Present Status and Future Trends of Natural-Derived Compounds Targeting T Helper (Th) 17 and Microsomal Prostaglandin E Synthase-1 (mPGES-1) as Alternative Therapies for Autoimmune and Inflammatory-Based Diseases
Abstract
1. Introduction
2. Selected Studies and Inclusion and Exclusion Criteria
3. Natural Compounds Targeting COX-2/mPGES-1/PGE2 Cascade in Inflammatory-Based Diseases
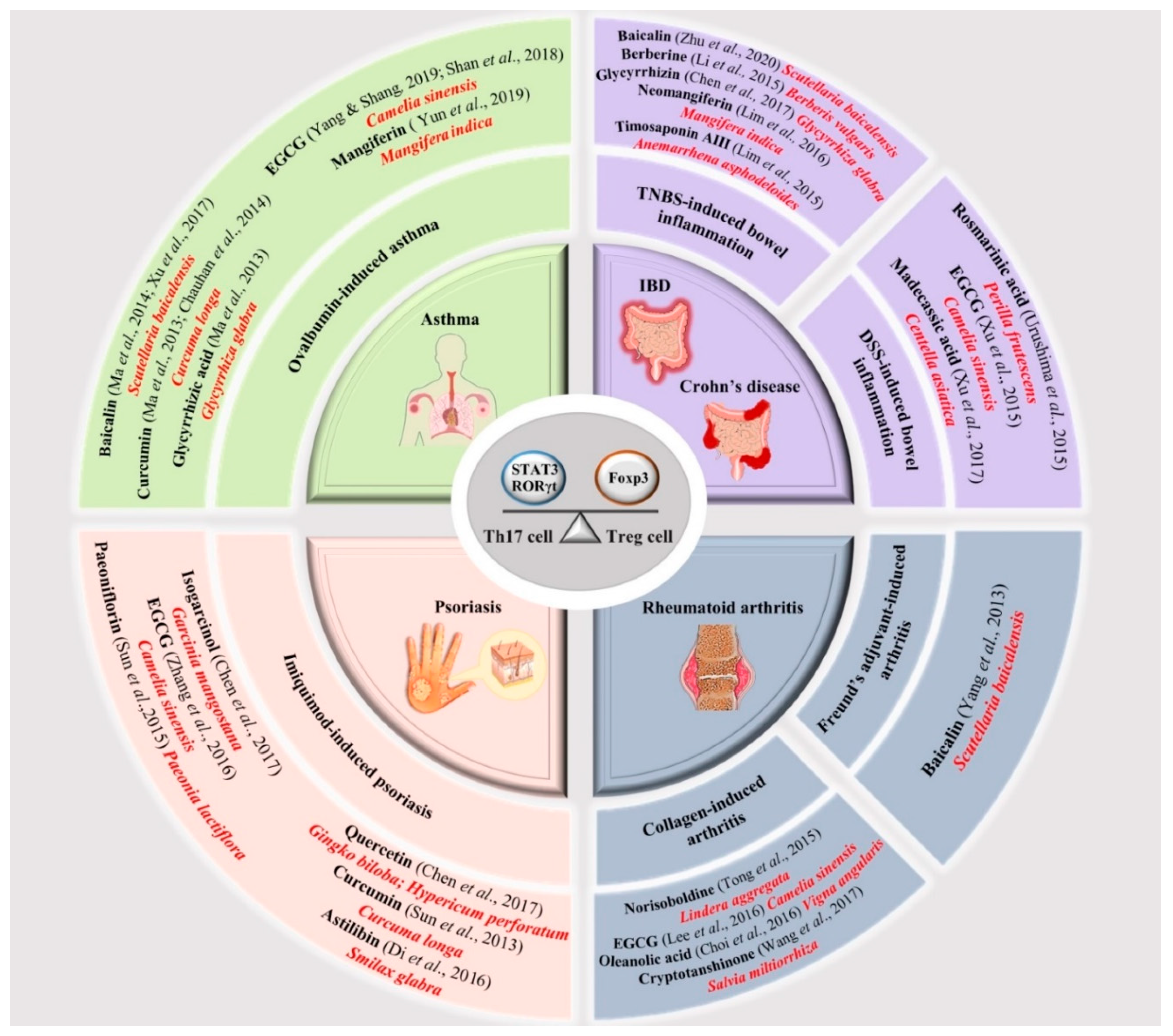
4. Natural Compounds Targeting Th17/Treg Axis in Immune-Mediated Inflammatory Diseases
5. Conclusions and Future Prospect
Author Contributions
Funding
Conflicts of Interest
References
- Cousyn, G.; Dalfrà, S.; Scarpa, B.; Geelen, J.; Anton, R.; Serafini, M.; Delmulle, L. Project BELFRIT: Harmonizing the Use of Plants in Food Supplements in the European Union: Belgium, France and Italy–AFirst Step. Eur. Food Feed Law 2013, 8, 187–196. [Google Scholar]
- Regulation (EC) No 258/97 of the European Parliament and of the Council of 27 January 1997 Concerning Novel Foods and Novel Food Ingredients; EC: Brussels, Belgium, 1997; pp. 1–6.
- 2008/558/EC: Commission Decision of 27 June 2008 Authorising the Placing on the Market of Refined Echium Oil as Novel Food Ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council; EC: Brussels, Belgium, 2008; pp. 17–19.
