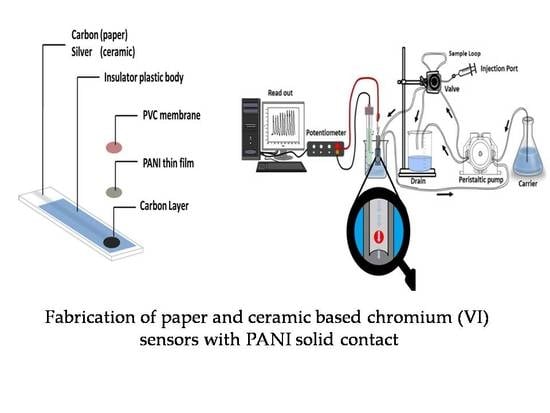
Paper Strip and Ceramic Potentiometric Platforms Modified with Nano-Sized Polyaniline (PANi) for Static and Hydrodynamic Monitoring of Chromium in Industrial Samples
Abstract
1. Introduction
2. Results and Discussions
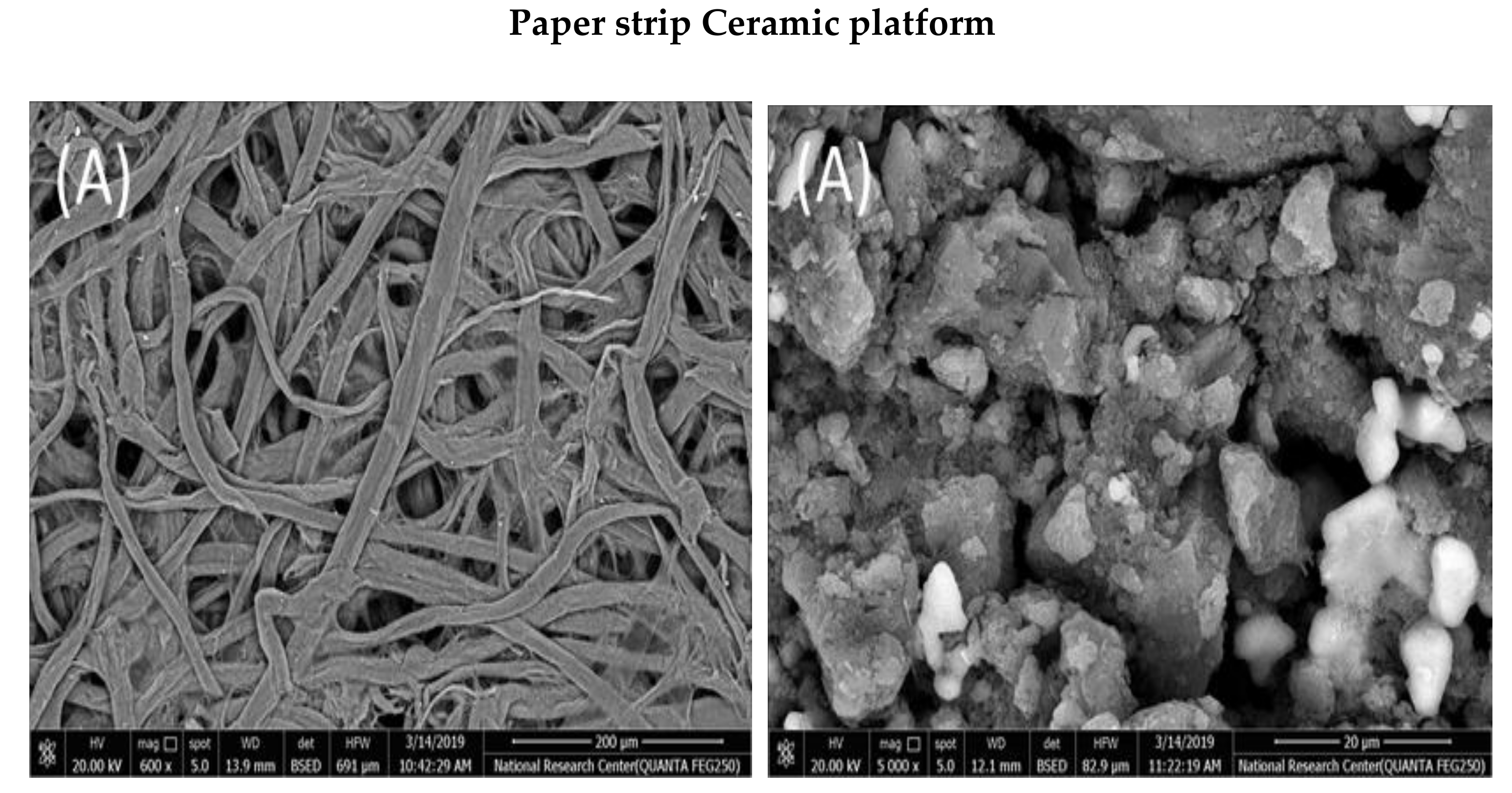
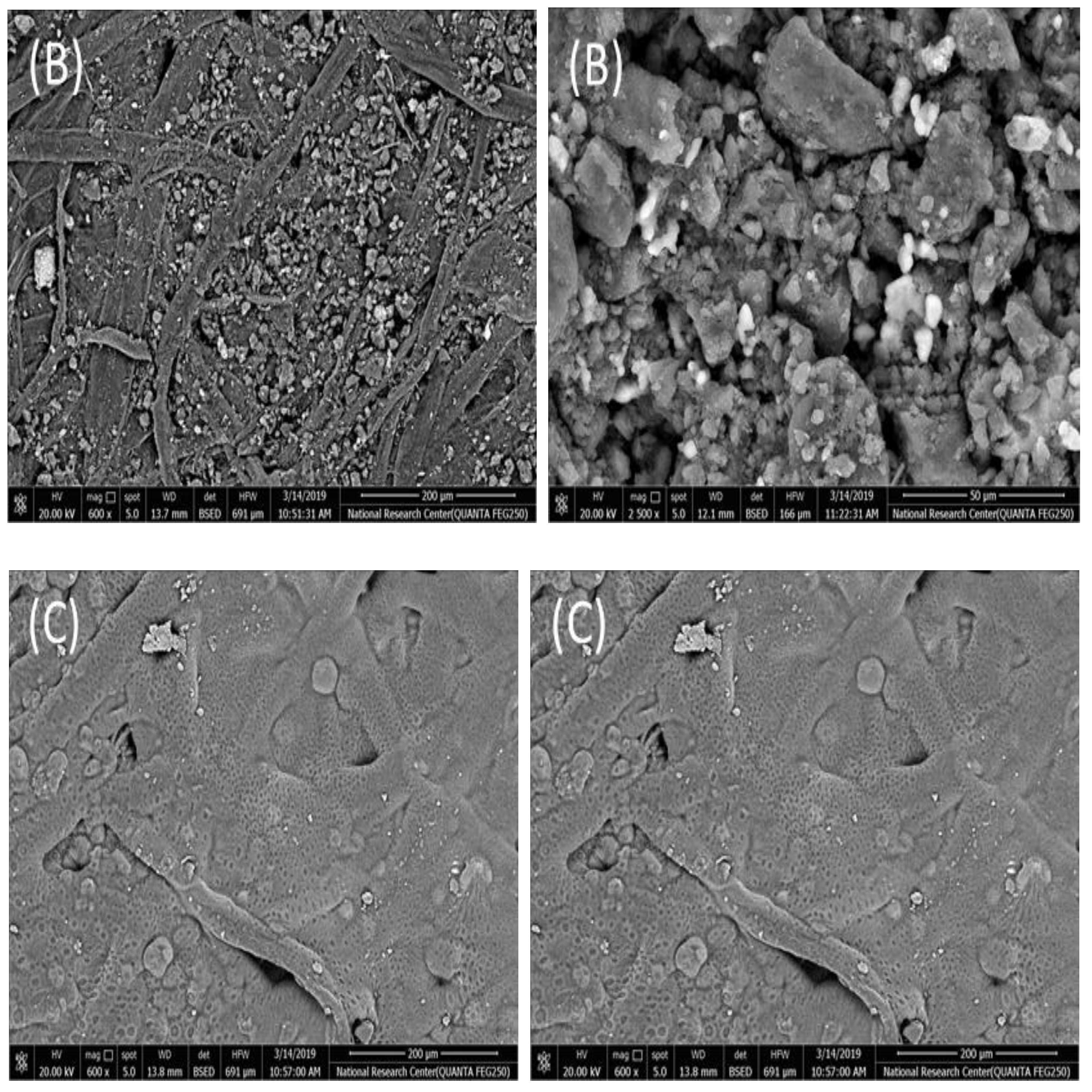
2.1. SEM and EDAX Measurements
2.2. Potentiometric Response
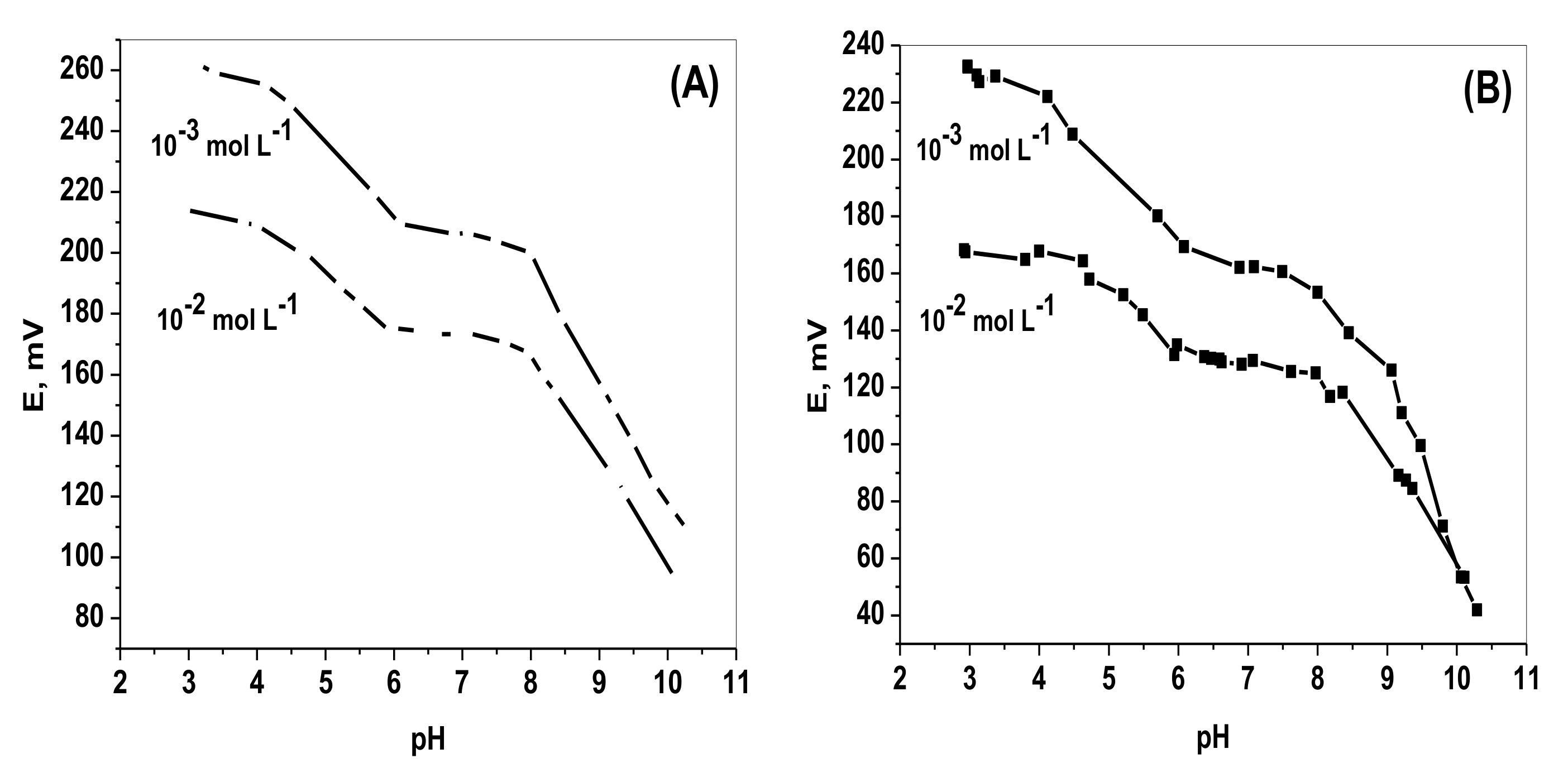
2.3. Effect of pH
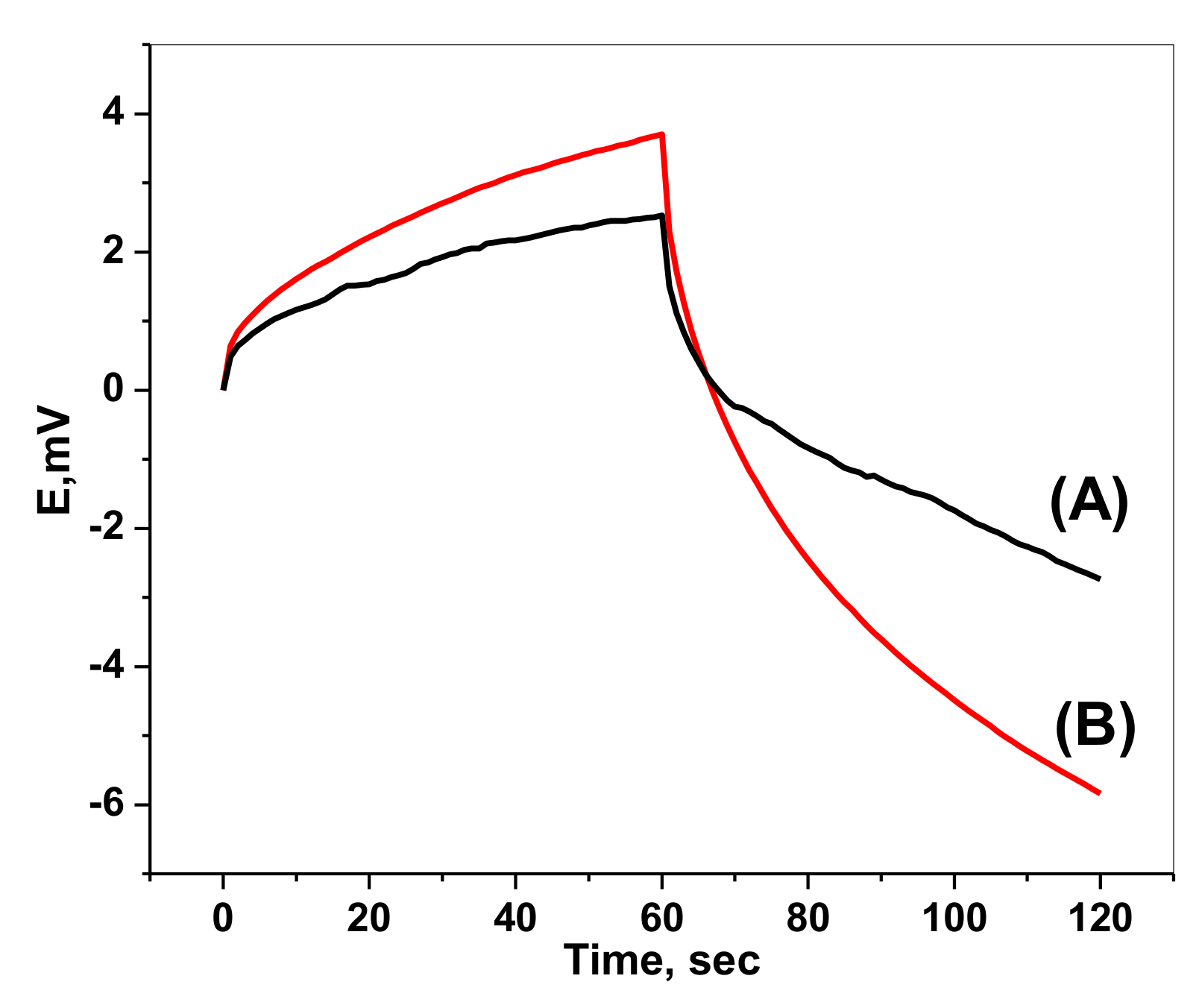
2.4. Chronopotentiometric Measurements
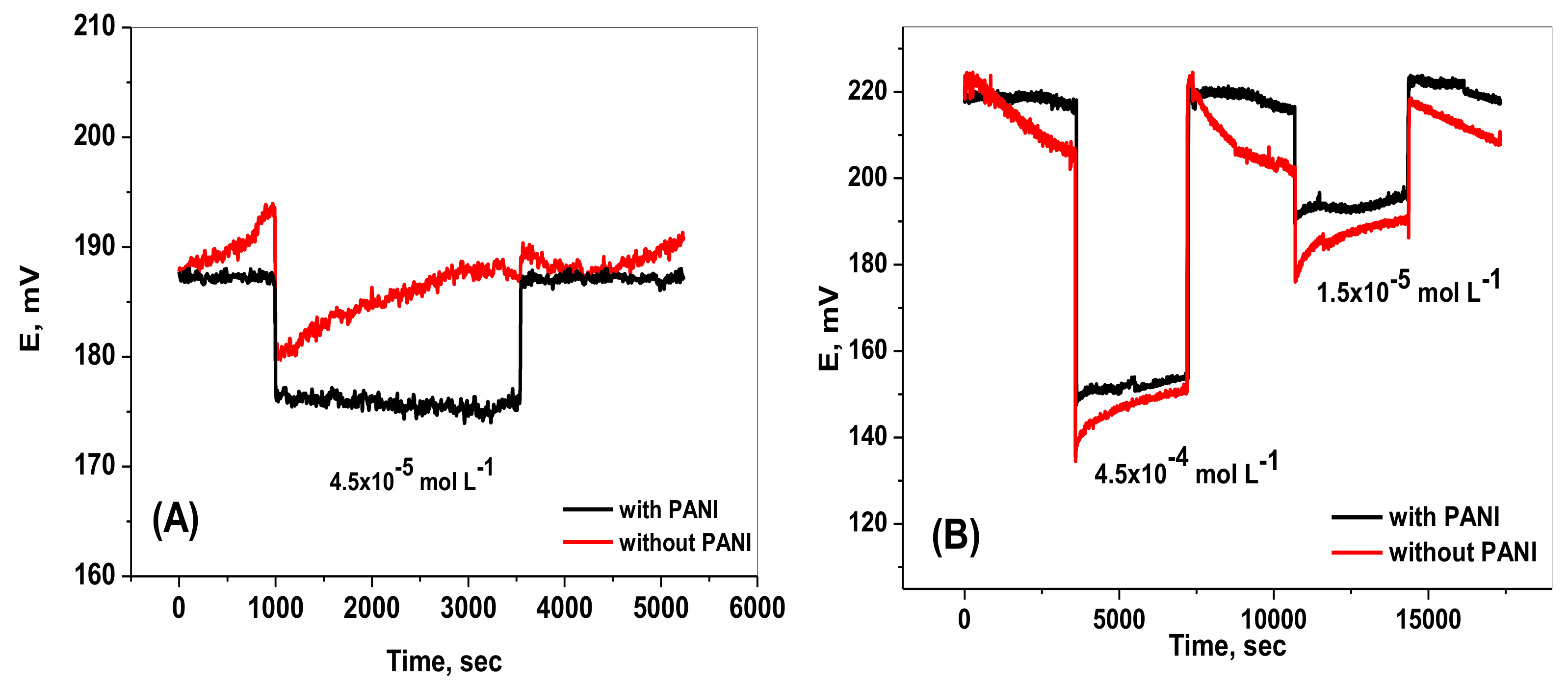
2.5. Water Layer Test
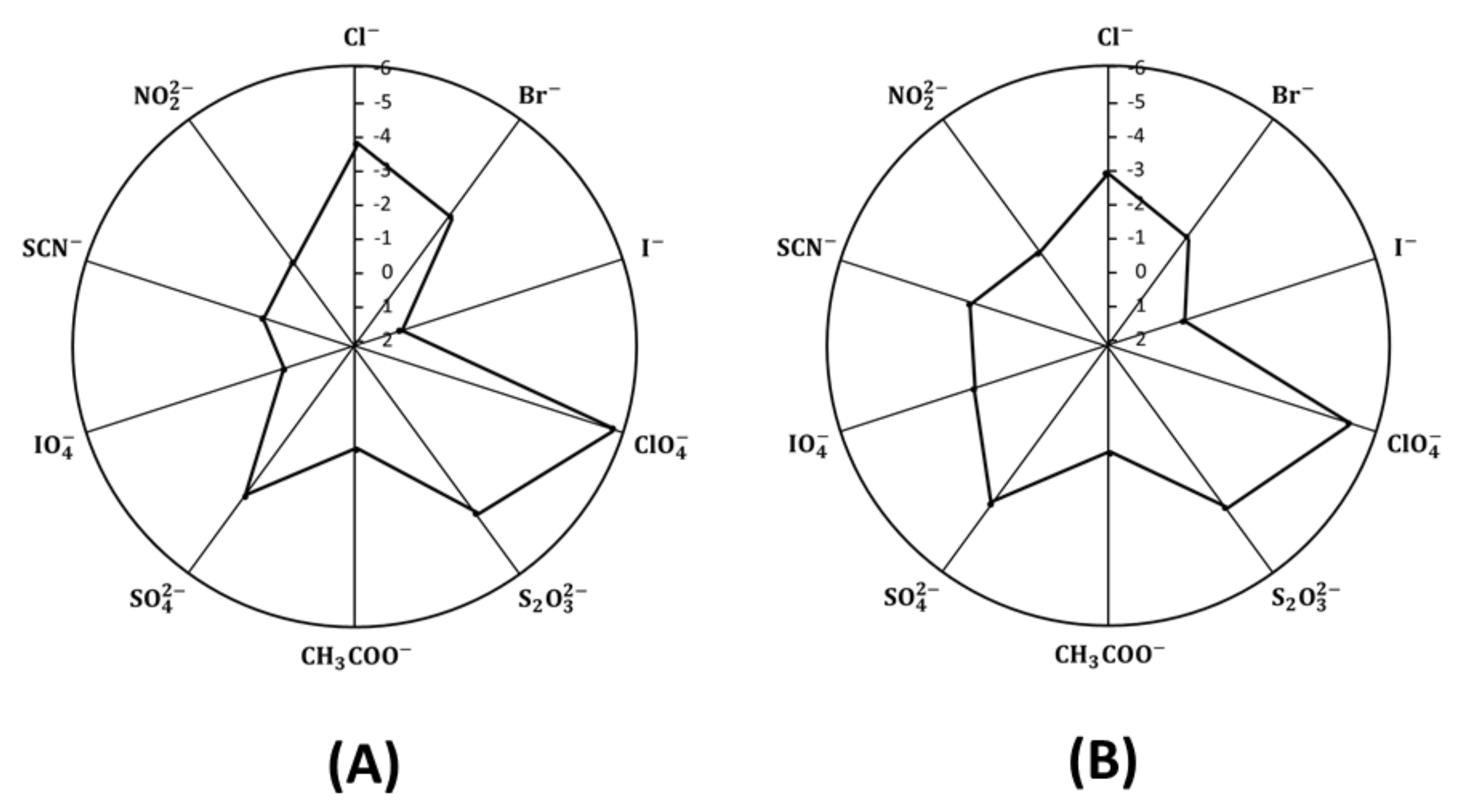
2.6. Sensor Selectivity
2.7. Batch and Continuous Determination of Chromium (III) and Cr(VI)
2.8. Determination of Chromium in Some Environmental and Industrial Samples
3. Materials and Methods
3.1. Apparatus
3.2. Materials and Reagents
3.3. Preparation of Solid Contact Cr(VI) Sensor
3.3.1. Fabrication of Paper and Ceramic-Based Sensors
3.3.2. Preparation of Polyaniline (PANI)
3.3.3. Preparation of Rhodamine-B/ Cr(VI) Membrane Sensor
3.4. Electrochemical Measurements
3.5. Sensor Selectivity
3.6. Flow Injection Setup and Continuous Measurements
3.7. Analysis of Real Chromium Containing Samples
3.7.1. Determination of Total Cr (VI) and Cr (III) in Leather Tanning Wastewater
3.7.2. Determination of Total Chromium Metal in Alloys
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schroeder, H.A.; Balassa, J.J.; Tipton, I.H. Abnormal trace metals in man-Chromium. J. Clin. Epidem. 1962, 15, 941–964. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Thacker, S.B.; Qualters, J.R.; Lee, L.M. Public Health Surveillance in the United States: Evolution and Challenges. MMWR Surveill Summ. 2012, 61, 3–9. [Google Scholar]
