Tailoring Photoluminescence from Si-Based Nanocrystals Prepared by Pulsed Laser Ablation in He-N2 Gas Mixtures
Abstract
1. Introduction
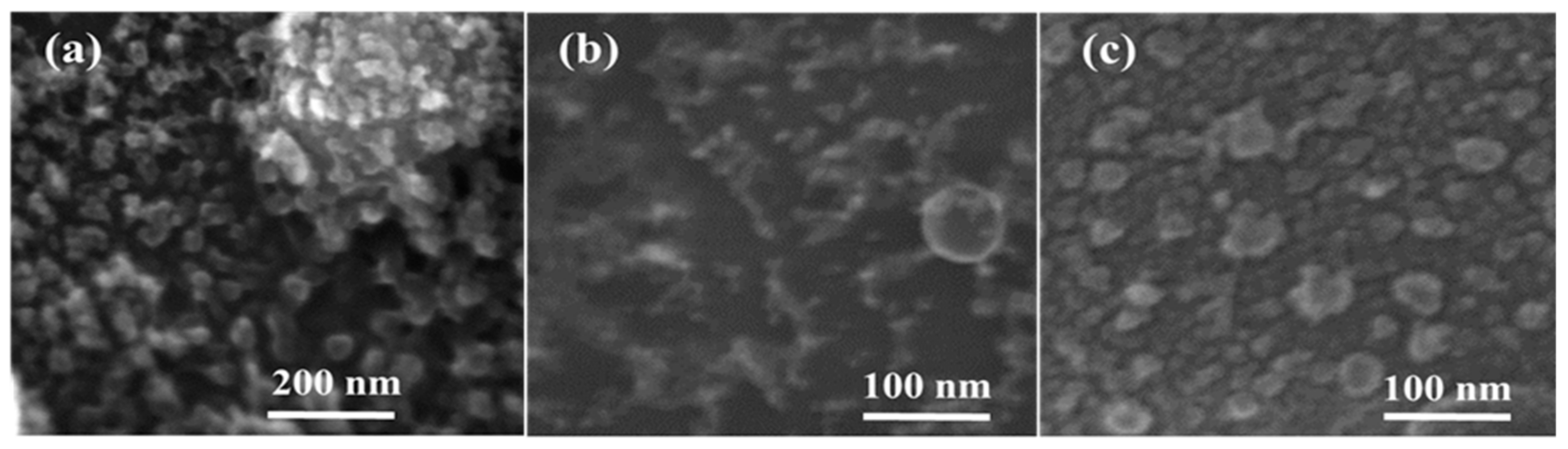
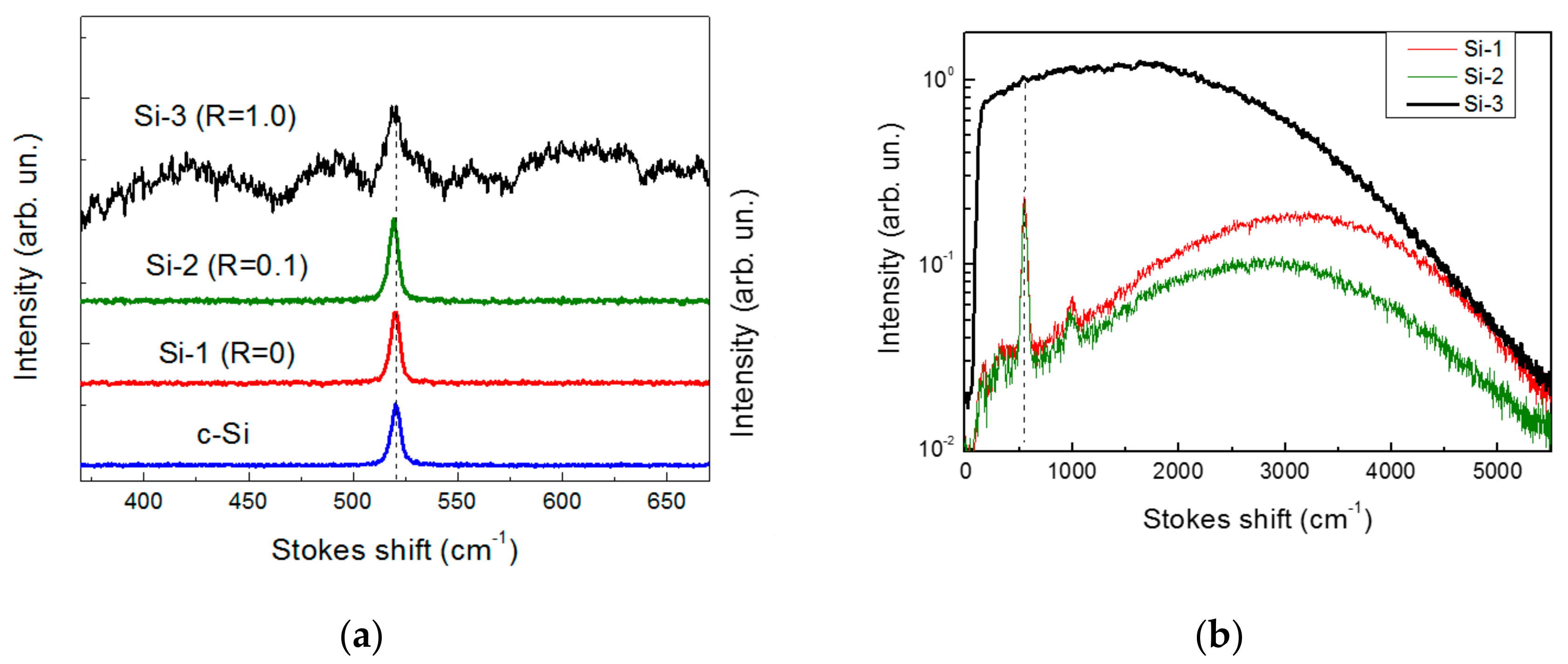
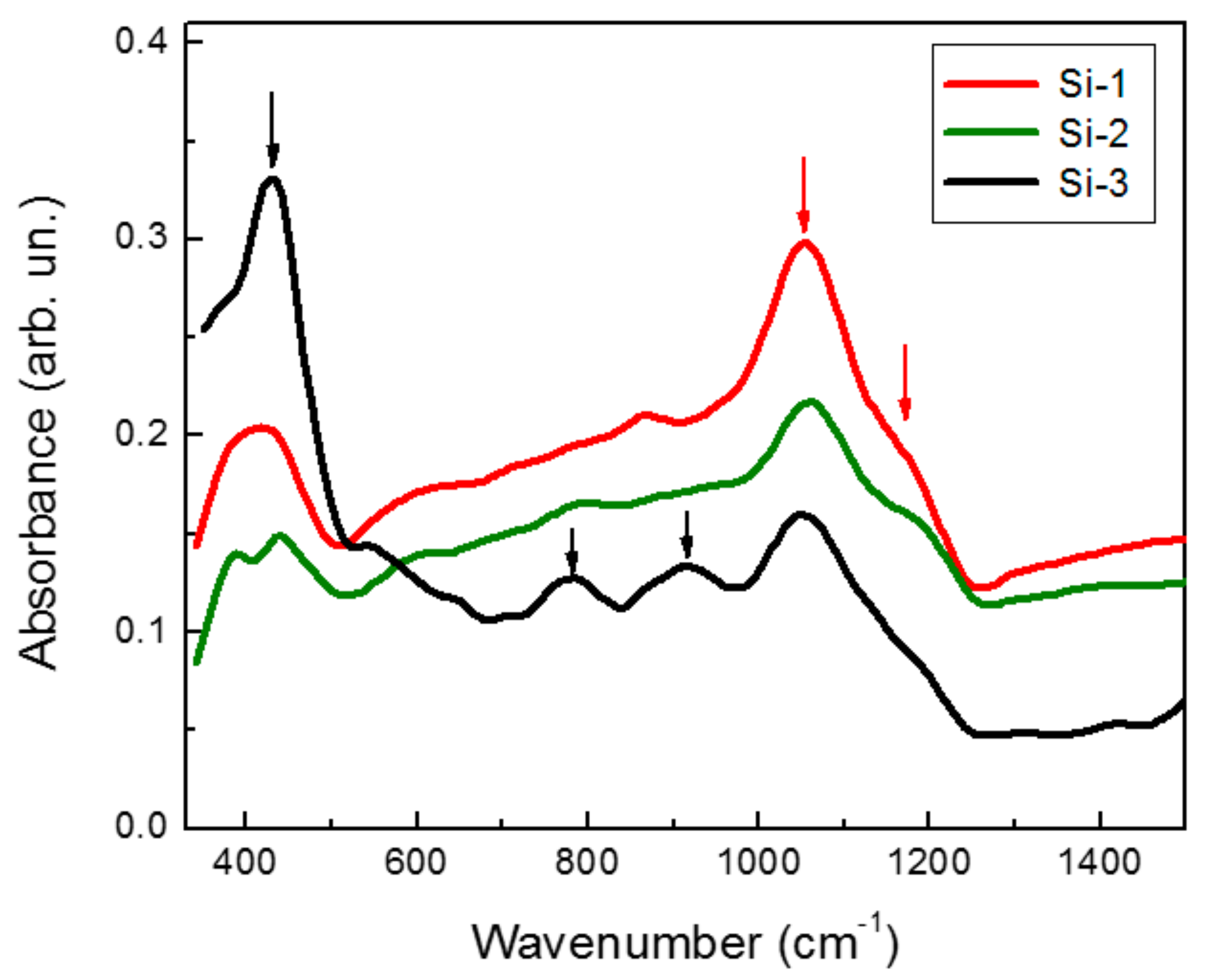
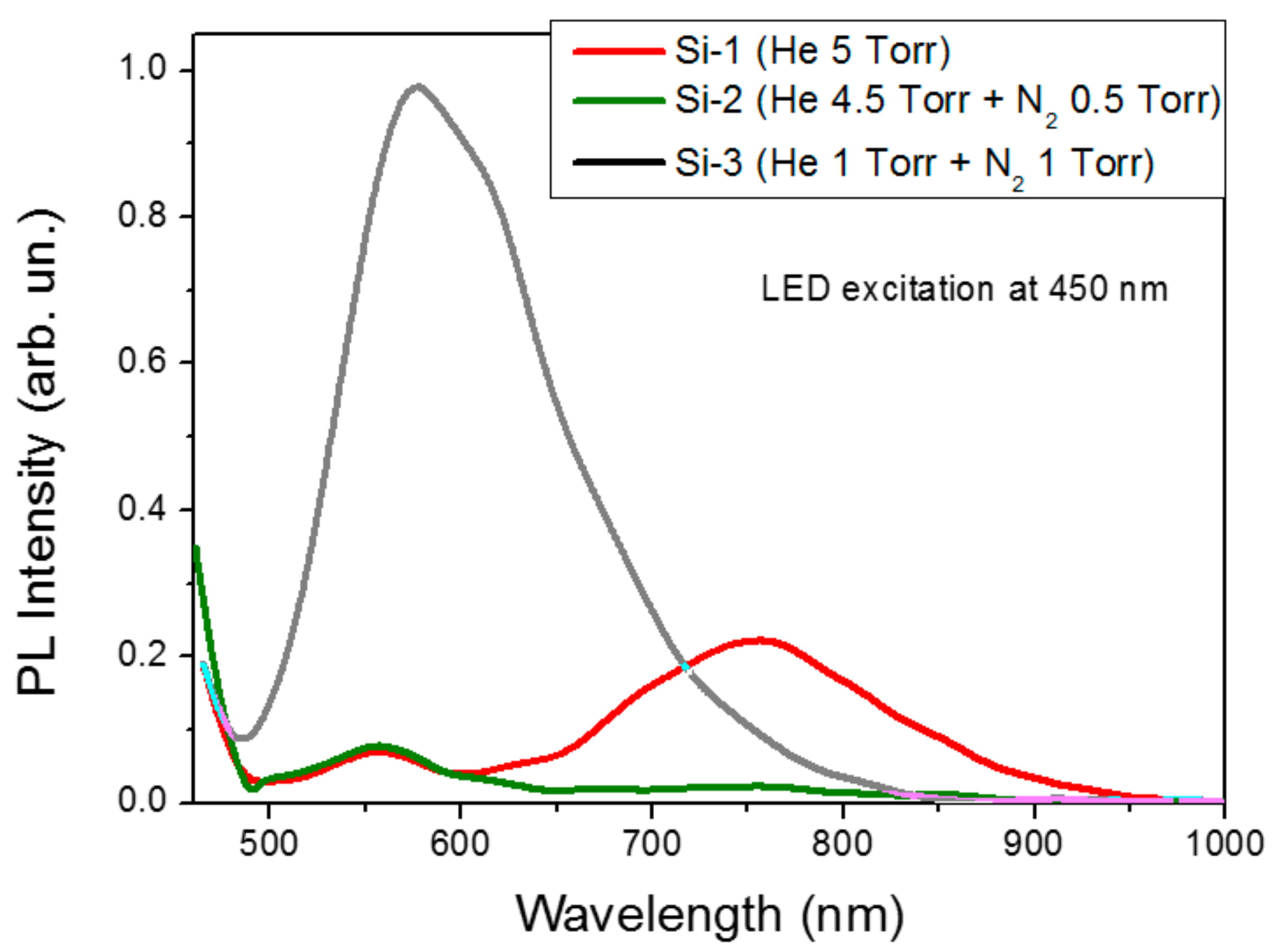
2. Results and Discussion
3. Materials and Methods
3.1. Materials
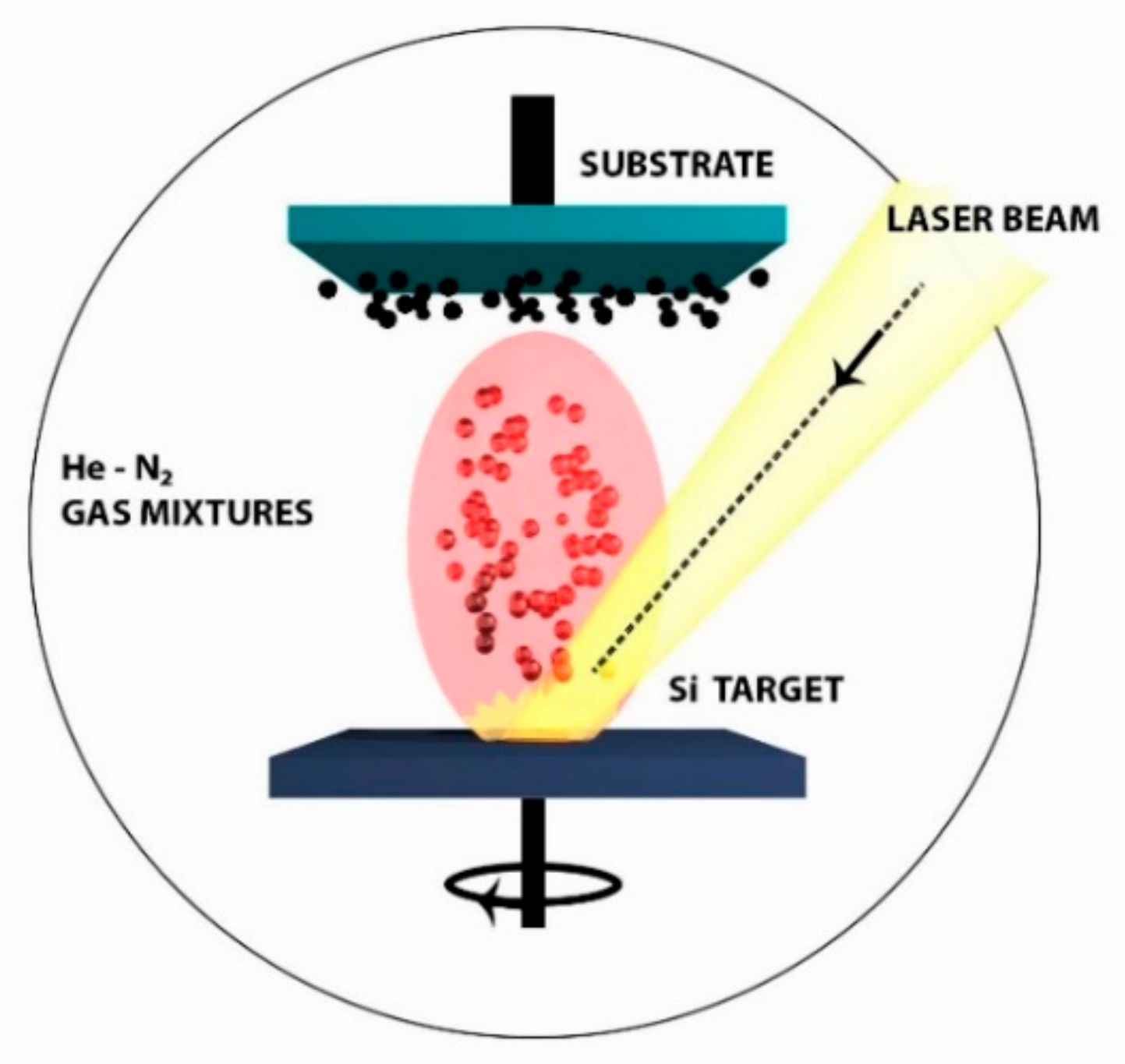
3.2. Pulsed Laser Ablation for the Deposition of Si-Based Nanostructured Films
3.3. Characterization of Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hull, R. Properties of Crystalline Silicon; Institution of Electrical Engineers: London, UK, 2006. [Google Scholar]
- Sailor, M.J. Porous Silicon in Practice Preparation, Characterization and Applications; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- He, Y. Silicon Nano-Biotechnology; Springer: Heidelberg, Germany, 2014. [Google Scholar]
- Canham, L.T. Bioactive Silicon Structure Fabrication Through Nanoetching Techniques. Adv. Mater. 1995, 7, 1033–1037. [Google Scholar] [CrossRef]
- Park, J.-H.; Gu, L.; von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable Luminescent Porous Silicon Nanoparticles for in Vivo Applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Baati, T.; Al-Kattan, A.; Esteve, M.A.; Njim, L.; Ryabchikov, Y.; Chaspoul, F.; Hammami, M.; Sentis, M.; Kabashin, A.V.; Braguer, D. Ultrapure Laser-Synthesized Si-Based Nanomaterials for Biomedical Applications: In Vivo Assessment of Safety and Biodistribution. Sci. Rep. 2016, 6, 25400. [Google Scholar] [CrossRef] [PubMed]
