New Cyclic Diarylheptanoids from Platycarya strobilacea
Abstract
1. Introduction
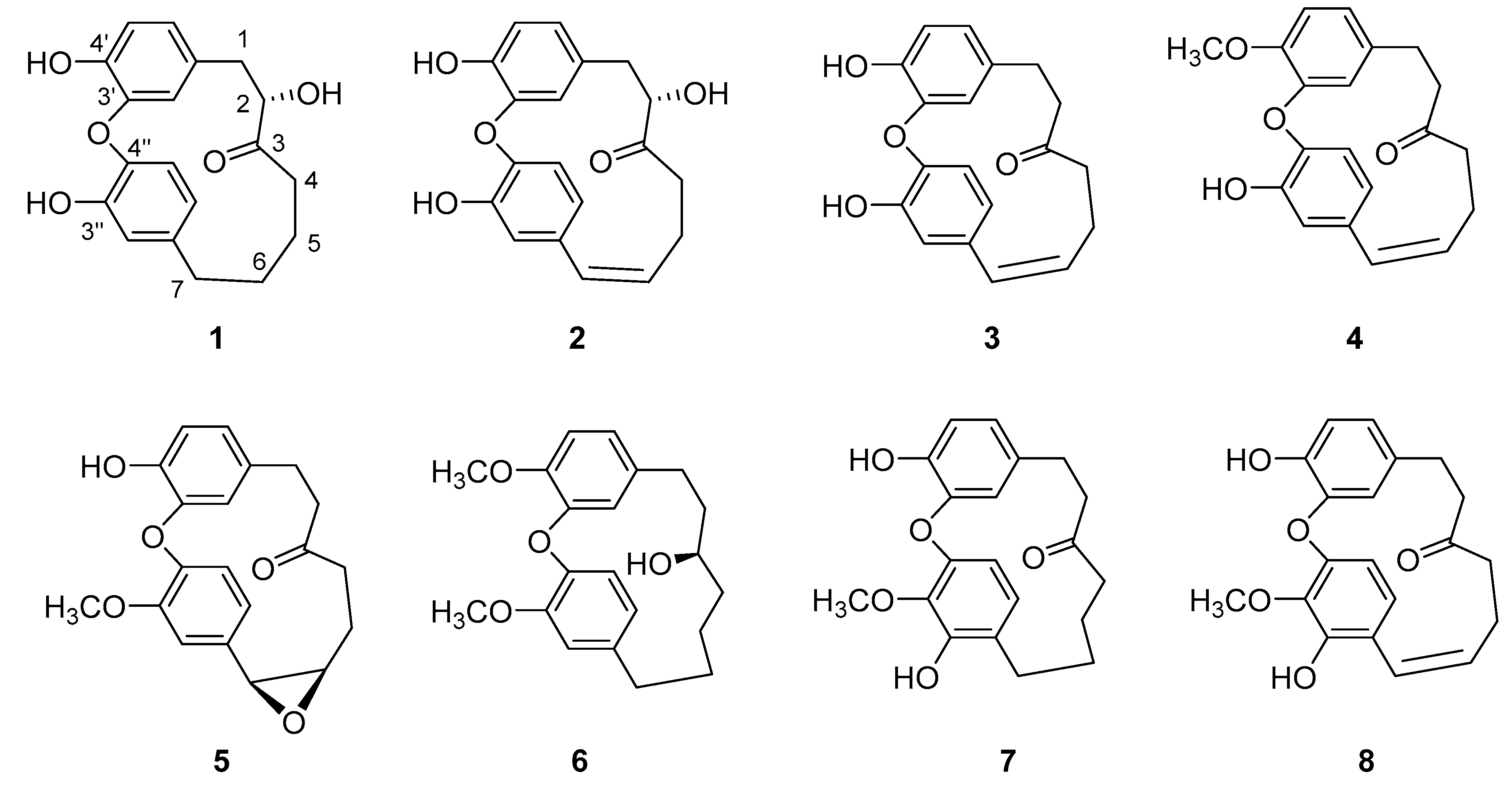
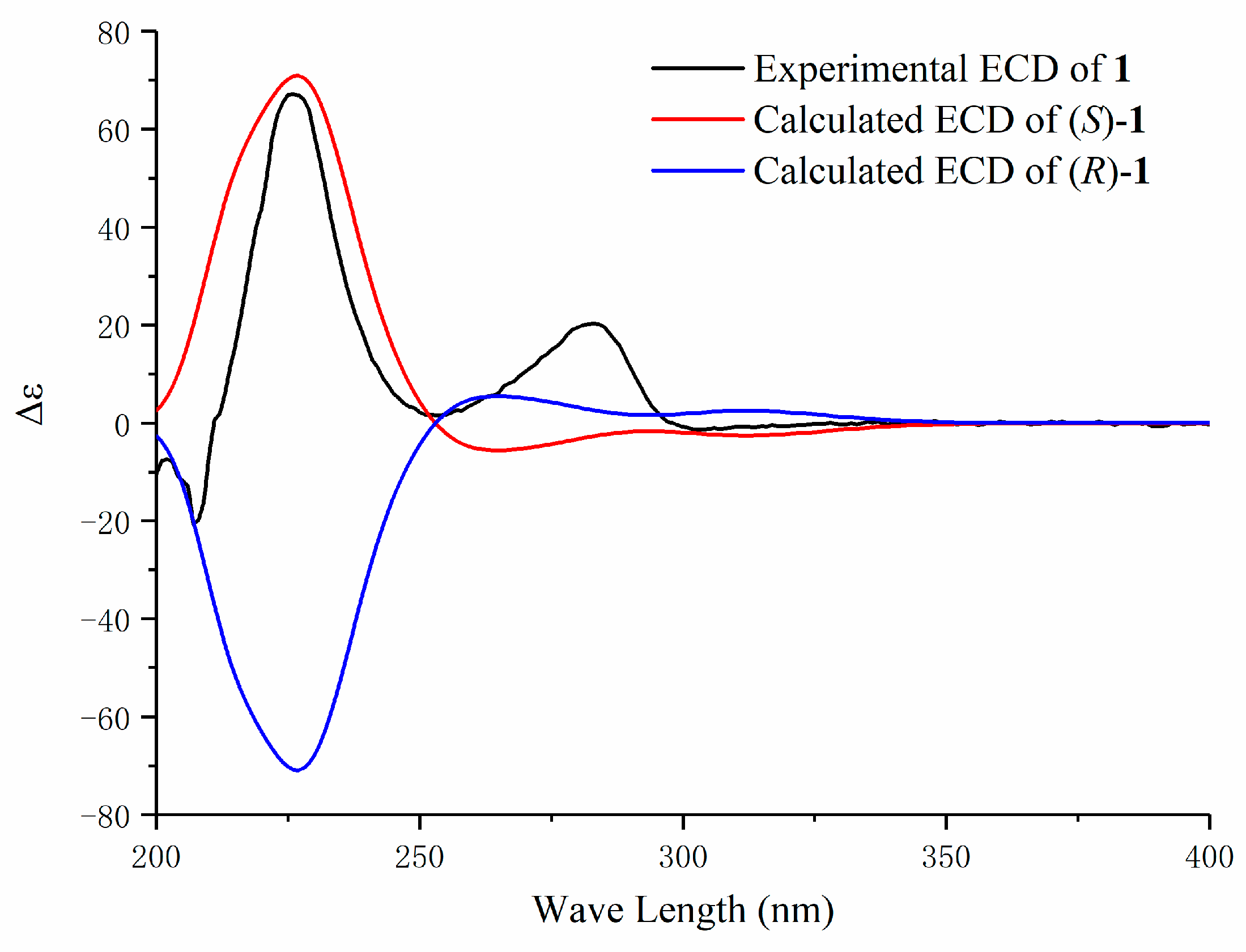
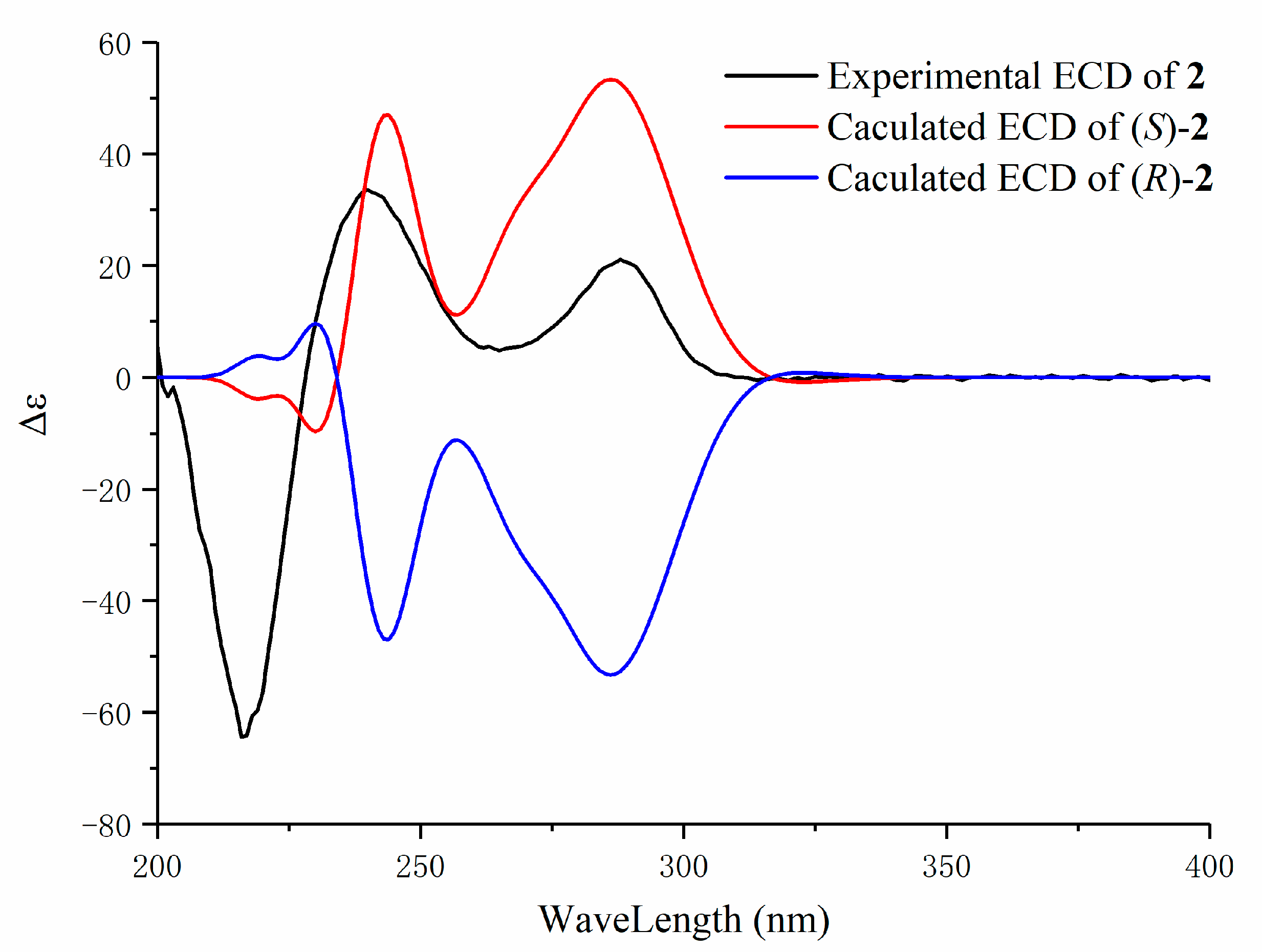
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Characterization of Compounds 1–5
3.5. Determination of Biological Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cerulli, A.; Masullo, M.; Montoro, P.; Montoro, P.; Hošek, J.; Pizza, C.; Piacente, S. Metabolite profiling of “green” extracts of Corylus avellana leaves by 1H-NMR spectroscopy and multivariate statistical analysis. J. Pharm. Biomed. 2018, 147, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Jahng, Y.; Park, J.G. Recent studies on cyclic 1, 7-diarylheptanoids: Their isolation, structures, biological activities, and chemical synthesis. Molecules 2018, 23, 3107. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, P.J.; Upson, T. Platycarya strobilacea, juglandaceae. Curtis’s Bot. Mag. 2006, 23, 77–83. [Google Scholar] [CrossRef]
- Tanaka, T.; Kirihara, S.; Nonaka, G.I.; Nishioka, I. Tannins and related compounds. CXXIV. Five new ellagitannins, Platycaryanins A, B, C, and D, and Platycariin, and a new complex tannin, Strobilanin, from the fruits and bark of Platycarya strobilacea SIEB et ZUCC., and biomimetic synthesis of C-glycosidic ellagitannins from glucopyranose-based ellagitannins. Chem. Pharm. Bull. 1993, 41, 1708–1716. [Google Scholar] [CrossRef]
