Review on the Properties of Boron-Doped Diamond and One-Dimensional-Metal-Oxide Based P-N Heterojunction
Abstract
1. Introduction
2. Heterojunctions with 1D Metal Oxides Semiconductors
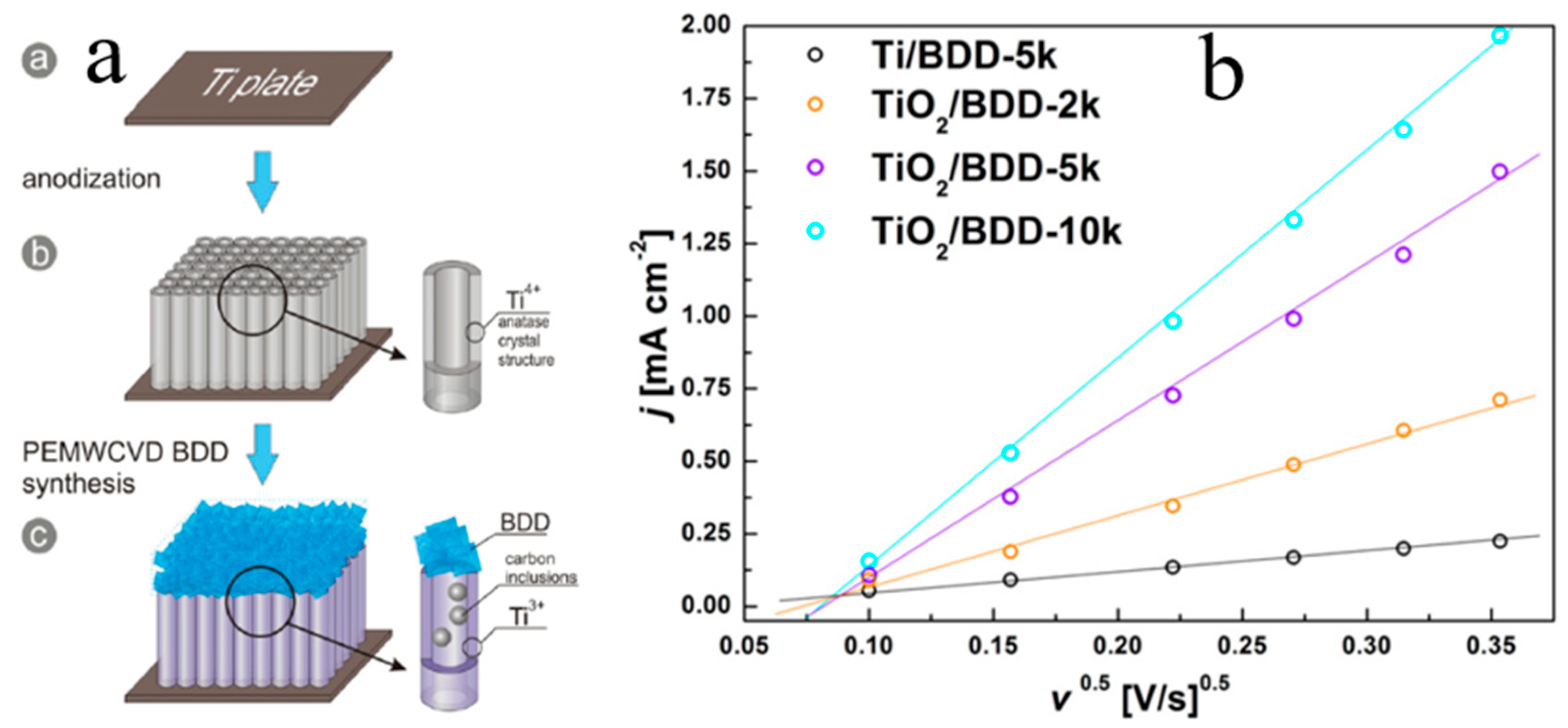
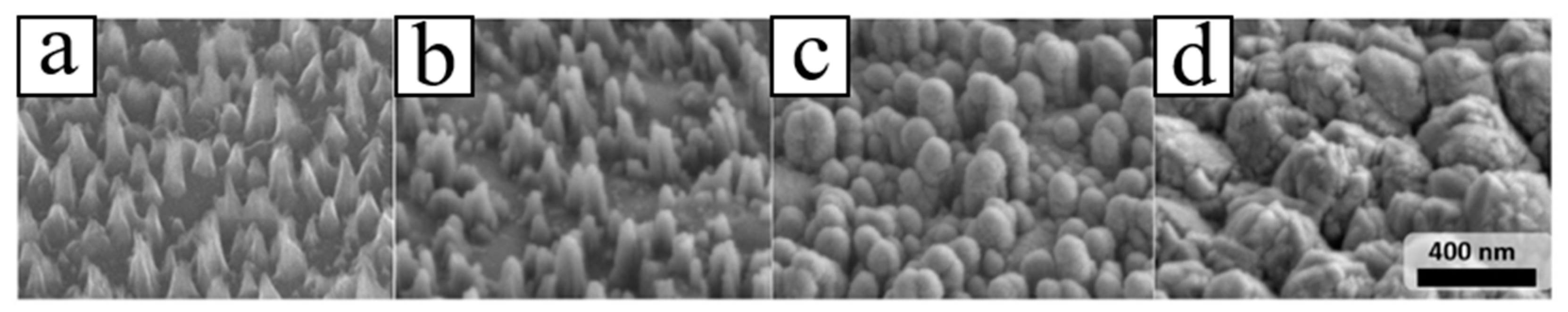
3. P-Type B-Doped Diamond Films
4. Diamond-Based 1D Metal Oxide Heterojunction Classes
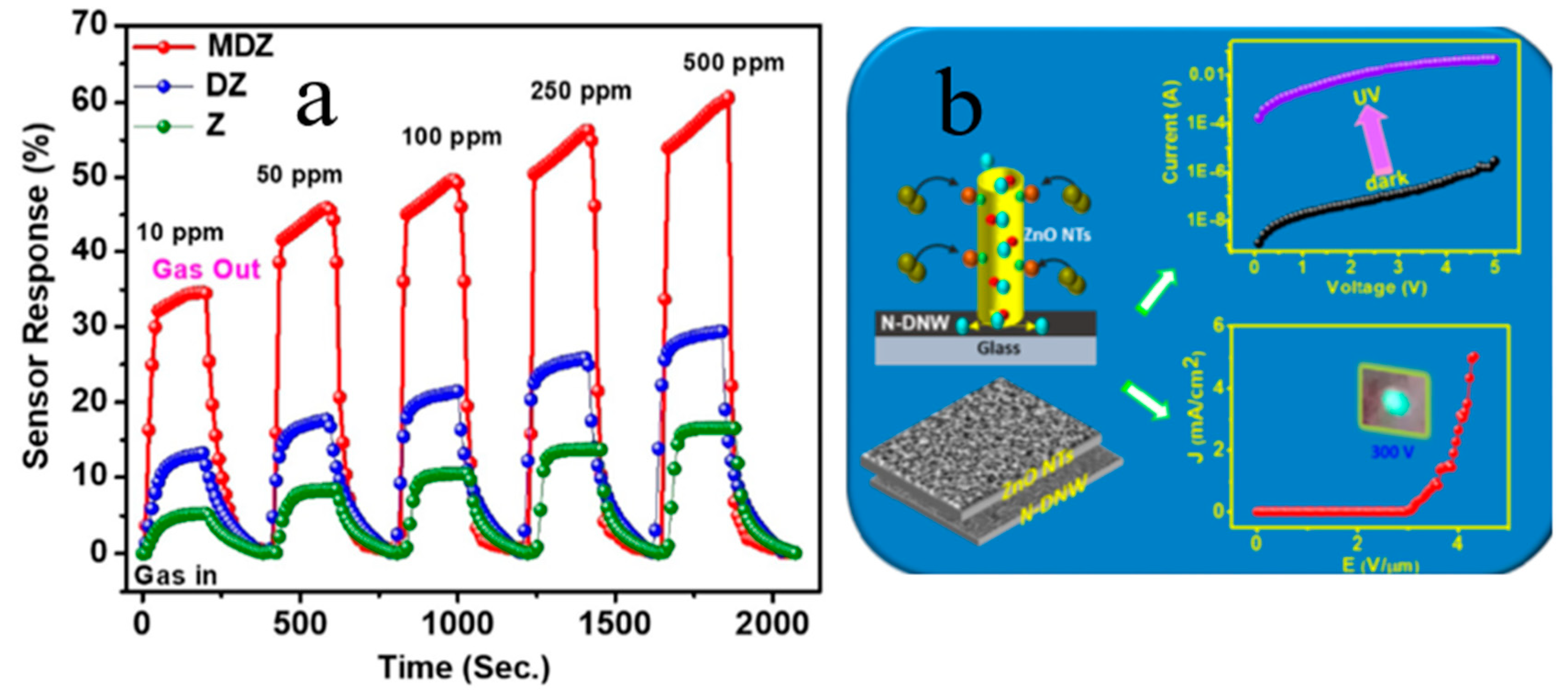
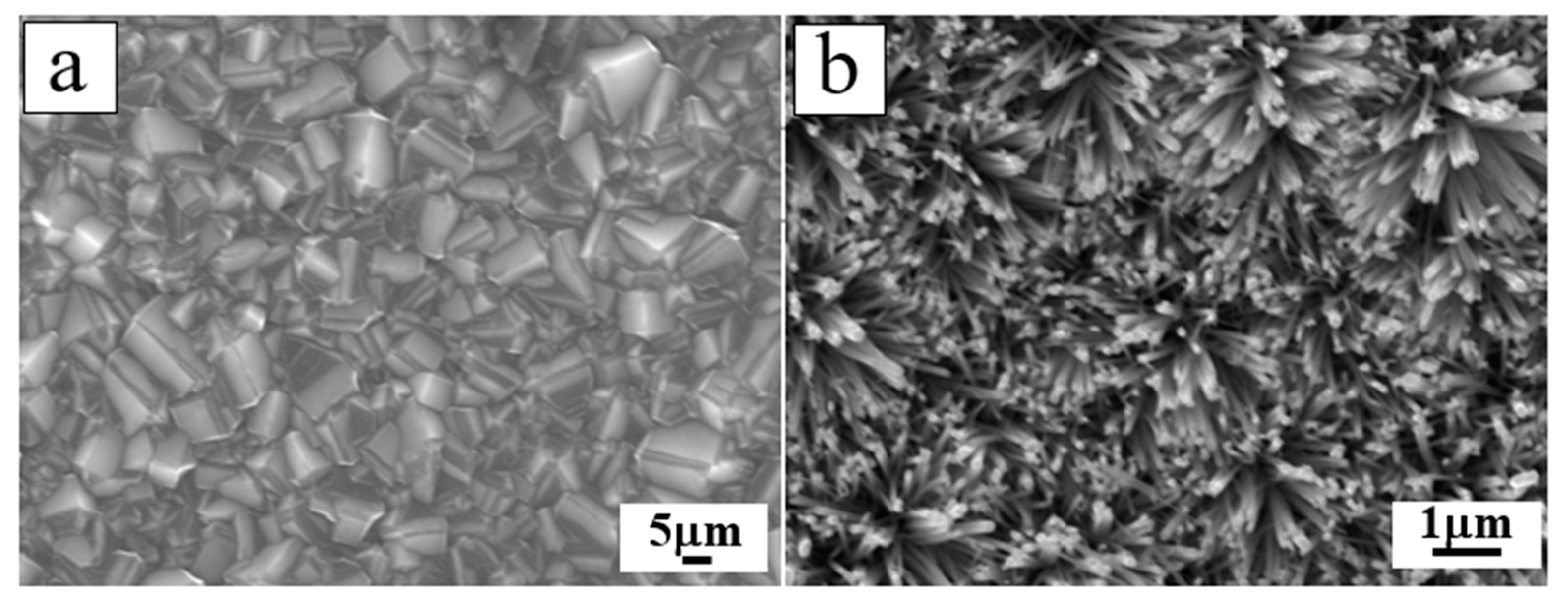
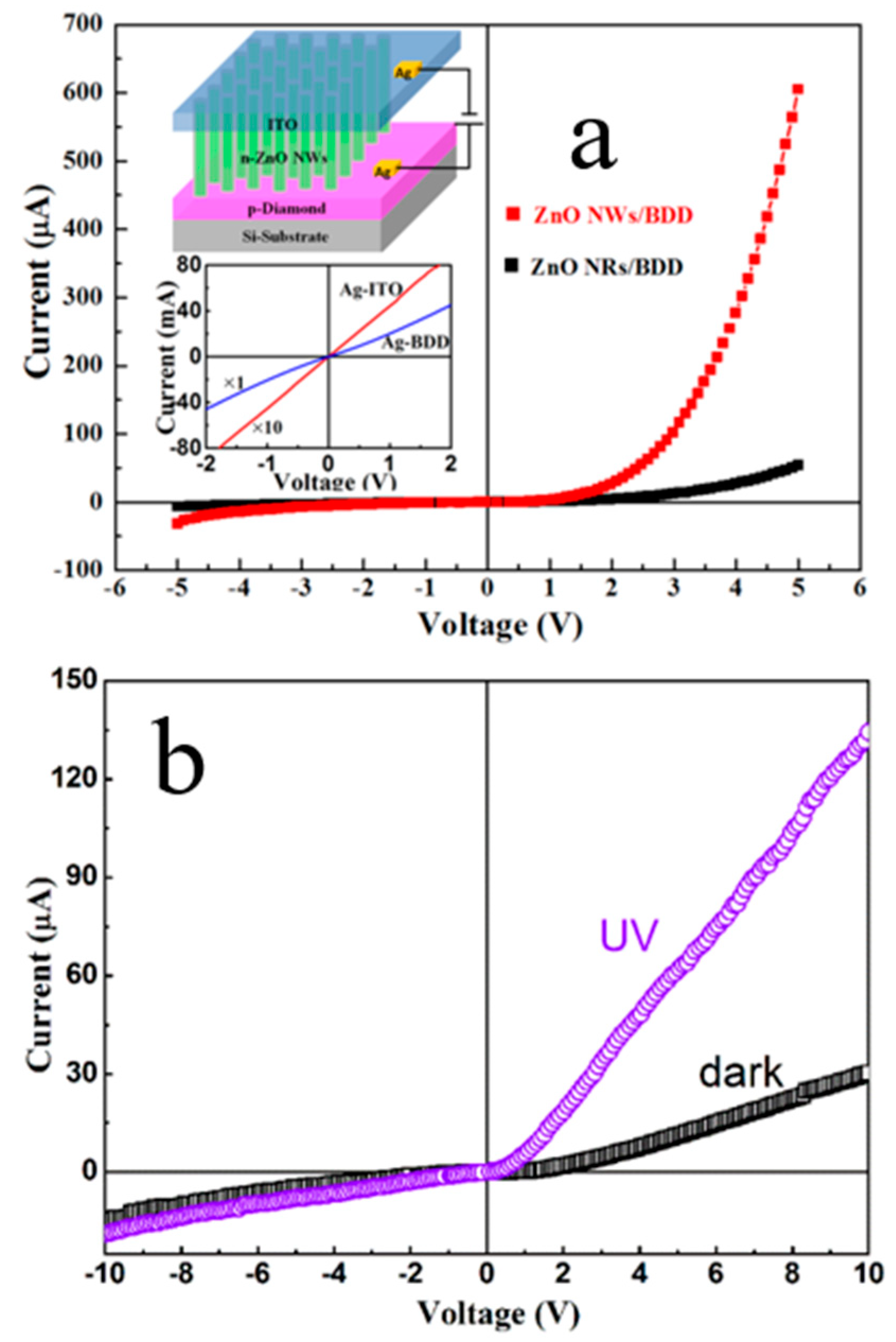
4.1. D N-ZnO/P-Diamond Heterojunction
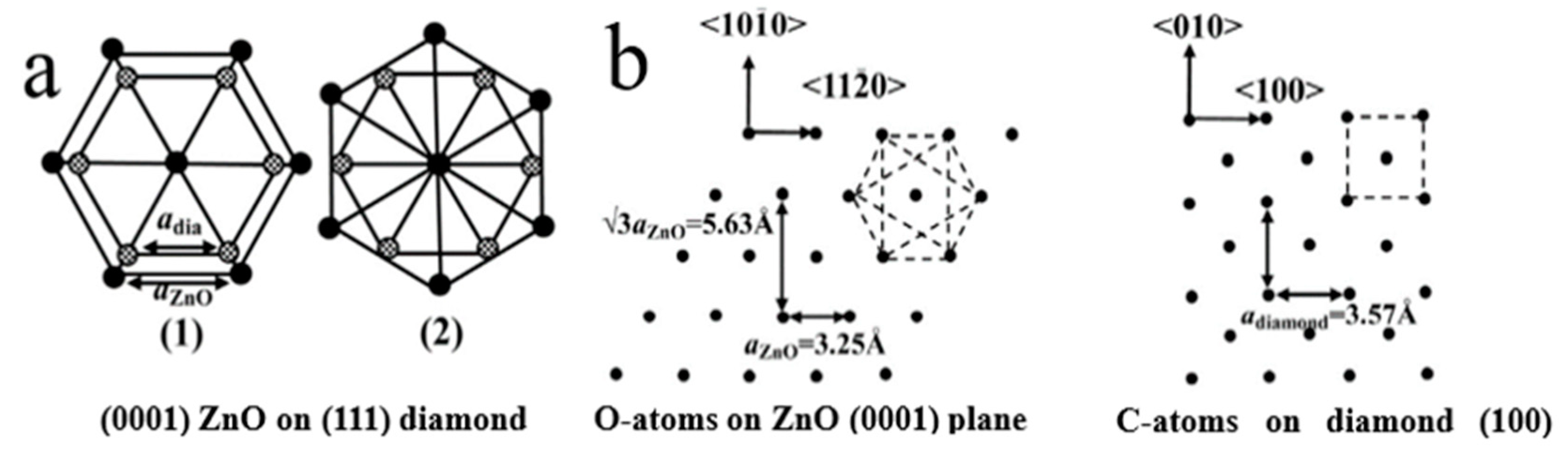
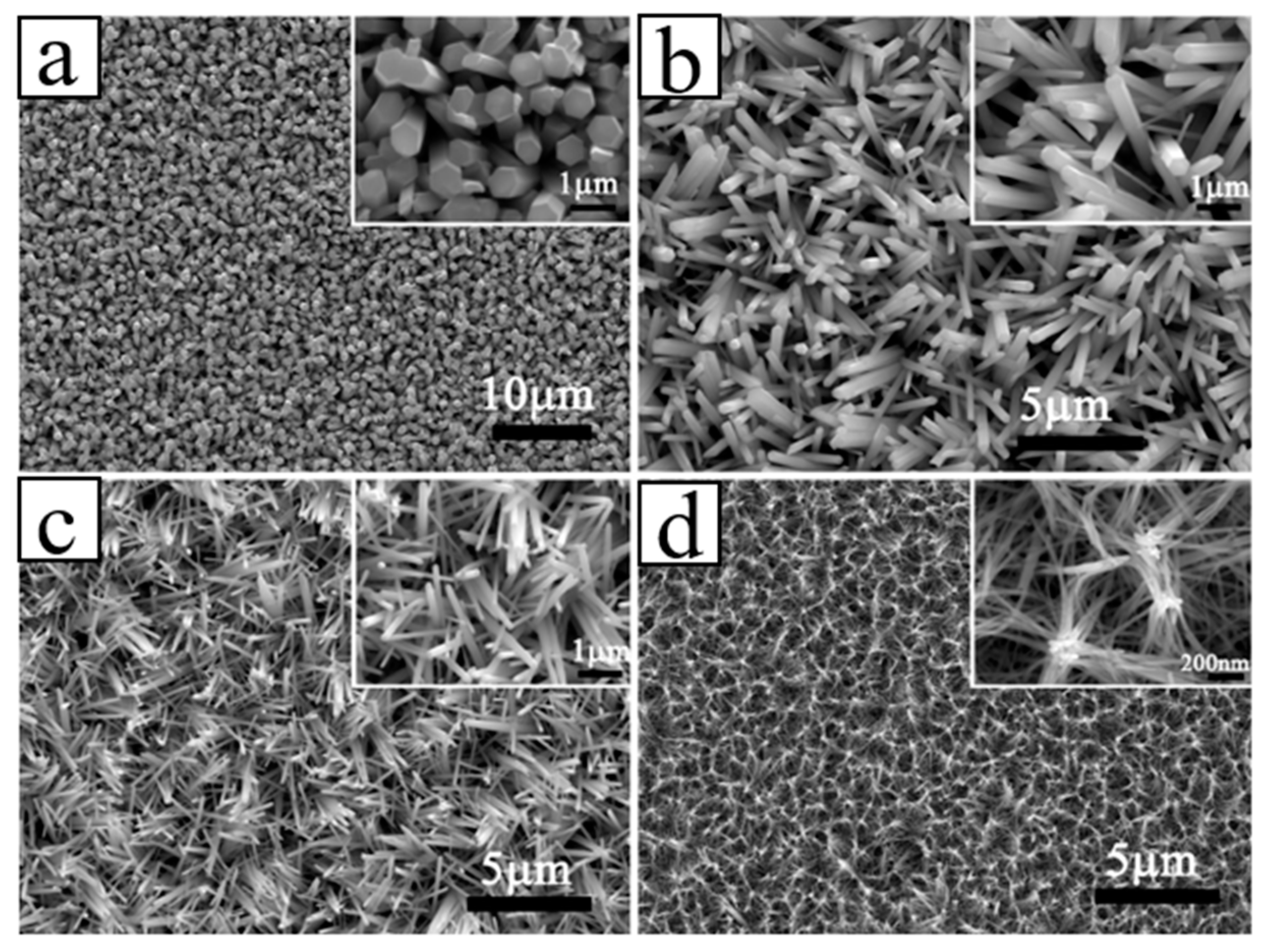
4.1.1. Epitaxial Growth of 1D ZnO NRs/Diamond Facet
4.1.2. 1 D N-ZnO/P-Diamond Related Optoelectronic Devices
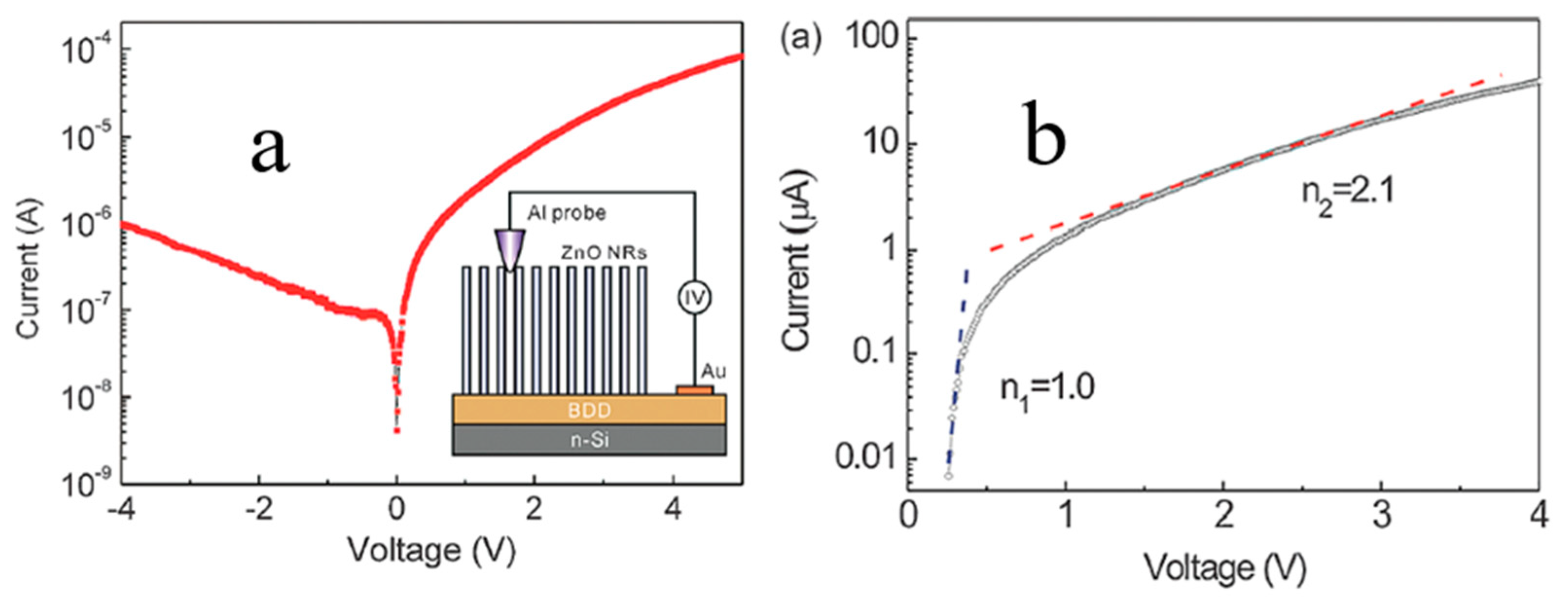
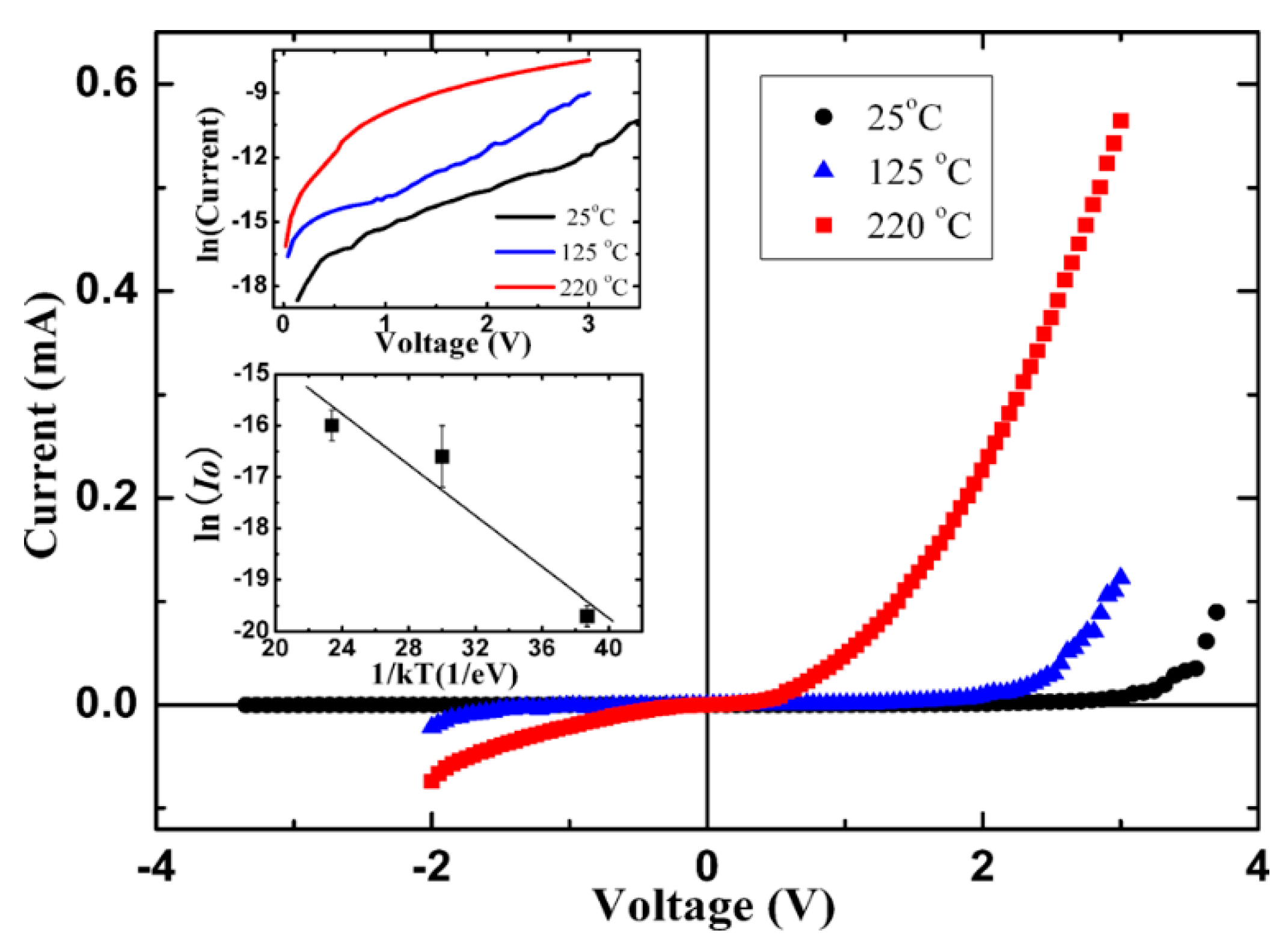
4.1.3. Electrical Transport Behavior of N-ZnO NRs/P-BDD Heterojunction at Elevated Temperatures
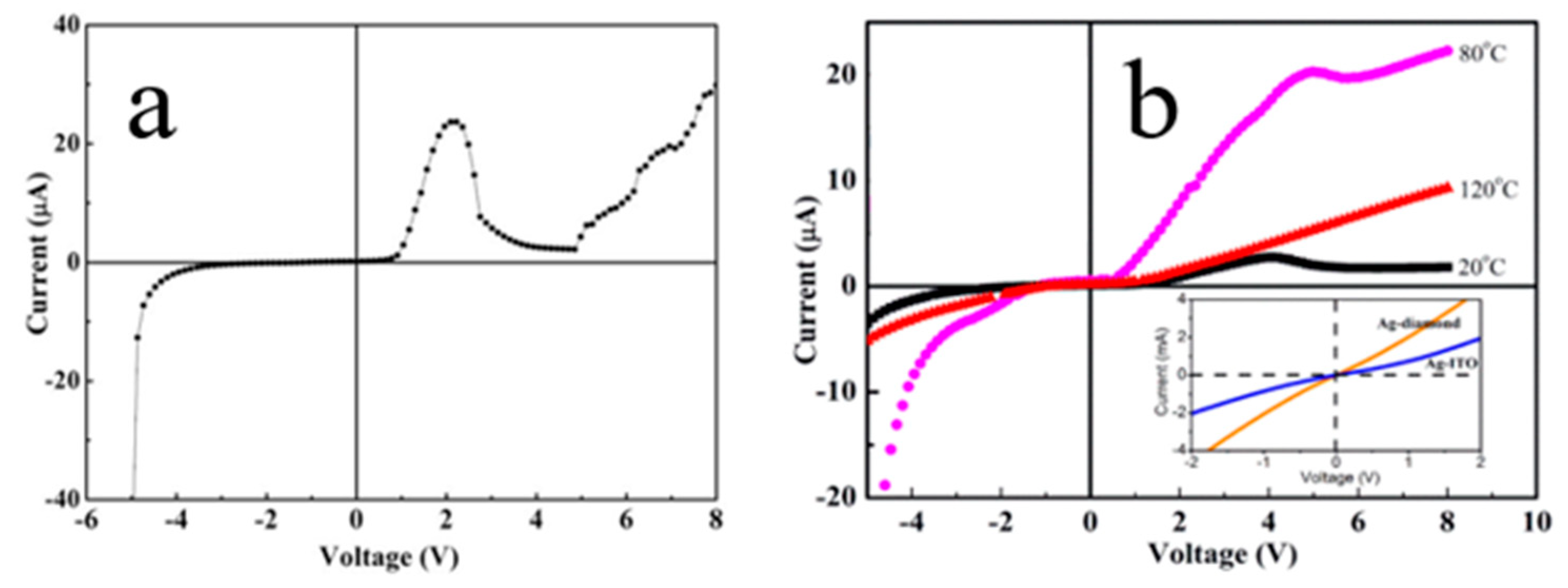
4.1.4. NDR for 1D N-ZnO/P-Degenerated BDD Heterojunction at Elevated Temperatures
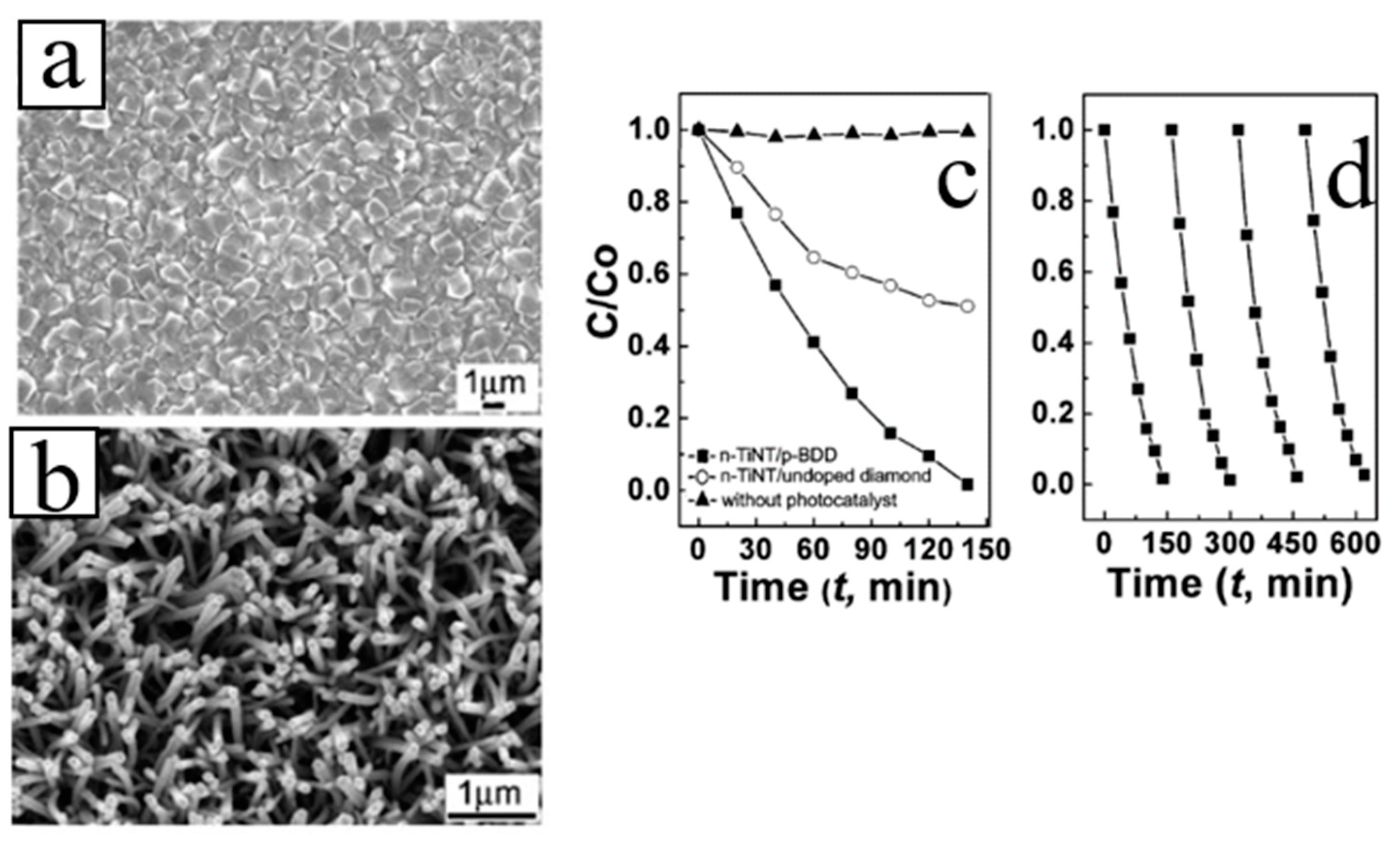
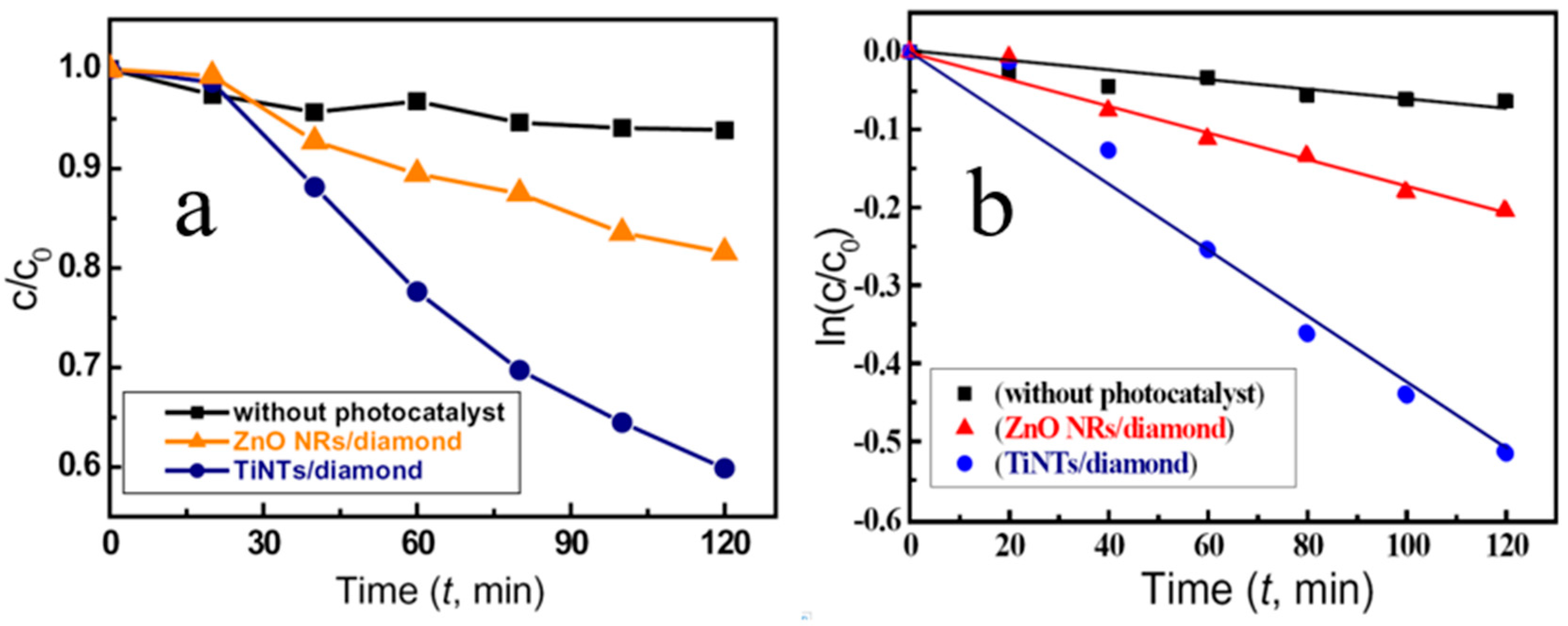
4.2. 1 D N-TiO2/P-Diamond Related Optoelectronic Devices
4.3. 1D N- WO3/P-BDD-Related Optoelectronic Devices
5. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Qi, J.; Wang, Q.; Huang, Y.; Liao, Q.; Zhang, Y. Single ZnO nanotetrapod-based sensors for monitoring localized UV irradiation. Nanoscale 2013, 5, 5981–5985. [Google Scholar] [CrossRef]
- Huo, N.; Yang, S.; Wei, Z.; Li, J. Synthesis of WO3 nanostructures and their ultraviolet photoresponse properties. J. Mater. Chem. C 2013, 1, 3999–4007. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, M.; Zhang, Y.; Chen, L.; Wang, Z. Fabrication of a high-brightness blue-light-emitting diode using a ZnO-Nanowire array grown on p-GaN thin film. Adv. Mater. 2009, 21, 2767–2770. [Google Scholar] [CrossRef]
- Jean, J.; Chang, S.; Brown, P.R.; Cheng, J.J.; Rekemeyer, P.H.; Bawendi, M.G.; Gradecak, S.; Bulovic, V. ZnO nanowire arrays for enhanced photocurrent in PbS quantum dot solar cells. Adv. Mater. 2013, 25, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Liu, B.; Zhai, J.; Fan, H.; Wang, D.; Lin, Y.; Xie, T. Synthesis of Zn-doped TiO2 microspheres with enhanced photovoltaic performance and application for dye-sensitized solar cells. Electrochim. Acta 2011, 56, 6517–6523. [Google Scholar] [CrossRef]
- Chai, G.; Lupan, O.; Chow, L.; Heinrich, H. Crossed zinc oxide nanorods for ultraviolet radiation detection. Sens. Actuators A Phys. 2009, 150, 184–187. [Google Scholar] [CrossRef]
- Wu, P.M.; Ishii, S.; Tanabe, K.; Munakata, K.; Hammond, R.H.; Tokiwa, K.; Geballe, T.H.; Beasley, M.R. Synthesis and ionic liquid gating of hexagonal WO3 thin films. Appl. Phys. Lett. 2015, 106, 042602. [Google Scholar] [CrossRef]
- Chebil, W.; Gokarna, A.; Fouzri, A.; Hamdaoui, N.; Nomenyo, K.; Lerondel, G. Study of the growth time effect on the structural, morphological and electrical characteristics of ZnO/p-Si heterojunction diodes grown by sol-gel assisted chemical bath deposition method. J. Alloys Compd. 2019, 771, 448–455. [Google Scholar] [CrossRef]
- Ko, K.B.; Ryu, B.D.; Han, M.; Hong, C.H.; Doan, T.A.; Cuong, T.V. Inserting dome shape microstructure for enhancement of ultraviolet photodetector performance of n-ZnO nanorods/p-Si heterojunction. J. Alloys Compd. 2020, 823, 153884. [Google Scholar] [CrossRef]
- Rahman, M.A.; Scott, J.A.; Gentle, A.; Phillips, M.R.; Ton-That, C. A facile method for bright, colour-tunable light-emitting diodes based on Ga-doped ZnO nanorods. Nanotechnology 2018, 29, 425707. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Wang, R.; Sun, J.; Si, Y.; Wang, H.; Pan, C.; Dai, Y. Ferroelectricity-induced performance enhancement of V-doped ZnO/Si photodetector by direct energy band modulation. Nano Energy 2019, 65, 104046. [Google Scholar] [CrossRef]