- Erogbogbo, F.; Yong, K.-T.; Roy, I.; Xu, G.; Prasad, P.N.; Swihart, M.T. Biocompatible Luminescent Silicon Quantum Dots for Imaging of Cancer Cells. ACS Nano 2008, 2, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Hall, D.J.; Qin, Z.; Anglin, E.; Joo, J.; Mooney, D.J.; Howell, S.B.; Sailor, M.J. In Vivo Time-Gated Fluorescence Imaging with Biodegradable Luminescent Porous Silicon Nanoparticles. Nat. Commun. 2013, 4, 2326. [Google Scholar] [CrossRef] [PubMed]
- Gongalsky, M.B.; Osminkina, L.A.; Pereira, A.; Manankov, A.A.; Fedorenko, A.A.; Vasiliev, A.N.; Solovyev, V.V.; Kudryavtsev, A.A.; Sentis, M.; Kabashin, A.V.; et al. Laser-Synthesized Oxide-Passivated Bright Si Quantum Dots for Bioimaging. Sci. Rep. 2016, 6, 24732. [Google Scholar] [CrossRef]
- Timoshenko, V.Y.; Kudryavtsev, A.A.; Osminkina, L.A.; Vorontsov, A.S.; Ryabchikov, Y.V.; Belogorokhov, I.A.; Kovalev, D.; Kashkarov, P.K. Silicon Nanocrystals as Photosensitizers of Active Oxygen for Biomedical Applications. JETP Lett. 2006, 83, 423–426. [Google Scholar] [CrossRef]
- Lee, C.; Kim, H.; Hong, C.; Kim, M.; Hong, S.S.; Lee, D.H.; Lee, W.I. Porous Silicon as an Agent for Cancer Thermotherapy Based on near-Infrared Light Irradiation. J. Mater. Chem. 2008, 18, 4790–4795. [Google Scholar] [CrossRef]
- Tamarov, K.P.; Osminkina, L.A.; Zinovyev, S.V.; Maximova, K.A.; Kargina, J.V.; Gongalsky, M.B.; Ryabchikov, Y.; Al-Kattan, A.; Sviridov, A.P.; Sentis, M.; et al. Radio Frequency Radiation-Induced Hyperthermia Using Si Nanoparticle-Based Sensitizers for Mild Cancer Therapy. Sci. Rep. 2014, 4, 7034. [Google Scholar] [CrossRef]
