Induced Hydrophilicity and In Vitro Preliminary Osteoblast Response of Polyvinylidene Fluoride (PVDF) Coatings Obtained via MAPLE Deposition and Subsequent Thermal Treatment
Abstract
1. Introduction
2. Results and Discussions
2.1. Physical and Chemical Characterization of the Polyvinylidene Fluoride Coatings
2.2. Elemental Composition
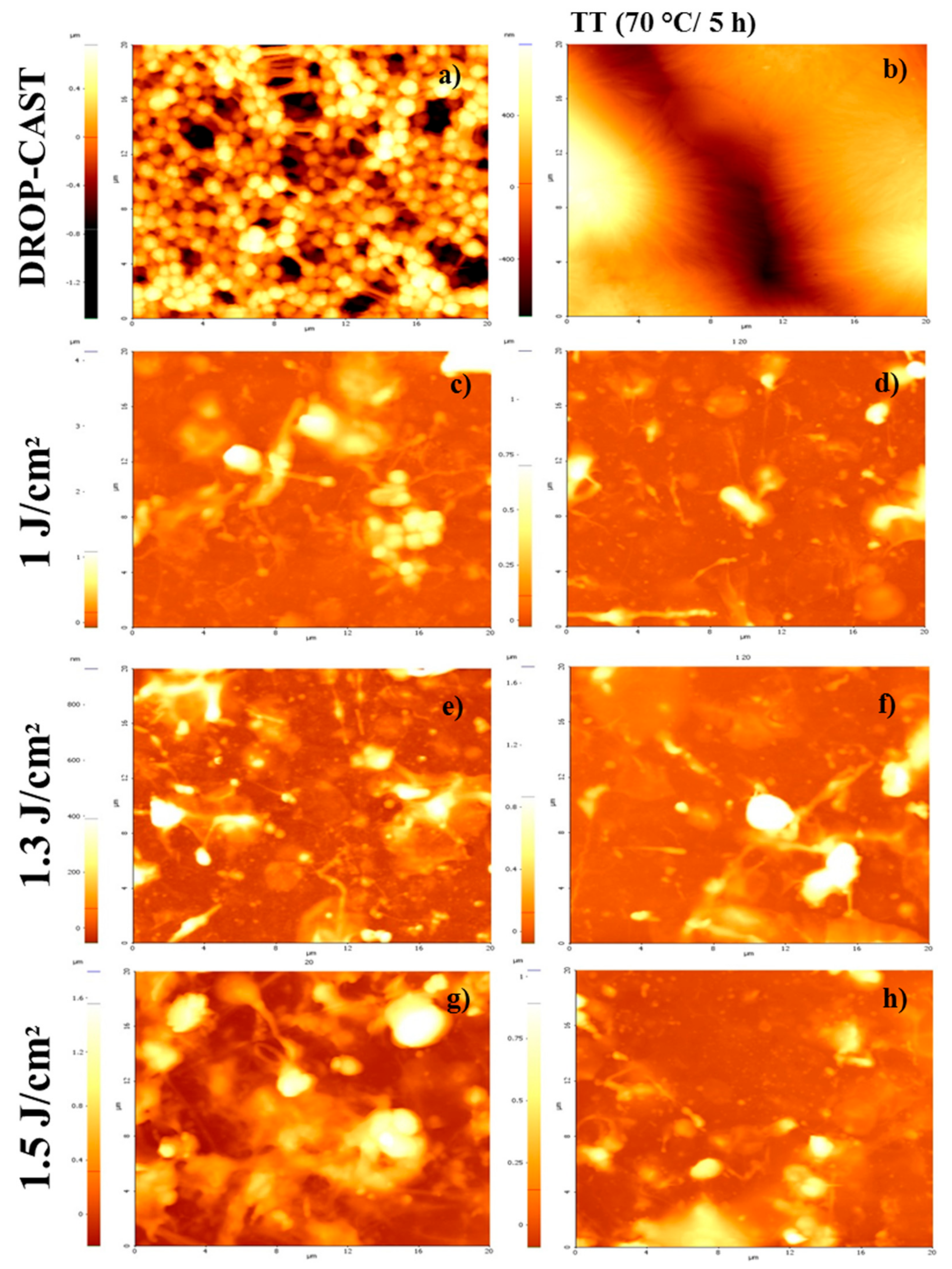
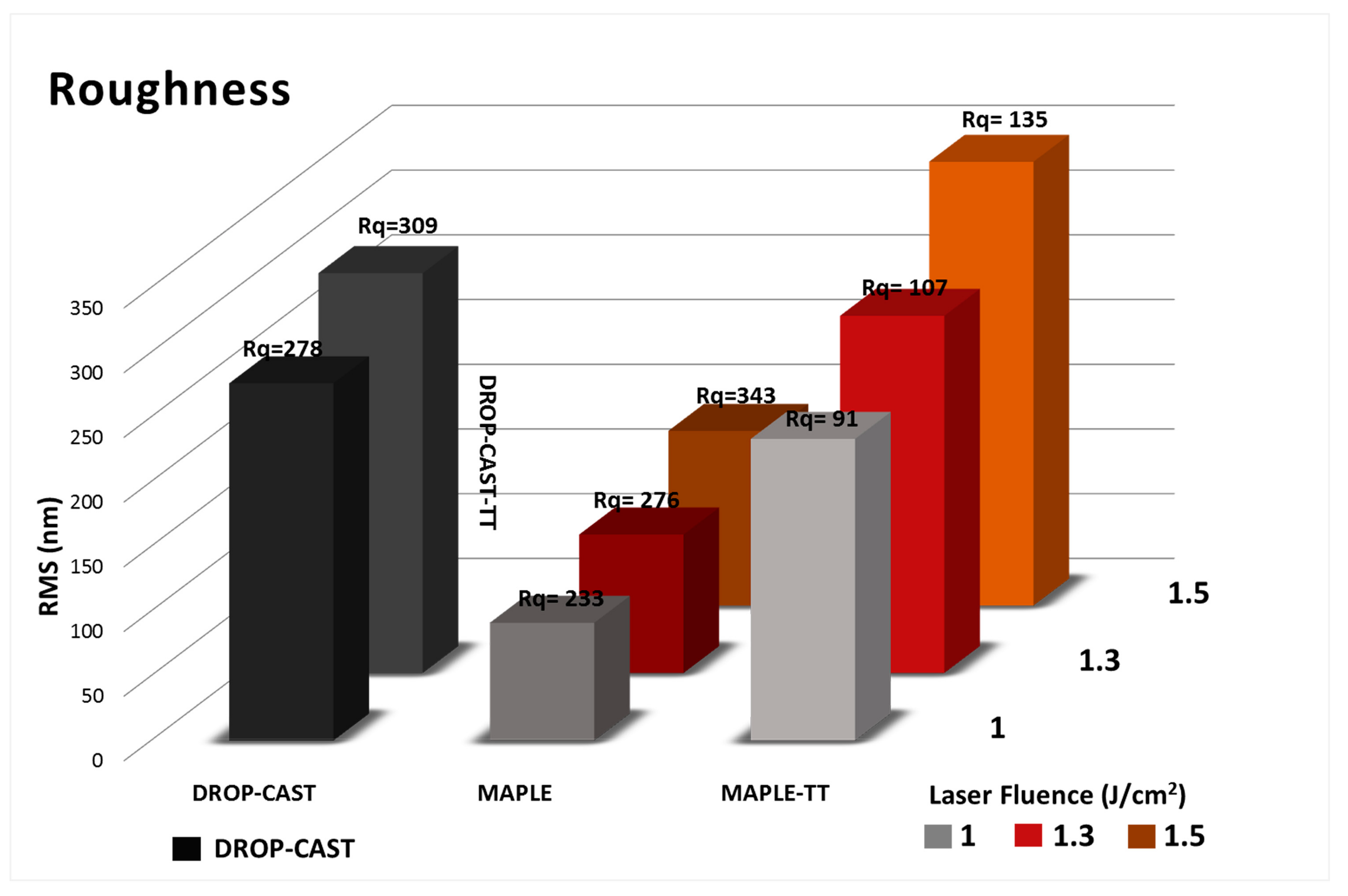
2.3. Surface Morphology
2.4. Wettability Measurements
2.5. In Vitro Preliminary Cell-Material Interaction
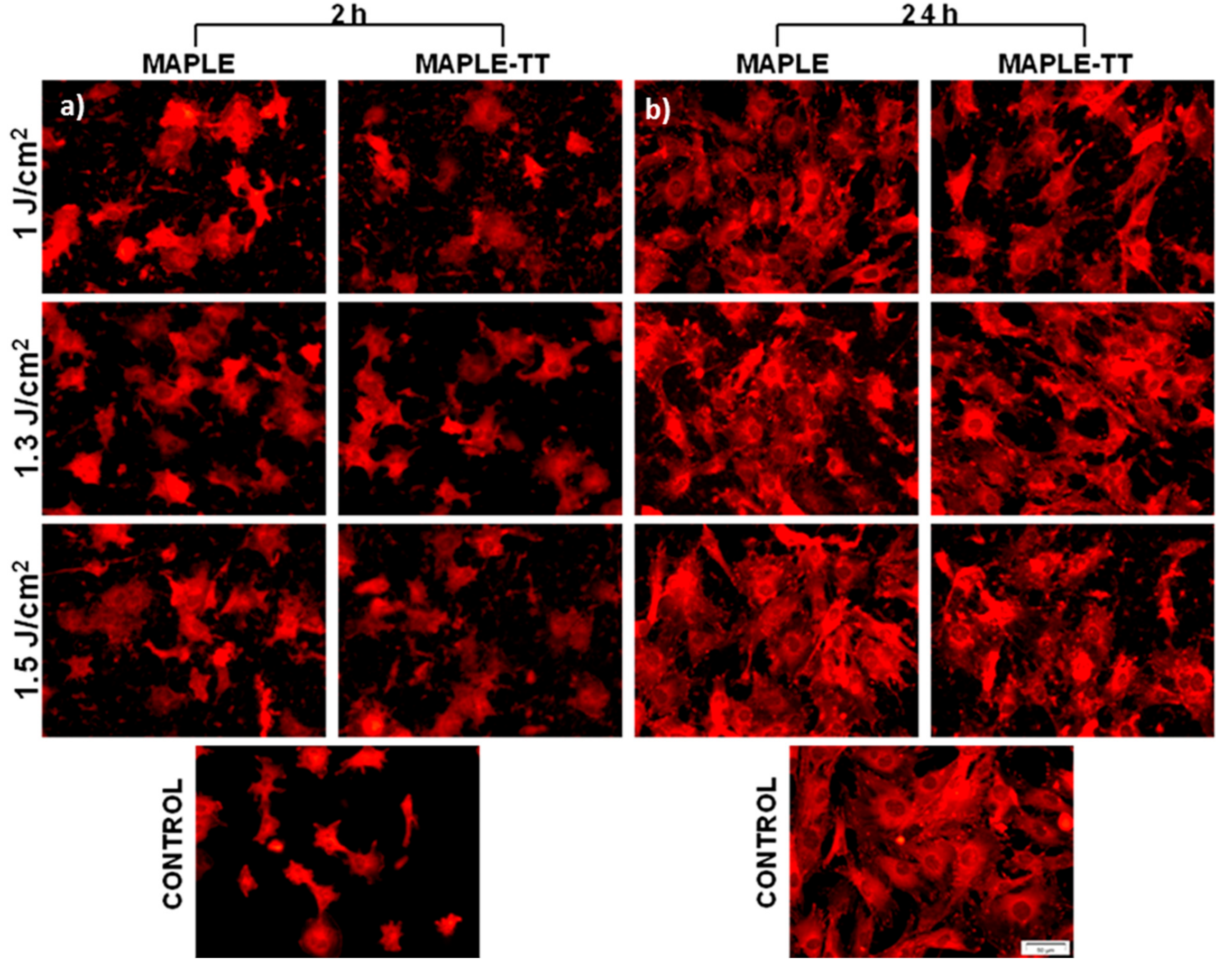
2.5.1. Cell Attachment Behaviour
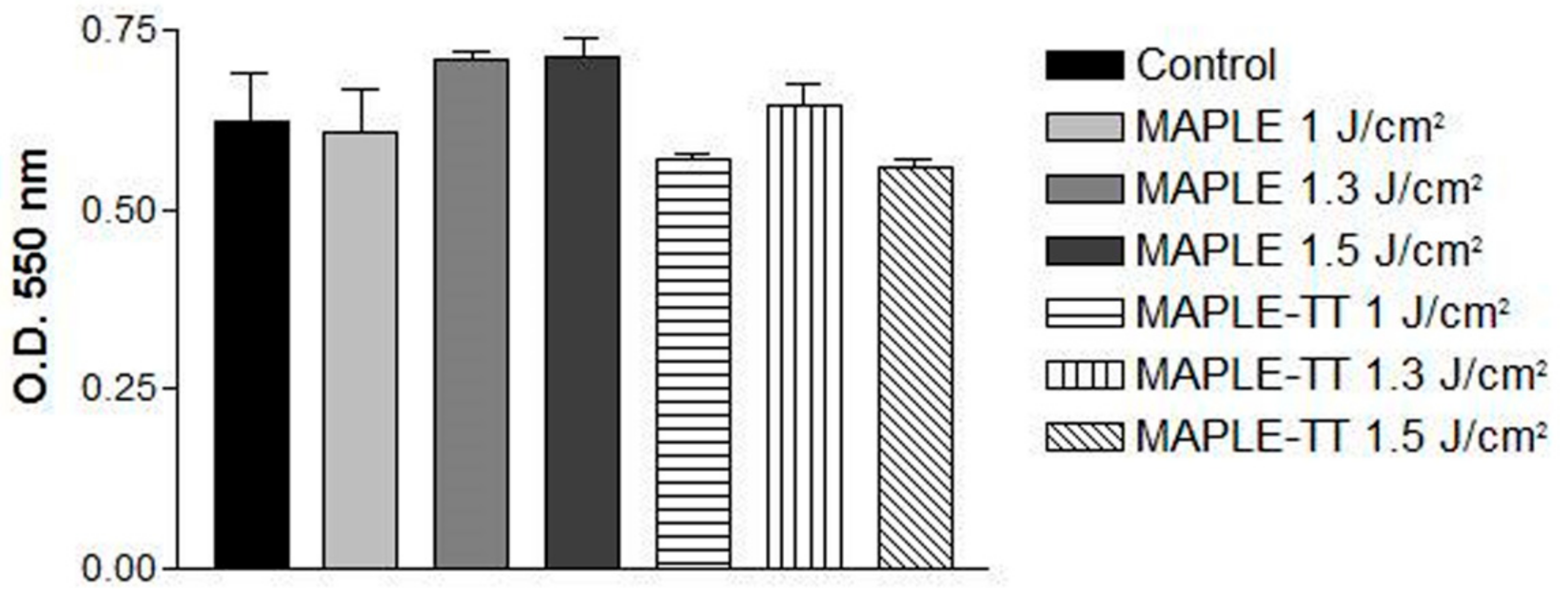
2.5.2. Cell Viability
3. Materials and Methods
3.1. Materials
3.2. Deposition Method: MAPLE Setup
3.3. Thermal Treatment (TT)
3.4. Polyvinylidene Fluoride Coatings Characterization
3.4.1. Thin Films Chemical Profile
3.4.2. Morphological Characterization of the Deposited Polyvinylidene Fluoride Coatings
3.4.3. Contact Angle Measurements
3.5. In Vitro Biocompatibility Assessment
3.5.1. Cell Culture
3.5.2. Investigation of the Cellular Attachment
3.5.3. Evaluation of Cell Viability
3.5.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mahdi, R.I.; Gan, W.C.; Abd Majid, W.H. Hot Plate Annealing at a Low Temperature of a Thin Ferroelectric P(VDF-TrFE) Film with an Improved Crystalline Structure for Sensors and Actuators. Sensors 2014, 14, 19115–19127. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hu, J.; Ren, W.; Xu, F.; Wu, X.; Shi, P.; Ye, Z.G. A new biosensor based on PVDF film for detection of nucleic acids. Ceram. Int. 2015, 41, S602–S606. [Google Scholar] [CrossRef]
- Tao, M.; Liu, F.; Ma, B.R.; Xue, L. Effect of solvent power on PVDF membrane polymorphism during phase inversion. Desalination 2013, 316, 137–145. [Google Scholar] [CrossRef]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2014, 84, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Ribeiro, C.; Lopes, A.C.; Martins, P.; Sencadas, V.; Soares, R.; Lanceros-Mendez, S. Osteoblast, fibroblast and in vivo biological response to poly(vinylidene fluoride) based composite materials. J. Mater. Sci. Mater. Med. 2013, 24, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Wang, J.K.; Xiong, G.M.; Luo, B.; Choo, C.C.; Yuan, S.; Tan, N.S.; Choong, C. Surface modification of PVDF using non-mammalian sources of collagen for enhancement of endothelial cell functionality. J. Mater. Sci. Mater. Med. 2016, 27, 45. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Minas, G.; Lanceros-Méndez, S. Multilayer spin-coating deposition of poly (vinylidene fluoride) films for controlling thickness and piezoelectric response. Sens. Actuator A Phys. 2013, 192, 76–80. [Google Scholar] [CrossRef]
