Production of 5-Hydroxymethylfurfural from Chitin Biomass: A Review
Abstract
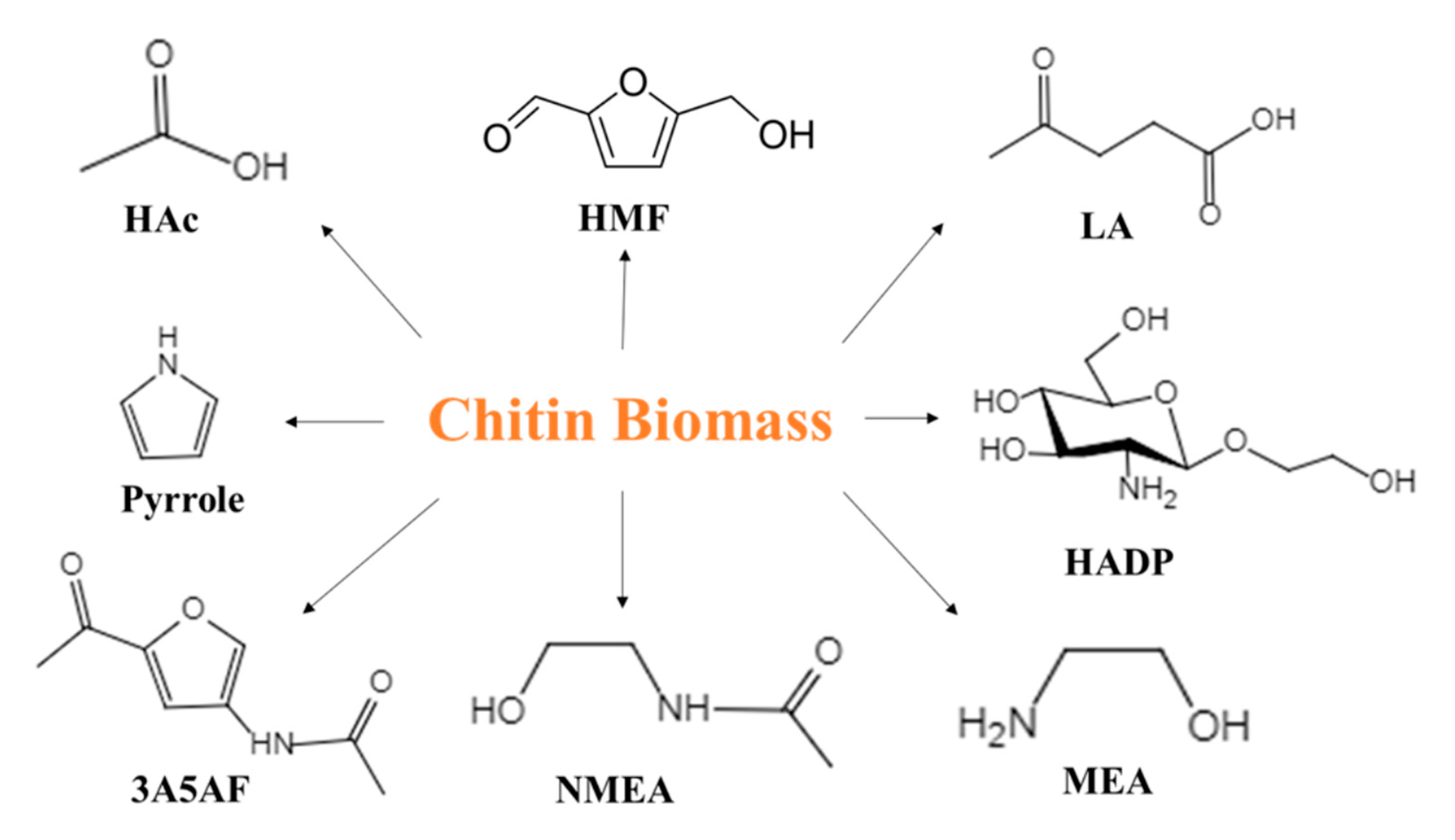
1. Introduction
2. Structure of Chitin Biomass
3. Chitin Biomass Pretreatment Techniques
3.1. Chemical and Biological Pretreatment
3.2. Physical-Assisted Pretreatment
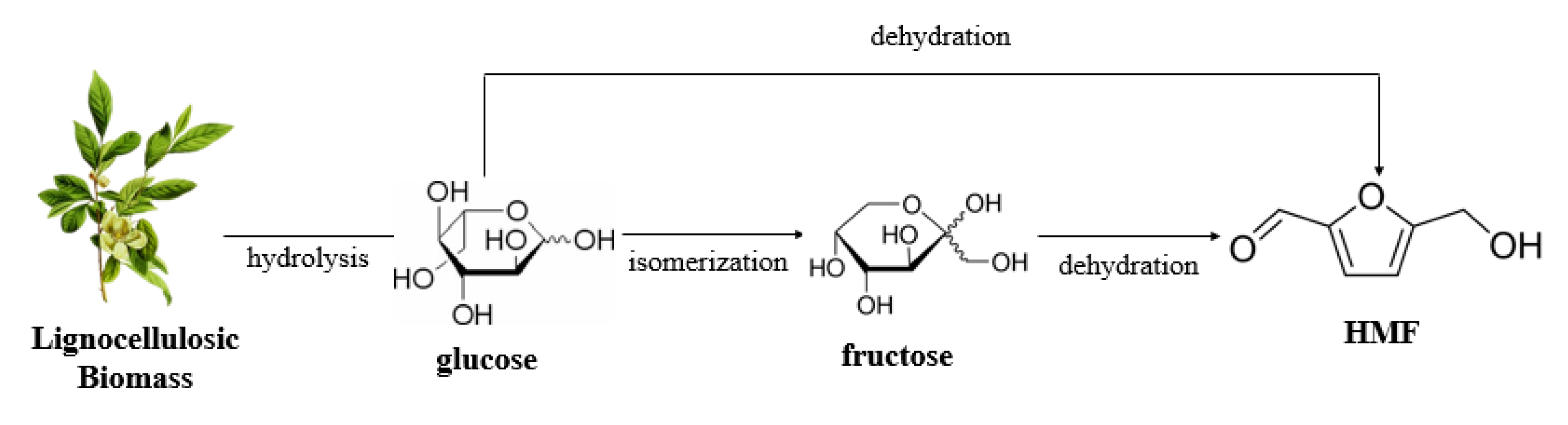
4. HMF Production from Chitin Biomass
4.1. HMF Production from Chitin Biomass by Brønsted Acids
4.2. HMF Production from Chitin Biomass by Lewis Acids
4.3. HMF Production from Chitin Biomass Using ILs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| DD | degree of deacetylation |
| DES | deep eutectic solvent |
| DMSO | dimethyl sulfoxide |
| GlcNAc | N-acetylglucosamine |
| GlcNH2 | glucosamine |
| HMF | 5-hydroxymethylfurfural |
| [[Hmim][HSO4]] | methyl imidazole hydrogen sulfate |
| [Hbim]Cl | benzimidazole chloride |
| IL | ionic liquid |
| LA | levulinic acid |
| [MIM]HSO4 | N-methylimidazole hydrogen sulfate |
| NADES | natural deep eutectic solvent |
| NH2SO3H | sulfamic acid |
References
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef]
- Menegazzo, F.; Ghedini, E.; Signoretto, M. 5-Hydroxymethylfurfural (HMF) Production from Real Biomasses. Molecules 2018, 23, 2201. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, B.; Wang, Y.; Yan, N. Valorization of Renewable Carbon Resources for Chemicals. CHIMIA Int. J. Chem. 2014, 69, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Delbecq, F.; Len, C. Recent Advances in the Microwave-Assisted Production of Hydroxymethylfurfural by Hydrolysis of Cellulose Derivatives-A Review. Molecules 2018, 23, 1973. [Google Scholar] [CrossRef]
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nat. News 2015, 524, 155. [Google Scholar] [CrossRef]
- Kerton, F.M.; Liu, Y.; Omari, K.W.; Hawboldt, K. Green chemistry and the ocean-based biorefinery. Green Chem. 2013, 15, 860–871. [Google Scholar] [CrossRef]
- Xi, C.; Ning, Y. Novel Catalytic Systems to Convert Chitin and Lignin into Valuable Chemicals. Catal. Surv. Asia 2014, 18, 164–176. [Google Scholar]
- Chen, X.; Yang, H.; Yan, N. Shell Biorefinery: Dream or Reality? Chem. Eur. J. 2016, 22, 13402–13421. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy’s "Top 10" revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Zhang, M.; Zang, H.; Yu, S.; Yan, B. Advances in conversion of chitin biomass into high-value chemicals. Chem. Ind. Eng. Prog. 2017, 36, 863–872, (In Chinese with English Abtract). [Google Scholar]
