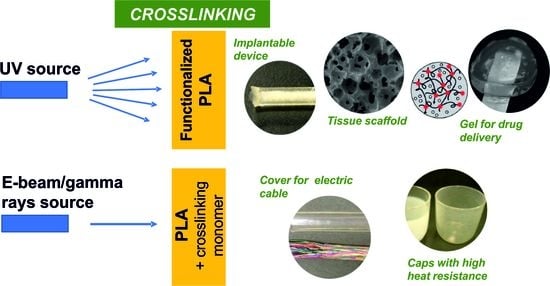
Crosslinking of Polylactide by High Energy Irradiation and Photo-Curing
Abstract
1. Introduction
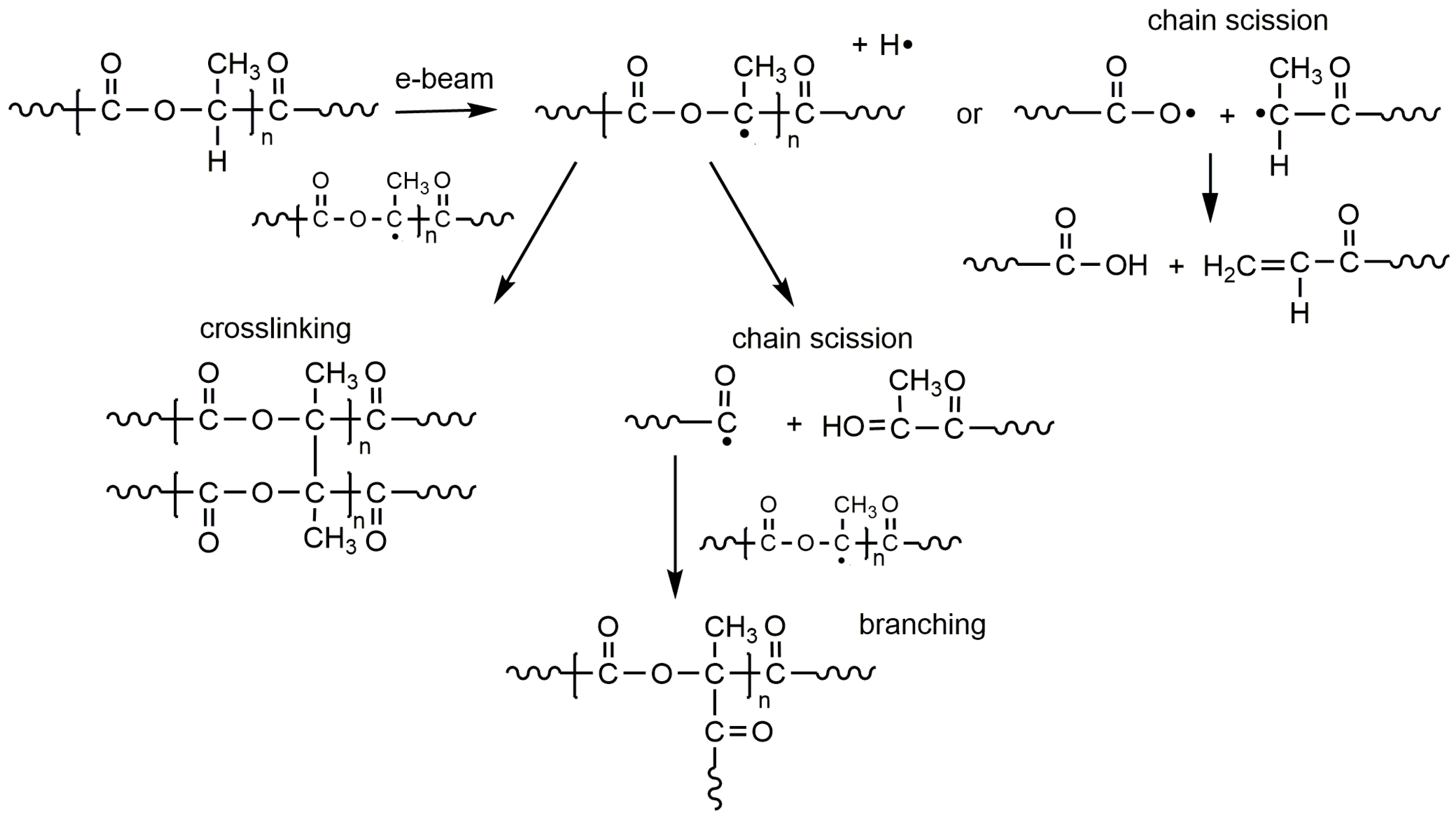
2. PLA Crosslinking by Electron Beam or Gamma Irradiation
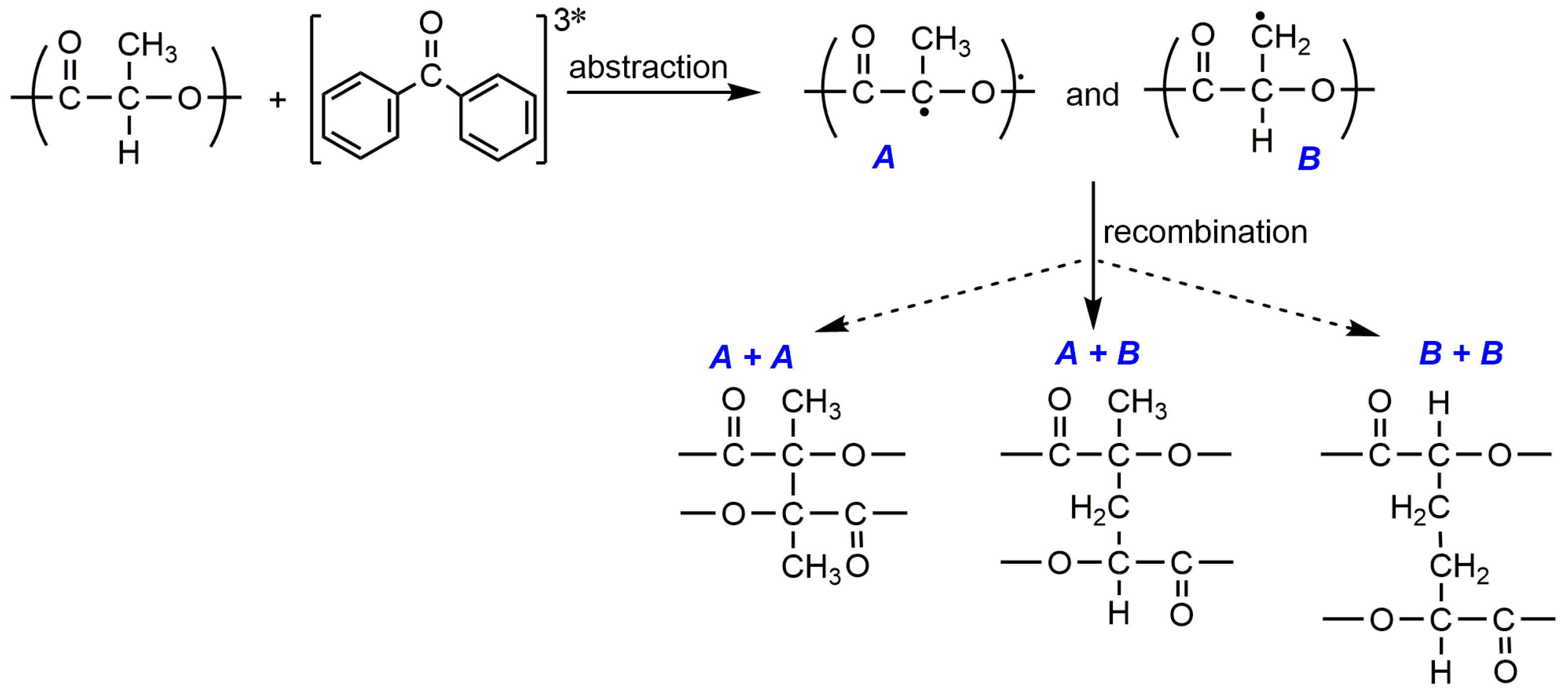
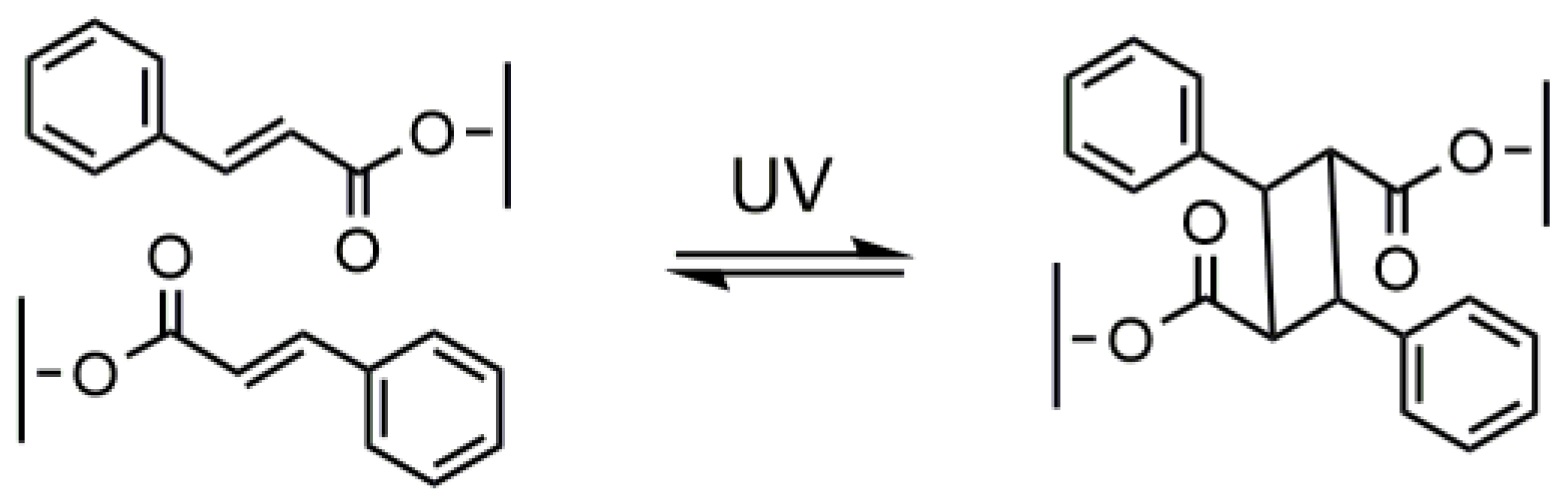
3. Photo-Crosslinked PLA
4. PLA-Based Materials by Photo- and High Energy Radiation Crosslinking
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Bawa, K.K.; Oh, J.K. Stimulus-Responsive Degradable Polylactide-Based Block Copolymer Nanoassemblies for Controlled/Enhanced Drug Delivery. Mol. Pharm. 2017, 14, 2460–2474. [Google Scholar] [CrossRef] [PubMed]
- Vacaras, S.; Baciut, M.; Lucaciu, O.; Dinu, C.; Baciut, G.; Crisan, L.; Hedesiu, M.; Crisan, B.; Onisor, F.; Armencea, G.; et al. Understanding the basis of medical use of poly-lactide-based resorbable polymers and composites – a review of the clinical and metabolic impact. Drug Metab. Rev. 2019, 51, 570–588. [Google Scholar] [CrossRef]
- Chatterjee, S.; Saxena, M.; Padmanabhan, D.; Jayachandra, M.; Pandya, H.J. Futuristic medical implants using bioresorbable materials and devices. Biosens. Bioelectron. 2019, 142, 111489. [Google Scholar] [CrossRef] [PubMed]
- Bano, K.; Pandey, R.; Jamal-e-Fatima and Roohi. New advancements of bioplastics in medical applications. Int. J. Pharm. Sci. Res. 2018, 9, 402–416. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Sharma, B.; Verma, A.; Tamulevicius, S.; Thakur, V.K. Sustainability of bioplastics: Opportunities and challenges. Curr. Opin. Green Sustain. Chem. 2018, 13, 68–75. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, R.J. PLA Plastic/Material: All You Need to Know in 2020. Available online: https://all3dp.com/1/pla-plastic-material-polylactic-acid/ (accessed on 11 January 2020).
