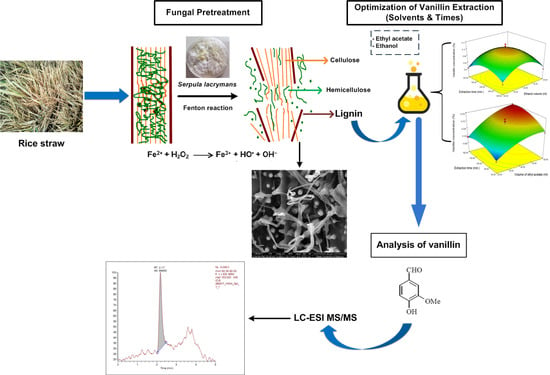
Extraction of Vanillin Following Bioconversion of Rice Straw and Its Optimization by Response Surface Methodology
Abstract
1. Introduction
2. Results and Discussion
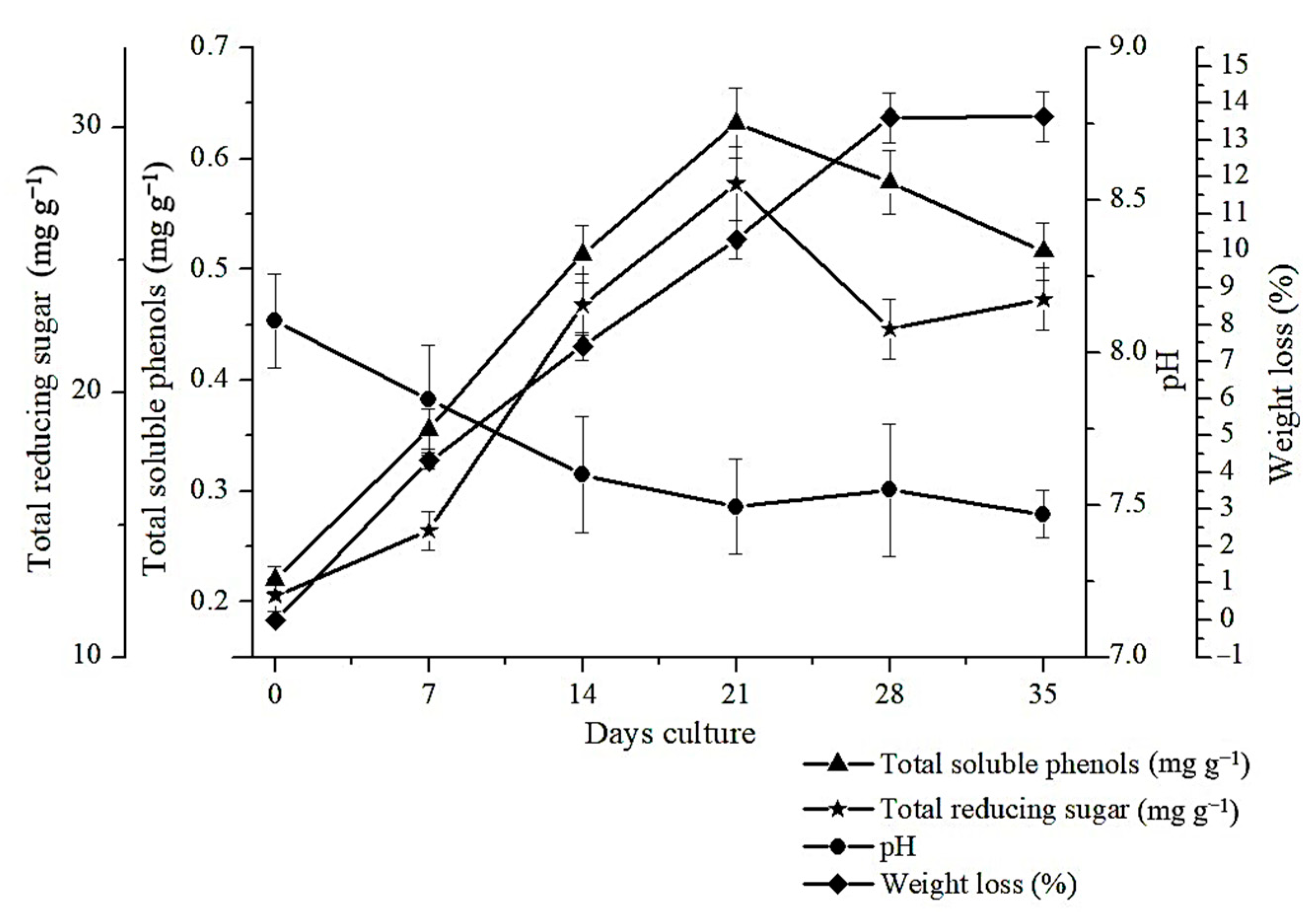
2.1. The Changes of Total Soluble Phenols, Total Reducing Sugars, pH and Weight Loss during Rice Straw SSF
2.2. Optimization of Pretreated Variables
2.3. Effect of Different Solvent on Vanillin Extraction
2.3.1. Ethanol
2.3.2. Ethyl Acetate
2.4. Optimization Concentrations and Yield of Vanillin Extract
2.5. Confirmation of Vanillin Content within Extracts
3. Materials and Methods
3.1. Fungal Strain and Medium
3.2. Solid State Fermentation (SSF)
3.3. Aqueous Extraction
3.4. Solvents Extraction
3.5. Response Surface Methodology (RSM) Design
3.6. Total Reducing Sugars and Soluble Phenols
3.7. Weight Loss (% Dry Weight)
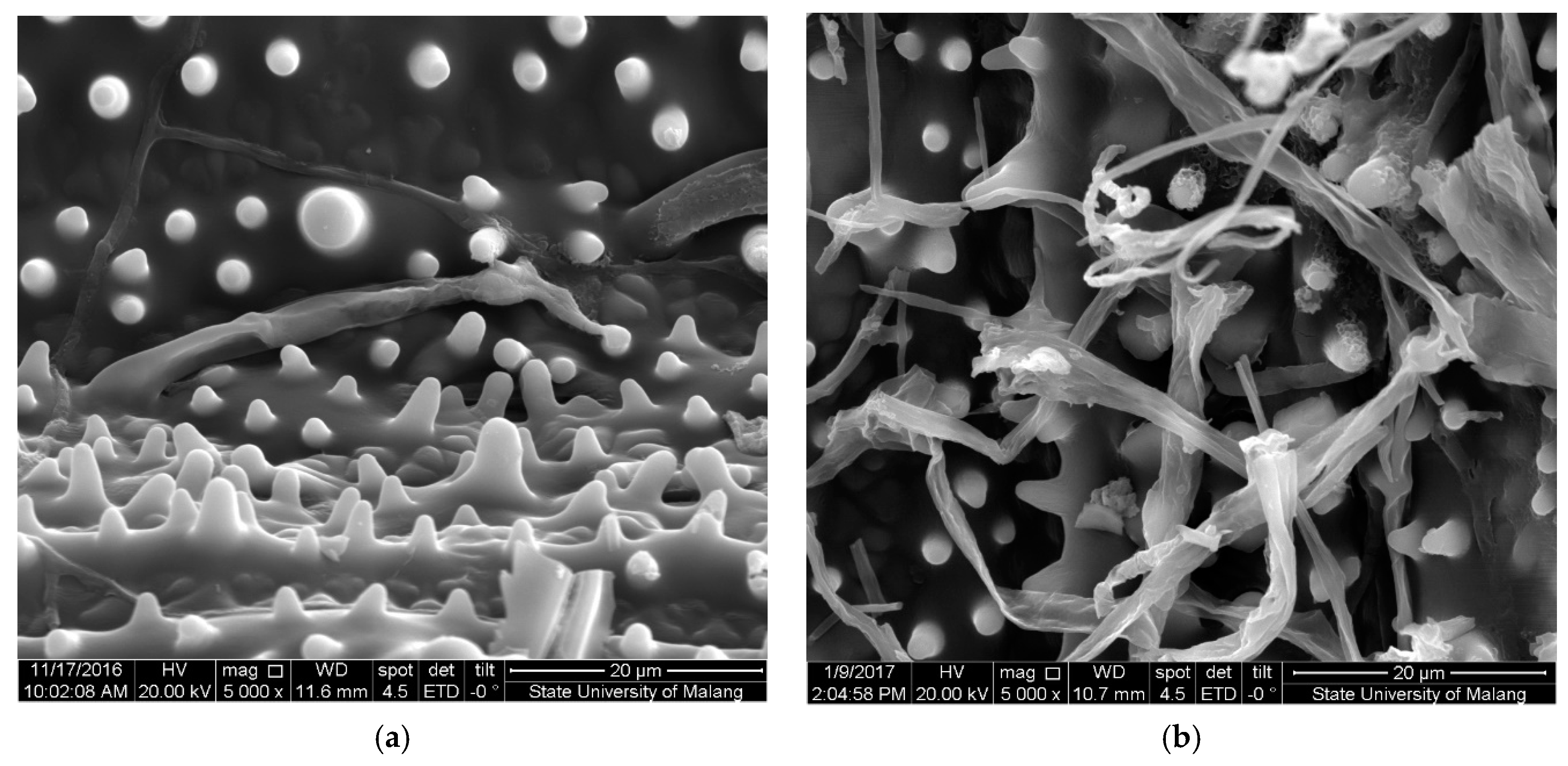
3.8. Scanning Electron Microscope (SEM)
3.9. Vanillin Concentration and Yield
- X = Sample solution concentration (ppm) obtained from the standard vanillin calibration curve
- Fp = Dilution factor
- M = Sample weight (mg)
3.10. Identification of Vanillin Compound Using Liquid Chromatography-Electrospray Ionization Tandem-Mass Spectrometry (Modification by Ong et al. [67])
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Howard, R.; Abotsi, E.; Jansen, V.R.E.; Howard, S. Lignocellulose biotechnology: Issues of bioconversion and enzyme production. Afr. J. Biotechnol. 2003, 2, 602–619. [Google Scholar] [CrossRef]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef] [PubMed]
- Garlapati, V.K.; Chandel, A.K.; Kumar, S.J.; Sharma, S.; Sevda, S.; Ingle, A.P.; Pant, D. Circular economy aspects of lignin: Towards a lignocellulose biorefinery. Renew. Sustain. Energy Rev. 2020, 130, 109977. [Google Scholar] [CrossRef]
- Lupoi, J.S.; Singh, S.; Parthasarathi, R.; Simmons, B.A.; Henry, R.J. Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin. Renew. Sustain. Energy Rev. 2015, 49, 871–906. [Google Scholar] [CrossRef]
- Güvenatam, B.; Heeres, E.H.; Pidko, E.A.; Hensen, E.J. Decomposition of lignin model compounds by Lewis acid catalysts in water and ethanol. J. Mol. Catal. A Chem. 2015, 410, 89–99. [Google Scholar] [CrossRef]
- Haghdan, S.; Renneckar, S.; Smith, G.D. Sources of Lignin. In Lignin in Polymer Composites; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 1–11. [Google Scholar]
- Fernández-Rodríguez, J.; Erdocia, X.; Sánchez, C.; Alriols, M.G.; Labidi, J. Lignin depolymerization for phenolic monomers production by sustainable processes. J. Energy Chem. 2017, 26, 622–631. [Google Scholar] [CrossRef]
