Poly (Vinyl Butyral-Co-Vinyl Alcohol-Co-Vinyl Acetate) Coating Performance on Copper Corrosion in Saline Environment
Abstract
1. Introduction
2. Results and Discussion
2.1. Open Circuit Potential Discussion
2.2. Electrochemical Impedance Spectroscopy (EIS)
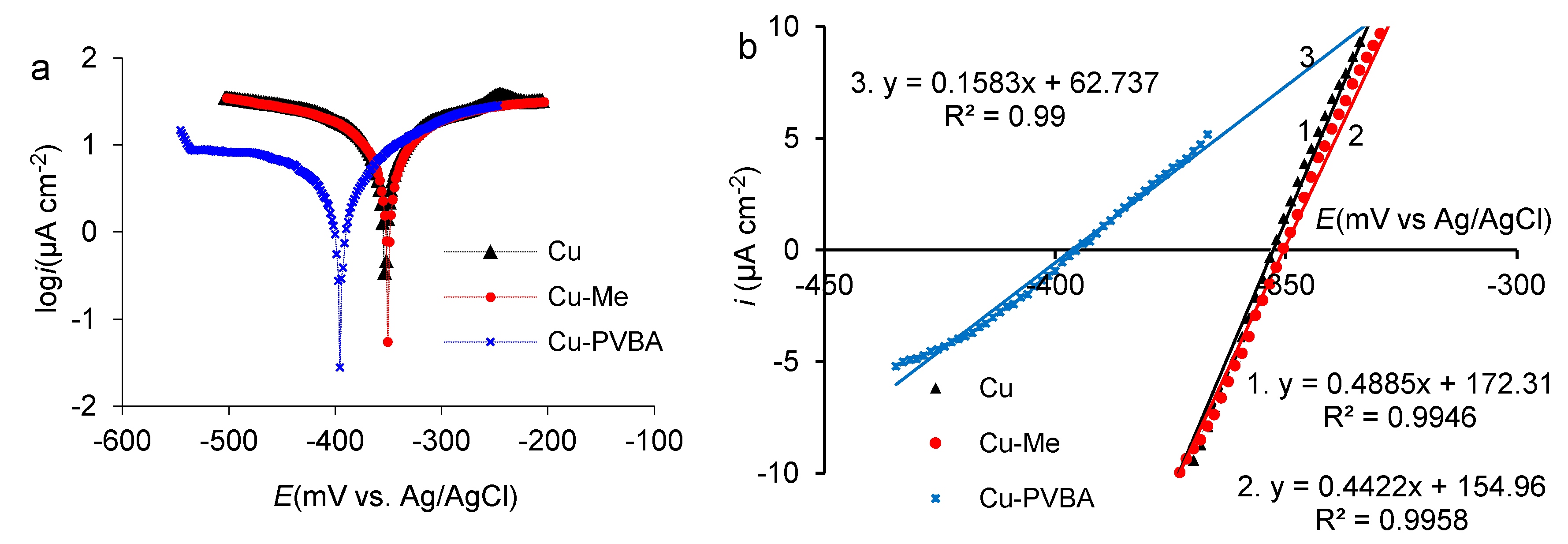
2.3. Potentiodynamic Polarization
2.4. PVBA Adsorption Mechanism
2.5. Atomic Force Microscopy
3. Materials and Methods
3.1. Materials
PVBA Coating Modeling
3.2. Corrosion Tests
3.2.1. Electrochemical Measurements
3.2.2. Atomic Force Microscopy (AFM)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PVBA | poly (vinyl butyral-co-vinyl alcohol-co-vinyl acetate) |
| Cu-Me | copper treated in methanol in the absence of PVBA |
| Cu-PVBA | copper treated in methanol in the presence of PVBA |
| EIS | electrochemical impedance spectroscopy |
| AFM | atomic force microscopy |
| OCP | open circuit potential |
| icorr | corrosion current density |
| Ecorr | corrosion potential |
| Rp | polarization resistance |
| Rct | charge transfer resistance |
| Cdl | double-layer capacitance |
| Rs | solution resistance |
| P% | coating protection performance |
| Sp | polarization conductance |
References
- Obot, I.B.; Solomon, M.M.; Umoren, S.A.; Suleiman, R.; Elanany, M.; Alanazi, N.M.; Sorour, A.A. Progress in the development of sour corrosion inhibitors: Past, present, and future perspectives. J. Ind. Eng. Chem. 2019, 79, 1–18. [Google Scholar] [CrossRef]
- Tiu, B.D.B.; Advincula, R.C. Polymeric corrosion inhibitors for the oil and gas industry: Design principles and mechanism. React. Funct. Polym. 2015, 95, 25–45. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M. Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review. Carbohydr. Polym. 2016, 140, 314–341. [Google Scholar] [CrossRef] [PubMed]
- Umoren, S.A.; Solomon, M.M. Protective polymeric films for industrial substrates: A critical review on past and recent applications with conducting polymers and polymer composites/nanocomposites. Prog. Mater. Sci. 2019, 104, 380–450. [Google Scholar] [CrossRef]