- Fukada, E. Piezoelectricity and pyroelectricity of biopolymers. In Ferroelectric Polymers: Chemistry: Physics, and Applications, 1st ed.; Nalwa, H.S., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 63–181. [Google Scholar]
- Szewczyk, P.K.; Metwally, S.; Krysiak, Z.J.; Kaniuk, Ł.; Karbowniczek, J.E.; Stachewicz, U. Enhanced osteoblasts adhesion and collagen formation on biomimetic polyvinylidene fluoride (PVDF) films for bone regeneration. Biomed. Mater. 2019, 14, 065006. [Google Scholar] [CrossRef]
- Kitsara, M.; Blanquer, A.; Murillo, G.; Humblot, V.; De Bragança Vieira, S.; Nogués, C.; Ibáñez, E.; Esteve, J.; Barrios, L. Permanently hydrophilic, piezoelectric PVDF nanofibrous scaffolds promoting unaided electromechanical stimulation on osteoblasts. Nanoscale 2019, 11, 8906–8917. [Google Scholar] [CrossRef]
- Fontananova, E.; Bahattab, M.A.; Aljlil, S.A.; Alowairdy, M.; Rinaldi, G.; Vuono, D.; Nagy, J.B.; Drioliac, E.; Di Profio, G. From hydrophobic to hydrophilic polyvinylidenefluoride (PVDF) membranes by gaining new insight into material’s properties. RSC Adv. 2015, 5, 56219–56231. [Google Scholar] [CrossRef]
- Niu, Y.; Bai, Y.; Yu, K.; Wang, Y.; Xiang, F.; Wang, H. Effect of the Modifier Structure on the Performance of Barium Titanate/Poly(vinylidene fluoride) Nanocomposites for Energy Storage Applications. ACS Appl. Mater. Interfaces 2015, 7, 24168–24176. [Google Scholar] [CrossRef] [PubMed]
- Pascu, M.; Nicolas, D.; Poncin-Epaillard, F.; Vasile, C. Surface modification of PVDF by plasma treatment for electroless metallization. J. Optoelectron. Adv. M. 2006, 8, 1062–1064. [Google Scholar]
- Miki, S.R.H.; Tsuchitani, S. Effect of Intermitted RIE Etching on the Beta-PVDF Film Micro Patterning. In Proceeding of the International Symposium on Micro-NanoMechatronics and Human Science; IEEE: Nagoyaty, Japan, 2017. [Google Scholar]
- Motamedi, A.S.; Mirzadeh, H.; Hajiesmaeilbaigi, F.; Bagheri-Khoulenjani, S.; Shokrgozar, M.A. Effect of electrospinning parameters on morphological properties of PVDF nanofibrous scaffolds. Prog. Biomater. 2017, 6, 113–123. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Cantu, T.; Macossay, J.; Kim, H. Fabrication of Poly (vinylidene fluoride) (PVDF) Nanofibers Containing Nickel Nanoparticles as Future Energy Server Materials. Sci Adv Mater. 2011, 3, 216–222. [Google Scholar] [CrossRef]
- Li, M.; Katsouras, I.; Pilieo, C.; Glasser, G.; Lieberwirth, I.; Blom, P.W.M.; de Leeuw, D.M. Controlling the microstructure of poly(vinylidene-fluoride) (PVDF) thin films for microelectronics. J. Mater. Chem. C 2013, 1, 7695–7702. [Google Scholar] [CrossRef]