- Sviridov, A.P.; Andreev, V.G.; Ivanova, E.M.; Osminkina, L.A.; Tamarov, K.P.; Timoshenko, V.Y. Porous Silicon Nanoparticles as Sensitizers for Ultrasonic Hyperthermia. Appl. Phys. Lett. 2013, 103, 193110. [Google Scholar] [CrossRef]
- Petriev, V.M.; Tischenko, V.K.; Mikhailovskaya, A.A.; Popov, A.A.; Tselikov, G.; Zelepukin, I.; Deyev, S.M.; Kaprin, A.D.; Ivanov, S.; Timoshenko, V.Y.; et al. Nuclear Nanomedicine Using Si Nanoparticles as Safe and Effective Carriers of 188Re Radionuclide for Cancer Therapy. Sci. Rep. 2019, 9, 2017. [Google Scholar] [CrossRef] [PubMed]
- Delerue, C.; Lannoo, M.; Allan, G. Excitonic and Quasiparticle Gaps in Si Nanocrystals. Phys. Rev. Lett. 2000, 84, 2457–2460. [Google Scholar] [CrossRef]
- Sangghaleh, F.; Sychugov, I.; Yang, Z.; Veinot, J.G.C.; Linnros, J. Near-Unity Internal Quantum Efficiency of Luminescent Silicon Nanocrystals with Ligand Passivation. ACS Nano 2015, 9, 7097–7104. [Google Scholar] [CrossRef]
- Li, Z.F.; Ruckenstein, E. Water-Soluble Poly(acrylic Acid) Grafted Luminescent Silicon Nanoparticles and Their Use as Fluorescent Biological Staining Labels. Nano Lett. 2004, 4, 1463–1467. [Google Scholar] [CrossRef]
- Koynov, S.; Pereira, R.N.; Crnolatac, I.; Kovalev, D.; Huygens, A.; Chirvony, V.; Stutzmann, M.; de Witte, P. Purification of Nano-Porous Silicon for Biomedical Applications. Adv. Eng. Mater. 2011, 13, B225–B233. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Swihart, M.T. Surface Functionalization of Silicon Nanoparticles Produced by Laser-Driven Pyrolysis of Silane followed by HF-HNO3 Etching. Langmuir 2004, 20, 4720–4727. [Google Scholar] [CrossRef] [PubMed]
