Advances in Synthesis of π-Extended Benzosilole Derivatives and Their Analogs
Abstract
1. Introduction
2. Synthesis of π-Extended Benzosiloles
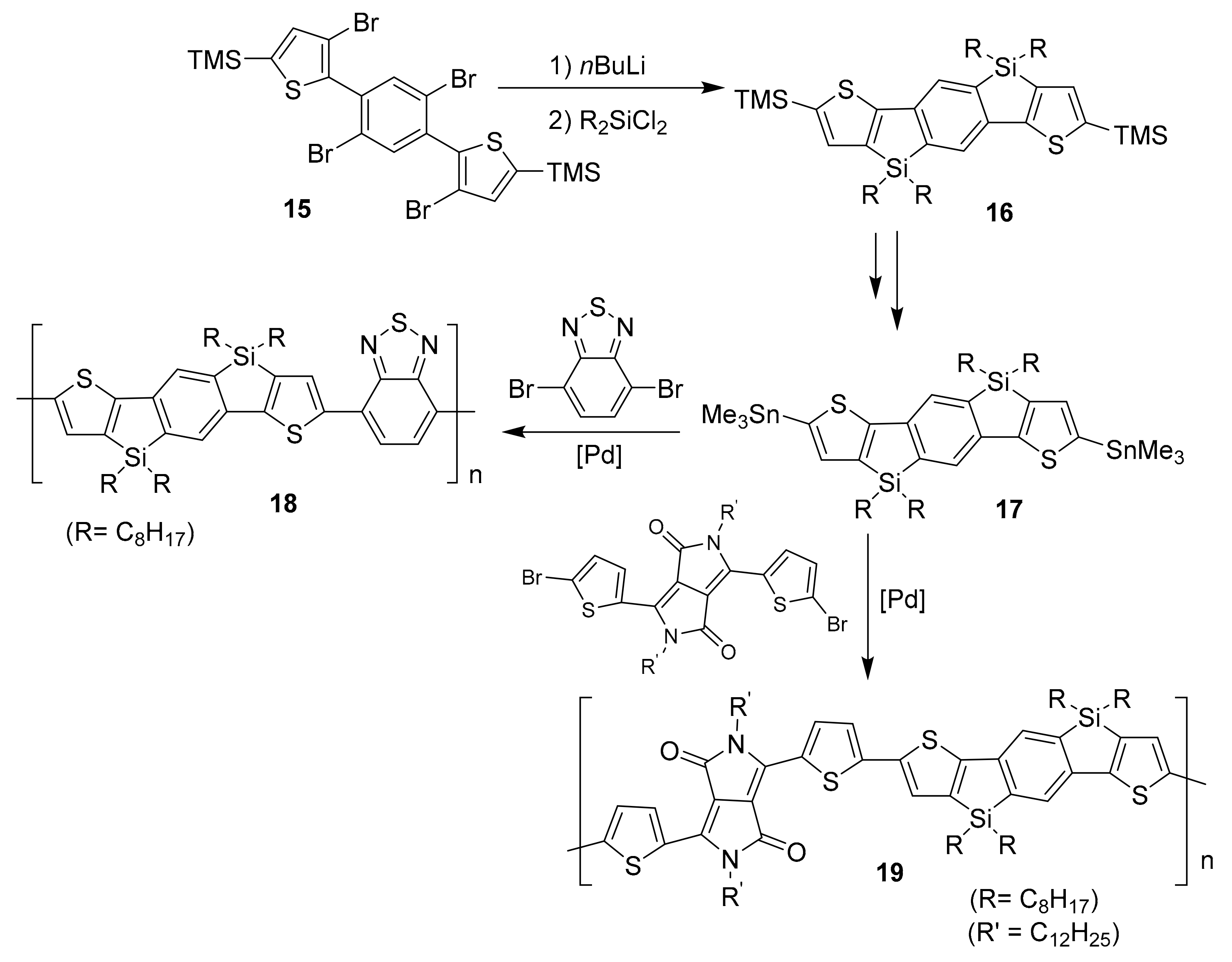
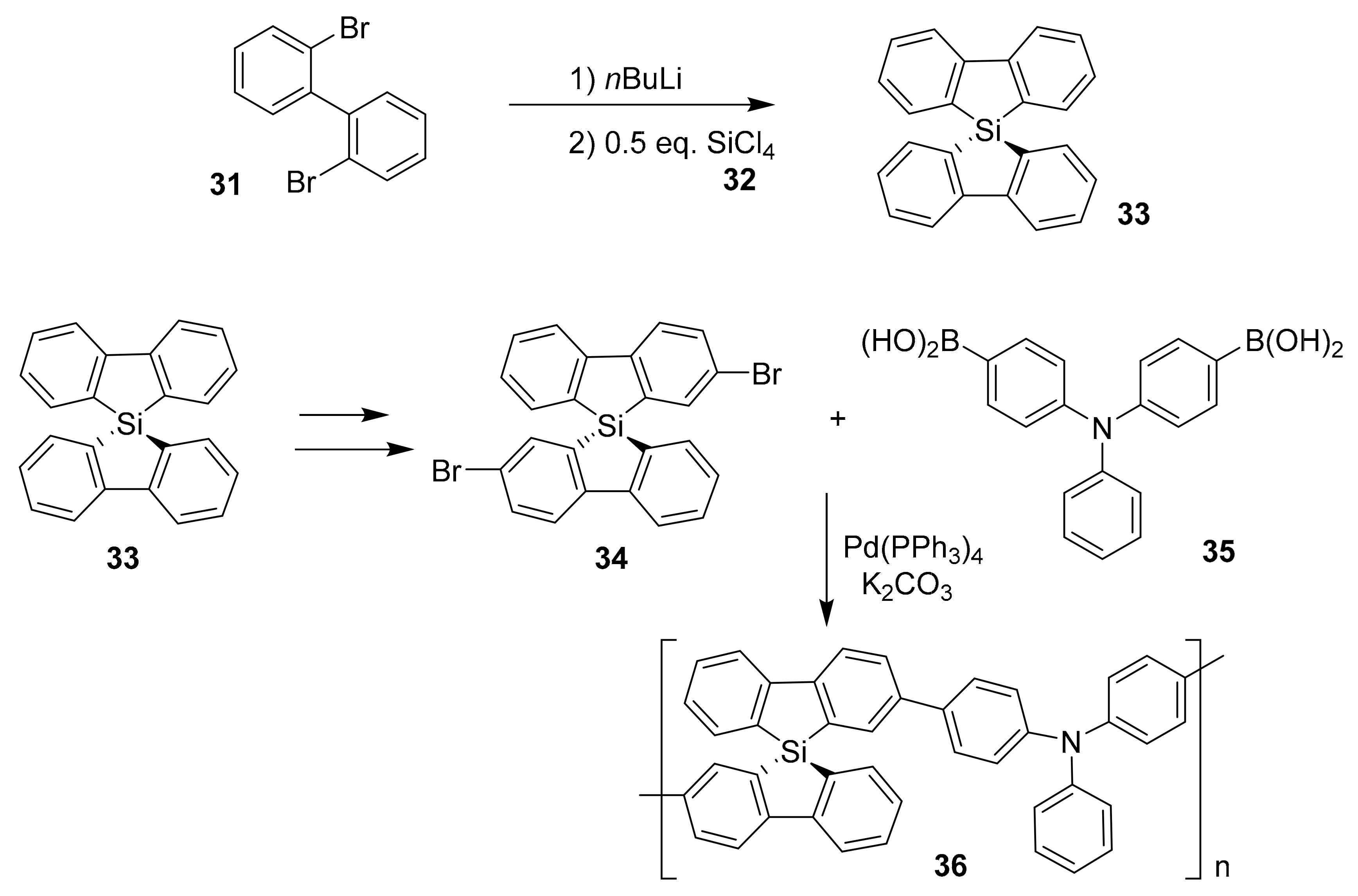
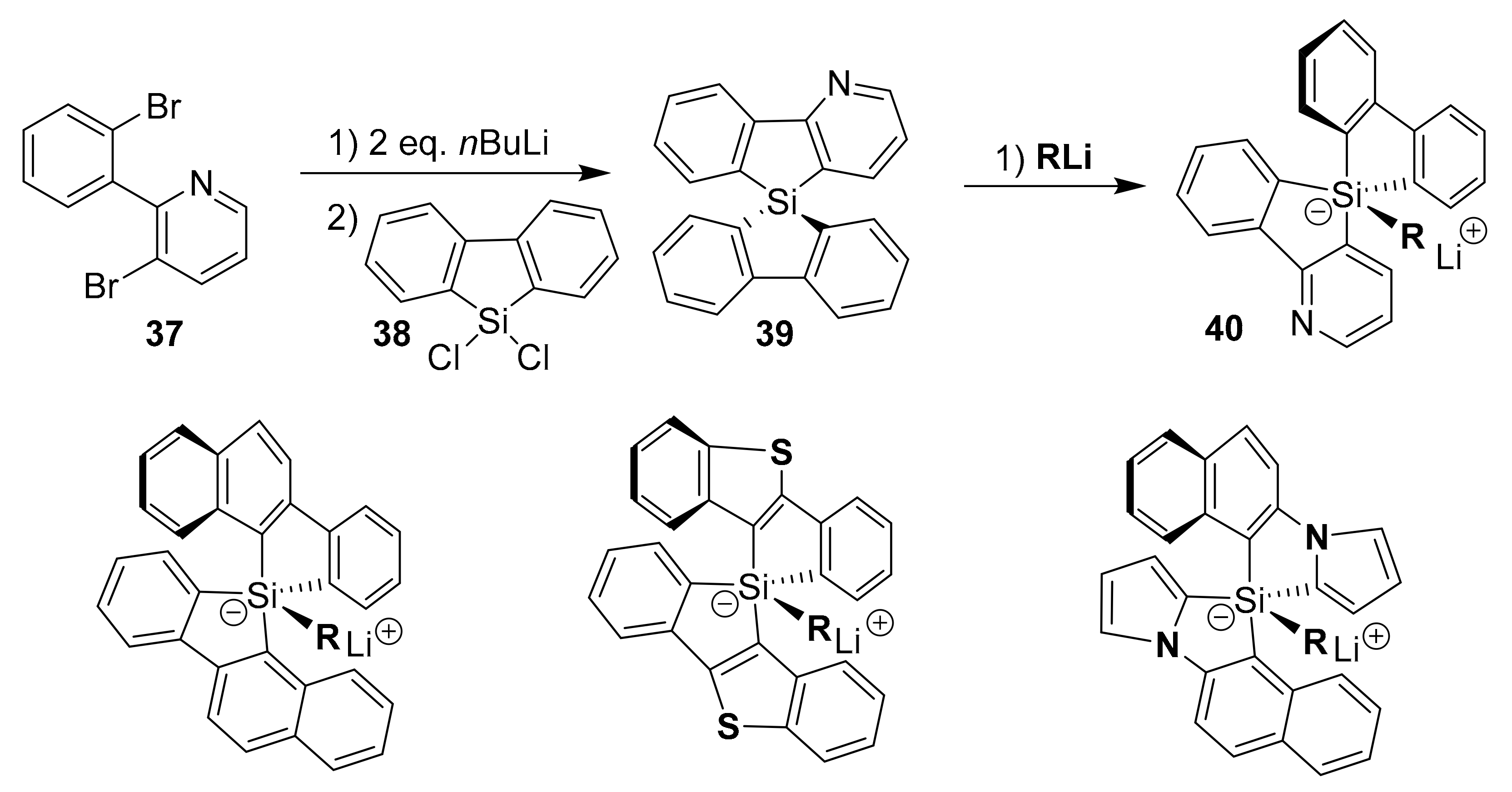
2.1. Conventional Metal-Halide Exchange Method
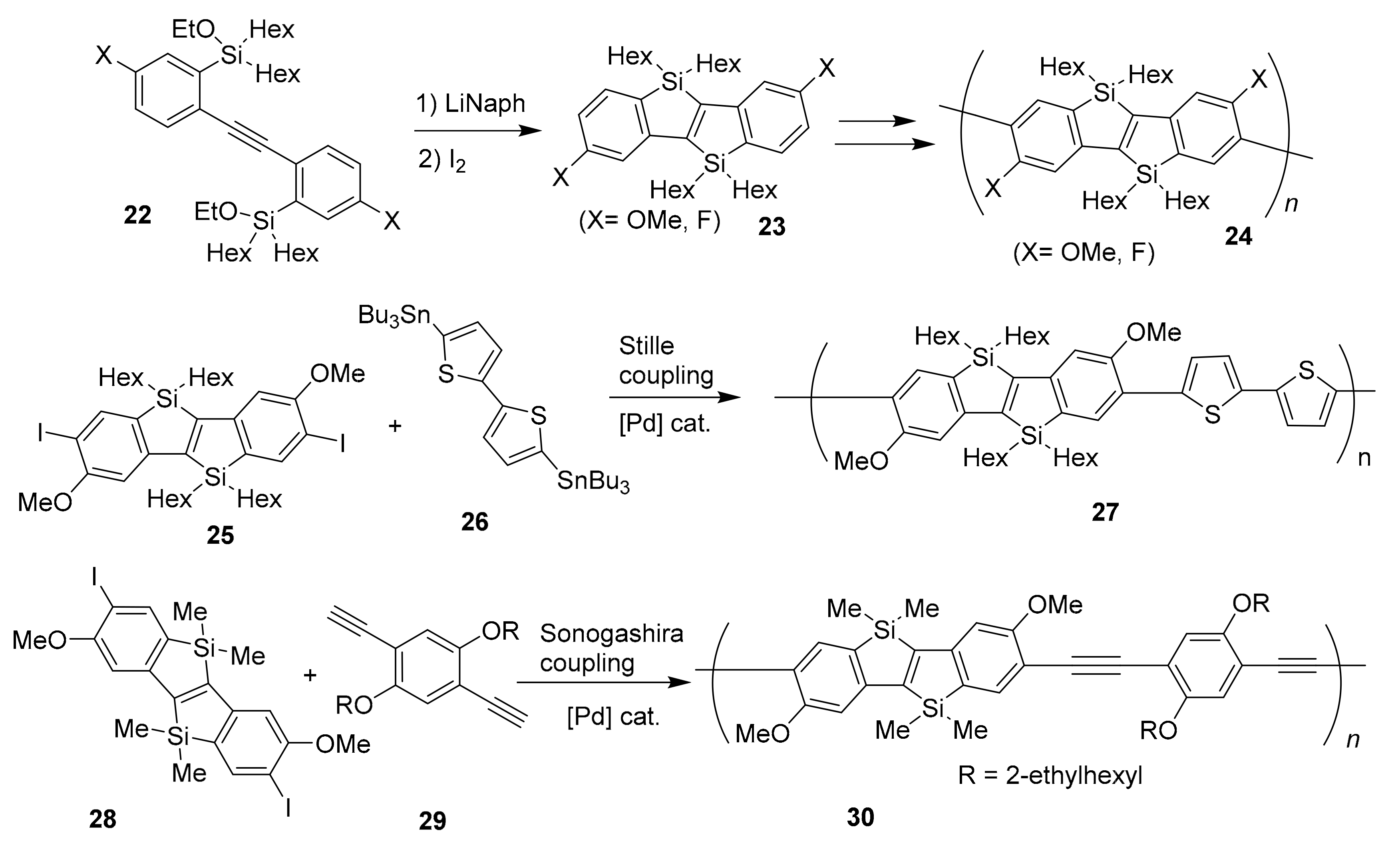
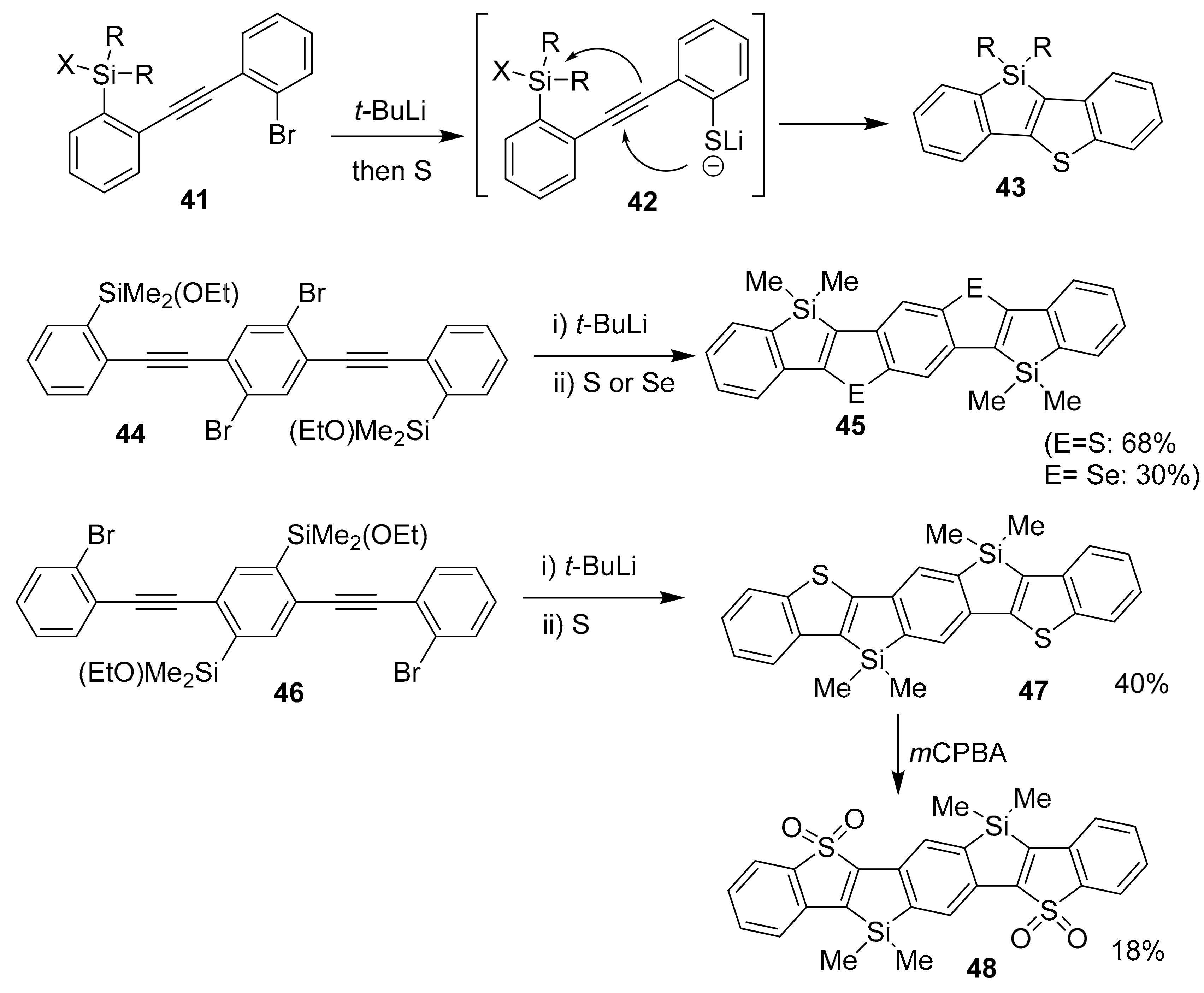
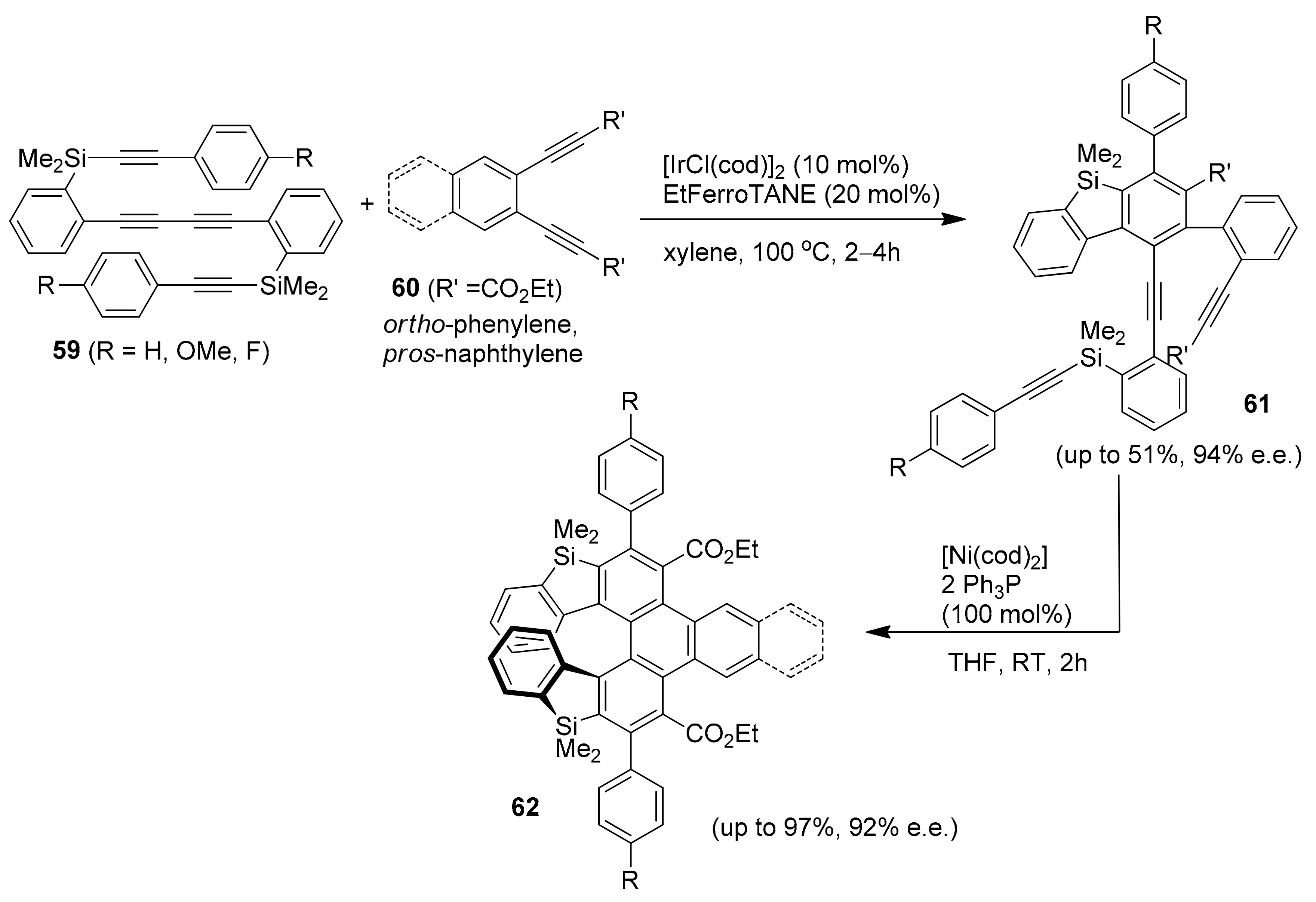
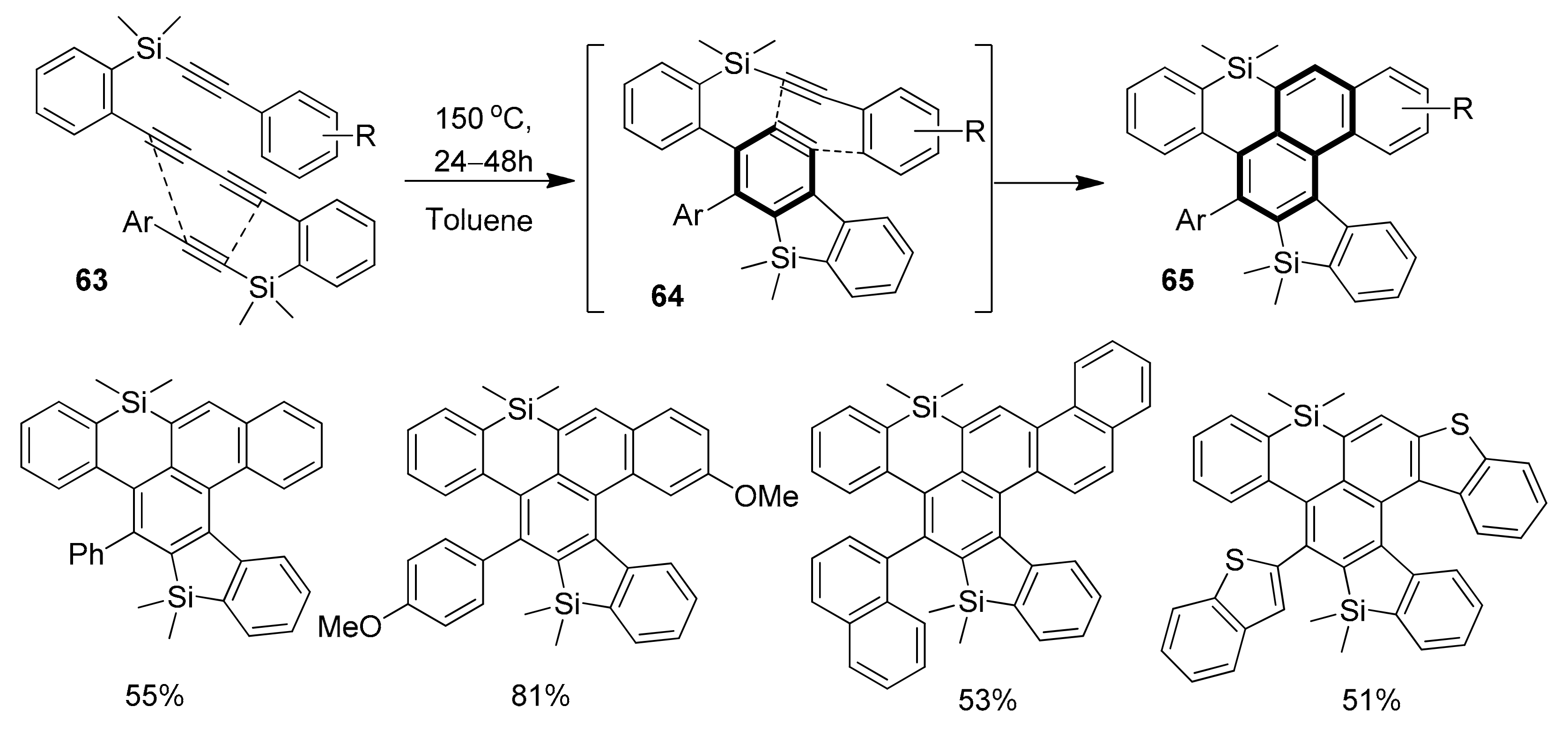
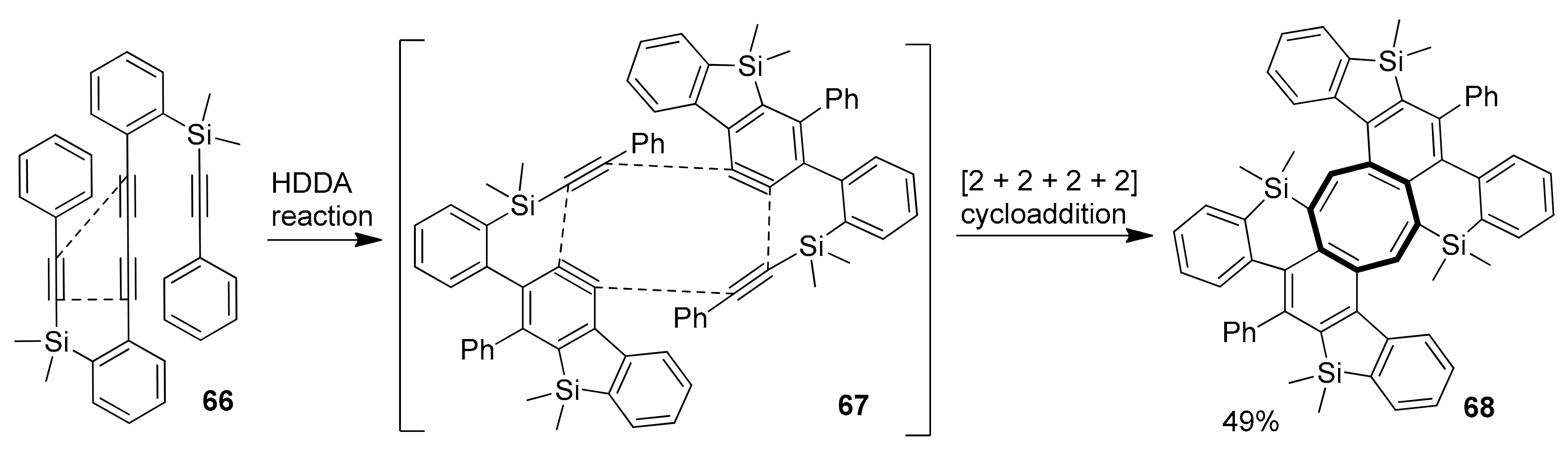
2.2. Metal-Catalyzed [2 + 2 + 2] Cycloadditions
2.3. Transition Metal Catalyzed C-Si Coupling via Cleavage of a C(sp3)-Si Bond
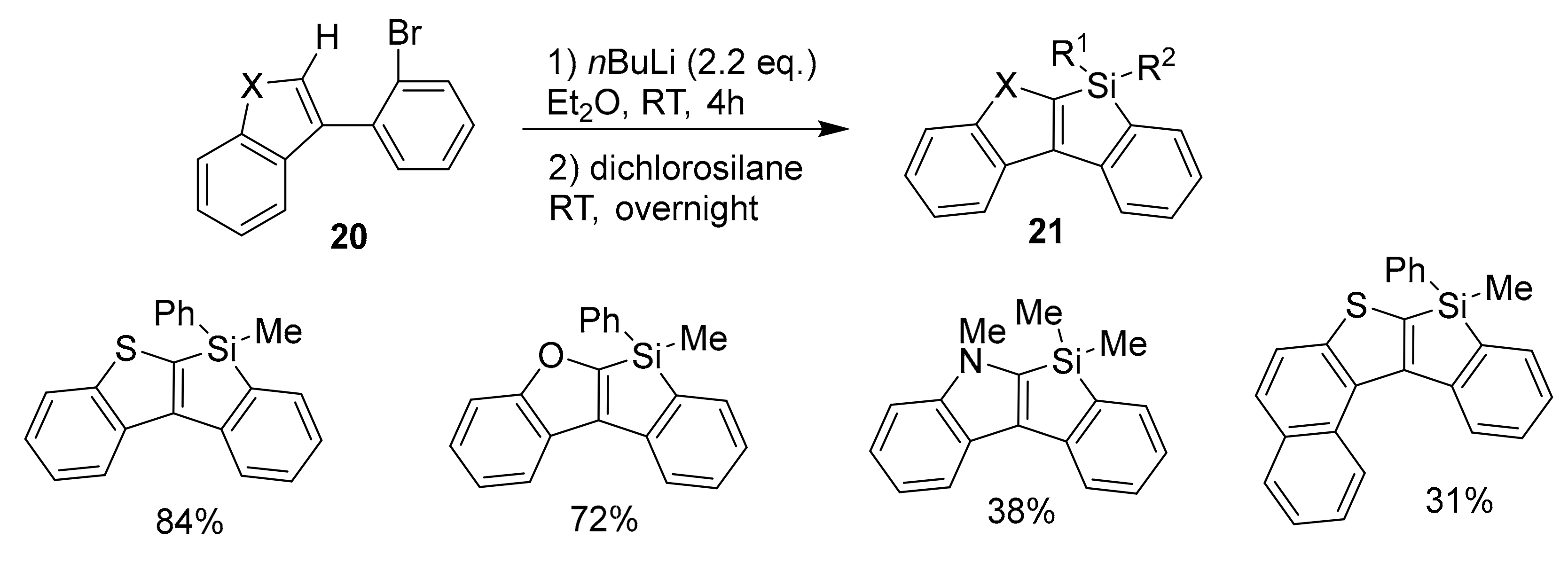
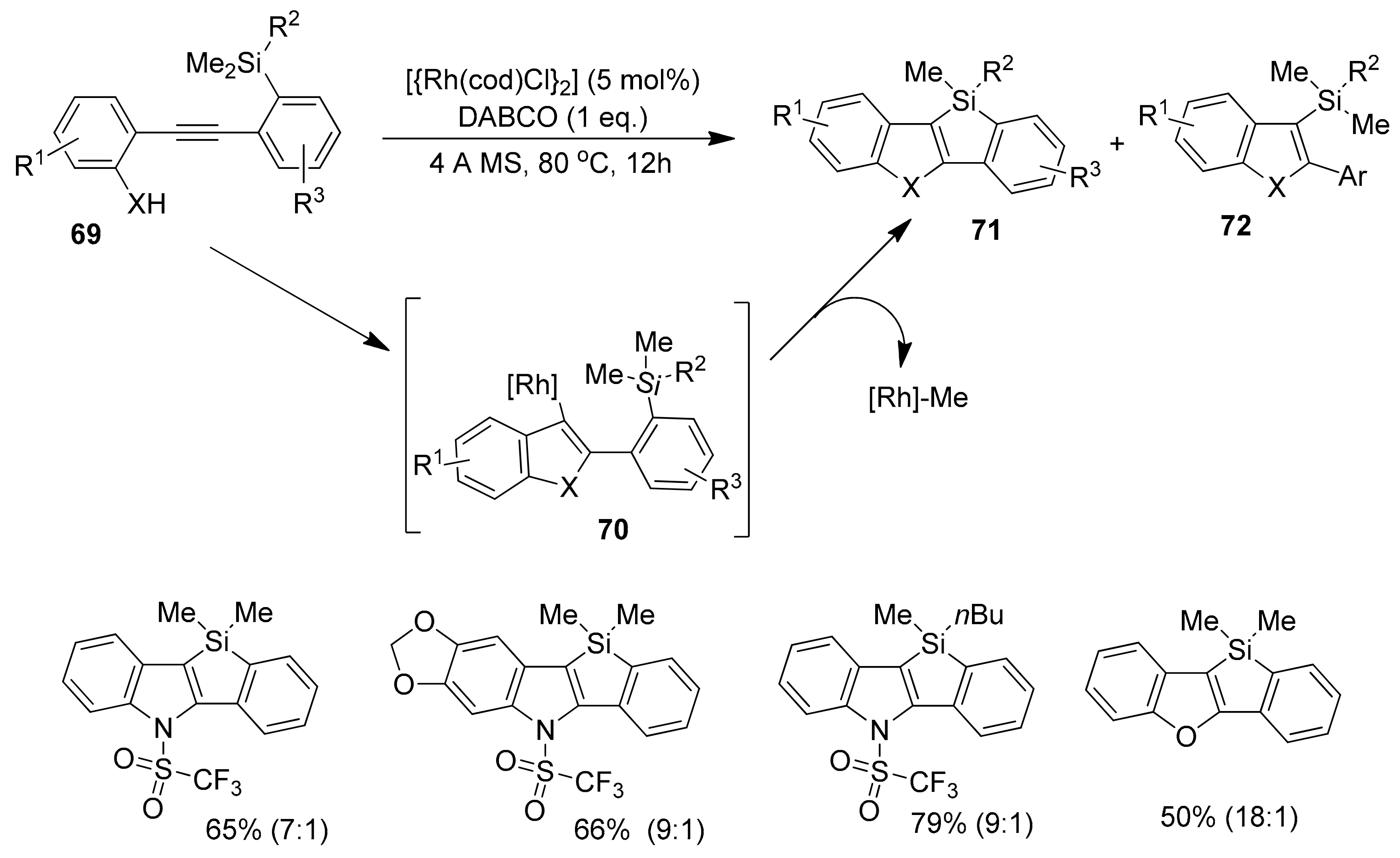
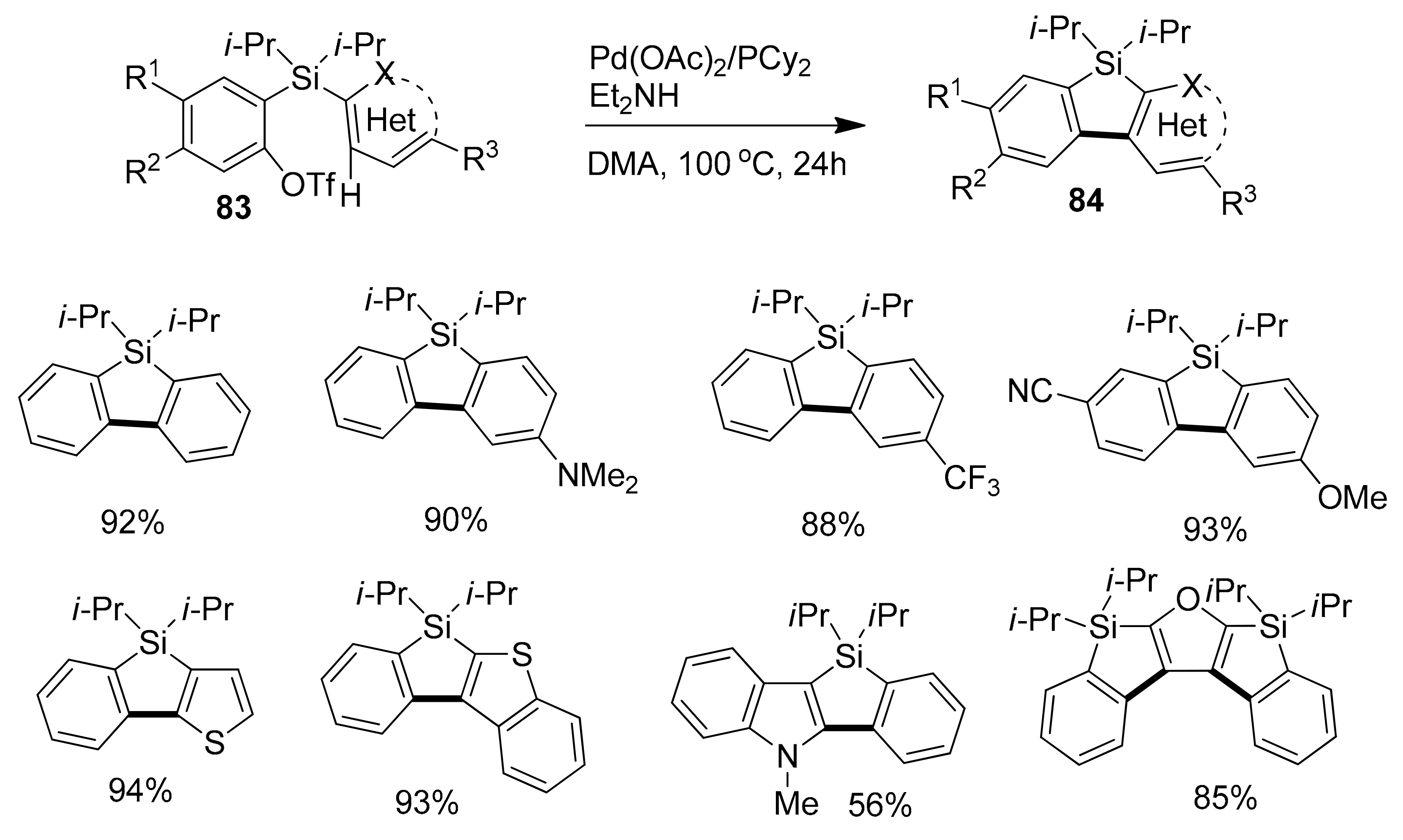
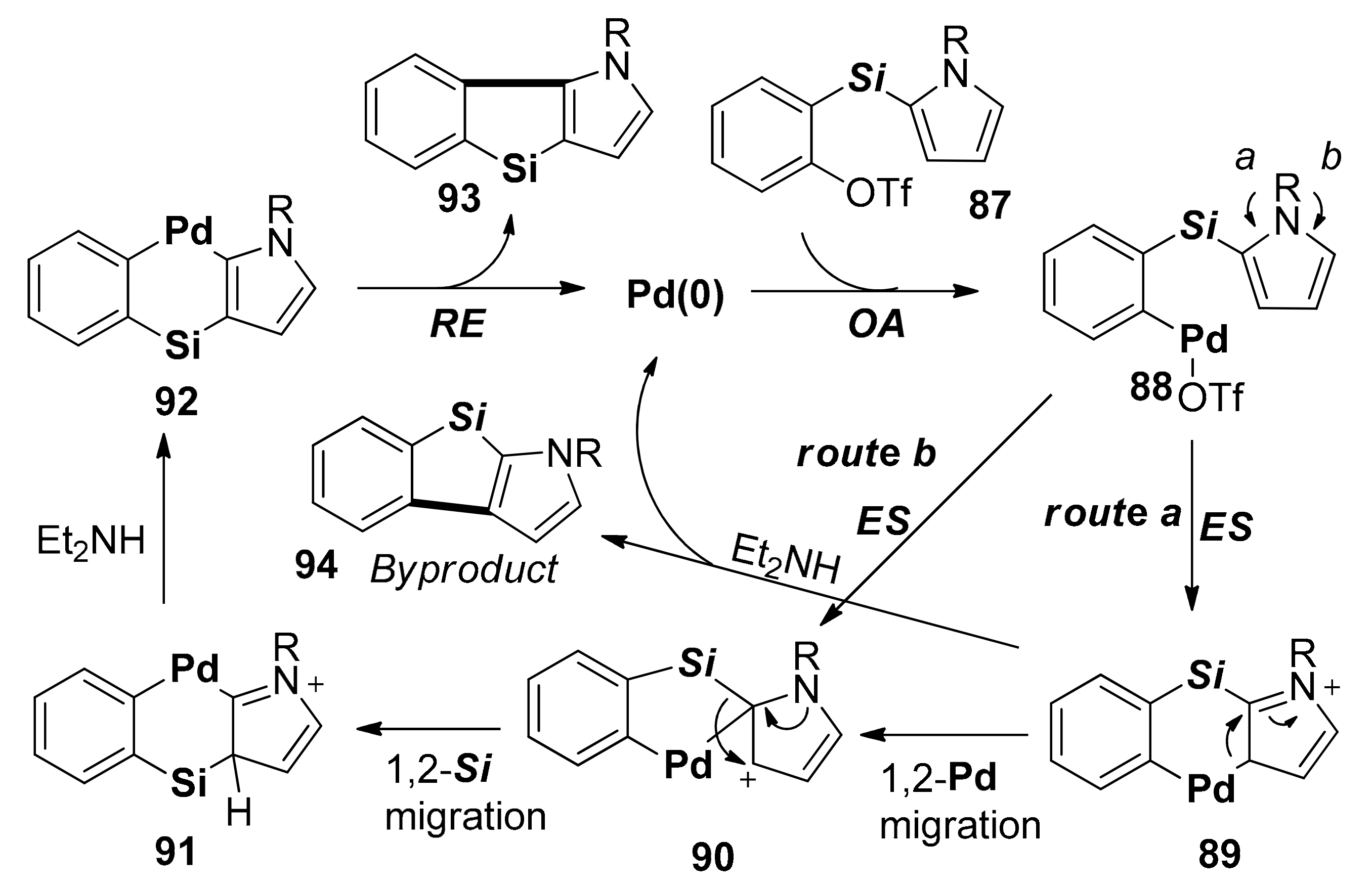
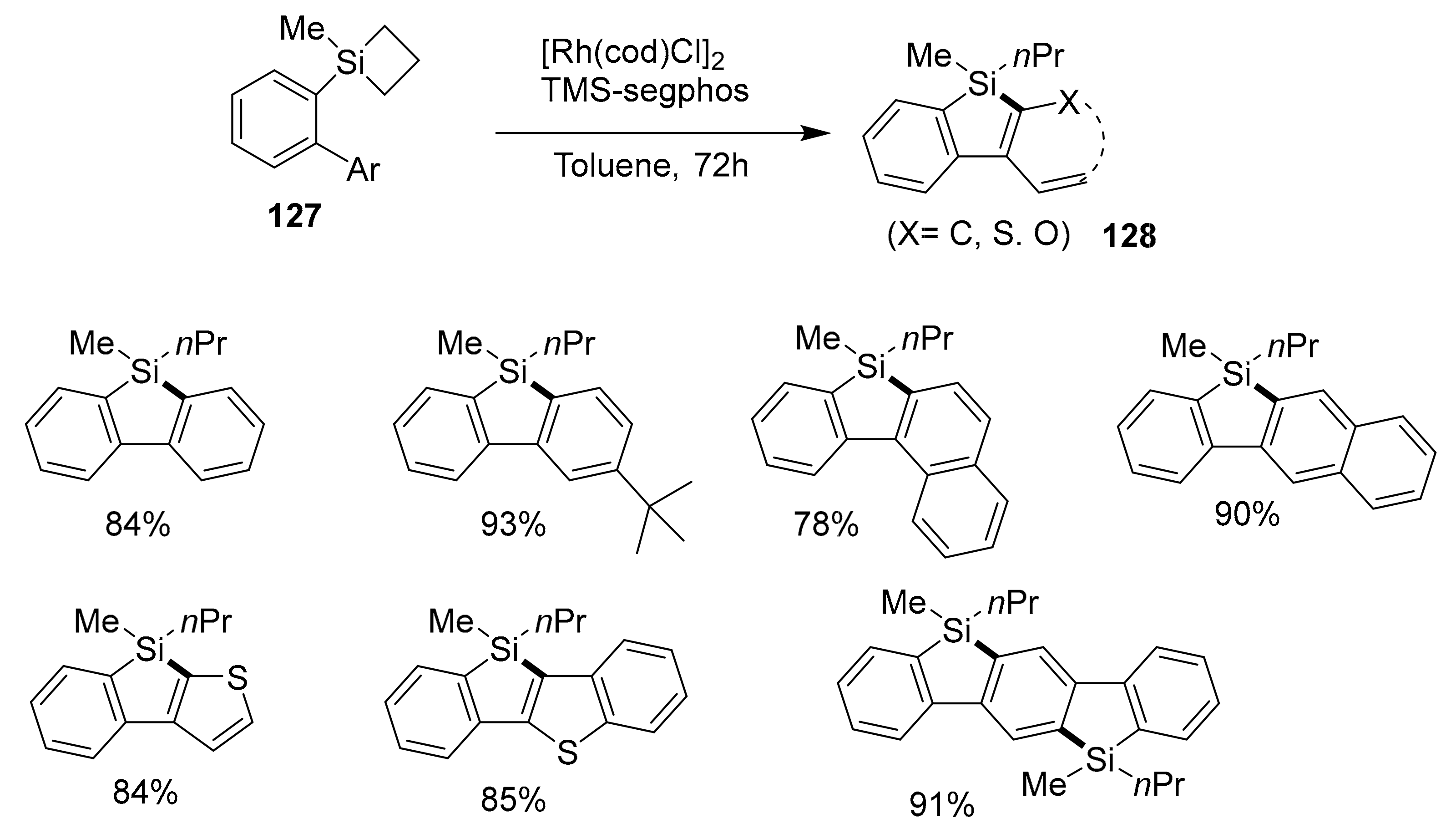
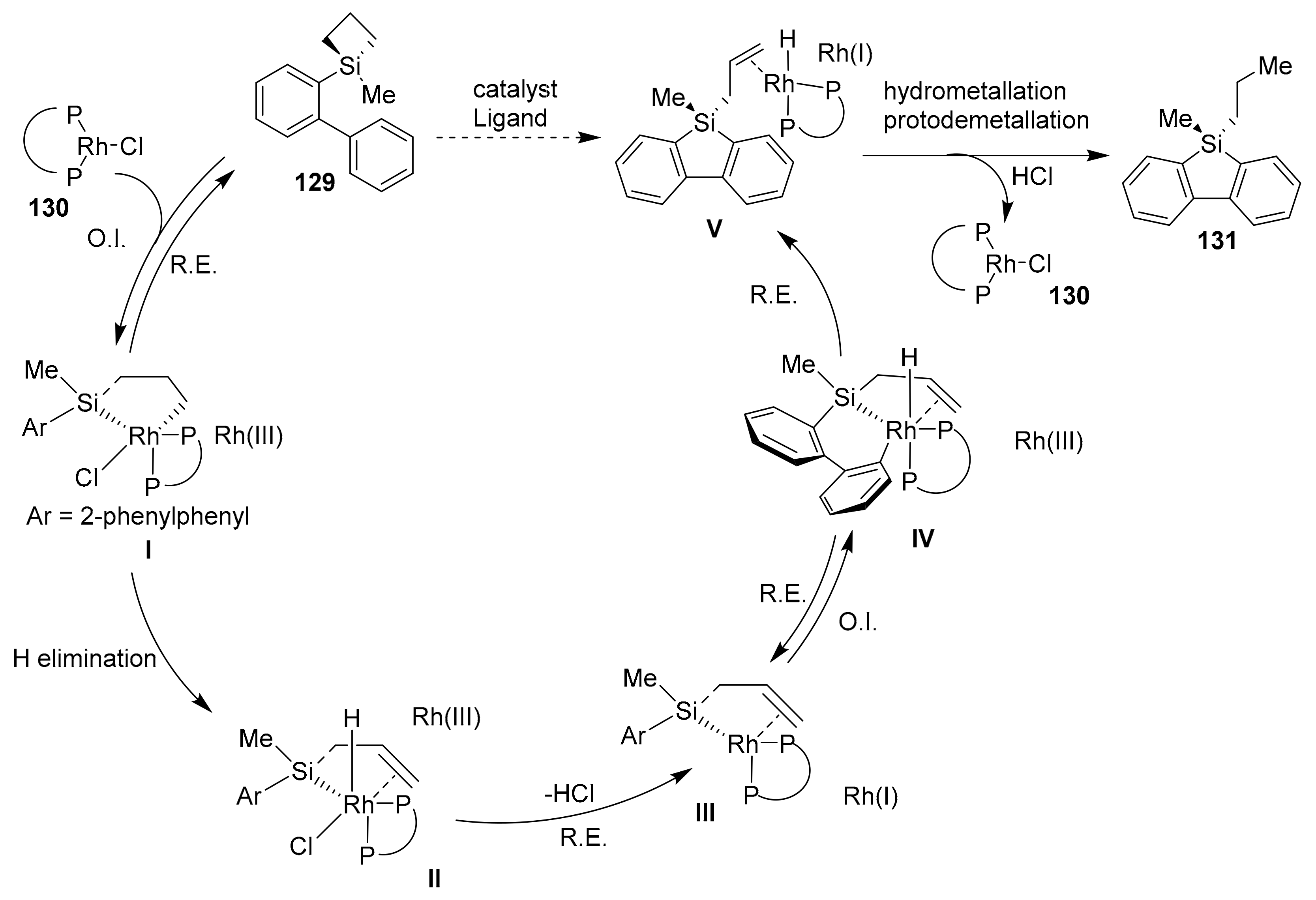
2.4. Metal-Catalyzed C–H Activations
2.5. C–H Silylations
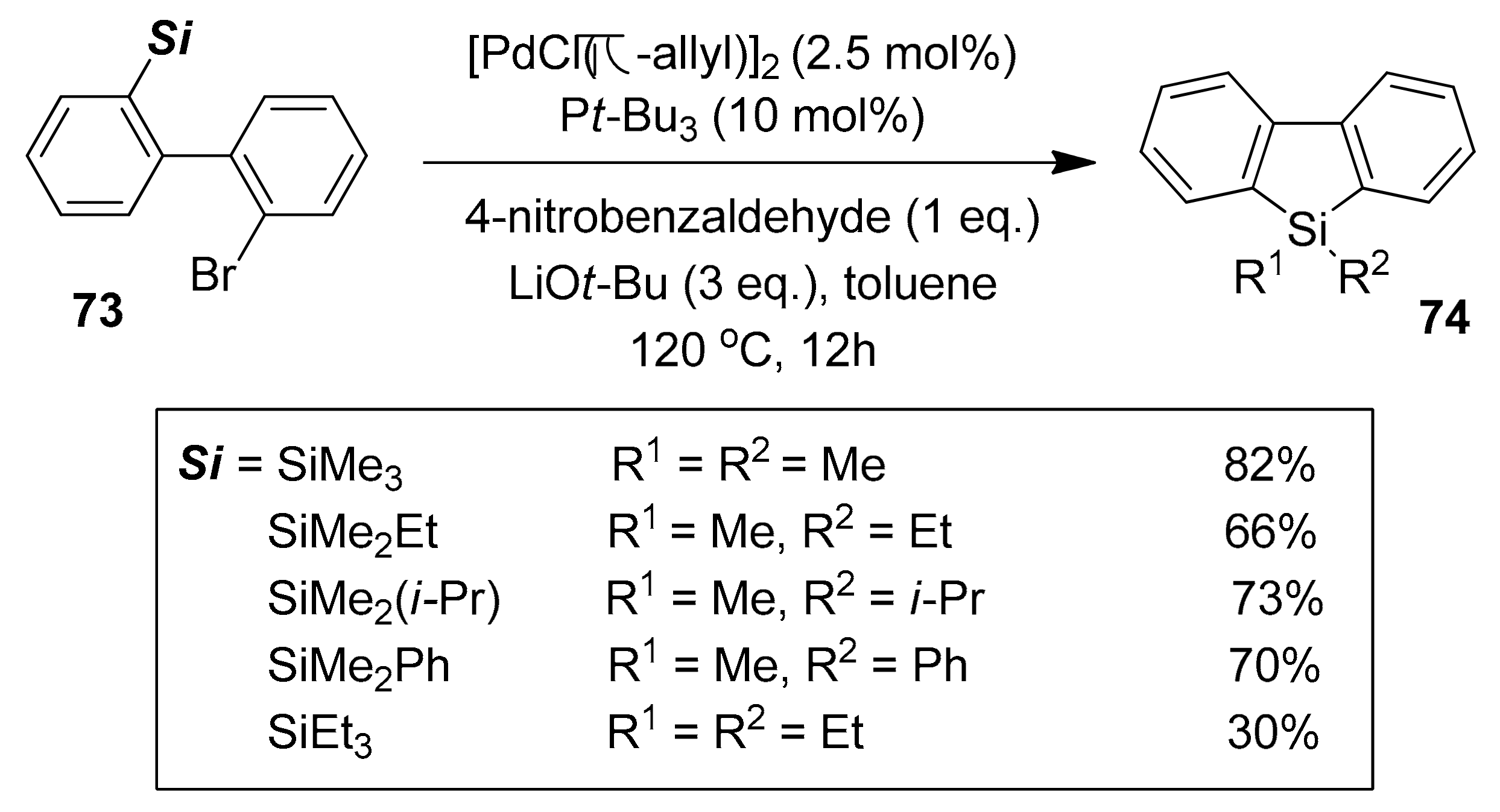
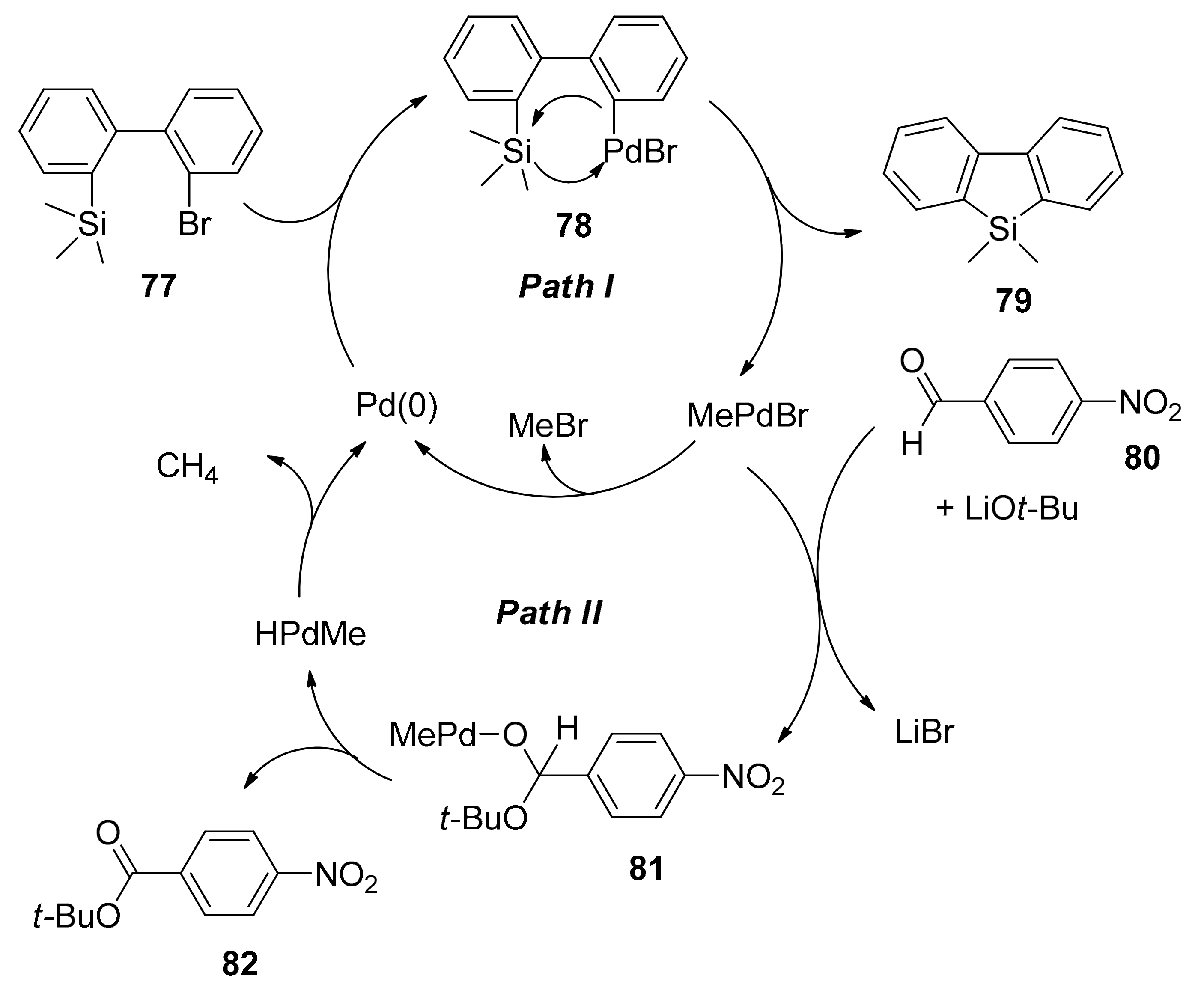
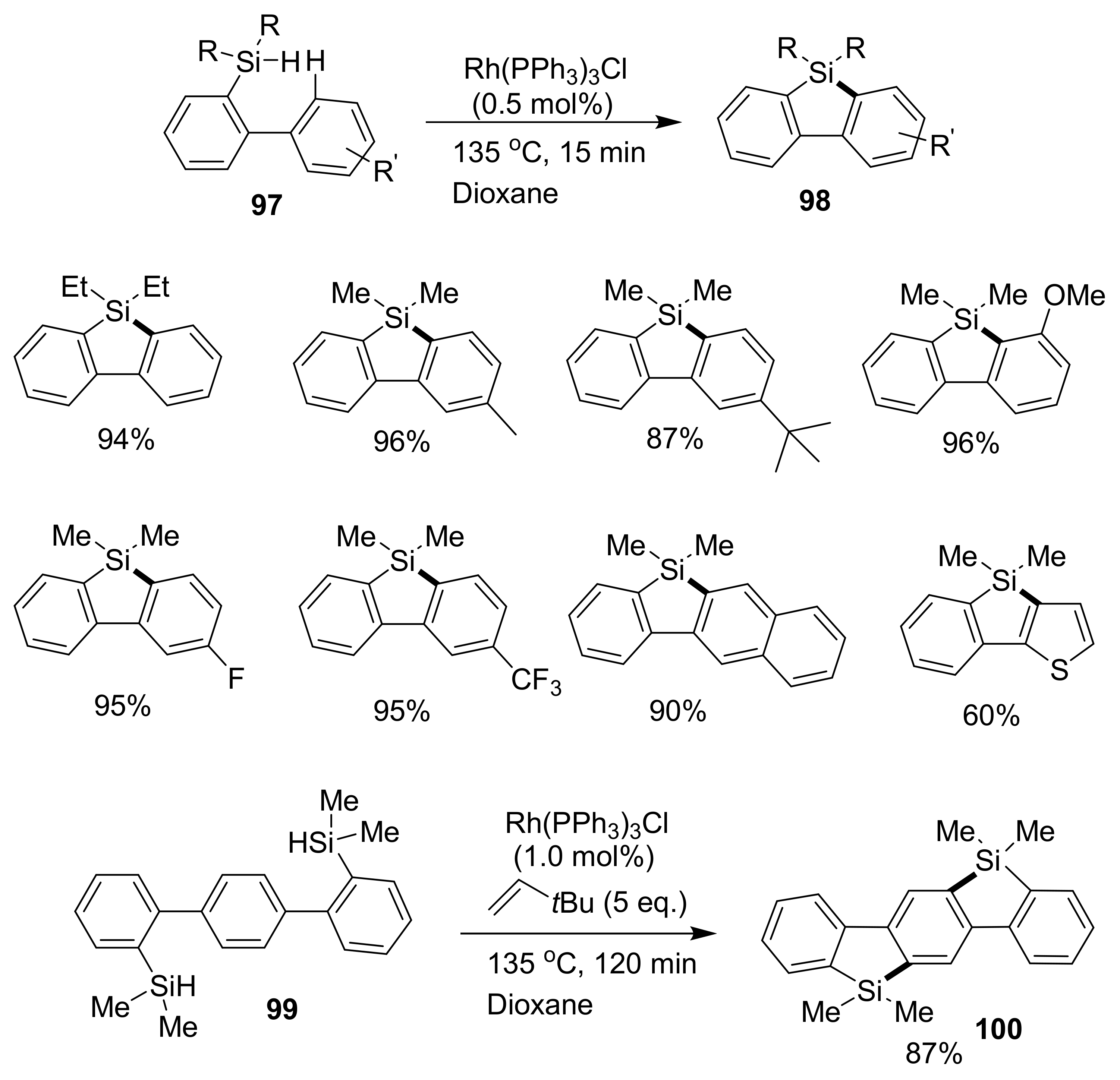
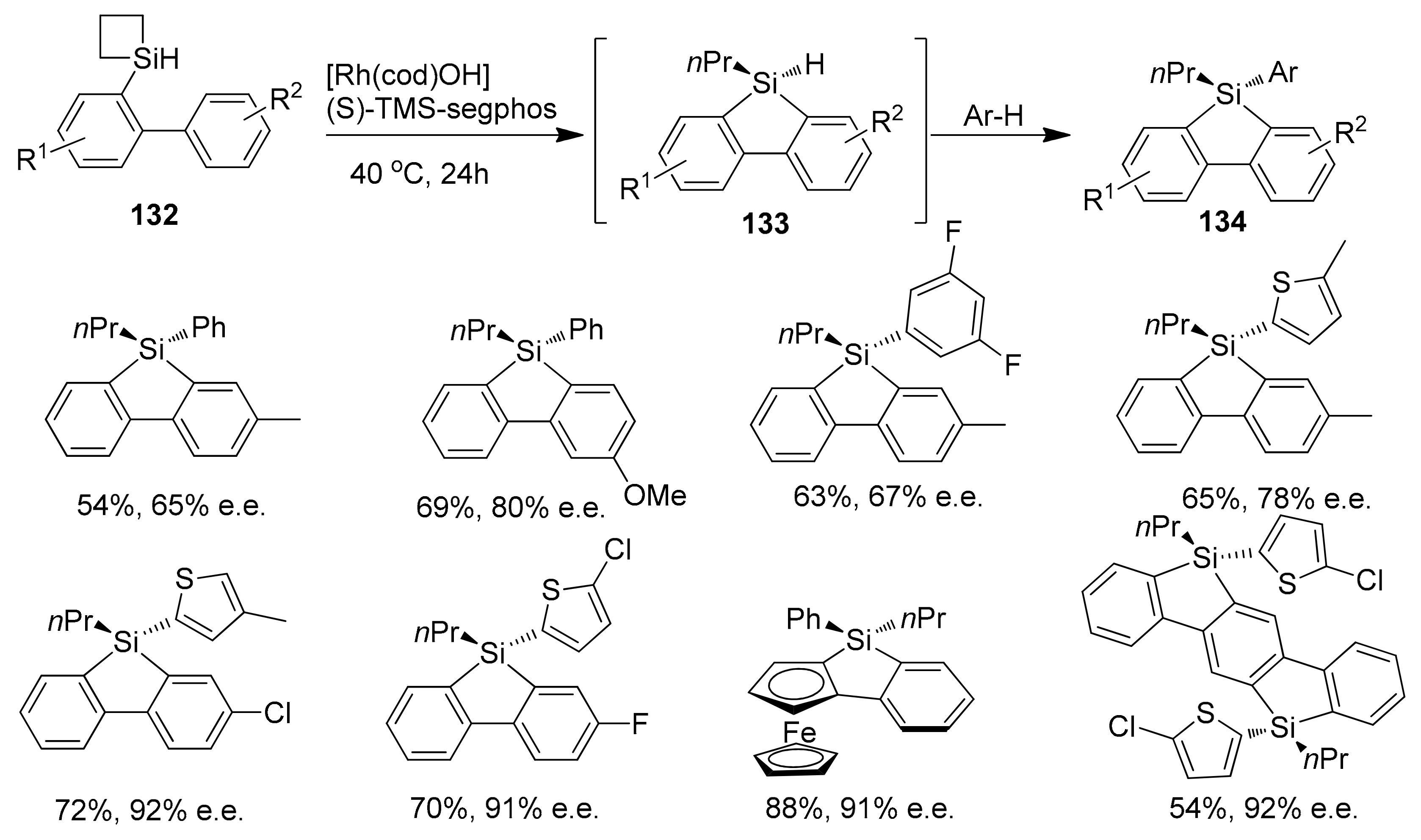
2.5.1. Transition Metal-Catalyzed C–H Silylations
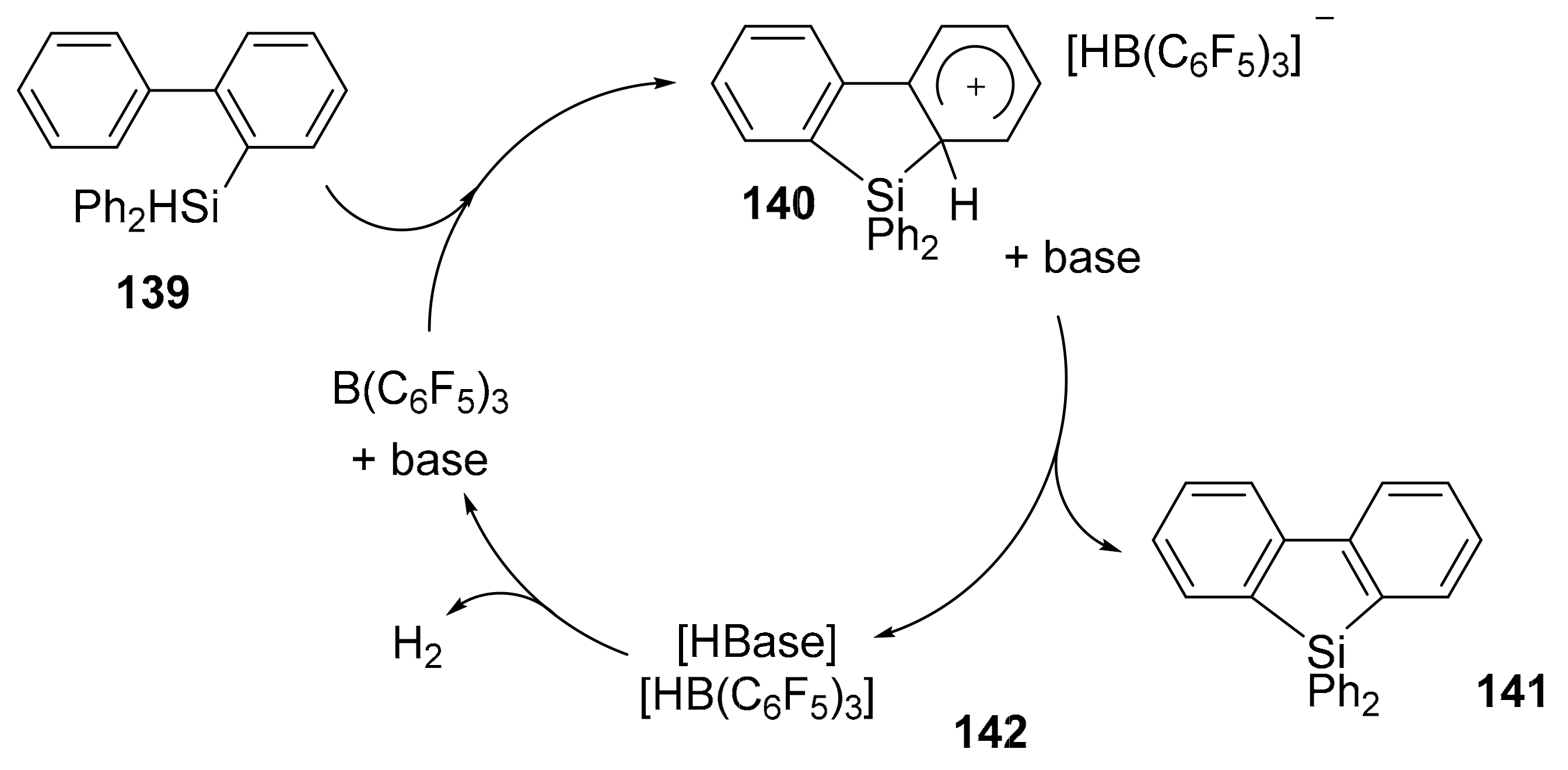
2.5.2. Synthesis of Dibenzosilole Derivatives via a sila-Friedel-Crafts Reaction
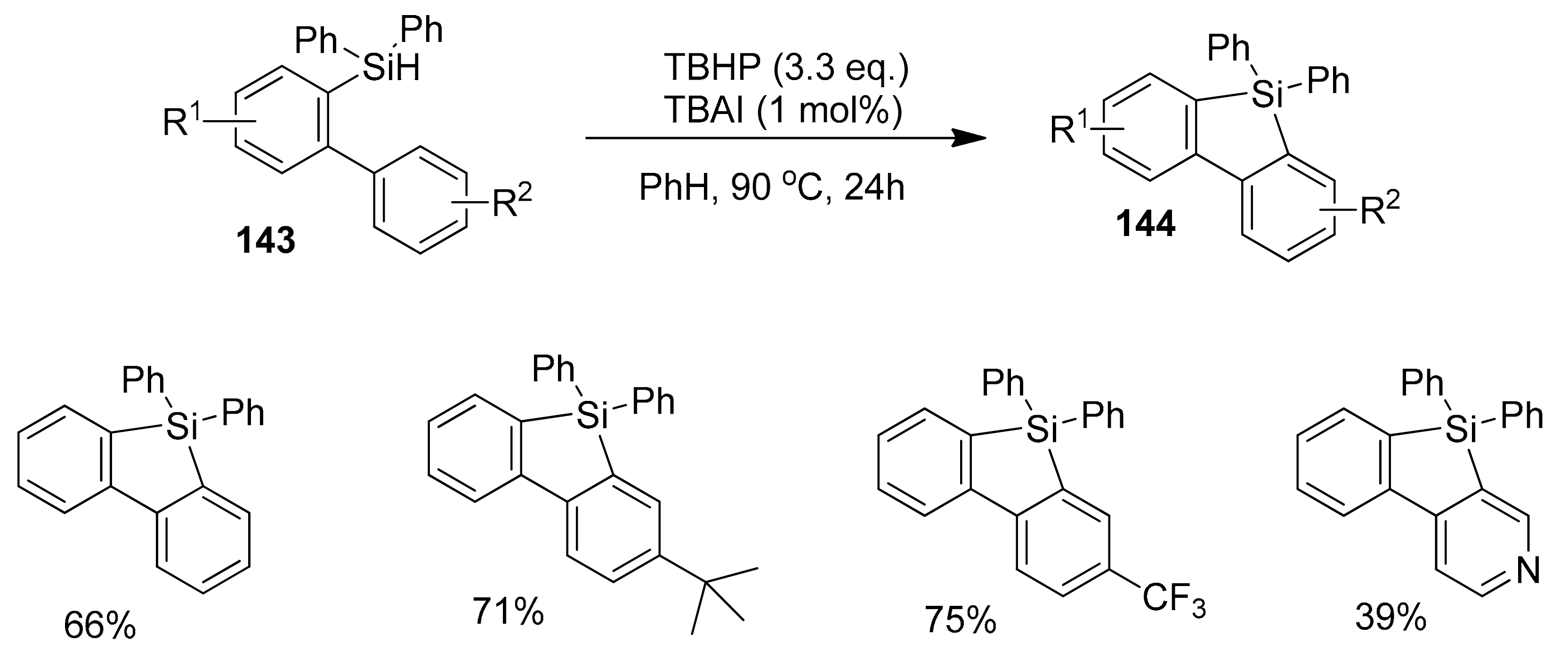
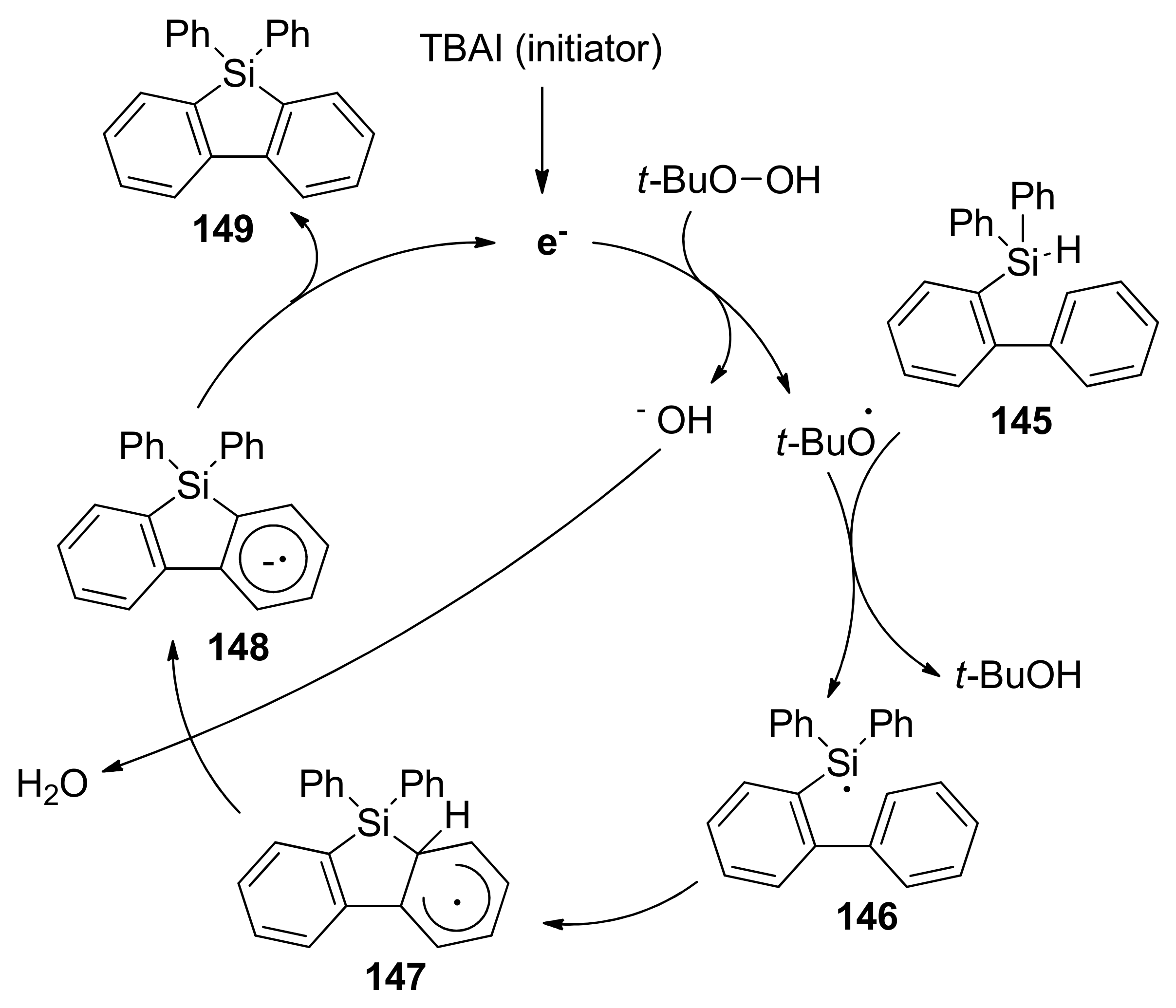
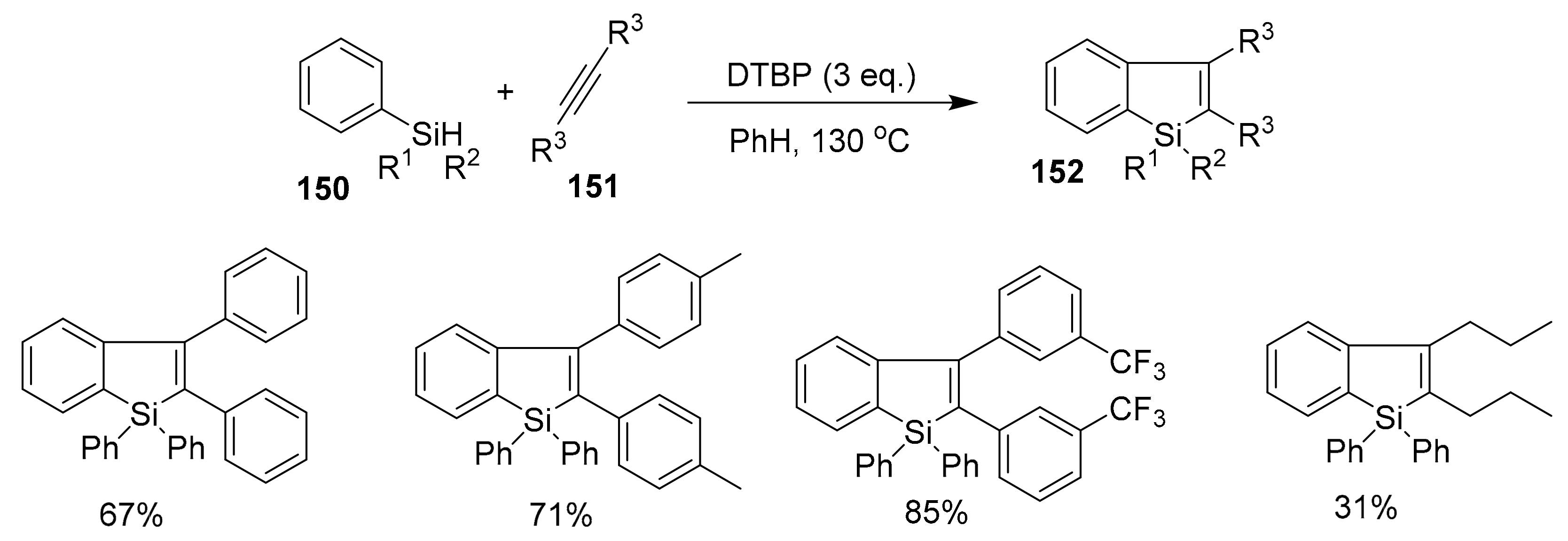
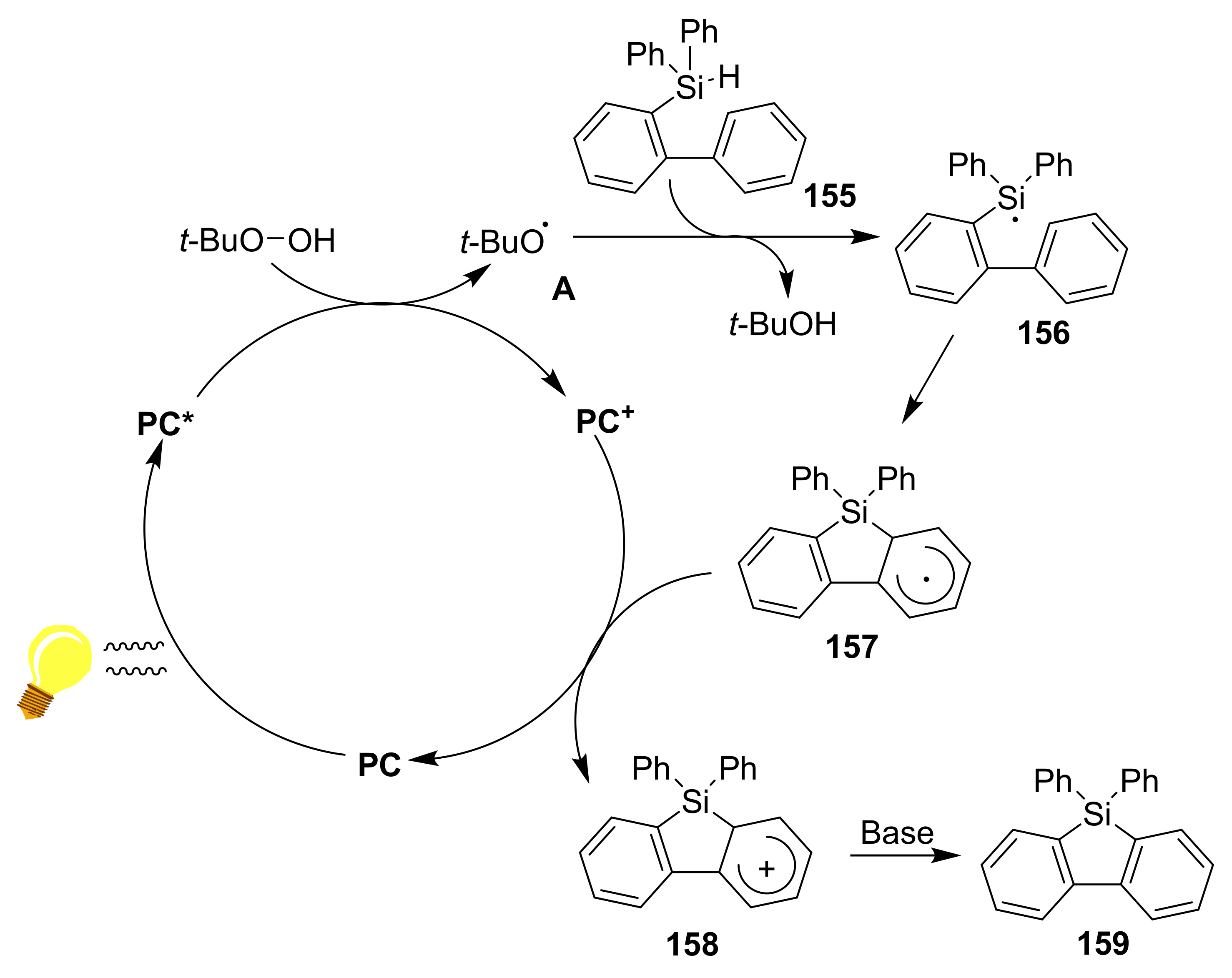
2.5.3. Cyclizations via Silyl Radicals
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, G.; Usta, H.; Risko, C.; Wang, L.; Facchetti, A.; Ratner, M.A.; Marks, T.J. Synthesis, characterization, and transistor response of semiconducting silole polymers with substantial hole mobility and air stability. Experiment and theory. J. Am. Chem. Soc. 2008, 130, 7670–7685. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.C.; Urbas, S.A.; Toal, S.J.; DiPasquale, A.G.; Rheingold, A.L.; Trogler, W.C. Catalytic Hydrosilylation Routes to Divinylbenzene Bridged Silole and Silafluorene Polymers. Applications to Surface Imaging of Explosive Particulates. Macromolecules 2008, 41, 1237–1245. [Google Scholar] [CrossRef]