- Li, H.; Zhao, W.; Liu, Y.; Liang, Y.; Ma, L.; Zhu, M.; Yi, C.; Xiong, L.; Gao, Y. High-level-Fe-doped p-type ZnO nanowire array/n-GaN film for ultraviolet-free white light-emitting diodes. Mater. Lett. 2019, 239, 45–47. [Google Scholar] [CrossRef]
- Khan, F.; Khan, W.; Kim, S.D. High-Performance Ultraviolet Light Detection Using nano-scale-fin isolation AlGaN/GaN heterostructures with ZnO nanorods. Nanomaterials 2019, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Choi, J.E.; Kim, Y.J.; Hwang, S.; Kim, S.K.; Kim, J.K.; Jeong, H.Y.; Hong, Y.J. Reverse-bias-driven dichromatic electroluminescence of n-ZnO wire arrays/p-GaN film heterojunction light-emitting diodes. Appl. Phys. Lett. 2016, 109, 101103. [Google Scholar] [CrossRef]
- Li, Y.; Wan, C.; Chang, C.; Tsai, W.; Huang, Y.; Wang, K.; Yang, P.; Cheng, H. Thickness effect of NiO on the performance of ultraviolet sensors with p-NiO/n-ZnO nanowire heterojunction structure. Vacuum 2015, 118, 48–54. [Google Scholar] [CrossRef]
- Fu, K.; Huang, J.; Yao, N.; Deng, X.; Xu, X.; Li, L. Hybrid nanostructures of TiO2 nanorod array/Cu2O with a CH3NH3PbI3 interlayer for enhanced photocatalytic activity and photoelectrochemical performance. RSC Adv. 2016, 6, S7695–S7700. [Google Scholar] [CrossRef]
- Perng, D.; Lin, H.; Hong, M. High-performance ultraviolet detection and visible-blind photodetector based on Cu2O/ZnO nanorods with poly-(N-vinylcarbazole) intermediate layer. Appl. Phys. Lett. 2015, 107, 241113. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Eftekhari, E.; Huo, Z.; Li, X.; Tade, M.O.; Yan, C.; Yan, Z.; Li, C.; Li, Q.; et al. High performance heterojunction photocatalytic membranes formed by embedding Cu2O and TiO2 nanowires in reduced graphene oxide. Catal. Sci. Technol. 2018, 8, 1704–1711. [Google Scholar] [CrossRef]
- El-Shaer, A.; Ismail, I.W.; Abdelfatah, M. Towards low cost fabrication of inorganic white light emitting diode based on electrodeposited Cu2O thin film/TiO2 nanorods heterojunction. Mater. Res. Bull. 2019, 116, 111–116. [Google Scholar] [CrossRef]
- Kwon, Y.; Kang, S.O.; Cheon, J.A.; Song, Y.; Lee, J.J. Fabrication of a Graphene/ZnO based p-n junction device and its ultraviolet photoresponse properties. Appl. Surf. Sci. 2017, 415, 2–7. [Google Scholar] [CrossRef]
- Weng, B.; Wu, J.; Zhang, N.; Xu, Y. Observing the role of graphene in boosting the Two-electron reduction of oxygen in graphene-WO3 nanorod photocatalysts. Langmuir 2014, 30, 5574–5584. [Google Scholar] [CrossRef] [PubMed]
- Sang, D.; Li, H.; Cheng, S.; Wang, Q.; Liu, J.; Wang, Q.; Wang, S.; Han, C.; Chen, K.; Pan, Y. Ultraviolet photoelectrical properties of a n-ZnO nanorods/p-diamond heterojunction. RSC Adv. 2015, 5, 49211–49215. [Google Scholar] [CrossRef]
- Li, H.; Sang, D.; Cheng, S.; Lu, J.; Zhai, X.; Chen, L.; Pei, X. Epitaxial growth of ZnO nanorods on diamond and negative differential resistance of n-ZnO nanorod/p-diamond heterojunction. Appl. Surf. Sci. 2013, 280, 201–206. [Google Scholar] [CrossRef]
- Sang, D.; Li, H.; Cheng, S.; Wang, Q.; Yu, Q.; Yang, Y. Electrical transport behavior of n-ZnO nanorods/p-diamond heterojunction device at higher temperatures. J. Appl. Phys. 2012, 112, 036101. [Google Scholar] [CrossRef]
- Davydova, M.; Laposa, A.; Smarhak, J.; Kromka, A.; Neykova, N.; Nahlik, J.; Kroutil, J.; Drahokoupil, J.; Voves, J. Gas-sensing behaviour of ZnO/diamond nanostructures. Beilstein J. Nanotechnol. 2018, 9, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, L.; Wang, Y.; Zhang, H.; Zhang, S. Photoelectrocatalytic activity of an ordered and vertically aligned TiO2 nanorod array/BDD heterojunction electrode. Sci. Bull. 2017, 62, 619–625. [Google Scholar] [CrossRef]
- Suzuki, N.; Okazaki, A.; Kuriyama, H.; Serizawa, I.; Hirami, Y.; Hara, A.; Hirano, Y.; Nakabayashi, Y.; Roy, N.; Terashima, C.; et al. Synergetic effect in water treatment with mesoporous TiO2/BDD Hybrid electrode. RSC Adv. 2020, 10, 1793–1798. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Kumar, R.T.R. Facile construction of vertically aligned ZnO nanorod/PEDOT: PSS hybrid heterojunction-based ultraviolet light sensors: Efficient performance and mechanism. Nanotechnology 2016, 27, 095304. [Google Scholar] [CrossRef]