- El-Sayed, S. Optical properties and dielectric relaxation of polyvinylidene fluoride thin films doped with gadolinium chloride. Physica B 2014, 454, 197–203. [Google Scholar] [CrossRef]
- Young, T.-H.; Cheng, L.-P.; Lin, D.-J.; Fane, L.; Chuang, W.Y. Mechanisms of PVDF membrane formation by immersion-precipitation in soft (1-octanol) and harsh (water) nonsolvents. Polymer 1999, 40, 5315–5323. [Google Scholar] [CrossRef]
- Chanmal, C.; Deo, M.; Jog, J. Enhanced dielectric permittivity in poly (vinylidene) fluoride/multiwalled carbon nanotubes nanocomposite thin films fabricated by pulsed laser deposition. Appl. Surf. Sci. 2011, 258, 1256–1260. [Google Scholar] [CrossRef]
- Sahu, N.; Parija, B.; Panigrahi, S. Fundamental understanding and modeling of spin coating process: Review. Indian J. Phys. 2009, 83, 493–502. [Google Scholar] [CrossRef]
- Braghirolli, D.I.; Zamboni, F.; Acasigua, G.A.; Pranke, P. Association of electrospinning with electrospraying: a strategy to produce 3D scaffolds with incorporated stem cells for use in tissue engineering. Int. J. Nanomedicine 2015, 10, 5159–5170. [Google Scholar] [CrossRef]
- Berro, S.; El Ahdab, R.; Hassan, H.H.; Khachfe, H.M.; Hajj-Hassan, M. From Plastic to Silicone: The Novelties in Porous Polymer Fabrications. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Kleea, D.; Ademovica, Z.; Bosserhoff, A.; Hoeckera, H.; Maziolisd, G.; Erli, H.J. Surface modification of poly(vinylidenefluoride) to improve the osteoblast adhesion. Biomaterials 2003, 24, 3663–3670. [Google Scholar] [CrossRef]
- Damaraju, S.M.; Wu, S.; Jaffe, M.; Arinzeh, T.L. Structural changes in PVDF fibers due to electrospinning and its effect on biological function. Biomed. Mater. 2013, 8, 045007. [Google Scholar] [CrossRef]
- Ribeiro, C.; Moreira, S.; Correia, V.; Sencadas, V.; Rocha, J.G.; Gama, F.M.; Gomez Ribelles, J.L.; Lanceros-Mendez, S. Enhanced proliferation of pre-osteoblastic cells by dynamic piezoelectric stimulation. RSC Adv. 2012, 2, 11504–11509. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J. Matrix-Assisted Pulsed Laser Evaporation (MAPLE) technique for deposition of hybrid nanostructures. Front. Nanosci. Nanotech. 2017, 3, 1–9. [Google Scholar] [CrossRef]
- McGill, R.A.; Chung, R.; Chrisey, D.B.; Dorsey, P.C.; Matthews, P.; Pique, A.; Mlsna, T.E.; Stepnowski, J.L. Performance optimization of surface acoustic wave chemical sensors. IEEE T. Ultrason. Ferr. Freq. Control. 1998, 45, 1370–1380. [Google Scholar] [CrossRef]