- Commission Decision 2000/196/EC of 22 February 2000 Refusing the Placing on the Market of Stevia Rebaudiana Bertoni: Plants and Dried Leaves’ as a Novel Food or Novel Food Ingredient under Regulation No 258/97; EC: Brussels, Belgium, 2000; p. 14.
- Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the Approximation of the Laws of the Member States Relating to Food Supplements; EC: Brussels, Belgium, 2002; pp. 51–57.
- Committee on Herbal Medicinal Products (HMPC) European Union Herbal Monograph on Hedera Helix L., Folium; 24 November 2015EMA/HMPC/586888/2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/HerbalHerbal_monograph/2016/01/WC500199890.pdf (accessed on 18 December 2020).
- Maione, F.; Russo, R.; Kha, H.; Mascolo, N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2016, 12, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Teixeira, M.; Alexander, S.P.H.; Cirino, G.; Docherty, J.R.; George, C.H.; Insel, P.A.; Ji, Y.; Kendall, D.A.; Panattieri, R.A.; et al. A practical guide for transparent reporting of research on natural products in the British Journal of Pharmacology: Reproducibility of natural product research. Br. J. Pharmacol. 2020, 10, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- López-Varela, S.; González-Gross, M.; Marcos, A. Functional foods and the immune system: A review. Eur. J. Clin. Nutr. 2002, 56, S29–S33. [Google Scholar] [CrossRef] [PubMed]
- Granado-Lorencio, F.; Hernández-Alvarez, E. Functional Foods and Health Effects: A Nutritional Biochemistry Perspective. Curr. Med. Chem. 2016, 23, 2929–2957. [Google Scholar] [CrossRef]
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and Functional Foods: The Foods for the Future World. Crit. Rev. Food Sci. Nutr. 2016, 16, 2617–2627. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Hoyles, L.; Vulevic, J. Diet, immunity and functional foods. Adv. Exp. Med. Biol. 2008, 635, 79–92. [Google Scholar] [CrossRef]
- Sattigere, V.D.; Kumar, P.R.; Prakash, V. Science-based regulatory approach for safe nutraceuticals. J. Sci. Food Agric. 2020, 14, 5079–5082. [Google Scholar] [CrossRef]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 9, 685–698. [Google Scholar] [CrossRef]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.J.; Varady, K.A. Are functional foods redefining nutritional requirements? Appl. Physiol. Nutr. Metab. 2008, 1, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Eussen, S.R.; Verhagen, H.; Klungel, O.H.; Garssen, J.; van Loveren, H.; van Kranen, H.J.; Rompelberg, C.J. Functional foods and dietary supplements: Products at the interface between pharma and nutrition. Eur. J. Pharmacol. 2011, 668, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, D.L.; Fernández-Ruiz, V.; Cámara, M. The frontier between nutrition and pharma: The international regulatory framework of functional foods, food supplements and nutraceuticals. Food Sci. Nutr. 2020, 10, 1738–1746. [Google Scholar] [CrossRef]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017, 1, 8–19. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. To Nutraceuticals and Back: Rethinking a Concept. Foods 2017, 6, 74. [Google Scholar] [CrossRef]
- Adefegha, S.A. Functional Foods and Nutraceuticals as Dietary Intervention in Chronic Diseases; Novel Perspectives for Health Promotion and Disease Prevention. J. Diet. Suppl. 2018, 6, 977–1009. [Google Scholar] [CrossRef]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 4, 659–672. [Google Scholar] [CrossRef]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert. Rev. Clin. Pharmacol. 2019, 12, 1–7. [Google Scholar] [CrossRef]
- Sultan, M.T.; Butt, M.S.; Qayyum, M.M.; Suleria, H.A. Immunity: Plants as effective mediators. Food Sci. Nutr. 2014, 10, 1298–1308. [Google Scholar] [CrossRef]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2019, 15, 3160. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010, 6, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ji, Y.; Kersten, S.; Qi, L. Mechanisms of inflammatory responses in obese adipose tissue. Annu. Rev. Nutr. 2012, 32, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Perretti, M.; D’Acquisto, F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 2009, 9, 62–70. [Google Scholar] [CrossRef] [PubMed]