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on Polylactic Acid (PLA) based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Mangeon, C.; Renard, E.; Thevenieau, F.; Langlois, V. Networks based on biodegradable polyesters: An overview of the chemical ways of crosslinking. Mater. Sci. Eng. C 2017, 80, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.C.; Deshmukh, V.G. Radiation effects on poly(lactic acid). Polymer (Guildf) 1983, 24, 827–830. [Google Scholar] [CrossRef]
- Manas, D.; Ovsik, M.; Mizera, A.; Manas, M.; Hylova, L.; Bednarik, M.; Stanek, M. The Effect of Irradiation on Mechanical and Thermal Properties of Selected Types of Polymers. Polymers (Basel) 2018, 10, 158. [Google Scholar] [CrossRef]
- Bednarek, M.; Borská, K.; Kubisa, P. New Polylactide -Based Materials by Chemical Crosslinking of Polylactide. Polymer Rev. 2020. submitted. [Google Scholar]
- Gilding, D.K.; Reed, A.M. Biodegradable polymers for use in surgery—poly(ethylene oxide) poly(ethylene terephthalate) (PEO/PET) copolymers: 1. Polymer (Guildf) 1979, 20, 1454–1458. [Google Scholar] [CrossRef]
- Birkinshaw, C.; Buggy, M.; Henn, G.G.; Jones, E. Irradiation of poly-d,l-lactide. Polym. Degrad. Stab. 1992, 38, 249–253. [Google Scholar] [CrossRef]
- Sintzel, M.B.; Merkli, A.; Tabatabay, C.; Gurny, R. Influence of Irradiation Sterilization on Polymers Used as Drug Carriers—A Review. Drug Dev. Ind. Pharm. 1997, 23, 857–878. [Google Scholar] [CrossRef]
- Yoshioka, S.; Aso, Y.; Otsuka, T.; Kojima, S. The effect of γ-irradiation on drug release from poly(lactide) microspheres. Radiat. Phys. Chem. 1995, 46, 281–285. [Google Scholar] [CrossRef]
- Babanalbandi, A.; Hill, D.J.T.; Whittaker, A.K. Volatile products and new polymer structures formed on 60Co γ-radiolysis of poly(lactic acid) and poly(glycolic acid). Polym. Degrad. Stab. 1997, 58, 203–214. [Google Scholar] [CrossRef]
- Babanalbandi, A.; Hill, D.J.T.; O’Donnell, J.H.; Pomery, P.J.; Whittaker, A. An electron spin resonance study on γ-irradiated poly(l-lactic acid) and poly(d,l-lactic acid). Polym. Degrad. Stab. 1995, 50, 297–304. [Google Scholar] [CrossRef]
- Milicevic, D.; Trifunovic, S.; Galovic, S.; Suljovrujic, E. Thermal and crystallization behaviour of gamma irradiated PLLA. Radiat. Phys. Chem. 2007, 76, 1376–1380. [Google Scholar] [CrossRef]
- Malinowski, R. Effect of high energy β-radiation and addition of triallyl isocyanurate on the selected properties of polylactide. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2016, 377, 59–66. [Google Scholar] [CrossRef]
- Adamus-Wlodarczyk, A.; Wach, R.; Ulanski, P.; Rosiak, J.; Socka, M.; Tsinas, Z.; Al-Sheikhly, M. On the Mechanisms of the Effects of Ionizing Radiation on Diblock and Random Copolymers of Poly(Lactic Acid) and Poly(Trimethylene Carbonate). Polymers (Basel) 2018, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.Y.; Han, D.H.; Narayan, R. Rheological and Thermal Properties of the PLA Modified by Electron Beam Irradiation in the Presence of Functional Monomer. J. Polym. Environ. 2010, 18, 558–566. [Google Scholar] [CrossRef]
- Chu, C.C. Degradation phenomena of two linear aliphatic polyester fibres used in medicine and surgery. Polymer (Guildf) 1985, 26, 591–594. [Google Scholar] [CrossRef]
- Nugroho, P.; Mitomo, H.; Yoshii, F.; Kume, T. Degradation of poly(l-lactic acid) by γ-irradiation. Polym. Degrad. Stab. 2001, 72, 337–343. [Google Scholar] [CrossRef]
- Mitomo, H.; Kaneda, A.; Quynh, T.M.; Nagasawa, N.; Yoshii, F. Improvement of heat stability of poly(l-lactic acid) by radiation-induced crosslinking. Polymer (Guildf) 2005, 46, 4695–4703. [Google Scholar] [CrossRef]
- Nagasawa, N.; Kaneda, A.; Kanazawa, S.; Yagi, T.; Mitomo, H.; Yoshii, F.; Tamada, M. Application of poly(lactic acid) modified by radiation crosslinking. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2005, 236, 611–616. [Google Scholar] [CrossRef]
- Quynh, T.M.; Mitomo, H.; Nagasawa, N.; Wada, Y.; Yoshii, F.; Tamada, M. Properties of crosslinked polylactides (PLLA & PDLA) by radiation and its biodegradability. Eur. Polym. J. 2007, 43, 1779–1785. [Google Scholar] [CrossRef]
- Quynh, T.M.; Mitomo, H.; Zhao, L.; Asai, S. The radiation crosslinked films based on PLLA/PDLA stereocomplex after TAIC absorption in supercritical carbon dioxide. Carbohydr. Polym. 2008, 72, 673–681. [Google Scholar] [CrossRef]
- Malinowski, R. Mechanical properties of PLA/PCL blends crosslinked by electron beam and TAIC additive. Chem. Phys. Lett. 2016, 662, 91–96. [Google Scholar] [CrossRef]
- Malinowski, R.; Rytlewski, P.; Żenkiewicz, M. Effects of electron radiation on properties of PLA. Arch. Mater. Sci. Eng. 2011, 49, 25–32. [Google Scholar]
- Jin, F.; Hyon, S.-H.; Iwata, H.; Tsutsumi, S. Crosslinking of Poly(L-lactide) by γ-Irradiation. Macromol. Rapid Commun. 2002, 23, 909–912. [Google Scholar] [CrossRef]
- Xia, X.; Shi, X.; Liu, W.; He, S.; Zhu, C.; Liu, H. Effects of gamma irradiation on properties of PLA/flax composites. Iran. Polym. J. 2020, 29, 581–590. [Google Scholar] [CrossRef]
- Hachana, N.; Wongwanchai, T.; Chaochanchaikul, K.; Harnnarongchai, W. Influence of Crosslinking Agent and Chain Extender on Properties of Gamma-Irradiated PLA. J. Polym. Environ. 2017, 25, 323–333. [Google Scholar] [CrossRef]
- Malinowski, R.; Janczak, K.; Moraczewski, K.; Raszkowska-Kaczor, A. Analysis of swelling degree and gel fraction of polylactide/poly(butylene adipate-co-terephthalate) blends crosslinked by radiation. Polimery 2018, 63, 25–30. [Google Scholar] [CrossRef]
- Malinowski, R.; Moraczewski, K.; Raszkowska-Kaczor, A. Studies on the Uncrosslinked Fraction of PLA/PBAT Blends Modified by Electron Radiation. Materials (Basel) 2020, 13, 1068. [Google Scholar] [CrossRef]
- Rytlewski, P.; Stepczyńska, M.; Gohs, U.; Malinowski, R.; Budner, B.; Żenkiewicz, M. Flax fibres reinforced polylactide modified by ionizing radiation. Ind. Crops Prod. 2018, 112, 716–723. [Google Scholar] [CrossRef]
- Salvatore, M.; Marra, A.; Duraccio, D.; Shayanfar, S.; Pillai, S.D.; Cimmino, S.; Silvestre, C. Effect of electron beam irradiation on the properties of polylactic acid/montmorillonite nanocomposites for food packaging applications. J. Appl. Polym. Sci. 2016, 133, 42219. [Google Scholar] [CrossRef]
- Kumar, A.; Venkatappa Rao, T.; Ray Chowdhury, S.; Ramana Reddy, S.V.S. Optimization of mechanical, thermal and hydrolytic degradation properties of Poly (lactic acid)/Poly (ethylene-co-glycidyl methacrylate)/Hexagonal boron nitride blend-composites through electron-beam irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2018, 428, 38–46. [Google Scholar] [CrossRef]
- Kodal, M.; Wis, A.A.; Ozkoc, G. The mechanical, thermal and morphological properties of γ-irradiated PLA/TAIC and PLA/OvPOSS. Radiat. Phys. Chem. 2018, 153, 214–225. [Google Scholar] [CrossRef]
- Seppälä, J.; Korhonen, H.; Hakala, R.; Malin, M. Photocrosslinkable Polyesters and Poly(ester anhydride)s for Biomedical Applications. Macromol. Biosci. 2011, 11, 1647–1652. [Google Scholar] [CrossRef]
