Figure 1.
Gas molecular models. The gas molecules are H2, CO, CO2, CH4, C2H2, C2H4, and C2H6.
Figure 1.
Gas molecular models. The gas molecules are H2, CO, CO2, CH4, C2H2, C2H4, and C2H6.
Figure 2.
H2 molecular models in oil. (a) Model diagram of H2 molecular models in natural ester. (b) Model diagram of H2 molecular models in mineral oil. The one circled by the pink line is H2.
Figure 2.
H2 molecular models in oil. (a) Model diagram of H2 molecular models in natural ester. (b) Model diagram of H2 molecular models in mineral oil. The one circled by the pink line is H2.
Figure 3.
The volume of gas molecules. The volume of (a) H2, (b) CO, and (c) C2H2 in natural ester. The volume of (d) H2, (e) CO, and (f) C2H2 in mineral oil.
Figure 3.
The volume of gas molecules. The volume of (a) H2, (b) CO, and (c) C2H2 in natural ester. The volume of (d) H2, (e) CO, and (f) C2H2 in mineral oil.
Figure 4.
Comparison diagram of the fractional free volume of gas molecules in natural ester and mineral oil.
Figure 4.
Comparison diagram of the fractional free volume of gas molecules in natural ester and mineral oil.
Figure 5.
The interaction energy of gas molecules and oil varies with time at 343 K. The interaction energy between (a) H2, (b) CO, and (c) C2H2 and natural ester. The interaction energy between (d) H2, (e) CO, and (f) C2H2 and mineral oil. The black line is Eint, the red line is Evdw, and the blue line is Eelec. Eint is the interaction energy between gas and oil, Evdw and Eelec, respectively, represent the Van der Waals action energy and electrostatic action energy of gas molecules and oil.
Figure 5.
The interaction energy of gas molecules and oil varies with time at 343 K. The interaction energy between (a) H2, (b) CO, and (c) C2H2 and natural ester. The interaction energy between (d) H2, (e) CO, and (f) C2H2 and mineral oil. The black line is Eint, the red line is Evdw, and the blue line is Eelec. Eint is the interaction energy between gas and oil, Evdw and Eelec, respectively, represent the Van der Waals action energy and electrostatic action energy of gas molecules and oil.
Figure 6.
Comparison diagram of the interaction energy between gas molecules and oil.
Figure 6.
Comparison diagram of the interaction energy between gas molecules and oil.
Figure 7.
The displacement of gas molecules in oil at 343 K. (a) H2, (b) CO, (c) CO2, (d) CH4, (e) C2H2, (f) C2H4, and (g) C2H6 in natural ester and mineral oil. The black line is the displacement of gas molecules in natural esters, and the red line is the displacement of gas molecules in mineral oil.
Figure 7.
The displacement of gas molecules in oil at 343 K. (a) H2, (b) CO, (c) CO2, (d) CH4, (e) C2H2, (f) C2H4, and (g) C2H6 in natural ester and mineral oil. The black line is the displacement of gas molecules in natural esters, and the red line is the displacement of gas molecules in mineral oil.
Figure 8.
The trajectory of gas molecules in oil at 343 K. (a) H2, (c) CO, (e) CO2, (g) CH4, (i) C2H2, (k) C2H4, and (m) C2H6 in natural ester. (b) H2, (d) CO, (f) CO2, (h) CH4, (j) C2H2, (l) C2H4, and (n) C2H6 in mineral oil.
Figure 8.
The trajectory of gas molecules in oil at 343 K. (a) H2, (c) CO, (e) CO2, (g) CH4, (i) C2H2, (k) C2H4, and (m) C2H6 in natural ester. (b) H2, (d) CO, (f) CO2, (h) CH4, (j) C2H2, (l) C2H4, and (n) C2H6 in mineral oil.
