Yellow Emission Obtained by Combination of Broadband Emission and Multi-Peak Emission in Garnet Structure Na2YMg2V3O12: Dy3+ Phosphor
Abstract
1. Introduction
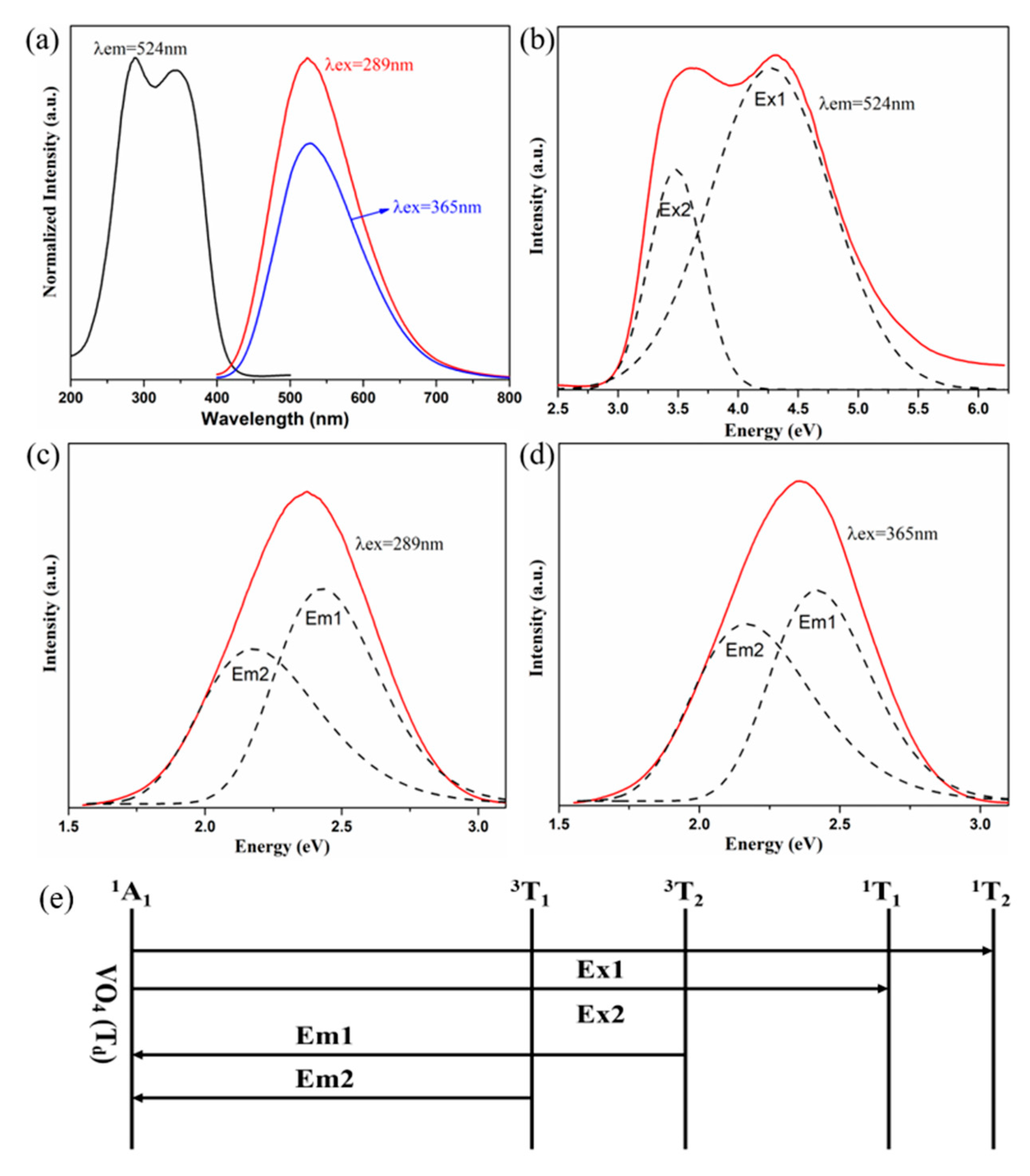
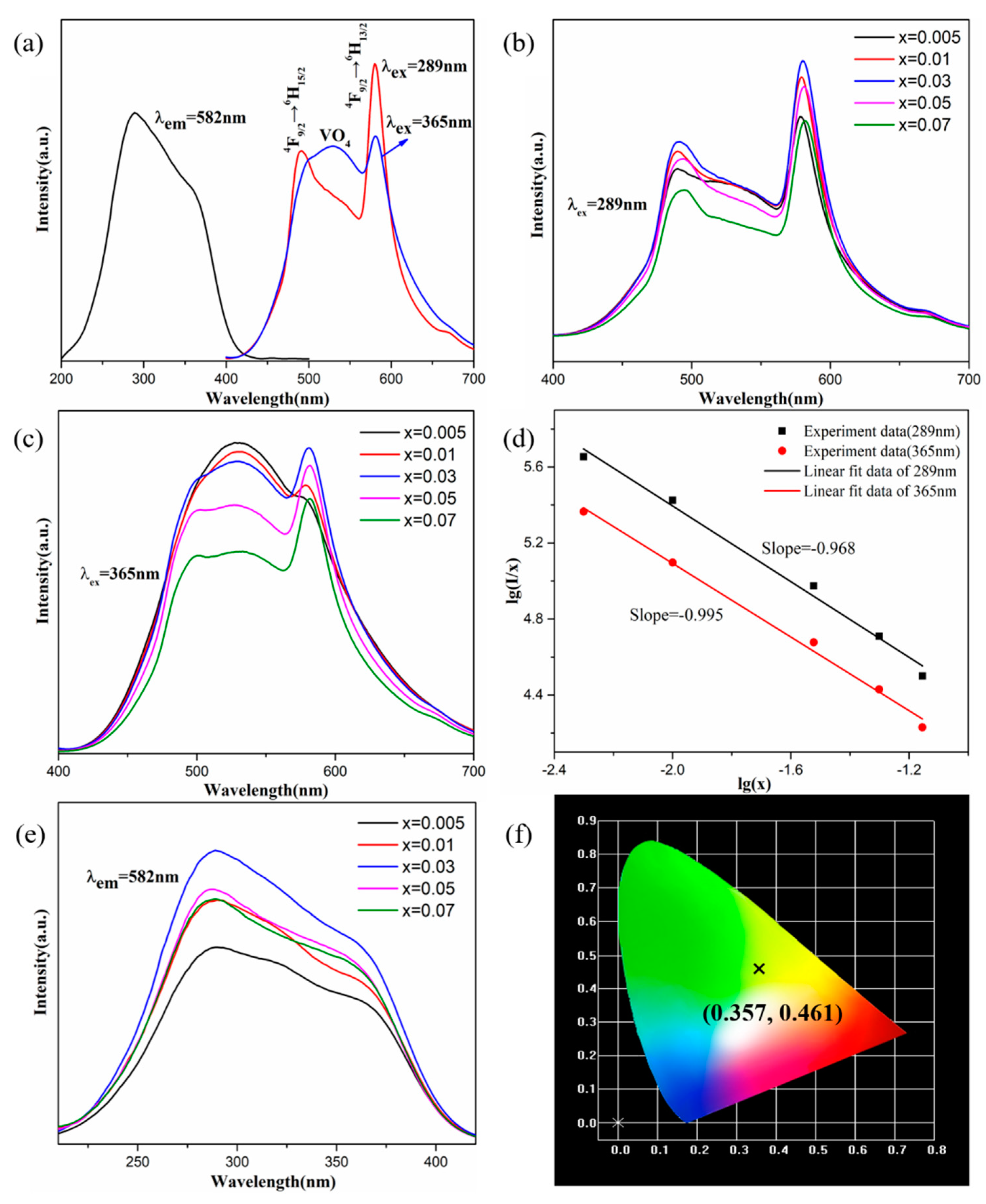
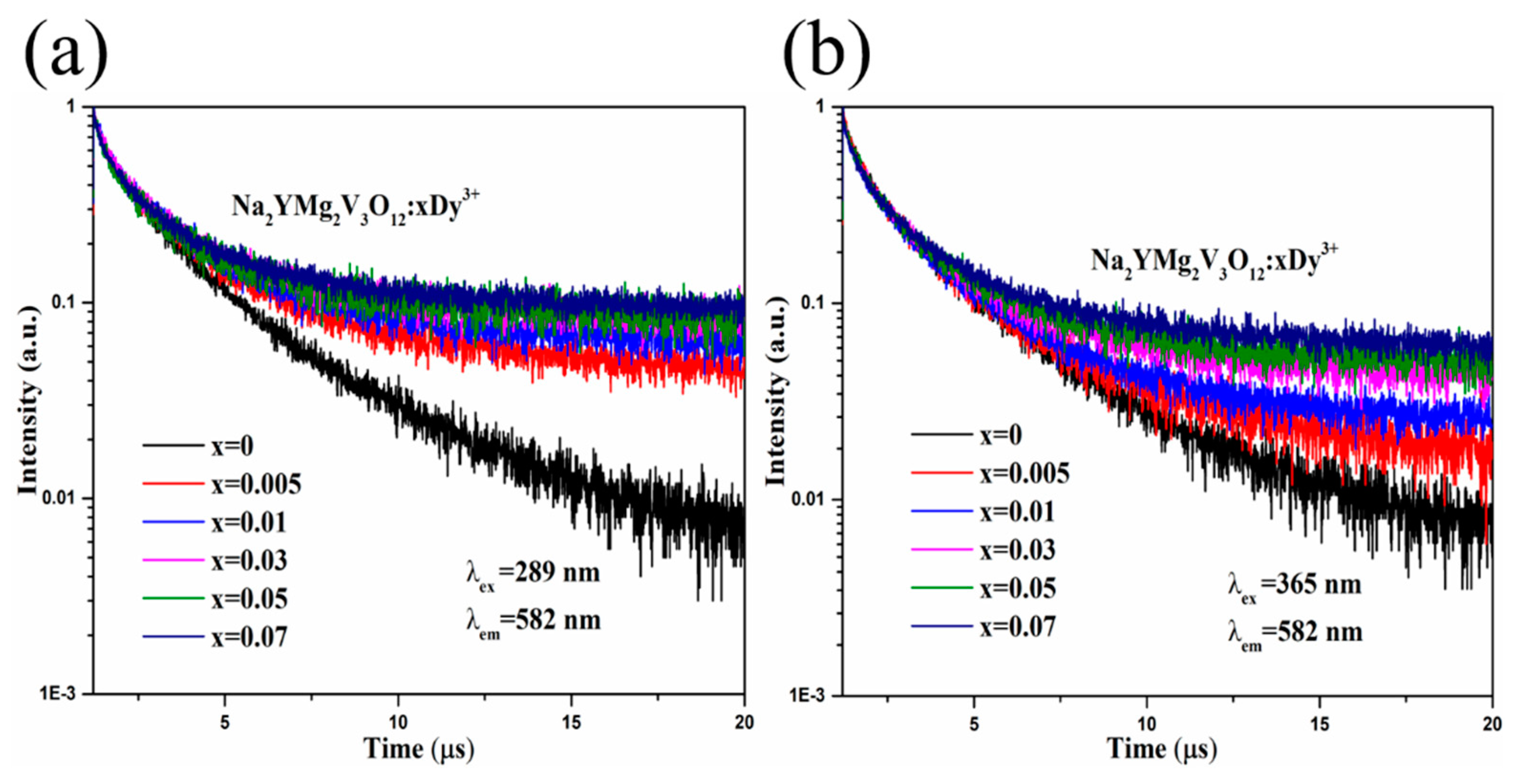
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Du, P.; Yu, J.S. Photoluminescence, cathodoluminescence and thermal stability of Sm3+-activated Sr3La(VO4)3 red-emitting phosphors. Luminescence 2017, 32, 1504–1510. [Google Scholar] [CrossRef]
- He, C.; Ji, H.; Huang, Z.; Wang, T.; Zhang, X.; Liu, Y.; Fang, M.; Wu, X.; Zhang, J.; Min, X. Red-shifted emission in Y3MgSiAl3O12: Ce3+ garnet phosphor for blue light-pumped white light-emitting diodes. J. Phys. Chem. C 2018, 122, 15659–15665. [Google Scholar] [CrossRef]
- Min, X.; Fang, M.; Huang, Z.; Liu, Y.G.; Tang, C.; Wu, X. Synthesis and optical properties of Pr3+-doped LaMgAl11O19—A novel blue converting yellow phosphor for white light emitting diodes. Ceram. Int. 2015, 41, 4238–4242. [Google Scholar] [CrossRef]
- Xie, R.-J.; Hirosaki, N. Silicon-based oxynitride and nitride phosphors for white LEDs—A review. Sci. Technol. Adv. Mater. 2007, 8, 588. [Google Scholar] [CrossRef]
- Xie, R.-J.; Hirosaki, N.; Suehiro, T.; Xu, F.-F.; Mitomo, M. A simple, efficient synthetic route to Sr2Si5N8: Eu2+-based red phosphors for white light-emitting diodes. Chem. Mater. 2006, 18, 5578–5583. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Fang, Y.-C.; Lin, H.-Y.; Chu, S.-Y. Photoluminescence properties and thermal stability of samarium-doped barium phosphate phosphors. Ceram. Int. 2017, 43, 4353–4356. [Google Scholar] [CrossRef]