- Wang, E.; Li, C.; Wenliu, Z.; Peng, J.; Cao, Y. High-efficiency red and green light-emitting polymers based on a novel wide bandgap poly(2,7-silafluorene). J. Mater. Chem. 2008, 18, 797–801. [Google Scholar] [CrossRef]
- Shimizu, M.; Tatsumi, H.; Mochida, K.; Oda, K.; Hiyama, T. Silicon-bridge effects on photophysical properties of silafluorenes. Chem. Asian J. 2008, 3, 1238–1247. [Google Scholar] [CrossRef]
- Chen, J.; Cao, Y. Silole-Containing Polymers: Chemistry and optoelectronic properties. Macromol. Rapid Commun. 2007, 28, 1714–1742. [Google Scholar] [CrossRef]
- Oshita, J.; Kurushima, Y.; Lee, K.-H.; Kunai, A.; Ooyama, Y.; Harima, Y. Synthesis of bis(diarylphosphino)dithienosilole derivatives as novel photo- and electroluminescence materials. Organometallics 2007, 26, 6591–6595. [Google Scholar] [CrossRef]
- Mouri, K.; Wakamiya, A.; Yamada, H.; Kajiwara, T.; Yamaguchi, S. Ladder Distyrylbenzenes with Silicon and Chalcogen Bridges: Synthesis, Structures, and Properties. Org. Lett. 2007, 9, 93–96. [Google Scholar] [CrossRef]
- Li, L.; Xiang, J.; Xu, C. Synthesis of Novel Ladder Bis-Silicon-Bridged p-Terphenyls. Org. Lett. 2007, 9, 4877–4879. [Google Scholar] [CrossRef]
- Boudreault, P.-L.T.; Michaud, A.; Leclerc, M. A New Poly(2,7-Dibenzosilole) Derivative in Polymer Solar Cells. Macromol. Rapid Commun. 2007, 28, 2176–2179. [Google Scholar] [CrossRef]
- Sanchez, J.C.; DiPasquale, A.G.; Rheingold, A.L.; Trogler, W.C. Synthesis, luminescence properties, and explosives sensing with 1,1-tetraphenylsilole- and 1,1-silafluorene-vinylene polymers. Chem. Mater. 2007, 19, 6459–6470. [Google Scholar] [CrossRef]
- Usta, H.; Lu, G.; Facchetti, A.; Marks, T.J. Dithienosilole- and dibenzosilole-thiophene copolymers as semiconductors for organic thin-film transistors. J. Am. Chem. Soc. 2006, 128, 9034–9035. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Li, C.; Mo, Y.; Zhang, Y.; Ma, G.; Shi, W.; Peng, J.; Yang, W.; Cao, Y. Poly(3,6-silafluorene-co-2,7-fluorene)-based high-efficiency and color-pure blue light-emitting polymers with extremely narrow band-width and high spectral stability. J. Mater. Chem. 2006, 16, 4133–4140. [Google Scholar] [CrossRef]
