Biosensors-on-Chip: An Up-to-Date Review
Abstract
1. Introduction
2. Design and Working Principles
3. Diagnostic Applications
3.1. Cancer Diagnosis
3.2. Microbial Infection Diagnosis
3.3. Neurodegenerative Disorder Diagnosis
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, N.I.; Song, E. Lab-on-a-Chip Systems for Aptamer-Based Biosensing. Micromachines 2020, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Husain, Q. Carbon nanotubes mediated immobilized glucose oxidase biosensors as an effective and sensitive analytical tool. Biointerface Res. Appl. Chem. 2018, 8, 3060–3074. [Google Scholar]
- Wegmann, M.; Scharr, M. Chapter 8—Synthesis of Magnetic Iron Oxide Nanoparticles. In Precision Medicine; Deigner, H.-P., Kohl, M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 145–181. [Google Scholar] [CrossRef]
- Kumar, N.; Upadhyay, L.S.B. 19—Polymeric gels for biosensing applications. In Polymeric Gels; Pal, K., Banerjee, I., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 487–503. [Google Scholar] [CrossRef]
- Muguruma, H. Biosensors: Enzyme Immobilization Chemistry. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; pp. 64–71. [Google Scholar] [CrossRef]
- Manikandan, R.; Charumathe, N.; Fariha Begum, A. Application of Biosensor. Bull. Sci. Res. 2019, 1, 34–40. [Google Scholar] [CrossRef][Green Version]
- Dai, Y.; Furst, A.; Liu, C.C. Strand displacement strategies for biosensor applications. Trends Biotechnol. 2019, 37, 1367–1382. [Google Scholar] [CrossRef]
- Mohamad, A.; Rizwan, M.; Keasberry, N.A.; Ahmed, M.U. Fabrication of label-free electrochemical food biosensor for the sensitive detection of ovalbumin on nanocomposite-modified graphene electrode. Biointerface Res. Appl. Chem. 2019, 9, 4655–4662. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Niri, A.D.; Faridi-Majidi, R.; Saber, R.; Khosravani, M.; Adabi, M. Electrospun carbon nanofiber-based electrochemical biosensor for the detection of hepatitis B virus. Biointerface Res. Appl. Chem. 2019, 9, 4022–4026. [Google Scholar] [CrossRef]
- Parkhey, P.; Mohan, S.V. Chapter 6.1—Biosensing Applications of Microbial Fuel Cell: Approach Toward Miniaturization. In Microbial Electrochemical Technology; Mohan, S.V., Varjani, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 977–997. [Google Scholar] [CrossRef]
- Malinee, M.; Kumar, A.; Dhiman, A.; Sharma, T.K. Chapter 8—Aptamer-Mediated Nanobiosensing for Health Monitoring. In Advanced Biosensors for Health Care Applications; Inamuddin, K.R., Mohammad, A., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 227–248. [Google Scholar] [CrossRef]
- Sadana, A.; Sadana, N.; Sadana, R. 3—Different Instrumentation Techniques. In A Fractal Analysis of Chemical Kinetics with Applications to Biological and Biosensor Interfaces; Sadana, A., Sadana, N., Sadana, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 43–67. [Google Scholar] [CrossRef]
- Carpenter, A.C.; Paulsen, I.T.; Williams, T.C. Blueprints for Biosensors: Design, Limitations, and Applications. Genes 2018, 9, 375. [Google Scholar] [CrossRef]
- Rajpoot, K. Recent Advances and Applications of Biosensors in Novel Technology. Biosens. J. 2017, 6, 1–12. [Google Scholar]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Sevenler, D.; Trueb, J.; Ünlü, M.S. Beating the reaction limits of biosensor sensitivity with dynamic tracking of single binding events. Proc. Natl. Acad. Sci. USA 2019, 116, 4129–4134. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Park, H.; Cho, H.; Siddique, R.H.; Narasimhan, V.; Yang, D.; Choo, H. Overcoming evanescent field decay using 3D-tapered nanocavities for on-chip targeted molecular analysis. Nat. Commun. 2020, 11, 2930. [Google Scholar] [CrossRef] [PubMed]
- Spitzberg, J.D.; Zrehen, A.; van Kooten, X.F.; Meller, A. Plasmonic-Nanopore Biosensors for Superior Single-Molecule Detection. Adv. Mater. 2019, 31, 1900422. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, V. Chapter 11—Receptor-based electrochemical biosensors for the detection of contaminants in food products. In Electrochemical Biosensors; Ensafi, A.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 307–365. [Google Scholar] [CrossRef]
- Francis, M.P.; Kemper, N.; Maghdouri-White, Y.; Thayer, N. 9—Additive manufacturing for biofabricated medical device applications. In Additive Manufacturing; Zhang, J., Jung, Y.-G., Eds.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 311–344. [Google Scholar] [CrossRef]
- Wibowo, D.; Zhao, C.-X.; He, Y. Chapter 2—Fluid properties and hydrodynamics of microfluidic systems. In Microfluidics for Pharmaceutical Applications; Santos, H.A., Liu, D., Zhang, H., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 37–77. [Google Scholar] [CrossRef]
