Cytotoxic and Anti-Inflammatory Effects of Ent-Kaurane Derivatives Isolated from the Alpine Plant Sideritis hyssopifolia
Abstract
1. Introduction
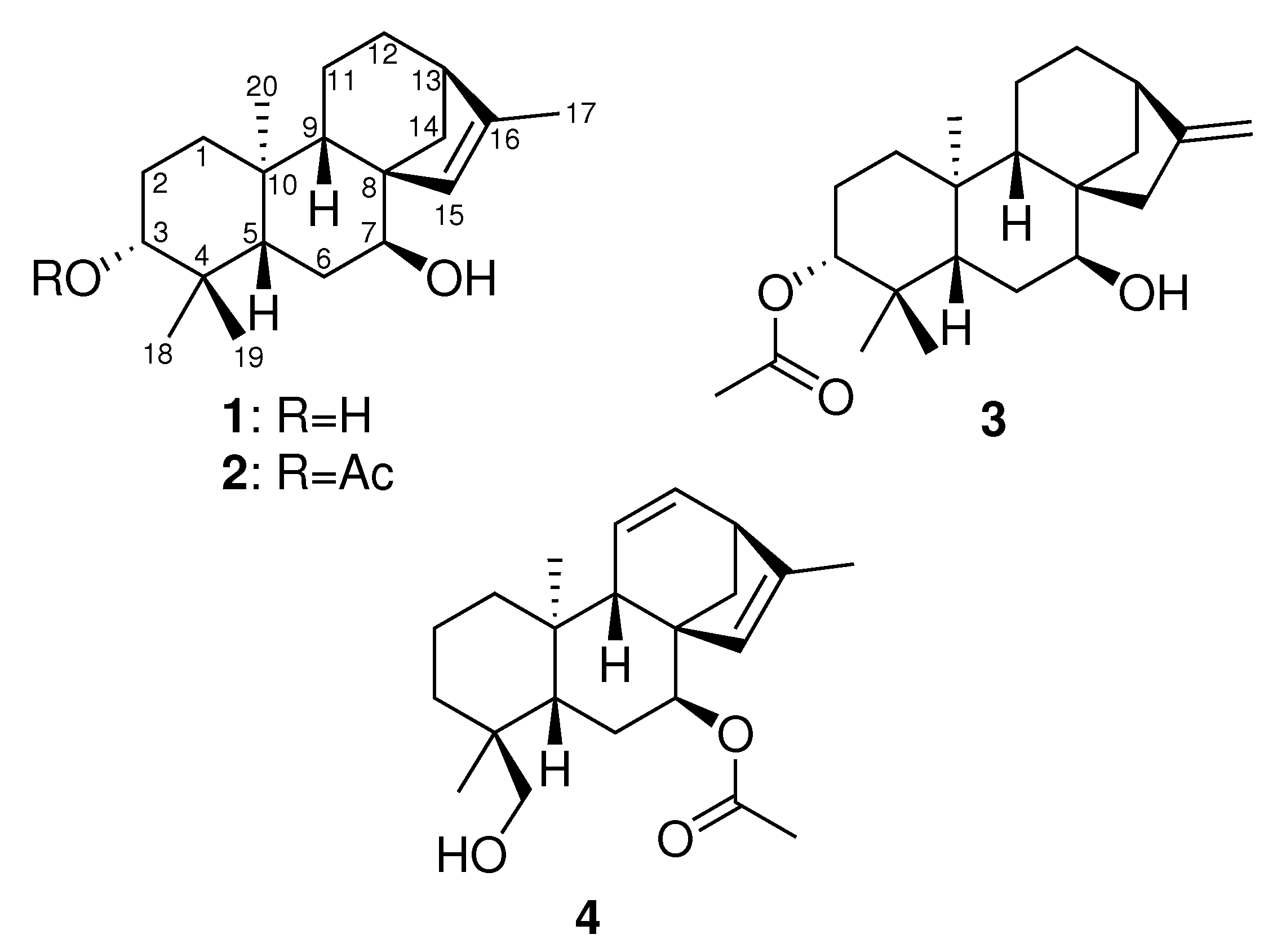
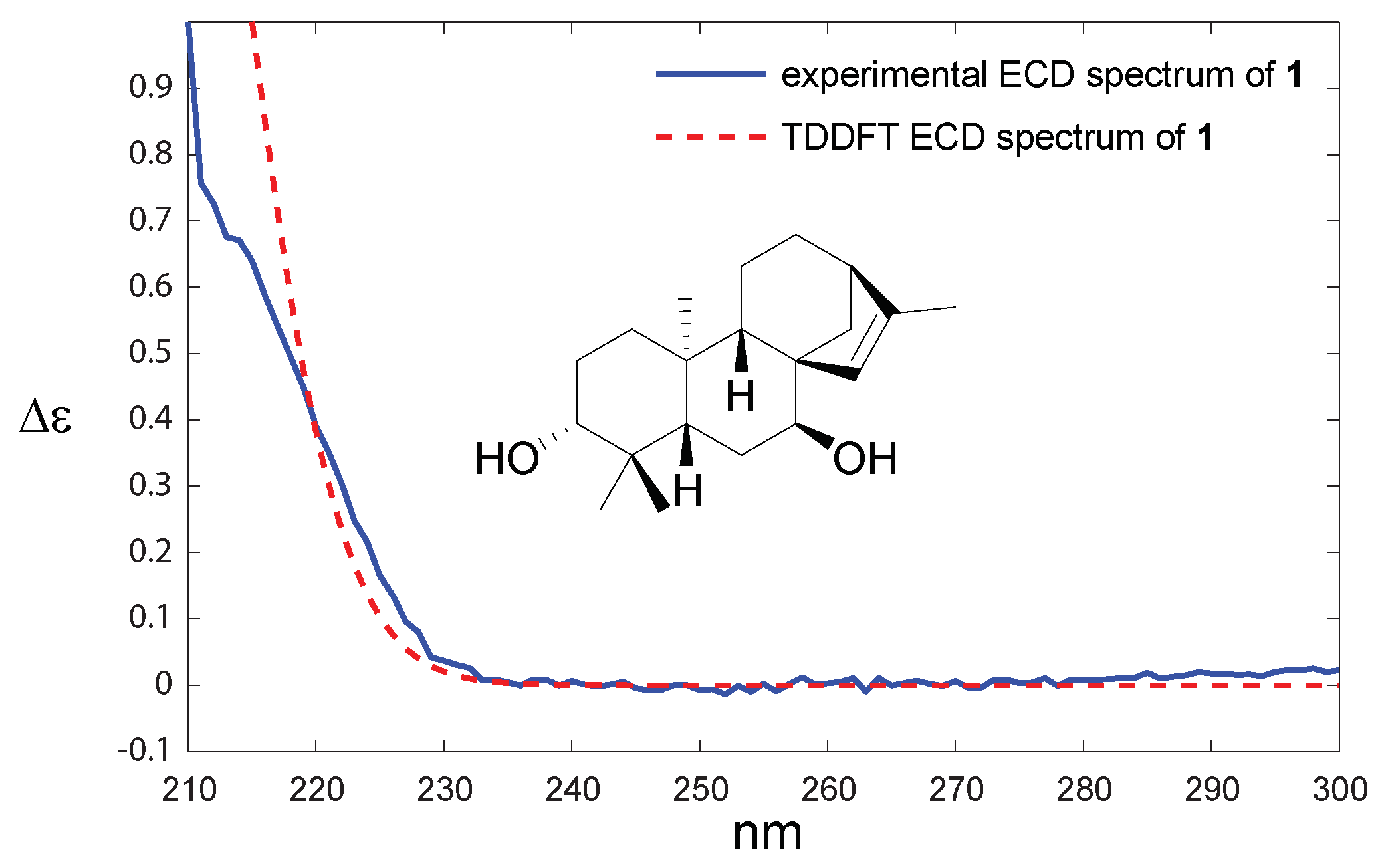
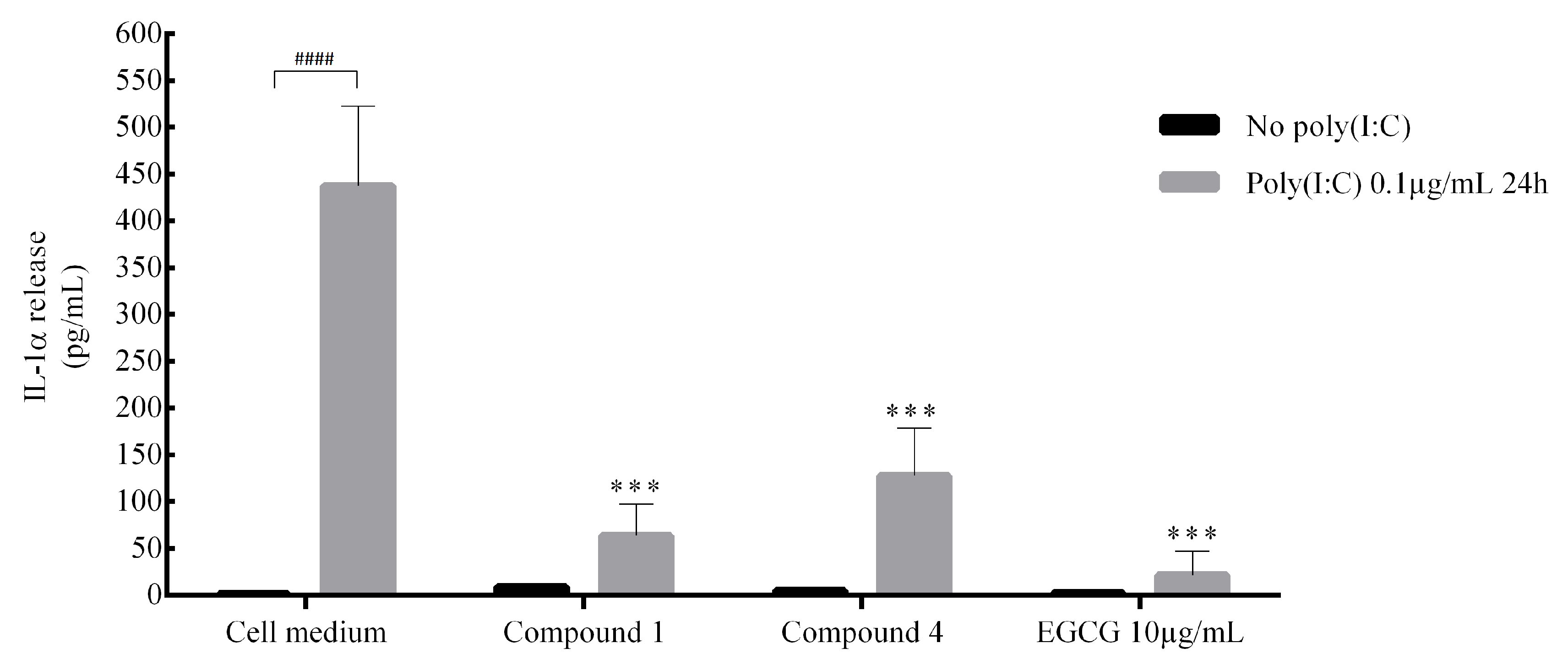
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Computational Details
3.5. Cell Experiments
3.6. Cell Viability Evaluation
3.7. Interleukin Release Measurement
3.8. Saponification of Compound 3
3.9. Statistical Analyses
3.10. Hyssopifoliol A (Compound 1)
3.11. Hyssopifoliol B (Compound 2)
3.12. 3-Acetoxy-ent-3-7-dihydroxykaur-16-ene (Compound 3)
3.13. 11,12-Didehydrosiderol (Compound 4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- González-Burgos, E.; Carretero, M.; Gómez-Serranillos, M. Sideritis spp.: Uses, chemical composition and pharmacological activities—A review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Piozzi, F.; Bruno, M.; Rosselli, S.; Maggio, A. The Diterpenoids from the Genus Sideritis. In Studies in Natural Products Chemistry; ur Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 33, pp. 493–540. [Google Scholar]
- Topçu, G.; Gören, A.C.; Yildiz, Y.K.; Tümen, G. Ent-Kaurene Diterpenes from Sideritis athoa. Nat. Prod. Lett. 1999, 14, 123–129. [Google Scholar] [CrossRef]