- Alrashed, M.M.; Jana, S.; Soucek, M.D. Corrosion performance of polyurethane hybrid coatings with encapsulated inhibitor. Prog. Org. Coat. 2019, 130, 235–243. [Google Scholar] [CrossRef]
- Yabuki, A.; Nagayama, Y.; Fathona, I.W. Porous anodic oxide film with self-healing ability for corrosion protection of aluminum. Electrochim. Acta 2019, 296, 662–668. [Google Scholar] [CrossRef]
- Okafor, P.A.; Singh-Beemat, J.; Iroh, J.O. Thermomechanical and corrosion inhibition properties of graphene/epoxy ester–siloxane–urea hybrid polymer nanocomposites. Prog. Org. Coat. 2015, 88, 237–244. [Google Scholar] [CrossRef]
- Othman, N.H.; Ismail, M.C.; Mustapha, M.; Sallih, N.; Kee, K.E.; Jaal, R.A. Graphene-based polymer nanocomposites as barrier coatings for corrosion protection. Prog. Org. Coat. 2019, 135, 82–99. [Google Scholar] [CrossRef]
- Jin, T.; Xie, Z.; Fullston, D.; Huang, C.; Zeng, R.; Bai, R. Corrosion resistance of copolymerization of acrylamide and acrylic acid grafted graphene oxide composite coating on magnesium alloy. Prog. Org. Coat. 2019, 136, 105222. [Google Scholar] [CrossRef]
- Li, L.Y.; Cui, L.Y.; Zeng, R.C.; Li, S.Q.; Chen, X.B.; Zheng, Y.; Kannan, M.B. Advances in functionalized polymer coatings on biodegradable magnesium alloys—A review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef]
- Bertuola, M.; Miñán, A.; Grillo, C.A.; Cortizo, M.C.; Fernández Lorenzo de Mele, M.A. Corrosion protection of AZ31 alloy and constrained bacterial adhesion mediated by a polymeric coating obtained from a phytocompound. Colloids Surf. B Biointerfaces 2018, 172, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yagoshi, K.; Koga, Y.; Sasaki, M.; Niidomea, T. Optimized polymer coating for magnesium alloy-based bioresorbable scaffolds for long-lasting drug release and corrosion resistance. Colloids Surf. B Biointerfaces 2018, 163, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ubaid, F.; Radwan, A.B.; Naeem, N.; Shakoor, R.A.; Ahmad, Z.; Montemor, M.F.; Kahraman, R.; Abdullah, A.M.; Soliman, A. Multifunctional self-healing polymeric nanocomposite coatings for corrosion inhibition of steel. Surf. Coat. Technol. 2019, 372, 121–133. [Google Scholar] [CrossRef]