- Li, H.; Cheng, S.; Li, J.; Song, J. A Review on the low-dimensional and hybridized nanostructured diamond films. J. Nanomater. 2015, 2015, 692562. [Google Scholar] [CrossRef]
- Sang, D.; Wang, Q.; Wang, Q.; Zhang, D.; Hu, H.; Wang, W.; Zhang, B.; Fan, Q.; Li, H. Improved electrical transport properties of an n-ZnO nanowire/p-diamond heterojunction. RSC Adv. 2018, 8, 28804–28809. [Google Scholar] [CrossRef]
- Sang, D.; Liu, J.; Wang, X.; Zhang, D.; Ke, F.; Hu, H.; Wang, W.; Zhang, B.; Li, H.; Liu, B.; et al. Negative differential resistance of n-ZnO nanorods/p-degenerated diamond heterojunction at high temperatures. Front. Chem. 2020, 8, 531. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Huang, B.R.; Chu, J.P.; Prasannan, A.; Tsai, H.C. Interface engineering of ultrananocrystalline diamond/MoS2-ZnO heterostructures and its highly enhanced hydrogen gas sensing properties. Sens. Actuators B Chem. 2019, 292, 70–79. [Google Scholar] [CrossRef]
- Yu, Q.; Li, J.; Li, H.; Wang, Q.; Cheng, S.; Li, L. Fabrication, structure, and photocatalytic activities of boron-doped ZnO nanorods hydrothermally grown on CVD diamond film. Chem. Phys. Lett. 2012, 539, 74–78. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, S.; Wu, C.; Pei, K.; Song, Y.P.; Li, H.; Wang, Q.; Sang, D. Fabrication and high temperature electronic behaviors of n-WO3 nanorods/p-diamond heterojunction. Appl. Phys. Lett. 2017, 110, 052106. [Google Scholar] [CrossRef]
- Yin, Z.; Tordjman, M.; Vardi, A.; Kalish, R.; Del Alamo, J.A. Diamond:H/WO3 metal–oxide–semiconductor field-effect transistor. IEEE Electr. Device L 2018, 39, 540–543. [Google Scholar] [CrossRef]
- Tordjman, M.; Weinfeld, K.; Kalish, R. Boosting surface charge-transfer doping efficiency and robustness of diamond with WO3 and ReO3. Appl. Phys. Lett. 2017, 111, 111601. [Google Scholar] [CrossRef]
- Suzuki, N.; Okazaki, A.; Kuriyama, H.; Serizawa, I.; Hara, A.; Hirano, Y.; Nakabayashi, Y.; Roy, N.; Terashima, C.; Nakata, K.; et al. Synthesis of mesoporous TiO2/boron-doped diamond photocatalyst and its photocatalytic activity under deep UV Light (λ= 222 nm) irradiation. Molecules 2018, 23, 3095. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Li, H.; Gao, S.; Lin, Y.; Li, H. A facile route to n-type TiO2-nanotube/p-type boron-doped-diamond heterojunction for highly efficient photocatalysts. Chem. Commun. 2010, 46, 3119–3121. [Google Scholar] [CrossRef]
- Yuan, J.; Li, H.; Wang, Q.; Cheng, S.; Zhang, X.; Yu, H.; Zhu, X.; Xie, Y. Fabrication, Characterization and optical properties of TiO2 nanotube arrays on boron-doped diamond film through liquid phase deposition. Chem. Res. Chin. Univ. 2014, 30, 18–22. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, D.; Min, T.; Wang, H. 3D TiO2/Diamond ultraviolet detector using back-to-back Pd schottky electrode. Phys. Status Solidi A 2020, 217, 2000218. [Google Scholar] [CrossRef]
- Behul, M.; Vojs, M.; Marton, M.; Michniak, P.; Mikolasek, M.; Kurniawan, M.; Honig, H.L.; Zyabkin, D.V.; Ramirez, M.O.; Spiess, L.; et al. Nanostructured boron doped diamond enhancing the photoelectrochemical performance of TiO2/BDD heterojunction anodes. Vacuum 2020, 117, 109006. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, Z.; Dai, Z. Nanobelts of semiconducting oxides. Science 2001, 291, 1947–1949. [Google Scholar] [CrossRef]
- Morales, A.M.; Lieber, C.M. A laser ablation method for the synthesis of crystalline semiconductor nanowires. Science 1998, 279, 208–211. [Google Scholar] [CrossRef]