- Ertaş, A.; Öztürk, M.; Boğa, M.; Topçu, G. Antioxidant and Anticholinesterase Activity Evaluation of ent-Kaurane Diterpenoids from Sideritis arguta. J. Nat. Prod. 2009, 72, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.H.; Chang, W.L.; Lu, T.M. Antityrosinase and Antioxidant Effects of ent-Kaurane Diterpenes from Leaves of Broussonetia papyrifera. J. Nat. Prod. 2008, 71, 1930–1933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, C.; Dai, W.; He, J.; Jiao, S.; Li, B. Anti-inflammatory ent-kaurenoic acids and their glycosides from Gochnatia decora. Phytochemistry 2017, 137, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lyon, M.L.; Díaz-Lanza, A.M.; Bernabé, M.; Villaescusa-Castillo, L. Flavone glycosides containing acetylated sugars from Sideritis hyssopifolia. Magn. Reson. Chem. 2000, 38, 684–687. [Google Scholar] [CrossRef]
- Adzet, T.; Cañigueral, S.; Monasterio, I.; Vila, R.; Ibáñez, C. The Essential Oil and Polyphenols of Sideritis hyssopifolia var pyrenaica. J. Essent. Oil Res. 1990, 2, 151–153. [Google Scholar] [CrossRef]
- Piozzi, F.; Venturella, P.; Bellino, A.; Mondelli, R. Diterpenes from Sideritis sicula ucria. Tetrahedron 1968, 24, 4073–4081. [Google Scholar] [CrossRef]
- Rodiguéz, B.; Peña, A.; Cuesta, R.; Peña, A. Diterpenes from three Sideritis species. Phytochemistry 1975, 14, 1670–1671. [Google Scholar] [CrossRef]
- Venturella, P.; Bellino, A.; Marino, M.L. Siderone, a diterpene from Sideritis syriaca. Phytochemistry 1983, 22, 2537–2538. [Google Scholar] [CrossRef]
- Rodriguez Gonzalez, B.; Valverde, S.R.J.M. Diterpenes from Sideritis candicans var eriocephala. Anal. Quim. 1970, 66, 503–511. [Google Scholar]
