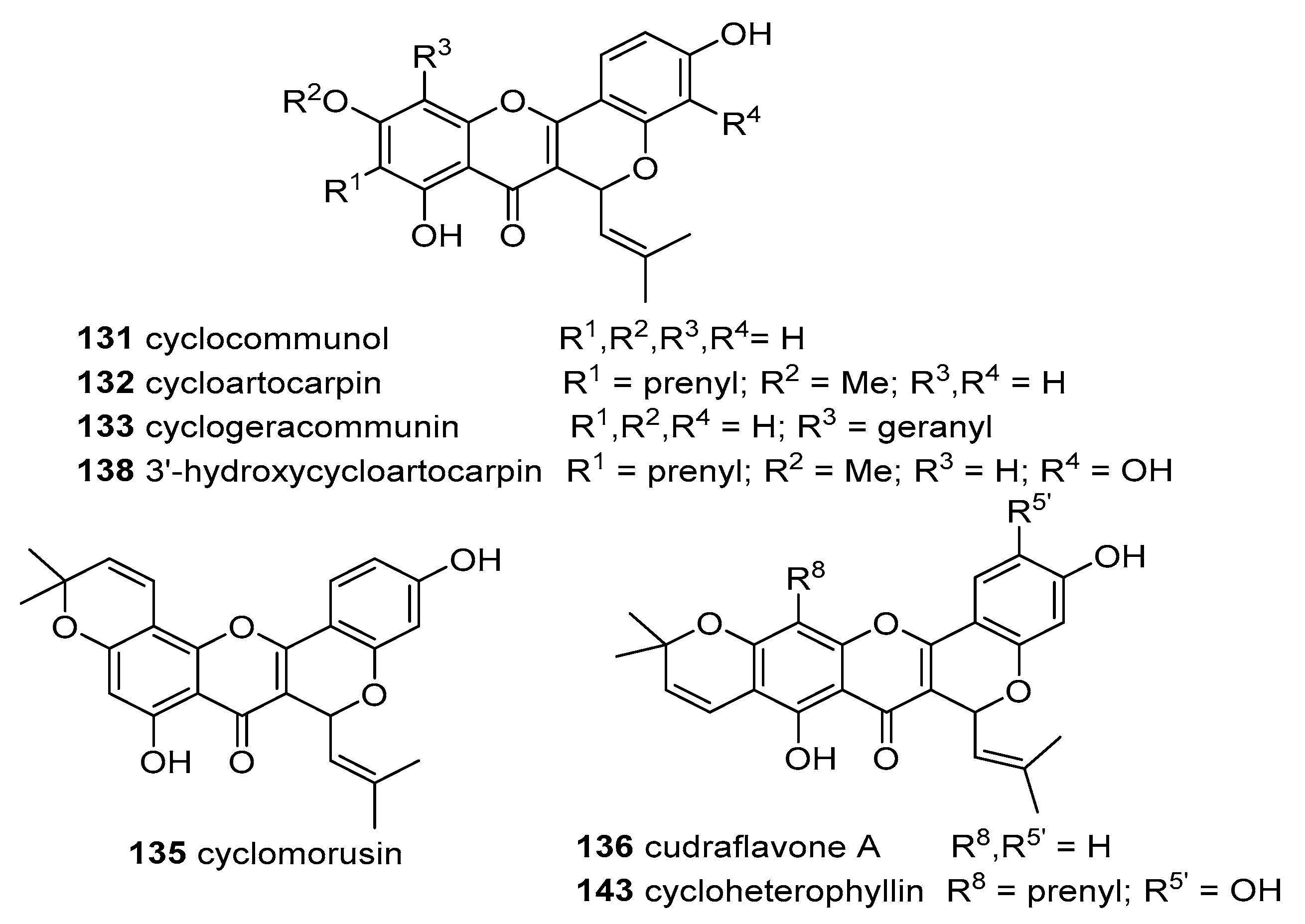
Lan et al. isolated 14 derivatives from the heartwood and cortex of
A. altilis and determined their DPPH scavenging effects. Seven flavones—artocarpin (
47), artoflavone A (
48), hydroxyartoflavone A (
49), artogomezianone (
50), 10-oxoartogomezianone (
51), 8-geranyl-3-(hydroxyprenyl)isoetin (
52), 8-geranylapigenin (
53)—and seven xanthone-type compounds—cyclocommunol (
131), cyclo-artocarpin (
132), cyclogeracommunin (
133), isocycloartobiloxanthone (
134), cyclomorusin (
135), cudraflavone A (
136) and artonin M (
137). Most of the tested compounds presented a weak scavenging effect with IC
50 > 300 μM, except for hydroxyartoflavone A (
49, IC
50 20.9 ± 2.1 μM) > isocycloartobiloxanthone (
134, IC
50 33.9 ± 1.5 μM) > artoflavone A (
48 (15), IC
50 53.5 ± 3.1 μM). Even so, these compounds are weaker scavengers than the positive control, quercetin (IC
50 10.2 ± 1.4 μM) [
35].