- El-Shahawi, M.S.; Hassan, S.S.M.; Othman, A.M.; Zyada, M.A.; El-Sonbati, M.A. Chemical speciation of chromium(III,VI) employing extractive spectrophotometry and tetraphenylarsonium chloride or tetraphenylphosphonium bromide as ion-pair reagent. Anal. Chim. Acta 2005, 534, 319–326. [Google Scholar] [CrossRef]
- Elshahawi, M.S.; Hassan, S.S.M.; Othman, A.M.; El Sonbate, M.A. Retention profile and subsequent chemical speciation of chromium (III) and (VI)) in industrial wastewater samples employing some onium cations loaded polyurethane foams. Microchem. J. 2008, 89, 13–19. [Google Scholar] [CrossRef]
- Matsuoka, S.; Tennichi, Y.; Takehara, K.; Yoshimura, K. Flow analysis of micro amounts of chromium(III) and (VI) in natural water by solid phase spectrophotometry using diphenylcarbazide. Analyst 1999, 124, 787–791. [Google Scholar] [CrossRef]
- Bittner, M.; Broekaert, J. Speciation of chromium by solid-phase extraction coupled to reversed-phase liquid chromatography with UV detection. Anal. Chim. Acta 1998, 364, 31–40. [Google Scholar] [CrossRef]
- Razek, T.M.; Spear, S.; Hassan, S.S.M.; Arnold, M.A. Selective measurement of chromium(VI) by fluorescence quenching of ruthenium. Talanta 1999, 48, 269–275. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Abdel-Shafi, A.A.; Mohammed, A.H.K. Flow injection fluorimetric determination of chromium(VI) in electroplating baths by luminescence quenching of tris(2,2’-bipyridyl) ruthenium(II). Talanta 2005, 67, 696–702. [Google Scholar] [CrossRef]