- Piozzi, F.; Venturella, P. Structure of sideritriol. Gazz. Chim. Ital. 1969, 99, 582–587. [Google Scholar]
- Piozzi, F.; Venturella, P.; Bellino, A.; Marino, M.L. Partial synthesis of ent-kaur-16-ene-15β,18-diol and ent-kaur-16-ene-7α,15β,18-triol. J. Chem. Soc. Perkin Trans. 1 Organ. Bio-Organ. Chem. 1973, 1, 1164–1166. [Google Scholar]
- Halfon, B.; Ceyhan Gören, A.; Ertaş, A.; Topçu, G. Complete 13C NMR assignments for ent-kaurane diterpenoids from Sideritis species. Magn. Reson. Chem. 2011, 49, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, D.; Wu, X. Synthesis and biological evaluation of novel exo-methylene cyclopentanone tetracyclic diterpenoids as antitumor agents. Bioorgan. Med. Chem. Lett. 2011, 21, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Feldmeyer, L.; Keller, M.; Niklaus, G.; Hohl, D.; Werner, S.; Beer, H.D. The Inflammasome Mediates UVB-Induced Activation and Secretion of Interleukin-1β by Keratinocytes. Curr. Biol. 2007, 17, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Harden, J.L.; Krueger, J.G.; Bowcock, A.M. The immunogenetics of Psoriasis: A comprehensive review. J. Autoimmun. 2015, 64, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Mailloux, C.M.; Gowan, K.; Riccardi, S.L.; LaBerge, G.; Bennett, D.C.; Fain, P.R.; Spritz, R.A. NALP1 in Vitiligo-Associated Multiple Autoimmune Disease. N. Engl. J. Med. 2007, 356, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Liu, W.; Luo, Y.; Tanaka, A.; Cai, X.; Norris, D.A.; Dinarello, C.A.; Fujita, M. Constitutively