- Pulikkalparambil, H.; Siengchin, S.; Parameswaranpillai, J. Corrosion protective self-healing epoxy resin coatings based on inhibitor and polymeric healing agents encapsulated in organic and inorganic micro and nanocontainers. Nano-Struct. Nano-Objects 2018, 16, 381–395. [Google Scholar] [CrossRef]
- Yabuki, A.; Tanabe, S.; Fathona, I.W. Self-healing polymer coating with the microfibers of superabsorbent polymers provides corrosion inhibition in carbon steel. Surf. Coat. Technol. 2018, 341, 71–77. [Google Scholar] [CrossRef]
- Singh, A.; Soni, N.; Deyuan, Y.; Kumar, A. A combined electrochemical and theoretical analysis of environmentally benign polymer for corrosion protection of N80 steel in sweet corrosive environment. Results Phys. 2019, 13, 102116. [Google Scholar] [CrossRef]
- Achary, G.; Naik, Y.A.; Kumar, S.V.; Venkatesha, T.V.; Sherigara, B.S. An electroactive co-polymer as corrosion inhibitor for steel in sulphuric acid medium. Appl. Surf. Sci. 2008, 254, 5569–5573. [Google Scholar] [CrossRef]
- Gopi, D.; Karthikeyan, P.; Kavitha, L.; Surendiran, M. Development of poly (3,4-ethylenedioxythiophene-co-indole-5-carboxylic acid) co-polymer coatings on passivated low-nickelstainless steel for enhanced corrosion resistance in the sulphuric acid medium. Appl. Surf. Sci. 2015, 357, 122–130. [Google Scholar] [CrossRef]
- Lin, Y.; Singh, A.; Ebenso, E.E.; Wu, Y.; Zhu, C.; Zhu, M. Effect of poly (methyl methacrylate-co-N-vinyl-2-pyrrolidone) polymer on J55 steel corrosion in 3.5% NaCl solution saturated with CO2. J. Taiwan Inst. Chem. Eng. 2015, 46, 214–222. [Google Scholar] [CrossRef]
- Mathew, A.M.; Predeep, P. Styrene butadiene co-polymer based conducting polymer composite as an effective corrosion protective coating. Prog. Org. Coat. 2012, 74, 14–18. [Google Scholar] [CrossRef]