- Vaithanomsat, P.; Apiwatanapiwat, W. Feasibility study on vanillin production from Jatropha curcas stem using steam explosion as a pretreatment. Inter. J. Chem. Biol. Engr. 2009, 3, 839–842. [Google Scholar]
- Di Gioia, D.; Luziatelli, F.; Negroni, A.; Ficca, A.G.; Fava, F.; Ruzzi, M. Metabolic engineering of Pseudomonas fluorescens for the production of vanillin from ferulic acid. J. Biotechnol. 2011, 156, 309–316. [Google Scholar] [CrossRef]
- Weerawatanakorn, M.; Wu, J.-C.; Pan, M.-H.; Ho, C.-T. Reactivity and stability of selected flavor compounds. J. Food Drug Anal. 2015, 23, 176–190. [Google Scholar] [CrossRef]
- Hua, D.; Ma, C.; Song, L.; Lin, S.; Zhang, Z.; Deng, Z.; Xu, P. Enhanced vanillin production from ferulic acid using adsorbent resin. Appl. Microbiol. Biotechnol. 2007, 74, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin, a key-intermediate of biobased polymers. Eur. Polym. J. 2015, 68, 488–502. [Google Scholar] [CrossRef]
- Eastwood, D.C.; Floudas, D.; Binder, M.; Majcherczyk, A.; Schneider, P.; Aerts, A.; Asiegbu, F.O.; Baker, S.E.; Barry, K.; Bendiksby, M.; et al. The Plant Cell Wall-Decomposing Machinery Underlies the Functional Diversity of Forest Fungi. Science 2011, 333, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, T.; Nakamura, M.; Hayashi, N.; Ishihara, M. Production of 2,5-dimethoxyhydroquinone by the brown-rot fungus Serpula lacrymans to drive extracellular Fenton reaction. Holzforsch. 2004, 58, 305–310. [Google Scholar] [CrossRef]
- Korripally, P.; Timokhin, V.I.; Houtman, C.J.; Mozuch, M.D.; Hammel, K.E. Evidence from Serpula lacrymans that 2,5-Dimethoxyhydroquinone Is a Lignocellulolytic Agent of Divergent Brown Rot Basidiomycetes. Appl. Environ. Microbiol. 2013, 79, 2377–2383. [Google Scholar] [CrossRef]
- Arantes, V.; Jellison, J.; Goodell, B. Peculiarities of brown-rot fungi and biochemical Fenton reaction with regard to their potential as a model for bioprocessing biomass. Appl. Microbiol. Biotechnol. 2012, 94, 323–338. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Selection of the Solvent and Extraction Conditions for Maximum Recovery of Antioxidant Phenolic Compounds from Coffee Silverskin. Food Bioprocess. Technol. 2014, 7, 1322–1332. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Dey, T.B.; Kuhad, R.C. Enhanced production and extraction of phenolic compounds from wheat by solid-state fermentation with Rhizopus oryzae RCK2012. Biotechnol. Rep. 2014, 4, 120–127. [Google Scholar] [CrossRef]