- Luo, S.K.; Berndt, H. Cr(III)/Cr(VI) determination in waste water by ICP/AES with on-line HPLC (HHPN) sample introduction. Fresenius’ J. Anal. Chem. 1998, 360, 545–549. [Google Scholar] [CrossRef]
- Andersen, J.E. Introduction of hydrogen peroxide as an oxidant in flow injection analysis: Speciation of Cr(III) and Cr(VI). Anal. Chim. Acta 1998, 361, 125–131. [Google Scholar] [CrossRef]
- Subramanian, K.S. Determination of chromium(III) and chromium(VI) by ammonium pyrrolidinecarbodithioate-methyl isobutyl ketone-furnace atomic absorption spectrometry. Anal. Chem. 1988, 60, 11–15. [Google Scholar]
- Milačič, R.; Štupar, J. Simultaneous determination of chromium(III) complexes and chromium(VI) by fast protein anion-exchange liquid chromatography–atomic absorption spectrometry. Analyst 1994, 119, 627–632. [Google Scholar] [CrossRef]
- Aranda, P.R.; Moyano, S.; Martinez, L.D.; De Vito, I.E. Determination of trace chromium(VI) in drinking water using X-ray fluorescence spectrometry after solid-phase extraction. Anal. Bioanal. Chem. 2010, 398, 1043–1048. [Google Scholar] [CrossRef]
- Harzdorf, C.; Janser, G. The determination of chromium (VI) in waste water and industrial effluents by differential pulse polarography. Anal. Chim. Acta 1984, 165, 201–207. [Google Scholar] [CrossRef]
- Zhu, X.; Hu, B.; Jiang, Z.; Wu, Y.; Xiong, S. Speciation of chromium(III) and chromium(VI) by in situ separation and sequential determination with electrothermal vaporization inductively coupled plasma atomic emission spectrometry. Anal. Chim. Acta 2002, 471, 121–126. [Google Scholar] [CrossRef]
