Manipulation of the Size and Phase Composition of Yttrium Iron Garnet Nanoparticles by Pulsed Laser Post-Processing in Liquid
Abstract
1. Introduction
2. Results and Discussion
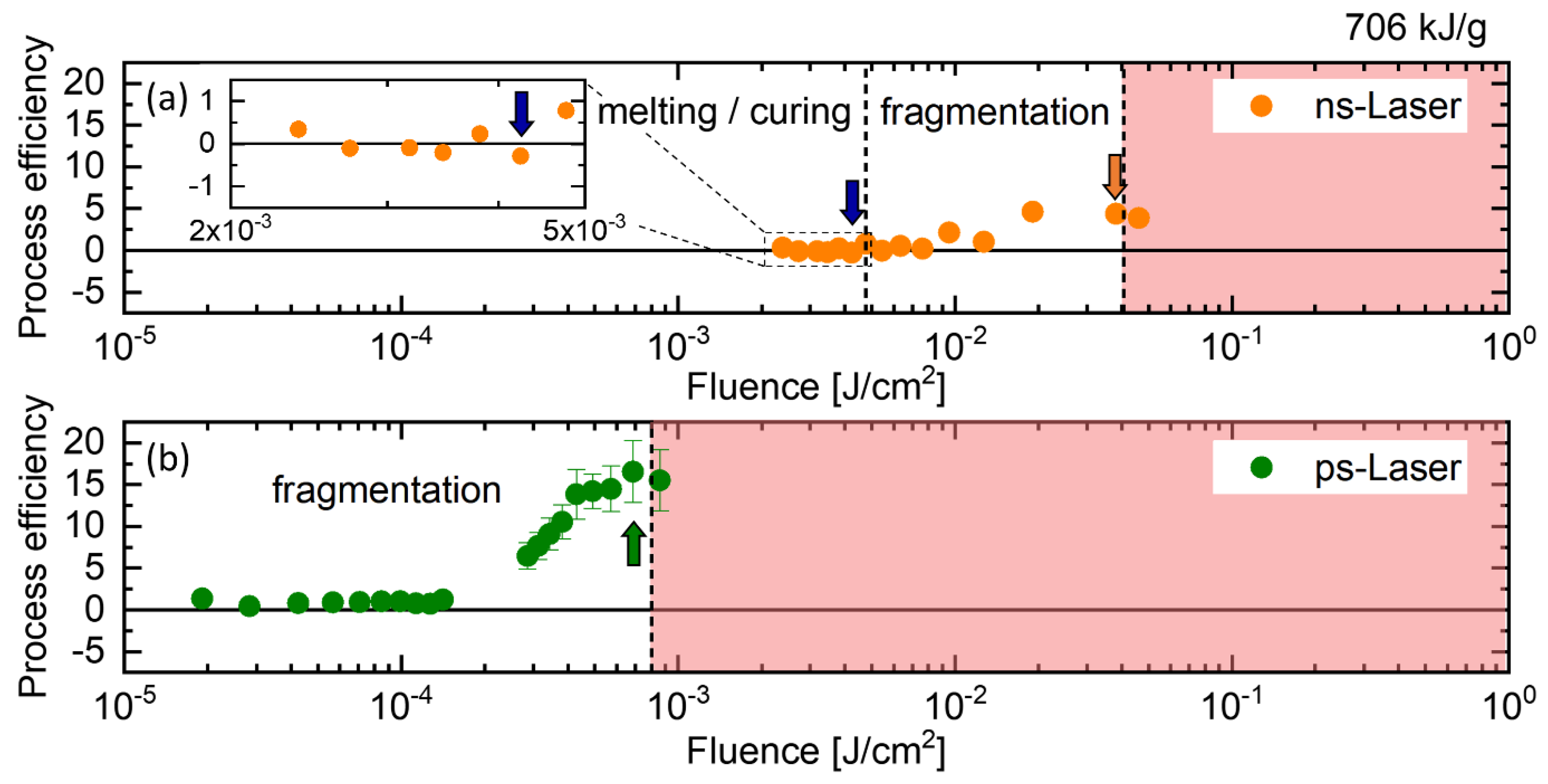
2.1. Nanoparticle Size Modification
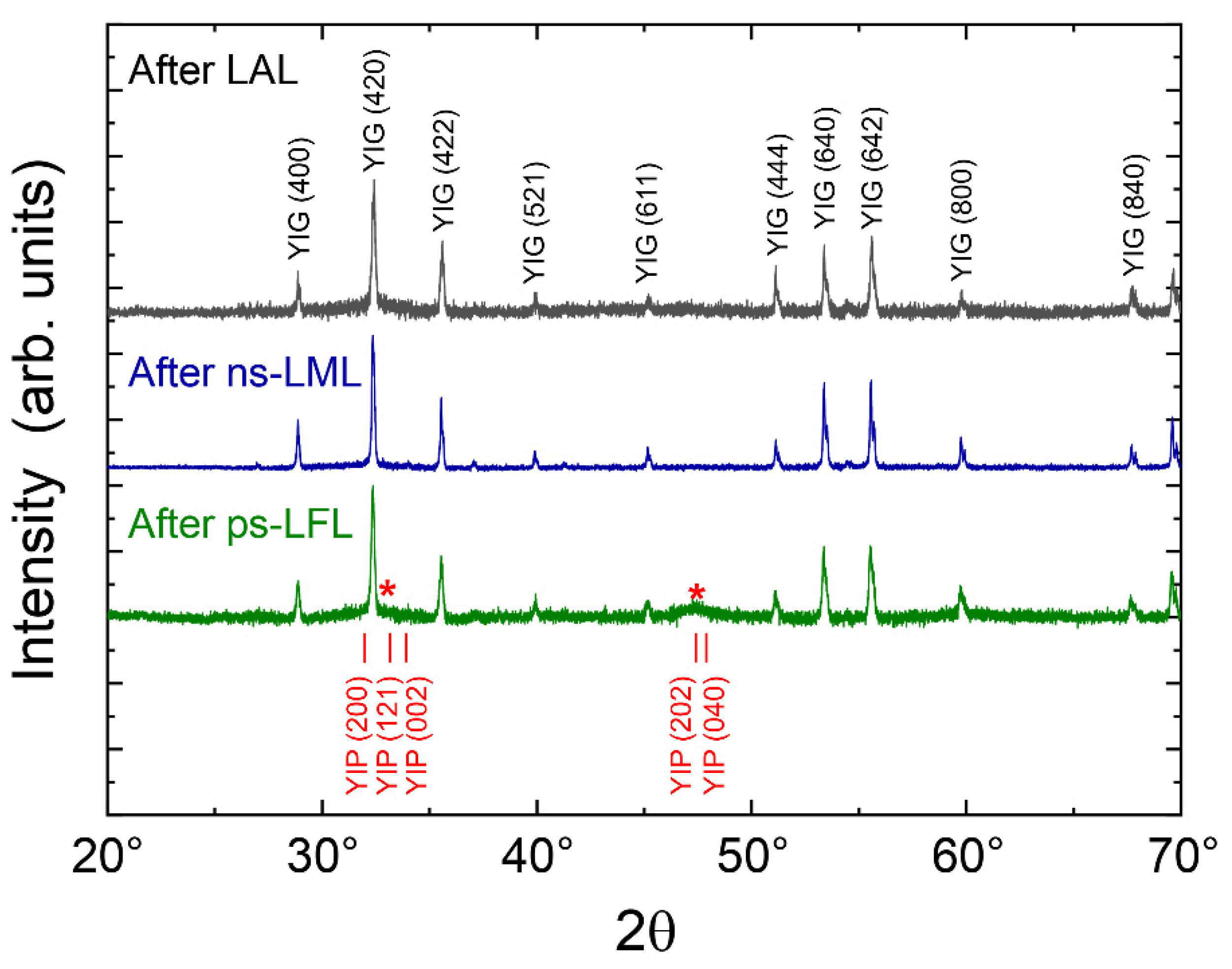
2.2. Structural Analysis
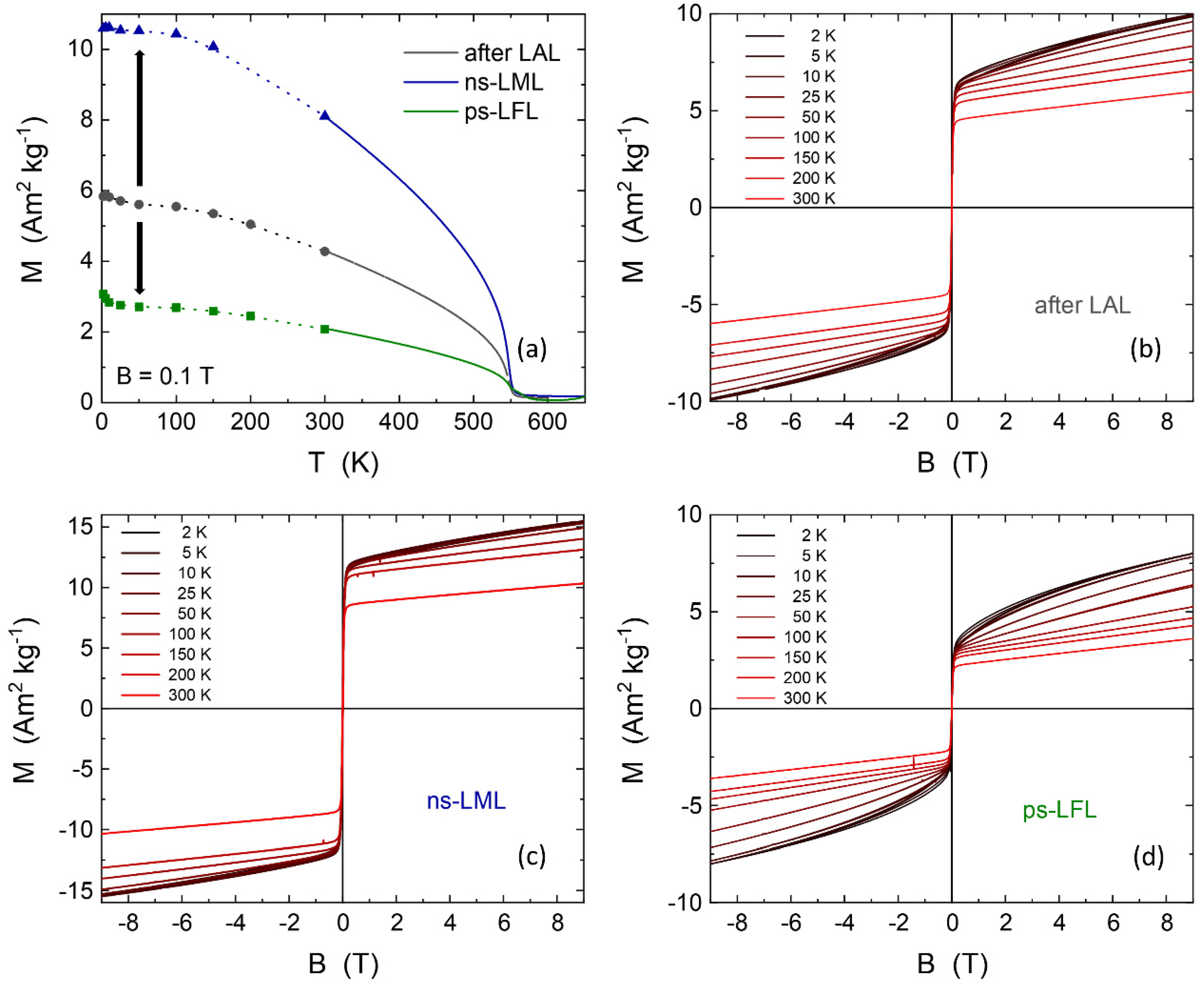
2.3. Magnetic Properties
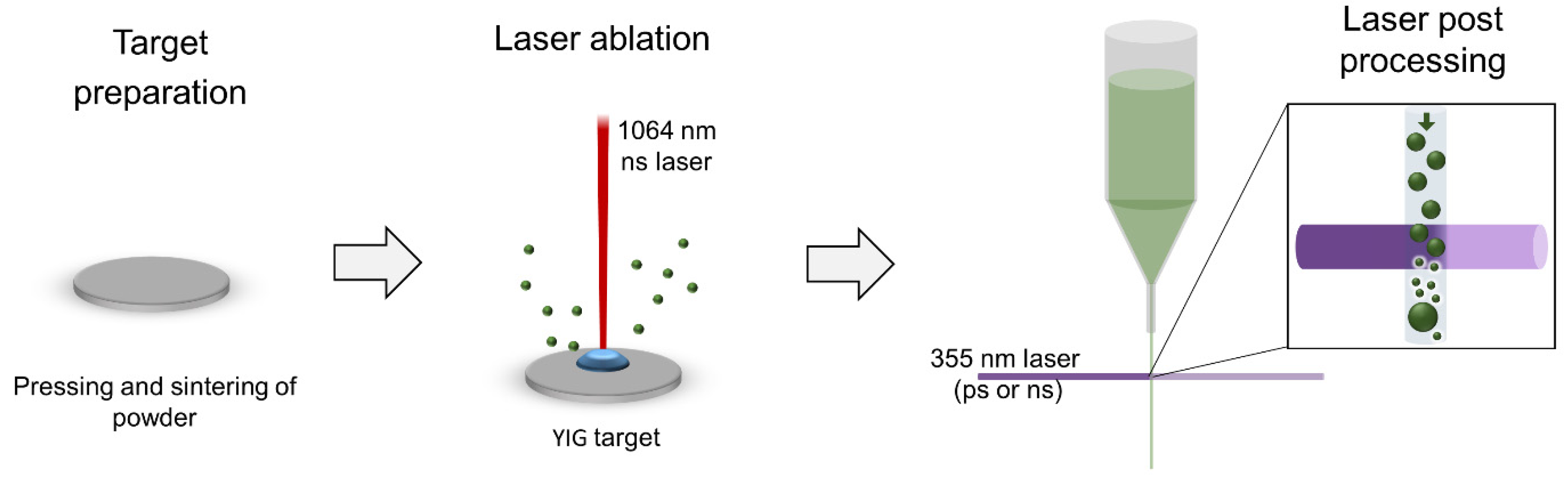
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pizzolato, E.; Scaramuzza, S.; Carraro, F.; Sartori, A.; Agnoli, S.; Amendola, V.; Bonchio, M.; Sartorel, A. Water oxidation electrocatalysis with iron oxide nanoparticles prepared via laser ablation. J. Energy Chem. 2016, 25, 246–250. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, C.; Roy, V.A.L. Recent progress in magnetic iron oxide-semiconductor composite nanomaterials as promising photocatalysts. Nanoscale 2015, 7, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Rosenweing, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Hu, Y.; Mignani, S.; Majoral, J.P.; Shen, M.; Shi, X. Construction of iron oxide nanoparticle-based hybrid platforms for tumor imaging and therapy. Chem. Soc. Rev. 2018, 47, 1874–1900. [Google Scholar] [CrossRef]
- Prasanna, S.R.V.S.; Balaji, K.; Pandey, S.; Rana, S. Metal Oxide Based Nanomaterials and Their Polymer Nanocomposites. Nanomater. Polym. Nanocompos. 2019, 123–144. [Google Scholar] [CrossRef]
- Streubel, R.; Wilms, M.B.; Doñate-Buendía, C.; Weisheit, A.; Barcikowski, S.; Schleifenbaum, J.H.; Gökce, B. Depositing laser-generated nanoparticles on powders for additive manufacturing of oxide dispersed strengthened alloy parts via laser metal deposition. Jpn. J. Appl. Phys. 2018, 57. [Google Scholar] [CrossRef]
- Wehner, M.; Truby, R.L.; Fitzgerald, D.J.; Mosadegh, B.; Whitesides, G.M.; Lewis, J.A.; Wood, R.J. An integrated design and fabrication strategy for entirely soft, autonomous robots. Nature 2016, 536, 451–455. [Google Scholar] [CrossRef]