- Dong, Z.; Gu, F.; Xu, F.; Wang, Q. Comparison of four kinds of extraction techniques and kinetics of microwave-assisted extraction of vanillin from Vanilla planifolia Andrews. Food Chem. 2014, 149, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Sciubba, L.; Di Gioia, D.; Fava, F.; Gostoli, C. Membrane-based solvent extraction of vanillin in hollow fiber contactors. Desalination 2009, 241, 357–364. [Google Scholar] [CrossRef]
- Ghosh, S.; Sachan, S.G.; Mitra, A. Degradation of ferulic acid by a white rot fungus Schizophyllum commune. World J. Microbiol. Biotechnol. 2005, 21, 385–388. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Sahoo, H.P. Standardization of cultural conditions for maximum vanillin production through ferulic acid degradation. Rep. Opin. 2009, 1, 49–51. [Google Scholar]
- Shakeel, F.; Haq, N.; Siddiqui, N.A. Solubility and thermodynamic function of vanillin in ten different environmentally benign solvents. Food Chem. 2015, 180, 244–248. [Google Scholar] [CrossRef]
- Cacace, J.; Mazza, G. Optimization of Extraction of Anthocyanins from Black Currants with Aqueous Ethanol. J. Food Sci. 2003, 68, 240–248. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave Assisted Extraction—An Innovative and Promising Extraction Tool for Medicinal Plant Research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Kumar, K.K.; Ananthakumar, A.A.; Ahmad, R.; Adhikari, S.; Variyar, P.; Sharma, A. Effect of gamma-radiation on major aroma compounds and vanillin glucoside of cured vanilla beans (Vanilla planifolia). Food Chem. 2010, 122, 841–845. [Google Scholar] [CrossRef]
- Zidi, C.; Tayeb, R.; Boukhili, N.; Dhahbi, M. A supported liquid membrane system for efficient extraction of vanillin from aqueous solutions. Sep. Purif. Technol. 2011, 82, 36–42. [Google Scholar] [CrossRef]
- Conde-Mejía, C.; Jiménez-Gutiérrez, A.; Elhalwagi, M.M. A comparison of pretreatment methods for bioethanol production from lignocellulosic materials. Process. Saf. Environ. Prot. 2012, 90, 189–202. [Google Scholar] [CrossRef]
- Yelle, D.J.; Ralph, J.; Lu, F.; Hammel, K.E. Evidence for cleavage of lignin by a brown rot basidiomycete. Environ. Microbiol. 2008, 10, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.; Gavioli, D.; Ferraz, A. Extracellular activities and wood component losses during Pinus taeda biodegradation by the brown-rot fungus Gloeophyllum trabeum. Int. Biodeterior. Biodegradation 2013, 82, 187–191. [Google Scholar] [CrossRef]
- Hibbett, D.S.; Donoghue, M.J. Analysis of Character Correlations Among Wood Decay Mechanisms, Mating Systems, and Substrate Ranges in Homobasidiomycetes. Syst. Biol. 2001, 50, 215–242. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.F.; Liang, J.B.; Rosfarizan, M.; Goh, Y.M.; Shokryazdan, P.; Ho, Y.W. Efficiency of rice straw lignocelluloses degradability by Aspergillus terreus ATCC 74135 in solid state fermentation. Afr. J. Biotechnol. 2011, 10, 4428–4435. [Google Scholar] [CrossRef]