- Nikolov, S.; Petrov, M.; Lymperakis, L.; Friak, M.; Sachs, C.; Fabritius, H.-O.; Raabe, D.; Neugebauer, J. Revealing the Design Principles of High-Performance Biological Composites Using Ab initio and Multiscale Simulations: The Example of Lobster Cuticle. Adv. Mater. 2010, 22, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, Y.; Yu, L.; Zhang, C.; Xu, X.; Xue, Y.; Li, Z.; Xue, C. Crystalline structure and thermal property characterization of chitin from Antarctic krill (Euphausia superba). Carbohydr. Polym. 2013, 92, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, H.; Velayutham, K.; Ravichandran, R. Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int. J. Biol. Macromol. 2018, 107, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Hajji, S.; Frachet, V.; Rinaudo, M.; Jellouli, K.; Nasri, M. Chitin extraction from shrimp shell using enzymatic treatment. Antitumor, antioxidant and antimicrobial activities of chitosan. Int. J. Biol. Macromol. 2014, 69, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wei, G.; Mo, X.; Zhou, N.; Chen, K.; Ouyang, P. Enzymatic hydrolysis of chitin pretreated by bacterial fermentation to obtain pure N-acetyl-D-glucosamine. Green Chem. 2018, 20, 2320–2327. [Google Scholar] [CrossRef]
- Pacheco, N.; Garnica-Gonzalez, M.; Gimeno, M.; Barzana, E.; Trombotto, S.; David, L.; Shirai, K. Structural Characterization of Chitin and Chitosan Obtained by Biological and Chemical Methods. Biomacromolecules 2011, 12, 3285–3290. [Google Scholar] [CrossRef]
- Setoguchi, T.; Kato, T.; Yamamoto, K.; Kadokawa, J.-i. Facile production of chitin from crab shells using ionic liquid and citric acid. Int. J. Biol. Macromol. 2012, 50, 861–864. [Google Scholar] [CrossRef]
- Barber, P.S.; Griggs, C.S.; Bonner, J.R.; Rogers, R.D. Electrospinning of chitin nanofibers directly from an ionic liquid extract of shrimp shells. Green Chem. 2013, 15, 601–607. [Google Scholar] [CrossRef]
- Qin, Y.; Lu, X.; Sun, N.; Rogers, R.D. Dissolution or extraction of crustacean shells using ionic liquids to obtain high molecular weight purified chitin and direct production of chitin films and fibers. Green Chem. 2010, 12, 968–971. [Google Scholar] [CrossRef]
- Ishii, D.; Ohashi, C.; Hayashi, H. Facile enhancement of the deacetylation degree of chitosan by hydrothermal treatment in an imidazolium-based ionic liquid. Green Chem. 2014, 16, 1764–1767. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents–Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic Liquids and Their Interaction with Cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef] [PubMed]