- Domínguez, O.; Arcos, M.J. Simultaneous determination of chromium(VI) and chromium(III) at trace levels by adsorptive stripping voltammetry. Anal. Chim. Acta 2002, 470, 241–252. [Google Scholar] [CrossRef]
- Cathum, S.; Brown, C.; Wong, W. Determination of Cr3+, CrO4 2–, and Cr2O72– in environmental matrixes by high-performance liquid chromatography with diode-array detection (HPLC–DAD). Anal. Bioanal. Chem. 2002, 373, 103–110. [Google Scholar] [CrossRef]
- Choi, Y.W.; Moon, S.H. Determination of Cr(VI) using an Ion Selective Electrode with SLMs containing Aliquat336. Envir. Monit. Asses. 2004, 92, 163–178. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Raheedayat, F. Chromium(III) Ion Selective Electrode Based on Oxalic Acid Bis(Cyclohexylidene Hydrazide). Electroanalysis 2004, 16, 1330–1335. [Google Scholar] [CrossRef]
- Abbaspour, A.; Izadyar, A. Chromium(III) ion-selective electrode based on 4-dimethylaminoazobenzene. Talanta 2001, 53, 1009–1013. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Abbas, M.N.; Moustafa, G.A.E. Hydrogen chromate PVC matrix membrane sensor for potentiometric determination of chromium(III) and chromium(VI) ions. Talanta 1996, 43, 797–804. [Google Scholar] [CrossRef]
- Cattrall, R.; Freiser, H. Coated wire ion-selective electrodes. Anal. Chem. 1971, 43, 1905–1906. [Google Scholar] [CrossRef]
- Buck, R.P.; Shepard, V.R., Jr. Reversible metal/salt interfaces and the relation of second kind and “all-solid-state” membrane electrodes. Anal. Chem. 1974, 46, 2097–2103. [Google Scholar] [CrossRef]