- Bohr, A.; Colombo, S.; Jensen, H. Chapter 15—Future of microfluidics in research and in the market. In Microfluidics for Pharmaceutical Applications; Santos, H.A., Liu, D., Zhang, H., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 425–465. [Google Scholar] [CrossRef]
- Nainys, J.; Milkus, V.; Mažutis, L. Chapter 13—Single-cell screening using microfluidic systems. In Microfluidics for Pharmaceutical Applications; Santos, H.A., Liu, D., Zhang, H., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 353–367. [Google Scholar] [CrossRef]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics Integrated Biosensors: A Leading Technology towards Lab-on-a-Chip and Sensing Applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [PubMed]
- Anik, Ü. 12—Electrochemical medical biosensors for POC applications. In Medical Biosensors for Point of Care (POC) Applications; Narayan, R.J., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 275–292. [Google Scholar] [CrossRef]
- Khalid, N.; Arif, S.; Kobayashi, I.; Nakajima, M. Chapter 14—Lab-on-a-chip techniques for high-throughput proteomics and drug discovery. In Microfluidics for Pharmaceutical Applications; Santos, H.A., Liu, D., Zhang, H., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 371–422. [Google Scholar] [CrossRef]
- Mukherji, S.; Mondal, D. 5—Lab-on-chip (LOC) devices for point of care (POC) applications. In Medical Biosensors for Point of Care (POC) Applications; Narayan, R.J., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 99–131. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Ali, M.A. Chapter 9—Microfluidic Biosensor. In Nanomaterials for Biosensors; Malhotra, B.D., Ali, M.A., Eds.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 263–293. [Google Scholar] [CrossRef]
- Loo, J.F.; Ho, A.H.; Turner, A.P.F.; Mak, W.C. Integrated Printed Microfluidic Biosensors. Trends Biotechnol. 2019, 37, 1104–1120. [Google Scholar] [CrossRef]
- Liao, Z.; Wang, J.; Zhang, P.; Zhang, Y.; Miao, Y.; Gao, S.; Deng, Y.; Geng, L. Recent advances in microfluidic chip integrated electronic biosensors for multiplexed detection. Biosens. Bioelectron. 2018, 121, 272–280. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, Y.; Li, Y.; Miao, Y.; Gao, S.; Lin, F.; Deng, Y.; Geng, L. Microfluidic chip coupled with optical biosensors for simultaneous detection of multiple analytes: A review. Biosens. Bioelectron. 2019, 126, 697–706. [Google Scholar] [CrossRef]
- Castaño-Álvarez, M.; Pozo-Ayuso, D.F.; Fernández-la-Villa, A. Integrated microfluidic electrochemical sensors to enhance automated flow analysis systems. In Laboratory Methods in Dynamic Electroanalysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–170. [Google Scholar]
- Choi, J.R. Development of Point-of-Care Biosensors for COVID-19. Front. Chem. 2020, 8, 517. [Google Scholar] [CrossRef]
- Manocha, A.; Bhargava, S. Emerging challenges in point-of-care testing. Curr. Med. Res. Pract. 2019, 9, 227–230. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Wu, L.; Huang, Y.; Zhang, Y.; Zhu, M.; Wang, Y.; Zhu, Z.; Yang, C. Trends in miniaturized biosensors for point-of-care testing. TrAC Trends Anal. Chem. 2020, 122, 115701. [Google Scholar] [CrossRef]
- Hochstetter, A. Lab-on-a-Chip Technologies for the Single Cell Level: Separation, Analysis, and Diagnostics. Micromachines 2020, 11, 468. [Google Scholar] [CrossRef]
- Jin, J.; Nguyen, N.-T. Manipulation schemes and applications of liquid marbles for micro total analysis systems. Microelectron. Eng. 2018, 197, 87–95. [Google Scholar] [CrossRef]
- Atalay, Y.T.; Vermeir, S.; Witters, D.; Vergauwe, N.; Verbruggen, B.; Verboven, P.; Nicolaï, B.M.; Lammertyn, J. Microfluidic analytical systems for food analysis. Trends Food Sci. Technol. 2011, 22, 386–404. [Google Scholar] [CrossRef]
- Cai, G.; Xue, L.; Zhang, H.; Lin, J.J.M. A review on micromixers. Micromachines 2017, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Nikoleli, G.-P.; Siontorou, C.G.; Nikolelis, D.P.; Bratakou, S.; Karapetis, S.; Tzamtzis, N. Chapter 13—Biosensors Based on Microfluidic Devices Lab-on-a-Chip and Microfluidic Technology. In Nanotechnology and Biosensors; Nikolelis, D.P., Nikoleli, G.-P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 375–394. [Google Scholar] [CrossRef]
- Keçili, R.; Büyüktiryaki, S.; Hussain, C.M. 8—Micro total analysis systems with nanomaterials. In Handbook of Nanomaterials in Analytical Chemistry; Mustansar, H.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 185–198. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Hou, C.-W.; Li, X.-J.; Jin, Z.-J.J.M. Actuation Mechanism of Microvalves: A Review. Micromachines 2020, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-S. Comparison of Chip Inlet Geometry in Microfluidic Devices for Cell Studies. Molecules 2016, 21, 778. [Google Scholar] [CrossRef] [PubMed]