Active Inflammasome in Human Melanoma Cells Mediating Autoinflammation via Caspase-1 Processing and Secretion of Interleukin-1β. J. Biol. Chem. 2010, 285, 6477–6488. [Google Scholar] [CrossRef] [PubMed]
- Audoin, C.; Zampalégré, A.; Blanchet, N.; Giuliani, A.; Roulland, E.; Laprévote, O.; Genta-Jouve, G. MS/MS-Guided Isolation of Clarinoside, a New Anti-Inflammatory Pentalogin Derivative. Molecules 2018, 23, 1237. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–4 are available from the authors. |


| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| No. | (m, J in Hz) | (m, J in Hz) | (m, J in Hz) | (m, J in Hz) |
| 1a | 1.84 (dt, 13.0, 3.5) | 1.82 (dt, 13.2, 3.5) | 1.84 (m) | 1.84 (m) |
| 1b | 0.95 (m) | 1.03 (m) | 1.05 (td, 13.0, 4.0) | 1.07 (dd, 12.5, 3.6) |
| 2a | 1.67 (m) | 1.70 (m) | 1.69 (m) | 1.65 (m) |
| 2b | 1.60 (m) | 1.62 (m) | 1.64 (m) | 1.52 (m) |
| 3a | 3.17 (dd, 11.6, 4.5) | 4.52 (dd, 11.6, 5.1) | 4.53 (dd, 11.6, 5.0) | 1.55 (m) |
| 3b | - | - | - | 1.27 (dd, 14.3, 5.5) |
| 4 | - | - | - | - |
| 5 | 1.40 (d, 12.0) | 1.49 (m) | 1.55 (m) | 1.86 (m) |
| 6a | 1.66 (m) | 1.65 (m) | 1.68 (m) | 1.74 (dd, 14.4, 3.8) |
| 6b | 1.62 (m) | 1.56 (m) | ||
| 7 | 3.54 (bs) | 3.63 (t, 2.9) | 3.62 (t, 3.1) | 4.82 (t, 3.0) |
| 8 | - | - | - | - |
| 9 | 1.28 (d, 4.7) | 1.30 (d, 7.5) | 1.42 (d, 6.6) | 1.86 (m) |
| 10 | - | - | - | - |
| 11 | 1.54 (m) | 1.49 (m) | 1.56 (m) | 5.36 (dd, 9.7, 3.5) |