- Borská, K.; Danko, M.; Mosnácek, J. Photodegradation and Photochemical Crosslinking of Polylactide. Chem. List. 2014, 108, 1030–1039. [Google Scholar]
- van Bochove, B.; Grijpma, D.W. Photo-crosslinked synthetic biodegradable polymer networks for biomedical applications. J. Biomater. Sci. Polym. Ed. 2019, 30, 77–106. [Google Scholar] [CrossRef]
- Sawhney, A.S.; Pathak, C.P.; Hubbell, J.A. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(.alpha.-hydroxy acid) diacrylate macromers. Macromolecules 1993, 26, 581–587. [Google Scholar] [CrossRef]
- Jansen, J.; Mihov, G.; Feijen, J.; Grijpma, D.W. Photo-Crosslinked Biodegradable Hydrogels Prepared from Fumaric Acid Monoethyl Ester-Functionalized Oligomers for Protein Delivery. Macromol. Biosci. 2012, 12, 692–702. [Google Scholar] [CrossRef]
- Burdick, J.A.; Frankel, D.; Dernell, W.S.; Anseth, K.S. An initial investigation of photocurable three-dimensional lactic acid based scaffolds in a critical-sized cranial defect. Biomaterials 2003, 24, 1613–1620. [Google Scholar] [CrossRef]
- Davis, K.A.; Burdick, J.A.; Anseth, K.S. Photoinitiated crosslinked degradable copolymer networks for tissue engineering applications. Biomaterials 2003, 24, 2485–2495. [Google Scholar] [CrossRef]
- Burdick, J.A.; Philpott, L.M.; Anseth, K.S. Synthesis and characterization of tetrafunctional lactic acid oligomers: A potential in situ forming degradable orthopaedic biomaterial. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 683–692. [Google Scholar] [CrossRef]
- Kim, B.S.; Hrkach, J.S.; Langer, R. Biodegradable photo-crosslinked poly(ether-ester) networks for lubricious coatings. Biomaterials 2000, 21, 259–265. [Google Scholar] [CrossRef]
- Shen, J.Y.; Pan, X.Y.; Lim, C.H.; Chan-Park, M.B.; Zhu, X.; Beuerman, R.W. Synthesis, Characterization, and In Vitro Degradation of a Biodegradable Photo-Cross-Linked Film from Liquid Poly(ε-caprolactone- co -lactide- co -glycolide) Diacrylate. Biomacromolecules 2007, 8, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Michlovská, L.; Vojtová, L.; Humpa, O.; Kučerík, J.; Žídek, J.; Jančář, J. Hydrolytic stability of end-linked hydrogels from PLGA–PEG–PLGA macromonomers terminated by α,ω-itaconyl groups. RSC Adv. 2016, 6, 16808–16816. [Google Scholar] [CrossRef]
- Sharifi, S.; Grijpma, D.W. Resilient Amorphous Networks Prepared by Photo-Crosslinking High-Molecular-Weight d,l-Lactide and Trimethylene Carbonate Macromers: Mechanical Properties and Shape-Memory Behavior. Macromol. Biosci. 2012, 12, 1423–1435. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A poly(d,l-lactide) resin for the preparation of tissue engineering scaffolds by stereolithography. Biomaterials 2009, 30, 3801–3809. [Google Scholar] [CrossRef]
- Grijpma, D.W.; Hou, Q.; Feijen, J. Preparation of biodegradable networks by photo-crosslinking lactide, ε-caprolactone and trimethylene carbonate-based oligomers functionalized with fumaric acid monoethyl ester. Biomaterials 2005, 26, 2795–2802. [Google Scholar] [CrossRef]
- Leclerc, E.; Furukawa, K.; Miyata, F.; Sakai, Y.; Ushida, T.; Fujii, T. Fabrication of microstructures in photosensitive biodegradable polymers for tissue engineering applications. Biomaterials 2004, 25, 4683–4690. [Google Scholar] [CrossRef]
- Aoyagi, T.; Miyata, F.; Nagase, Y. Preparation of cross-linked aliphatic polyester and application to thermo-responsive material. J. Control. Release 1994, 32, 87–96. [Google Scholar] [CrossRef]
- Amsden, B.G. Biodegradable elastomers in drug delivery. Expert Opin. Drug Deliv. 2008, 5, 175–187. [Google Scholar] [CrossRef]
- Chen, F.; Hochleitner, G.; Woodfield, T.; Groll, J.; Dalton, P.D.; Amsden, B.G. Additive Manufacturing of a Photo-Cross-Linkable Polymer via Direct Melt Electrospinning Writing for Producing High Strength Structures. Biomacromolecules 2016, 17, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hayami, J.W.S.; Amsden, B.G. Electrospun Poly(d,l-lactide- co -acryloyl carbonate) Fiber Scaffolds With a Mechanically Stable Crimp Structure For Ligament Tissue Engineering. Biomacromolecules 2014, 15, 1593–1601. [Google Scholar] [CrossRef]
- Amsden, B.; Misra, G.; Marshall, M.; Turner, N. Synthesis and Characterization of Biodegradable Networks Providing Saturated-Solution Prolonged Delivery. J. Pharm. Sci. 2008, 97, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Chapanian, R.; Amsden, B.G. Combined and sequential delivery of bioactive VEGF165 and HGF from poly(trimethylene carbonate) based photo-cross-linked elastomers. J. Control. Release 2010, 143, 53–63. [Google Scholar] [CrossRef]
- Amsden, B.G.; Marecak, D. Long-Term Sustained Release from a Biodegradable Photo-Cross-Linked Network for Intraocular Corticosteroid Delivery. Mol. Pharm. 2016, 13, 3004–3012. [Google Scholar] [CrossRef] [PubMed]
- Chapanian, R.; Tse, M.Y.; Pang, S.C.; Amsden, B.G. Osmotic Release of Bioactive VEGF from Biodegradable Elastomer Monoliths is the Same In Vivo As In Vitro. J. Pharm. Sci. 2012, 101, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Karikari, A.S.; Edwards, W.F.; Mecham, J.B.; Long, T.E. Influence of Peripheral Hydrogen Bonding on the Mechanical Properties of Photo-Cross-Linked Star-Shaped Poly(d,l-lactide) Networks. Biomacromolecules 2005, 6, 2866–2874. [Google Scholar] [CrossRef] [PubMed]
- Tanodekaew, S.; Channasanon, S.; Uppanan, P. Preparation and degradation study of photocurable oligolactide-HA composite: A potential resin for stereolithography application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 604–611. [Google Scholar] [CrossRef]
- Koroleva, A.; Gill, A.A.; Ortega, I.; Haycock, J.W.; Schlie, S.; Gittard, S.D.; Chichkov, B.N.; Claeyssens, F. Two-photon polymerization-generated and micromolding-replicated 3D scaffolds for peripheral neural tissue engineering applications. Biofabrication 2012, 4, 025005. [Google Scholar] [CrossRef]
- Timashev, P.; Kuznetsova, D.; Koroleva, A.; Prodanets, N.; Deiwick, A.; Piskun, Y.; Bardakova, K.; Dzhoyashvili, N.; Kostjuk, S.; Zagaynova, E.; et al. Novel biodegradable star-shaped polylactide scaffolds for bone regeneration fabricated by two-photon polymerization. Nanomedicine 2016, 11, 1041–1053. [Google Scholar] [CrossRef]
- Shashkova, V.T.; Matveeva, I.A.; Glagolev, N.N.; Zarkhina, T.S.; Cherkasova, A.V.; Kotova, S.L.; Timashev, P.S.; Solovieva, A.B. Synthesis of polylactide acrylate derivatives for the preparation of 3D structures by photo-curing. Mendeleev Commun. 2016, 26, 418–420. [Google Scholar] [CrossRef]
- Kuznetsova, D.; Ageykin, A.; Koroleva, A.; Deiwick, A.; Shpichka, A.; Solovieva, A.; Kostjuk, S.; Meleshina, A.; Rodimova, S.; Akovanceva, A.; et al. Surface micromorphology of cross-linked tetrafunctional polylactide scaffolds inducing vessel growth and bone formation. Biofabrication 2017, 9, 025009. [Google Scholar] [CrossRef] [PubMed]