- Kim, Y.; Yuk, H.; Zhao, R.; Chester, S.A.; Zhao, X. Printing ferromagnetic domains for untethered fast-transforming soft materials. Nature 2018, 558, 274–279. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Q.; Yao, Y.; Liu, L.; Liu, Y.; Leng, J. Direct-write fabrication of 4D active shape-changing structures based on a shape memory polymer and its nanocomposite. ACS Appl. Mater. Interfaces 2017, 9, 876–883. [Google Scholar] [CrossRef]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef] [PubMed]
- Streubel, R.; Barcikowski, S.; Gökce, B. Continuous multigram nanoparticle synthesis by high-power, high-repetition-rate ultrafast laser ablation in liquids. Opt. Lett. 2016, 41, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Jendrzej, S.; Gökce, B.; Epple, M.; Barcikowski, S. How Size Determines the Value of Gold: Economic Aspects of Wet Chemical and Laser-Based Metal Colloid Synthesis. ChemPhysChem 2017, 18, 1012–1019. [Google Scholar] [CrossRef]
- Rehbock, C.; Jakobi, J.; Gamrad, L.; van der Meer, S.; Tiedemann, D.; Taylor, U.; Kues, W.; Rath, D.; Barcikowski, S. Current state of laser synthesis of metal and alloy nanoparticles as ligand-free reference materials for nano-toxicological assays. Beilstein J. Nanotechnol. 2014, 5, 1523–1541. [Google Scholar] [CrossRef] [PubMed]
- Yang, G. Laser ablation in liquids: Applications in the synthesis of nanocrystals. Prog. Mater. Sci. 2007, 52, 648–698. [Google Scholar] [CrossRef]
- Barcikowski, S.; Baranowski, T.; Durmus, Y.; Wiedwald, U.; Gökce, B. Solid solution magnetic FeNi nanostrand–polymer composites by connecting-coarsening assembly. J. Mater. Chem. C 2015, 3, 10699–10704. [Google Scholar] [CrossRef]
- Kohsakowski, S.; Gökce, B.; Tanabe, R.; Wagener, P.; Plech, A.; Ito, Y.; Barcikowski, S. Target geometry and rigidity determines laser-induced cavitation bubble transport and nanoparticle productivity – a high-speed videography study. Phys. Chem. Chem. Phys. 2016, 18, 16585–16593. [Google Scholar] [CrossRef]