- Lee, S.H.; Jang, B.-B.; Kafafi, Z.H. Highly Fluorescent Solid-State Asymmetric Spirosilabifluorene Derivatives. J. Am. Chem. Soc. 2005, 127, 9071–9078. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.; McKiernan, M.J.; Towns, C.R.; Holmes, A.B. Poly(2,7-dibenzosilole): A Blue Light Emitting Polymer. J. Am. Chem. Soc. 2005, 127, 7662–7663. [Google Scholar] [CrossRef]
- Xu, C.; Wakamiya, A.; Yamaguchi, S. Ladder Oligo(p-phenylenevinylene)s with Silicon and Carbon Bridges. J. Am. Chem. Soc. 2005, 127, 1638–1639. [Google Scholar] [CrossRef]
- McCulloch, I.; Ashraf, R.S.; Biniek, L.; Bronstein, H.; Combe, C.; Donaghey, J.E.; James, D.I.; Nielsen, C.B.; Schroeder, B.C.; Zhang, W. Design of Semiconducting indacenodithiophene polymers for high performance transistors and solar cells. Acc. Chem. Res. 2012, 45, 714–722. [Google Scholar] [CrossRef]
- Chen, X.-K.; Zou, L.-Y.; Ren, A.-M.; Fan, J.-X. How dual bridging atoms tune structural and optoelectronic properties of ladder-type heterotetracenes? a theoretical study. Phys. Chem. Chem. Phys. 2011, 13, 19490–19498. [Google Scholar] [CrossRef]
- Richter, S.C.; Oestreich, M. Emerging strategies for C–H silylation. Trends Chem. 2019, 2, 13–17. [Google Scholar] [CrossRef]
- Cheng, C.; Hartwig, J.F. Catalytic silylation of unactivated C–H bonds. Chem. Rev. 2015, 115, 8946–8975. [Google Scholar] [CrossRef]
- Shang, X.; Liu, Z.-Q. Recent developments in free-radical-promoted C–Si formation via selective C-H/Si-H functionalization. Org. Biomol. Chem. 2016, 14, 7829–7831. [Google Scholar] [CrossRef]
- Dubac, J.; Laporterie, A.; Manuel, G. Group 14 metalloles. 1. Synthesis, organic chemistry, and physicochemical data. Chem. Rev. 1990, 90, 215–263. [Google Scholar] [CrossRef]
- Hudrlik, P.F.; Dai, D.; Hudrlik, A.M. Reactions of dilithiobutadienes with monochlorosilanes: Observation of facile loss of organic groups from silicon. J. Organomet. Chem. 2006, 691, 1257–1264. [Google Scholar] [CrossRef]