- del Monte, F.; Carriazo, D.; Serrano, M.C.; Gutierrez, M.C.; Ferrer, M.L. Deep Eutectic Solvents in Polymerizations: A Greener Alternative to Conventional Syntheses. Chemsuschem 2014, 7, 999–1009. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jerome, F. Deep eutectic solvents: syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, Z.; Hong, S.; Lian, H. One-pot production of chitin with high purity from lobster shells using choline chloride–malonic acid deep eutectic solvent. Carbohydr. Polym. 2017, 177, 217–223. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, Y.; Yang, Q.; Zhu, P.; Lian, H. Versatile acid base sustainable solvent for fast extraction of various molecular weight chitin from lobster shell. Carbohydr. Polym. 2018, 201, 211–217. [Google Scholar] [CrossRef]
- Huang, W.-C.; Zhao, D.; Guo, N.; Xue, C.; Mao, X. Green and Facile Production of Chitin from Crustacean Shells Using a Natural Deep Eutectic Solvent. J. Agric. Food. Chem. 2018, 66, 11897–11901. [Google Scholar] [CrossRef]
- Tan, Y.T.; Chua, A.S.M.; Ngoh, G.C. Deep eutectic solvent for lignocellulosic biomass fractionation and the subsequent conversion to bio-based products—A review. Bioresour. Technol. 2019, 122522. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, M.; Lu, X.; Shi, C.; Li, X.; Xin, J.; Yue, G.; Zhang, S. Base-free preparation of low molecular weight chitin from crab shell. Carbohydr. Polym. 2018, 190, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yang, Q.; Yuan, Y.; Chen, L.; Song, Y.; Lian, H. Sustainable co-solvent induced one step extraction of low molecular weight chitin with high purity from raw lobster shell. Carbohydr. Polym. 2019, 205, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Dhamodharan, R. Pretreatment in Hot Glycerol for Facile and Green Separation of Chitin from Prawn Shell Waste. ACS Sustain. Chem. Eng. 2017, 6, 846–853. [Google Scholar] [CrossRef]
- Liu, C.; Wang, G.; Sui, W.; An, L.; Si, C. Preparation and Characterization of Chitosan by a Novel Deacetylation Approach Using Glycerol as Green Reaction Solvent. ACS Sustain. Chem. Eng. 2017, 5, 4690–4698. [Google Scholar] [CrossRef]
- Yang, H.; Gozaydin, G.; Nasaruddin, R.R.; Har, J.R.G.; Chen, X.; Wang, X.; Yan, N. Toward the Shell Biorefinery: Processing Crustacean Shell Waste Using Hot Water and Carbonic Acid. ACS Sustain. Chem. Eng. 2019, 7, 5532–5542. [Google Scholar] [CrossRef]
- Vasil’eva, T.M.; Lopatin, S.A.; Varlamov, V.P. Production of the low-molecular-weight chitin and chitosan forms in electron-beam plasma. High Energy Chem. 2016, 50, 150–154. [Google Scholar] [CrossRef]
- Birolli, W.G.; de Moura Delezuk, J.A.; Campana-Filho, S.P. Ultrasound-assisted conversion of alpha-chitin into chitosan. Appl. Acoust. 2016, 103, 239–242. [Google Scholar] [CrossRef]
- Fiamingo, A.; de Moura Delezuk, J.A.; Trombotto, S.; David, L.; Campana-Filho, S.P. Extensively deacetylated high molecular weight chitosan from the multistep ultrasound-assisted deacetylation of beta-chitin. Ultrason. Sonochem. 2016, 32, 79–85. [Google Scholar] [CrossRef]