- D’Acquisto, F.; Maione, F.; Pederzoli-Ribeil, M. From IL-15 to IL-33: The never-ending list of new players in inflammation. Is it time to forget the humble aspirin and move ahead? Biochem. Pharmacol. 2010, 79, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.D.; Alder, M.N. The evolution of adaptive immune systems. Cell 2006, 124, 815–822. [Google Scholar] [CrossRef]
- Varela, M.L.; Mogildea, M.; Moreno, I.; Lopes, A. Acute Inflammation and Metabolism. Inflammation 2018, 4, 1115–1127. [Google Scholar] [CrossRef]
- Perretti, M.; Cooper, D.; Dalli, J.; Norling, L.V. Immune resolution mechanisms in inflammatory arthritis. Nat. Rev. Rheumatol. 2017, 13, 87–99. [Google Scholar] [CrossRef]
- Ge, Y.; Huang, M.; Yao, Y.M. Autophagy and Proinflammatory Cytokines: Interactions and Clinical Implications. Cytokine Growth Factor Rev. 2018, 43, 38–46. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 7203, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Higgs, G.A.; Moncada, S.; Vane, J.R. Eicosanoids in inflammation. Ann. Clin. Res. 1984, 16, 287–299. [Google Scholar] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015, 3, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Naraba, H.; Tanioka, T.; Semmyo, N.; Nakatani, Y.; Kojima, F.; Ikeda, T.; Fueki, M.; Ueno, A.; Oh, S. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 2000, 32783–33279. [Google Scholar] [CrossRef]
- Stichtenoth, D.O.; Thorén, S.; Bian, H.; Peters-Golden, M.; Jakobsson, P.J.; Crofford, L.J. Microsomal prostaglandin E synthase is regulated by proinflammatory cytokines and glucocorticoids in primary rheumatoid synovial cells. J. Immunol. 2001, 167, 469–474. [Google Scholar] [CrossRef]
- Saegusa, M.; Murakami, M.; Nakatani, Y.; Yamakawa, K.; Katagiri, M.; Matsuda, K.; Kawaguchi, H. Contribution of membrane-associated prostaglandin E2 synthase to bone resorption. J. Cell. Physiol. 2003, 197, 348–356. [Google Scholar] [CrossRef]
- Li, X.; Afif, H.; Cheng, S.; Martel-Pelletier, J.; Pelletier, J.P.; Ranger, P.; Fahmi, H. Expression and regulation of microsomal prostaglandin E synthase-1 in human osteoarthritic cartilage and chondrocytes. J. Rheumatol. 2005, 32, 887–895. [Google Scholar]
- Samuelsson, B.; Morgenstern, R.; Jakobsson, P.J. Membrane prostaglandin E synthase-1: A novel therapeutic target. Pharmacol. Rev. 2007, 3, 207–224. [Google Scholar] [CrossRef]
- Koeberle, A.; Werz, O. Perspective of microsomal prostaglandin E2 synthase-1 as drug target in inflammation-related disorders. Biochem. Pharmacol. 2015, 1, 1–15. [Google Scholar] [CrossRef]
- Raucci, F.; Iqbal, A.J.; Saviano, A.; Minosi, P.; Piccolo, M.; Irace, C.; Caso, F.; Scarpa, R.; Pieretti, S.; Mascolo, N.; et al. IL-17A neutralizing antibody regulates monosodium urate crystal-induced gouty inflammation. Pharmacol. Res. 2019, 147, 104351. [Google Scholar] [CrossRef] [PubMed]
- Maione, F.; Casillo, G.M.; Raucci, F.; Iqbal, A.J.; Mascolo, N. The functional link between microsomal prostaglandin E synthase-1 (mPGES-1) and peroxisome proliferator-activated receptor γ (PPARγ) in the onset of inflammation. Pharmacol. Res. 2020, 157, 104807. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Meuillet, E.J. Identification and development of mPGES-1 inhibitors: Where we are at? Future Med. Chem. 2011, 15, 1909–1934. [Google Scholar] [CrossRef]
- Koeberle, A.; Werz, O. Inhibitors of the microsomal prostaglandin E (2) synthase-1 as alternative to non steroidal anti-inflammatory drugs (NSAIDs)—A critical review. Curr. Med. Chem. 2009, 32, 4274–4496. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, F.; Morgenstern, R.; Jakobsson, P.J. A review on mPGES-1 inhibitors: From preclinical studies to clinical applications. Prostaglandins Other Lipid Mediat. 2020, 147, 106383. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, A.; Bauer, J.; Verhoff, M.; Hoffmann, M.; Northoff, H.; Werz, O. Green tea epigallocatechin-3-gallate inhibits microsomal prostaglandin E(2) synthase-1. Biochem. Biophys. Res. Comm. 2009, 2, 350–354. [Google Scholar] [CrossRef]