- Cao, Y.; Ding, J.; Ding, X.; Wang, X.; Wang, Y. Tunable white light of multi-cation-site Na2BaCa(PO4)2: Eu, Mn phosphor: Synthesis, structure and PL/CL properties. J. Mater. Chem. C 2017, 5, 1184–1194. [Google Scholar] [CrossRef]
- Dang, P.; Liang, S.; Li, G.; Wei, Y.; Cheng, Z.; Lian, H.; Shang, M.; Al Kheraif, A.A.; Lin, J. Full Color Luminescence Tuning in Bi3+/Eu3+-Doped LiCa3MgV3O12 Garnet Phosphors Based on Local Lattice Distortion and Multiple Energy Transfers. Inorg. Chem. 2018, 57, 9251–9259. [Google Scholar] [CrossRef]
- Ji, H.; Cho, Y.; Wang, L.; Hirosaki, N.; Molokeev, M.S.; Huang, Z.; Xie, R.-J. Phase formation of (Y,Ce)2BaAl4SiO12 yellow microcrystal-glass phosphor for blue LED pumped white lighting. Ceram. Int. 2017, 43, 6425–6429. [Google Scholar] [CrossRef]
- Lee, S.H.; Du, P.; Bharat, L.K.; Yu, J.S. Ultraviolet radiation excited strong red-emitting LaAlO3: Eu3+ nanophosphors: Synthesis and luminescent properties. Ceram. Int. 2017, 43, 4599–4605. [Google Scholar] [CrossRef]
- Qiao, J.; Xia, Z.; Zhang, Z.; Hu, B.; Liu, Q. Near UV-pumped yellow-emitting Sr9MgLi(PO4)7:Eu2+ phosphor for white-light LEDs. Sci. China Mater. 2018, 61, 985–992. [Google Scholar] [CrossRef]
- Liu, S.; Liu, S.; Wang, J.; Sun, P.; Zhong, Y.; Jeong, J.H.; Deng, B.; Yu, R. Preparation and investigation of Dy3+-doped Ca9LiGd2/3(PO4)7 single-phase full-color phosphor. Mater. Res. Bull. 2018, 108, 275–280. [Google Scholar] [CrossRef]