- Lee, H.; Choi, Y.J.; Jin, J.H. Extractives from the bark of Platycarya strobilacea. J. Korean Soc. For. Sci. 2007, 96, 408–413. [Google Scholar]
- Maeda, H.; Kakoki, N.; Ayabe, M.; Koga, Y.; Oribe, T.; Matsuo, Y.; Tanaka, T.; Kouno, I. Ent-eudesmane sesquiterpenoids, galloyl esters of the oak lactone precursor, and a 3-O-methylellagic acid glycoside from the wood of Platycarya strobilacea. Phytochemistry 2011, 72, 796–803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morikawa, H.; Saito, Y.; Matsuo, Y.; Maeda, H.; Tanaka, T. Eudesmane Sesquiterpenoids from the Wood of Platycarya strobilacea. Nat. Prod. Commun. 2016, 11, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Jiang, Z.H.; Kouno, I. Distribution of ellagic acid derivatives and a diarylheptanoid in wood of Platycarya strobilacea. Phytochemistry 1998, 47, 851–854. [Google Scholar] [CrossRef]
- Morihara, M.; Sakurai, N.; Inoue, T.; Kawai, K.I.; Nagai, M. Two novel diarylheptanoid glucosides from myrica gale var. tomentosa and absolute structure of plane-chiral galeon. Chem. Pharm. Bull. 1997, 45, 820–823. [Google Scholar] [CrossRef]