- Koeberle, A.; Northoff, H.; Werz, O. Curcumin blocks prostaglandin E2 biosynthesis through direct inhibition of the microsomal prostaglandin E2 synthase-1. Mol. Cancer Ther. 2009, 8, 2348–2355. [Google Scholar] [CrossRef]
- Siemoneit, U.; Koeberle, A.; Rossi, A.; Dehm, F.; Verhoff, M.; Reckel, S.; Maier, T.J.; Jauch, J.; Northoff, H.; Bernhard, F.; et al. Inhibition of microsomal prostaglandin E2 synthase-1 as a molecular basis for the anti-inflammatory actions of boswellic acids from frankincense. Br. J. Pharmacol. 2011, 162, 147–162. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 11, 1618. [Google Scholar] [CrossRef]
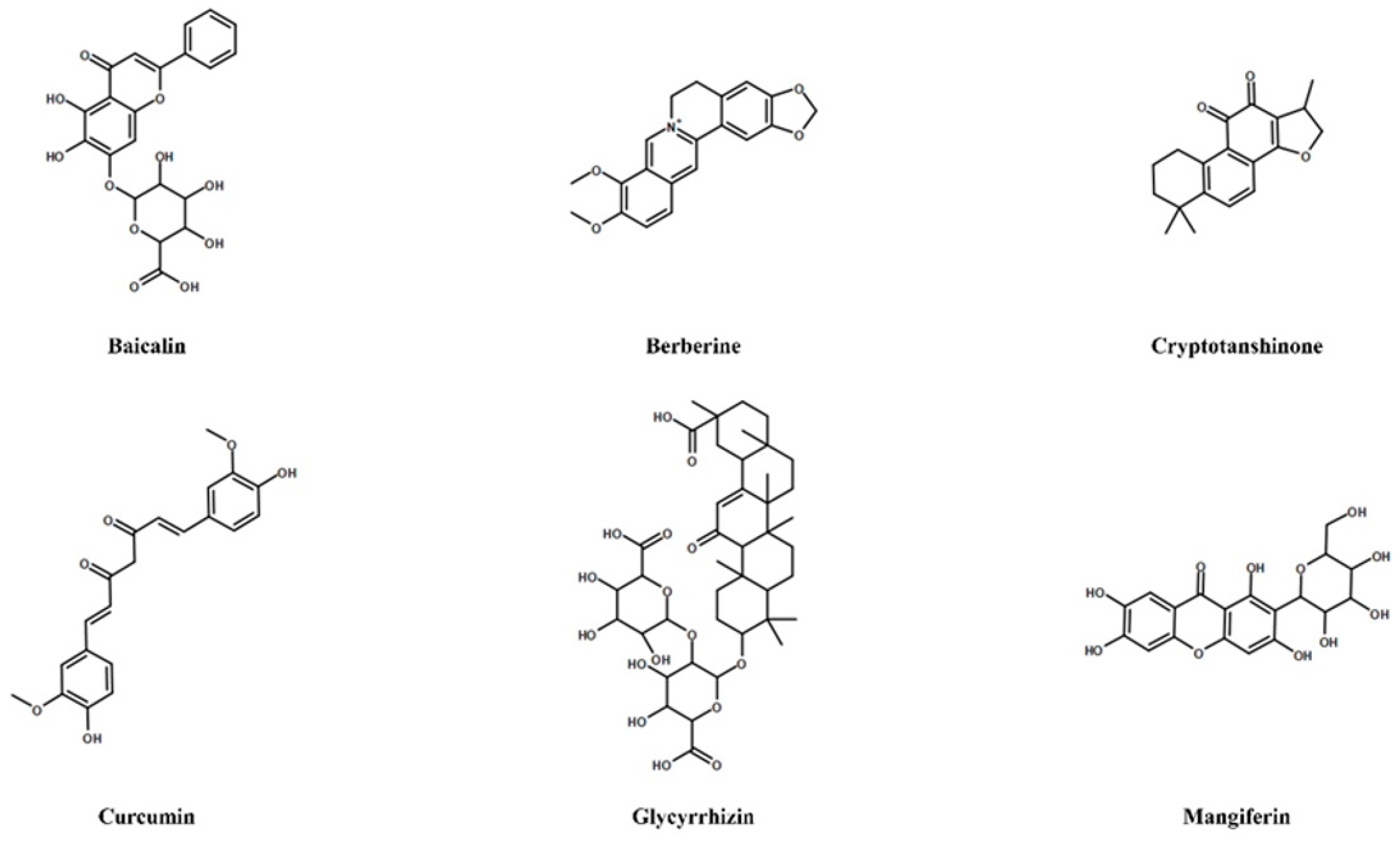
- Cui, L.; Feng, L.; Zhang, Z.H.; Jia, X.B. The anti-inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF-κB pathway activation. Int. Immunopharmacol. 2014, 23, 294–303. [Google Scholar] [CrossRef]
- Wang, C.; Song, Y.; Wang, X.; Mao, R.; Song, L. Baicalin Ameliorates Collagen-Induced Arthritis through the Suppression of Janus Kinase 1 (JAK1)/Signal Transducer and Activator of Transcription 3 (STAT3) Signaling in Mice. Med. Sci. Monit. 2018, 24, 9213–9222. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Surh, Y.J.; Do, S.G.; Shin, E.; Shim, K.S.; Lee, C.K.; Na, H.K.J. Baicalein Inhibits Dextran Sulfate Sodium-induced Mouse Colitis. Cancer Prev. 2019, 24, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, H.; Ou, G.; Ren, L.; Yang, X.; Zeng, M. Curcumin attenuates MSU crystal-induced inflammation by inhibiting the degradation of IκBα and blocking mitochondrial damage. Arthritis Res. Ther. 2019, 2, 193. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Kim, M.O.; Choi, Y.H.; Park, Y.M.; Kim, G.Y. Curcumin attenuates inflammatory response in IL-1beta-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int. Immunopharmacol. 2010, 10, 605–610. [Google Scholar] [CrossRef]
- He, W.; Li, Y.; Liu, M.; Yu, H.; Chen, Q.; Chen, Y.; Ruan, J.; Ding, Z.; Zhang, Y.; Wang, T. Citrus aurantium L. and Its Flavonoids Regulate TNBS-Induced Inflammatory Bowel Disease through Anti-Inflammation and Suppressing Isolated Jejunum Contraction. Int. J. Mol. Sci. 2018, 19, 3057. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Cárdeno, A.; de la Lastra, C.A. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem. Pharmacol. 2011, 82, 737–745. [Google Scholar] [CrossRef]
- Marín, M.; Giner, R.M.; Ríos, J.L.; Recio Mdel, C. Protective effect of apocynin in a mouse model of chemically-induced colitis. Planta Med. 2013, 79, 1392–1400. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Nam, S.J.; Chun, W.; Kim, S.I.; Park, S.C.; Kang, C.D.; Lee, S.J. Anti-inflammatory effects of apocynin on dextran sulfate sodium-induced mouse colitis model. PLoS ONE 2019, 14, e0217642. [Google Scholar] [CrossRef]
- Yang, L.; Shen, L.; Li, Y.; Li, Y.; Yu, S.; Wang, S. Hyperoside attenuates dextran sulfate sodium-induced colitis in mice possibly via activation of the Nrf2 signalling pathway. J. Inflamm. 2017, 14, 25. [Google Scholar] [CrossRef]
- Dou, W.; Zhang, J.; Ren, G.; Ding, L.; Sun, A.; Deng, C.; Wu, X.; Wei, X.; Mani, S.; Wang, Z. Mangiferin attenuates the symptoms of dextran sulfate sodium-induced colitis in mice via NF-κB and MAPK signaling inactivation. Int. Immunopharmacol. 2014, 23, 170–178. [Google Scholar] [CrossRef]