- Ishikawa, M.; Tabohashi, T.; Sugisawa, H.; Nishimura, K.; Kumada, M. Chemistry of siloles. The reactions of siloles with organolithium reagents. J. Organomet. Chem. 1983, 250, 109–119. [Google Scholar] [CrossRef]
- Kira, M.; Sakamoto, K.; Sakurai, H. Photochemistry of dibenzo-1,1,2,2-tetramethyl-1,2-disilacyclohexa-3,5-diene and the germanium analog. Exclusive extrusion of the divalent species. J. Am. Chem. Soc. 1983, 105, 7469–7470. [Google Scholar] [CrossRef]
- Gilman, H.; Gorsich, R.D. A silicon analog of 9,9-diphenyl fluorence. J. Am. Chem. Soc. 1955, 77, 6380–6381. [Google Scholar] [CrossRef]
- Mo, Y.; Tian, R.; Shi, W.; Cao, Y. Ultraviolet-emitting conjugated polymer poly(9,9′-alkyl-3,6-silafluorene) with a wide band gap of 4.0 eV. Chem. Commun. 2005, 4925–4926. [Google Scholar] [CrossRef]
- Chan, K.L.; Watkins, S.E.; Mak, C.S.K.; McKiernan, M.J.; Towns, C.R.; Pascu, S.I.; Holmes, A.B. Poly(9,9-dialkyl-3,6-dibenzosilole)-a high energy gap host for phosphorescent light emitting devices. Chem. Commun. 2005, 5766–5768. [Google Scholar] [CrossRef]
- Chen, R.-F.; Fan, Q.-L.; Zheng, C.; Huang, W. A General strategy for the facile synthesis of 2,7-dibromo-9-heterofluorenes. Org. Lett. 2006, 8, 203–205. [Google Scholar] [CrossRef]
- Ashraf, R.S.; Chen, Z.; Leem, D.S.; Bronstein, H.; Zhang, W.; Schroeder, B.C.; Geerts, Y.; Smith, J.; Watkins, S.; Anthopoulos, T.D.; et al. Silaindacenodithiophene semiconducting polymers for efficient solar cells and high mobility ambipolar transistors. Chem. Mater. 2011, 23, 768–770. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Hau, S.K.; Yip, H.-L.; Davies, J.A.; Chen, K.-S.; Zhang, Y.; Sun, Y.; Jen, A.K.-Y. Benzobis(silolothiophene)-based low bandgap polymers for efficient polymer solar cells. Chem. Mater. 2011, 23, 765–767. [Google Scholar] [CrossRef]
- Chen, W.; Ijadi-Maghsoodi, S.; Barton, T.J. Synthesis and study of conjugated polymers containing silole units. Polym. Prepr. 1997, 38, 189–190. [Google Scholar]