- Bardakova, K.N.; Grebenik, E.A.; Istranova, E.V.; Istranov, L.P.; Gerasimov, Y.V.; Grosheva, A.G.; Zharikova, T.M.; Minaev, N.V.; Shavkuta, B.S.; Dudova, D.S.; et al. Reinforced Hybrid Collagen Sponges for Tissue Engineering. Bull. Exp. Biol. Med. 2018, 165, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Bardakova, K.N.; Grebenik, E.A.; Minaev, N.V.; Churbanov, S.N.; Moldagazyeva, Z.; Krupinov, G.E.; Kostjuk, S.V.; Timashev, P.S. Tailoring the collagen film structural properties via direct laser crosslinking of star-shaped polylactide for robust scaffold formation. Mater. Sci. Eng. C 2020, 107, 110300. [Google Scholar] [CrossRef]
- Petchsuk, A.; Submark, W.; Opaprakasit, P. Development of crosslinkable poly(lactic acid-co-glycidyl methacrylate) copolymers and their curing behaviors. Polym. J. 2013, 45, 406–412. [Google Scholar] [CrossRef]
- Rahman, M.; Thananukul, K.; Supmak, W.; Petchsuk, A.; Opaprakasit, P. Synthesis and quantitative analyses of acrylamide-grafted poly(lactide-co-glycidyl methacrylate) amphiphilic copolymers for environmental and biomedical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117447. [Google Scholar] [CrossRef]
- Koo, G.-H.; Jang, J. Preparation of melting-free poly(lactic acid) by amorphous and crystal crosslinking under UV irradiation. J. Appl. Polym. Sci. 2013, 127, 4515–4523. [Google Scholar] [CrossRef]
- Poplata, S.; Tröster, A.; Zou, Y.-Q.; Bach, T. Recent Advances in the Synthesis of Cyclobutanes by Olefin [2 + 2] Photocycloaddition Reactions. Chem. Rev. 2016, 116, 9748–9815. [Google Scholar] [CrossRef]
- Habault, D.; Zhang, H.; Zhao, Y. Light-triggered self-healing and shape-memory polymers. Chem. Soc. Rev. 2013, 42, 7244–7256. [Google Scholar] [CrossRef]
- Kaur, G.; Johnston, P.; Saito, K. Photo-reversible dimerisation reactions and their applications in polymeric systems. Polym. Chem. 2014, 5, 2171–2186. [Google Scholar] [CrossRef]
- Nagata, M.; Inaki, K. Synthesis and characterization of photocrosslinkable poly(l-lactide)s with a pendent cinnamate group. Eur. Polym. J. 2009, 45, 1111–1117. [Google Scholar] [CrossRef]
- Nagata, M.; Sato, Y. Photocurable biodegradable polyesters from poly(L-lactide) diols. Polym. Int. 2005, 54, 386–391. [Google Scholar] [CrossRef]
- Hu, X.; Chen, X.; Cheng, H.; Jing, X. Cinnamate-functionalized poly(ester-carbonate): Synthesis and its UV irradiation-induced photo-crosslinking. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 161–169. [Google Scholar] [CrossRef]
- Gangolphe, L.; Déjean, S.; Bethry, A.; Hunger, S.; Pinese, C.; Garric, X.; Bossard, F.; Nottelet, B. Degradable multi(aryl azide) star copolymer as universal photo-crosslinker for elastomeric scaffolds. Mater. Today Chem. 2019, 12, 209–221. [Google Scholar] [CrossRef]
- Rupp, B.; Ebner, C.; Rossegger, E.; Slugovc, C.; Stelzer, F.; Wiesbrock, F. UV-induced crosslinking of the biopolyester poly(3-hydroxybutyrate)-co-(3-hydroxyvalerate). Green Chem. 2010, 12, 1796–1802. [Google Scholar] [CrossRef]
- Chapanian, R.; Tse, M.Y.; Pang, S.C.; Amsden, B.G. Long term in vivo degradation and tissue response to photo-cross-linked elastomers prepared from star-shaped prepolymers of poly(ε-caprolactone- co -d,l-lactide). J. Biomed. Mater. Res. Part A 2009, 92A, 830–842. [Google Scholar] [CrossRef]
- Gu, F.; Neufeld, R.; Amsden, B. Maintenance of vascular endothelial growth factor and potentially other therapeutic proteins bioactivity during a photo-initiated free radical cross-linking reaction forming biodegradable elastomers. Eur. J. Pharm. Biopharm. 2007, 66, 21–27. [Google Scholar] [CrossRef]
- Gu, F.; Neufeld, R.; Amsden, B. Sustained release of bioactive therapeutic proteins from a biodegradable elastomeric device. J. Control. Release 2007, 117, 80–89. [Google Scholar] [CrossRef]
- Gu, F.; Younes, H.M.; El-Kadi, A.O.S.; Neufeld, R.J.; Amsden, B.G. Sustained interferon-γ delivery from a photocrosslinked biodegradable elastomer. J. Control. Release 2005, 102, 607–617. [Google Scholar] [CrossRef]
- Ilagan, B.G.; Amsden, B.G. Macroporous photocrosslinked elastomer scaffolds containing microposity: Preparation and in vitro degradation properties. J. Biomed. Mater. Res. Part A 2009, 93A, 211–218. [Google Scholar] [CrossRef]
- Vyner, M.C.; Liu, L.; Sheardown, H.D.; Amsden, B.G. The effect of elastomer chain flexibility on protein adsorption. Biomaterials 2013, 34, 9287–9294. [Google Scholar] [CrossRef] [PubMed]
- Chapanian, R.; Amsden, B.G. Osmotically driven protein release from photo-cross-linked elastomers of poly(trimethylene carbonate) and poly(trimethylene carbonate-co-d,l-lactide). Eur. J. Pharm. Biopharm. 2010, 74, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Timbart, L.; Tse, M.Y.; Pang, S.; Amsden, B.G. Tissue Response to, and Degradation Rate of, Photocrosslinked Trimethylene Carbonate-Based Elastomers Following Intramuscular Implantation. Materials (Basel) 2010, 3, 1156–1171. [Google Scholar] [CrossRef]
- Hayami, J.W.S.; Waldman, S.D.; Amsden, B.G. A Photocurable Hydrogel/Elastomer Composite Scaffold with Bi-Continuous Morphology for Cell Encapsulation. Macromol. Biosci. 2011, 11, 1672–1683. [Google Scholar] [CrossRef]
- Hayami, J.W.S.; Waldman, S.D.; Amsden, B.G. Injectable, High Modulus, And Fatigue Resistant Composite Scaffold for Load-Bearing Soft Tissue Regeneration. Biomacromolecules 2013, 14, 4236–4247. [Google Scholar] [CrossRef] [PubMed]
- Hayami, J.W.S.; Surrao, D.C.; Waldman, S.D.; Amsden, B.G. Design and characterization of a biodegradable composite scaffold for ligament tissue engineering. J. Biomed. Mater. Res. Part A 2009, 92A, 1407–1420. [Google Scholar] [CrossRef]
- Amsden, B.; Qi, B. Anti-atherosclerotic peptide delivery from a photocrosslinkable biodegradable network. Int. J. Pharm. 2010, 388, 32–39. [Google Scholar] [CrossRef]
- Shinyama, K. Influence of Electron Beam Irradiation on Electrical Insulating Properties of PLA with Soft Resin Added †. Polymers (Basel) 2018, 10, 898. [Google Scholar] [CrossRef]
- Oyj, S.E. Method of Use of Polylactide and Manufacturing a Heat-Sealed Paper or Board Container or Package. U.S. Patent 10 414 105B2, 24 January 2014. Available online: https://patentswarm.com/patents/US10414105B2. (accessed on 22 October 2020).
- Oyj, S.E. Heat-Sealable Biodegradable Packaging Material, a Method for Its Manufacture, and a Product Package Made from the Material. U.S. Patent 9181010B2, 11 May 2011. Available online: https://patents.google.com/patent/EP2544957A1. (accessed on 22 October 2020).
- Oyj, S.E. Method for Improving the Heat Sealibility of Packaging Material and Method for Manufacturing Heat-Sealed Container or Package. International Application No. PCT/FI2011/050381; U.S. Patent Application No. 13695496, U.S. Patent 20130137562 3 November 2011. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2011135182. (accessed on 22 October 2020).
- Lai, W.-J.; Huang, C.-H. Wei Mon Ind Co Polylactide-Coated Paperboard. U.S. Patent 20100209636A1, 9 April 2009. Available online: https://patents.google.com/patent/US20100209636A1. (accessed on 22 October 2020).
- Taleyarkhan, R.; Bakken, A.C.; Fisher, K.F.; Hagen, A.R.; Kostry, N.P. Polylactic Acid Adhesive Compositions and Methods for Their Preparation and Use. U.S. Patent 10442966B2, 15 November 2013. Available online: https://patents.google.com/patent/WO2014078720A1 (accessed on 22 October 2020).