- Sambyal, P.; Ruhi, G.; Dhawan, R.; Dhawan, S.K. Designing of smart coatings of conducting polymer poly (aniline-co-phenetidine)/SiO2 composites for corrosion protection in marine environment. Surf. Coat. Technol. 2016, 303, 362–371. [Google Scholar] [CrossRef]
- Bustos-Terrones, V.; Serratos, I.N.; Castañeda-Villa, N.; Escobar, J.O.V.; Romo, M.A.R.; Córdoba, G.; Chavarín, J.U.; Campos, C.M.; Schulz, J.M.E.; Ortiz, A.D. Functionalized coatings based on organic polymer matrix against the process of corrosion of mild steel in neutral medium. Prog. Org. Coat. 2018, 119, 221–229. [Google Scholar] [CrossRef]
- Kaur, H.; Sharma, J.; Jindal, D.; Arya, R.K.; Ahuja, S.K.; Arya, S.B. Crosslinked polymer doped binary coatings for corrosion protection. Prog. Org. Coat. 2018, 125, 32–39. [Google Scholar] [CrossRef]
- Azzam, E.M.S.; El-Salam, H.M.A.; Mohamed, R.A.; Shaban, S.M.; Shokry, A. Control the corrosion of mild steel using synthesized polymers based on polyacrylamide. Egypt. J. Pet. 2018, 27, 897–910. [Google Scholar] [CrossRef]
- Biswas, A.; Pal, S.; Udayabhanu, G. Experimental and theoretical studies of xanthan gum and its graft co-polymer as corrosion inhibitor for mild steel in 15% HCl. Appl. Surf. Sci. 2015, 353, 173–183. [Google Scholar] [CrossRef]
- Madhankumar, A.; Rajendran, N. A promising copolymer of p-phenylendiamine and o-aminophenol: Chemical and electrochemical synthesis, characterization and its corrosion protection aspect on mild steel. Synth. Met. 2012, 162, 176–185. [Google Scholar] [CrossRef]
- Aly, K.I.; Younis, O.; Mahross, M.H.; Orabi, E.A.; Hakim, M.A.; Tsutsumi, O.; Mohamed, M.G.; Sayed, M.M. Conducting copolymers nanocomposite coatings with aggregation controlled luminescence and efficient corrosion inhibition properties. Prog. Org. Coat. 2019, 135, 525–535. [Google Scholar] [CrossRef]
- Samide, A.; Bratulescu, G.; Merisanu, C.; Cioatera, N. Anticorrosive coating based on poly (vinyl acetate) formed by electropolymerization on the copper surface. J. App. Polym. Sci. 2019, 136, 47320. [Google Scholar] [CrossRef]