- Cui, Y.; Lieber, C.M. Functional nanoscale electronic devices assembled using silicon nanowire building blocks. Science 2001, 291, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Kempa, T.J.; Lieber, C.M. Single nanowire photovoltaics. Chem. Soc. Rev. 2009, 38, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Rasch, F.; Postica, V.; Schutt, F.; Mishra, Y.K.; Nia, A.S.; Lohe, M.R.; Feng, X.L.; Adelung, R.; Lupan, O. Highly selective and ultra-low power consumption metal oxide based hydrogen gas sensor employing graphene oxide as molecular sieve. Sens. Actuators B Chem. 2020, 320, 128363. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, M.; Zhao, L.; Guo, J.; Zhang, B.; Gu, G.; Cheng, G.; Du, Z. The high-speed ultraviolet photodetector of ZnO nanowire Schottky barrier based on the triboelectric-nanogenerator-powered surface-ionic-gate. Nano Energy 2019, 60, 680–688. [Google Scholar] [CrossRef]
- Ouyang, B.S.; Zhao, H.; Wang, Z.; Yang, Y. Dual-polarity response in self-powered ZnO NWs/Sb2Se3 film heterojunction photodetector array for optical communication. Nano Energy 2020, 68, 104312. [Google Scholar] [CrossRef]
- Xu, X.; Chen, J.; Cai, S.; Long, Z.; Zhang, Y.; Su, L.; He, S.; Tang, C.; Liu, P.; Peng, H.; et al. Real-Time Wearable UV-Radiation monitor based on a high-performance p-CuZnS/n-TiO2 photodetector. Adv. Mater. 2018, 30, 1803165. [Google Scholar] [CrossRef]
- Chen, A.; Zhu, H.; Wu, Y.; Chen, M.; Zhu, Y.; Gui, X.; Tang, Z. Beryllium-Assisted p-Type doping for ZnO homojunction light-emitting devices. Adv. Funct. Mater. 2016, 26, 3696–3702. [Google Scholar] [CrossRef]
- Chen, D.; Chen, S.; Yue, S.; Lu, B.; Pan, X.; He, H.; Ye, Z. N-ZnO nanorod arrays/p-GaN light-emitting diodes with graphene transparent electrode. J. Lumin. 2019, 216, 116719. [Google Scholar] [CrossRef]
- Bai, Z.; Fu, M.; Zhang, Y. Vertically aligned and ordered ZnO/CdS nanowire arrays for self-powered UV-visible photosensing. J. Mater. Sci. 2017, 52, 1308–1317. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Nengzi, L.; Li, B.; Gou, J.; Cheng, X. Synthesis of SnS/TiO2 nano-tube arrays photoelectrode and its high photoelectrocatalytic performance for elimination of 2,4,6-trichlorophenol. Sep. Purif. Technol. 2019, 228, 115742. [Google Scholar] [CrossRef]
- Yan, H.; Liu, L.; Wang, R.; Zhu, W.; Ren, X.; Luo, L.; Zhang, X.; Luo, S.; Ai, X.; Wang, J. Binary composite MoS2/TiO2 nanotube arrays as a recyclable and efficient photocatalyst for solar water disinfection. Chem. Eng. J. 2020, 401, 126052. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, H.; Ji, J.; Pei, X. Fabrication of WO3 nanorod/graphene/BiV1−xMoxO4 heterojunction photoelectrode for efficient photoelectrochemical water splitting. J. Mater. Sci. Mater. Electron. 2020, 31, 3323–3331. [Google Scholar] [CrossRef]
- Das, A.; Kushwaha, A.; Sivasayan, R.K.; Chakraborty, S.; Dutta, H.S.; Karmakar, A.; Chattopadhyay, S.; Chi, D.Z.; Dalapati, G.K. Temperature-dependent electrical characteristics of CBD/CBD grown n-ZnO nanowire/p-Si heterojunction diodes. J. Phys. D Appl. Phys. 2016, 49, 145105. [Google Scholar] [CrossRef]
- Singh, S.K.; Hazra, P. Performance of RF sputtered p-Si/n-ZnO nanoparticle thin film heterojunction diodes in high temperature environment. Appl. Surf. Sci. 2016, 400, 206–211. [Google Scholar] [CrossRef]
- Makhlouf, M.M.; El-Nahass, M.M.; Zeyada, M.H. Fabrication, temperature dependent current-voltage characteristics and photoresponse properties of Au/α-PbO2/p-Si/Al heterojunction photodiode. Mater. Sci. Semicond. Process. 2017, 58, 68–75. [Google Scholar] [CrossRef]
- Angus, J.C.; Hayman, C.C. Low-Pressure, Metastable growth of diamond and “diamondlike” phases. Science 1988, 241, 913–921. [Google Scholar] [CrossRef]
- Reggiani, L.; Bosi, S.; Canali, C.; Nava, F.; Kozlov, S.F. Hole-drift velocity in natural diamond. Phys. Rev. B 1981, 23, 3050–3057. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Li, L.; Lu, X.; Li, B.; Jin, Z.; Zou, G. Investigation on crystalline structure, boron distribution, and residual stresses in freestanding boron-doped CVD diamond films. J. Cryst. Growth 2010, 312, 1986–1991. [Google Scholar] [CrossRef]