- Amsden, B.G.; Misra, G.; Gu, F.; Younes, H.M. Synthesis and Characterization of a Photo-Cross-Linked Biodegradable Elastomer. Biomacromolecules 2004, 5, 2479–2486. [Google Scholar] [CrossRef]
- Kim, K.; Yeatts, A.; Dean, D.; Fisher, J.P. Stereolithographic Bone Scaffold Design Parameters: Osteogenic Differentiation and Signal Expression. Tissue Eng. Part B Rev. 2010, 16, 523–539. [Google Scholar] [CrossRef]
- Concellón, A.; Asín, L.; González-Lana, S.; de la Fuente, J.M.; Sánchez-Somolinos, C.; Piñol, M.; Oriol, L. Photopolymers based on ethynyl-functionalized degradable polylactides by thiol-yne ‘Click Chemistry’. Polymer (Guildf) 2017, 117, 259–267. [Google Scholar] [CrossRef]
- Ronca, A.; Ambrosio, L.; Grijpma, D.W. Preparation of designed poly(d,l-lactide)/nanosized hydroxyapatite composite structures by stereolithography. Acta Biomater. 2013, 9, 5989–5996. [Google Scholar] [CrossRef] [PubMed]
- Akopova, T.A.; Timashev, P.S.; Demina, T.S.; Bardakova, K.N.; Minaev, N.V.; Burdukovskii, V.F.; Cherkaev, G.V.; Vladimirov, L.V.; Istomin, A.V.; Svidchenko, E.A.; et al. Solid-state synthesis of unsaturated chitosan derivatives to design 3D structures through two-photon-induced polymerization. Mendeleev Commun. 2015, 25, 280–282. [Google Scholar] [CrossRef]
- Bryant, S.J.; Nuttelman, C.R.; Anseth, K.S. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polym. Ed. 2000, 11, 439–457. [Google Scholar] [CrossRef]
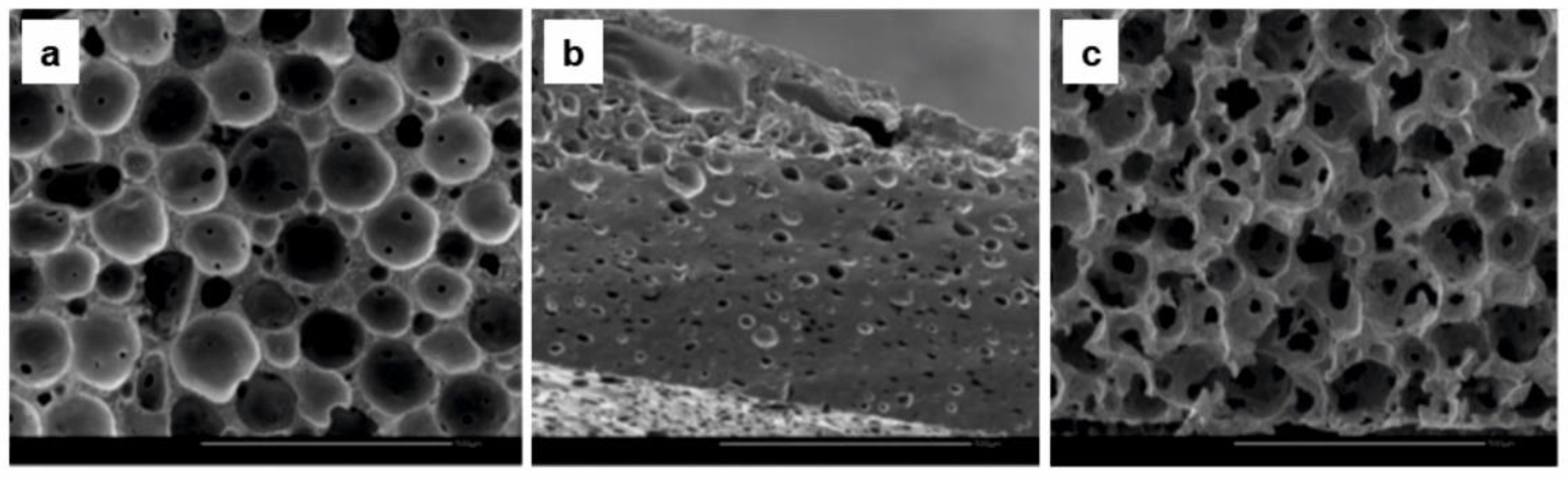
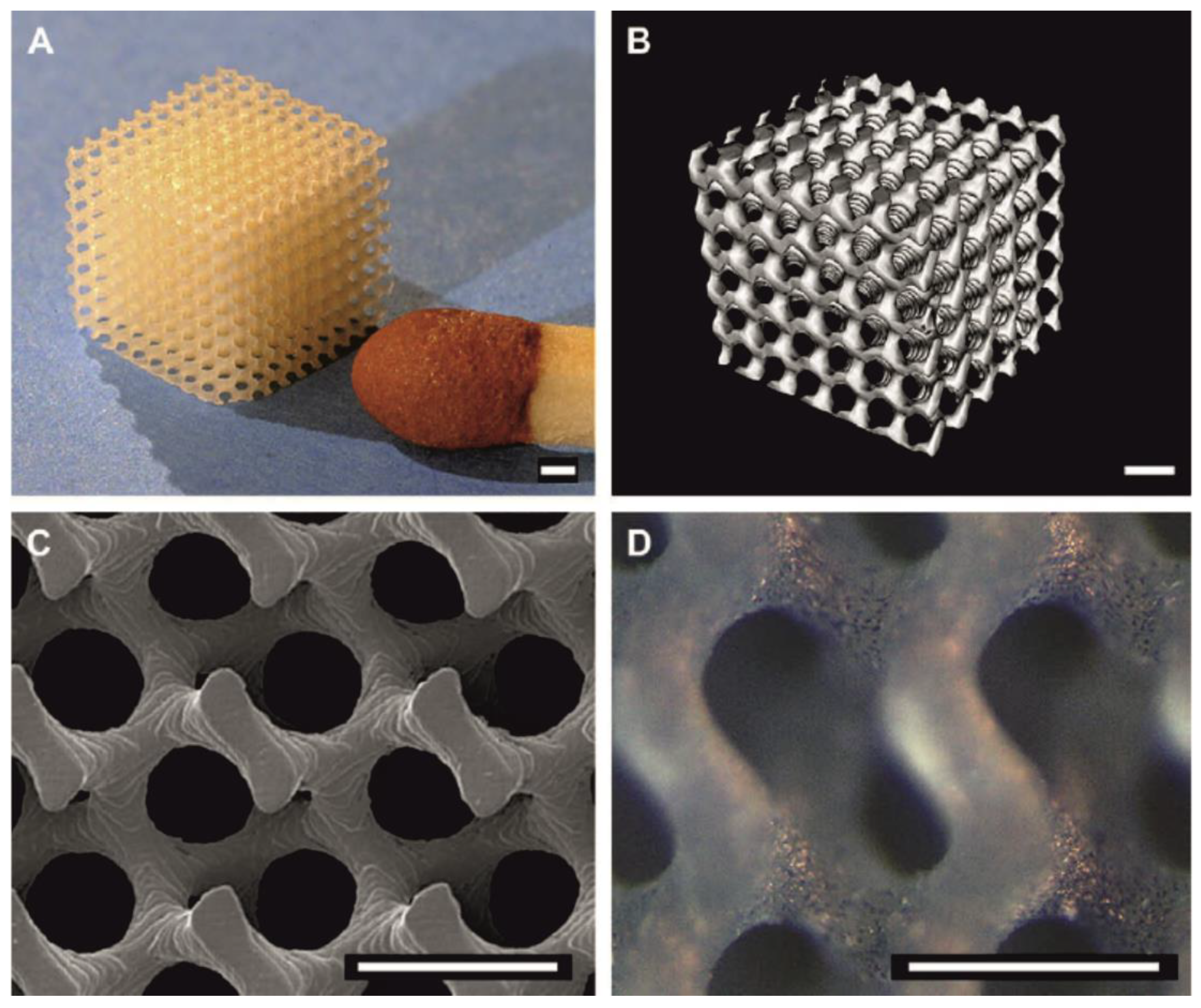
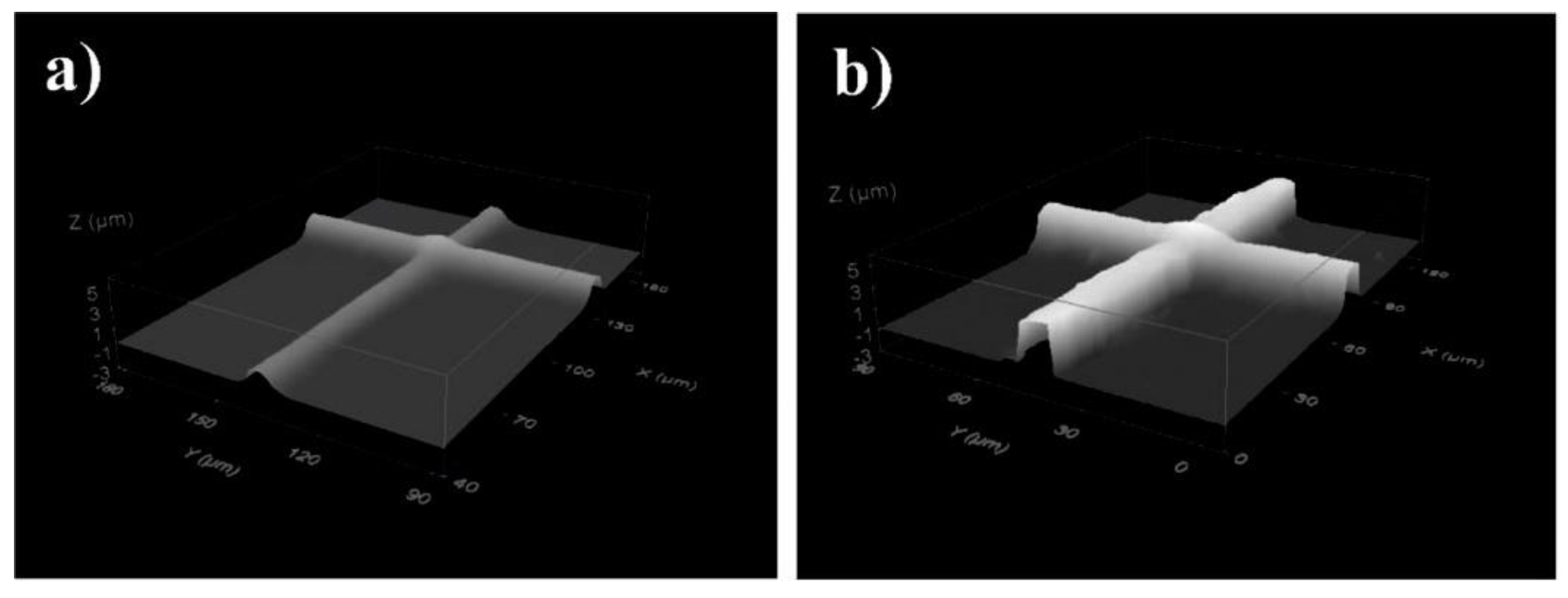
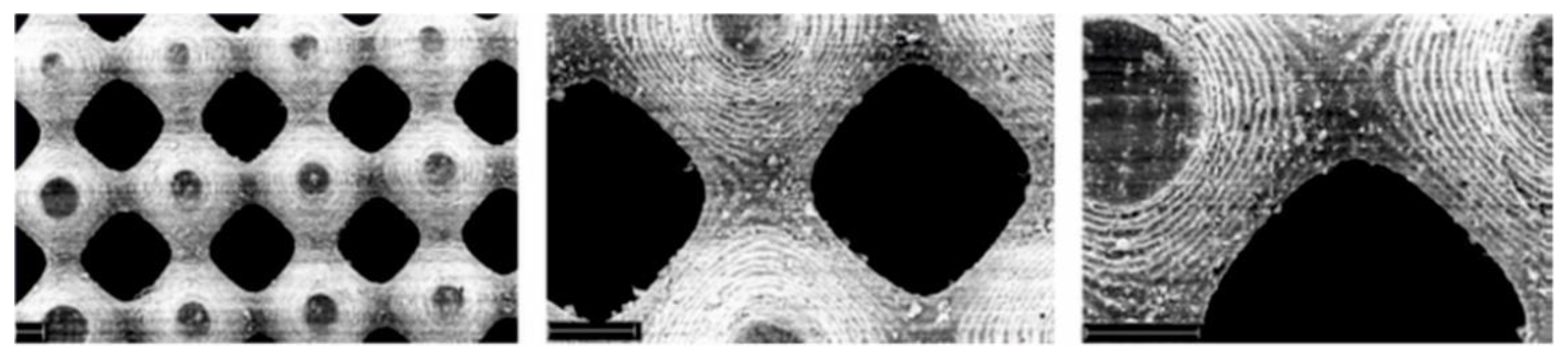
| Polylactide-Based Polymer (Mn, g·mol−1) | Radiation Type, Dose | Curing Co-Agent | Gel Content, % | Achieved Results | Reference |
|---|---|---|---|---|---|
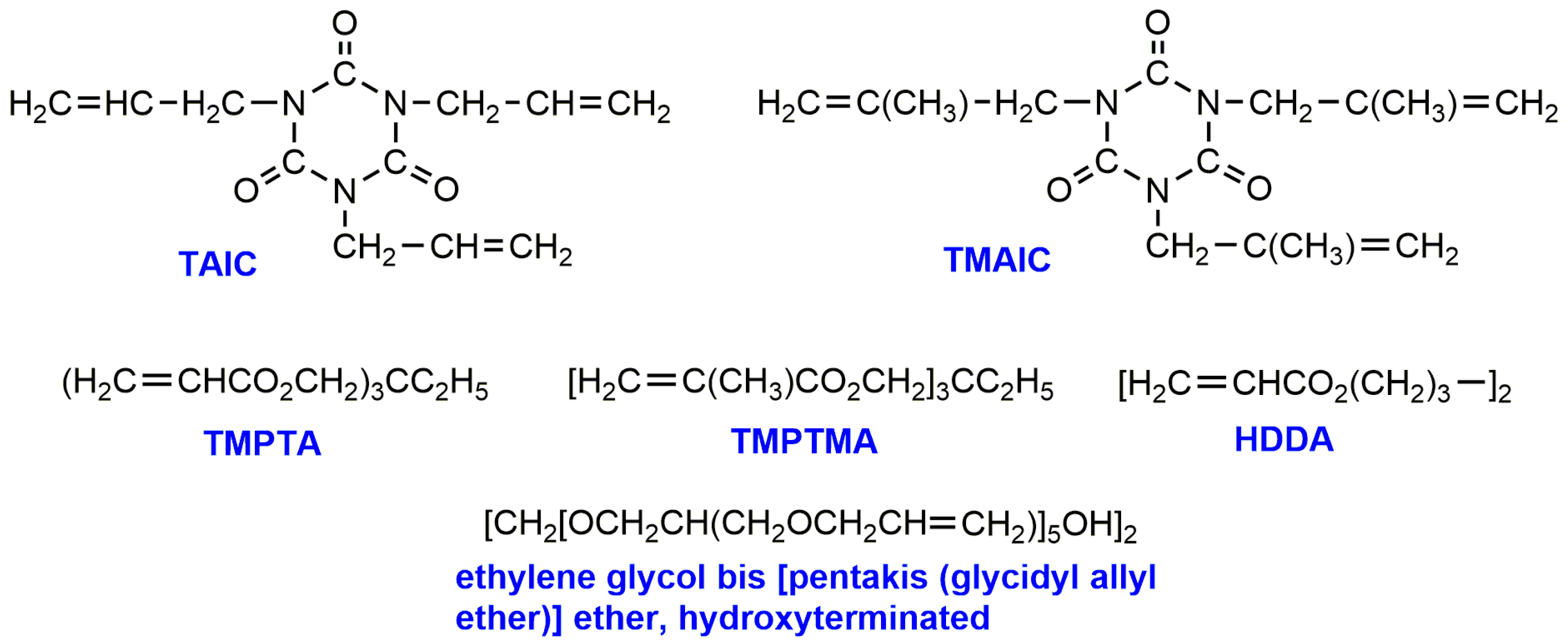
| PLLA (99,000) | Electron beam, 0–100 kGy | TAIC, TMAIC, TMPTA, TMPTMA, HDDA, derivative of EG | 0.1–88 | Together with annealing improved heat stability above Tg until Tm; lower solubility in any solvents; retarded enzymatic degradation. | [30] |
| PLLA | Electron beam, 0–50 kGy | TAIC, TMAIC, TMPTA, TMPTMA, HDDA, derivative of EG | 10–83 | Stability at higher melting temperature; application of the crosslinked PLLA on heat-shrinkable tubes, cups and plates. | [31] |
| PLLA (115,100), PDLLA (197,000) | Electron beam, 0–50 kGy | TAIC | ~40–100 | Shifts of Tcc to higher and Tm to lower temperatures; increase in tensile strength, young’s modulus and decrease in elongation at break; the crosslinked PLA samples were harder and more brittle at low temperature, but rubbery and soft, then stable at higher temperature (over Tm); decreased rate of enzymatic hydrolysis. | [32] |
| Equimolar blend of PLLA and PDLA | Electron beam, 0–50 kGy | TAIC + supercritical CO2 | ~30–90 | Shift of Tm of homo crystals to lower temp.; improved toughness and tensile strength. | [33] |
| PLLA (155,500) | Electron beam 0–90 kGy | TAIC | NA | Pristine PLA: Only degradation was observed; PLA/TAIC: Increase of Tg (69–75 °C), decrease of melt flow and water vapor permeability. | [35] |
| PLA (155,500) | Electron beam, 200–1000 kGy | TAIC | 68.2–89.4 | For neat PLA: only degradation; PLA/TAIC: decrease of gel content with increasing radiation dose; optimum crosslinking obtained at radiation dose of 40–200 kGy and 3–5 wt % of TAIC. | [25] |
| PLA (155,500) /PCL (82,500) blend | Electron beam, 0–90 kGy | TAIC | NA | PLA: Increase of flexural modulus, tensile strength, flexural strength, decrease of elongation at break; PLA/PCL blend: partial degradation of PLA phase, mechanical properties depending on ratios of the polymeric components. | [34] |
| PLA (91,000)/PBAT (35,000) blend | Electron beam, 0–90 kGy | TAIC | 40–90 | Crosslinking and degradation after irradiation mostly in PLA phase, PBAT less susceptible to radiation influence. | [39,40] |
| PLLA + Reinforced by flax fiber (20 wt %) | Electron beam, 0–40kGy | TAIC | 7.6–62.5 | Increase of tensile strength of about 20% in the presence of TAIC at 40kGy of irradiation dose; irradiation in the presence of TAIC led to reduced enzymatic degradation; decrease of interfacial adhesion of flax fibers and PLA matrix in the presence of TAIC. | [41] |
| PLA (210,000) + MMT (1,3,5 wt %) | Electron beam, 1 and 10 kGy | - | NA | Increase of Tg, crystallinity and young modulus, decrease of elongation at brake and oxygen permeability. | [42] |
| PLA/PEGM/HBN blend composite | Electron beam, 0–100 kGy | - | NA | At low doses: partial branching and crosslinking for neat PLA and PLA/PEGM; at higher doses: chain scission dominates. increase of Tg, notched impact strength and heat deflection temp. with radiation of blend-composites with higher amount of HBN; accelerated hydrolytic degradation of irradiated blend and blend-composites. | [43] |