- Tengerdy, R.; Szakacs, G. Bioconversion of lignocellulose in solid substrate fermentation. Biochem. Eng. J. 2003, 13, 169–179. [Google Scholar] [CrossRef]
- Umasaravanan, D.; Jayapriya, J.; Rajendran, R.B. Comparison of lignocellulose biodegradation in solid state fermentation of sugarcane bagasse and rice straw by Aspergillus tamarii. Cey. J. Sci. (Bio. Sci.) 2011, 40, 65. [Google Scholar] [CrossRef]
- Surthikanthi, D.; Suranto; Susilowati, A. Bioconversion of lignocellulosic complex from water hyacinth (Eichorrnia crassipes (Martz) Solms for the production of reducing sugar by Phanerochaete chrysosporium. BioSmart 2005, 7, 17–22. [Google Scholar]
- Hastrup, A.C.S.; Green, F.; Lebow, P.K.; Jensen, B. Enzymatic oxalic acid regulation correlated with wood degradation in four brown-rot fungi. Int. Biodeterior. Biodegradation 2012, 75, 109–114. [Google Scholar] [CrossRef]
- Yoon, J.-J.; Cha, C.-J.; Kim, Y.-S.; Son, D.-W.; Kim, Y.-K. The brown-rot basidiomycete Fomitopsis palustris has the endo-glucanases capable of degrading microcrystalline cellulose. J. Microbiol. Biotechnol. 2007, 17, 800–805. [Google Scholar]
- Mansour, M.M.A.; Hamed, S.A.E.-K.M.; Salem, M.Z.M.; Ali, H.M. Illustration of the Effects of Five Fungi on Acacia saligna Wood Organic Acids and Ultrastructure Alterations in Wood Cell Walls by HPLC and TEM Examinations. Appl. Sci. 2020, 10, 2886. [Google Scholar] [CrossRef]
- Liang, Y.-S.; Yuan, X.; Zeng, G.-M.; Hu, C.-L.; Zhong, H.; Huang, D.-L.; Tang, L.; Zhao, J.-J. Biodelignification of rice straw by Phanerochaete chrysosporium in the presence of dirhamnolipid. Biogeochem. 2010, 21, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Osińska-Jaroszuk, M.; Wlizło, K.; Szałapata, K.; Jarosz-Wilkołazka, A. Correlation between the production of exopolysaccharides and oxalic acid secretion by Ganoderma applanatum and Tyromyces palustris. World J. Microbiol. Biotechnol. 2014, 30, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Ritschkoff, A. Decay Mechanisms of Brown-Rot Fungi; Valtion Teknillinen Tutkimuskeskus (VTT): Helsinki, Finland, 1996; ISBN 9513849260. [Google Scholar]
- Espejo, E.; Agosin, E. Production and Degradation of Oxalic Acid by Brown Rot Fungi. Appl. Environ. Microbiol. 1991, 57, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Antai, S.P.; Crawford, D.L. Applied Microbiology and Biotechnology Degradation of Extractive-free Lignoeelluloses by Coriolus versicolor and Poria placenta. Eur. J. Appl. Microbiol. Biotechnol. 1982, 14, 165–168. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, M.; Zhou, Z.; Zhao, M.; Li, Y. Selective removal of lignin in steam-exploded rice straw by Phanerochaete chrysosporium. Int. Biodeterior. Biodegradation 2012, 75, 89–95. [Google Scholar] [CrossRef]