- Xia, Z.; Meijerink, A. Ce3+-Doped garnet phosphors: Composition modification, luminescence properties and applications. Chem. Soc. Rev. 2017, 46, 275–299. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Liu, W.-R.; Chen, T.-M. Single-phased white-light phosphors Ca9Gd(PO4)7: Eu2+, Mn2+ under near-ultraviolet excitation. J. Phys. Chem. C 2010, 114, 18698–18701. [Google Scholar] [CrossRef]
- Miao, S.; Xia, Z.; Zhang, J.; Liu, Q. Increased Eu2+ content and codoping Mn2+ induced tunable full-color emitting phosphor Ba1.55Ca0.45SiO4: Eu2+, Mn2+. Inorg. Chem. 2014, 53, 10386–10393. [Google Scholar] [CrossRef]
- Min, X.; Fang, M.; Huang, Z.; Liu, Y.; Tang, C.; Wu, X. Luminescent properties of white-light-emitting phosphor LaMgAl11O19:Dy3+. Mater. Lett. 2014, 125, 140–142. [Google Scholar] [CrossRef]
- Song, D.; Guo, C.; Li, T. Luminescence of the self-activated vanadate phosphors Na2LnMg2V3O12 (Ln=Y, Gd). Ceram. Int. 2015, 41, 6518–6524. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Y.; Ju, G.; Zhang, S.; Xue, F. Photoluminescence of a novel Na3Y(VO4)2:Eu3+ red phosphor for near ultraviolet light emitting diodes application. J. Mater. Sci. Mater. Electron. 2016, 28, 2529–2537. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J. Nanosci. Nanotechnol. 2006, 6, 1159–1166. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Wang, S.; Joly, A.G. Investigation of water-soluble x-ray luminescence nanoparticles for photodynamic activation. Appl. Phys. Lett. 2008, 92, 043901. [Google Scholar] [CrossRef]