| PLLA | γ-rays 2.5–50 kGy | TAIC | 10–100 | Decrease of swelling with increasing gel content, decrease in elongation (75%), maintenance of tensile strength, decrease of crystallinity (from 36 to 10%) and Tm (from 182 to 165 °C). | [36] |
| PLA + Flax fiber (5 wt %) | γ-rays 0–20 kGy | TAIC | 70–90 | Increase of the gel fraction in PLA/ flax composite with the radiation dose, degradation at higher doses; improvement of tensile strength and toughness with the increase in the radiation dose, decrease of elongation at break. | [37] |
| PLA (106,000) | γ-rays 0–100 kGy | TAIC, Ov-POSS | Up to 80 | Higher degree of crosslinking for PLA/OvPOSS in comparison to PLA/TAIC; irradiated composites exhibited decrease of crystallinity, lower elongation at break and higher E-modulus, higher thermal stability and heat deflection temp. than that of neat PLA | [44] |
| PLA (72,000) | γ-rays 0–20 kGy | TAIC as crosslinking agent (CA), Epoxy functional acrylic oligomer (Joncryl® ADR 4368) as chain extender (CE) | 1.2–46.2 | Considerable gel formation was observed for PLA/CA at high irradiation dose; addition of CA or CE increased the shear viscosity of neat and irradiated PLA; addition of CA and CE enhanced Tc and decreased crystallinity; improvement of tensile properties was higher for CA. | [38] |
| PLA Structure (Mn, g·mol−1) | Crosslinking Group | Photoinitiator | Gel Content a, % | Achieved Results | Ref. |
|---|---|---|---|---|---|
| PDLLA-b-PEG-b-PDLLA (1000–20,000) | Acrylate end group | 2,2-dimethoxy-2-phenylacetophenone (Irgacure 651) | 65–74 | Degradation rate increased with increasing Mn of precursor; materials used in the sustained release of proteins. | [48] |
| PDLLA-b-PEG-b-PDLLA Or: P(DLLA-co-TMC)-b-PEG-b-P(DLLA-co-TMC (4500–5500) | Fumarate end group | 2,2-dimethoxy-2-phenylacetophenone | >90 | Hydrogels prepared in N-vinylpyrrolidone were used for the study of model protein release; the degradation behavior could be controlled by changing the composition of the hydrophobic segments. | [49] |
| PDLLA-b-PEG-b-PDLLA (~1600) | Acrylate end group | 2,2-dimethoxy-2-phenyl acetophenone | Preparation of porous scaffolds for the study of the growth factor encapsulation and release and implantation in the case of cranial defect. | [50] | |
| PDLLA-b-PEG-b-PDLLA (990–1240) | Acrylate end group | camphorquinone/ethyl-4-N,N-dimethylaminobenzoate | 89–100 | Modification of hydrophobicity (contact angle 123°–142°); Tg = 1.8–26 °C depending on the composition and crosslinking density; tensile modulus in the range 0.92–3.67 MPa and strain at break 0.19–0.65; preparation of scaffolds with various pore sizes by salt-leaching method. | [52] |
| PDLLA-b-PEG-b-PDLLA (1120–10,720) | Acrylate end group | 2,2-dimethoxy-2-phenylacetophenone | 78–100 | Both lower crosslinking density (higher Mn of macromer) and the lower crystallinity (lower Mn) increased the degradation rate of the networks; the maximum improvement in penetration force, lubricant property, over control was 41% in the needle coated with PPG-based polymer network. | [53] |
| PDLLA-b-PPG-b-PDLLA (1150–4720) | Acrylate end group | 2,2-dimethoxy-2-phenylacetophenone | 93–99 | ||
| PDLLA-b-PTMG-b-PDLLA (1370–3620) | Acrylate end group | 2,2-dimethoxy-2-phenylacetophenone | 95–97 | ||
| PDLLA-b-PCL (1570–2390) | Methacrylate end group | camphorquinone/ethyl-4-dimethylaminobenzoate | Highly crosslinked | Decrease of Tg with increasing CL content (Tg in the range −30 to 60 °C). Storage moduli in the glassy regime were similar, in the rubbery regime dependent on crosslinking density; highly cross-linked scaffolds were cellularly compatible and promoted osteoblast attachment. | [51] |
| P(CL-co-LLA-co-GA) (1870–10,190) | Acrylate end group | 2,2-dimethoxy-2-phenylacetophenone | >95 | Increase of Tg of 2.8–14.9 °C, similar ultimate strength (σ = 2.39–3.76 MPa); Young’s modulus (E = 1.66–12.29 MPa and maximum strain (ε = 21–176%); Excellent biocompatibility of films with smooth muscle cells. | [54] |
| P(LDLA-co-GA)-b-PEG-b- P(LDLA-co-GA) (~5300) | Itaconic end groups | camphorquinone | 94–98 b | Swelling properties depended on crosslinking time, thus crosslinking density; with longer UV exposure better hydrolytic stability of hydrogel was observed. | [55] |
| P(DLLA-co-TMC) (27,000–29,000) | Methacrylate end group | Irgacure 2959 | 74–90 | Depending on the DLLA /TMC ratio, amorphous networks with Tg of 13 to 51 °C and elastic modulus from 3.6 MPa to 2.7 GPa were obtained; networks of more than 40 mol% of TMC are tough, flexible and elastomeric at r.t. with elongations at break of up to 800%. When DLLA:TMC = 60:40, Tg is between 25 and 37 °C, thus elastic medical devices with SM properties could be implanted in a temporary shape. | [56] |