- Mangolini, L.; Thimsen, E.; Kortshagen, U. High-yield Plasma Synthesis of Luminescent Silicon Nanocrystals. Nano Lett. 2005, 5, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Kabashin, A.V.; Timoshenko, V.Y. What Theranostic Applications Could Ultrapure Laser-Synthesized Si Nanoparticles Have in Cancer? Nanomedicine 2016, 11, 2247–2250. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Singh, A.; Swihart, M.T.; Zavestovskaya, I.N.; Prasad, P.N. Laser-Processed Nanosilicon: A Multifunctional Nanomaterial for Energy and Healthcare. ACS Nano 2019, 13, 9841–9867. [Google Scholar] [CrossRef] [PubMed]
- Geohegan, D.B.; Puretzky, A.A.; Duscher, G.; Pennycook, S.J. Photoluminescence from Gas-Suspended SiOx Nanoparticles Synthesized by Laser Ablation. Appl. Phys. Lett. 1998, 73, 438–440. [Google Scholar] [CrossRef]
- Itina, T.E.; Gouriet, K.; Zhigilei, L.V.; Noel, S.; Hermann, J.; Sentis, M. Mechanisms of small clusters production by short and ultra-short laser ablation, Appl. Surf. Sci. 2007, 253, 7656–7661. [Google Scholar] [CrossRef]
- Itina, T.E. On nanoparticle formation by laser ablation in liquids. J. Phys. Chem. C 2010, 115, 5044–5048. [Google Scholar] [CrossRef]
- Rioux, D.; Laferriere, M.; Douplik, A.; Shah, D.; Lilge, L.; Kabashin, A.V.; Meunier, M.M. Silicon Nanoparticles Produced by Femtosecond Laser Ablation in Water as Novel Contamination-Free Photosensitizers. J. Biomed. Opt. 2009, 14, 021010. [Google Scholar] [CrossRef] [PubMed]