- Rahman, S.; Mondal, I.H.; Yeasmin, M.S.; Abu Sayeed, M.; Hossain, A.; Ahmed, M.B. Conversion of Lignocellulosic Corn Agro-Waste into Cellulose Derivative and Its Potential Application as Pharmaceutical Excipient. Processes 2020, 8, 711. [Google Scholar] [CrossRef]
- Mukherjee, A.; Banerjee, S.; Halder, G. Parametric optimization of delignification of rice straw through central composite design approach towards application in grafting. J. Adv. Res. 2018, 14, 11–23. [Google Scholar] [CrossRef]
- Nurika, I.; Suhartini, S.; Barker, G.C. Biotransformation of Tropical Lignocellulosic Feedstock Using the Brown rot Fungus Serpula lacrymans. Waste Biomass Valori. 2019, 11, 2689–2700. [Google Scholar] [CrossRef]
- Peralbo-Molina, Á.; Priego-Capote, F.; De Castro, M.D.L. Comparison of extraction methods for exploitation of grape skin residues from ethanol distillation. Talanta 2012, 101, 292–298. [Google Scholar] [CrossRef]
- Qu, W.; Pan, Z.; Ma, H. Extraction modeling and activities of antioxidants from pomegranate marc. J. Food Eng. 2010, 99, 16–23. [Google Scholar] [CrossRef]
- Dağdelen, A.; Dağdelen, A.F. Extraction of vanillin from sugared vanillin used in bakery products: An optimization of conventional soxhlet extraction and development of ultrasound assisted extraction. J. Agric. Fac. Uludag Univ. 2016, 30, 436–439. [Google Scholar]
- Rasoamandrary, N.; Fernandes, A.M.; Bashari, M. Improved extraction of vanillin 4-hydroxy-3-methoxybenzaldehyde from cured vanilla beans using ultrasound-assisted extraction: A comparison of ultrasound-assisted and hot water bath extraction. Akad. Gıda 2013, 11, 6–12. [Google Scholar]
- Anuar, N.; Adnan, A.F.M.; Saat, N.; Aziz, N.; Taha, R.M. Optimization of Extraction Parameters by Using Response Surface Methodology, Purification, and Identification of Anthocyanin Pigments in Melastoma malabathricum Fruit. Sci. World J. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, D.; Rekha, B.N.; Gogate, P.R.; Rathod, V.K. Extraction of vanillin from vanilla pods: A comparison study of conventional soxhlet and ultrasound assisted extraction. J. Food Eng. 2009, 93, 421–426. [Google Scholar] [CrossRef]
- Copeland, K.A.F. Design and Analysis of Experiments, 5th Ed. J. Qual. Technol. 2001, 33, 382–383. [Google Scholar] [CrossRef]
- Kaur, B.; Chakraborty, D. Statistical media and process optimization for biotransformation of rice bran to vanillin using Pediococcus acidilactici. Indian J. Exp. Boil. 2013, 51, 935–943. [Google Scholar]