| 2,3- and 6-arm PDLLA (6600–34,200) | Methacrylate end group | 2-hydroxy-1-[4- (hydroxyethoxy)phenyl]-2-methyl-1-propanone (Irgacure 2959) | 96 | Tg (55–76 °C) dependent on macromer chain length; mechanical properties similar to HMW PDLLA- suitable for stereolithography; mouse pre-osteoblasts readily adhered and proliferated well on networks. | [57] |
| 3-arm P(TMC-co-DLLA) (3100–4000) | Fumaric acid monoethyl ester | 2,2-dimethoxy-2-phenylacetophenone | 67–81c | The E modulus decrease with TMC content, tensile strength and elongation at break unaffected. Relative low values of tensile strength (1–2 MPa), and E modulus (1–10 MPa) in comparison with HMW PDLLA and PTMC. | [58] |
| 4-arm PDLLA-co-PCL (5000–10,000) | Acrylate end group | 1-hydroxycyclohexylphenylketone (irgacure 184) | NA | Fabrication of microstructures by soft lithography. Possibility of using studied materials to culture mammalian cells. | [59] |
| 4-arm P(LLA-b-CL) (Mn ~3200–12,000) | Methacrylate end group | Camphorquinone d | NA | Transition temperatures depended on the length of poly-CL segments. Decrease of Tm and crystallinity with increasing Mn. Thermo-responsive properties as permeability of a drug. | [60] |
| 3-arm P(CL-co-DLLA) (1250–7800) | Acrylate end group | 2,2-dimethoxy-2-phenylacetophenone | >95 | Tg of elastomers below physiological temperature (even below 0 °C). The Young’s modulus and stress at break inversely proportional but strain at break-proportional to the prepolymer Mn. The ability of elastomeric devices to encapsulate (glyco)proteins and release them according to an osmotic pressure delivery mechanism; confirmed ability to degradation in vitro and in vivo. Preparation of porous scaffolds capable to degradation with mechanical properties dependent on prepolymers Mn. Ability to adsorb proteins and to cell proliferation; dependence of adsorbed protein layer on the material stiffness. | [87,88,89,90,91,92] |
| 3-arm poly(CL-co- DLLA) (1250, 2700 and 3900) | Acrylate end group and co-photo-crosslinker poly(ethylene glycol)diacrylate (PEGDA) (4000 and 24,000) | 2,2-dimethoxy-2-phenylacetophenone | 95–98 | Tg, Tm and ∆Hf varied with prepolymer Mn, co-photo-crosslinker amount and Mn. Networks without PEGDA were amorphous, with PEGDA indicated melting; preparation of cylindrical elastomeric devices able to encapsulate Vitamin B12. | [64] |
| 3-arm poly(TMC-co-DLLA) (7800–8500) | Acrylate end group | 2,2-dimethoxy-2-phenylacetophenone | 79–88 | With increasing amount of DLLA increase of Young’s, stress at break, Tg and decrease of elongation at break. The possibility of osmotic pressure driven release of proteins. Study of the behavior of elastomers implanted into rats. | [93,94] |
| 3-arm poly(TMC-DLLA-CL) (2300–7800) | Acrylate end group and co-photo-crosslinker poly(ethylene glycol)diacrylate (PEGDA) | 2,2-dimethoxy-2-phenylacetophenone | 86–99 | Tg (−18 to 2 °C) varied with the monomer composition and the Mn of PEGDA. Preparation of cylindrical elastomeric devices able to swell and to encapsulate corticosteroid and growth factors utilizing the osmotic pressure mechanism. | [65,66,67] |
| Poly(LLA-co- CL-acryolyl carbonate) (17,900–22,600) | Pendant acrylate group | 2,2-dimethoxy-2-phenylacetophenone | 90 | Preparation of fibrous scaffolds by melt electrospinning writing; Stiffness of the scaffolds increased significantly (up to ∼10-fold) after crosslinking with UV compared with un-crosslinked scaffolds; the preservation of stiffness upon repetitive loading. | [62] |
| Poly(L-lactide-co-acryolyl carbonate) (55,900–72,100) | Pendant acrylate group | NA | 84–94 | Increase of Tg, decrease of Tm and degree of crystallinity after crosslinking; Electrospun and photo-crosslinked polymer resulted in scaffolds with increased tensile modulus in comparison with uncrosslinked fibrous scaffolds; good cytocompatibility toward fibroblasts of crimp-stabilized scaffolds. | [63] |
| 3-arm Poly(DLLA-co-CL) (Mw 4800–10,900) | Acrylate end group and co-photo-crosslinker N-methacrylated glycol chitosan (MGC) | Irgacure 2959 | 98–100 | Preparation of bi-continuous two-phase (elastomer /hydrogel) cell delivery device for the repair and/or replacement of load-bearing soft tissues. Decrease of elastic modulus with increasing content of MGC; using electrospinning for scaffold preparation. | [95,96,97] |
| 3-arm Poly(DLLA-co-CL) (2700 and 5000) | Acrylate end group and co-photo-crosslinker diacrylate oligo(d,l-lactide)-b-poly(ethylene glycol)-b-oligo(d,l-lactide) | 2,2-dimethoxy-2-phenylacetophenone | >95 | Enhancing the degradation rate by introducing PEG fragment; regulation of the degradation rate and peptide release by Mn of PEG and Mn of prepolymer. | [98] |