- Pique, A.; McGill, R.A.; Chrisey, D.B.; Leonhardt, D.; Mslna, T.E.; Spargo, B.J.; Callahan, J.H.; Vachet, R.W.; Chung, R.; Bucaro, M.A. Growth of organic thin films by the matrix assisted pulsed laser evaporation (MAPLE) technique. Thin Solid Films 1999, 355, 536–541. [Google Scholar] [CrossRef]
- Chrisey, D.B.; Pique, A.; McGill, R.A.; Horwitz, J.S.; Ringeisen, B.R.; Bubb, D.M.; Wu, P.K. Laser deposition of polymer and biomaterial films. Chem. Rev. 2003, 103, 553–576. [Google Scholar] [CrossRef]
- Wang, Y.; Jeong, H.; Chowdhury, M.; Arnold, C.B.; Priestley, R.D. Exploiting physical vapor deposition for morphological control in semi-crystalline polymer films. Polym. Crystallization 2018, 1, e10021. [Google Scholar] [CrossRef]
- Dinca, V.; Sima, L.E.; Rusen, L.; Bonciu, A.; Lippert, T.; Dinescu, M.; Farsari, M. Bio-interfaces engineering using laser-based methods for controlled regulation of mesenchymal stem cell response in vitro. In Recent Advances in Biopolymers; Perveen, F.K., Ed.; IntechOpen: London, UK, 2016; pp. 221–251. [Google Scholar]
- Piqué, A. The Matrix-Assisted Pulsed Laser Evaporation (MAPLE) process: origins and future directions. Appl. Phys. A 2011, 105, 517–528. [Google Scholar] [CrossRef]
- Caricato, A.P.; Arima, V.; Cesaria, M.; Martino, M.; Tunno, T.; Rinaldi, R.; Zacheo, A. Solvent-related effects in MAPLE mechanism. Appl. Phys. B 2013, 3, 463–471. [Google Scholar] [CrossRef]
- Ayzner, A.L.; Tassone, C.J.; Tolbert, S.H.; Schwartz, B.J. Reappraising the Need for Bulk Heterojunctions in Polymer−Fullerene Photovoltaics: The Role of Carrier Transport in All-Solution-Processed P3HT/PCBM Bilayer Solar Cells. J. Phys. Chem. C 2009, 113, 20050–20060. [Google Scholar] [CrossRef]
- Caricato, A.P.; Ge, W.; Stiff-Roberts, A.D. UV- and RIR-MAPLE: Fundamentals and Applications. In Advances in the Application of Lasers in Materials Science; Ossi, P.M., Ed.; Springer International Publishing: New York, NY, USA, 2018; Volume 274, pp. 273–275. [Google Scholar]
- Palla-Papavlu, A.; Dinca, V.; Dinescu, M.; Di Pietrantonio, F.; Cannatà, D.; Benetti, M.; Verona, E. Matrix-assisted pulsed laser evaporation of chemoselective polymers. Appl. Phys. A 2011, 105, 651–659. [Google Scholar] [CrossRef]
- Caricato, A.P.; Arima, V.; Catalano, M.; Cesaria, M.; Cozzoli, P.D.; Martino, M.; Taurinoc, A.; Rella, R.; Scarfiello, R.; Tunno, T.; et al. MAPLE deposition of nanomaterials. Appl. Surf. Sci. 2014, 302, 92–98. [Google Scholar] [CrossRef]