- Converti, A.; Aliakbarian, B.; Domínguez, J.M.; Vázquez, G.B.; Perego, P. Microbial production of biovanillin. Braz. J. Microbiol. 2010, 41, 519–530. [Google Scholar] [CrossRef]
- Dong, L.; Lü, L.-B.; Lai, R. Molecular cloning of Tupaia belangeri chinensis neuropeptide Y and homology comparison with other analogues from primates. Zool. Res. 2013, 33, 75–78. [Google Scholar] [CrossRef]
- Dal Bello, E. Vanillin production from ferulic acid with Pseudomonas fluorescens BF13-1p4. Ph.D. Thesis, Università di Bologna, Bologna, Italy, 2013. [Google Scholar]
- Nazila, M.; Ismail, M.; Forough, N. Bioconversion of ferulic acid to vanillin by combined action of Aspergillus niger K8 and Phanerochaete crysosporium ATCC 24725. Afr. J. Biotechnol. 2013, 12, 6618–6624. [Google Scholar] [CrossRef]
- Lun, O.K.; Wai, T.B.; Ling, L.S. Pineapple cannery waste as a potential substrate for microbial biotranformation to produce vanillic acid and vanillin. Int. Food Res. J. 2014, 21, 953–958. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 2005, 31, 426–428. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Barbosa, E.d.S.; Perrone, D.; Vendramini, A.L.d.A.; Leite, S.G.F. Vanillin production by Phanerochaete chrysosporium grown on green coconut agro-industrial husk in solid state fermentation. BioResources 2008, 3, 1042–1050. [Google Scholar]
- Ong, E.S.; Heng, M.Y.; Tan, S.N.; Yong, J.W.; Koh, H.; Teo, C.C.; Hew, C.S. Determination of gastrodin and vanillyl alcohol in Gastrodia elata Blume by pressurized liquid extraction at room temperature. J. Sep. Sci. 2007, 30, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, I.S.C.; Basri, M.; Masoumi, H.R.F.; Chee, W.J.; Ashari, S.E.; Ismail, M. Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem. Central J. 2017, 11, 1–11. [Google Scholar] [CrossRef]

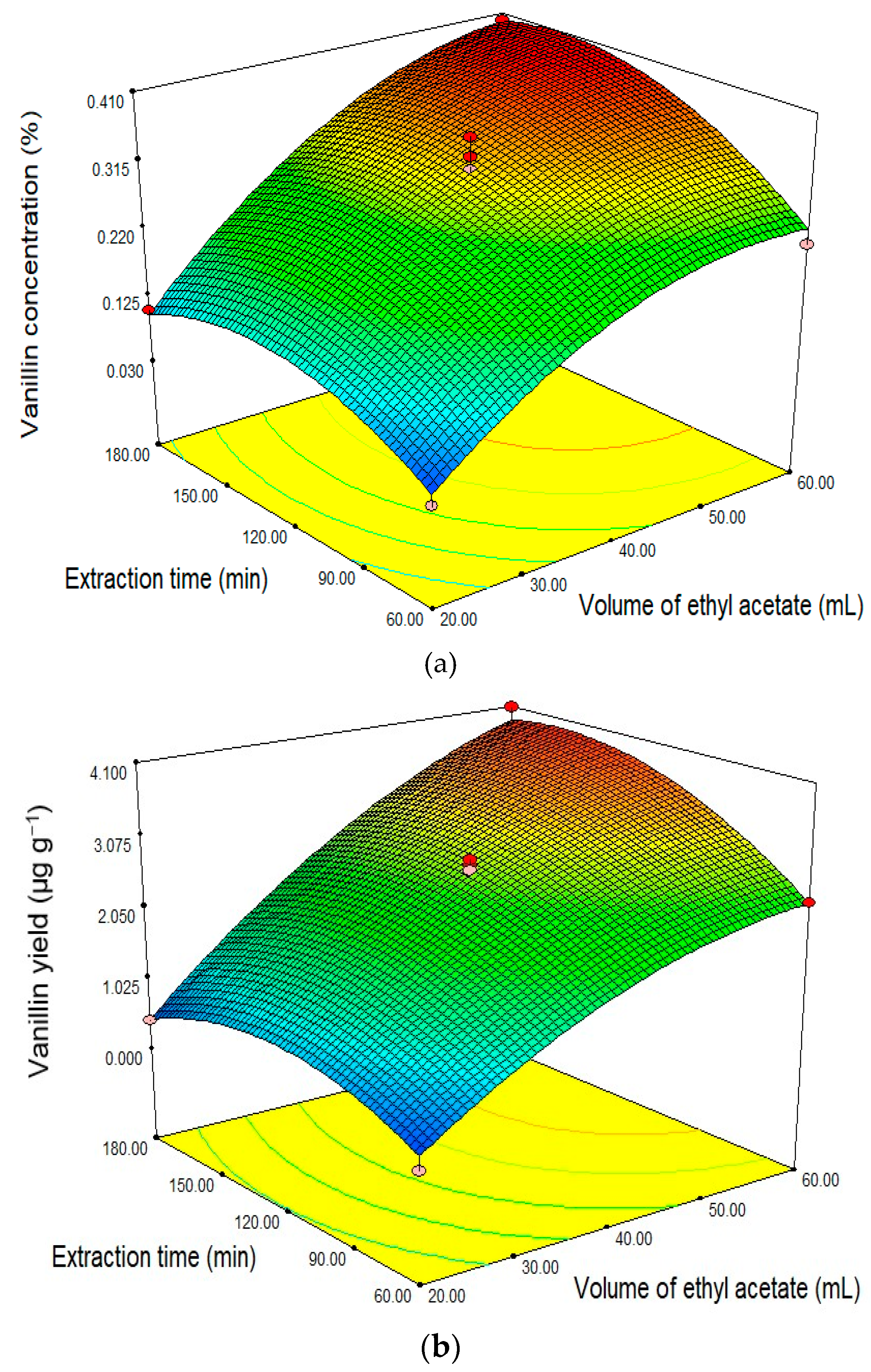
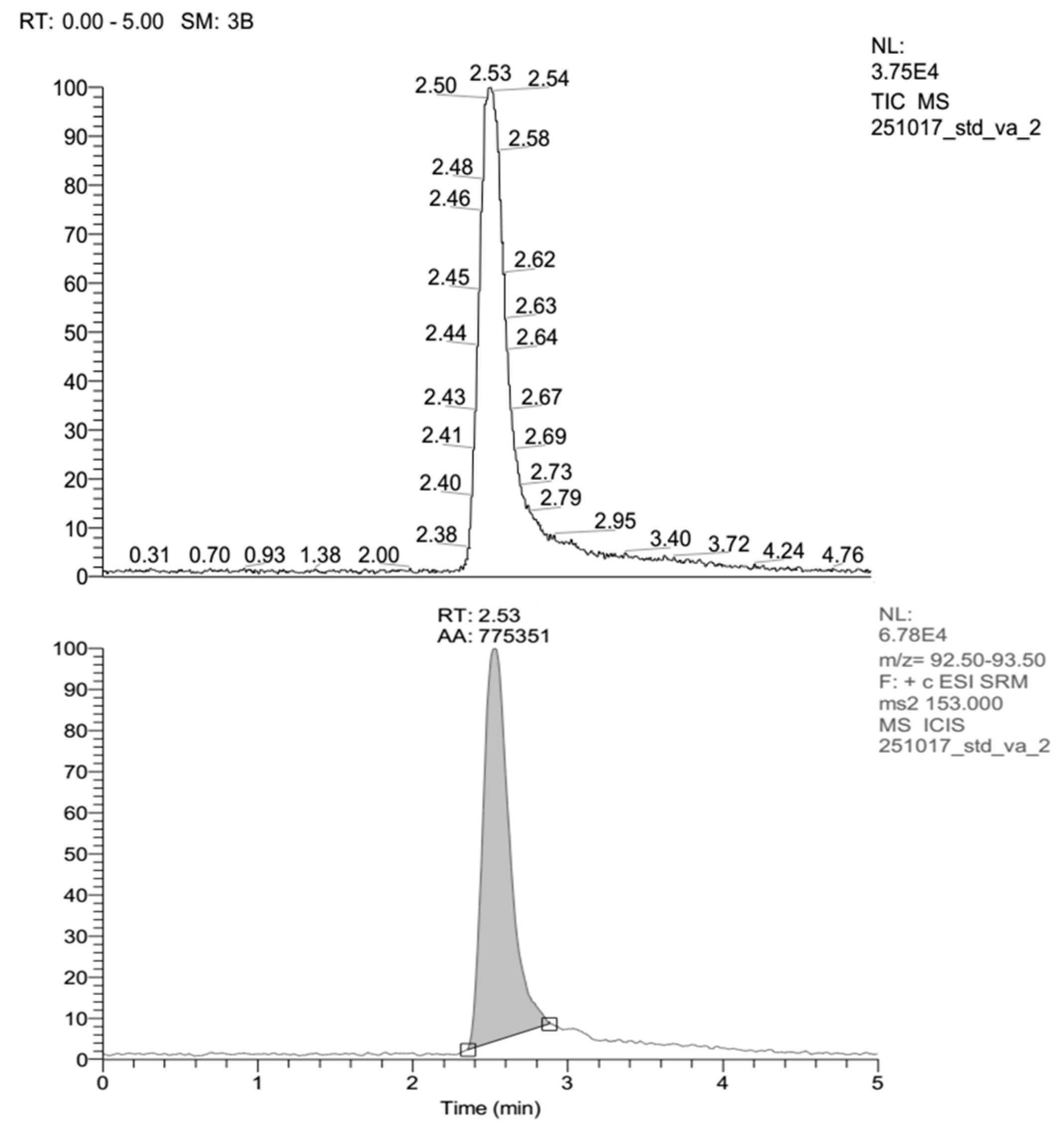
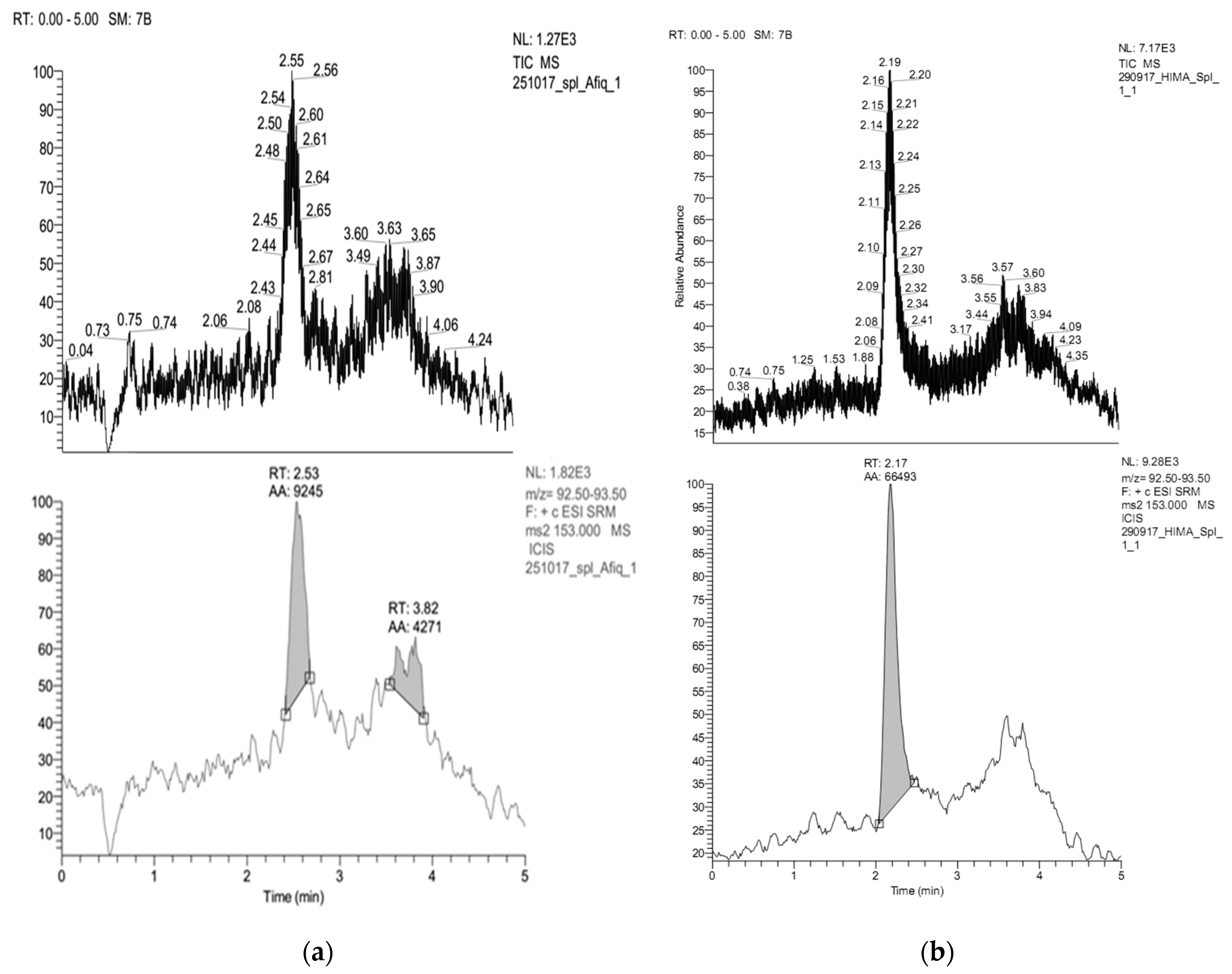
| Run | Code Variable | Actual Variable | Response | |||
|---|---|---|---|---|---|---|
| X1 (Solvent Volume) | X2 (Extraction Time) | Volume Ethanol (mL) | Extraction Time (min) | Vanillin Concentration (%) | Vanillin Yield (µg g−1) | |
| 1 | −1 | −1 | 20 | 120 | 0.014 | 0.121 |
| 2 | 1 | −1 | 60 | 120 | 0.014 | 0.130 |
| 3 | −1 | 1 | 20 | 360 | 0.020 | 0.178 |
| 4 | 1 | 1 | 60 | 360 | 0.018 | 0.163 |
| 5 | −1.414 | 0 | 11.72 | 240 | 0.019 | 0.162 |
| 6 | 1.414 | 0 | 68.28 | 240 | 0.014 | 0.114 |
| 7 | 0 | −1.414 | 40 | 70.29 | 0.033 | 0.685 |
| 8 | 0 | 1.414 | 40 | 409.71 | 0.067 | 1.207 |
| 9 | 0 | 0 | 40 | 240 | 0.124 | 2.412 |
| 10 | 0 | 0 | 40 | 240 | 0.161 | 2.995 |