- Whulanza, Y.; Hakim, A.T.; Utomo, S.M.; Irwansyah, R.; Charmet, J.J.E. Design and Characterization of Finger-Controlled Micropump for Lab-on-a-Chip Devices. Evergreen 2019, 6, 108–113. [Google Scholar] [CrossRef]
- Guevara-Pantoja, P.E.; Jiménez-Valdés, R.J.; García-Cordero, J.L.; Caballero-Robledo, G.A. Pressure-actuated monolithic acrylic microfluidic valves and pumps. Lab Chip 2018, 18, 662–669. [Google Scholar] [CrossRef]
- Agrawal, P.; Gandhi, P.S.; Majumder, M.; Kumar, P. Insight into the Design and Fabrication of a Leaf-Mimicking Micropump. Phys. Rev. App. 2019, 12, 031002. [Google Scholar] [CrossRef]
- Eluru, G.; Adhikari, J.V.; Chanda, P.; Gorthi, S.S. Hand-Powered Elastomeric Pump for Microfluidic Point-of-Care Diagnostics. Micromachines 2020, 11, 67. [Google Scholar] [CrossRef]
- Avvari, R.K. A novel two-indenter based micro-pump for lab-on-a-chip application: Modeling and characterizing flows for a non-Newtonian fluid. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ferraro, D.; Serra, M.; Ferrante, I.; Viovy, J.L.; Descroix, S. Microfluidic valve with zero dead volume and negligible back-flow for droplets handling. Sens. Actuators B Chem. 2018, 258, 1051–1059. [Google Scholar] [CrossRef]
- Zhang, X.; Oseyemi, A.E.J.M. Microfluidic Passive Valve with Ultra-Low Threshold Pressure for High-Throughput Liquid Delivery. Chem. Eng. Process. Process. Intensif. 2019, 10, 798. [Google Scholar] [CrossRef] [PubMed]
- Bayareh, M.; Ashani, M.N.; Usefian, A. Active and passive micromixers: A comprehensive review. Chem. Eng. Process. Process. Intensif. 2020, 147, 107771. [Google Scholar] [CrossRef]
- Raza, W.; Hossain, S.; Kim, K.-Y. A Review of Passive Micromixers with a Comparative Analysis. Micromachines 2020, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Fu, L.-M. Recent advances and applications of micromixers. Sens. Actuators B Chem. 2018, 259, 677–702. [Google Scholar] [CrossRef]
- Liu, X.; Lin, B. Materials Used in Microfluidic Devices. In Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer: Boston, MA, USA, 2008; pp. 1065–1068. [Google Scholar] [CrossRef]
- Hosseini, M.-S.; Amjadi, I.; Mozafari, M. Chapter 18—State-of-the-art and future perspectives of functional polymers. In Advanced Functional Polymers for Biomedical Applications; Mozafari, M., Singh Chauhan, N.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 383–395. [Google Scholar] [CrossRef]
- Gao, B.; Li, X.; Yang, Y.; Chu, J.; He, B. Emerging paper microfluidic devices. Analyst 2019, 144, 6497–6511. [Google Scholar] [CrossRef]
- Böhm, A.; Biesalski, M. Paper-based microfluidic devices: A complex low-cost material in high-tech applications. MRS Bull. 2017, 42, 356–364. [Google Scholar] [CrossRef]
- Soum, V.; Park, S.; Brilian, A.I.; Kwon, O.-S.; Shin, K.J.M. Programmable paper-based microfluidic devices for biomarker detections. Micromachines 2019, 10, 516. [Google Scholar] [CrossRef]
- Strong, E.B.; Schultz, S.A.; Martinez, A.W.; Martinez, N.W. Fabrication of Miniaturized Paper-Based Microfluidic Devices (MicroPADs). Sci. Rep. 2019, 9, 7. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Current trends and challenges in bioelectrochemistry for non-invasive and early diagnosis. Curr. Opin. Electrochem. 2018, 12, 81–91. [Google Scholar] [CrossRef]
- Cheng, S.-J.; Hsieh, K.Y.; Chen, S.-L.; Chen, C.-Y.; Huang, C.-Y.; Tsou, H.-I.; Kumar, P.V.; Hsieh, J.C.-H.; Chen, G.-Y. Microfluidics and Nanomaterial-based Technologies for Circulating Tumor Cell Isolation and Detection. Sensors 2020, 20, 1875. [Google Scholar] [CrossRef] [PubMed]
- Sierra, T.; Crevillen, A.G.; Escarpa, A. Electrochemical detection based on nanomaterials in CE and microfluidic systems. Electrophoresis 2019, 40, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chen, Z.; Wu, M.; Zhang, Y. Nanomaterial-based Microfluidic Chips for the Capture and Detection of Circulating Tumor Cells. Nanotheranostics 2017, 1, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Persichetti, G.; Bernini, R. Optofluidic Approaches for Enhanced Microsensor Performances. Sensors 2015, 15, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lu, M.; Jin, H.; Chen, T.; Bond, T.C. Nanomaterials for Optical Sensing and Sensors: Plasmonics, Raman, and Optofluidics. J. Nanomater. 2015, 2015, 162537. [Google Scholar] [CrossRef]
- Liu, S.; Hawkins, A.R.; Schmidt, H. Optofluidic devices with integrated solid-state nanopores. Mikrochim. Acta 2016, 183, 1275–1287. [Google Scholar] [CrossRef]
- Farmani, A.; Soroosh, M.; Mozaffari, M.; Daghoghi, T. Optical nanosensors for cancer and virus detections. Nanosens. Smart Cities 2020. [Google Scholar] [CrossRef]