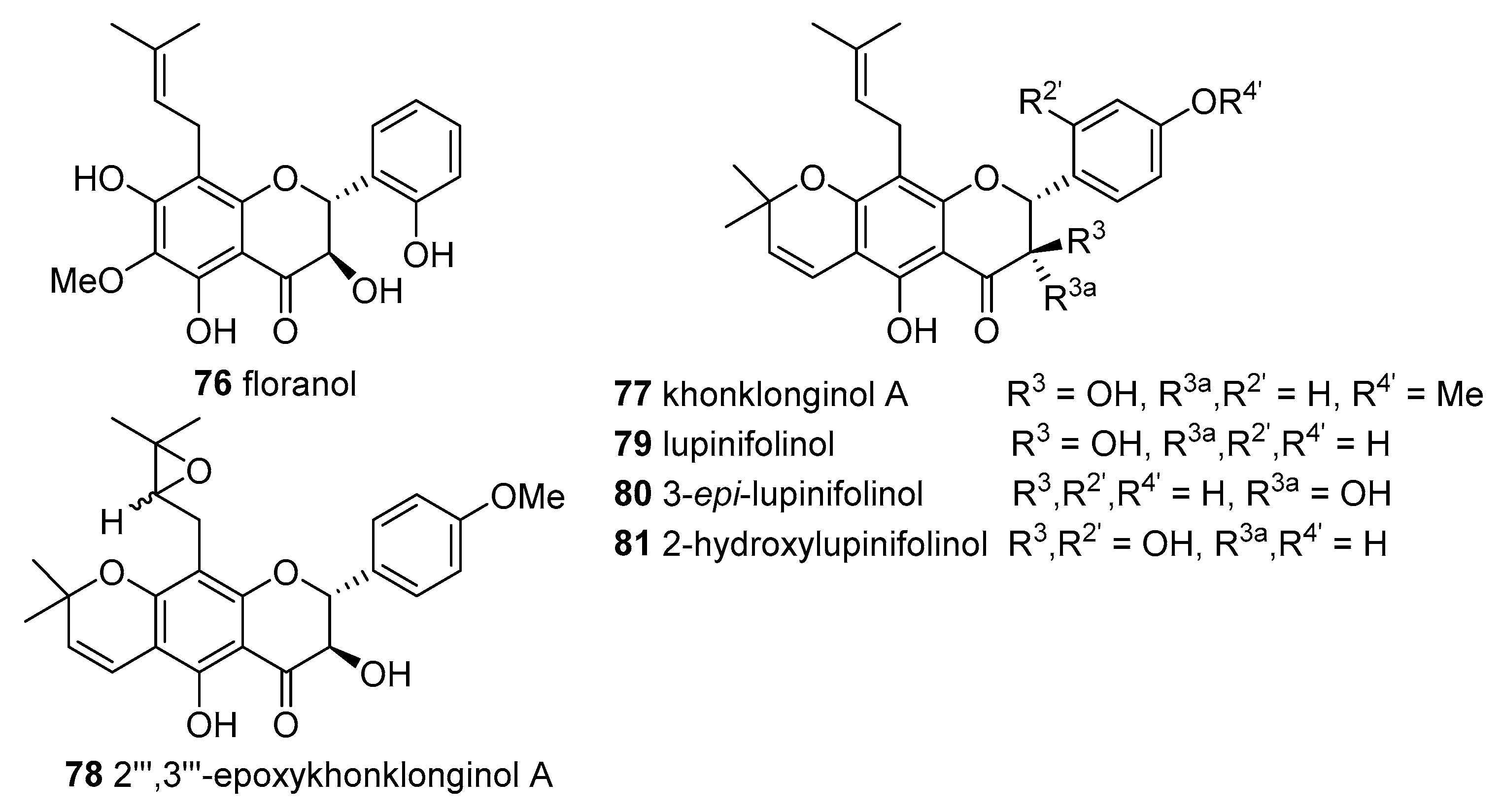
From the roots of
E. chinense collected at Ubonratchathani Province, Thailand, was isolated one flavonol 3,5,2′,4′-tetrahydroxy-6″,6″-dimethylpyrano(2″,3″:7,6)-8-(3‴,3‴-dimethylallyl)flavone (
69) and six flavanones—khonklonginol A (
77), 2‴,3‴-epoxykhonklonginol A (
78), lupinifolinol (
79), 3-
epi-lupinifolinol (
80), 2-hydroxylupinifolinol (
81) and flemichin D (
82)—and their antioxidant activity was investigated using the DPPH
● scavenging system. Flavonol
69 was the most active compound showing an IC
50 value of 35 ± 1 μM, similar to the obtained for BHT (IC
50 39 ± 1 μM). The order of efficacy for the remaining compounds were:
81 (252 ± 1.1) >
82 (538 ± 24) >
80 (681 ± 11) >
79 (1768 ± 210) >
78 (2553 ± 207) >
77 (7919 ± 55) μM [
40].
Elastichalcone B (
45) and cycloartocarpesin B (
56) 3 isolated from the leaves of
A. elasticus collected from Ulu Langat, Malaysia exhibited DPPH
● scavenging activity with IC
50 values of 11.30 and 11.89 μg/mL, respectively. No data concerning any positive control was given by the authors [
30].
Three isoflavones were isolated from the aerial parts of
Azorella madreporica collected in Chile: alpinum isoflavone (
119), angustone C (
130) and 4′-acetylalpinum isoflavone (
129). These compounds demonstrated modest DPPH
● scavenging activity in the following order:
130 (IC
50 134.61 ± 0.67 μM) >
119 (IC
50 160.75 ± 0.41 μM) >
129 (IC
50 309.87 ± 0.90 μM). Quercetin was used as positive control and reached an IC
50 value of 24.93 ± 0.03 μM [
53].
The chalcone xanthohumol (
1) extracted from hop pellets (
Humulus lupulus) did not show any scavenging effect on DPPH
● scavenging system [
17]. One chalcone isobavachalcone (
14), two flavones—4′,5-dihydroxy-6,7-(2,2-dimethylpyrano)-2′-methoxy-8-(γ,γ-dimethyl)allylflavone (
54) and artocarpin (
47)—one flavanone: 5,7-dihydroxy-4′-methoxy-8-prenylflavanone (
88), three xanthones—3′-hydroxycycloartocarpin (
138), pyranocycloartobiloxanthone A (
139) and cycloartocarpin (
132)—and the miscellaneous derivative chaplashin (
153) were isolated for the first time from the leaves and the heartwoods of
A. anisophyllus Miq collected in Malasya. Pyranocycloartobiloxanthone A (
139), artocarpin (
47) and 3′-hydroxycycloartocarpin (
138) were the most active DPPH
● scavengers with SC
50 (concentration required to produce 50% stimulation) values of 20.2, 140.0 and 152.9 μg/mL, respectively. Isobavachalcone (
14), flavone
54 and chaplashin (
153) were poor scavengers with SC
50 values superior to 400 μg/mL and no scavenging activity was recorded for flavanone
88 and cycloartocarpin (
132). BHA was used as positive control, with a SC
50 value of 17.5 μg/mL [
18].