- Lee, I.A.; Hyun, Y.J.; Kim, D.H. Berberine ameliorates TNBS-induced colitis by inhibiting lipid peroxidation, enterobacterial growth and NF-κB activation. Eur. J. Pharmacol. 2010, 648, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.W.; Bonet, I.J.M.; Tambeli, C.H.; de-Faria, F.M.; Parada, C.A. Local administration of mangiferin prevents experimental inflammatory mechanical hyperalgesia through CINC-1/epinephrine/PKA pathway and TNF-α inhibition. Eur. J. Pharmacol. 2018, 830, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, J.; Zhao, J.M.; Guan, J.; Li, W.; Xie, Q.M.; Zhao, Y.Q. Cedrol attenuates collagen-induced arthritis in mice and modulates the inflammatory response in LPS-mediated fibroblast-like synoviocytes. Food Funct. 2020, 11, 4752–4764. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Duan, C.; Chen, H.; Wang, C.; Liu, X.; Qiu, M.; Tang, H.; Zhang, F.; Zhou, X.; Yang, J. Inhibition of COX-2/mPGES-1 and 5-LOX in macrophages by leonurine ameliorates monosodium urate crystal-induced inflammation. Toxicol. Appl. Pharmacol. 2018, 351, 1–11. [Google Scholar] [CrossRef]
- Li, H.; Gong, X.; Zhang, L.; Zhang, Z.; Luo, F.; Zhou, Q.; Chen, J.; Wan, J. Madecassoside attenuates inflammatory response on collagen-induced arthritis in DBA/1 mice. Phytomedicine 2009, 16, 538–546. [Google Scholar] [CrossRef]
- Song, J.H.; Lee, J.W.; Shim, B.; Lee, C.Y.; Choi, S.; Kang, C.; Sohn, N.W.; Shin, J.W. Glycyrrhizin alleviates neuroinflammation and memory deficit induced by systemic lipopolysaccharide treatment in mice. Molecules 2013, 18, 15788–15803. [Google Scholar] [CrossRef]
- Wang, H.L.; Li, Y.X.; Niu, Y.T.; Zheng, J.; Wu, J.; Shi, G.J.; Ma, L.; Niu, Y.; Sun, T.; Yu, J.Q. Observing Anti-inflammatory and Anti-nociceptive Activities of Glycyrrhizin Through Regulating COX-2 and Pro-inflammatory Cytokines Expressions in Mice. Inflammation 2015, 38, 2269–2278. [Google Scholar] [CrossRef]
- Maione, F.; Piccolo, M.; De Vita, S.; Chini, M.G.; Cristiano, C.; De Caro, C.; Lippiello, P.; Miniaci, M.C.; Santamaria, R.; Irace, C.; et al. Down regulation of pro-inflammatory pathways by tanshinone IIA and cryptotanshinone in a non-genetic mouse model of Alzheimer’s disease. Pharmacol. Res. 2018, 129, 482–490. [Google Scholar] [CrossRef]
- Zhang, F.X.; Xu, R.S. Juglanin ameliorates LPS-induced neuroinflammation in animal models of Parkinson’s disease and cell culture via inactivating TLR4/NF-κB pathway. Biomed. Pharmacother. 2018, 97, 1011–1019. [Google Scholar] [CrossRef]
- Halawany, A.M.E.; Sayed, N.S.E.; Abdallah, H.M.; Dine, R.S.E. Protective effects of gingerol on streptozotocin-induced sporadic Alzheimer’s disease: Emphasis on inhibition of β-amyloid, COX-2, alpha-, beta-secretases and APH1a. Sci. Rep. 2017, 7, 2902. [Google Scholar] [CrossRef]
- Maione, F.; Cantone, V.; Pace, S.; Chini, M.G.; Bisio, A.; Romussi, G.; Pieretti, S.; Werz, O.; Koeberle, A.; Mascolo, N.; et al. Anti-inflammatory and analgesic activity of carnosol and carnosic acid in vivo and in vitro and in silico analysis of their target interactions. Br. J. Pharmacol. 2017, 174, 1497–1508. [Google Scholar] [CrossRef]
- Chen, J.; Si, M.; Wang, Y.; Liu, L.; Zhang, Y.; Zhou, A.; Wei, W. Ginsenoside metabolite compound K exerts anti-inflammatory and analgesic effects via downregulating COX2. Inflammopharmacology 2019, 27, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Noack, M.; Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 2014, 13, 668–677. [Google Scholar] [CrossRef]
- Zhu, J.; Paul, W.E. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol. Rev. 2010, 1, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Li, M.O.; Rudensky, A.Y. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol. 2016, 4, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor ROR gammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 6, 1121–1133. [Google Scholar] [CrossRef]
- Bettelli, E.; Korn, T.; Oukka, M.; Kuchroo, V.K. Induction and effector functions of Th17 cells. Nature 2008, 453, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Samson, M.; Audia, S.; Janikashvili, N.; Ciudad, M.; Trad, M.; Fraszczak, J.; Ornetti, P.; Maillefert, J.F.; Miossec, P.; Bonnotte, B. Brief report: Inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum. 2012, 64, 2499–2503. [Google Scholar] [CrossRef]