- Li, J.; Xu, K.P.; Zou, Z.X.; Zou, H.; Long, H.P.; Tan, L.H.; Liu, R.H.; Wang, Y.K.; Xu, P.S.; Tan, G.S. Two new compounds from the green peel of Juglans mandshurica. J. Asian Nat. Prod. Res. 2017, 19, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xu, M.L.; Choi, H.G.; Lee, S.H.; Jahng, Y.J.; Lee, C.S.; Moon, D.C.; Woo, M.H.; Son, J.K. Four New Diarylheptanoids from the Roots of Juglans mandshurica. Chem. Pharm. Bull. 2003, 51, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, G.O.; Regentin, R.; Piagentini, M.; Rascher, A.; McDaniel, R.; Galazzo, J.L.; Licari, P.J. Production of 8-demethylgeldanamycin and 4,5-Epoxy-8-demethylgeldanamycin from a Recombinant Strain of Streptomyces hygroscopicus. J. Nat. Prod. 2005, 68, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Lewis, P.A.; Jacobs, H. 3,4-epoxy-8,9-dihydropiplartine. A new imide from Piper verrucosum. J. Nat. Prod. 1996, 59, 436–437. [Google Scholar] [CrossRef]
- Li, Y.F.; Luo, Z.P. Desipramine antagonized corticosterone-induced apoptosis in cultured PC12 cells. Acta. Pharmacol. Sin. 2002, 23, 311–314. [Google Scholar] [PubMed]
- Guo, Y.; Zhang, N.; Chen, C.; Huang, J.; Li, X.N.; Liu, J.; Zhu, H.; Tong, Q.; Zhang, J.; Luo, Z.; et al. Tricyclic polyprenylated acylphloroglucinols from St John’s Wort, Hypericum perforatum. J. Nat. Prod. 2017, 80, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Malich, G.; Markovic, B.; Winder, C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997, 124, 179–192. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Park, E.J.; Rostama, B.; Pezzuto, J.M.; Chang, L.C. Inhibition of nitric oxide (NO) production in lipopolysaccharide (LPS)-activated murine macrophage RAW 264.7 cells by the norsesterterpene peroxide, Epimuqubilin A. Mar. Drugs 2010, 8, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.P.; Liu, Y.M.; Le, L.; Li, Z.Y.; Si, J.Y.; Liu, X.M.; Chang, Q.; Pan, R.L. Cajaninstilbene acid prevents corticosterone-induced apoptosis in PC12 cells by inhibiting the mitochondrial apoptotic pathway. Cell Physiol. Biochem. 2014, 34, 1015–1026. [Google Scholar] [CrossRef] [PubMed]

| No. | 1 a | 2 a | 3 a | 4 b | 5 b |
|---|---|---|---|---|---|
| 1 | 3.07 (dd, 15.1, 2.0) | 3.18 (d, 15.8) | 2.87 (m) | 3.00 (m) | 3.25 (m) |
| 2.82 (dd, 15.1, 7.6) | 2.91 (dd, 15.8, 7.6) | 2.80 (m) | 2.85 (dd, 16.4, 8.3) | 2.51 (dd, 16.1, 5.4) | |
| 2 | 3.98 (dd, 7.6, 2.0) | 4.06 (dd, 7.7, 1.9) | 2.35–2.39 (m) | 2.40 (dd, 17.4, 9.6) | 2.27 (dd, 17.7, 5.4) |
| 2.33 (dd, 16.8, 7.7) | 2.46 (m) | ||||
| 4 | 2.12 (m) | 2.36 (m) | 2.18–2.24 (m) | 2.22 (m) | 2.40 (m) |
| 1.64 (m) | 2.27 (m) | 2.09 (m) | 1.52 (dd, 19.0, 11.5) | ||
| 5 | 1.46–1.56 (m) | 2.55 (m) | 2.38 (m) | 2.52 (m) | 2.06 (m) |
| 2.14 (m) | 2.31 (m) | 2.28 (m) | 1.71 (m) | ||
| 6 | 1.75 (m) | 5.99 (dt, 11.2, 7.2) | 5.97 (dt, 11.5, 7.3) | 5.97 (dt, 11.2, 7.2) | 3.26 (m, overlapped) |