| 12a | 1.51 (m) | 1.49 (m) | 1.70 (m) | 6.25 (dd, 9.6, 6.5) |
| 12b | 1.49 (m) | - | ||
| 13 | 2.33 (bs) | 2.37 (bs) | 2.68 (m) | 2.56 (m) |
| 14a | 1.96 (d, 9.9) | 1.89 d (10.1) | 1.82 (m) | 2.01 (d, 9.4) |
| 14b | 1.35 (dd, 10.1, 5.3) | 1.36 (d, 10.2, 5.2) | 1.17 (dd, 11.4, 5.0) | 1.50 (m) |
| 15 | 5.52 (s) | 5.47 (s) | 2.25 (bs) | 5.09 (s) |
| 16 | - | - | - | - |
| 17a | 1.71 (s) | 1.72 (bs) | 4.83 (bs) | 1.77 (d, 1.1) |
| 17b | - | - | 4.80 (bs) | - |
| 18 | 0.96 (s) | 0.84 (s) | 0.88 (s) | 0.72 (s) |
| 19a | 0.76 (s) | 0.85 (s) | 0.86 (s) | 3.36 (d, 10.8) |
| 19b | - | - | - | 3.04 (d, 10.8) |
| 20 | 1.07 (s) | 1.05 (s) | 1.05 (s) | 1.04 (s) |
| 21 | - | - | - | - |
| 22 | - | 2.04 (s) | 2.04 (s) | 2.09 (s) |
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| No. | ||||
| 1 | 40.1 | 38.4 | 38.4 | 39.2 |
| 2 | 28.1 | 23.6 | 23.8 | 17.9 |
| 3 | 79.8 | 80.8 | 81.0 | 35.1 |
| 4 | 39.4 | 37.2 | 37.4 | 37.1 |
| 5 | 46.1 | 45.1 | 45.6 | 38.8 |
| 6 | 27.7 | 26.7 | 27.4 | 23.9 |
| 7 | 76.2 | 75.0 | 77.2 | 78.0 |
| 8 | 54.5 | 53.3 | 48.3 | 50.5 |
| 9 | 45.2 | 43.7 | 50.3 | 50.9 |
| 10 | 40.3 | 39.1 | 38.9 | 38.8 |
| 11 | 19.6 | 18.4 | 18.0 | 126.1 |
| 12 | 25.9 | 24.8 | 33.7 | 134.4 |
| 13 | 46.1 | 44.7 | 43.9 | 45.5 |
| 14 | 43.5 | 42.1 | 38.6 | 40.2 |
| 15 | 131.9 | 129.7 | 45.8 | 126.6 |
| 16 | 144.2 | 144.3 | 154.9 | 154.0 |
| 17 | 15.5 | 15.5 | 103.8 | 15.9 |
| 18 | 162 | 16.6 | 16.9 | 17.6 |
| 19 | 28.6 | 28.0 | 28.3 | 71.3 |
| 20 | 18.2 | 17.6 | 17.6 | 17.5 |