- Zou, X.; Yao, M.; Ma, L.; Hossu, M.; Han, X.; Juzenas, P.; Chen, W. X-ray-induced nanoparticle-based photodynamic therapy of cancer. Nanomedicine 2014, 9, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.K.; Giang, T.T.H.; Yu, J.S. UV excitation band induced novel Na3Gd(VO4)2:RE3+ (RE3+ = Eu3+ or Dy3+ or Sm3+) double vanadate phosphors for solid-state lightning applications. J. Alloys Compd. 2018, 739, 218–226. [Google Scholar] [CrossRef]
- Huang, X.; Guo, H. LiCa3MgV3O12:Sm3+: A new high-efficiency white-emitting phosphor. Ceram. Int. 2018, 44, 10340–10344. [Google Scholar] [CrossRef]
- Duke John David, A.; Muhammad, G.S.; Sivakumar, V. Synthesis and photoluminescence properties of Sm3+ substituted glaserite-type orthovanadates K3Y[VO4]2 with monoclinic structure. J. Lumin. 2016, 177, 104–110. [Google Scholar] [CrossRef]
- Kang, F.; Yang, X.; Peng, M.; Wondraczek, L.; Ma, Z.; Zhang, Q.; Qiu, J. Red photoluminescence from Bi3+ and the influence of the oxygen-vacancy perturbation in ScVO4: A combined experimental and theoretical study. J. Phys. Chem. C 2014, 118, 7515–7522. [Google Scholar] [CrossRef]
- Yu, M.; Lin, J.; Fang, J. Silica Spheres Coated with YVO4: Eu3+ Layers via sol–gel process: A simple method to obtain spherical core–shell phosphors. Chem. Mater. 2005, 17, 1783–1791. [Google Scholar] [CrossRef]
- Li, K.; Lian, H.; Shang, M.; Lin, J. A novel greenish yellow-orange red Ba3Y4O9: Bi3+, Eu3+ phosphor with efficient energy transfer for UV-LEDs. Dalton Trans. 2015, 44, 20542–20550. [Google Scholar] [CrossRef]