- Grecu, R.; Samide, A.; Iacobescu, G.E.; Cioateră, N.; Popescu, A. Copper Corrosion Inhibitors Based on Polyvinyl alcohol and Silver nanoparticles. Chem. Ind. Chem. Eng. Q. 2019, 25, 267–275. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B.; Dobrițescu, A.; Ilea, P.; Vladu, A.C.; Tigae, C. Electrochemical and theoretical study of metronidazole drug as inhibitor for copper corrosion in hydrochloric acid solution. Int. J. Electrochem. Sci. 2016, 11, 5520–5534. [Google Scholar] [CrossRef]
- Farahati, R.; Ghaffarinejad, A.; Rezania, H.J.; Mousavi-Khoshdel, S.M.; Behzadi, H. Sulfonated aromatic polyamide as water-soluble polymeric corrosion inhibitor of copper in HCl. Colloids Surf. A 2019, 578, 123626. [Google Scholar] [CrossRef]
- Mouaden, K.E.; Ibrahimi, B.E.; Oukhrib, R.; Bazzi, L.; Hammouti, B.; Jbara, O.; Tara, A.; Chauhan, D.S.; Quraishi, M.A. Chitosan polymer as a green corrosion inhibitor for copper in sulfide-containing synthetic seawater. Int. J. Biol. Macromol. 2018, 119, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Bertuola, M.; Grillo, C.A.; Pissinis, D.E.; Prieto, E.D.; Lorenzo de Mele, M.F. Is the biocompatibility of copper with polymerized natural coating dependent on the potential selected for the electropolymerization process? Colloids Surf. B Biointerfaces 2017, 159, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Chen, Y.; Wang, Q.; Wang, T. Poly (vinyl butyral) based polymer networks with dual-responsive shape memory and self-healing properties. J. Mater. Chem. A 2014, 2, 9169–9177. [Google Scholar] [CrossRef]
- Kumar, P.; Khan, N.; Kumar, D. Polyvinyl butyral (PVB), versetile template for designing nanocomposite/composite materials: A review. Green Chem. Technol. Lett. 2016, 2, 185–194. [Google Scholar] [CrossRef]
- Jiang, Z.C.; Xiao, Y.Y.; Kang, Y.; Pan, M.; Li, B.J.; Zhang, S. Shape memory polymers based on supramolecular interactions. ACS Appl. Mater. Interfaces 2017, 9, 20276–20293. [Google Scholar] [CrossRef]