- Baehr, S.; Ogasawara, H.; Yamaguchi, S.; Oestreich, M. An expedient produre for the synthesis of benzo[4,5]silolo[2,3-b]thiophenes and related systems. Organometallics 2017, 36, 4013–4019. [Google Scholar] [CrossRef]
- Xu, C.; Yamada, H.; Wakamiya, A.; Yamaguchi, S.; Tamao, K. Ladder bis-silicon-bridged stilbenes as a new building unit for fluorescent π-conjugated polymers. Macromolecules 2004, 37, 8978–8983. [Google Scholar] [CrossRef]
- Gilman, H.; Gorsich, R.D. Cyclic organosilicon compounds. I. Synthesis of compounds containing the dibenzosilole nucleus. J. Am. Chem. Soc. 1958, 80, 1883–1886. [Google Scholar]
- Xiao, H.; Leng, B.; Tian, H. Hole transport triphenylamine-spirosilabifluorene alternating copolymer: Synthesis and optical, electrochemical and electroluminescent properties. Polymer 2005, 46, 5707–5713. [Google Scholar] [CrossRef]
- Van der Boon, L.J.P.; Gelderen, L.; de Groot, T.R.; Lutz, M.; Slootweg, C.J.; Ehlers, A.W.; Lammertsma, K. Chiral control in pentacoordinate systems: The case of organosilicates. Inorg. Chem. 2018, 57, 12697–12708. [Google Scholar] [CrossRef]
- Van der Boon, L.J.P.; Hendriks, J.H.; Roolvink, D.; O’Kennedy, S.J.; Lutz, M.; Slootweg, C.J.; Ehlers, A.W.; Lammertsma, K. Dynamic conformational behavier in stable pentaorganosilicates. Eur. J. Inorg. Chem. 2019, 2019, 3318–3328. [Google Scholar] [CrossRef]
- Matsuda, T.; Kadowaki, S.; Goya, T.; Murakami, M. Synthesis of silafluorenes by Iridium-catalyzed [2+2+2] cycloaddition of silicon-bridged diynes with alkynes. Org. Lett. 2007, 9, 133–136. [Google Scholar] [CrossRef]
- Shibata, T.; Uchiyama, T.; Yoshinami, Y.; Takayasu, S.; Tsuchikama, K.; Endo, K. Highly enantioselective synthesis of silahelicenes using Ir-catalyzed [2+2+2] cycloaddition. Chem. Commun. 2012, 48, 1311–1313. [Google Scholar] [CrossRef]
- Mitake, A.; Nagai, R.; Sekine, A.; Takano, H.; Sugimura, N.; Kanyiva, K.S.; Shibata, T. Consecutive HDDA and TDDA reactions of silicontethered tetraynes for the synthesis of dibenzosilole-fused polycyclic compounds and their unique reactivity. Chem. Sci. 2019, 10, 6715–6720. [Google Scholar] [CrossRef]