| 11 | 0 | 0 | 40 | 240 | 0.116 | 2.190 |
| 12 | 0 | 0 | 40 | 240 | 0.150 | 2.773 |
| 13 | 0 | 0 | 40 | 240 | 0.132 | 2.596 |
| Run | Code Variable | Actual Variable | Response | |||
|---|---|---|---|---|---|---|
| X1 (Solvent Volume) | X2 (Extraction Time) | Volume Ethyl Acetate (mL) | Extraction Time (min) | Vanillin Concentration (%) | Vanillin Yield (µg g−1) | |
| 1 | −1 | −1 | 20 | 60.00 | 0.044 | 0.176 |
| 2 | 1 | −1 | 60 | 60.00 | 0.234 | 2.481 |
| 3 | −1 | 1 | 20 | 180.00 | 0.105 | 0.418 |
| 4 | 1 | 1 | 60 | 180.00 | 0.399 | 4.077 |
| 5 | −1.414 | 0 | 11.72 | 120.00 | 0.032 | 0.075 |
| 6 | 1.414 | 0 | 68.28 | 120.00 | 0.387 | 3.722 |
| 7 | 0 | −1.414 | 40 | 35.15 | 0.164 | 1.310 |
| 8 | 0 | 1.414 | 40 | 204.85 | 0.263 | 2.105 |
| 9 | 0 | 0 | 40 | 120.00 | 0.337 | 2.826 |
| 10 | 0 | 0 | 40 | 120.00 | 0.364 | 2.912 |
| 11 | 0 | 0 | 40 | 120.00 | 0.291 | 2.654 |
| 12 | 0 | 0 | 40 | 120.00 | 0.313 | 2.718 |
| 13 | 0 | 0 | 40 | 120.00 | 0.318 | 2.761 |
| Term | Coefficient | Standard Error | F-Value | p-Value |
|---|---|---|---|---|
| Vanillin Concentration | ||||
| Intercept | 0.14 | 0.0078 | ||
| X1 | −0.0012 | 0.0062 | 0.036 | 0.8555 |
| X2 | 0.0073 | 0.0062 | 1.39 | 0.2767 |
| X1X2 | −0.0007 | 0.0087 | 0.0065 | 0.9381 |
| X12 | −0.064 | 0.0066 | 94.56 | <0.0001 |
| X22 | −0.047 | 0.0066 | 51.57 | 0.0002 |
| R2 = 0.95 | ||||
| Vanillin Yield | ||||
| Intercept | 2.59 | 0.15 | ||
| X1 | −0.0092 | 0.12 | 0.0063 | 0.9387 |
| X2 | 0.10 | 0.12 | 0.80 | 0.4015 |
| X1X2 | −0.006 | 0.16 | 0.001339 | 0.9718 |
| X12 | −1.33 | 0.12 | 113.77 | <0.0001 |
| X22 | −0.92 | 0.12 | 55.01 | 0.0001 |
| R2 = 0.96 |
| Term | Sum of Square | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Vanillin Concentration | |||||
| Model | 0.040 | 5 | 0.0080 | 26.31 | 0.0002 |
| Residual | 0.0021 | 7 | 0.0003 | ||
| Lack of fit | 0.0007 | 3 | 0.0002 | 0.71 | 0.5947 |
| Pure error | 0.0014 | 4 | 0.0003 | ||
| Total | 0.042 | 12 | |||
| Vanillin Yield | |||||
| Model | 16.29 | 5 | 3.26 | 30.30 | 0.0001 |
| Residual | 0.75 | 7 | 0.11 | ||
| Lack of fit | 0.36 | 3 | 0.12 | 1.25 | 0.4041 |
| Pure error | 0.39 | 4 | 0.097 | ||
| Total | 17.04 | 12 |
| Term | Coefficient | Standard Error | F-Value | p-Value |
|---|---|---|---|---|
| Vanillin Concentration | ||||
| Intercept | 0.32 | 0.011 | ||
| X1 | 0.12 | 0.0090 | 189.47 | <0.0001 |
| X2 | 0.046 | 0.0090 | 26.10 | 0.0014 |
| X1X2 | 0.026 | 0.013 | 4.22 | 0.0792 |
| X12 | −0.062 | 0.009603 | 41.08 | 0.0004 |
| X22 | −0.060 | 0.009603 | 38.46 | 0.0004 |
| R2 = 0.9764 | ||||
| Vanillin Yield | ||||
| Intercept | 2.77 | 0.073 | ||
| X1 | 1.39 | 0.057 | 585.49 | <0.0001 |
| X2 | 0.37 | 0.057 | 41.54 | 0.0004 |