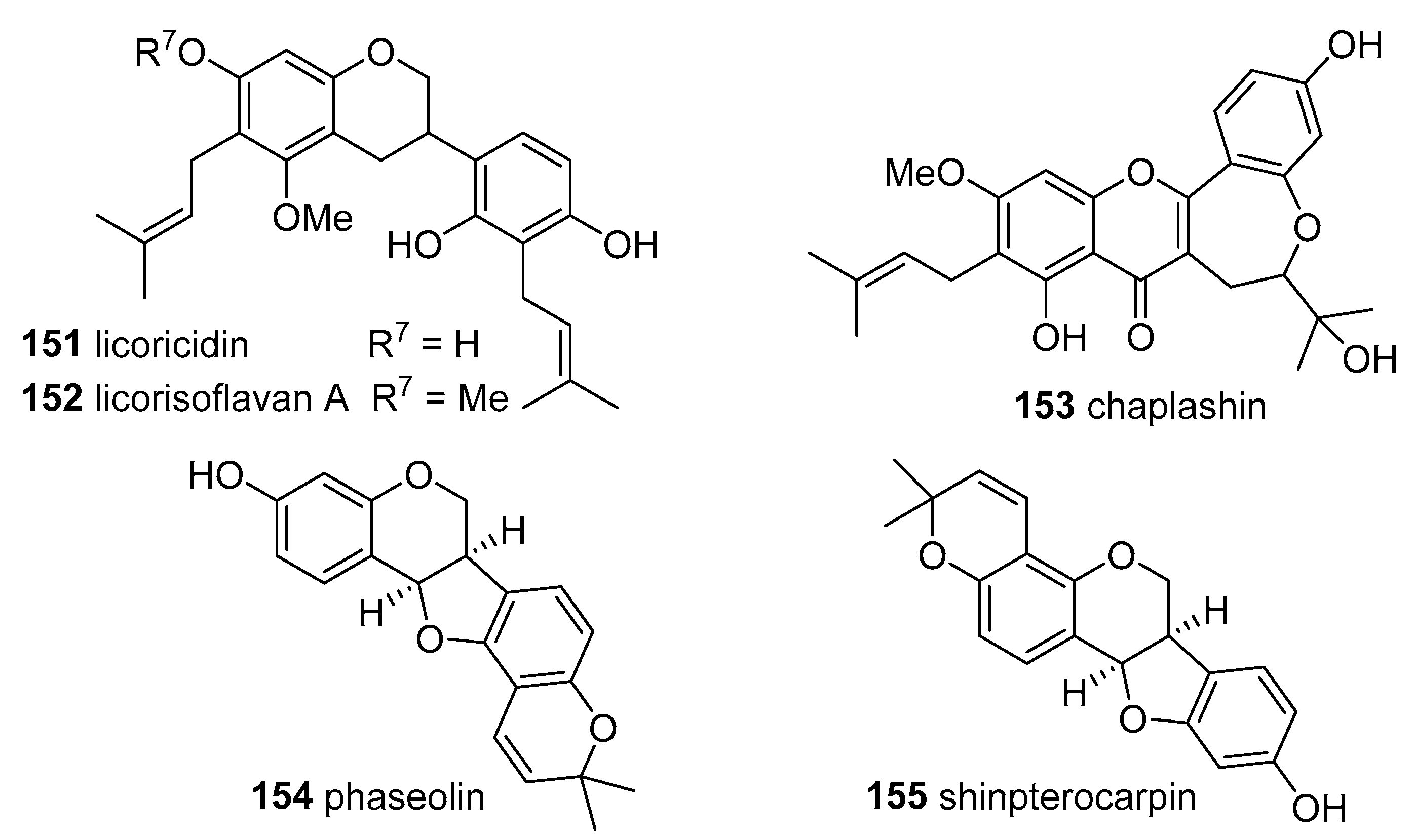
Two prenylated pterocarpans—phaseollin (
154) and shinpterocarpin (
155)—the flavanone 4′-
O-methyllicoflavanone (
83) and two isoflavones, alpinum isoflavone (
119) and 8-prenyldaidzein (
120) have been isolated from the stem bark of
E. orientalis collected in East Java, Indonesia. The results pointed out that antioxidant activity against DPPH
● scavenging by phaseollin (
154, IC
50 241.9 μM) and 8-prenyldaidzein (
120, IC
50 174.2 μM) were more effective than the positive control, ascorbic acid (IC
50 329.0 μM). The remaining compounds
83,
119 and
155 showed moderate activity with IC
50 values of 648.1, 708.5 and 909.8 μM, respectively [
46].