- Wagener, P.; Jakobi, J.; Rehbock, C.; Chakravadhanula, V.S.K.; Thede, C.; Wiedwald, U.; Bartsch, M.; Kienle, L.; Barcikowski, S. Solvent-surface interactions control the phase structure in laser-generated iron-gold core-shell nanoparticles. Sci. Rep. 2016, 6, 23352. [Google Scholar] [CrossRef]
- Zhang, D.; Gökce, B.; Notthoff, C.; Barcikowski, S. Layered Seed-Growth of AgGe Football-like Microspheres via Precursor-Free Picosecond Laser Synthesis in Water. Sci. Rep. 2015, 5, 13661. [Google Scholar] [CrossRef]
- Hu, S.; Tian, M.; Ribeiro, E.L.; Duscher, G.; Mukherjee, D. Tandem laser ablation synthesis in solution-galvanic replacement reaction (LASiS-GRR) for the production of PtCo nanoalloys as oxygen reduction electrocatalysts. J. Power Sources 2016, 306, 413–423. [Google Scholar] [CrossRef]
- Zhang, J.; Oko, D.N.; Garbarino, S.; Imbeault, R.; Chaker, M.; Tavares, A.C.; Guay, D.; Ma, D. Preparation of PtAu alloy colloids by laser ablation in solution and their characterization. J. Phys. Chem. C 2012, 116, 13413–13420. [Google Scholar] [CrossRef]
- Marzun, G.; Levish, A.; Mackert, V.; Kallio, T.; Barcikowski, S.; Wagener, P. Laser synthesis, structure and chemical properties of colloidal nickel-molybdenum nanoparticles for the substitution of noble metals in heterogeneous catalysis. J. Colloid Interface Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Neumeister, A.; Jakobi, J.; Rehbock, C.; Moysig, J.; Barcikowski, S. Monophasic ligand-free alloy nanoparticle synthesis determinants during pulsed laser ablation of bulk alloy and consolidated microparticles in water. Phys. Chem. Chem. Phys. 2014, 16, 23671–23678. [Google Scholar] [CrossRef] [PubMed]