| 1.60 (m) | |||||
| 7 | 2.75 (m) | 6.75 (d, 11.2) | 6.71 (d, 11.5) | 6.71 (d, 11.2) | 4.18 (d, 4.3) |
| 2.55 (m) | |||||
| 2′ | 5.72 (d, 2.1) | 5.57 (br s) | 5.38 (d, 2.0) | 5.45 (d, 2.0) | 5.16 (d, 2.2) |
| 5′ | 6.72 (d, 8.1) | 6.76 (overlapped) | 6.73 (d, 8.1) | 6.82 (d, 8.1) | 6.84 (d, 8.3) |
| 6′ | 6.57 (dd, 8.1, 2.1) | 6.59 (dd, 8.2, 2.1) | 6.57 (dd, 8.1, 2.0) | 6.73 (dd, 8.1, 2.0) | 6.63 (dd, 8.3, 2.2) |
| 2″ | 6.82 (d, 2.0) | 6.87 (d, 1.9) | 6.83 (d, 1.9) | 6.90 (d, 2.0) | 6.97 (d, 1.7) |
| 5″ | 6.83 (d, 8.2) | 6.96 (d, 8.2) | 6.92 (d, 8.1) | 6.99 (d, 8.2) | 7.06 (overlapped) |
| 6″ | 6.77 (dd, 8.2, 2.0) | 6.73 (overlapped) | 6.74 (dd, 8.1, 1.9) | 6.83 (dd, 8.2, 2.0) | 7.06 (overlapped) |
| 4′-OCH3 | 3.91 (s) | ||||
| 3″-OCH3 | 3.72 (s, 3H) |
| NO. | 1 a | 2 a | 3 a | 4 b | 5 b |
|---|---|---|---|---|---|
| 1 | 38.5 | 37.9 | 27.7 | 26.7 | 26.7 |
| 2 | 77.3 | 76.6 | 40.8 | 39.9 | 40.0 |
| 3 | 215.5 | 214.3 | 211.4 | 208.5 | 207.3 |
| 4 | 42.7 | 39.0 | 42.9 | 42.1 | 37.9 |
| 5 | 20.8 | 20.4 | 20.0 | 18.8 | 20.9 |
| 6 | 28.6 | 132.2 | 132.4 | 131.6 | 56.2 |
| 7 | 36.8 | 133.2 | 133.1 | 131.9 | 55.9 |
| 1′ | 141.2 | 138.4 | 138.5 | 137.3 | 134.4 |
| 2′ | 119.6 | 118.4 | 118.4 | 116.5 | 110.8 |
| 3′ | 151.5 | 151.7 | 151.7 | 149.2 | 152.5 |
| 4′ | 144.1 | 144.1 | 143.3 | 141.3 | 144.0 |