- Ivaska, A. Analytical applications of conducting polymers. Electroanalysis 1991, 3, 247–254. [Google Scholar] [CrossRef]
- Kamel, A.H.; Amr, A.E.; Abdalla, N.S.; El-Naggar, M.; Al-Omar, M.A.; Alkahtani, H.M.; Sayed, A.Y.A. Novel Solid-State Potentiometric Sensors Using Polyaniline (PANI) as A Solid-Contact Transducer for Flucarbazone Herbicide Assessment. Polymers 2019, 11, 1796. [Google Scholar] [CrossRef]
- Lindfors, T.; Aarnio, H.; Ivaska, A. Potassium-Selective Electrodes with Stable and Geometrically Well-Defined Internal Solid Contact Based on Nanoparticles of Polyaniline and Plasticized Poly(vinyl chloride). Anal. Chem. 2007, 79, 8571–8577. [Google Scholar] [CrossRef]
- Michalska, A.J.; Appaih-Kusi, C.; Heng, L.Y.; Walkiewicz, S.; Hall, E.A. An Experimental Study of Membrane Materials and Inner Contacting Layers for Ion-Selective K+ Electrodes with a Stable Response and Good Dynamic Range. Anal. Chem. 2004, 76, 2031–2039. [Google Scholar] [CrossRef]
- Crespo, G.N.A.; Macho, S.; Bobacka, J.; Rius, F.X. Transduction Mechanism of Carbon Nanotubes in Solid-Contact Ion-Selective Electrodes. Anal. Chem. 2008, 81, 676–681. [Google Scholar] [CrossRef]
- Lewenstam, A.; Bobacka, J.; Ivaska, A. Mechanism of ionic and redox sensitivity of p-type conducting polymers: Part Theory. J. Electroanal. Chem. 1994, 368, 23–31. [Google Scholar] [CrossRef]
- Tandon, R.; Crisp, P.; Ellis, J.; Baker, R. Effect of pH on chromium(VI) species in solution. Talanta 1984, 31, 227–228. [Google Scholar] [CrossRef]