- Stelzig, S.H.; Menneking, C.; Hoffmann, M.S.; Eisele, K.; Barcikowski, S.; Klapper, M.; Müllen, K. Compatibilization of laser generated antibacterial Ag- and Cu-nanoparticles for perfluorinated implant materials. Eur. Polym. J. 2011, 47, 662–667. [Google Scholar] [CrossRef]
- Zhang, D.; Gökce, B. Perspective of laser-prototyping nanoparticle-polymer composites. Appl. Surf. Sci. 2017, 392, 991–1003. [Google Scholar] [CrossRef]
- Jakobi, J.; Petersen, S.; Menéndez-Manjón, A.; Wagener, P.; Barcikowski, S. Magnetic alloy nanoparticles from laser ablation in cyclopentanone and their embedding into a photoresist. Langmuir 2010, 26, 6892–6897. [Google Scholar] [CrossRef]
- Menéndez-Manjón, A.; Schwenke, A.; Steinke, T.; Meyer, M.; Giese, U.; Wagener, P.; Barcikowski, S. Ligand-free gold-silver nanoparticle alloy polymer composites generated by picosecond laser ablation in liquid monomer. Appl. Phys. A Mater. Sci. Process 2013, 110, 343–350. [Google Scholar] [CrossRef]
- Lau, M.; Waag, F.; Barcikowski, S. Direct Integration of Laser-Generated Nanoparticles into Transparent Nail Polish: The Plasmonic “goldfinger”. Ind. Eng. Chem. Res. 2017, 56, 3291–3296. [Google Scholar] [CrossRef]
- Amans, D.; Malaterre, C.; Diouf, M.; Mancini, C.; Chaput, F.; Ledoux, G.; Breton, G.; Guillin, Y.; Dujardin, C.; Masenelli-Varlot, K.; et al. Synthesis of Oxide Nanoparticles by Pulsed Laser Ablation in Liquids Containing a Complexing Molecule: Impact on Size Distributions and Prepared Phases. J. Phys. Chem. C 2011, 115, 5131–5139. [Google Scholar] [CrossRef]
- Schmitz, T.; Wiedwald, U.; Dubs, C.; Gökce, B. Ultrasmall Yttrium Iron Garnet Nanoparticles with High Coercivity at Low Temperature Synthesized by Laser Ablation and Fragmentation of Pressed Powders. ChemPhysChem 2017, 18, 1125–1132. [Google Scholar] [CrossRef]
- Chemin, A.; Lam, J.; Laurens, G.; Trichard, F.; Motto-Ros, V.; Ledoux, G.; Jarý, V.; Laguta, V.; Nikl, M.; Dujardin, C.; et al. Doping nanoparticles using pulsed laser ablation in a liquid containing the doping agent. Nanoscale Adv. 2019. [Google Scholar] [CrossRef]