Akter et al. isolated from stem bark of
E. stricta Roxb., collected in India, four isoflavones—alpinum isoflavone (
119), erynone (
121), wighteone (
122) and luteone (
123)—and accessed their antioxidant activity using dot-lot and DPPH
● staining methods. Erynone (
121) was the most active compound while luteone (
123) possessed moderate antioxidant activity. Derivatives
122 and
123 were completely inactive against DPPH
● scavenging activity. The authors suggested that the high effect of erynone (
121) is due to its highest number (six) of free phenolic hydroxyl groups and therefore high ability to donate phenolic hydrogens to DPPH
●, when compared to the related structures
119,
122 and
123 [
50].
Stompor et al. synthesized isoxanthohumol (
102) and two acetylated derivatives
117 and
118 and evaluated their DPPH
● scavenging properties. Acylation of
102 decreased the antioxidant activity of its analogues in the order: isoxanthohumol (
102) > 4′-
O-acetylisoxanthohumol (
117) > 7,4′-di-
O-acetylisoxanthohumol (
118). Thus,
102 (EC
50 7.6 mM) is about 8-fold stronger antioxidant than its monoacyl derivative
117 (EC
50 59.7 mM) and 10-fold stronger than the diacyl derivative
118 (EC
50 73.5 mM) due to the presence of two free hydroxyl groups in the molecule [
49].
A series of eight chalcones were obtained by synthesis: xanthohumol (
1), 4-
O-acetyl-xanthohumol (
25), 4,4′-di-
O-acetylxanthohumol (
26), 4-
O-decanoylxanthohumol (
27), 4-
O-dodecanoylxanthohumol (
28), 4,4′-di-
O-dodecanoylxanthohumol (
29), 4-
O-pivaloylxanthohumol (
30) and 4,4′-di-
O-pivaloylxanthohumol (
31). All the compounds were tested for their ability to scavenge DPPH
● and the results expressed as Trolox equivalents, defined as the concentration of Trolox (μM) having the same activity as 1 g of the tested compound. Thus, the most active compound was
25 (15 μM TEAC/g), a two-fold stronger scavenger than
31 (37 μM TEAC/g) and
1 (38 μM TEAC/g). The values for the remaining compounds
25-30 ranged from 44 to 70 μM TEAC/g [
27].
From the leaves and stem barks of
A. scortechinii King collected at Pahang, Malaysia one chalcone flemichapparin A (
15), four flavones—artocarpin (
47), 4′,5-dihydroxy-6,7-(2,2-dimethylpyrano)-2′-methoxy-8-(γ,γ-dimethyl)allyflavone (
54), artonin E (
55) and macakurzin C (
64)—and two xanthones—cudraflavone A (
136) and cycloartobiloxanthone (
140)—were isolated and their DPPH
● scavenging activity evaluated. It was not possible to determine the IC
50 value of compounds
47,
54,
64 and
136 while derivatives
15,
55 and
140 reached IC
50 values of 131.0, 151.6 and 196.0 μM, respectively. Two positive controls were used in this assay being BHA a stronger scavenger (IC
50 49.87 μM), better than the analyzed compounds, and BHT considerably weaker, with an IC
50 value of 231.6 μM [
19].