- Chen, H.M.; Wang, L.; Zhou, S.B. Recent progress in shape memory polymers for biomedical applications. Chin. J. Polym. Sci. 2018, 36, 905–917. [Google Scholar] [CrossRef]
- Samide, A.; Stoean, R.; Stoean, C.; Tutunaru, B.; Grecu, R.; Cioatera, N. Investigation of Polymer Coatings Formed by Polyvinyl Alcohol and Silver Nanoparticles on Copper Surface in Acid Medium by Means of Deep Convolutional Neural Networks. Coatings 2019, 9, 105. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B. Quinine sulfate: A pharmaceutical product as effective corrosion inhibitor for carbon steel in hydrochloric acid solution. Cent. Eur. J. Chem. 2014, 12, 901–908. [Google Scholar] [CrossRef]
- Samide, A.; Ilea, P.; Vladu, A.C. Metronidazole Performance as Corrosion Inhibitor for Carbon Steel, 304L Stainless Steel and Aluminum in Hydrochloric Acid Solution. Int. J. Electrochem. Sci. 2017, 12, 5964–5983. [Google Scholar] [CrossRef]
- Samide, A.; Iacobescu, G.E.; Tutunaru, B.; Grecu, R.; Tigae, C.; Spînu, C. Inhibitory Properties of Neomycin Thin Film Formed on Carbon Steel in Sulfuric Acid Solution: Electrochemical and AFM Investigation. Coatings 2017, 7, 181. [Google Scholar] [CrossRef]
- Samide, A.; Grecu, R.; Tutunaru, B.; Tigae, C.; Spînu, C. Cisplatin-chemotherapeutic Drug Interactions with the Surface of Some Metal Bioimplants in Physiological Serum. Int. J. Electrochem. Sci. 2017, 12, 11316–11329. [Google Scholar] [CrossRef]
- Samide, A.; Iacobescu, G.E.; Tutunaru, B.; Tigae, C. Electrochemical and AFM Study of Inhibitory Properties of Thin Film Formed by Tartrazine Food Additive on 304L Stainless Steel in Saline Solution. Int. J. Electrochem. Sci. 2017, 12, 2088–2101. [Google Scholar] [CrossRef]
- Carrot, C.; Bendaoud, A.; Pillon, C. Chapter 3. Polyvinyl Butyral. In Handbook of Thermoplastics; CRC Press: Boca Raton, FL, USA, 2015; p. 128. [Google Scholar]