- Wang, H.; Odawara, O.; Wada, H. Facile and Chemically Pure Preparation of YVO 4: Eu 3 + Colloid with Novel Nanostructure via Laser Ablation in Water. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Adam, J.D.; Davis, L.E.; Dionne, G.F.; Schloemann, E.F.; Stitzer, S.N. Ferrite devices and materials. IEEE Trans. Microw. Theory Tech. 2002, 50, 721–737. [Google Scholar] [CrossRef]
- Fu, H.P.; Hong, R.Y.; Wu, Y.J.; Di, G.Q.; Xu, B.; Zheng, Y.; Wei, D.G. Preparation and Faraday rotation of Bi-YIG/PMMA nanocomposite. J. Magn. Magn. Mater. 2008, 320, 2584–2590. [Google Scholar] [CrossRef]
- Kim, T.Y.; Yamazaki, Y.; Hirano, T. Magneto-optical properties of Bi-YIG nanoparticle with polymethacrylate matrix materials. Phys. Status Solidi Basic Res. 2004, 241, 1601–1604. [Google Scholar] [CrossRef]
- Kucera, M.; Bok, J.; Nitsch, K. Faraday rotation and MCD in Ce doped yig. Solid State Commun. 1989, 69, 1117–1121. [Google Scholar] [CrossRef]
- Serga, A.A.; Chumak, A.V.; Hillebrands, B. YIG magnonics. J. Phys. D Appl. Phys. 2010, 43, 264002. [Google Scholar] [CrossRef]
- Doñate-Buendía, C.; Frömel, F.; Wilms, M.B.; Streubel, R.; Tenkamp, J.; Hupfeld, T.; Nachev, M.; Gökce, E.; Weisheit, A.; Barcikowski, S.; et al. Oxide dispersion-strengthened alloys generated by laser metal deposition of laser-generated nanoparticle-metal powder composites. Mater. Des. 2018, 154, 360–369. [Google Scholar] [CrossRef]
- Shen, H.; Xu, J.; Wu, A.; Zhao, J.; Shi, M. Magnetic and thermal properties of perovskite YFeO3 single crystals. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2009, 157, 77–80. [Google Scholar] [CrossRef]
- Rajendran, M.; Deka, S.; Joy, P.A.; Bhattacharya, A.K. Size-dependent magnetic properties of nanocrystalline yttrium iron garnet powders. J. Magn. Magn. Mater. 2006, 301, 212–219. [Google Scholar] [CrossRef]