Zakaria et al. isolated from heartwood of
A. integer (Thunb.) Merr., collected in Indonesia, the flavones cudraflavone C (
59) and artocarpin (
47) and the xanthone tephrosin (
146) and subjected them to the DPPH
● decoloration method at λ
max 500 nm. The flavones
59 and
47 were the most active, with IC
50 values of 3.35 μg/mL and 4.70 μg/mL, respectively, while the xanthone
146 was 10-fold weaker with an IC
50 value of 55.58 μg/mL. Ascorbic acid (IC
50 2.79 μg/mL) was used as positive control [
36].
The rhizomes of
Ficus tikoua collected in Yunnan Province, China, furnished five isoflavones—alpinum isoflavone (
119), 4′-
O-methylalpinum isoflavone (
125), ficusin A (
126), ficusin C (
127) and 6-[(1
R*,6
R*)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5,7,4′-trihydroxyisoflavone (
128)—and their ability to scavenge DPPH
● was investigated. All compounds exhibited moderate antioxidant activity with the EC
50 values of 54.8 ± 9.7, 83.6 ± 12.5, 42.4 ± 6.6, 49.3 ± 7.8 and 43.3 ± 6.9 μM, respectively. Propyl gallate used as positive control presented an EC
50 value of 1.8 ± 0.5 μM [
52].
From the leaves of
Macaranga pruinosa collected in Samarinda, Indonesia the flavone glyasperin A (
71) was isolated and its antioxidant potential tested using a DPPH
● scavenging system. The IC
50 value of
71 (443.0 ± 8.0 μg/mL) was almost twice higher than that of the positive control, kaempferol (IC
50 238.0 ± 3.3 μg/mL) [
41].
From
Macaranga gigantea (Euphorbiaceae) leaves collected in Indonesia the flavones glyasperin A (
71) and broussoflavonol F (
72) were isolated. The antioxidant activity was evaluated by their ability to scavenge DPPH
●, showing IC
50 values of 125.10 and 708.54 μM, respectively. In addition, the results pointed out that
71 was twice as active as the positive control ascorbic acid (329.01 μM) [
42].
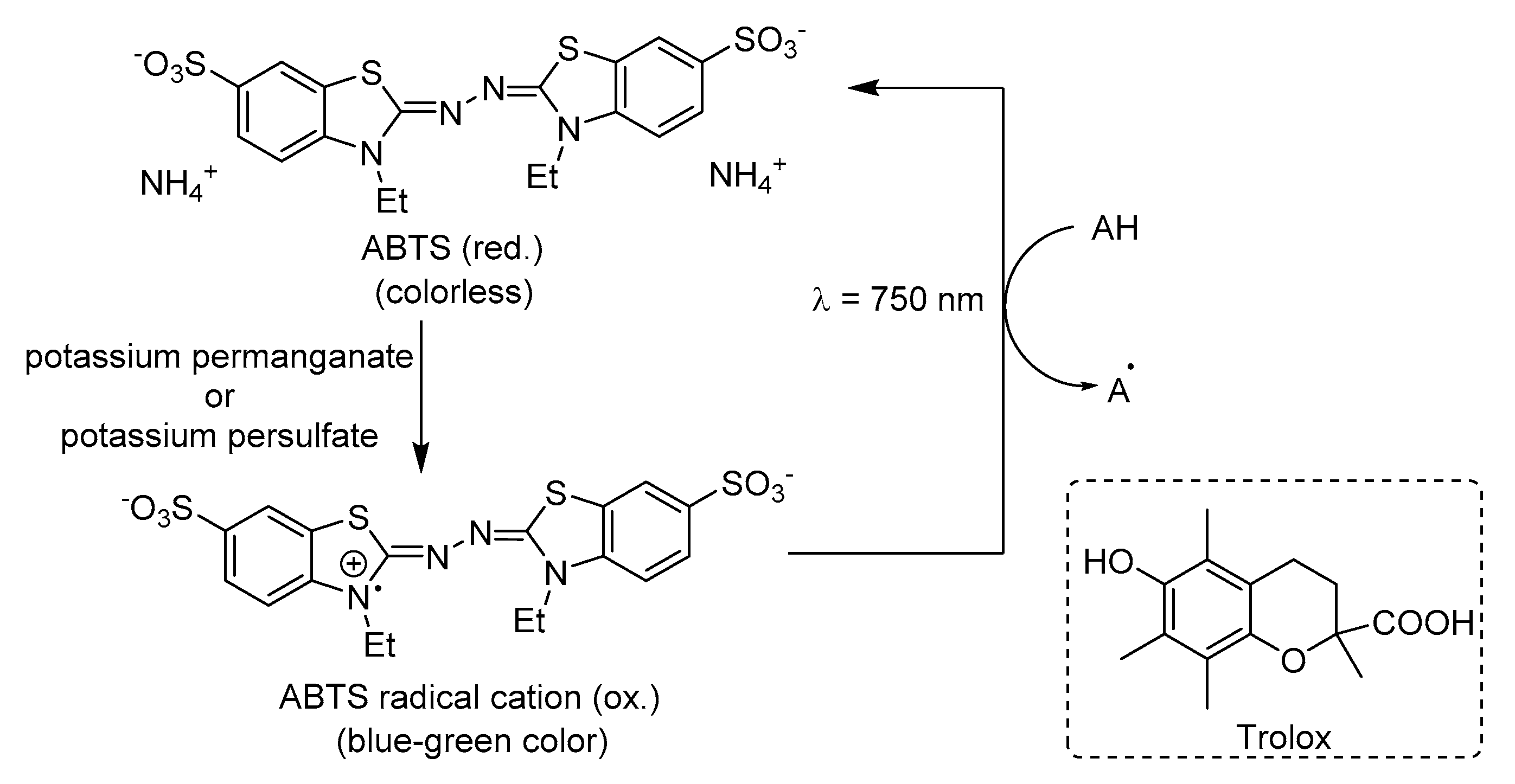
ABTS Radical Cation Scavenging Activity (TEAC Method)
Rajendran et al. verified the suppression of the absorbance of ABTS
●+ in a concentration-dependent manner for artocarpin (
47) and cycloartocarpin (