- Intartaglia, R.; Barchanski, A.; Bagga, K.; Genovese, A.; Das, G.; Wagener, P.; Di Fabrizio, E.; Diaspro, A.; Brandi, F.; Barcikowski, S. Bioconjugated silicon quantum dots from one-step green synthesis. Nanoscale 2012, 4, 1271–1274. [Google Scholar] [CrossRef]
- Al-Kattan, A.; Ryabchikov, Y.V.; Baati, T.; Chirvony, V.; Sánchez-Royo, J.F.; Sentis, M.; Braguer, D.; Timoshenko, V.Y.; Estève, M.-A.; Kabashin, A.V. Ultrapure Laser-Synthesized Si Nanoparticles with Variable Oxidation States for Biomedical Applications. J. Mater. Chem. B 2016, 4, 7852–7858. [Google Scholar] [CrossRef]
- Kharin, A.Y.; Lysenko, V.V.; Rogov, A.; Ryabchikov, Y.V.; Geloen, A.; Tishchenko, I.; Marty, O.; Sennikov, P.G.; Kornev, R.A.; Zavestovskaya, I.N.; et al. Bi-Modal Nonlinear Optical Contrast from Si Nanoparticles for Cancer Theranostics. Adv. Opt. Mater. 2019, 7, 1801728. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Meunier, M. Visible Photoluminescence from Nanostructured Si-Based Layers Produced by Air Optical Breakdown on Silicon. Appl. Phys. Lett. 2003, 82, 1619–1621. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Meunier, M. Laser-Induced Treatment of Silicon in Air and Formation of Si/SiOx Photoluminescent Nanostructured Layers. Mater. Sci. Eng. B 2003, 101, 60–64. [Google Scholar] [CrossRef]