- Wu, T.; Wu, C.; Xiang, Y.; Huang, J.; Luan, L.; Chen, S.; Hu, Y. Kinetics and mechanism of degradation of chitosan by combining sonolysis with H2O2/ascorbic acid. RSC Adv. 2016, 6, 76280–76287. [Google Scholar] [CrossRef]
- Rathi, A.K.; Gawande, M.B.; Zboril, R.; Varma, R.S. Microwave-assisted synthesis—Catalytic applications in aqueous media. Coord. Chem. Rev. 2015, 291, 68–94. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.-J. Author Response to Comment on: Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review. Materials 2019, 12, 3640. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Goswami, P.; Bora, U. Microwave mediated rapid synthesis of chitosan. J. Mater. Sci. Mater. Med. 2009, 20, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Samar, M.M.; El-Kalyoubi, M.H.; Khalaf, M.M.; El-Razik, M.M.A. Physicochemical, functional, antioxidant and antibacterial properties of chitosan extracted from shrimp wastes by microwave technique. Ann. Agric. Sci. 2013, 58, 33–41. [Google Scholar] [CrossRef]
- El Knidri, H.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and efficient extraction of chitin and chitosan for scale-up production: Effect of process parameters on deacetylation degree and molecular weight. Int. J. Biol. Macromol. 2019, 139, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Al Sagheer, F.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- El Knidri, H.; El Khalfaouy, R.; Laajeb, A.; Addaou, A.; Lahsini, A. Eco-friendly extraction and characterization of chitin and chitosan from the shrimp shell waste via microwave irradiation. Process Saf. Environ. Prot. 2016, 104, 395–405. [Google Scholar] [CrossRef]
- Kleine, T.; Buendia, J.; Bolm, C. Mechanochemical degradation of lignin and wood by solvent-free grinding in a reactive medium. Green Chem. 2013, 15, 160–166. [Google Scholar] [CrossRef]
- Di Nardo, T.; Hadad, C.; Van Nhien, A.N.; Moores, A. Synthesis of high molecular weight chitosan from chitin by mechanochemistry and aging. Green Chem. 2019, 21, 3271–3285. [Google Scholar] [CrossRef]
- Margoutidis, G.; Parsons, V.H.; Bottaro, C.S.; Yan, N.; Kerton, F.M. Mechanochemical Amorphization of alpha-Chitin and Conversion into Oligomers of N-Acetyl-D-glucosamine. ACS Sustain. Chem. Eng. 2018, 6, 1662–1669. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.; Zhong, Z.; Yan, N. Base-catalysed, one-step mechanochemical conversion of chitin and shrimp shells into low molecular weight chitosan. Green Chem. 2017, 19, 2783–2792. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, W.-C.; Guo, N.; Zhang, S.; Xue, C.; Mao, X. Two-Step Separation of Chitin from Shrimp Shells Using Citric Acid and Deep Eutectic Solvents with the Assistance of Microwave. Polymers 2019, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, D.G.; Caratzoulas, S. The roles of catalysis and reaction engineering in overcoming the energy and the environment crisis. Chem. Eng. Sci. 2010, 65, 18–29. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W. Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and underlying mechanisms. Bioresour. Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, S.G.; Alonso, D.M.; Guerbuez, E.I.; Dumesic, J.A. A roadmap for conversion of lignocellulosic biomass to chemicals and fuels. Curr. Opin. Chem. Eng. 2012, 1, 218–224. [Google Scholar] [CrossRef]
- Omari, K.W.; Besaw, J.E.; Kerton, F.M. Hydrolysis of chitosan to yield levulinic acid and 5-hydroxymethylfurfural in water under microwave irradiation. Green Chem. 2012, 14, 1480–1487. [Google Scholar] [CrossRef]
- Lee, S.-B.; Jeong, G.-T. Catalytic Conversion of Chitosan to 5-Hydroxymethylfurfural Under Low Temperature Hydrothermal Process. Appl. Biochem. Biotechnol. 2015, 176, 1151–1161. [Google Scholar] [CrossRef]
- Jeong, G.-T. Production of levulinic acid from glucosamine by dilute-acid catalyzed hydrothermal process. Ind. Crops Prod. 2014, 62, 77–83. [Google Scholar] [CrossRef]
- Rackemann, D.W.; Bartley, J.P.; Doherty, W.O.S. Methanesulfonic acid-catalyzed conversion of glucose and xylose mixtures to levulinic acid and furfural. Ind. Crops Prod. 2014, 52, 46–57. [Google Scholar] [CrossRef]
- Rackemann, D.W.; Bartley, J.P.; Harrison, M.D.; Doherty, W.O.S. The effect of pretreatment on methanesulfonic acid-catalyzed hydrolysis of bagasse to levulinic acid, formic acid, and furfural. RSC Adv. 2016, 6, 74525–74535. [Google Scholar] [CrossRef]
- Park, M.-R.; Kim, H.S.; Kim, S.-K.; Jeong, G.-T. Thermo-chemical conversion for production of levulinic and formic acids from glucosamine. Fuel Process. Technol. 2018, 172, 115–124. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, M.-R.; Kim, S.-K.; Jeong, G.-T. Valorization of chitosan into levulinic acid by hydrothermal catalytic conversion with methanesulfonic acid. Korean J. Chem. Eng. 2018, 35, 1290–1296. [Google Scholar] [CrossRef]
- Savitri, E.; Juliastuti, R.; Handaratri, A.; Sumarno; Roesyadi, A. Degradation of chitosan by sonication in very-low-concentration acetic acid. Polym. Degrad. Stab. 2014, 110, 344–352. [Google Scholar] [CrossRef]
- Deng, T.; Cui, X.; Qi, Y.; Wang, Y.; Hou, X.; Zhu, Y. Conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by ZnCl2 in water. Chem. Commun. 2012, 48, 5494–5496. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.J.; Deshpande, S.V.; Kennedy, J.F. Metal complexation by chitosan and its derivatives: A review. Carbohydr. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Liu, H. Preparation, characterization and antimicrobial activity of chitosan–Zn complex. Carbohydr. Polym. 2004, 56, 21–26. [Google Scholar] [CrossRef]