- Liu, D.; Hao, L.; Chen, Z.; Zhao, W.; Shen, Y.; Bian, Y.; Tang, K.; Ye, J.; Zhu, S.; Zhang, R.; et al. Sulfur regulation of boron doping and growth behavior for high-quality diamond in microwave plasma chemical vapor deposition. Appl. Phys. Lett. 2020, 117, 022101. [Google Scholar] [CrossRef]
- Wei, K.; Wang, R.; Li, J.; Liu, B.; Wei, Q.; Wu, R.; Wu, S.; Hu, W.; Wang, H. Secondary electron emission properties of double-layer B-doped diamond films. Diam. Relat. Mater. 2020, 106, 107826. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, F.; Li, D.; Niu, J.; Pan, F. Deposition of high-quality zinc oxide thin films on diamond substrates for high-frequency surface acoustic wave filter applications. Thin Solid Films 2005, 485, 257–261. [Google Scholar] [CrossRef]
- Tang, K.; Wang, L.; Huang, J.; Xu, R.; Lai, J.; Wang, J.; Min, J.; Shi, W.; Xia, Y. Preparation of ZnO thin films on free-standing diamond substrates. Plasma Sci. Technol. 2009, 11, 587–591. [Google Scholar]
- Sun, J.; Bai, Y.; Sun, J.; Du, G.; Jiang, X. Structural and electrical properties of ZnO films on freestanding thick diamond films. Chin. Sci. Bull. 2008, 53, 2931–2934. [Google Scholar] [CrossRef]
- Wang, C.; Yang, G.; Liu, H.; Han, Y.; Luo, J.; Gao, C.; Zou, G. Experimental analysis and theoretical model for anomalously high ideality factors in ZnO/diamond pn junction diode. Appl. Phys. Lett. 2004, 84, 2427–2429. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, D.; Zhi, J. A novel tyrosinase biosensor based on biofunctional ZnO nanorod microarrays on the nanocrystalline diamond electrode for detection of phenolic compounds. Bioelectrochemistry 2009, 75, 44–49. [Google Scholar] [CrossRef]
- Siuzdak, K.; Bogdanowicz, R.; Sawczak, M.; Sobaszek, M. Enhanced capacitance of composite TiO2 nanotube/boron-doped diamond electrodes studied by impedance spectroscopy. Nanoscale 2015, 7, 551–558. [Google Scholar] [CrossRef]
- Bensmaine, S.; Le Brizoual, L.; Elmazria, O.; Fundenberger, J.J.; Belmahi, M.; Benyoucef, B. SAW devices based on ZnO inclined c-axis on diamond. Diam. Relat. Mater. 2008, 17, 1420–1423. [Google Scholar] [CrossRef]
- Hikavyy, A.; Clauws, P.; Vanbesien, K.; De Visschere, P.; Williams, O.A.; Daenen, M.; Haenen, K.; Butler, J.E.; Feygelson, T. Atomic layer deposition of ZnO thin films on boron-doped nanocrystalline diamond. Diam. Relat. Mater. 2007, 16, 983–986. [Google Scholar] [CrossRef]
- Saw, K.G.; Tneh, S.S.; Pung, S.Y.; Ng, S.S.; Yam, F.K.; Hassan, Z. Ultraviolet photoresponse properties of Zinc Oxide nanorods on heavily boron-doped diamond heterostructure. Adv. Mater. Res. 2014, 832, 172. [Google Scholar] [CrossRef]
- Dixit, T.; Palani, I.A.; Singh, V. Insights into non-noble metal based nanophotonics: Exploration of Cr-coated ZnO nanorods for optoelectronic applications. RSC Adv. 2018, 8, 6820–6833. [Google Scholar] [CrossRef]
- Li, W.; Gao, S.; Li, L.; Jiao, S.; Li, H.; Wang, J.; Yu, Q.; Zhang, Y.; Wang, D.; Zhao, L. Hydrothermal synthesis of a 3D double-sided comb-like ZnO nanostructure and its growth mechanism analysis. Chem. Commun. 2016, 52, 8231–8234. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Liu, Y.H. Field emission properties of Al-doped ZnO nanosheet based on field emitter device with UV exposure. RSC Adv. 2017, 7, 14219–14223. [Google Scholar] [CrossRef]
- Soci, C.; Zhang, A.; Xiang, B.; Dayeh, S.A.; Aplin, D.; Park, J.; Bao, X.; Lo, Y.H.; Wang, D. ZnO nanowire UV photodetector with high internal gain. Nano Lett. 2007, 7, 1003–1009. [Google Scholar] [CrossRef]
- Li, Y.; Della Valle, F.; Simonnet, M.; Yamada, I.; Delaunay, J.J. High-performance UV detector made of ultra-long ZnO bridging nanowires. Nanotechnology 2009, 20, 045501. [Google Scholar] [CrossRef]
- Galdamez-Martinez, A.; Santana, G.; Guell, F.; Martinez-Alanis, P.R.; Dutt, A. Photoluminescence of ZnO nanowires: A Review. Nanomaterials 2020, 10, 857. [Google Scholar] [CrossRef]
- Tennant, S. On the Nature of the Diamond. FRS Philos. Trans. R. Soc. Lond. 1797, 87, 123–127. [Google Scholar]