- Rickard, J.J.S.; Di-Pietro, V.; Smith, D.J.; Davies, D.J.; Belli, A.; Oppenheimer, P.G. Rapid optofluidic detection of biomarkers for traumatic brain injury via surface-enhanced Raman spectroscopy. Nat. Biomed. Eng. 2020, 4, 610–623. [Google Scholar] [CrossRef]
- Garcia-Cordero, J.L.; Maerkl, S.J. Microfluidic systems for cancer diagnostics. Curr. Opin. Biotechnol. 2020, 65, 37–44. [Google Scholar] [CrossRef]
- Silva, A.C.Q.; Vilela, C.; Santos, H.A.; Silvestre, A.J.D.; Freire, C.S.R. Recent trends on the development of systems for cancer diagnosis and treatment by microfluidic technology. App. Mater. Today 2020, 18, 100450. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Wu, H.; Punyadeera, C.; Warkiani, M.E. The Use of Microfluidic Technology for Cancer Applications and Liquid Biopsy. Micromachines 2018, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, H.; Zhou, W.; Ma, L.; Perez, S.; Ibarra, A.; Xu, F.; Zhan, S.; Li, X. Recent advances in microfluidic platforms for single-cell analysis in cancer biology, diagnosis and therapy. TrAC Trends Anal. Chem. 2019, 117, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Cetin, D.; Okan, M.; Bat, E.; Kulah, H. A comparative study on EpCAM antibody immobilization on gold surfaces and microfluidic channels for the detection of circulating tumor cells. Colloids Surf. B Biointerfaces 2020, 188. [Google Scholar] [CrossRef]
- Chan, K.M.; Vasilev, K.; Shirazi, H.S.; McNicholas, K.; Li, J.; Gleadle, J.; MacGregor, M. Biosensor device for the photo-specific detection of immuno-captured bladder cancer cells using hexaminolevulinate: An ex-vivo study. Photodiagn. Photodyn. Ther. 2019, 28, 238–247. [Google Scholar] [CrossRef]
- Nguyen, N.V.; Yang, C.H.; Liu, C.J.; Kuo, C.H.; Wu, D.C.; Jen, C.P. An aptamer-based capacitive sensing platform for specific detection of lung carcinoma cells in the microfluidic chip. Biosensors 2018, 8, 98. [Google Scholar] [CrossRef]
- Anu Prathap, M.U.; Castro-Pérez, E.; Jiménez-Torres, J.A.; Setaluri, V.; Gunasekaran, S. A flow-through microfluidic system for the detection of circulating melanoma cells. Biosens. Bioelectron. 2019, 142. [Google Scholar] [CrossRef]
- Lunelli, L.; Barbaresco, F.; Scordo, G.; Potrich, C.; Vanzetti, L.; Marasso, S.L.; Cocuzza, M.; Pirri, C.F.; Pederzolli, C. PDMS-based microdevices for the capture of MicroRNA biomarkers. Appl. Sci. 2020, 10, 3867. [Google Scholar] [CrossRef]
- Kutluk, H.; Bruch, R.; Urban, G.A.; Dincer, C. Impact of assay format on miRNA sensing: Electrochemical microfluidic biosensor for miRNA-197 detection. Biosens. Bioelectron. 2020, 148. [Google Scholar] [CrossRef]
- Fakhri, N.; Abarghoei, S.; Dadmehr, M.; Hosseini, M.; Sabahi, H.; Ganjali, M.R. Paper based colorimetric detection of miRNA-21 using Ag/Pt nanoclusters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227. [Google Scholar] [CrossRef]
- Tian, T.; Li, L.; Zhang, Y.; Liu, H.; Zhang, L.; Yan, M.; Yu, J. Dual-mode fluorescence biosensor platform based on T-shaped duplex structure for detection of microRNA and folate receptor. Sensors Actuators B Chem. 2018, 261, 44–50. [Google Scholar] [CrossRef]
- Bruch, R.; Baaske, J.; Chatelle, C.; Meirich, M.; Madlener, S.; Weber, W.; Dincer, C.; Urban, G.A. CRISPR/Cas13a-Powered Electrochemical Microfluidic Biosensor for Nucleic Acid Amplification-Free miRNA Diagnostics. Adv. Mater. 2019, 31. [Google Scholar] [CrossRef] [PubMed]
- Veselinovic, J.; Li, Z.; Daggumati, P.; Seker, E. Electrically guided DNA immobilization and multiplexed DNA detection with nanoporous gold electrodes. Nanomaterials 2018, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Garrido-Maestu, A.; Guerreiro, J.R.L.; Carvalho, S.; Abalde-Cela, S.; Prado, M.; Diéguez, L. Amplification-free SERS analysis of DNA mutation in cancer cells with single-base sensitivity. Nanoscale 2019, 11, 7781–7789. [Google Scholar] [CrossRef]
- Wu, L.; Teixeira, A.; Garrido-Maestu, A.; Muinelo-Romay, L.; Lima, L.; Santos, L.L.; Prado, M.; Diéguez, L. Profiling DNA mutation patterns by SERS fingerprinting for supervised cancer classification. Biosens. Bioelectron. 2020, 165. [Google Scholar] [CrossRef]
- Wang, Y.; Ali, M.A.; Chow, E.K.C.; Dong, L.; Lu, M. An optofluidic metasurface for lateral flow-through detection of breast cancer biomarker. Biosens. Bioelectron. 2018, 107, 224–229. [Google Scholar] [CrossRef]
- Bahavarnia, F.; Saadati, A.; Hassanpour, S.; Hasanzadeh, M.; Shadjou, N.; Hassanzadeh, A. Paper based immunosensing of ovarian cancer tumor protein CA 125 using novel nano-ink: A new platform for efficient diagnosis of cancer and biomedical analysis using microfluidic paper-based analytical devices (μPAD). Int. J. Biol. Macromol. 2019, 138, 744–754. [Google Scholar] [CrossRef]