| 5′ | 125.3 | 125.5 | 125.5 | 123.8 | 124.0 |
| 6′ | 122.8 | 121.7 | 121.6 | 121.3 | 119.6 |
| 1″ | 129.4 | 129.5 | 133.8 | 133.8 | 132.4 |
| 2″ | 116.6 | 115.7 | 113.5 | 112.9 | 111.6 |
| 3″ | 149.4 | 149.3 | 149.4 | 148.7 | 147.5 |
| 4″ | 145.6 | 145.2 | 144.6 | 146.4 | 143.0 |
| 5″ | 117.0 | 117.2 | 117.2 | 112.1 | 115.3 |
| 6″ | 124.9 | 124.6 | 122.9 | 122.5 | 122.4 |
| 4′-OCH3 | 56.1 | ||||
| 3″-OCH3 | 56.2 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.-b.; Zhao, R.-y.; Li, G.-h.; Liu, B.-l.; He, H.-l.; Qiu, L.; Xue, J.; Li, Y.-z. New Cyclic Diarylheptanoids from Platycarya strobilacea. Molecules 2020, 25, 6034. https://doi.org/10.3390/molecules25246034
Ding W-b, Zhao R-y, Li G-h, Liu B-l, He H-l, Qiu L, Xue J, Li Y-z. New Cyclic Diarylheptanoids from Platycarya strobilacea. Molecules. 2020; 25(24):6034. https://doi.org/10.3390/molecules25246034
Chicago/Turabian StyleDing, Wen-bing, Rui-yuan Zhao, Guan-hua Li, Bing-lei Liu, Hua-liang He, Lin Qiu, Jin Xue, and You-zhi Li. 2020. "New Cyclic Diarylheptanoids from Platycarya strobilacea" Molecules 25, no. 24: 6034. https://doi.org/10.3390/molecules25246034
APA StyleDing, W.-b., Zhao, R.-y., Li, G.-h., Liu, B.-l., He, H.-l., Qiu, L., Xue, J., & Li, Y.-z. (2020). New Cyclic Diarylheptanoids from Platycarya strobilacea. Molecules, 25(24), 6034. https://doi.org/10.3390/molecules25246034