132). The results demonstrate that the reaction with these compounds show small inhibitory effects even up to 4 min of reaction, when compared with the reaction of the positive control quercetin which is completed within 1 min. Artocarpin (
47) has two hydroxyl groups in the B ring at 4′ and 6′and registered a TEAC value of 910 μM while cycloartocarpin (
132) has a fused partially saturated six member heterocyclic ring between rings C and B and had a TEAC value of 690 μM. Meanwhile, the control quercetin possesses a catechol structure in the B ring, a C2=C3 double bond in conjunction a 3-OH and 4-carbonyl groups, allowing resonance stabilization for electron delocalization and therefore, an higher TEAC value (1230 μM) when compared with
47 and
132. These results demonstrate the importance of electron delocalization across the molecule for stabilization of the aryloxyl radical [
32].
The two prenylated flavones cycloartocarpesin B (
56) and cudraflavone B (
65) and three flavanones—euchrestaflavanone B (
94), euchrestaflavanone C (
95) and a novel flavanone A (
96)—had similar ABTS
●+ scavenging activity (IC
50 4.2–8.3 μM) to that of quercetin (IC
50 4.0 μM). In addition, the most active compound
65 had the same TEAC value (expresses the numbers of μmols of Trolox having an antioxidant capacity corresponding to 1.0 μmol of the test substance) than quercetin (TEAC value of 5.0). These results point out the importance of the prenyl group for the antioxidant effect against ABTS system [
38].
Wu et al. synthesized three baicalein derivatives
73–
75 possessing different prenylated chains at C-7 and evaluated their ABTS
●+ scavenging activity. The order of potency was: parent baicalein (IC
50 5.5 ± 0.40 μM) > 7-prenylbaicalein (
73, IC
50 8.8 ± 0.11 μM) ≈7-geranylbaicalein (
74, IC
50 8.7 ± 0.28 μM) > 7-farnesylbaicalein (
75, IC
50 10.6 ± 0.50 μM). Looking at the results, substitution of 7-hydroxyl group of baicalein by terpenoid groups led to a decrease in the scavenging activity and that the largest farnesyl group lead to the weakest scavenger. Even so, the scavenging activities of baicalein and its derivatives
73–
75 were higher than that of Trolox (IC
50 12.6 ± 0.21 μM), the water-soluble vitamin E analogue with one hydroxyl group [
43].