- Nunna, B.B.; Mandal, D.; Lee, J.U.; Zhuang, S.; Lee, E.S. Sensitivity Study of Cancer Antigens (CA-125) Detection Using Interdigitated Electrodes Under Microfluidic Flow Condition. BioNanoScience 2019, 9, 203–214. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, L.; Li, L.; Zong, S.; Wang, Z.; Cui, Y. Simultaneous and highly sensitive detection of multiple breast cancer biomarkers in real samples using a SERS microfluidic chip. Talanta 2018, 188, 507–515. [Google Scholar] [CrossRef]
- Gao, R.; Lv, Z.; Mao, Y.; Yu, L.; Bi, X.; Xu, S.; Cui, J.; Wu, Y. SERS-Based Pump-Free Microfluidic Chip for Highly Sensitive Immunoassay of Prostate-Specific Antigen Biomarkers. ACS Sens. 2019, 4, 938–943. [Google Scholar] [CrossRef]
- Guo, L.; Shi, Y.; Liu, X.; Han, Z.; Zhao, Z.; Chen, Y.; Xie, W.; Li, X. Enhanced fluorescence detection of proteins using ZnO nanowires integrated inside microfluidic chips. Biosens. Bioelectron. 2018, 99, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.; Marrugo-Ramírez, J.; Rodriguez-Trujillo, R.; Mir, M.; Samitier, J. Sensor-Integrated Microfluidic Approaches for Liquid Biopsies Applications in Early Detection of Cancer. Sensors 2020, 20, 1317. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, R.; Shamloo, A.; Ahadian, S.; Amirifar, L.; Akbari, J.; Goudie, M.J.; Lee, K.; Ashammakhi, N.; Dokmeci, M.R.; Di Carlo, D.; et al. Microfluidic-Based Approaches in Targeted Cell/Particle Separation Based on Physical Properties: Fundamentals and Applications. Small 2020, 16, 2000171. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiang, Z.; Wang, J.; Ren, Y.; Wu, A. Microfluidic applications on circulating tumor cell isolation and biomimicking of cancer metastasis. Electrophoresis 2020, 41, 933–951. [Google Scholar] [CrossRef]
- Davaran, S.; Sadeghinia, M.; Jamalpoor, Z.; Raeisdasteh Hokmabad, V.; Doosti-Telgerd, M.; Karimian, A.; Sadeghinia, Z.; Khalilifard, J.; keramt, A.; Moradikhah, F.; et al. Multiple functions of microfluidic platforms: Characterization and applications in tissue engineering and diagnosis of cancer. Electrophoresis 2020, 41, 1081–1094. [Google Scholar] [CrossRef]
- Liang, W.; Yang, X.; Wang, J.; Wang, Y.; Yang, W.; Liu, L. Determination of Dielectric Properties of Cells using AC Electrokinetic-based Microfluidic Platform: A Review of Recent Advances. Micromachines 2020, 11, 513. [Google Scholar] [CrossRef]
- Liang, W.; Liu, J.; Yang, X.; Zhang, Q.; Yang, W.; Zhang, H.; Liu, L. Microfluidic-based cancer cell separation using active and passive mechanisms. Microfluid. Nanofluid. 2020, 24. [Google Scholar] [CrossRef]
- Macgregor-Ramiasa, M.; McNicholas, K.; Ostrikov, K.; Li, J.; Michael, M.; Gleadle, J.M.; Vasilev, K. A platform for selective immuno-capture of cancer cells from urine. Biosens. Bioelectron. 2017, 96, 373–380. [Google Scholar] [CrossRef]
- Iliescu, F.S.; Poenar, D.P.; Yu, F.; Ni, M.; Chan, K.H.; Cima, I.; Taylor, H.K.; Cima, I.; Iliescu, C. Recent advances in microfluidic methods in cancer liquid biopsy. Biomicrofluidics 2019, 13. [Google Scholar] [CrossRef]
- Narang, R.; Mohammadi, S.; Ashani, M.M.; Sadabadi, H.; Hejazi, H.; Zarifi, M.H.; Sanati-Nezhad, A. Sensitive, Real-time and Non-Intrusive Detection of Concentration and Growth of Pathogenic Bacteria using Microfluidic-Microwave Ring Resonator Biosensor. Sci. Rep. 2018, 8, s41598. [Google Scholar] [CrossRef]
- He, P.J.W.; Katis, I.N.; Kumar, A.J.U.; Bryant, C.A.; Keevil, C.W.; Somani, B.K.; Mahobia, N.; Eason, R.W.; Sones, C.L. Laser-patterned paper-based sensors for rapid point-of-care detection and antibiotic-resistance testing of bacterial infections. Biosens. Bioelectron. 2020, 152. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cherukury, H.; Labanieh, L.; Zhao, W.; Kang, D.K. Rapid detection of β-lactamase-producing bacteria using the integrated comprehensive droplet digital detection (Ic 3d) system. Sensors 2020, 20, 4667. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Chang, H.Y.; Wu, J.K.; Tseng, F.G. Ultra-sensitive electrochemical detection of bacteremia enabled by redox-active gold nanoparticles (raGNPs) in a nano-sieving microfluidic system (NS-MFS). Biosens. Bioelectron. 2019, 133, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Savas, S.; Ersoy, A.; Gulmez, Y.; Kilic, S.; Levent, B.; Altintas, Z. Nanoparticle enhanced antibody and DNA biosensors for sensitive detection of Salmonella. Materials 2018, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Thiha, A.; Ibrahim, F.; Muniandy, S.; Dinshaw, I.J.; Teh, S.J.; Thong, K.L.; Leo, B.F.; Madou, M. All-carbon suspended nanowire sensors as a rapid highly-sensitive label-free chemiresistive biosensing platform. Biosens. Bioelectron. 2018, 107, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, L.; Zhao, J.; Zhu, Y.; Yang, J.; Yang, F. Enhanced binding efficiency of microcantilever biosensor for the detection of yersinia. Sensors 2019, 19, 3326. [Google Scholar] [CrossRef]