- Dinca, V.; Florian, P.E.; Sima, L.E.; Rusen, L.; Constantinescu, C.; Evans, R.W.; Dinescu, M.; Roseanu, A. MAPLE based method to obtain biodegradable hybrid polymeric thin films with embedded antitumoral agents. Biomed. Microdevices 2014, 16, 11–21. [Google Scholar] [CrossRef]
- Constantinescu, C.; Rotaru, A.; Nedelcea, A.; Dinescu, M. Thermal behaviour and thin film deposition by MAPLE technique of functional polymeric materials with potential use in optoelectronics. Mater. Sci. Semicond. Process. 2015, 30, 242–249. [Google Scholar] [CrossRef]
- Constantinescu, C.; Scarisoreanu, N.; Moldovan, A.; Dinescu, M.; Vasiliu, C. Thin films of polyaniline deposited by MAPLE technique. Appl. Surf. Sci. 2007, 253, 7711–7714. [Google Scholar] [CrossRef]
- Rusen, L.; Dinca, V.; Mustaciosu, C.; Icriverzi, M.; Sima, L.E.; Bonciu, A.; Brajnicov, S.; Mihailescu, N.; Dumitrescu, N.; Popovici, A.I.; et al. Smart Thermoresponsive Surfaces Based on pNIPAm Coatings and Laser Method for Biological Applications. In Modern Technologies for Creating the Thin-film Systems and Coatings; Nikitenkov, N., Ed.; IntechOpen: London, UK, 2017; pp. 172–191. [Google Scholar]
- Califano, V.; Bloisi, F.; Aronne, A.; Federici, S.; Nasti, L.; Depero, L.E.; Vicari, L.R.M. Biosensor Applications of MAPLE Deposited Lipase. Biosensors 2014, 4, 329–339. [Google Scholar] [CrossRef]
- Constantinescu, C.; Palla-Papavlu, A.; Rotaru, A.; Florian, P.; Chelu, F.; Icriverzi, M.; Nedelcea, A.; Dincă, V.; Roşeanu, A.; Dinescu, M. Multifunctional thin films of lactoferrin for biochemical use deposited by MAPLE technique. Appl. Surf. Sci. 2009, 255, 5491–5495. [Google Scholar] [CrossRef]
- Rotaru, A.; Moanţă, A.; Constantinescu, C.; Dumitru, M.; Manolea, H.O.; Andrei, A.; Dinescu, M. Thermokinetic study of CODA azoic liquid crystal and thin films deposition by matrix-assisted pulsed laser evaporation. J. Therm. Anal. Calorim. 2017, 128, 89–105. [Google Scholar] [CrossRef]
- Rotaru, A.; Constantinescu, C.; Rotaru, P.; Moanţă, A.; Dumitru, M.; Socaciu, M.; Dinescu, M.; Segal, E. Thermal analysis and thin films deposition by matrix assisted pulsed laser evaporation of a 4CN type azomonoether. J. Therm. Anal. Calorim. 2008, 92, 279–284. [Google Scholar] [CrossRef]
- Shepard, K.B.; Priestley, R.D. MAPLE Deposition of Macromolecules. Macromol. Chem. Phys. 2013, 214, 857–956. [Google Scholar] [CrossRef]
- Rotaru, A.; Constantinescu, C.; Mandruleanu, A.; Rotaru, P.; Moldovan, A.; Gyoryova, K.; Dinescu, M.; Balek, V. Matrix assisted pulsed laser evaporation of zinc benzoate for ZnO thin films and non-isothermal decomposition kinetics. Thermochim. Acta 2010, 498, 81–91. [Google Scholar] [CrossRef]