- Tobisu, M.; Onoe, M.; Kita, Y.; Chatani, N. Rhodium-Ccatalyzed coupling of 2-silylphenylboronic acids with alkynes leading to benzosiloles: Catalytic cleavage of C-Si bond in trialkylsilyl groups. J. Am. Chem. Soc. 2009, 131, 7506–7507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; An, K.; He, W. Rhodium-catalyzed tandem cyclization/Si-C activation reaction for the synthesis of siloles. Angew. Chem. Int. Ed. 2014, 53, 5667–5671. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, S.; Xi, Z. Palladium-catalyzed synthesis of Benzosilolo[2,3-b]indoles via Cleavage of a C(sp3)-Si Bond and Consequent Intramolecular C(sp2)-Si Coupling. J. Am. Chem. Soc. 2011, 133, 9204–9207. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Mochida, K.; Hiyama, T. Modular Approach to Silicon-Bridged Biaryls: Palladium-Catalyzed Intramolecular Coupling of 2-(Arylsilyl)aryl Triflates. Angew. Chem. Int. Ed. 2008, 47, 9760–9764. [Google Scholar] [CrossRef] [PubMed]
- Masaki, S.; Kenji, M.; Tamejiro, H. Highly efficient blue fluorescence from 3,20-silylene-bridged 2-phenylindoles in the solid state. J. Phys. Chem. C. 2011, 115, 11265–11274. [Google Scholar]
- Mochida, K.; Shimizu, M.; Hiyama, T. Palladium-catalyzed intramolecular coupling of 2-[(2-Pyrrolyl)silyl]aryl triflates through 1,2-silicon migration. J. Am. Chem. Soc. 2009, 131, 8350–8351. [Google Scholar] [CrossRef]
- Ureshino, T.; Yoshida, T.; Kuninobu, Y.; Takai, K. Rhodium-satalyzed synthesis of silafluorene derivatives via cleavage of silicon-hydrogen and carbon-hydrogen bonds. J. Am. Chem. Soc. 2010, 132, 14324–14326. [Google Scholar] [CrossRef]
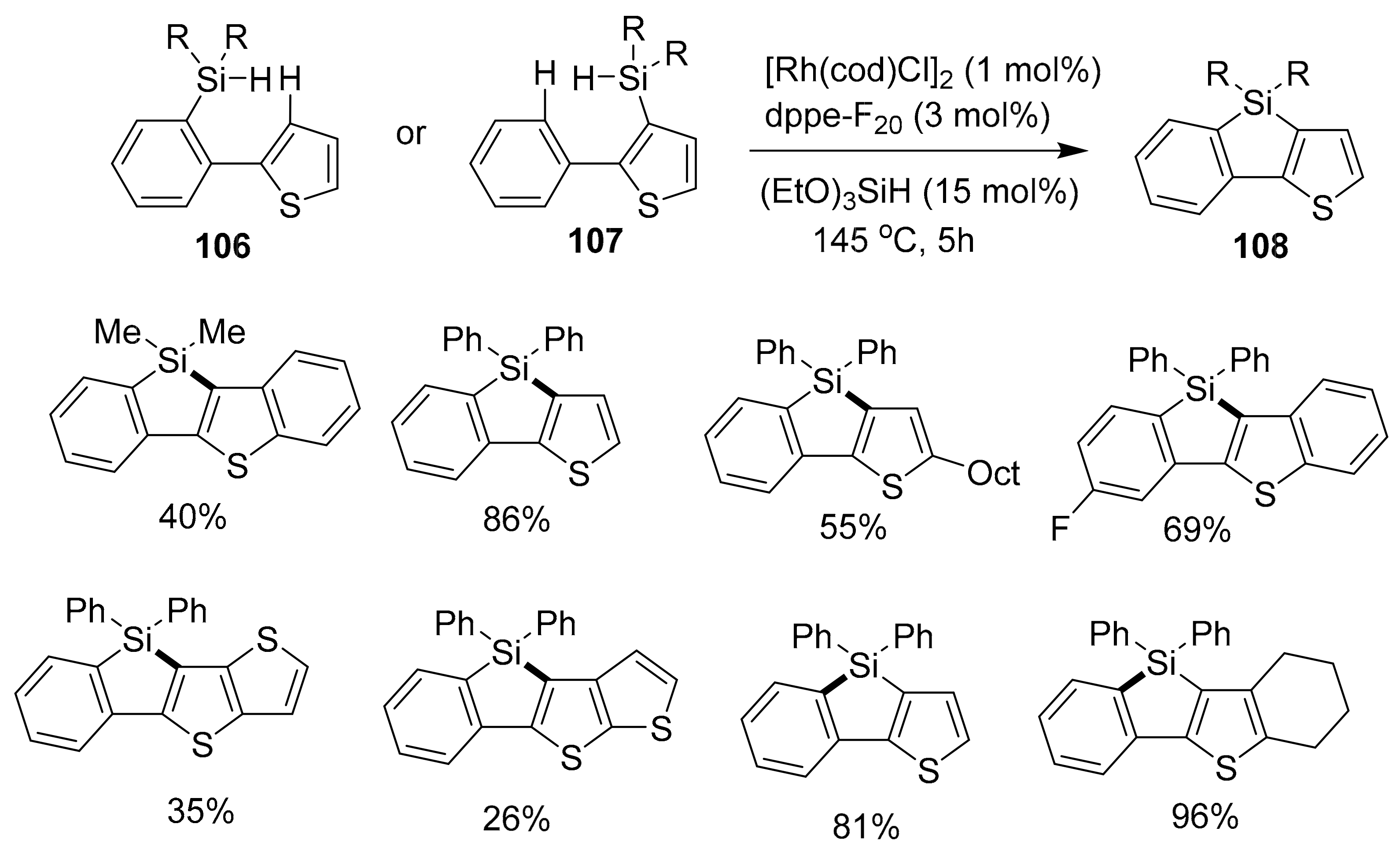
- Mitsudo, K.; Tanaka, S.; Isobuchi, R.; Inada, T.; Mandai, H.; Korenaga, T.; Wakamiya, A.; Murata, Y.; Suga, S. Rh-catalyzed dehydrogenative cyclization leading to benzosilolothiophene derivatives via Si–H/C–H bond cleavage. Org. Lett. 2017, 19, 2564–2567. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; An, K.; Liu, L.-C.; Yue, Y.; He, W. Rhodium-catalyzed enantioselective intramolecular C-H Silylation for the Syntheses of Planar-Chiral Metallocene Siloles. Angew. Chem. Int. Ed. 2015, 54, 6918–6921. [Google Scholar] [CrossRef]