- Jing, L.; Liu, X.; Li, Y.; Wang, Y. Green-to-red tunable luminescence of Eu3+-doped K3Y(VO4)2 phosphors. J. Mater. Sci. 2016, 51, 903–910. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, C.; Li, T.; Su, X.; Zhang, N.; Chen, J. Synthesis, electronic structure and photoluminescence properties of Ba2BiV3O11: Eu3+ red phosphor. Dyes Pigments 2016, 132, 159–166. [Google Scholar] [CrossRef]
- Biswas, P.; Kumar, V.; Agarwal, G.; Ntwaeaborwa, O.; Swart, H. NaSrVO4: Sm3+—An n-UV convertible phosphor to fill the quantum efficiency gap for LED applications. Ceram. Int. 2016, 42, 2317–2323. [Google Scholar] [CrossRef]
- Nakajima, T.; Isobe, M.; Tsuchiya, T.; Ueda, Y.; Manabe, T. Correlation between luminescence quantum efficiency and structural properties of vanadate phosphors with chained, dimerized, and isolated VO4 tetrahedra. J. Phys. Chem. C 2010, 114, 5160–5167. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Y.M.; Tsuboi, T.; Seo, H.J. Novel yellow-emitting phosphors of Ca5M4(VO4)6 (M=Mg, Zn) with isolated VO4 tetrahedra. Opt. Express 2012, 20, 4360–4368. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Townsend, P.D. Potential problems in collection and data processing of luminescence signals. J. Lumin. 2013, 142, 202–211. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, L.; Fan, F.; Bai, Y.; Ouyang, B.; Chen, W.; Li, Y.; Huang, L. Near UV-pumped yellow-emitting Ca3TeO6:Dy3+ phosphor for white light-emitting diodes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 223, 117343. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Ye, S.; Wang, D.; Li, K.; Wang, Y.; Liu, Z.; Wang, H. Surface modification of SrAl2O4: Eu2+, Dy3+ and Sr4Al14O25: Eu2+, Dy3+ long lasting phosphors and their application in water-borne paint. J. Chin. Ceram. Soc 2015, 2, 17–23. [Google Scholar]