- Gao, S.; Jiao, S.; Lei, B.; Li, H.; Wang, J.; Yu, Q.; Wang, D.; Guo, F.; Zhao, L. Efficient photocatalyst based on ZnO nanorod arrays/p-type boron-doped-diamond heterojunction. J. Mater. Sci. Mater. Electron. 2015, 26, 1018–1022. [Google Scholar] [CrossRef]
- Wang, C.D.; Jha, S.K.; Chen, Z.H.; Ng, T.W.; Liu, Y.K.; Yuen, M.F.; Lu, Z.Z.; Kwok, S.Y.; Zapien, J.A.; Bello, I.; et al. Construction and evaluation of high-quality n-ZnO nanorod/p-diamond heterojunctions. J. Nanosci. Nanotechnol. 2012, 12, 4560–4563. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Li, M.; Li, C.; Lei, Y.; Sun, D.; Yang, B. Fabrication and catalytic activities of anodes consisting of ZnO nanorods on boron-doped diamond film. J. Alloys Compd. 2018, 743, 187–195. [Google Scholar] [CrossRef]
- Huang, B.; Ke, W.; Peng, Y.; Liou, R. Low temperature annealing effect on photoresponse of the bilayer structures of ZnO nanorod/nanodiamond films based on ultraviolet photodetector. Thin Solid Films 2016, 605, 243–247. [Google Scholar] [CrossRef]
- Huang, B.R.; Chu, J.P.; Saravanan, A.; Yenesew, M.M.; Bonninghoff, N.; Chang, C.H. High-performance sensor based on thin-film metallic glass/ultra-nanocrystalline diamond/ZnO Nanorod heterostructures for detection of hydrogen gas at room temperature. Chem. Eur. J. 2019, 25, 10385–10393. [Google Scholar] [CrossRef]
- Huang, B.R.; Saravanan, A.; Kathiravan, D.; Liou, R.H.; Keiser, G. Role of conductive nitrogen incorporated diamond nanowires for enhancing the UV detection and field emission properties of ZnO nanotubes. Mater. Des. 2018, 154, 130–139. [Google Scholar] [CrossRef]
- Reddy, N.K.; Ahsanulhaq, Q.; Kim, J.H.; Hahn, Y.B. Behavior of n-ZnO nanorods/p-Si heterojunction devices at higher temperatures. Appl. Phys. Lett. 2008, 92, 043127. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 Nanotubes: Recent advances in synthesis and gas sensing properties. Sensors 2013, 13, 14813–14838. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. Engl. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Sun, H.; Tian, W.; Cao, F.; Xiong, J.; Li, L. Ultrahigh-performance self-powered flexible double-twisted fibrous broadband perovskite photodetector. Adv. Mater. 2018, 30, 1706986. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, S.; Poddar, P. Template-free fabrication of highly-oriented singlecrystalline 1d-rutile TiO2-MWCNT composite for enhanced photoelectrochemical activity. J. Phys. Chem. C 2014, 118, 19363–19373. [Google Scholar] [CrossRef]
- Sun, Z.; Kim, J.H.; Liao, T.; Zhao, Y.; Bijarbooneh, F.; Malgras, V.; Dou, S. Continually adjustable oriented 1D TiO2 nanostructure arrays with controlled growth of morphology and their application in dye-sensitized solar cells. Cryst. Eng. Comm. 2012, 14, 5472–5478. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Wang, B.; Yu, J.; Hu, Y.; Xia, C.; Zhang, J.; Liu, R. Significant enhancement of power conversion efficiency for dye sensitized solar cell using 1D/3D network nanostructures as photoanodes. Sci. Rep. UK 2015, 5, 9305. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.S.; Zhang, K.; Yang, Y. Self-Powered UV Photodetector array based on P3HT/ZnO nanowire array heterojunction. Adv. Mater. Technol. US 2017, 2, 1700208. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Song, J.; Kulinich, S.A.; Yan, J.; Li, Z.; He, J.; Kan, C.; Zeng, H. Epitaxial ZnO nanowire-on-nanoplate structures as efficient and transferable field emitters. Adv. Mater. 2013, 25, 5750. [Google Scholar] [CrossRef]
- Andronic, L.; Isac, L.; Miralles-Cuevas, S.; Visa, M.; Oller, I.; Duta, A.; Malato, S. Pilot-plant evaluation of TiO2 and TiO2-based hybrid photocatalysts for solar treatment of polluted water. J. Hazard. Mater. 2016, 320, 469–478. [Google Scholar] [CrossRef]
- Wang, X.; Ni, Q.; Zeng, D.; Liao, G.; Xie, C. Charge separation in branched TiO2 nanorod array homojunction aroused by quantum effect for enhanced photocatalytic decomposition of gaseous benzene. Appl. Surf. Sci. 2016, 389, 165–172. [Google Scholar] [CrossRef]