| 4-arm PDLLA (1500–9500) | Methacrylate end group (methacrylic anhydride or 2-isocyanatoethyl methacrylate) | 2,2-dimethoxy-2-phenylacetophenone | 90–99 | Increasing of Tg with decreasing Mn of precursors; networks based on low Mn oligomers were generally more rigid, those based on high Mn exhibited higher elongation; mechanical properties differ with type of precursors methacrylate end group. | [68] |
| PDLLA (1310) + TEGDMA as reactive diluent + Hydroxyapatite (HA) as bioactive filler | Methacrylate end group | Camphorquinone/ N,N’-dimethylaminoethyl Methacrylate | 77–100 | Tg (38–55 °C), flexural strength (3.5–94 MPa) and flexural modulus (75-3980 MPa) were dependent on composition of polymer resin and an amount of HA; increasing thermal stability with increasing amount of filler. Higher gel content and higher concentration of HA led to decreased rate of degradation; higher HA content resulted in the less cytotoxic sample. | [69] |
| 4-arm Poly(d,l-lactide) (2600 or 2400 or 450–820) | Methacrylate end group | 4,4′-bis(dimethylamino)benzophenone e | NA | Preparation of scaffolds with Young’s modulus even bigger than 4 GPa for the mesenchymal stem cells osteogenic differentiation; Independently—collagen reinforcement: about one order of magnitude increased Young’s modulus for the hybrid matrix without affecting its cytotoxicity; | [72,73,74,75] |
| 4-arm Poly(L-lactide) (Mw 1250) | Methacrylate end group | Irgacure 369 e | NA | Preparation of scaffolds for supporting Schwann cell growth—neural scaffolds in nerve repair. | [70] |
| Poly(LLA-co-GMA) (1650–3260) | Pendant methacrylate group | Camphorquinone/ N,N′-dimethylaminoethyl methacrylate | 72–95 | With increasing content of GMA (9.5–19.2 mol%) the increase of gel content, compressive stress (3–25.5 MPa) and the decrease of degree of swelling was observed; Increase of Tg by 15–20 °C in comparison with original copolymer. | [76,77] |
| PLLA (MV 276,500) | - | Benzophenone | 38–98.5 | Slight increase of Tg in comparison with pristine PLA, decrease of Tm and crystallinity; improvement of thermal stability; with increase of gel fraction—increase of storage modulus (from 5.4 to 9.6 GPa at 0 °C), tensile strength (from 48 to 81 MPa), modulus (from 1.8 to 3.1 GPa), toughness (from 67 to 82 MPa) and decrease of strain (from 3.9 to 1.6%). | [78] |
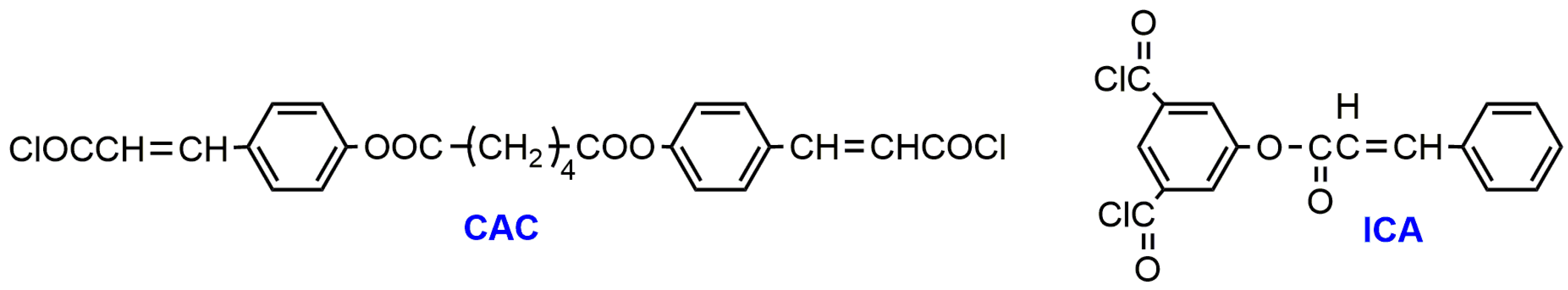
| PLLA - diacyl of 5-cinnamoyloxyisophthalic acid (ICA) (10,000–34,500) | Pendant 3-phenylprop-2-ene group | - | 50–100 | Slight increase of Tg (from 50 to 53 °C), decrease of crystallinity (from 10 to 3 %), slight decreases of Tm, thermal decomposition Td, increase of ultimate tensile strength (from 13 to 23 MPa), decrease in elongation (from 12 to 5.2%), increase of Young’s modulus E (from 483 to 830 MPa); Decrease of degradation rate. | [82] |
| PLLA- diacyl of 4,4′-(adipoyldioxy)dicinnamic acid (8700–43,500) f | Main-chain 3-phenylprop-2-ene group | - | 9–86 | Increase of Tg (from 51 to 53 °C); decrease of ∆Hm (from 4.8 to 0.1 J.g−1), small decrease of Tm (from 150 to 147 °C), increase of thermal decomposition Td; increase of tensile strength and tensile modulus and decrease of elongation at break with increasing photocuring time and gel content; decrease of degradation rate. | [83] |
| P(LLA-co-MC) (12,900–65,100) f | Pendant phenylprop-2-ene group | - | NA | The kinetic of UV crosslinking was studied by FT IR spectroscopy. | [84] |
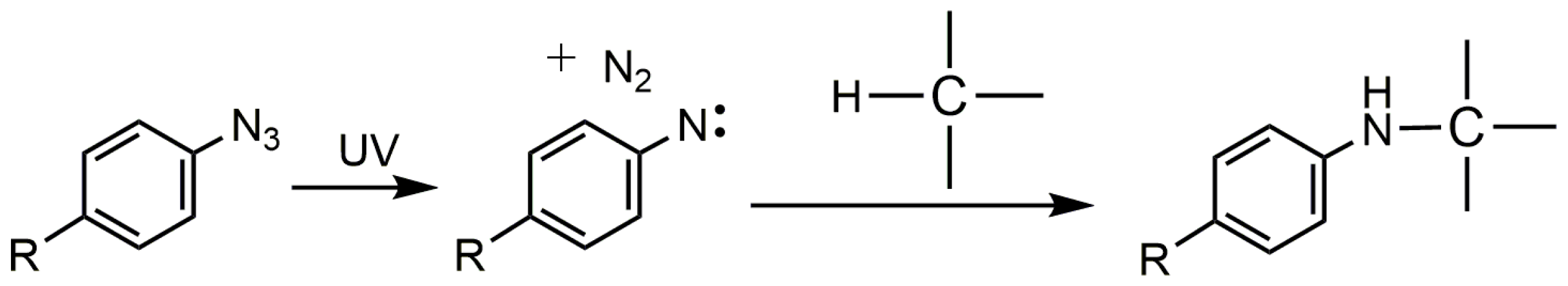
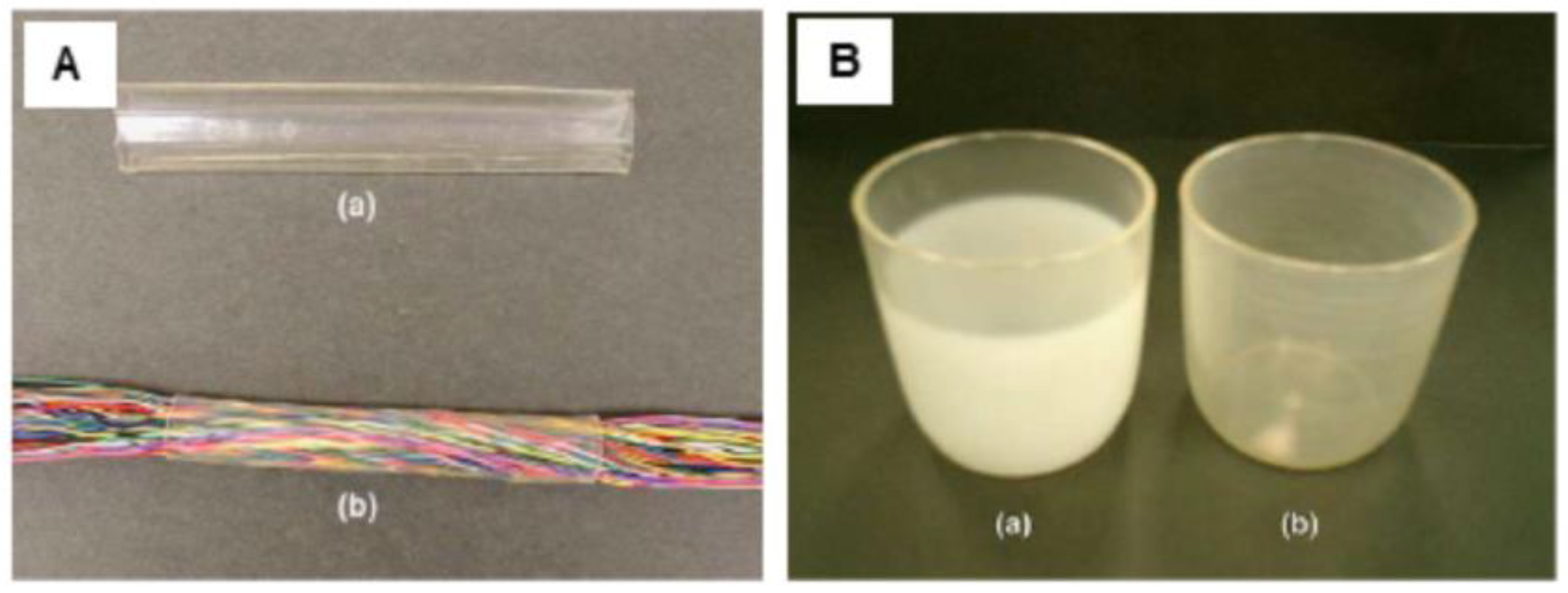
| PLA50-Pluronic®-PLA50 (50,000-200,000) | -C-H- bond in polymer chain | Aryl-azide group | Up to 55 | Preparation of elastic microfibers (elastic limit–εy up to 182 %) for soft tissues by electrospinning. | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarek, M.; Borska, K.; Kubisa, P. Crosslinking of Polylactide by High Energy Irradiation and Photo-Curing. Molecules 2020, 25, 4919. https://doi.org/10.3390/molecules25214919
Bednarek M, Borska K, Kubisa P. Crosslinking of Polylactide by High Energy Irradiation and Photo-Curing. Molecules. 2020; 25(21):4919. https://doi.org/10.3390/molecules25214919
Chicago/Turabian StyleBednarek, Melania, Katarina Borska, and Przemysław Kubisa. 2020. "Crosslinking of Polylactide by High Energy Irradiation and Photo-Curing" Molecules 25, no. 21: 4919. https://doi.org/10.3390/molecules25214919
APA StyleBednarek, M., Borska, K., & Kubisa, P. (2020). Crosslinking of Polylactide by High Energy Irradiation and Photo-Curing. Molecules, 25(21), 4919. https://doi.org/10.3390/molecules25214919