- Li, S.; Wu, Z.; Li, L.; Liu, X. Interleukin-6 IL-6 receptor antagonist protects against rheumatoid arthritis. Int. Med. J. Exp. Clin. Res. 2016, 22, 2113–2118. [Google Scholar] [CrossRef]
- Fasching, P.; Stradner, M.; Graninger, W.; Dejaco, C.; Fessler, J. Therapeutic potential of targeting the Th17/Treg axis in autoimmune disorders. Molecules 2017, 22, 134. [Google Scholar] [CrossRef]
- Cristiano, C.; Volpicelli, F.; Lippiello, P.; Buono, B.; Raucci, F.; Piccolo, M.; Iqbal, A.J.; Irace, C.; Miniaci, M.C.; Perrone Capano, C.; et al. Neutralization of IL-17 rescues amyloid-β-induced neuroinflammation and memory impairment. Br. J. Pharmacol. 2019, 18, 3544–3557. [Google Scholar] [CrossRef] [PubMed]
- Raucci, F.; Iqbal, A.J.; Saviano, A.; Casillo, G.M.; Russo, M.; Lezama, D.; Mascolo, N.; Maione, F. In-depth immunophenotyping data relating to IL-17Ab modulation of circulating Treg/Th17 cells and of in situ infiltrated inflammatory monocytes in the onset of gouty inflammation. Data Brief. 2019, 25, 104381. [Google Scholar] [CrossRef] [PubMed]
- Littman, D.R.; Rudensky, A.Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 2010, 6, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 7090, 235–238. [Google Scholar] [CrossRef]
- Mangan, P.R.; Harrington, L.E.; O’Quinn, D.B.; Helms, W.S.; Bullard, D.C.; Elson, C.O.; Hatton, R.D.; Wahl, S.M.; Schoeb, T.R.; Weaver, C.T. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 2006, 7090, 231–234. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Zhou, L.; Littman, D.R. Transcriptional regulation of Th17 cell differentiation. Semin. Immunol. 2007, 6, 409–417. [Google Scholar] [CrossRef]
- Zhou, L.; Chong, M.M.; Littman, D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity 2009, 30, 646–655. [Google Scholar] [CrossRef]
- Komatsu, N.; Okamoto, K.; Sawa, S.; Nakashima, T.; Oh-hora, M.; Kodama, T.; Tanaka, S.; Bluestone, J.A.; Takayanagi, H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 2014, 20, 62–68. [Google Scholar] [CrossRef]
- Gagliani, N.; Amezcua Vesely, M.C.; Iseppon, A.; Brockmann, L.; Xu, H.; Palm, N.W.; de Zoete, M.R.; Licona-Limon, P.; Paiva, R.S.; Ching, T.; et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 2015, 523, 221–225. [Google Scholar] [CrossRef]
- Boissier, M.C.; Assier, E.; Biton, J.; Denys, A.; Falgarone, G.; Bessis, N. Regulatory T Cells (Treg) in Rheumatoid Arthritis. J. Bone Spine. 2009, 76, 10–14. [Google Scholar] [CrossRef]
- Chabaud, M.; Durand, J.M.; Buchs, N.; Fossiez, F.; Page, G.; Frappart, L.; Miossec, P. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999, 42, 963–970. [Google Scholar] [CrossRef]
- Wehrens, E.J.; Prakken, B.J.; van Wijk, F. T cells out of control—Impaired immune regulation in the inflamed joint. Nat. Rev. Rheumatol. 2013, 9, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jung, Y.O.; Ryu, J.G.; Oh, H.J.; Son, H.J.; Lee, S.H.; Kwon, J.E.; Kim, E.K.; Park, M.K.; Park, S.H.; et al. Epigallocatechin-3-gallate ameliorates autoimmune arthritis by reciprocal regulation of T helper-17 regulatory T cells and inhibition of osteoclastogenesis by inhibiting STAT3 signaling. J. Leukoc. Biol. 2016, 3, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Mei, L.; Wang, H.; Fang, F. Epigallocatechin-3-gallate (EGCG) inhibits imiquimod-induced psoriasis-like inflammation of BALB/c mice. BMC Complement. Altern. Med. 2016, 1, 334. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Kang, X.; Liu, F.; Cai, X.; Han, X.; Shang, Y. Epigallocatechin gallate improves airway inflammation through TGF-β1 signaling pathway in asthmatic mice. Mol. Med. Rep. 2018, 18, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wei, C.; Zhang, R.U.; Yao, J.; Zhang, D.; Wang, L. Epigallocatechin-3-gallate-induced inhibition of interleukin-6 release and adjustment of the regulatory T/T helper 17 cell balance in the treatment of colitis in mice. Exp. Ther. Med. 2015, 6, 2231–2238. [Google Scholar] [CrossRef][Green Version]