| 21 | - | 170.9 | 171.1 | 170.5 |
| 22 | - | 21.3 | 21.5 | 21.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aimond, A.; Calabro, K.; Audoin, C.; Olivier, E.; Dutot, M.; Buron, P.; Rat, P.; Laprévote, O.; Prado, S.; Roulland, E.; et al. Cytotoxic and Anti-Inflammatory Effects of Ent-Kaurane Derivatives Isolated from the Alpine Plant Sideritis hyssopifolia. Molecules 2020, 25, 589. https://doi.org/10.3390/molecules25030589
Aimond A, Calabro K, Audoin C, Olivier E, Dutot M, Buron P, Rat P, Laprévote O, Prado S, Roulland E, et al. Cytotoxic and Anti-Inflammatory Effects of Ent-Kaurane Derivatives Isolated from the Alpine Plant Sideritis hyssopifolia. Molecules. 2020; 25(3):589. https://doi.org/10.3390/molecules25030589
Chicago/Turabian StyleAimond, Axelle, Kevin Calabro, Coralie Audoin, Elodie Olivier, Mélody Dutot, Pauline Buron, Patrice Rat, Olivier Laprévote, Soizic Prado, Emmanuel Roulland, and et al. 2020. "Cytotoxic and Anti-Inflammatory Effects of Ent-Kaurane Derivatives Isolated from the Alpine Plant Sideritis hyssopifolia" Molecules 25, no. 3: 589. https://doi.org/10.3390/molecules25030589
APA StyleAimond, A., Calabro, K., Audoin, C., Olivier, E., Dutot, M., Buron, P., Rat, P., Laprévote, O., Prado, S., Roulland, E., Thomas, O. P., & Genta-Jouve, G. (2020). Cytotoxic and Anti-Inflammatory Effects of Ent-Kaurane Derivatives Isolated from the Alpine Plant Sideritis hyssopifolia. Molecules, 25(3), 589. https://doi.org/10.3390/molecules25030589