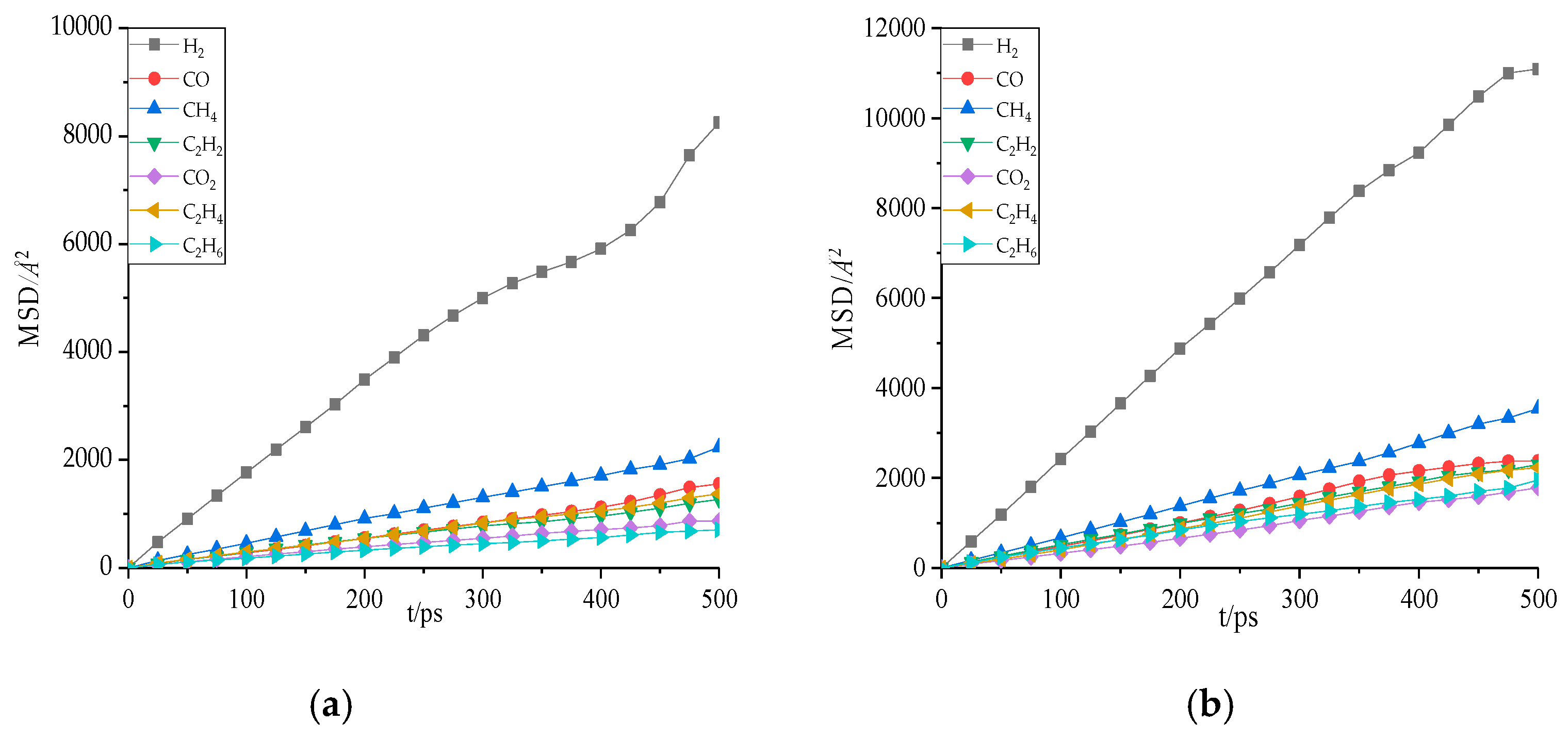
Figure 9.
The mean square displacement (MSD) of gas molecules in oil at 343 K. (a) Gas molecules in natural ester. (b) Gas molecules in mineral oil.
Figure 9.
The mean square displacement (MSD) of gas molecules in oil at 343 K. (a) Gas molecules in natural ester. (b) Gas molecules in mineral oil.
Figure 10.
Comparison diagram of the diffusion coefficient of gas molecules in oil.
Figure 10.
Comparison diagram of the diffusion coefficient of gas molecules in oil.
Figure 11.
The free volume of a gas molecule varies with temperature. (a) H2, (b) CO, (c) CO2, (d) CH4, (e) C2H2, (f) C2H4, and (g) C2H6.
Figure 11.
The free volume of a gas molecule varies with temperature. (a) H2, (b) CO, (c) CO2, (d) CH4, (e) C2H2, (f) C2H4, and (g) C2H6.
Figure 12.
The interaction energy between the gas molecule and oil varies with temperature. (a) H2, (b) CO, (c) CO2, (d) CH4, (e) C2H2, (f) C2H4, and (g) C2H6.
Figure 12.
The interaction energy between the gas molecule and oil varies with temperature. (a) H2, (b) CO, (c) CO2, (d) CH4, (e) C2H2, (f) C2H4, and (g) C2H6.