- Jiang, X.; Parmeter, J.E.; Estrada, C.A.; Wayne Goodman, D. The adsorption and decomposition of methanol on copper on the Rh(100) surface. Surf. Sci. 1991, 249, 44–60. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
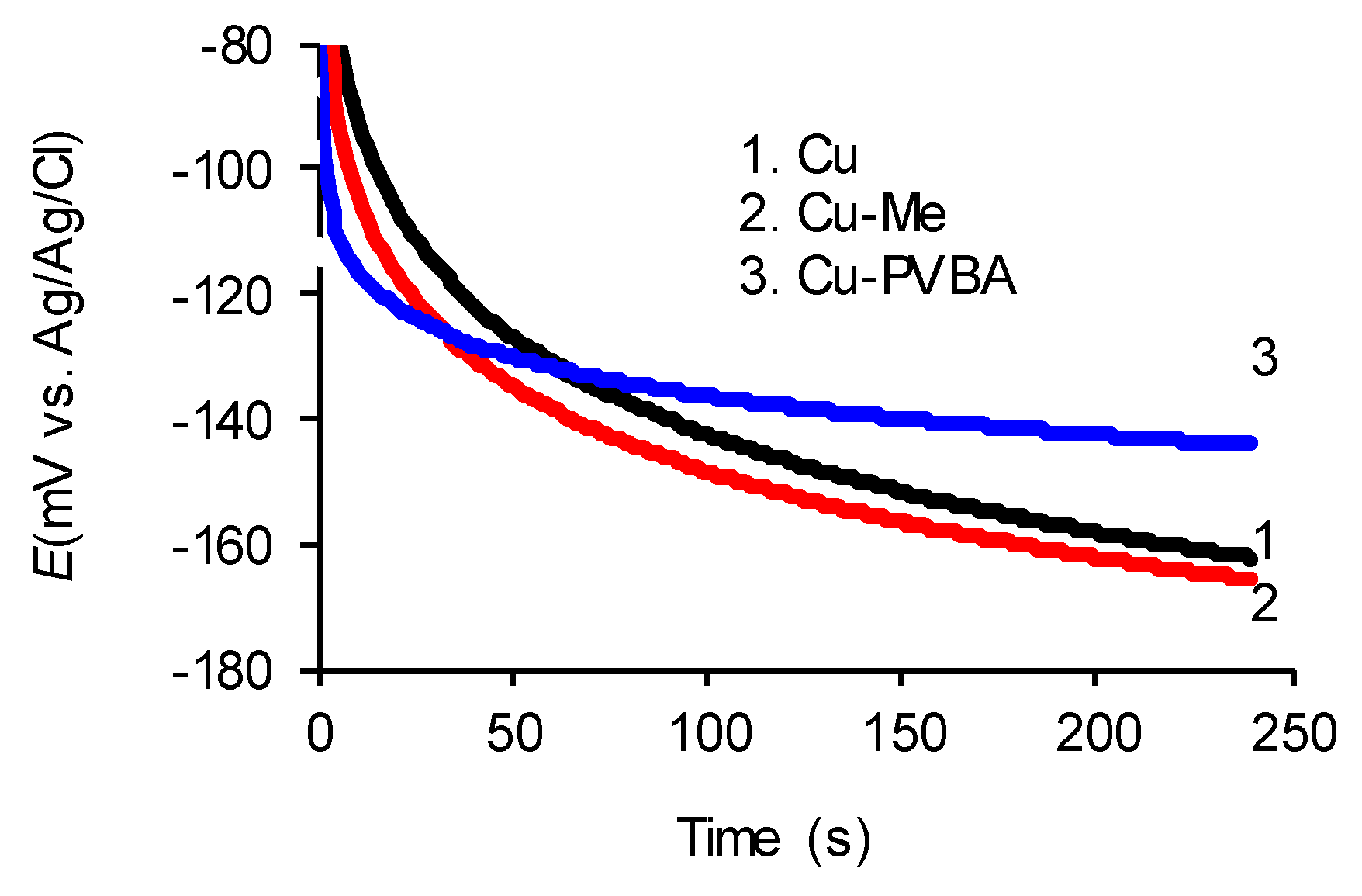

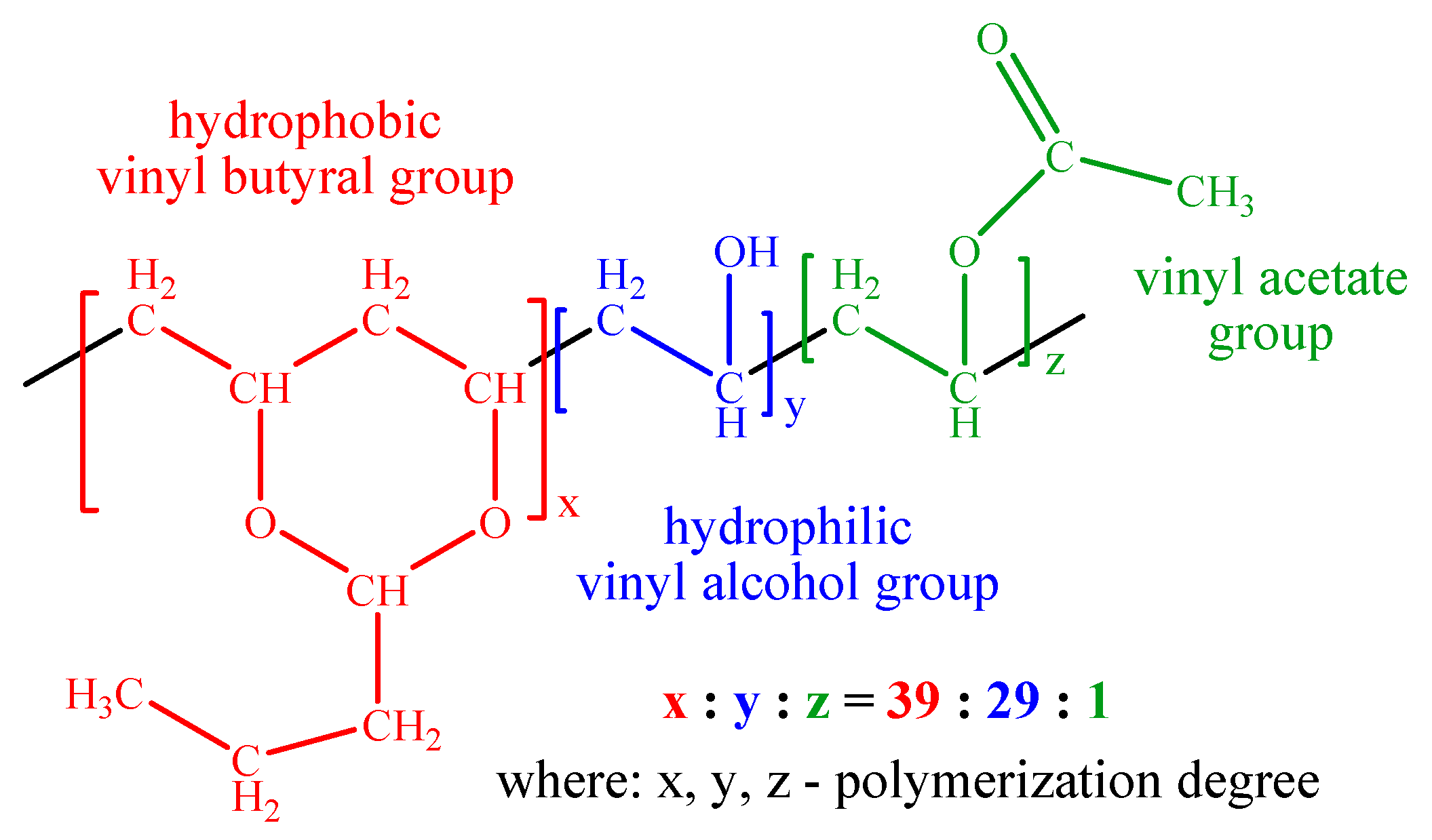


| Sample | EOCP (mV vs. Ag/AgCl) | Rs (Ω cm2) | Rct (Ω cm2) | Cdl (µF cm−2) | logZ (Ω cm2) | Z (Ω cm2) | │Phase│ (degree) | P (%) |
|---|---|---|---|---|---|---|---|---|
| Standard | −164 | 245 | 515 | 845 | 2.71 | 513 | 46.3/peak 44.5/plateau | - |
| Cu-Me | −167 | 132 | 650 | 612 | 2.84 | 692 | 48.8/plateau | - |
| Cu-PVBA | −147 | 64 | 2763 | 342 | 3.43 | 2692 | 57.1/peak | 80.9 |
| Sample | Ecorr (mV vs. Ag/AgCl) | icorr (µA cm−2) | ba (mV dec−1) | bc (mV dec−1) | Sp 103 (S cm−2) | Rp (Ω cm2) | P (%) |
|---|---|---|---|---|---|---|---|
| Standard | −353 | 10.2 | 179 | −172 | 0.4885 | 2047 | - |
| Cu-Me | −350 | 11.3 | 228 | −232 | 0.4422 | 2261 | - |
| Cu-PVBA | −395 | 2.03 | 133 | −260 | 0.1583 | 6317 | 80.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samide, A.; Merisanu, C.; Tutunaru, B.; Iacobescu, G.E. Poly (Vinyl Butyral-Co-Vinyl Alcohol-Co-Vinyl Acetate) Coating Performance on Copper Corrosion in Saline Environment. Molecules 2020, 25, 439. https://doi.org/10.3390/molecules25030439
Samide A, Merisanu C, Tutunaru B, Iacobescu GE. Poly (Vinyl Butyral-Co-Vinyl Alcohol-Co-Vinyl Acetate) Coating Performance on Copper Corrosion in Saline Environment. Molecules. 2020; 25(3):439. https://doi.org/10.3390/molecules25030439
Chicago/Turabian StyleSamide, Adriana, Claudia Merisanu, Bogdan Tutunaru, and Gabriela Eugenia Iacobescu. 2020. "Poly (Vinyl Butyral-Co-Vinyl Alcohol-Co-Vinyl Acetate) Coating Performance on Copper Corrosion in Saline Environment" Molecules 25, no. 3: 439. https://doi.org/10.3390/molecules25030439
APA StyleSamide, A., Merisanu, C., Tutunaru, B., & Iacobescu, G. E. (2020). Poly (Vinyl Butyral-Co-Vinyl Alcohol-Co-Vinyl Acetate) Coating Performance on Copper Corrosion in Saline Environment. Molecules, 25(3), 439. https://doi.org/10.3390/molecules25030439