- Barcikowski, S.; Amendola, V.; Marzun, G.; Rehbock, C.; Reichenberger, S.; Zhang, D.; Gökce, B. Handbook of Laser Synthesis of Colloids; DueEPublico: Duisburg-Essen, Germany, 2016. [Google Scholar] [CrossRef]
- Letzel, A.; Reich, S.; Rolo, T.D.S.; Kanitz, A.; Hoppius, J.; Rack, A.; Olbinado, M.P.; Ostendorf, A.; Gökce, B.; Plech, A.; et al. Time and Mechanism of Nanoparticle Functionalization by Macromolecular Ligands during Pulsed Laser Ablation in Liquids. Langmuir 2019, 35, 3038–3047. [Google Scholar] [CrossRef] [PubMed]
- Letzel, A.; Gökce, B.; Wagener, P.; Ibrahimkutty, S.; Menzel, A.; Plech, A.; Barcikowski, S. Size Quenching during Laser Synthesis of Colloids Happens Already in the Vapor Phase of the Cavitation Bubble. J. Phys. Chem. C 2017, 121, 5356–5365. [Google Scholar] [CrossRef]
- Merk, V.; Rehbock, C.; Becker, F.; Hagemann, U.; Nienhaus, H.; Barcikowski, S. In situ non-DLVO stabilization of surfactant-free, plasmonic gold nanoparticles: Effect of Hofmeister’s anions. Langmuir 2014, 30, 4213–4222. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.; Barcikowski, S. Quantification of mass-specific laser energy input converted into particle properties during picosecond pulsed laser fragmentation of zinc oxide and boron carbide in liquids. Appl. Surf. Sci. 2015, 348, 22–29. [Google Scholar] [CrossRef]
- Jendrzej, S.; Gökce, B.; Barcikowski, S. Colloidal Stability of Metal Nanoparticles in Engine Oil under Thermal and Mechanical Load. Chem. Eng. Technol. 2017, 40, 1569–1576. [Google Scholar] [CrossRef]
- Gökce, B.; Zand, D.D.V.; Menéndez-Manjón, A.; Barcikowski, S. Ripening kinetics of laser-generated plasmonic nanoparticles in different solvents. Chem. Phys. Lett. 2015, 626, 96–101. [Google Scholar] [CrossRef]