Figure 13.
The fitting curve of gas molecular diffusion coefficient with temperature (a) H2, (b) CO, (c) C2H2.
Figure 13.
The fitting curve of gas molecular diffusion coefficient with temperature (a) H2, (b) CO, (c) C2H2.
Table 1.
Fatty acid compositions of natural ester.
Table 1.
Fatty acid compositions of natural ester.
| Saturated Fatty Acid | Unsaturated Fatty Acid | Polyunsaturated Fatty Acids |
|---|
| Diunsaturated Fatty Acid | Triunsaturated Fatty Acid |
|---|
| 16 wt.% | 24 wt.% | 50 wt.% | 10 wt.% |
Table 2.
Compositions of mineral oil.
Table 2.
Compositions of mineral oil.
| Chain Hydrocarbons | Alkanes |
|---|
| A Cycloalkane | Bicyclic Alkanes | Tricyclic Alkanes | Tetracyclic Alkanes |
|---|
| 11.6 wt.% | 15.5 wt.% | 28.5 wt.% | 23.3 wt.% | 9.7 wt.% |
Table 3.
FFV (fraction of free volume) of gas molecules in natural ester at 343 K.
Table 3.
FFV (fraction of free volume) of gas molecules in natural ester at 343 K.
| | H2 | CO | CH4 | C2H2 | CO2 | C2H4 | C2H6 |
|---|
| Vo (Å3) | 19463 | 20638 | 20867 | 20972 | 21003 | 21175 | 21296 |
| Vf (Å3) | 2711 | 1419 | 1190 | 1085 | 1054 | 882 | 761 |
| FFV (%) | 12.29 | 6.43 | 5.40 | 4.92 | 4.78 | 3.99 | 3.45 |
Table 4.
FFV of gas molecules in mineral oil at 343 K.
Table 4.
FFV of gas molecules in mineral oil at 343 K.
| | H2 | CO | CH4 | C2H2 | CO2 | C2H4 | C2H6 |
|---|
| Vo (Å3) | 15253 | 16448 | 16681 | 16793 | 16842 | 17002 | 17125 |
| Vf (Å3) | 2574.67 | 1379.23 | 1146.38 | 1034.11 | 985.46 | 825.37 | 701.84 |
| FFV (%) | 14.44 | 7.74 | 6.43 | 5.80 | 5.53 | 4.63 | 3.94 |
Table 5.
The average interaction energy between gas molecules and natural ester at 343 K.
Table 5.
The average interaction energy between gas molecules and natural ester at 343 K.
| | H2 | CO | CH4 | C2H2 | CO2 | C2H4 | C2H6 |
|---|
| Eint (kcal/mol) | −35.56 | −177.51 | −124.70 | −171.39 | −168.34 | −190.66 | −206.34 |
| Evdw (kcal/mol) | −35.56 | −176.38 | −124.55 | −166.53 | −67.06 | −187.12 | −205.98 |
| Eelec (kcal/mol) | 0 | −1.13 | −0.15 | −4.86 | −1.28 | −3.54 | −0.36 |
Table 6.
The average interaction energy between gas molecules and mineral oil at 343 K.
Table 6.
The average interaction energy between gas molecules and mineral oil at 343 K.
| | H2 | CO | CH4 | C2H2 | CO2 | C2H4 | C2H6 |
|---|
| Eint (kcal/mol) | −33.36 | −155.80 | −121.31 | −145.21 | −158.10 | −172.27 | −187.26 |
| Evdw (kcal/mol) | −33.36 | −155.72 | −121.23 | −144.48 | −158.06 | −171.82 | −187.1 |
| Eelec (kcal/mol) | 0 | −0.08 | −0.07 | −0.73 | −0.04 | −0.45 | −0.16 |
Table 7.
The relative molecular mass of gas molecules.
Table 7.
The relative molecular mass of gas molecules.
| | H2 | CO | CH4 | C2H2 | CO2 | C2H4 | C2H6 |
|---|
| Relative molecular mass | 2 | 30 | 16 | 30 | 48 | 32 | 34 |
Table 8.
Diffusion coefficients of seven gas molecules in natural ester at 343 K.
Table 8.