- Murai, M.; Matsumoto, K.; Takeuchi, Y.; Takai, K. Rhodium-catalyzed synthesis of benzosilolometallocenes via the dehydrogenative silylation of C(sp2)–H bonds. Org. Lett. 2015, 17, 3102–3105. [Google Scholar] [CrossRef]
- Shibata, T.; Shizuno, T.; Sasaki, T. Enantioselective Synthesis of Planar-chiral benzosiloloferrocenes by Rh-catalyzed intramolecular C-H silylation. Chem. Commun. 2015, 51, 7802–7804. [Google Scholar] [CrossRef] [PubMed]
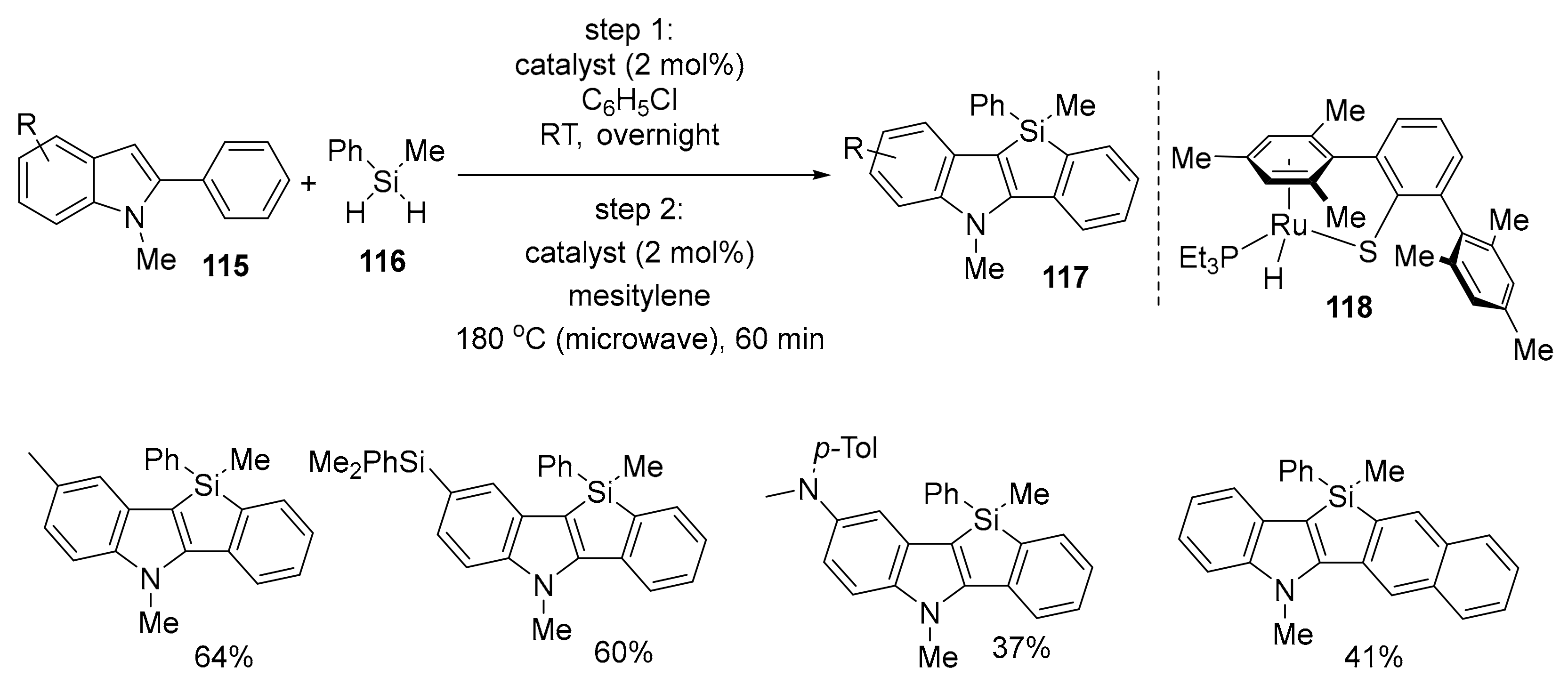
- Omann, L.; Oestreich, M. Catalytic Access to Indole-Fused Benzosiloles by 2-Fold Electrophilic C–H silylation with dihydrosilanes. Organometallics 2017, 36, 767–776. [Google Scholar] [CrossRef]
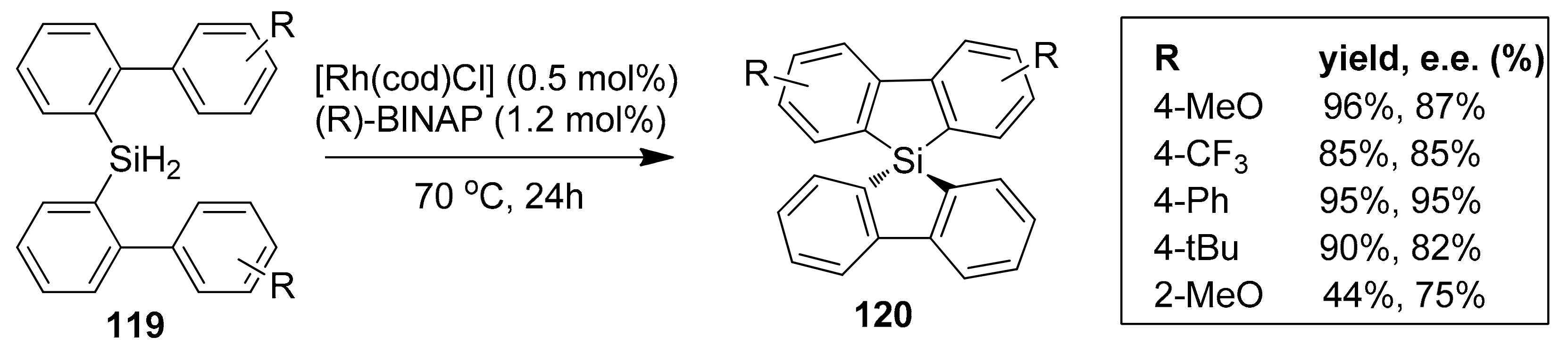
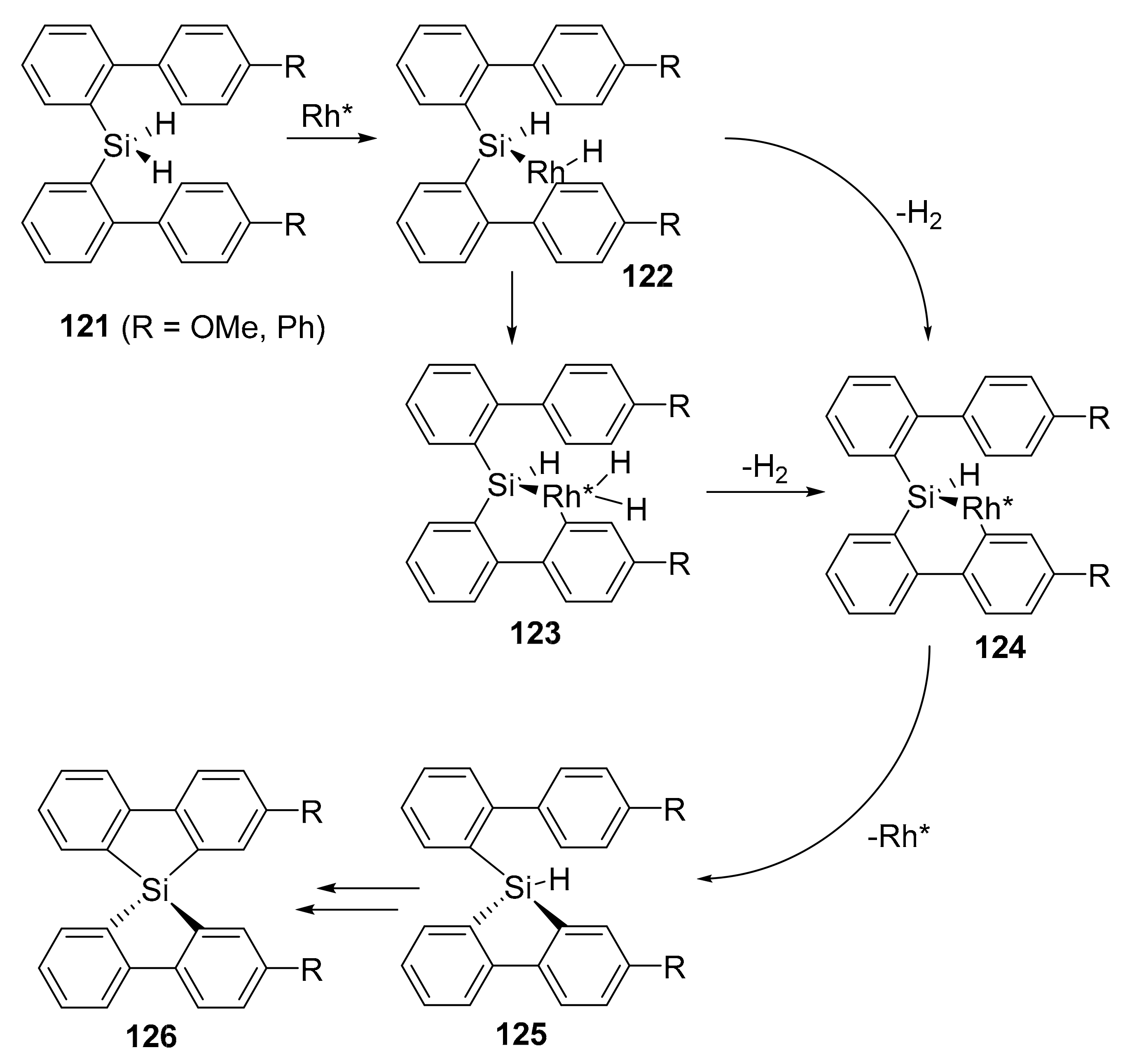
- Kuninobu, Y.; Yamauchi, K.; Tamura, N.; Seiki, T.; Takai, K. Rhodium-catalyzed asymmetric synthesis of spirosilabifluorene derivatives. Angew. Chem. Int. Ed. 2013, 52, 1520–1522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; An, K.; Liu, L.-C.; Guo, S.; Jiang, C.; Guo, H.; He, W. Rhodium-catalyzed intramolecular C-H silylation by silacyclobutanes. Angew. Chem. Int. Ed. 2016, 55, 6319–6323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; An, K.; Liu, L.-C.; Zhang, Q.; Guo, H.; He, W. Construction of chiral tetraorganosilicons by tandem desymmetrization of silacyclobutanes/intermolecular dehydrogenative silylation. Angew. Chem. Int. Ed. 2016, 55, 1125–1129. [Google Scholar]
- Sollott, G.P.; Peterson, W.R. Silylation of ferrocene by chloro- and aminosilanes under Friedel-Crafts conditions. J. Am. Chem. Soc. 1967, 89, 5054–5056. [Google Scholar] [CrossRef]
- Simchen, G.; Frick, U. elektrophile silylierung elektronenreicher heteroaromaten. Synthesis 1984, 928–929. [Google Scholar]
- Furukawa, S.; Kobayashi, J.; Kawashima, T. Development of a sila-Friedel-Crafts reaction and its application to the synthesis of dibenzosilole derivatives. J. Am. Chem. Soc. 2009, 131, 14192–14193. [Google Scholar] [CrossRef]