- Patrone, L.; Nelson, D.; Safarov, V.I.; Sentis, M.; Marine, W.; Giorgio, S. Photoluminescence of Silicon Nanoclusters with Reduced Size Dispersion Produced by Laser Ablation. J. Appl. Phys. 2000, 87, 3829–3837. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Meunier, M.; Leonelli, R. Photoluminescence Characterization of Si-Based Nanostructured Films Produced by Pulsed Laser Ablation. J. Vac. Sci. Technol. B 2001, 19, 2217–2222. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Sylvestre, J.-P.; Patskovsky, S.; Meunier, M. Correlation between Photoluminescence Properties and Morphology of Laser-Ablated Si/SiOx Nanostructured Films. J. Appl. Phys. 2002, 91, 3248–3254. [Google Scholar] [CrossRef]
- Richter, H.; Wang, Z.P.; Ley, L. The one phonon Raman spectrum in microcrystalline silicon. Solid State Comm. 1981, 39, 625–629. [Google Scholar] [CrossRef]
- Alykova, A.F.; Zavestovskaya, I.N.; Yakunin, V.G.; Timoshenko, V.Y. Raman Diagnostics of Silicon Nanocrystals Dissolution in Aqueous Medium. J. Phys. Conf. Ser. 2018, 945, 012002. [Google Scholar] [CrossRef]
- Degenhardt, U.; Stegner, F.; Liebscher, C.; Glatzel, U.; Berroth, K.; Krenkel, W.; Motza, G. Sintered Silicon Nitride/Nano-Silicon Carbide Materials Based on Preceramic Polymers and Ceramic Powder. J. Europ. Ceram. Soc. 2012, 32, 1893–1899. [Google Scholar] [CrossRef]
- Oliphanta, C.J.; Arendsea, C.J.; Mullera, T.F.G.; Knoesena, D. Characterization of Silicon Nitride Thin Films Deposited by Hot-wire CVD at Low Gas Flow Rates. Appl. Surf. Sci. 2013, 285P, 440–449. [Google Scholar] [CrossRef][Green Version]