- Dash, S.; Choudhary, R.N.P.; Goswami, M.N. Enhanced dielectric and ferroelectric properties of PVDF-BiFeO3 composites in 0e3 connectivity. J. Alloy Compd. 2017, 715, 29–36. [Google Scholar] [CrossRef]
- Ruan, L.; Yao, X.; Chang, Y.; Zhou, L.; Qin, G.; Zhang, X. Properties and Applications of the Phase Poly(vinylidene fluoride). Polymers 2018, 10, 228. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly (vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, X.; Peng, G.; Liu, W.; Liu, K.; Zhan, Z. Investigation on crystalline structure and dielectric relaxation behaviors of hot pressed poly(vinylidene fluoride) film. Curr. Appl. Phys. 2017, 17, 15–23. [Google Scholar] [CrossRef]
- Bai, H.; Wang, X.; Zhou, Y.; Zhang, L. Preparation and characterization of poly(vinylidene fluoride) composite membranes blended with nano-crystalline cellulose. Prog. Nat. Sci-Mater. 2012, 22, 250–257. [Google Scholar] [CrossRef]
- Wan, C.; Bowen, C.R. Multiscale-structuring of polyvinylidene fluoride for energy harvesting: the impact of molecular-, micro- and macro-structure. J. Mater. Chem. A 2017, 5, 3091. [Google Scholar] [CrossRef]
- Rusen, L.; Mustaciosu, C.; Mitu, B.; Filipescu, M.; Dinescu, M.; Dinca, V. Protein-resistant polymer coatings obtained by matrix assisted pulsed laser evaporation. Appl. Surf. Sci. 2013, 278, 198–202. [Google Scholar] [CrossRef]
- Leveugle, E.; Sellinger, A.; Fitz-Gerald, J.M.; Zhigilei, L.V. Making Molecular Balloons in Laser-Induced Explosive Boiling of Polymer Solutions. Phys. Rev. Lett. 2007, 98, 216101. [Google Scholar] [CrossRef] [PubMed]
- Zhigilei, L.V.; Volkov, A.N.; Leveugle, E.; Tabetah, M. The effect of the target structure and composition on the ejection and transport of polymer molecules and carbon nanotubes in matrix-assisted pulsed laser evaporation. Appl. Phys. A 2011, 105, 529–546. [Google Scholar] [CrossRef]
- Awaja, F.; Zhang, S.; James, N.; McKenzie, D.R. Enhanced autohesive bonding of polyetheretherketone (PEEK) for biomedical applications using a methane/oxygen plasma treatment. Plasma Process. Polym. 2010, 7, 1010–1021. [Google Scholar] [CrossRef]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of polymers. Prog. Polym. Sci. 2009, 34, 948–968. [Google Scholar] [CrossRef]
- Chen, Y.; Cho, M.R.; Mak, A.F.T.; Li, J.S.; Wang, M.; Sun, S. Morphology and adhesion of mesenchymal stem cells on PLLA, apatite and apatite/collagen surfaces. J. Mater. Sci. Mater. Med. 2008, 19, 2563–2567. [Google Scholar] [CrossRef] [PubMed]
- Huag, H.-S.; Chou, S.-H.; Don, T.-M.; Lai, W.-C.; Cheng, L.-P. Formation of microporous poly(hydroxybutyric acid) membranes for culture of osteoblast and fibroblast. Polym Advan. Technol. 2009, 20, 1082–1090. [Google Scholar] [CrossRef]