- Wang, Y.; Pedersen, C.M.; Deng, T.; Qiao, Y.; Hou, X. Direct conversion of chitin biomass to 5-hydroxymethylfurfural in concentrated ZnCl2 aqueous solution. Bioresour. Technol. 2013, 143, 384–390. [Google Scholar] [CrossRef]
- Yu, S.; Zang, H.; Chen, S.; Jiang, Y.; Yan, B.; Cheng, B. Efficient conversion of chitin biomass into 5-hydroxymethylfurfural over metal salts catalysts in dimethyl sulfoxide -water mixture under hydrothermal conditions. Polym. Degrad. Stab. 2016, 134, 105–114. [Google Scholar] [CrossRef]
- Rostami, A.; Yari, A. Sulfamic acid as a recyclable and green catalyst for rapid and highly efficient synthesis of 2-arylbenzothiazoles in water at room temperature. J. Iran. Chem. Soc. 2012, 9, 489–493. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, X.; Shen, Y.; Yi, Y.; Wang, B.; Xu, F.; Sun, R. Conversion of bamboo fiber into 5-hydroxymethylfurfural catalyzed by sulfamic acid with microwave assistance in biphasic system. Ind. Crops Prod. 2015, 70, 266–271. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, M.-R.; Jeon, Y.J.; Kim, S.-K.; Hong, Y.-K.; Jeong, G.-T. Valorization of Chitosan as Food Waste of Aquatic Organisms into 5-Hydroxymethylfurfural by Sulfamic Acid-Catalyzed Conversion Process. Energy Technol. 2018, 6, 1747–1754. [Google Scholar] [CrossRef]
- Agarwal, B.; Kailasam, K.; Sangwan, R.S.; Elumalai, S. Traversing the history of solid catalysts for heterogeneous synthesis of 5-hydroxymethylfurfural from carbohydrate sugars: A review. Renew. Sustain. Energy Rev. 2018, 82, 2408–2425. [Google Scholar] [CrossRef]
- Dai, J.H.; Zhu, L.F.; Tang, D.Y.; Fu, X.; Tang, J.Q.; Guo, X.W.; Hu, C.W. Sulfonated polyaniline as a solid organocatalyst for dehydration of fructose into 5-hydroxymethylfurfural. Green Chem. 2017, 19, 1932–1939. [Google Scholar] [CrossRef]
- Huang, F.M.; Su, Y.W.; Tao, Y.; Sun, W.; Wang, W.T. Preparation of 5-hydroxymethylfurfural from glucose catalyzed by silica-supported phosphotungstic acid heterogeneous catalyst. Fuel 2018, 226, 417–422. [Google Scholar] [CrossRef]
- Wang, J.J.; Tan, Z.C.; Zhu, C.C.; Miao, G.; Kong, L.Z.; Sun, Y.H. One-pot catalytic conversion of microalgae (Chlorococcum sp.) into 5-hydroxymethylfurfural over the commercial H-ZSM-5 zeolite. Green Chem. 2016, 18, 452–460. [Google Scholar] [CrossRef]
- Bhaumik, P.; Dhepe, P.L. Influence of properties of SAPO’s on the one-pot conversion of mono-, di- and poly-saccharides into 5-hydroxymethylfurfural. RSC Adv. 2013, 3, 17156–17165. [Google Scholar] [CrossRef]
- Yan, L.L.; Liu, N.A.; Wang, Y.; Machida, H.; Qi, X.H. Production of 5-hydroxymethylfurfural from corn stalk catalyzed by corn stalk-derived carbonaceous solid acid catalyst. Bioresour. Technol. 2014, 173, 462–466. [Google Scholar] [CrossRef]