- Del Prado, A.; Martil, I.; Fernandez, M.; Ganzalez-Diaz, G. Full Composition Range Silicon Oxynitride Films Deposited by ECR-PECVD at Room Temperature. Thin Solid Films 1999, 343–344, 437–440. [Google Scholar] [CrossRef]
- Szepvolgy, J.; Mohai, I.; Gubicza, J. Atmospheric Aging of Nanosized Silicon Nitride Powder. J. Mat. Chem. 2001, 11, 859–863. [Google Scholar] [CrossRef][Green Version]
- Rebib, F.; Tomasella, E.; Bêche, E.; Cellier, J.; Jacquet, M. FTIR and XPS Investigations of a-SiOxNy Thin Films Structure. J. Phys. Conf. Ser. 2008, 100, 082034. [Google Scholar] [CrossRef]
- Mohamed, H. FTIR and Spectroscopic Ellipsometry Iinvestigations of the Electron Beam Evaporated Silicon Oxynitride Thin Films. Physica B 2011, 406, 211–215. [Google Scholar] [CrossRef]
- Kistner, J.; Chen, X.; Weng, Y.; Strunk, H.P.; Schubert, M.B.; Werner, J.H. Photoluminescence from Silicon Nitride—No Quantum Effect. J. Appl. Phys. 2011, 110, 023520. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Wu, Y.; Wang, S.; Ge, X. High Photoluminescence Quantum Yields Generated from N-Si-O Bonding States in Amorphous Silicon Oxynitride Films. Opt. Express 2018, 26, 31617. [Google Scholar] [CrossRef] [PubMed]
- Morozov, M.O.; Zavestovskaya, I.N.; Kabashin, A.V.; Timoshenko, V.Y. Photoluminescence Properties of Silicon Nanocrystals Grown by Nanosecond Laser Ablation of Solid-state Targets in an Inert Gas Atmosphere, Bull. Lebedev Phys. Inst. 2017, 44, 353–356. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Notation of Sample | PHe, Torr | PN2, Torr | R = PN2/PHe |
|---|---|---|---|
| Si-1 | 5.0 | 0 | 0 |
| Si-2 | 5.0 | 0.5 | 0.1 |