- Jin, C.E.; Lee, T.Y.; Koo, B.; Sung, H.; Kim, S.H.; Shin, Y. Rapid virus diagnostic system using bio-optical sensor and microfluidic sample processing. Sens. Actuators B Chem. 2018, 255, 2399–2406. [Google Scholar] [CrossRef]
- Srisomwat, C.; Teengam, P.; Chuaypen, N.; Tangkijvanich, P.; Vilaivan, T.; Chailapakul, O. Pop-up paper electrochemical device for label-free hepatitis B virus DNA detection. Sens. Actuators B Chem. 2020, 316. [Google Scholar] [CrossRef]
- Ruiz-Vega, G.; Arias-Alpízar, K.; de la Serna, E.; Borgheti-Cardoso, L.N.; Sulleiro, E.; Molina, I.; Fernàndez-Busquets, X.; Sánchez-Montalvá, A.; del Campo, F.J.; Baldrich, E. Electrochemical POC device for fast malaria quantitative diagnosis in whole blood by using magnetic beads, Poly-HRP and microfluidic paper electrodes. Biosens. Bioelectron. 2020, 150. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Forouzanfar, F.; Roshanravan, B.; Samarghandian, S. Curcumin effect on non-amyloidogenic pathway for preventing alzheimer’s disease. Biointerface Res. Appl. Chem. 2019, 9, 4085–4089. [Google Scholar] [CrossRef]
- Moya-Alvarado, G.; Gershoni-Emek, N.; Perlson, E.; Bronfman, F.C. Neurodegeneration and Alzheimer’s disease (AD). What Can Proteomics Tell Us About the Alzheimer’s Brain? Mol. Cell. Proteom. 2016, 15, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Zetterberg, H. Biomarkers for Alzheimer’s disease: Current status and prospects for the future. J. Intern. Med. 2018, 284, 643–663. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, H.; Blennow, K. From Cerebrospinal Fluid to Blood: The Third Wave of Fluid Biomarkers for Alzheimer’s Disease. J. Alzheimer Dis. 2018, 64, S271–S279. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; Verheul, C.; Willemse, E.A.J. Chapter 1—The use of cerebrospinal fluid in biomarker studies. In Handbook of Clinical Neurology; Deisenhammer, F., Teunissen, C.E., Tumani, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 146, pp. 3–20. [Google Scholar]
- Altuna-Azkargorta, M.; Mendioroz-Iriarte, M. Blood biomarkers in Alzheimer’s disease. Neurología 2020. [Google Scholar] [CrossRef]
- Hampel, H.; O’Bryant, S.E.; Molinuevo, J.L.; Zetterberg, H.; Masters, C.L.; Lista, S.; Kiddle, S.J.; Batrla, R.; Blennow, K. Blood-based biomarkers for Alzheimer disease: Mapping the road to the clinic. Nat. Rev. Neurol. 2018, 14, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, H.; Burnham, S.C. Blood-based molecular biomarkers for Alzheimer’s disease. Mol. Brain 2019, 12, 26. [Google Scholar] [CrossRef]
- Gabelli, C. Blood and cerebrospinal fluid biomarkers for Alzheimer’s disease. J. Lab. Precis. Med. 2020, 5. [Google Scholar] [CrossRef]
- Toombs, J.; Zetterberg, H. In the blood: Biomarkers for amyloid pathology and neurodegeneration in Alzheimer’s disease. Brain Commun. 2020, 2. [Google Scholar] [CrossRef]
- O’Bryant, S.E. Blood Biomarkers for Use in Alzheimer Disease—Moving From “If” to “How?”. JAMA Neurol. 2019, 76, 1009–1010. [Google Scholar] [CrossRef]
- Mattsson, N.; Cullen, N.C.; Andreasson, U.; Zetterberg, H.; Blennow, K. Association Between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients with Alzheimer Disease. JAMA Neurol. 2019, 76, 791–799. [Google Scholar] [CrossRef]
- Weston, P.S.J.; Poole, T.; Ryan, N.S.; Nair, A.; Liang, Y.; Macpherson, K.; Druyeh, R.; Malone, I.B.; Ahsan, R.L.; Pemberton, H.; et al. Serum neurofilament light in familial Alzheimer disease. J. Neurol. 2017, 89, 2167–2175. [Google Scholar] [CrossRef]
- Ashton, N.J.; Ide, M.; Zetterberg, H.; Blennow, K. Salivary Biomarkers for Alzheimer’s Disease and Related Disorders. Neurol. Ther. 2019, 8, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Farah, R.; Haraty, H.; Salame, Z.; Fares, Y.; Ojcius, D.M.; Said Sadier, N. Salivary biomarkers for the diagnosis and monitoring of neurological diseases. Biomed. J. 2018, 41, 63–87. [Google Scholar] [CrossRef] [PubMed]
- González-Sánchez, M.; Bartolome, F.; Antequera, D.; Puertas-Martín, V.; González, P.; Gómez-Grande, A.; Llamas-Velasco, S.; San Martín, A.H.; Pérez-Martínez, D.; Villarejo-Galende, A. Decreased salivary lactoferrin levels are specific to Alzheimer’s disease. Res. Square 2020. [Google Scholar] [CrossRef] [PubMed]