- Cruywagen, J.; Heyns, J.; Rohwer, E. New spectrophotometric evidence for the existence of HCrO4−. Polyhedron 1998, 17, 1741–1746. [Google Scholar] [CrossRef]
- Bobacka, J. Potential Stability of All-Solid-State Ion-Selective Electrodes Using Conducting Polymers as Ion-to-Electron Transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; de Rooij, N.F.; Pretsch, E. Potential Drifts of Solid-Contacted Ion-Selective Electrodes Due to Zero-Current Ion Fluxes Through the Sensor Membrane. Electroanalysis 2000, 12, 1286–1292. [Google Scholar] [CrossRef]
- Bakker, E. Determination of Improved Selectivity Coefficients of Polymer Membrane Ion-Selective Electrodes by Conditioning with a Discriminated Ion. J. Electrochem. Soc. 1996, 143, L83–L85. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E.; Bühlmann, P. Selectivity of Potentiometric Ion Sensors. Anal. Chem. 2000, 72, 1127–1133. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J.; Li, G.; Cheng, Q.; Zhou, Y.; Liu, Z.; Tao, C. Highly efficient oxidation of chromium (III) with hydrogen peroxide in alkaline medium. Water Sci. Tech. 2019, 79, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.M. Organic Analysis Using Atomic Absorption Spectrometry; E. Horwood: London, UK, 1984. [Google Scholar]
- Stejskal, J.; Gilbert, R. Polyaniline. Preparation of a conducting polymer(IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 857–867. [Google Scholar] [CrossRef]
- Bakker, E. Determination of Unbiased Selectivity Coefficients of Neutral Carrier-Based Cation-Selective Electrodes. Anal. Chem. 1997, 69, 1061–1069. [Google Scholar] [CrossRef]