- Lu, N.; Su, Y.; Li, J.; Yu, H.; Quan, X. Fabrication of quantum-sized CdS-coated TiO2 nanotube array with efficient photoelectrochemical performance using modified successive ionic layer absorption and reaction (SILAR) method. Sci. Bull. 2015, 60, 1281–1286. [Google Scholar] [CrossRef]
- Liu, J.; Han, L.; Ma, H.; Tian, H.; Yang, J.; Zhang, Q.; Seligmann, B.J.; Wang, S.; Liu, J. Template-free synthesis of carbon doped TiO2 mesoporous microplates for enhanced visible light photodegradation. Sci. Bull. 2016, 61, 1543–1550. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X. Design of BDD-TiO2 hybrid electrode with P-N function for photoelectrocatalytic degradation of organic contaminants. Environ. Sci. Technol. 2008, 42, 4934–4939. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Li, H.; Wang, Q.; Cheng, S.; Zhang, X.; Yin, Z.; Yu, H.; Zhu, X.; Xie, Y. Fabrication, characterisation and photocatalytic activity of TiO2 nanorods on boron doped diamond film through hydrothermal method. Mater. Technol. 2015, 30, 101–105. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Q.; Liu, J.; Ai, T.; Li, H. Fabrication and characteristics of TiO2 nanotubes on hemispherical diamond films using ZnO nanorods as template. Nano 2015, 10, 1550083. [Google Scholar] [CrossRef]
- Sobaszek, M.; Siuzdak, K.; Sawczak, M.; Ryl, J.; Bogdanowicz, R. Fabrication and characterization of composite TiO2 nanotubes/boron-doped diamond electrodes towards enhanced supercapacitors. Thin Solid Films 2016, 601, 35–40. [Google Scholar] [CrossRef]
- Sawczak, M.; Sobaszek, M.; Siuzdak, K.; Ryl, J.; Bogdanowicz, R.; Darowicki, K.; Gazda, M.; Ceniana, A. Formation of highly conductive boron-doped diamond on TiO2 nanotubes composite for supercapacitor or energy storage devices. J. Electrochem. Soc. 2015, 162, 2085–2092. [Google Scholar] [CrossRef]
- Feng, X.; Chen, Y.; Qin, Z.; Wang, M.; Guo, L. Facile fabrication of sandwich structured WO3 nanoplate arrays for efficient photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2016, 8, 18089–18096. [Google Scholar] [CrossRef]
- Coelho, D.; Gaudencio, J.P.R.S.; Carminati, S.A.; Ribeiro, F.W.P.; Nogueira, A.F.; Mascaro, L.H. Bi electrodeposition on WO3 photoanode to improve the photoactivity of the WO3/BiVO4 heterostructure to water splitting. Chem. Eng. J. 2020, 399, 125836. [Google Scholar] [CrossRef]
- Han, X.; Yao, B.; Li, K.; Zhu, W.; Zhang, X. Preparation and photocatalytic performances of WO3/TiO2 composite nanofibers. J. Chem. N. Y. 2020, 2020, 2390486. [Google Scholar]
- Hong, D.; Wang, W.; Chen, Y.; Sun, J.; Shen, B. In-situ observation of self-regulated switching behavior in WO3-x based resistive switching devices. Appl. Phys. Lett. 2014, 105, 113504. [Google Scholar] [CrossRef]
- Zeb, S.; Sun, G.X.; Nie, Y.; Cui, Y.; Jiang, X. Synthesis of highly oriented WO3 nanowire bundles decorated with Au for gas sensing application. Sens. Actuators B Chem. 2020, 321, 128439. [Google Scholar] [CrossRef]
- Hu, Q.; He, J.; Chang, J.; Gao, J.; Huang, J.; Feng, L. Needle-shaped WO3 nanorods for triethylamine gas sensing. ACS Appl. Nano Mater. 2020, 3, 9045–9054. [Google Scholar] [CrossRef]
- Sze, S.M.; Kwok, K.N. Physics of Semiconductor Devices, 3rd ed.; Wiley: New York, NY, USA, 2007. [Google Scholar]
- Chung, D.S.; Lee, D.H.; Yang, C.; Hong, K.; Park, C.E.; Park, J.W.; Kwon, S.K. Origin of high mobility within an amorphous polymeric semiconductor: Space-charge-limited current and trap distribution. Appl. Phys. Lett. 2008, 93, 033303. [Google Scholar] [CrossRef]
- Burrows, P.E.; Shen, Z.; Bulovic, V.; McCarty, D.M.; Forrest, S.R.; Cronin, J.A.; Thompsom, M.E. Relation between electroluminesce and current transport in organic heterojunction light emitting devices. J. Appl. Phys. 1996, 79, 7991–8006. [Google Scholar] [CrossRef]
- Vuong, N.M.; Hieu, H.N.; Kim, D. An edge-contacted pn-heterojunction of a p-SWCNT/n-WO3 thin film. J. Mater. Chem. C 2013, 1, 5153–5160. [Google Scholar] [CrossRef]