- Falcon, C.; Monté-Rubio, G.C.; Grau-Rivera, O.; Suárez-Calvet, M.; Sánchez-Valle, R.; Rami, L.; Bosch, B.; Haass, C.; Gispert, J.D.; Molinuevo, J.L. CSF glial biomarkers YKL40 and sTREM2 are associated with longitudinal volume and diffusivity changes in cognitively unimpaired individuals. NeuroImage Clin. 2019, 23, 101801. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Xu, Y.; Zhuo, R.; Wang, T.; Wang, K.; Huang, R.; Wang, D.; Gao, Y.; Zhu, Y.; Sheng, X.; et al. Soluble TREM2 ameliorates pathological phenotypes by modulating microglial functions in an Alzheimer’s disease model. Nat. Commun. 2019, 10, 1365. [Google Scholar] [CrossRef] [PubMed]
- Llorens, F.; Thüne, K.; Tahir, W.; Kanata, E.; Diaz-Lucena, D.; Xanthopoulos, K.; Kovatsi, E.; Pleschka, C.; Garcia-Esparcia, P.; Schmitz, M.; et al. YKL-40 in the brain and cerebrospinal fluid of neurodegenerative dementias. Mol. Neurodegener. 2017, 12, 83. [Google Scholar] [CrossRef]
- Bălașa, A.F.; Chircov, C.; Grumezescu, A.M. Marine Biocompounds for Neuroprotection—A Review. Mar. Drugs 2020, 18, 290. [Google Scholar] [CrossRef]
- Bălașa, A.; Șerban, G.; Chinezu, R.; Hurghiș, C.; Tămaș, F.; Manu, D. The Involvement of Exosomes in Glioblastoma Development, Diagnosis, Prognosis, and Treatment. Brain Sci. 2020, 10, 553. [Google Scholar] [CrossRef]
- Balasa, R.; Barcutean, L.; Balasa, A.; Motataianu, A.; Roman-Filip, C.; Manu, D. The action of TH17 cells on blood brain barrier in multiple sclerosis and experimental autoimmune encephalomyelitis. Hum. Immunol. 2020, 81, 237–243. [Google Scholar] [CrossRef]
- Bălaşa, R.; Maier, S.; Bajko, Z.; Motataianu, A.; Crişan, A.; Bălaşa, A. Longitudinally extensive transverse myelitis in neuromyelitis optica: A prospective study of 13 Caucasian patients and literature review. Acta Neurol. Belg. 2015, 115, 635–642. [Google Scholar] [CrossRef]
- Brazaca, L.C.; Sampaio, I.; Zucolotto, V.; Janegitz, B.C. Applications of biosensors in Alzheimer’s disease diagnosis. Talanta 2020, 210, 120644. [Google Scholar] [CrossRef] [PubMed]
- Shui, B.; Tao, D.; Florea, A.; Cheng, J.; Zhao, Q.; Gu, Y.; Li, W.; Jaffrezic-Renault, N.; Mei, Y.; Guo, Z. Biosensors for Alzheimer’s disease biomarker detection: A review. Biochimie 2018, 147, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Tarutani, A.; Hasegawa, M. Chapter Eighteen—Prion-like propagation of α-synuclein in neurodegenerative diseases. In Progress in Molecular Biology and Translational Science; Teplow, D.B., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 168, pp. 323–348. [Google Scholar]
- Meade, R.M.; Fairlie, D.P.; Mason, J.M. Alpha-synuclein structure and Parkinson’s disease—Lessons and emerging principles. Mol. Neurodegener. 2019, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, F.G.; Himmelberg, M.M.; Laursen, B.; Siebner, H.R.; Wade, A.R.; Christensen, K.V. Classification of α-synuclein-induced changes in the AAV α-synuclein rat model of Parkinson’s disease using electrophysiological measurements of visual processing. Sci. Rep. 2020, 10, 11869. [Google Scholar] [CrossRef] [PubMed]
- Mahul-Mellier, A.-L.; Burtscher, J.; Maharjan, N.; Weerens, L.; Croisier, M.; Kuttler, F.; Leleu, M.; Knott, G.W.; Lashuel, H.A. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 4971–4982. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Casalini, S.; Parkula, V.; Selvaraj, M.; Saygin, G.D.; Greco, P.; Biscarini, F.; Mas-Torrent, M. Label-free immunodetection of α-synuclein by using a microfluidics coplanar electrolyte-gated organic field-effect transistor. Biosens. Bioelectron. 2020, 167. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Deng, P.; Que, L. Rapid multiplexed detection of beta-amyloid and total-tau as biomarkers for Alzheimer’s disease in cerebrospinal fluid. Nanomedicine 2018, 14, 1845–1852. [Google Scholar] [CrossRef]
- Antunes, A.P.; Schiefecker, A.J.; Beer, R.; Pfausler, B.; Sohm, F.; Fischer, M.; Dietmann, A.; Lackner, P.; Hackl, W.O.; Ndayisaba, J.-P.; et al. Higher brain extracellular potassium is associated with brain metabolic distress and poor outcome after aneurysmal subarachnoid hemorrhage. Crit. Care 2014, 18, R119. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Wang, Y.; Pan, L.; Lin, P.; Zhang, B.; Zheng, Y.; Xu, Y.; Liao, H.; Ko, G.; et al. A sensitive and specific nanosensor for monitoring extracellular potassium levels in the brain. Nat. Nanotechnol. 2020, 15, 321–330. [Google Scholar] [CrossRef]
- Serafín, V.; Gamella, M.; Pedrero, M.; Montero-Calle, A.; Razzino, C.A.; Yáñez-Sedeño, P.; Barderas, R.; Campuzano, S.; Pingarrón, J.M. Enlightening the advancements in electrochemical bioanalysis for the diagnosis of Alzheimer’s disease and other neurodegenerative disorders. J. Pharm. Biomed. Anal. 2020, 189, 113437. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
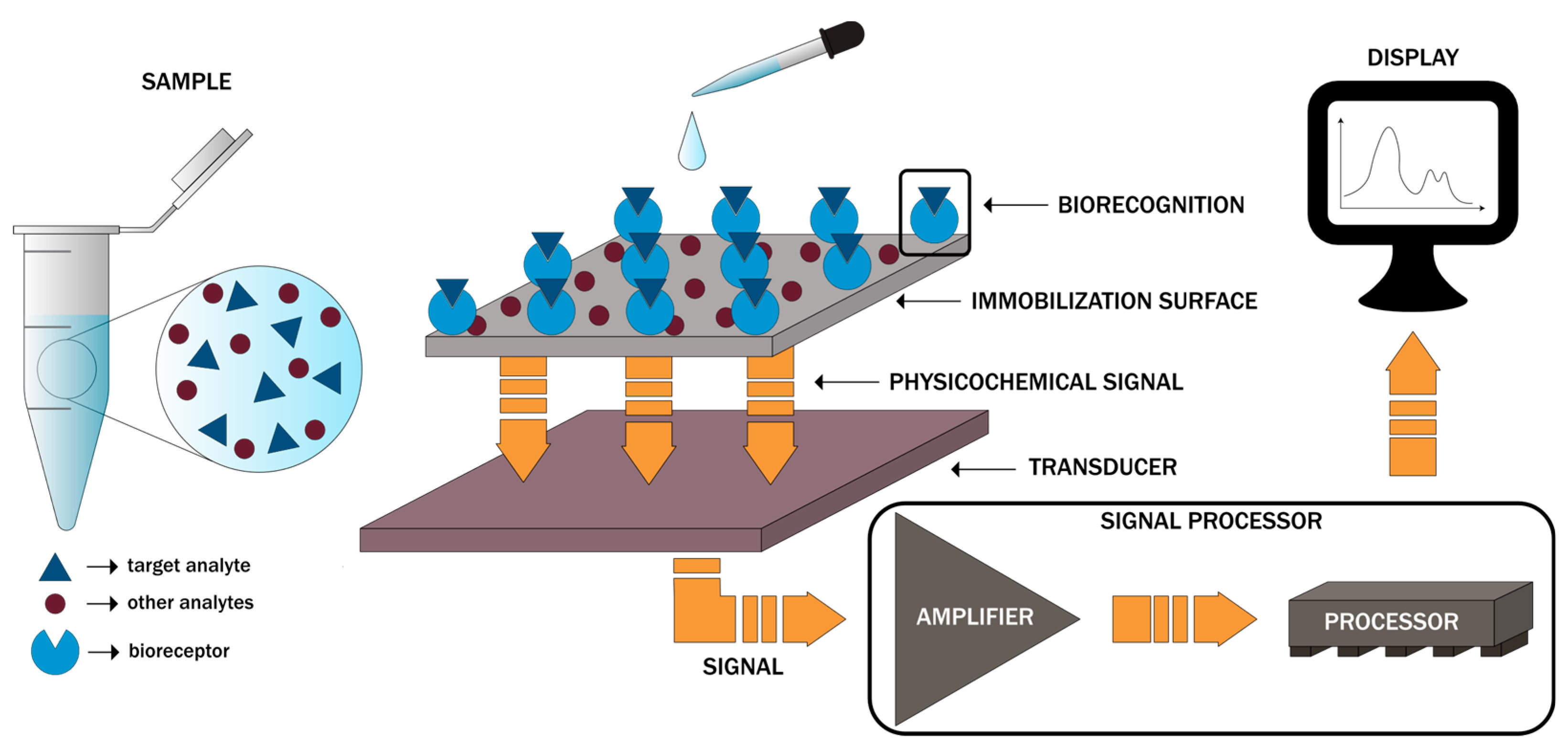
| Biomarker Type | Biosensor Type | Measuring Principle | Target | Capture Molecule | Cancer Model | Limit of Detection | Ref |