Among eight flavonoids isolated from
S. flavescens, the chalcones kuraridin (
18) and kuraridinol (
19) were the most potent ABTS
●+ scavengers with IC
50 values of 10.45 ± 0.07 and 11.90 ± 2.77 μM, respectively. The remaining compounds (the flavonol kushenol C (
70) and the flavanones leachianone (
89), kushenol E (
90), sophoraflavanone G (
91), kurarinone (
92) and kurarinol (
93)) revealed IC
50 values in the range of 14.08 to 28.84 μM, while the positive controls Trolox and L-ascorbic acid exhibited IC
50 values of 24.57 ± 0.11 and 28.86 ± 0.02 μM, respectively. In addition,
70,
18 and
19 possessed TEAC values of 1.88, 2.45, and 2.44, respectively, whereas those of the flavanones (
89,
90,
91,
92 and
93) were 1.28, 1.76, 1.05, 1.56, and 1.39, respectively [
22].
Lan et al. isolated 14 flavonoids from
A. altilis, but only five were evaluated in an ABTS
●+ scavenging assay. Isocycloartobiloxanthone (
134) showed a concentration-dependent scavenging behavior and was the most efficient scavenger, with an IC
50 value of 7.2 ± 1.6 μM, similar to the positive control quercetin (IC
50 7.8 ± 2.1 μM). The order to potency for the other derivatives was: artogomezianone (
50, IC
50 36.9 ± 2.3 μM) > 8-geranyl-3-(hydroxyprenyl)isoetin (
52, IC
50 156.9 ± 5.3 μM) > artocarpin (
47, IC
50 265.1 ± 4.3 μM). No activity was found for cyclocommunol (
131, IC
50 > 500 μM) [
35].
Xanthohumol (
1) had a maximum inhibition of ABTS
●+ of 47% at 60 μM concentration and a TEAC value of 0.32 ± 0.09 μM [
17]. In another study involving xanthohumol (
1) and seven synthetic ester derivatives, 4-
O-acetylxanthohumol (
25, 160 μM TEAC/g) and 4-
O-decanoylxanthohumol (
27, 170 μM TEAC/g) displayed higher antioxidant effect than xanthohumol (
1, 190 μM TEAC/g). The other tested compounds showed comparable or weaker ABTS scavenger properties than
1 (>200 μM TEAC/g) [
27]. The ABTS radical scavenging profile of the chalcone desmethylxanthohumol (
3) was expressed as the half-maximal effective concentration (EC
50), presenting an EC
50 of 0.54 ± 0.09 μM, slightly less than that of gallic acid (0.35 ± 0.01 μM) [
29].
Isoxanthohumol (
21) and 2′,4′,6′-trihydroxy-3′-prenylchalcone (
22) displayed the highest TEAC values (4529.01 ± 2.44; 4170.66 ± 6.72) μM Trolox equivalents/g, respectively, among the flavonoids isolated from
H. teretifolium. Isoglabranin (
99), glabranin (
100) and 7-methoxyisoglabranin (
101) presented modest ABTS
●+ scavenging effects and heliteretifolin (
20) was completely inactive [
24].
From the seven flavonoids isolated from
A. scortechinii King it was only possible to determine the ABTS
●+ scavenging potential of flemichapparin A (
15, IC
50 199.7 μM), artonin E (
55, IC
50 145.0 μM) and cycloartobiloxanthone (
140, IC
50 269.0 μM). As in the DPPH
● assay, the positive control BHA used in this assay was a stronger scavenger than the analyzed compounds (IC
50 91.01 μM) [
19].
Glyasperin A (
71 (IC
50 210.0 ± 2.7 μM) isolated from
M. pruinosa leaf showed almost half the antioxidant potential than the positive control kaempferol (IC
50 111.0 ± 1.6 μM) in the ABTS
●+ scavenging assay [
41].