- Yang, N.; Shang, Y.X. Epigallocatechin gallate ameliorates airway inflammation by regulating Treg/Th17 imbalance in an asthmatic mouse model. Int. Immunopharmacol. 2019, 72, 422–428. [Google Scholar] [CrossRef]
- Zou, Y.; Dai, S.X.; Chi, H.G.; Li, T.; He, Z.W.; Wang, J.; Ye, C.; Huang, G.L.; Zhao, B.; Li, W.Y.; et al. Baicalin attenuates TNBS-induced colitis in rats by modulating the Th17/Treg paradigm. Arch. Pharm. Res. 2015, 10, 1873–1887. [Google Scholar] [CrossRef]
- Hu, C.A.; Hou, Y.; Yi, D.; Qiu, Y.; Wu, G.; Kong, X.; Yin, Y. Autophagy and tight junction proteins in the intestine and intestinal diseases. Anim. Nutr. 2015, 1, 123–127. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, L.Z.; Zhao, S.; Shen, Z.F.; Shen, H.; Zhan, L.B. Protective effect of baicalin on the regulation of Treg/Th17 balance, gut microbiota and short-chain fatty acids in rats with ulcerative colitis. Appl. Microbiol. Biotechnol. 2020, 104, 5449–5460. [Google Scholar] [CrossRef]
- Ma, C.; Ma, Z.; Fu, Q.; Ma, S. Anti-asthmatic effects of baicalin in a mouse model of allergic asthma. Phytother. Res. 2014, 28, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, J.; Zhang, Y.; Zhao, P.; Zhang, X.J. Regulatory effect of baicalin on the imbalance of Th17/Treg responses in mice with allergic asthma. J. Ethnopharmacol. 2017, 208, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, J.; Zou, H. Baicalin inhibits IL-17-mediated joint inflammation in murine adjuvant-induced arthritis. Clin. Dev. Immunol. 2013, 2013, 268065. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ma, Z.; Liao, X.L.; Liu, J.; Fu, Q.; Ma, S.J. Immunoregulatory effects of glycyrrhizic acid exerts anti-asthmatic effects via modulation of Th1/Th2 cytokines and enhancement of CD4(+)CD25(+)Foxp3+ regulatory T cells in ovalbumin-sensitized mice. J. Ethnopharmacol. 2013, 3, 755–762. [Google Scholar] [CrossRef]
- Chen, X.; Fang, D.; Li, L.; Chen, L.; Li, Q.; Gong, F.; Fang, M. Glycyrrhizin ameliorates experimental colitis through attenuating interleukin-17-producing T cell responses via regulating antigen-presenting cells. Immunol. Res. 2017, 3, 666–680. [Google Scholar] [CrossRef]
- Yun, C.; Chang, M.; Hou, G.; Lan, T.; Yuan, H.; Su, Z.; Zhu, D.; Liang, W.; Li, Q.; Zhu, H.; et al. Mangiferin suppresses allergic asthma symptoms by decreased Th9 and Th17 responses and increased Treg response. Mol. Immunol. 2019, 114, 233–242. [Google Scholar] [CrossRef]
- Lim, S.M.; Kang, G.D.; Jeong, J.J.; Choi, H.S.; Kim, D.H. Neomangiferin modulates the Th17/Treg balance and ameliorates colitis in mice. Phytomedicine 2016, 23, 131–140. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Dash, D.; Singh, R. Intranasal curcumin attenuates airway remodeling in murine model of chronic asthma. Int. Immunopharmacol. 2014, 21, 63–75. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Y.; Hu, J. Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1beta and IL-6 production in mice. PLoS ONE 2013, 8, e67078. [Google Scholar] [CrossRef]
- Ma, C.; Ma, Z.; Fu, Q.; Ma, S. Curcumin attenuates allergic airway inflammation by regulation of CD4+CD25+ regulatory T cells (Tregs)/Th17 balance in ovalbumin-sensitized mice. Fitoterapia 2013, 87, 57–64. [Google Scholar] [CrossRef]
- Chen, H.; Lu, C.; Liu, H.; Wang, M.; Zhao, H.; Yan, Y.; Han, L. Quercetin ameliorates imiquimod-induced psoriasis-like skin inflammation in mice via the NF-κB pathway. Int. Immunopharmacol. 2017, 48, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, C.; Gao, H.; Li, C.; Li, D.; Liu, P.; Huang, M.; Shen, X.; Liu, L. Therapeutic effect of Cryptotanshinone on experimental rheumatoid arthritis through downregulating p300 mediated-STAT3 acetylation. Biochem. Pharmacol. 2017, 138, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Kim, S.W.; Kim, D.S.; Lee, J.Y.; Lee, S.; Oh, H.M.; Ha, Y.S.; Yoo, J.; Park, P.H.; Shin, T.Y.; et al. Oleanolic acid acetate inhibits rheumatoid arthritis by modulating T cell immune responses and matrix-degrading enzymes. Toxicol. Appl. Pharmacol. 2016, 290, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.; Dou, Y.; Wang, T.; Yu, J.; Wu, X.; Lu, Q.; Chou, G.; Wang, Z.; Kong, L.; Dai, Y.; et al. Norisoboldine ameliorates collagen-induced arthritis through regulating the balance between Th17 and regulatory T cells in gut-associated lymphoid tissues. Toxicol. Appl. Pharmacol. 2015, 28, 90–99. [Google Scholar] [CrossRef]