- Rodrigues, M.T.; Gomes, M.E.; Mano, J.F.; Reis, R.L. β-PVDF Membranes Induce Cellular Proliferation and Differentiation in Static and Dynamic Conditions. Mater. Sci. Forum 2008, 587–588, 72–76. [Google Scholar] [CrossRef]
- Ribeiro, C.; Panadero, J.A.; Sencadas, V.; Lanceros-Méndez, S.; Tamaño, M.N.; Moratal, D.; Salmerón-Sánchez, M.; Gómez Ribelles, J.L. Fibronectin adsorption and cell response on electroactive poly(vinylidene fluoride) films. Biomed. Mater. 2012, 7, 1–10. [Google Scholar] [CrossRef]
- Nunes-Pereira, J.; Ribeiro, S.; Ribeiro, C.; Gombek, C.J.; Gama, F.M.; Gomes, A.C.; Patterson, D.A.; Lanceros-Méndez, S. Poly(vinylidene fluoride) and copolymers as porous membranes for tissue engineering applications. Polymer Testing 2015, 44, 234–241. [Google Scholar] [CrossRef]
- Fukada, E.; Yasuda, I. On the Piezoelectric Effect of Bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Rizzi, S.C.; Heath, D.T.; Coombes, A.G.A.; Bock, N.; Textor, M.; Downes, S. Biodegradable polymer/hydroxyapatite composites: surface analysis and initial attachment of human osteoblasts. J. Biomed. Mater. Res. 2001, 55, 475–486. [Google Scholar] [CrossRef]
- Dasgupta, S.; Tarafder, S.; Bandyopadhyay, A.; Bose, S. Effect of grain size on mechanical, surface and biological properties of microwave sintered hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Meyer, T.; Pfau, B.; Hofmann, T.; Tan, T.W.; Jones, D. Rapid vinculin exchange dynamics at focal adhesions in primary osteoblasts following shear flow stimulation. J. Musculoskel. Neuron. 2010, 10, 92–99. [Google Scholar]
- Weber, N.; Lee, Y.S.; Shanmugasundaram, S.; Jaffe, M.; Arinzeh, T.L. Characterization and in vitro cytocompatibility of piezoelectric electrospun scaffolds. Acta Biomater. 2010, 6, 3550–3556. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Arinzeh, T.L. The influence of piezoelectric scaffolds on neural differentiation of human neural stem/progenitor cells. Tissue Eng. Part A 2012, 18, 2063–2072. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Collard, D.M. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials 2004, 25, 5947–5954. [Google Scholar] [CrossRef]
- Kunzler, T.P.; Drobek, T.; Schuler, M.; Spencer, N.D. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials 2007, 13, 2175–2182. [Google Scholar] [CrossRef]
- Kroeze, R.J.; Helder, M.N.; Govaert, L.E.; Smit, T.H. Biodegradable Polymers in Bone Tissue Engineering. Materials 2009, 3, 833–856. [Google Scholar] [CrossRef]
Sample Availability: Samples of the PVDF coatings are available from the authors. |