- Ling, Z.; Leach, C.; Freer, R. NO2 Sensitivity of a heterojunction sensor based on WO3 and doped SnO2. J. Eur. Ceram. Soc. 2003, 23, 1881–1891. [Google Scholar] [CrossRef]
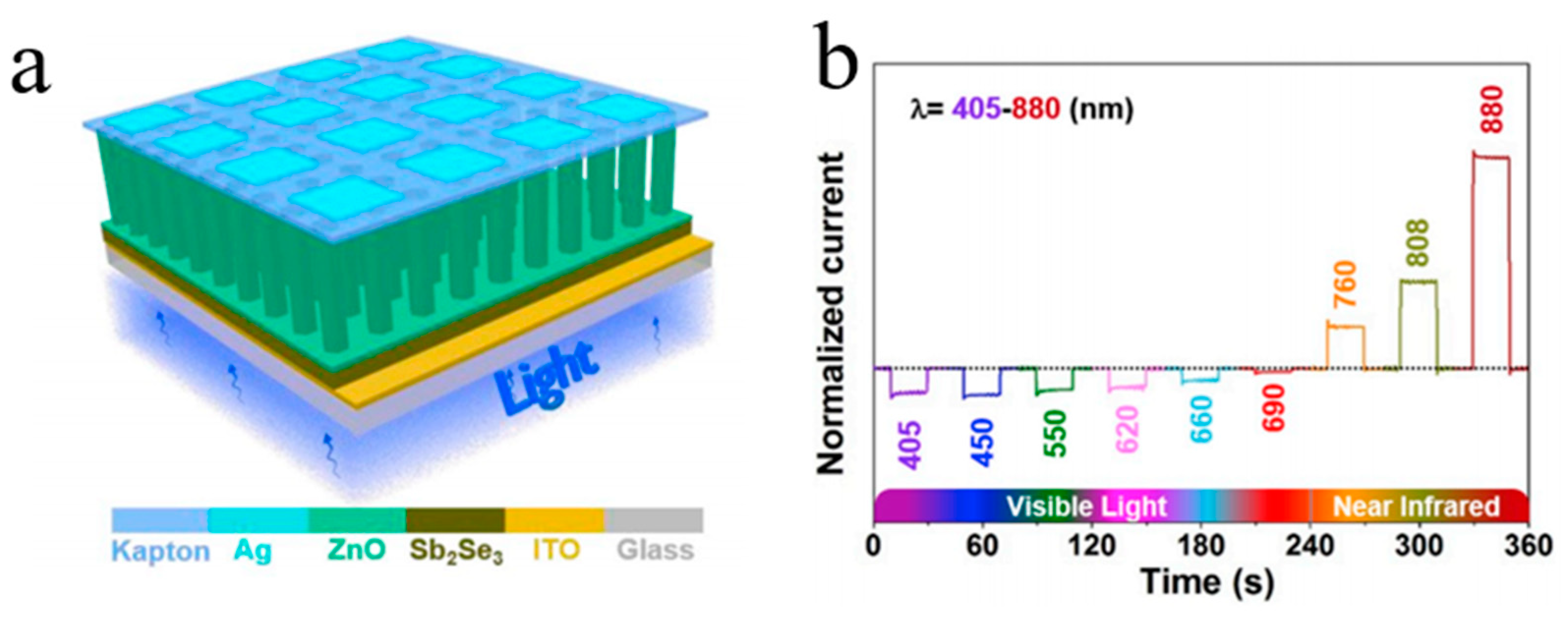
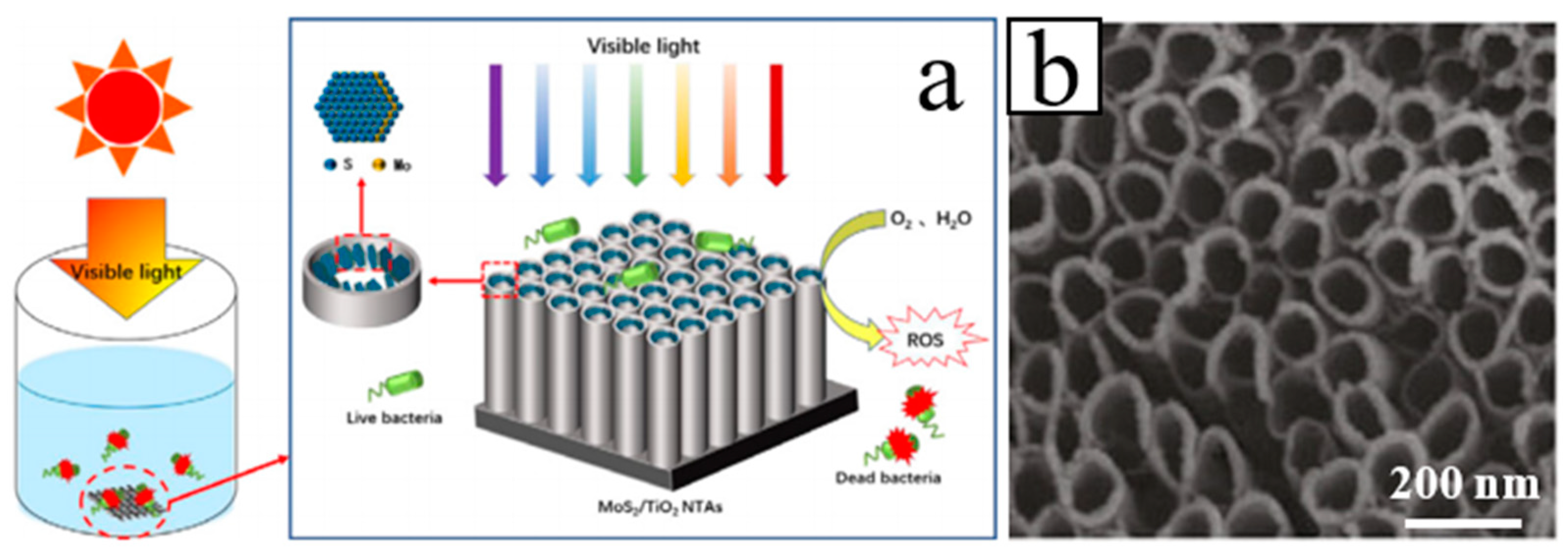
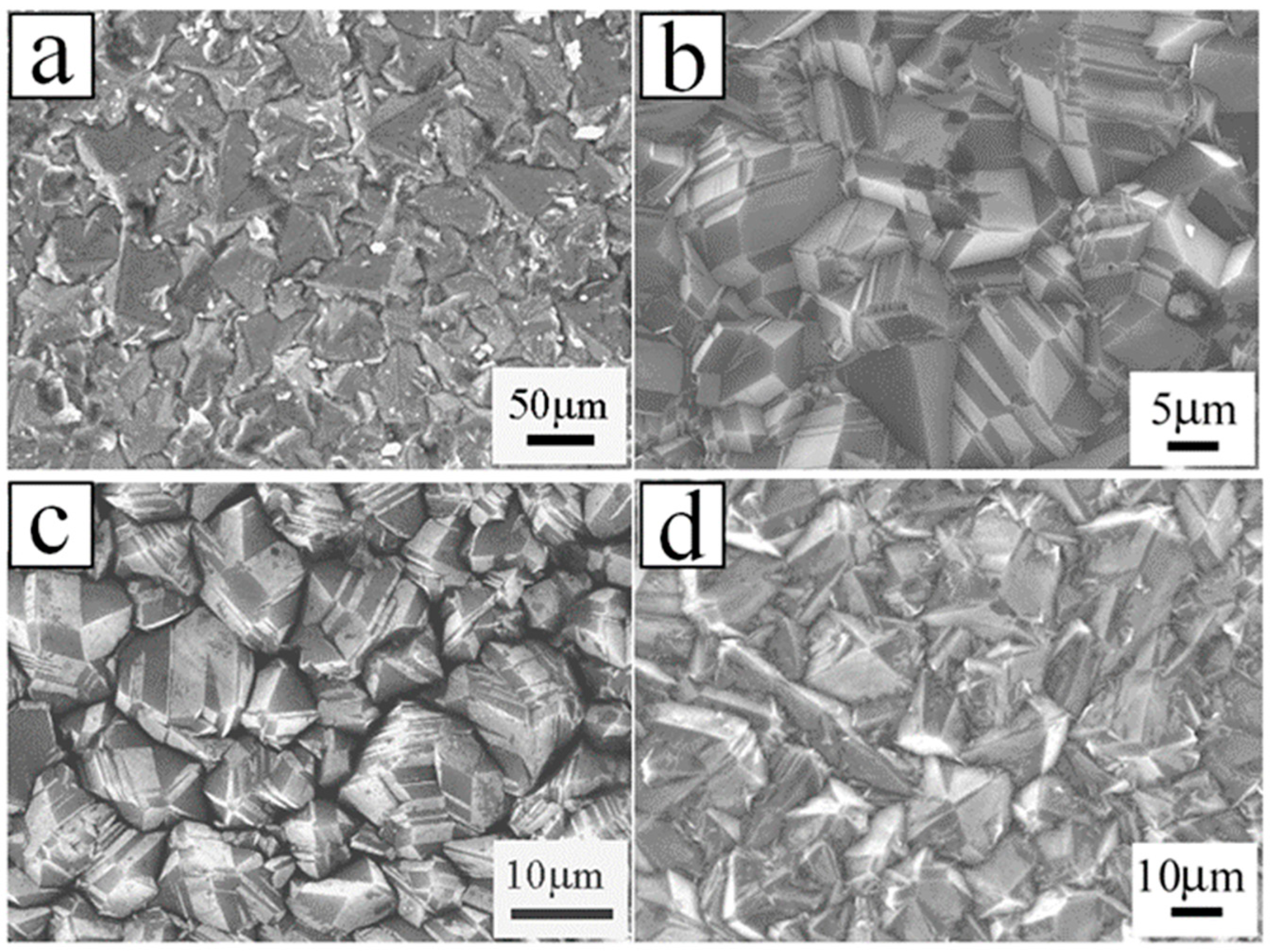
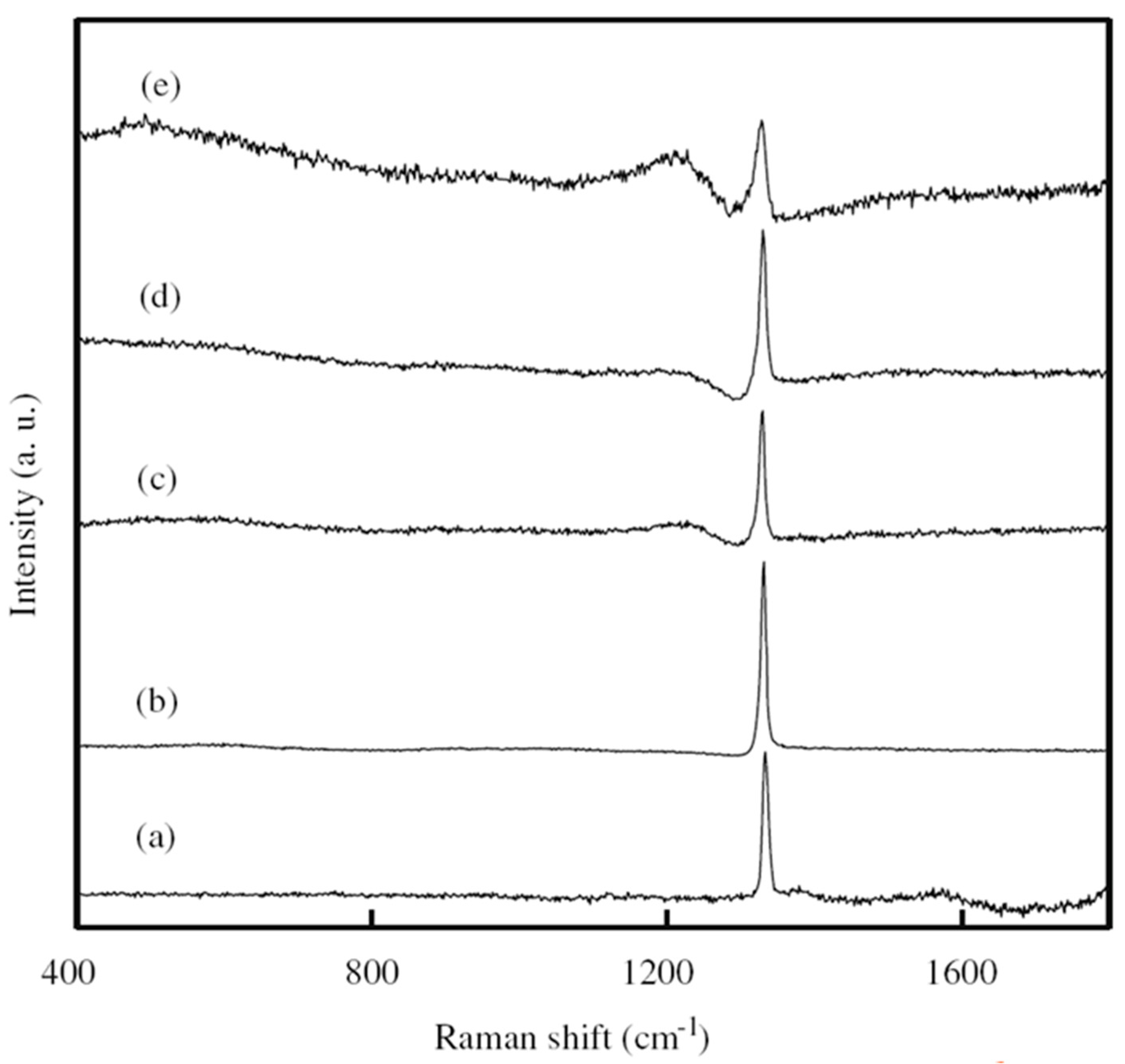
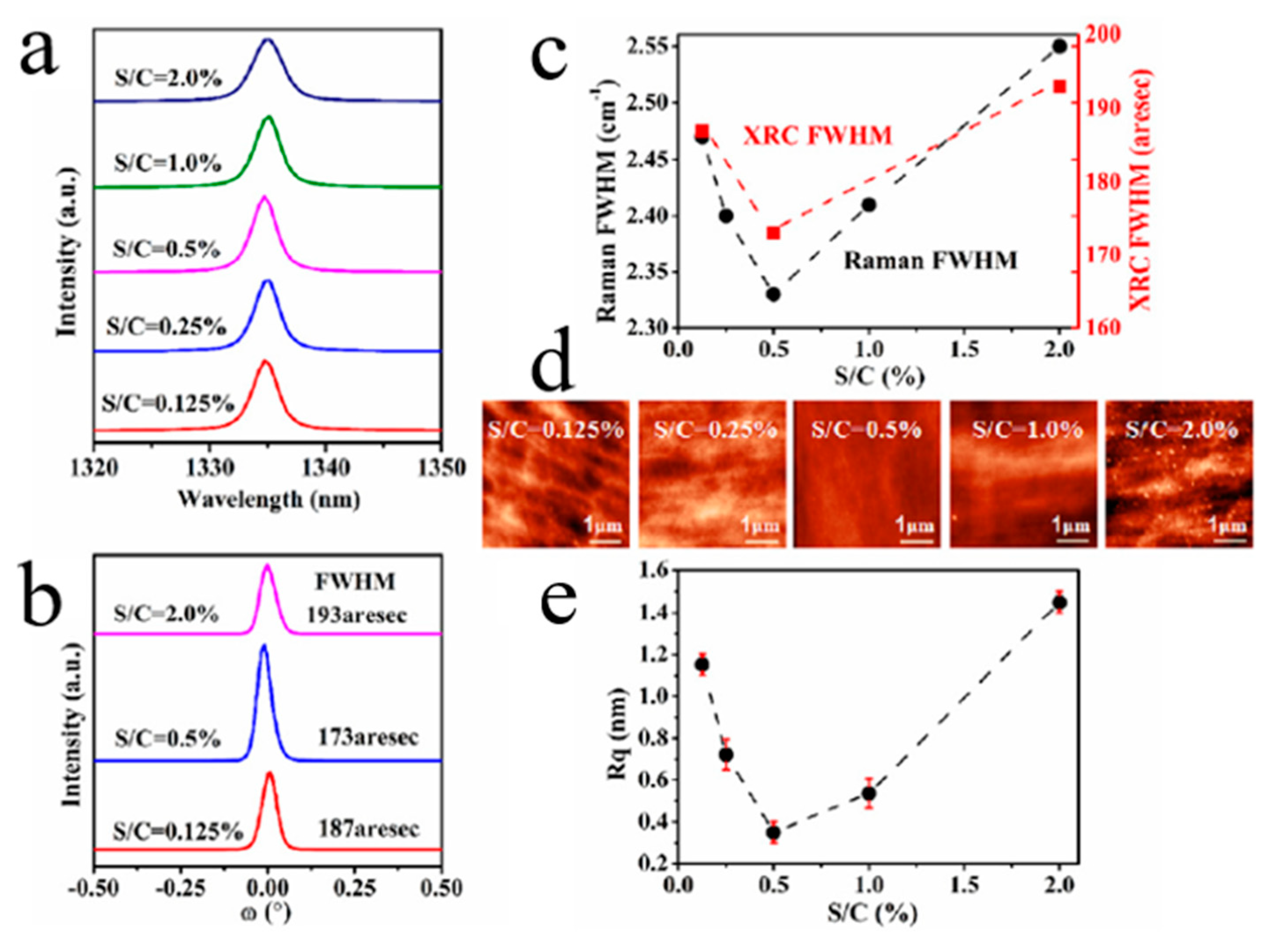
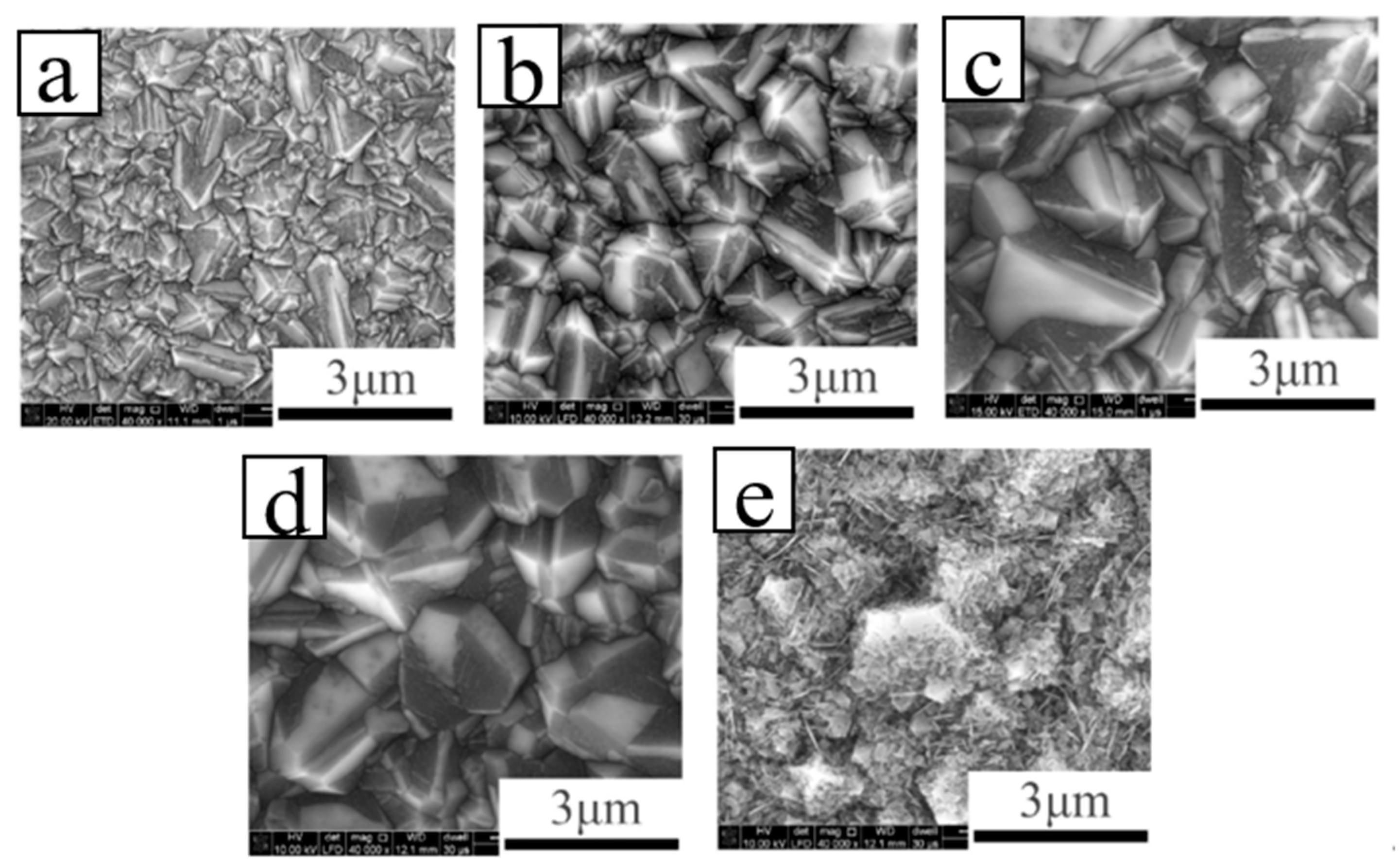
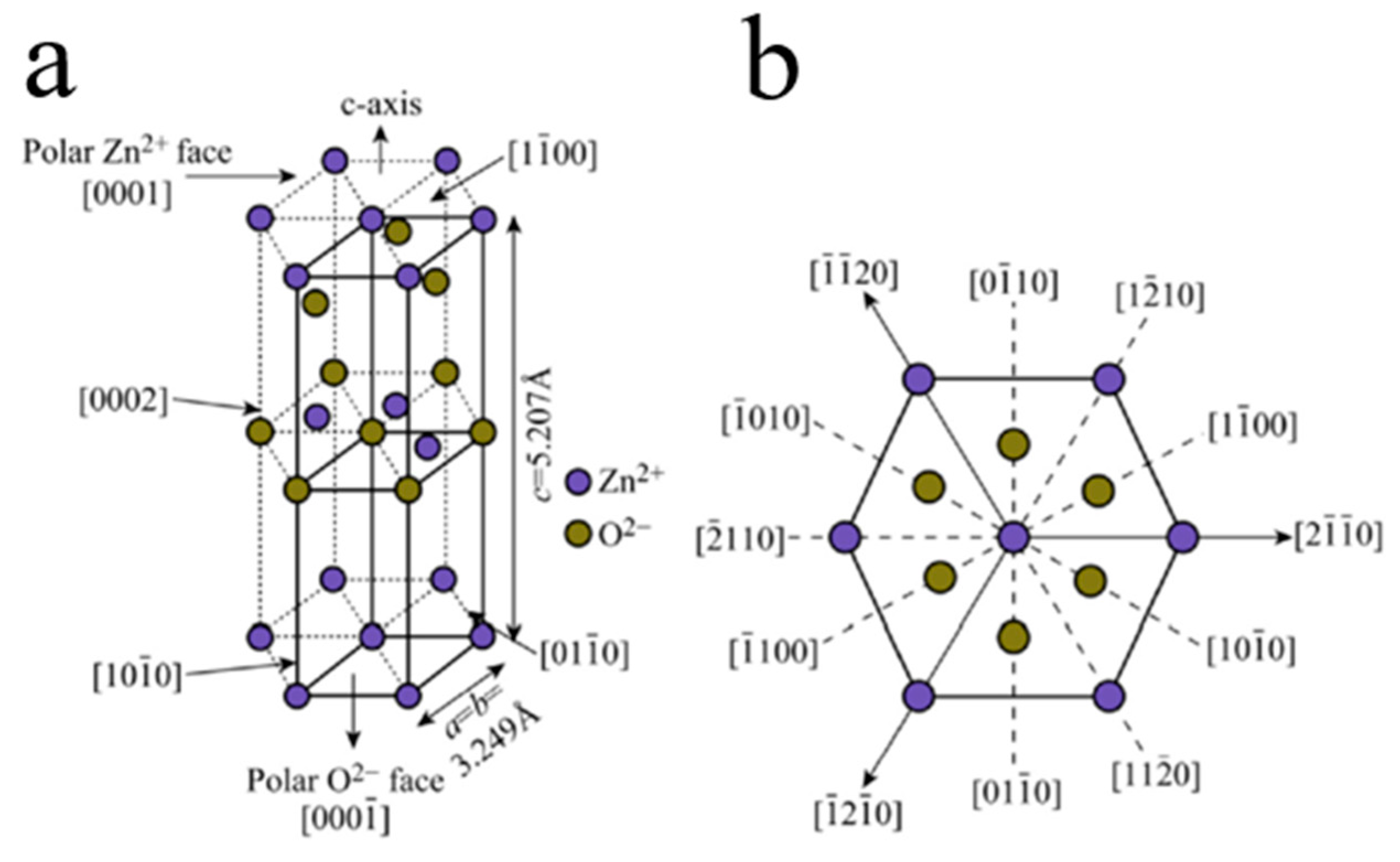
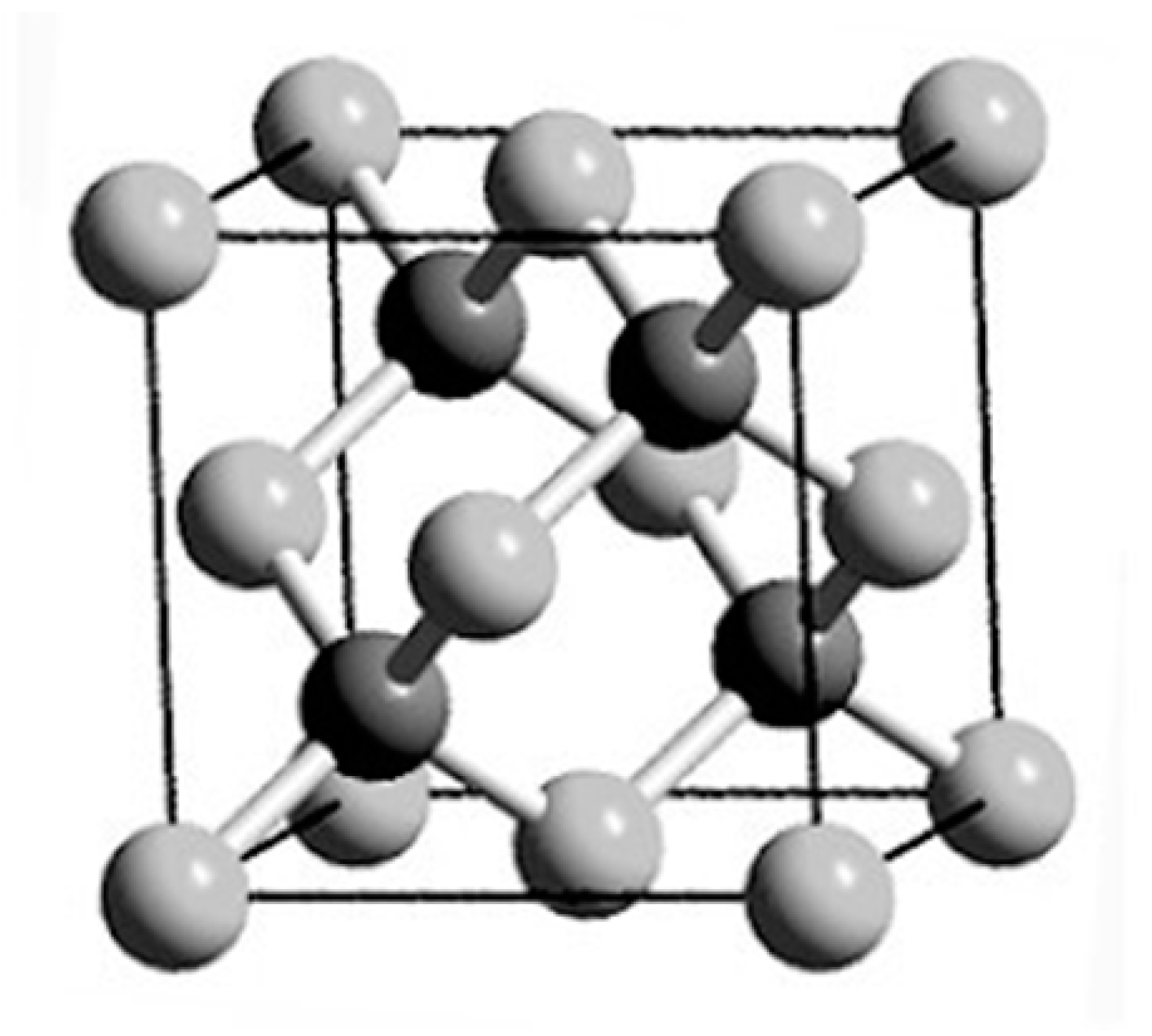
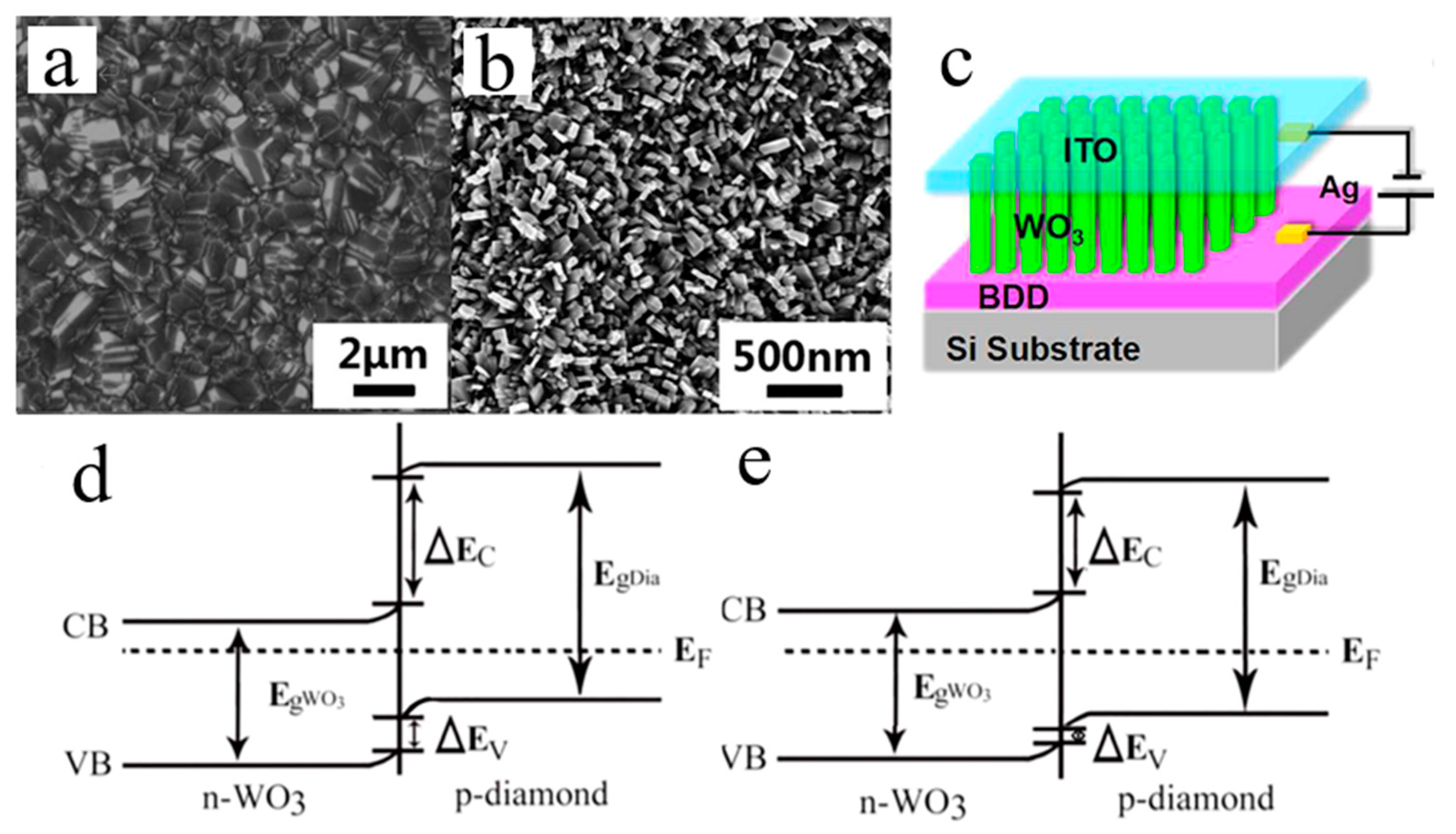
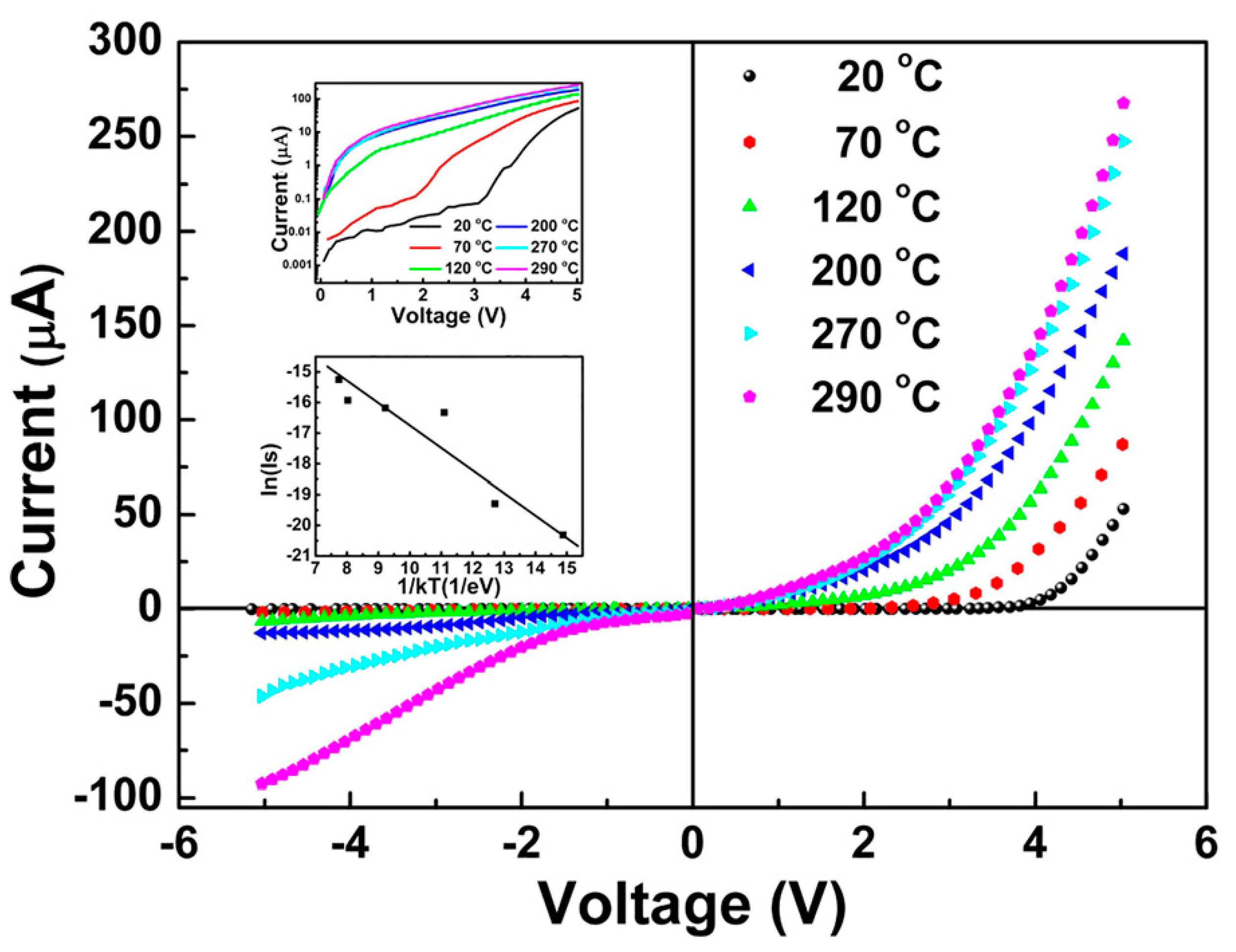
| Heterojunction Morphology | Synthesis Route | Applications (Properties) | Ref. |
|---|---|---|---|
| ZnO film/diamond | Magnetron sputtering | Surface acoustic wave | [65] |
| ZnO film/diamond | Magnetron sputtering | Films’ quality | [66] |
| ZnO film/diamond | MOCVD | Electrical properties | [67] |
| ZnO film/diamond | Atomic Layer Chemical Vapour Deposition | Electrical properties | [72] |
| ZnO film/diamond | Magnetron Sputtering | Heterojunction diode | [68] |
| ZnO NRs/diamond | Low-temperature solution | Tyrosinase biosensor | [69] |
| ZnO NRs/diamond | Hydrothermal method | Photocatalytic activities | [33] |
| ZnO NRs/diamond | Hydrothermal method | Photocatalytic activities | [81] |
| ZnO NRs/diamond | Low-temperature solution | Electrical properties | [82] |
| ZnO NRs/diamond | Hydrothermal method | Photoelectric anodes | [83] |
| ZnO NRs/diamond | Hydrothermal method | UV photodetector | [84] |
| ZnO NRs/diamond | Sol-gel method | Hydrogen gas sensors | [85] |
| ZnO NTs/diamond | Hydrothermal method | UV detection and field emission | [86] |
| ZnO NWs/diamond | Hydrothermal method | Electrical transport properties | [30] |
| ZnO NRs/diamond | Thermal evaporation method | NDR properties | [31] |
| ZnO NRs/diamond | Thermal evaporation method | UV photoelectrical properties | [22] |
| ZnO NRs/diamond | Thermal evaporation method | Electrical transport behavior | [24] |
| ZnO NRs/diamond | Thermal evaporation method | NDR properties | [23] |
| Heterojunction Morphology | Synthesis Route | Applications (Properties) | Ref. |
|---|---|---|---|
| TiO2 film/diamond | Sol-gel method | Photoelectrocatalytic activities | [102] |
| TiO2 NRs/diamond | Hydrothermal method | Photocatalytic activities | [103] |
| TiO2 NTs/diamond | Liquid phase deposition method | Photocatalytic activities | [38] |
| TiO2 NTs/diamond | Liquid phase deposition method | Photoelectronic nanodevices | [39] |
| TiO2 NTs/diamond | Liquid phase deposition method | Photocatalytic devices | [104] |
| TiO2 NTs/diamond | Anodization method | Hybrid electrode | [105] |
| TiO2 NTs/diamond | Anodization method | Supercapacitor or Energy Storage Devices | [106] |
| TiO2 NTs/diamond | Anodization method | Supercapacitor | [70] |
| TiO2 film/diamond | Radio Frequency sputtering | Photoelectrochemical performance | [41] |
| TiO2 film/diamond | Sol-gel method | Photocatalytic activities | [37] |
| TiO2 film/diamond | Sol-gel method | Hybrid electrode | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Sang, D.; Duan, S.; Wang, Q.; Liu, C. Review on the Properties of Boron-Doped Diamond and One-Dimensional-Metal-Oxide Based P-N Heterojunction. Molecules 2021, 26, 71. https://doi.org/10.3390/molecules26010071
Yao Y, Sang D, Duan S, Wang Q, Liu C. Review on the Properties of Boron-Doped Diamond and One-Dimensional-Metal-Oxide Based P-N Heterojunction. Molecules. 2021; 26(1):71. https://doi.org/10.3390/molecules26010071
Chicago/Turabian StyleYao, Yu, Dandan Sang, Susu Duan, Qinglin Wang, and Cailong Liu. 2021. "Review on the Properties of Boron-Doped Diamond and One-Dimensional-Metal-Oxide Based P-N Heterojunction" Molecules 26, no. 1: 71. https://doi.org/10.3390/molecules26010071
APA StyleYao, Y., Sang, D., Duan, S., Wang, Q., & Liu, C. (2021). Review on the Properties of Boron-Doped Diamond and One-Dimensional-Metal-Oxide Based P-N Heterojunction. Molecules, 26(1), 71. https://doi.org/10.3390/molecules26010071