- Waag, F.; Gökce, B.; Kalapu, C.; Bendt, G.; Salamon, S.; Landers, J.; Hagemann, U.; Heidelmann, M.; Schulz, S.; Wende, H.; et al. Adjusting the catalytic properties of cobalt ferrite nanoparticles by pulsed laser fragmentation in water with defined energy dose. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Sakaki, S.; Saitow, K.I.; Sakamoto, M.; Wada, H.; Swiatkowska-Warkocka, Z.; Ishikawa, Y.; Koshizaki, N. Comparison of picosecond and nanosecond lasers for the synthesis of TiN sub-micrometer spherical particles by pulsed laser melting in liquid. Appl. Phys. Express 2018, 11, 4–8. [Google Scholar] [CrossRef]
- Sakaki, S.; Ishikawa, Y.; Koshizaki, N. Heating process control of pulsed-laser melting in liquid via a burst-mode laser. Appl. Phys. Express 2019, 12. [Google Scholar] [CrossRef]
- Zhang, G.B.; Lau, D.; Lu, M.; Barcikowski, S.; Gökce, B. Germanium Sub-Microspheres Synthesized by Picosecond Pulsed Laser Melting in Liquids: Educt Size Effects. Sci. Rep. 2017, 7, 40355. [Google Scholar] [CrossRef]
- Widatallah, H.M.; Johnson, C.; Al-Harthi, S.H.; Gismelseed, A.M.; Al-Rawas, A.D.; Stewart, S.J.; Elzain, M.E.; Al-Omari, I.A.; Yousif, A.A. A structural and mössbauer study of Y3Fe5O12 nanoparticles prepared with high energy ball milling and subsequent sintering. Hyperfine Interact. 2008, 183, 87–92. [Google Scholar] [CrossRef]
- Niyaifar, M.; Mohammadpour, H.; Dorafshani, M.; Hasanpour, A. Size dependence of non-magnetic thickness in YIG nanoparticles. J. Magn. Magn. Mater. 2016, 409, 104–110. [Google Scholar] [CrossRef]
- Sharma, V.; Saha, J.; Patnaik, S.; Kuanr, B.K. Synthesis and characterization of yttrium iron garnet (YIG) nanoparticles—Microwave material. AIP Adv. 2017, 7. [Google Scholar] [CrossRef]
- Choi, J.; Cha, J.; Lee, J.K. Synthesis of various magnetite nanoparticles through simple phase transformation and their shape-dependent magnetic properties. RSC Adv. 2013, 3, 8365–8371. [Google Scholar] [CrossRef]
- Nagrare, B.S.; Kekade, S.S.; Thombare, B.; Reddy, R.V.; Patil, S.I. Hyperfine interaction, Raman and magnetic study of YFeO3 nanocrystals. Solid State Commun. 2018, 280, 32–38. [Google Scholar] [CrossRef]