- Li, K.; Zhang, Y.; Li, X.; Shang, M.; Lian, H.; Lin, J. Host-sensitized luminescence in LaNbO4: Ln3+ (Ln3+ = Eu3+/Tb3+/Dy3+) with different emission colors. Phys. Chem. Chem. Phys. 2015, 17, 4283–4292. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Zhou, J.; Mao, Z. Near UV-pumped green-emitting Na3(Y,Sc)Si3O9: Eu2+ phosphor for white-emitting diodes. J. Mater. Chem. C 2013, 1, 5917–5924. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, Y.; Molokeev, M.S.; Atuchin, V.V. Structural and luminescence properties of yellow-emitting NaScSi2O6: Eu2+ phosphors: Eu2+ site preference analysis and generation of red emission by codoping Mn2+ for white-light-emitting diode applications. J. Phys. Chem. C 2013, 117, 20847–20854. [Google Scholar] [CrossRef]
- Lakshmi Devi, L.; Jayasankar, C.K. Spectroscopic investigations on high efficiency deep red-emitting Ca2SiO4:Eu3+ phosphors synthesized from agricultural waste. Ceram. Int. 2018, 44, 14063–14069. [Google Scholar] [CrossRef]
- Nakano, H.; Kamimoto, K.; Yokoyama, N.; Fukuda, K. The Effect of Heat Treatment on the Emission Color of P-Doped Ca2SiO4 Phosphor. Materials 2017, 10, 1000. [Google Scholar] [CrossRef]