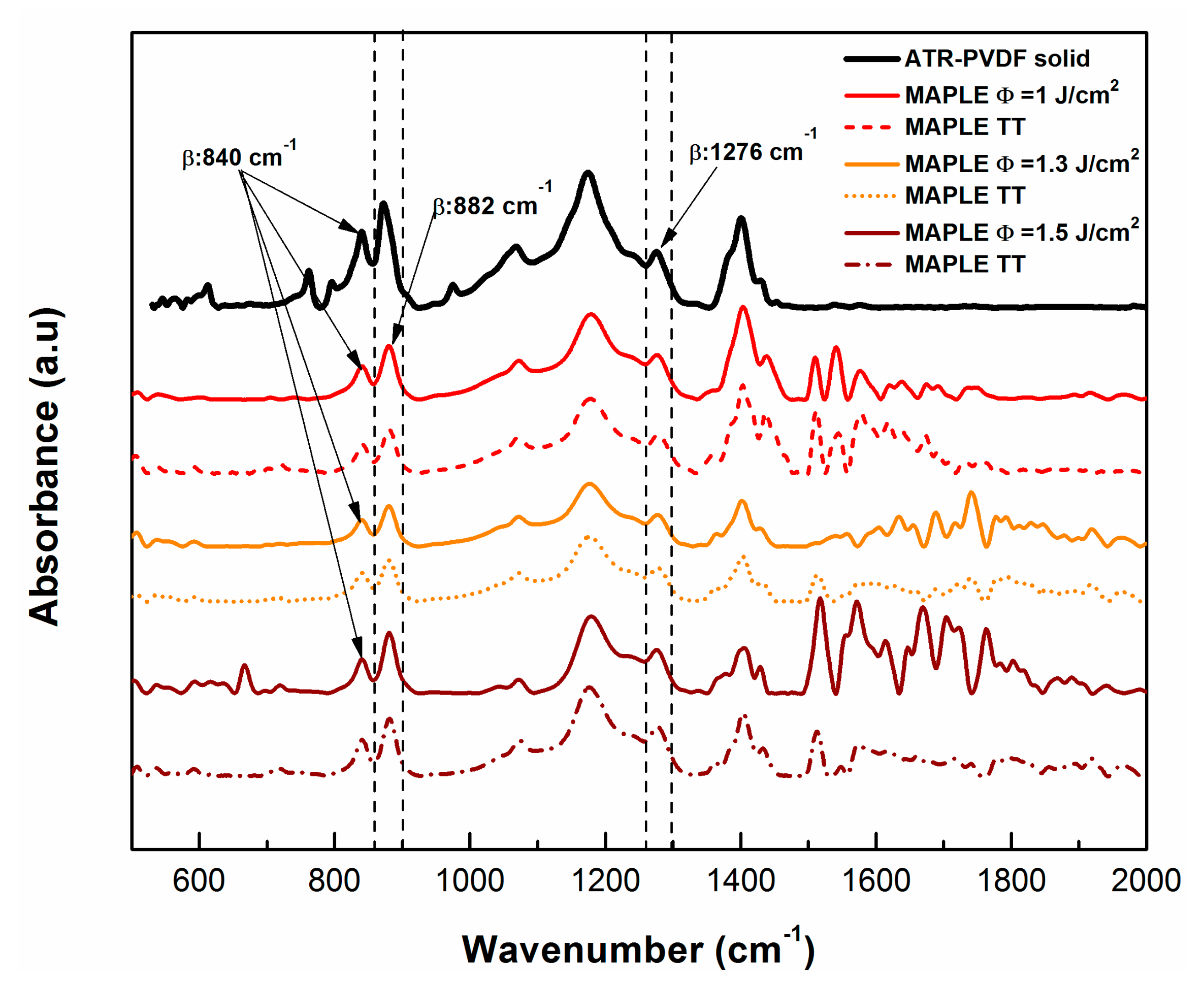


| Characteristic Bands from PVDF (CH2CHF)n | |||
|---|---|---|---|
| Peak Assignment of PVDF MAPLE | MAPLE TT | ||
| 1400 cm−1 | ωCH2 wagging | 1400 cm−1 | ωCH2 wagging |
| 1276 cm−1 | Can be assigned to the long trans-sequence of the ferroelectric β-phase of PVDF | 1279 cm−1 | β phase |
| 1068 & 1178 cm−1 | νCF2 symmetrical stretching of -CF2 group; β-phase | 1072 cm−1 | CH2 wagging |
| 1178 cm−1 | Symmetrical stretching of -CF2 group; β-phase | ||
| 882 cm−1 | C-F (stretching vibration); β phase | 882 cm−1 | β phase; C-F stretching vibration |
| 879 cm−1 | νsymCC-C (asymmetric stretching vibration) | 879 cm−1 | νsymCC-C (asymmetric stretching vibration) |
| 840 cm−1 | β-phase; C-F (stretching vibration); Deformation C-F from PVDF | 840 cm−1 | β-phase; C-F (stretching vibration); Deformation C-F from PVDF |
| Characteristic bands from DMSO (CH3)2SO | |||
| 1500–2000 cm−1 | Fingerprint of DMSO | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitrescu, L.N.; Neacsu, P.; Necula, M.G.; Bonciu, A.; Marascu, V.; Cimpean, A.; Moldovan, A.; Rotaru, A.; Dinca, V.; Dinescu, M. Induced Hydrophilicity and In Vitro Preliminary Osteoblast Response of Polyvinylidene Fluoride (PVDF) Coatings Obtained via MAPLE Deposition and Subsequent Thermal Treatment. Molecules 2020, 25, 582. https://doi.org/10.3390/molecules25030582
Dumitrescu LN, Neacsu P, Necula MG, Bonciu A, Marascu V, Cimpean A, Moldovan A, Rotaru A, Dinca V, Dinescu M. Induced Hydrophilicity and In Vitro Preliminary Osteoblast Response of Polyvinylidene Fluoride (PVDF) Coatings Obtained via MAPLE Deposition and Subsequent Thermal Treatment. Molecules. 2020; 25(3):582. https://doi.org/10.3390/molecules25030582
Chicago/Turabian StyleDumitrescu, Luminita Nicoleta, Patricia Neacsu, Madalina G. Necula, Anca Bonciu, Valentina Marascu, Anisoara Cimpean, Antoniu Moldovan, Andrei Rotaru, Valentina Dinca, and Maria Dinescu. 2020. "Induced Hydrophilicity and In Vitro Preliminary Osteoblast Response of Polyvinylidene Fluoride (PVDF) Coatings Obtained via MAPLE Deposition and Subsequent Thermal Treatment" Molecules 25, no. 3: 582. https://doi.org/10.3390/molecules25030582
APA StyleDumitrescu, L. N., Neacsu, P., Necula, M. G., Bonciu, A., Marascu, V., Cimpean, A., Moldovan, A., Rotaru, A., Dinca, V., & Dinescu, M. (2020). Induced Hydrophilicity and In Vitro Preliminary Osteoblast Response of Polyvinylidene Fluoride (PVDF) Coatings Obtained via MAPLE Deposition and Subsequent Thermal Treatment. Molecules, 25(3), 582. https://doi.org/10.3390/molecules25030582