Diffusion coefficients of seven gas molecules in natural ester at 343 K.
| | H2 | CO | CH4 | C2H2 | CO2 | C2H4 | C2H6 |
|---|
| Slope a | 14.9801 | 2.9812 | 4.2021 | 2.3690 | 1.7128 | 2.1208 | 1.3335 |
| Fitting correlation coefficient R2 | 0.99 | 0.98 | 0.99 | 0.99 | 0.97 | 0.99 | 0.98 |
| Diffusion coefficient D/×10−8 m2/s | 2.4967 | 0.4969 | 0.7003 | 0.3948 | 0.2855 | 0.3535 | 0.2223 |
Table 9.
Diffusion coefficients of seven gas molecules in mineral oil at 343 K.
Table 9.
Diffusion coefficients of seven gas molecules in mineral oil at 343 K.
| | H2 | CO | CH4 | C2H2 | CO2 | C2H4 | C2H6 |
|---|
| Slope a | 23.2031 | 5.2672 | 7.0346 | 4.6477 | 3.1398 | 4.3617 | 3.9591 |
| Fitting correlation coefficient R2 | 0.99 | 0.99 | 0.98 | 0.99 | 0.99 | 0.99 | 0.97 |
| Diffusion coefficient D/×10−8 m2/s | 3.8677 | 0.8779 | 1.1724 | 0.7746 | 0.5233 | 0.7270 | 0.6598 |
Table 10.
Pearson correlation coefficient in oil.
Table 10.
Pearson correlation coefficient in oil.
| | D-E | D-FFV | D-R |
|---|
| Natural ester | 0.9793 | 0.9558 | −0.8554 |
| Mineral oil | 0.9488 | 0.9543 | −0.8499 |
Table 11.
FFV of gas molecules in natural ester at different temperatures.
Table 11.
FFV of gas molecules in natural ester at different temperatures.
| | FFV/% |
|---|
| T/K | H2 | CO | CH4 | C2H2 | CO2 | C2H4 | C2H6 |
|---|
| 283 K | 5.93 | 2.26 | 197 | 1.74 | 1.56 | 1.22 | 0.98 |
| 303 K | 6.48 | 2.90 | 2.43 | 2.21 | 2.03 | 1.87 | 1.63 |
| 323 K | 7.37 | 3.24 | 2.72 | 2.46 | 2.31 | 2.11 | 1.92 |
| 343 K | 12.29 | 6.43 | 5.40 | 4.92 | 4.78 | 3.99 | 3.45 |
| 363 K | 17.22 | 8.72 | 7.13 | 6.77 | 6.52 | 5.48 | 4.97 |
Table 12.
FFV of gas molecules in mineral oil at different temperatures.
Table 12.
FFV of gas molecules in mineral oil at different temperatures.
| | FFV/% |
|---|
| T/K | H2 | CO | CH4 | C2H2 | CO2 | C2H4 | C2H6 |
|---|
| 283 K | 9.79 | 5.07 | 4.27 | 3.94 | 3.71 | 3.32 | 2.96 |
| 303 K | 12.97 | 5.95 | 4.84 | 4.34 | 4.17 | 3.46 | 3.21 |
| 323 K | 13.28 | 6.43 | 5.21 | 4.95 | 4.88 | 4.58 | 3.44 |
| 343 K | 14.44 | 7.74 | 6.43 | 5.80 | 5.53 | 4.63 | 3.94 |
| 363 K | 20.90 | 13.71 | 12.11 | 11.31 | 10.98 | 9.81 | 8.85 |
Table 13.
The interaction energy between gas molecules and mineral oil at different temperatures.
Table 13.
The interaction energy between gas molecules and mineral oil at different temperatures.
| | Eint/(kcal/mol) |
|---|
| T/K | H2 | CO | CH4 | C2H2 | CO2 | C2H4 | C2H6 |
|---|
| 283 K | −43.38 | −196.98 | −148.83 | −189.77 | −183.04 | −210.01 | 238.86 |
| 303 K | −42.56 | −196.00 | −136.14 | −187.69 | −171.69 | −207.08 | 228.09 |
| 323 K | −38.45 | −183.55 | −139.87 | −179.22 | −170.23 | −195.12 | −212.16 |
| 343 K | −35.56 | −177.51 | −124.70 | −171.39 | −168.34 | −190.66 | −206.34 |
| 363 K | −32.61 | −165.22 | −122.43 | −170.94 | −156.82 | −186.68 | −195.96 |
Table 14.
The interaction energy between gas molecules and mineral oil at different temperatures.
Table 14.