- Rojas-Hernandez, R.E.; Rubio-Marcos, F.; Serrano, A.; Salas, E.; Hussainova, I.; Fernandez, J.F. Towards Blue Long-Lasting Luminescence of Eu/Nd-Doped Calcium-Aluminate Nanostructured Platelets via the Molten Salt Route. Nanomaterials 2019, 9, 1473. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, Z.; Wang, Z.; Guo, Q. White-light-emitting diodes of UV-based Sr3Y2(BO3)4:Dy3+ and luminescent properties. Mater. Lett. 2008, 62, 1455–1457. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Liu, Y.; Wang, L.; Zhang, N.; Wang, X.; Huang, Z.; Ji, C. Sol-gel synthesis and luminescent properties of a novel KBaY(MoO4)3:Dy3+ phosphor for white light emission. J. Lumin. 2019, 211, 218–226. [Google Scholar] [CrossRef]
- Rao, B.V.; Jang, K.; Lee, H.S.; Yi, S.-S.; Jeong, J.-H. Synthesis and photoluminescence characterization of RE3+ (=Eu3+, Dy3+)-activated Ca3La(VO4)3 phosphors for white light-emitting diodes. J. Alloys Compd. 2010, 496, 251–255. [Google Scholar] [CrossRef]
- Ratnam, B.V.; Jayasimhadri, M.; Jang, K.; Sueb Lee, H.; Yi, S.-S.; Jeong, J.-H. White Light Emission from NaCaPO4:Dy3+ Phosphor for Ultraviolet-Based White Light-Emitting Diodes. J. Am. Ceram. Soc. 2010, 93, 3857–3861. [Google Scholar] [CrossRef]
- Rojas-Hernandez, R.E.; Barradas, N.P.; Alves, E.; Santos, L.F.; Almeida, R.M. Up-conversion emission of aluminosilicate and titania films doped with Er3+/Yb3+ by ion implantation and sol-gel solution doping. Surf. Coat. Technol. 2018, 355, 162–168. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


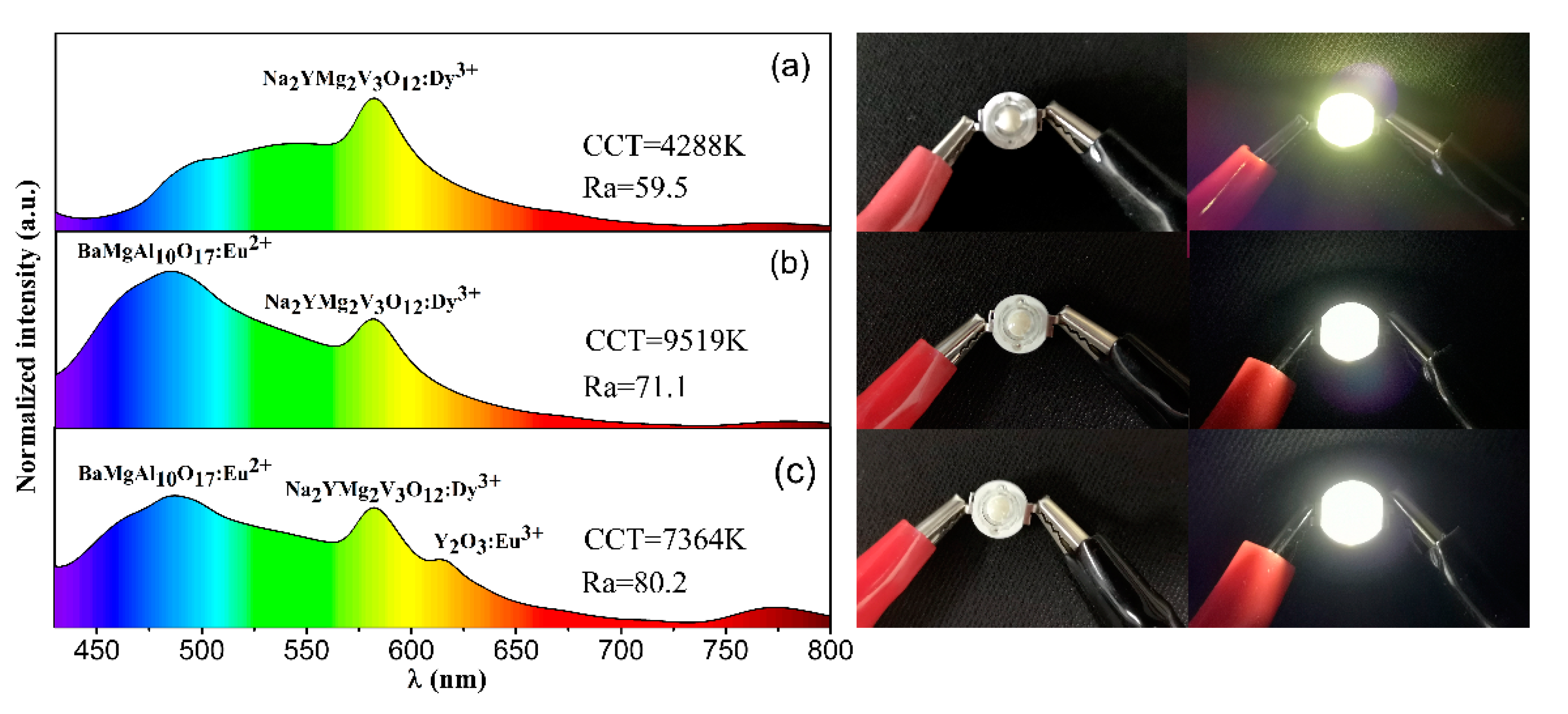
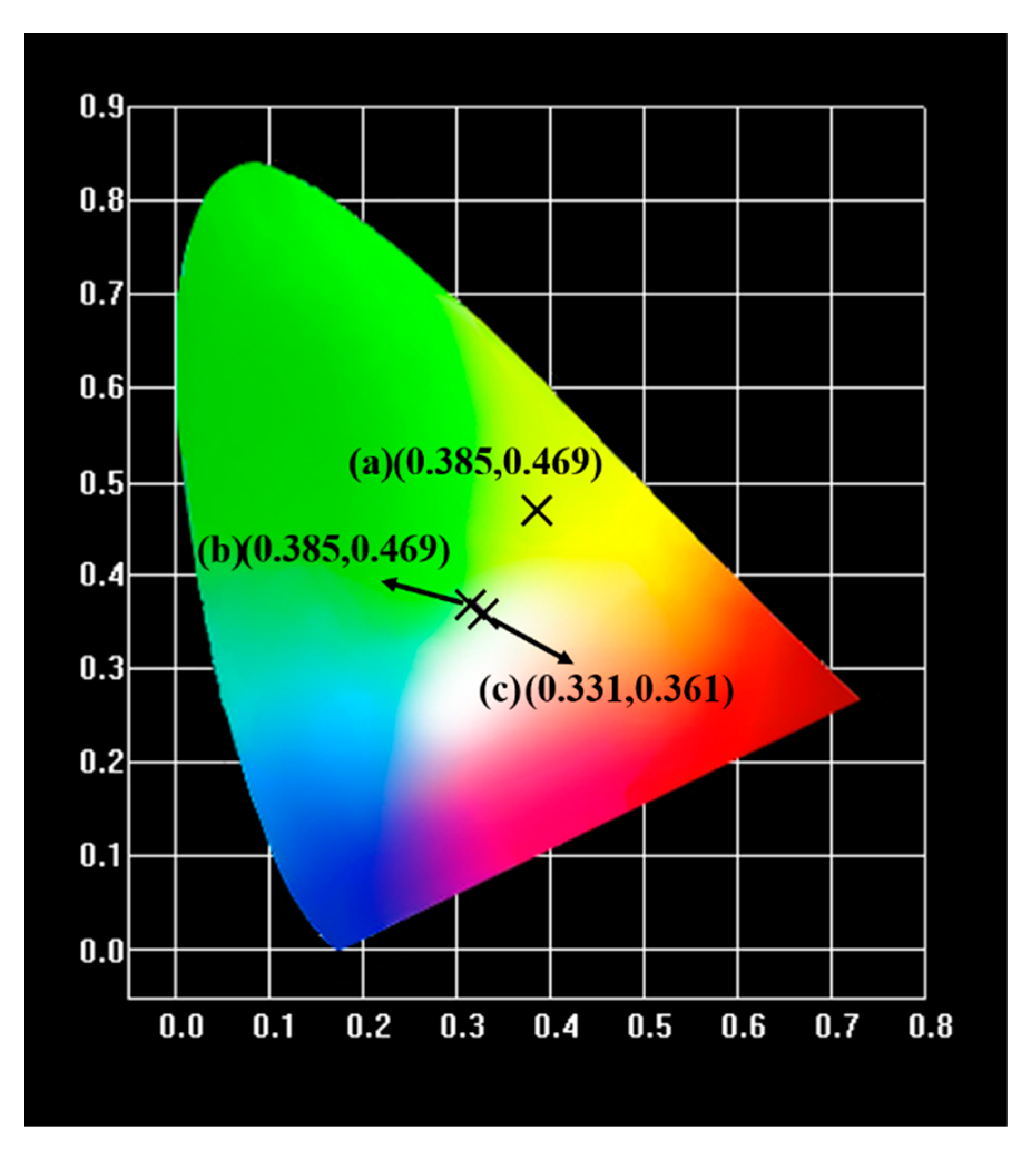
| Sample | (x, y) | CCT | Lifetimes | Reference |
|---|---|---|---|---|
| Na2YMg2V3O12: Dy3+ | (0.357, 0.461) | 4288 | 1.50 | Present work |
| Sr3Y2(BO3)4: Dy3+ | (0.300, 0.314) | 5896 | - | [42] |