- Kalane, N.D.; Krishnan, R.A.; Yadav, V.D.; Jain, R.; Dandekar, P. Synergistic effect of hetero- and homo-catalysts on the "green’ synthesis of 5-hydroxymethylfurfural from chitosan biomass. Cellulose 2019, 26, 2805–2819. [Google Scholar] [CrossRef]
- Weerachanchai, P.; Leong, S.S.J.; Chang, M.W.; Ching, C.B.; Lee, J.M. Improvement of biomass properties by pretreatment with ionic liquids for bioconversion process. Bioresour. Technol. 2012, 111, 453–459. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, S.P.; Xu, W.J.; Qi, W.; Yan, Y.J.; Xu, Q.L. Efficient saccharification by pretreatment of bagasse pith with ionic liquid and acid solutions simultaneously. Energy Convers. Manag. 2015, 89, 120–126. [Google Scholar] [CrossRef]
- Dutta, S.; Pal, S. Promises in direct conversion of cellulose and lignocellulosic biomass to chemicals and fuels: Combined solvent–nanocatalysis approach for biorefinary. Biomass Bioenergy 2014, 62, 182–197. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. Thermo-kinetic assessment of glucose decomposition to 5-hydroxymethyl furfural and levulinic acid over acidic functionalized ionic liquid. Chem. Eng. 2018, 335, 221–230. [Google Scholar] [CrossRef]
- Li, C.Z.; Zhao, Z.B.K.; Cai, H.L.; Wang, A.Q.; Zhang, T. Microwave-promoted conversion of concentrated fructose into 5-hydroxymethylfurfural in ionic liquids in the absence of catalysts. Biomass Bioenergy 2011, 35, 2013–2017. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, Z.H.; Wang, X.; Liu, B.; Lin, J.T. Hydrolysis of cellulose in ionic liquids catalyzed by a magnetically-recoverable solid acid catalyst. Chem. Eng. 2014, 235, 349–355. [Google Scholar] [CrossRef]
- Feng, J.; Zang, H.; Yan, B.; Li, M.; Cheng, B. Conversion of Chitosan into 5-Hydroxymethylfurfural via Hydrothermal Synthesis. Adv. Mater. Res. 2015, 1095, 411–414. [Google Scholar]
- Li, M.; Zang, H.; Feng, J.; Yan, Q.; Yu, N.; Shi, X.; Cheng, B. Efficient conversion of chitosan into 5-hydroxymethylfurfural via hydrothermal synthesis in ionic liquids aqueous solution. Polym. Degrad. Stab. 2015, 121, 331–339. [Google Scholar] [CrossRef]
- Zang, H.; Yu, S.; Yu, P.; Ding, H.; Du, Y.; Yang, Y.; Zhang, Y. Hydrothermal conversion of N-acetyl-D-glucosamine to 5-hydroxymethylfurfural using ionic liquid as a recycled catalyst in a water-dimethyl sulfoxide mixture. Carbohydr. Res. 2017, 442, 1–8. [Google Scholar] [CrossRef]
- Zhang, M.; Zang, H.; Ma, B.; Zhang, X.; Xie, R.; Cheng, B. Green Synthesis of 5-Hydroxymethylfurfural from Chitosan Biomass Catalyzed by Benzimidazole-Based Ionic Liquids. Chemistryselect 2017, 2, 10323–10328. [Google Scholar] [CrossRef]