- Curless, L.D.; Ingleson, M.J. B(C6F5)3-catalyzed synthesis of benzofused-Siloles. Organometallics 2014, 33, 7241–7246. [Google Scholar] [CrossRef]
- Leifert, D.; Studer, A. 9-Silafluorenes via base-promoted homolytic aromatic substitution (BHAS)—The electron as a catalyst. Org. Lett. 2015, 17, 386–389. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, S.; Li, P. Synthesis of silafluorenes and silaindenes via silyl radicals from arylhydrosilanes: Intramolecular cyclization and intermolecular annulation with alkynes. Org. Chem. Front. 2015, 2, 459–463. [Google Scholar] [CrossRef]
- Narayanam, J.M.R.; Stephenson, C.R.J. Visible light photoredox catalysis: Applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, J.; Li, J.; Ma, W.; An, K.; He, W.; Jiang, C. Visible-light induced radical silylation for the synthesis of dibenzosiloles via dehydrogenative cyclization. Adv. Synth. Catal. 2018, 17, 3049–3054. [Google Scholar] [CrossRef]





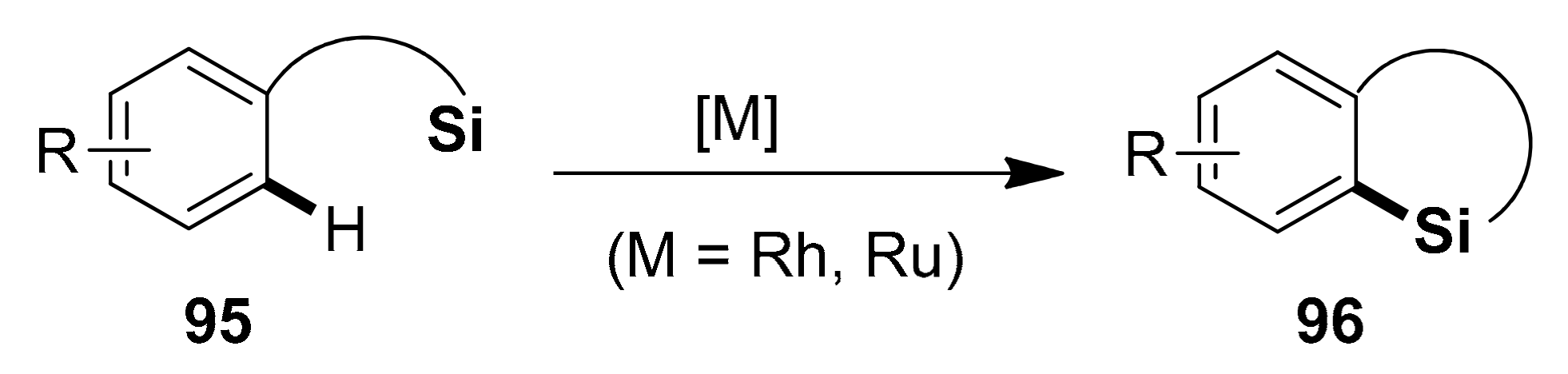





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, T.T.; Nguyen, H.M.T.; Nguyen, H.; Dung, T.N.; Nguyen, M.T.; Dehaen, W. Advances in Synthesis of π-Extended Benzosilole Derivatives and Their Analogs. Molecules 2020, 25, 548. https://doi.org/10.3390/molecules25030548
Dang TT, Nguyen HMT, Nguyen H, Dung TN, Nguyen MT, Dehaen W. Advances in Synthesis of π-Extended Benzosilole Derivatives and Their Analogs. Molecules. 2020; 25(3):548. https://doi.org/10.3390/molecules25030548
Chicago/Turabian StyleDang, Tuan Thanh, Hue Minh Thi Nguyen, Hien Nguyen, Tran Ngoc Dung, Minh Tho Nguyen, and Wim Dehaen. 2020. "Advances in Synthesis of π-Extended Benzosilole Derivatives and Their Analogs" Molecules 25, no. 3: 548. https://doi.org/10.3390/molecules25030548
APA StyleDang, T. T., Nguyen, H. M. T., Nguyen, H., Dung, T. N., Nguyen, M. T., & Dehaen, W. (2020). Advances in Synthesis of π-Extended Benzosilole Derivatives and Their Analogs. Molecules, 25(3), 548. https://doi.org/10.3390/molecules25030548