The interaction energy between gas molecules and mineral oil at different temperatures.
| | Eint/(kcal/mol) |
|---|
| T/K | H2 | CO | CH4 | C2H2 | CO2 | C2H4 | C2H6 |
|---|
| 283 K | −39.01 | −175.62 | −132.75 | −159.30 | −168.59 | −195.16 | −207.63 |
| 303 K | −36.25 | −172.51 | −129.59 | −157.70 | −167.48 | −182.73 | −195.24 |
| 323 K | −34.91 | −160.68 | −123.69 | −147.93 | −159.18 | −176.81 | −189.75 |
| 343 K | −33.36 | −155.80 | −121.31 | −145.21 | −158.10 | −172.27 | −187.26 |
| 363 K | −32.17 | −149.44 | −114.72 | −136.42 | −150.58 | −157.78 | −176.52 |
Table 15.
Diffusion coefficients of gas molecules in natural ester at different temperatures.
Table 15.
Diffusion coefficients of gas molecules in natural ester at different temperatures.
| | Diffusion Coefficients D/×10−8 m2/s |
|---|
| T/K | H2 | CO | CH4 | C2H2 | CO2 | C2H4 | C2H6 |
|---|
| 283 K | 1.0657 | 0.1915 | 0.2943 | 0.1613 | 0.1227 | 0.1457 | 0.1216 |
| 303 K | 1.2222 | 0.2652 | 0.3592 | 0.2349 | 0.2005 | 0.2161 | 0.1981 |
| 323 K | 1.5351 | 0.3296 | 0.4631 | 0.2916 | 0.2582 | 0.2689 | 0.2167 |
| 343 K | 2.4967 | 0.4969 | 0.7003 | 0.3948 | 0.2855 | 0.3535 | 0.2723 |
| 363 K | 3.9543 | 0.6503 | 1.0189 | 0.5512 | 0.4664 | 0.5462 | 0.4004 |
Table 16.
Diffusion coefficients of gas molecules in mineral oil at different temperatures.
Table 16.
Diffusion coefficients of gas molecules in mineral oil at different temperatures.
| | Diffusion Coefficients D/×10−8 m2/s |
|---|
| T/K | H2 | CO | CH4 | C2H2 | CO2 | C2H4 | C2H6 |
|---|
| 283 K | 1.7678 | 0.3827 | 0.5849 | 0.3819 | 0.2834 | 0.3042 | 0.2927 |
| 303 K | 2.5852 | 0.5779 | 0.6082 | 0.5558 | 0.4288 | 0.4933 | 0.4594 |
| 323 K | 3.3513 | 0.6750 | 0.8987 | 0.6554 | 0.4599 | 0.5711 | 0.5358 |
| 343 K | 3.8677 | 0.8779 | 1.1724 | 0.7746 | 0.5233 | 0.7270 | 0.6598 |
| 363 K | 5.5090 | 1.2009 | 1.4043 | 1.0747 | 0.6264 | 0.8890 | 0.8664 |
Table 17.
The fitting formula of the diffusion coefficient and temperature in natural ester.
Table 17.
The fitting formula of the diffusion coefficient and temperature in natural ester.
| Gas Molecules | Fitting Formula | R2 |
|---|
| H2 | D = 1516.15487e−2174.98083/T | 0.95 |
| CO | D = 67.01119e−1685.98837/T | 0.98 |
| CH4 | D = 177.88902e−1885.69621/T | 0.97 |
| C2H2 | D = 47.60204e−1628.25238/T | 0.98 |
| CO2 | D = 41.14987e−1647.59101/T | 0.95 |
| C2H4 | D = 72.30426e−1790.25078/T | 0.97 |
| C2H6 | D = 21.70519e−1467.3526/T | 0.95 |
Table 18.
The fitting formula of the diffusion coefficient and temperature in mineral oil.
Table 18.
The fitting formula of the diffusion coefficient and temperature in mineral oil.
| Gas Molecules | Fitting Formula | R2 |
|---|
| H2 | D = 262.1956e−1415.01326/T | 0.97 |
| CO | D = 63.09076e−1448.72382/T | 0.98 |
| CH4 | D = 50.27274e−1297.76786/T | 0.97 |
| C2H2 | D = 35.09568e−1279.70589/T | 0.97 |
| CO2 | D = 6.99573e−878.38744/T | 0.95 |
| C2H4 | D = 27.95973e−1251.07713/T | 0.98 |
| C2H6 | D = 30.18511e−1296.12329/T | 0.98 |