- Jiang, Y.; Zang, H.; Han, S.; Yan, B.; Yu, S.; Cheng, B. Direct conversion of chitosan to 5-hydroxymethylfurfural in water using Bronsted-Lewis acidic ionic liquids as catalysts. RSC Adv. 2016, 6, 103774–103781. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Natural Resource | Reaction Solvent | Product | Condition |
|---|---|---|---|
| glycerol | hot glycerol | chitin | - |
| Glycerol + 5–7% HCl | 55 kDa chitin 57 kDa chitin | 7% HCl, 120 °C, 2 h 5% HCl, 150 °C, 2 h | |
| Glycerol + 30% NaOH | chitosan | 180 °C, 12 h, liquid–solid = 40 |
| Substrates | Acid Catalyst | Reaction Conditions | HMF (%) | References |
|---|---|---|---|---|
| Chitosan | 2.2% H2SO4 | 174 °C, 36.9 min | 12.1 (wt.) | [56] |
| GlcNH2 | 3% H2SO4 | 175 °C, 5 min | 1.8 (wt.) | [57] |
| GlcNH2 | 0.1 M MSA | 160 °C, 40 min | 2.3 (wt.) | [60] |
| Chitosan | 0.1 M MSA | 200 °C,15 min | 15.0 (wt.) | [61] |
| Chitosan | 0.12 mmol SnCl4⋅5H2O | MW200 °C, 30 min | 10.0 (wt.) | [55] |
| GlcNH2 | 67% ZnCl2 | 120 °C, 90 min | 21.9 (mol) | [66] |
| GlcNAc | FeCl2⋅4H2O | 190 °C, 6 h DMSO-water solvent | 33.9 (mol) | [67] |
| Chitosan | 26.6 (mol) | |||
| Chitosan | 0.7 M NH2SO3H | 200 °C, 2 min | 21.5 (wt.) | [70] |
| Substrates | ILs | Reaction Conditions | HMF (%) | References |
|---|---|---|---|---|
| Chitosan | [BMIM]HSO4 | 180 °C, 5 h 100 mol% AlCl3⋅6H2O | 25.2 (mol) | [84] |
| Chitosan | [MIM]HSO4 | 180 °C, 5 h | 29.5 (mol) | [85] |
| Chitin | 19.3 (mol) | |||
| Chitin | [[Hmim][HSO4]] | 180 °C, 6 h | 25.7 (mol) | [86] |
| Chitosan | DMSO-water solvent | 34.7 (mol) | ||
| Chitosan | 180 °C, 6 h | 30.8 (mol) | ||
| Chitosan | [Hbim]Cl | 180 °C, 3 h DMSO-water solvent | 34.9 (mol) | [87] |
| Chitosan | [Hmim][HSO4]−0.5FeCl2 | 180 °C, 4 h | 44.1 (mol) | [88] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Shen, D.; Lu, W.; Song, T.; Wang, M.; Feng, H.; Shentu, J.; Long, Y. Production of 5-Hydroxymethylfurfural from Chitin Biomass: A Review. Molecules 2020, 25, 541. https://doi.org/10.3390/molecules25030541
Zhou D, Shen D, Lu W, Song T, Wang M, Feng H, Shentu J, Long Y. Production of 5-Hydroxymethylfurfural from Chitin Biomass: A Review. Molecules. 2020; 25(3):541. https://doi.org/10.3390/molecules25030541
Chicago/Turabian StyleZhou, Dan, Dongsheng Shen, Wenjing Lu, Tao Song, Meizhen Wang, Huajun Feng, Jiali Shentu, and Yuyang Long. 2020. "Production of 5-Hydroxymethylfurfural from Chitin Biomass: A Review" Molecules 25, no. 3: 541. https://doi.org/10.3390/molecules25030541
APA StyleZhou, D., Shen, D., Lu, W., Song, T., Wang, M., Feng, H., Shentu, J., & Long, Y. (2020). Production of 5-Hydroxymethylfurfural from Chitin Biomass: A Review. Molecules, 25(3), 541. https://doi.org/10.3390/molecules25030541