| X1X2 | 0.34 | 0.081 | 17.36 | 0.0042 |
| X12 | −0.44 | 0.062 | 51.37 | 0.0002 |
| X22 | −0.54 | 0.062 | 75.99 | <0.0001 |
| R2 = 0.9908 |
| Term | Sum of Square | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Vanillin Concentration | |||||
| Model | 0.19 | 5 | 0.037 | 58.03 | <0.0001 |
| Residual | 0.0045 | 7 | 0.0006 | ||
| Lack of fit | 0.0015 | 3 | 0.0005 | 0.65 | 0.6214 |
| Pure error | 0.0030 | 4 | 0.0007 | ||
| Total | 0.19 | 12 | |||
| Vanillin Yield | |||||
| Model | 20.00 | 5 | 4.00 | 151.48 | <0.0001 |
| Residual | 0.18 | 7 | 0.026 | ||
| Lack of fit | 0.15 | 3 | 0.048 | 4.91 | 0.0791 |
| Pure error | 0.039 | 4 | 0.0099 | ||
| Total | 20.19 | 12 |
| Criteria | Parameter | Standard Prediction |
|---|---|---|
| Factor | Volume of ethanol (mL) | 39.86 |
| Factor | Extraction time (min) | 247.98 |
| Response | Vanilin concentration (%) | 0.165 |
| Response | Yield vanilin (μg g−1) | 2.596 |
| Desirability | - | 0.848 |
| Criteria | Parameter | Standard Prediction |
|---|---|---|
| Factor | Volume of ethyl acetate (mL) | 60 |
| Factor | Extraction time (min) | 159.56 |
| Response | Vanilin concentration (%) | 0.408 |
| Response | Yield vanilin (μg g−1) | 3.957 |
| Desirability | - | 0.985 |
| Fix Variable | Symbol | Level | ||||
|---|---|---|---|---|---|---|
| −1.414 | −1 | 0 | +1 | +1.414 | ||
| Ethanol | ||||||
| Solvent volume (mL) | X1 | 11.72 | 20 | 40 | 60 | 68.28 |
| Time extraction (min) | X2 | 70.29 | 120 | 240 | 360 | 409.71 |
| Ethyl acetate | ||||||
| Solvent volume (mL) | X1 | 11.72 | 20 | 40 | 60 | 68.28 |
| Time extraction (min) | X2 | 35.15 | 60 | 120 | 180 | 204.85 |
Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurika, I.; Suhartini, S.; Azizah, N.; Barker, G.C. Extraction of Vanillin Following Bioconversion of Rice Straw and Its Optimization by Response Surface Methodology. Molecules 2020, 25, 6031. https://doi.org/10.3390/molecules25246031
Nurika I, Suhartini S, Azizah N, Barker GC. Extraction of Vanillin Following Bioconversion of Rice Straw and Its Optimization by Response Surface Methodology. Molecules. 2020; 25(24):6031. https://doi.org/10.3390/molecules25246031
Chicago/Turabian StyleNurika, Irnia, Sri Suhartini, Nurul Azizah, and Guy C. Barker. 2020. "Extraction of Vanillin Following Bioconversion of Rice Straw and Its Optimization by Response Surface Methodology" Molecules 25, no. 24: 6031. https://doi.org/10.3390/molecules25246031
APA StyleNurika, I., Suhartini, S., Azizah, N., & Barker, G. C. (2020). Extraction of Vanillin Following Bioconversion of Rice Straw and Its Optimization by Response Surface Methodology. Molecules, 25(24), 6031. https://doi.org/10.3390/molecules25246031