- Li, C.; Xi, Y.; Li, S.; Zhao, Q.; Cheng, W.; Wang, Z.; Zhong, J.; Niu, X.; Chen, G. Berberine ameliorates TNBS induced colitis by inhibiting inflammatory responses and Th1/Th17 differentiation. Mol. Immunol. 2015, 67, 444–454. [Google Scholar] [CrossRef]
- Urushima, H.; Nishimura, J.; Mizushima, T.; Hayashi, N.; Maeda, K.; Ito, T. Perilla frutescens extract ameliorates DSS-induced colitis by suppressing proinflammatory cytokines and inducing anti-inflammatory cytokines. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G32–G41. [Google Scholar] [CrossRef]
- Lim, S.M.; Jeong, J.J.; Kang, G.D.; Kim, K.A.; Choi, H.S.; Kim, D.H. Timosaponin AIII and its metabolite sarsasapogenin ameliorate colitis in mice by inhibiting NF-κB and MAPK activation and restoring Th17/Treg cell balance. Int. Immunopharmacol. 2015, 25, 493–503. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Wei, Z.; Wei, W.; Zhao, P.; Tong, B.; Xia, Y.; Dai, Y. Madecassic acid, the contributor to the anti-colitis effect of madecassoside, enhances the shift of Th17 toward Treg cells via the PPARγ/AMPK/ACC1 pathway. Cell Death Dis. 2017, 8, e2723. [Google Scholar] [CrossRef]
- Chen, S.; Han, K.; Li, H.; Cen, J.; Yang, Y.; Wu, H.; Wei, Q.J. Isogarcinol Extracted from Garcinia mangostana L. Ameliorates Imiquimod-Induced Psoriasis-like Skin Lesions in Mice. Agric. Food Chem. 2017, 65, 846–857. [Google Scholar] [CrossRef]
- Di, T.T.; Ruan, Z.T.; Zhao, J.X.; Wang, Y.; Liu, X.; Wang, Y.; Li, P. Astilbin inhibits Th17 cell differentiation and ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice via Jak3/Stat3 signaling pathway. Int. Immunopharmacol. 2016, 32, 32–38. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Huo, R.; Zhai, T.; Li, H.; Wu, P.; Zhu, X.; Zhou, Z.; Shen, B.; Li, N. Paeoniflorin inhibits skin lesions in imiquimod-induced psoriasis-like mice by downregulating inflammation. Int. Immunopharmacol. 2015, 24, 392–399. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saviano, A.; Raucci, F.; Casillo, G.M.; Indolfi, C.; Pernice, A.; Foreste, C.; Iqbal, A.J.; Mascolo, N.; Maione, F. Present Status and Future Trends of Natural-Derived Compounds Targeting T Helper (Th) 17 and Microsomal Prostaglandin E Synthase-1 (mPGES-1) as Alternative Therapies for Autoimmune and Inflammatory-Based Diseases. Molecules 2020, 25, 6016. https://doi.org/10.3390/molecules25246016
Saviano A, Raucci F, Casillo GM, Indolfi C, Pernice A, Foreste C, Iqbal AJ, Mascolo N, Maione F. Present Status and Future Trends of Natural-Derived Compounds Targeting T Helper (Th) 17 and Microsomal Prostaglandin E Synthase-1 (mPGES-1) as Alternative Therapies for Autoimmune and Inflammatory-Based Diseases. Molecules. 2020; 25(24):6016. https://doi.org/10.3390/molecules25246016
Chicago/Turabian StyleSaviano, Anella, Federica Raucci, Gian Marco Casillo, Chiara Indolfi, Alessia Pernice, Carmen Foreste, Asif Jilani Iqbal, Nicola Mascolo, and Francesco Maione. 2020. "Present Status and Future Trends of Natural-Derived Compounds Targeting T Helper (Th) 17 and Microsomal Prostaglandin E Synthase-1 (mPGES-1) as Alternative Therapies for Autoimmune and Inflammatory-Based Diseases" Molecules 25, no. 24: 6016. https://doi.org/10.3390/molecules25246016
APA StyleSaviano, A., Raucci, F., Casillo, G. M., Indolfi, C., Pernice, A., Foreste, C., Iqbal, A. J., Mascolo, N., & Maione, F. (2020). Present Status and Future Trends of Natural-Derived Compounds Targeting T Helper (Th) 17 and Microsomal Prostaglandin E Synthase-1 (mPGES-1) as Alternative Therapies for Autoimmune and Inflammatory-Based Diseases. Molecules, 25(24), 6016. https://doi.org/10.3390/molecules25246016