| KBaY(MoO4)3: Dy3+ | (0.431, 0.457) | 3988 | 0.125 | [43] |
| Na3Gd(VO4)2:Dy3+ | (0.664, 0.335) | - | 0.234 | [22] |
| Ca3TeO6:Dy3+ | (0.417, 0.460) | 3730 | 0.506 | [35] |
| NaLa(PO3)4: Dy3+ | (0.292, 0.336) | - | 0.78 | [44] |
| NaCaPO4:Dy3+ | (0.32, 0.37) | 5962 | 0.604 | [45] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; He, C.; Wu, X.; Huang, X.; Lin, F.; Liu, Y.; Fang, M.; Min, X.; Huang, Z. Yellow Emission Obtained by Combination of Broadband Emission and Multi-Peak Emission in Garnet Structure Na2YMg2V3O12: Dy3+ Phosphor. Molecules 2020, 25, 542. https://doi.org/10.3390/molecules25030542
Zhang W, He C, Wu X, Huang X, Lin F, Liu Y, Fang M, Min X, Huang Z. Yellow Emission Obtained by Combination of Broadband Emission and Multi-Peak Emission in Garnet Structure Na2YMg2V3O12: Dy3+ Phosphor. Molecules. 2020; 25(3):542. https://doi.org/10.3390/molecules25030542
Chicago/Turabian StyleZhang, Weiyi, Can He, Xiaowen Wu, Ximing Huang, Fankai Lin, Yan’gai Liu, Minghao Fang, Xin Min, and Zhaohui Huang. 2020. "Yellow Emission Obtained by Combination of Broadband Emission and Multi-Peak Emission in Garnet Structure Na2YMg2V3O12: Dy3+ Phosphor" Molecules 25, no. 3: 542. https://doi.org/10.3390/molecules25030542
APA StyleZhang, W., He, C., Wu, X., Huang, X., Lin, F., Liu, Y., Fang, M., Min, X., & Huang, Z. (2020). Yellow Emission Obtained by Combination of Broadband Emission and Multi-Peak Emission in Garnet Structure Na2YMg2V3O12: Dy3+ Phosphor. Molecules, 25(3), 542. https://doi.org/10.3390/molecules25030542