| Si-3 | 1.0 | 1.0 | 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fronya, A.A.; Antonenko, S.V.; Kharin, A.Y.; Muratov, A.V.; Aleschenko, Y.A.; Derzhavin, S.I.; Karpov, N.V.; Dombrovska, Y.I.; Garmash, A.A.; Kargin, N.I.; et al. Tailoring Photoluminescence from Si-Based Nanocrystals Prepared by Pulsed Laser Ablation in He-N2 Gas Mixtures. Molecules 2020, 25, 440. https://doi.org/10.3390/molecules25030440
Fronya AA, Antonenko SV, Kharin AY, Muratov AV, Aleschenko YA, Derzhavin SI, Karpov NV, Dombrovska YI, Garmash AA, Kargin NI, et al. Tailoring Photoluminescence from Si-Based Nanocrystals Prepared by Pulsed Laser Ablation in He-N2 Gas Mixtures. Molecules. 2020; 25(3):440. https://doi.org/10.3390/molecules25030440
Chicago/Turabian StyleFronya, Anastasiya A., Sergey V. Antonenko, Alexander Yu. Kharin, Andrei V. Muratov, Yury A. Aleschenko, Sergey I. Derzhavin, Nikita V. Karpov, Yaroslava I. Dombrovska, Alexander A. Garmash, Nikolay I. Kargin, and et al. 2020. "Tailoring Photoluminescence from Si-Based Nanocrystals Prepared by Pulsed Laser Ablation in He-N2 Gas Mixtures" Molecules 25, no. 3: 440. https://doi.org/10.3390/molecules25030440
APA StyleFronya, A. A., Antonenko, S. V., Kharin, A. Y., Muratov, A. V., Aleschenko, Y. A., Derzhavin, S. I., Karpov, N. V., Dombrovska, Y. I., Garmash, A. A., Kargin, N. I., Klimentov, S. M., Timoshenko, V. Y., & Kabashin, A. V. (2020). Tailoring Photoluminescence from Si-Based Nanocrystals Prepared by Pulsed Laser Ablation in He-N2 Gas Mixtures. Molecules, 25(3), 440. https://doi.org/10.3390/molecules25030440