- Meier, P.C. Two-parameter Debye-Htickel approximation for the evaluation of mean activity coefficients of 109 electrolytes. Anal. Chim. Acta 1982, 136, 363–368. [Google Scholar] [CrossRef]
- Bruce, G.R.; Gill, P.S. Estimates of Precision in a Standard Additions Analysis. J. Chem. Educ. 1999, 76, 805–807. [Google Scholar] [CrossRef]
Sample Availability: Samples containing chromium are collected by the authors. |
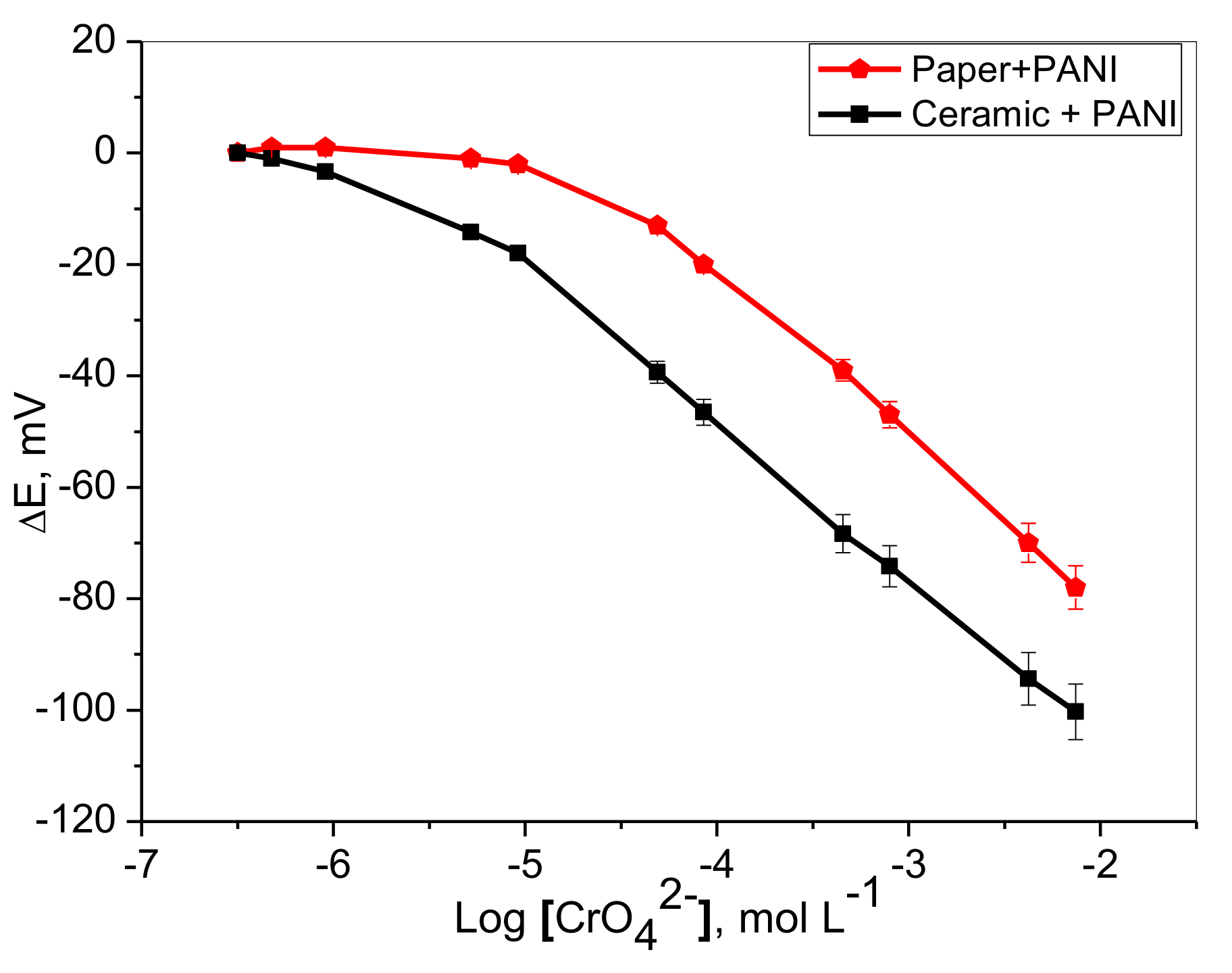

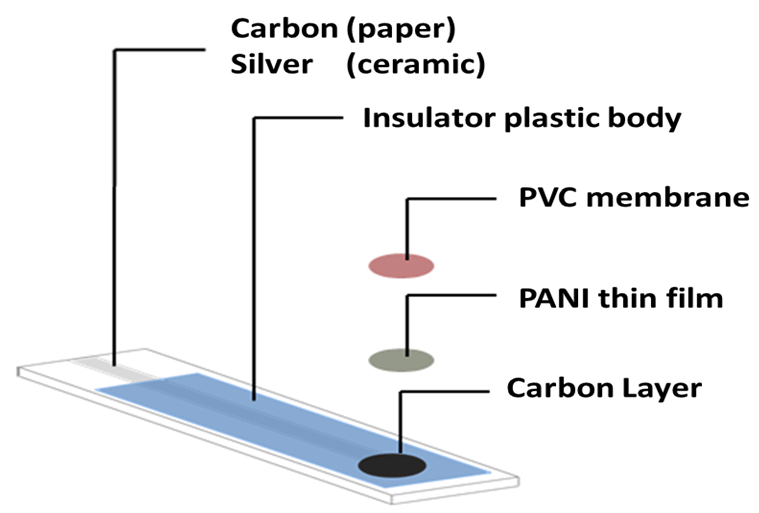
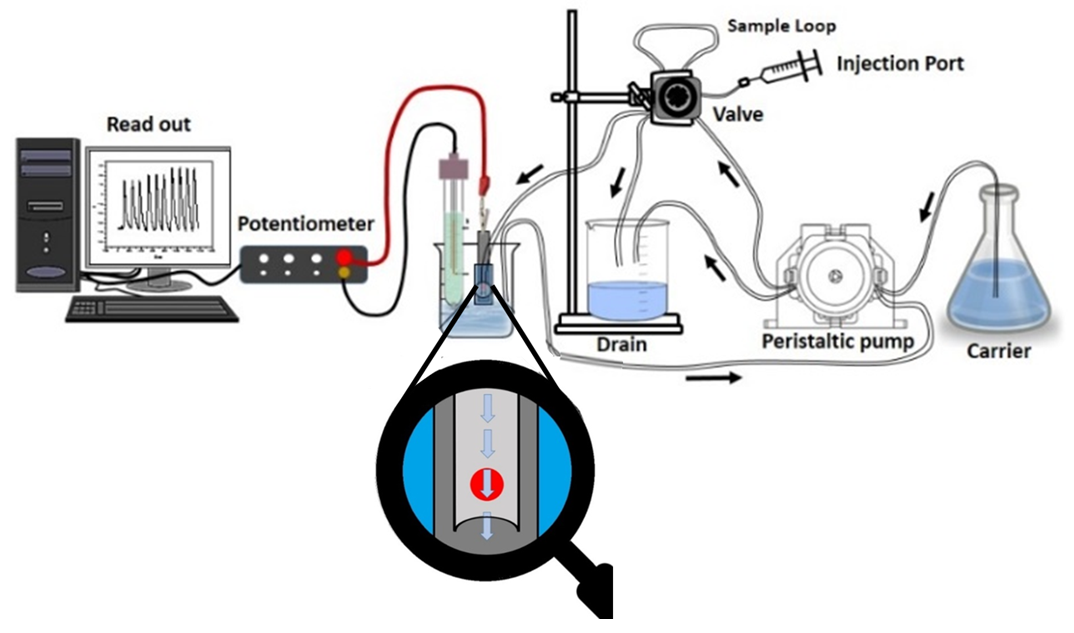
| Parameter | Paper Based Cr(VI) Sensor | Ceramic Based Cr(VI) Sensor |
|---|---|---|
| Slope, (mVdecade−1) | −29.7 ± 0.5 | −28.6 ± 0.3 |
| Correlation coefficient, (r2) | 0.9977 | 0.9996 |
| Detection limit, (mol L−1) | 2.5 × 10−5 | 2.4 × 10−6 |
| Linear range, (mol L−1) | 7.5 × 10−3–5.0 × 10−5 | 7.5 × 10−3–1.0 × 10−5 |
| Working pH range (pH) | 6.0–8.0 | 6.0–8.0 |
| Response time (s) | <10 | <10 |
| Repeatability (% mV) | 0.9 | 1.2 |
| Reproducibility (% mV) | 1.2 | 0.8 |
| Accuracy (%) | 98.4 | 99.3 |
| Sample No. | [Chromium], mg L−1 | ||||
|---|---|---|---|---|---|
| AAS | Paper Based Sensor | Ceramic Based Sensor | |||
| Batch | Flow Injection | Batch | Flow Injection | ||
| (1) | 71.2 ± 0.4 | 70.9 ± 0.4 | 71.1 ± 0.5 | 72.5 ± 1.0 | 69.5 ± 0.9 |
| (2) | 86.6 ± 0.5 | 85.9 ± 0.6 | 86.1 ± 0.6 | 86.4 ± 0.7 | 87.1 ± 0.8 |
| (3) | 192.2 ± 0.7 | 191.8 ± 1.1 | 193.1 ± 0.9 | 192.8 ± 0.8 | 189.3 ± 0.7 |
| Sample No. | [Chromium], mg g−1 | ||||
|---|---|---|---|---|---|
| AAS | Paper Based Sensor | Ceramic Based Sensor | |||
| Batch | Flow Injection | Batch | Flow Injection | ||