- Popkov, V.I.; Almjasheva, O.V.; Semenova, A.S.; Kellerman, D.G.; Nevedomskiy, V.N.; Gusarov, V.V. Magnetic properties of YFeO3 nanocrystals obtained by different soft-chemical methods. J. Mater. Sci. Mater. Electron. 2017, 28, 7163–7170. [Google Scholar] [CrossRef]
- Jendrzej, S.; Gökce, B.; Amendola, V.; Barcikowski, S. Barrierless growth of precursor-free, ultrafast laser-fragmented noble metal nanoparticles by colloidal atom clusters—A kinetic in situ study. J. Colloid Interface Sci. 2016, 463, 299–307. [Google Scholar] [CrossRef]
- Siebeneicher, S.; Waag, F.; Castillo, M.E.; Shvartsman, V.V.; Lupascu, D.C.; Gökce, B. Laser fragmentation synthesis of colloidal bismuth ferrite particles. Nanomaterials 2020, 10, 359. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hupfeld, T.; Stein, F.; Barcikowski, S.; Gökce, B.; Wiedwald, U. Manipulation of the Size and Phase Composition of Yttrium Iron Garnet Nanoparticles by Pulsed Laser Post-Processing in Liquid. Molecules 2020, 25, 1869. https://doi.org/10.3390/molecules25081869
Hupfeld T, Stein F, Barcikowski S, Gökce B, Wiedwald U. Manipulation of the Size and Phase Composition of Yttrium Iron Garnet Nanoparticles by Pulsed Laser Post-Processing in Liquid. Molecules. 2020; 25(8):1869. https://doi.org/10.3390/molecules25081869
Chicago/Turabian StyleHupfeld, Tim, Frederic Stein, Stephan Barcikowski, Bilal Gökce, and Ulf Wiedwald. 2020. "Manipulation of the Size and Phase Composition of Yttrium Iron Garnet Nanoparticles by Pulsed Laser Post-Processing in Liquid" Molecules 25, no. 8: 1869. https://doi.org/10.3390/molecules25081869
APA StyleHupfeld, T., Stein, F., Barcikowski, S., Gökce, B., & Wiedwald, U. (2020). Manipulation of the Size and Phase Composition of Yttrium Iron Garnet Nanoparticles by Pulsed Laser Post-Processing in Liquid. Molecules, 25(8), 1869. https://doi.org/10.3390/molecules25081869