|---|---|---|---|---|---|---|---|
| CTCs | immunosensor | fluorometry | EpCAM | EpCAM antibody | MCF-7 breast cancer cells | n.r. | [74] |
| immunosensor | fluorometry | EpCAM | EpCAM antibody | EJ138, HT1376, HT1197, and RT4 human bladder cancer cells | n.r. | [75] | |
| aptasensor | electrochemical impedance | A549 cells | DNA aptamer | A549 human lung carcinoma cells | 1.5 × 104 cells/mL | [76] | |
| immunosensor | electrochemical impedance | MC1R | MC1R antibody | SK-MEL-2 human melanoma cells | 10 cells/10 mL | [77] | |
| miRNA | aptasensor | fluorometry | miRNA-1246-TAMRA | 3-aminopropyltrimethoxysilane | non-small cell lung cancer | n.r. | [78] |
| aptasensor | amperometry | miRNA-197 | complementary single-stranded DNA | - | 1.28 nM | [79] | |
| aptasensor | colorimetry | miRNA-21 | complementary single-stranded DNA | - | 4.1 pM | [80] | |
| aptasensor | fluorometry | miRNA-21 | thiol-modified hairpin DNA probe | MCF-7 breast cancer cells | 0.0033 fM | [81] | |
| enzyme-based sensor | chronoamperometry | miRNA-19b and miRNA-20a | Cas13a effector | brain cancer | 10 pM | [82] | |
| DNA | aptasensor | chronoamperometry | BRCA1, BRCA2, and p53 breast cancer genes | thiolated 19-mer BRCA1, 17-mer BRCA2, and 17-mer p53 | breast cancer | - | [83] |
| aptasensor | SERS | KRAS gene | molecular beacon probes | MDA-MB-435 and SW480 | 50 fM | [84] | |
| aptasensor | SERS | KRAS gene | molecular beacon probes | colorectal cancer | 10 fM | [85] | |
| immunosensor | reflectometry | ErbB2 gene | anti-ErbB2 antibody | breast cancer | 0.7 ng/mL | [86] | |
| proteins | immunosensor | chronoamperometry | CA 125 | CA 125 antibody | ovarian cancer | 0.78 U/mL | [87] |
| immunosensor | capacitance measurement | CA 125 | CA 125 antibody | ovarian cancer | - | [88] | |
| immunosensor | SERS | CA 125, CA 153, carcinoembryonic antigen | CA 125, CA 153, carcinoembryonic antigen antibodies | breast cancer | 0.0001 U/mL | [89] | |
| immunosensor | SERS | prostate specific antigen | prostate specific antigen antibody | prostate cancer | 0.01 ng/mL | [90] | |
| immunosensor | fluorometry | α-fetoprotein and carcinoembryonic antigen | α-fetoprotein and carcinoembryonic antibodies | - | 1 pg/mL and 100 fg/mL | [91] |
| Microbial Type | Microbial Strain | Biosensor Type | Measuring Principle | Target | Capture Molecule | Limit of Detection | Ref |
|---|---|---|---|---|---|---|---|
| bacteria | Escherichia coli | microwave-based sensor | vector network analysis | Escherichia coli | - | n.r. | [100] |
| Escherichia coli | optical sensor | colorimetry | Escherichia coli | - | n.r. | [101] | |
| ampicillin-resistant Escherichia coli, ampicillin-susceptible Escherichia coli, and Bacillus subtilis | enzyme-based sensor | fluorometry | β-lactamase | - | 1 × 104 cells per well | [102] | |
| Pseudomonas aeruginosa and Staphylococcus aureus | immunosensor | amperometry | Pseudomonas aeruginosa and Staphylococcus aureus | mouse anti-Pseudomonas aeruginosa antibody and mouse anti-Staphylococcus aureus antibody | 10 CFU/mL | [103] | |
| Salmonella typhimurium | immunosensor and aptasensor | amperometry | Salmonella typhimurium and target DNA | anti-Salmonella antibody and DNA probe | 2.7 × 101 CFU/mL and 0.94 nM | [104] | |
| Salmonella typhimurium | aptasensor | chemiresistive detection | - | amine-ended aptamers | 10 CFU/mL | [105] | |
| Yersinia pestis | immunosensor | microcantilever detection | Yersinia pestis | Yersinia-specific antibodies | n.r. | [106] | |
| viruses | human adenovirus | aptasensor | fluorimetry | extracted viral DNA | DNA probe | 10 virus copies | [107] |
| hepatitis B virus | aptasensor | differential pulse voltammetry | target DNA | DNA probe | 1.45 pM | [108] | |
| parasites | Plasmodium falciparum | immunosensor | magnetometry | Plasmodium falciparum lactate dehydrogenase | Anti- Plasmodium lactate dehydrogenase monoclonal capture and c-MAb and bd-MAb detection antibodies | 200 ng/mL | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M.; Andronescu, E. Biosensors-on-Chip: An Up-to-Date Review. Molecules 2020, 25, 6013. https://doi.org/10.3390/molecules25246013
Chircov C, Bîrcă AC, Grumezescu AM, Andronescu E. Biosensors-on-Chip: An Up-to-Date Review. Molecules. 2020; 25(24):6013. https://doi.org/10.3390/molecules25246013
Chicago/Turabian StyleChircov, Cristina, Alexandra Cătălina Bîrcă, Alexandru Mihai Grumezescu, and Ecaterina Andronescu. 2020. "Biosensors-on-Chip: An Up-to-Date Review" Molecules 25, no. 24: 6013. https://doi.org/10.3390/molecules25246013
APA StyleChircov, C., Bîrcă, A. C., Grumezescu, A. M., & Andronescu, E. (2020). Biosensors-on-Chip: An Up-to-Date Review. Molecules, 25(24), 6013. https://doi.org/10.3390/molecules25246013