| (1) | 197.5 ± 1.2 | 192.6 ± 1.1 | 202.5 ± 0.8 | 195.6 ± 0.7 | 207.4 ± 0.6 |
| (2) | 162.9 ± 1.5 | 153.1 ± 1.2 | 148.2 ± 1.5 | 167.9 ± 1.1 | 172.8 ± 0.9 |
| (3) | 138.3 ± 0.9 | 133.3 ± 1.0 | 128.4 ± 1.6 | 143.2 ± 0.9 | 148.1 ± 1.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.S.M.; Kamel, A.H.; Amr, A.E.-G.E.; Abdelwahab Fathy, M.; Al-Omar, M.A. Paper Strip and Ceramic Potentiometric Platforms Modified with Nano-Sized Polyaniline (PANi) for Static and Hydrodynamic Monitoring of Chromium in Industrial Samples. Molecules 2020, 25, 629. https://doi.org/10.3390/molecules25030629
Hassan SSM, Kamel AH, Amr AE-GE, Abdelwahab Fathy M, Al-Omar MA. Paper Strip and Ceramic Potentiometric Platforms Modified with Nano-Sized Polyaniline (PANi) for Static and Hydrodynamic Monitoring of Chromium in Industrial Samples. Molecules. 2020; 25(3):629. https://doi.org/10.3390/molecules25030629
Chicago/Turabian StyleHassan, Saad S. M., Ayman H. Kamel, Abd El-Galil E. Amr, M. Abdelwahab Fathy, and Mohamed A. Al-Omar. 2020. "Paper Strip and Ceramic Potentiometric Platforms Modified with Nano-Sized Polyaniline (PANi) for Static and Hydrodynamic Monitoring of Chromium in Industrial Samples" Molecules 25, no. 3: 629. https://doi.org/10.3390/molecules25030629
APA StyleHassan, S. S. M., Kamel, A. H., Amr, A. E.-G. E., Abdelwahab Fathy, M., & Al-Omar, M. A. (2020). Paper Strip and Ceramic Potentiometric Platforms Modified with Nano-Sized Polyaniline (PANi) for Static and Hydrodynamic Monitoring of Chromium in Industrial Samples. Molecules, 25(3), 629. https://doi.org/10.3390/molecules25030629