A Survey of Synthetic Routes and Antitumor Activities for Benzo[g]quinoxaline-5,10-diones
Abstract
1. Introduction
- Condensations of 2,3-diamino-1,4-naphthoquinone with 1,2-dicarbonyl derivatives.
- Cycloaddition via generated in situ N-substituted-quinolinedione intermediates.
- Diels–Alder cyclocondensation reactions.
2. Discussion
2.1. Synthesis of Benzo[g]quinoxaline-5,10-diones and Their Uses in Heterocyclic Chemistry
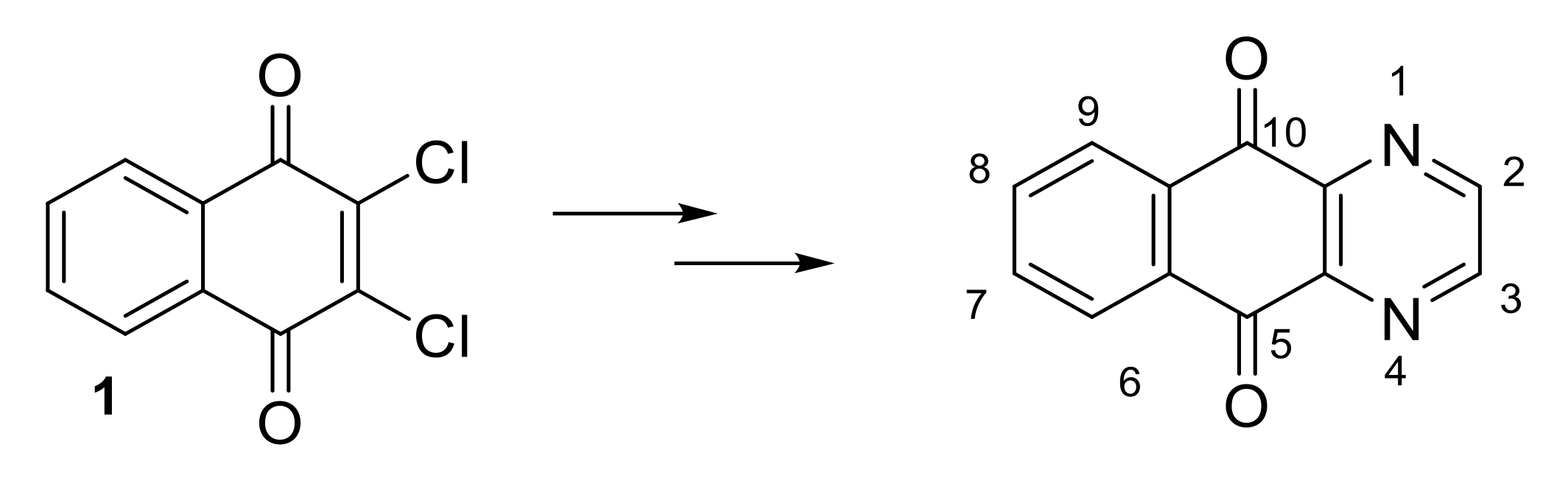
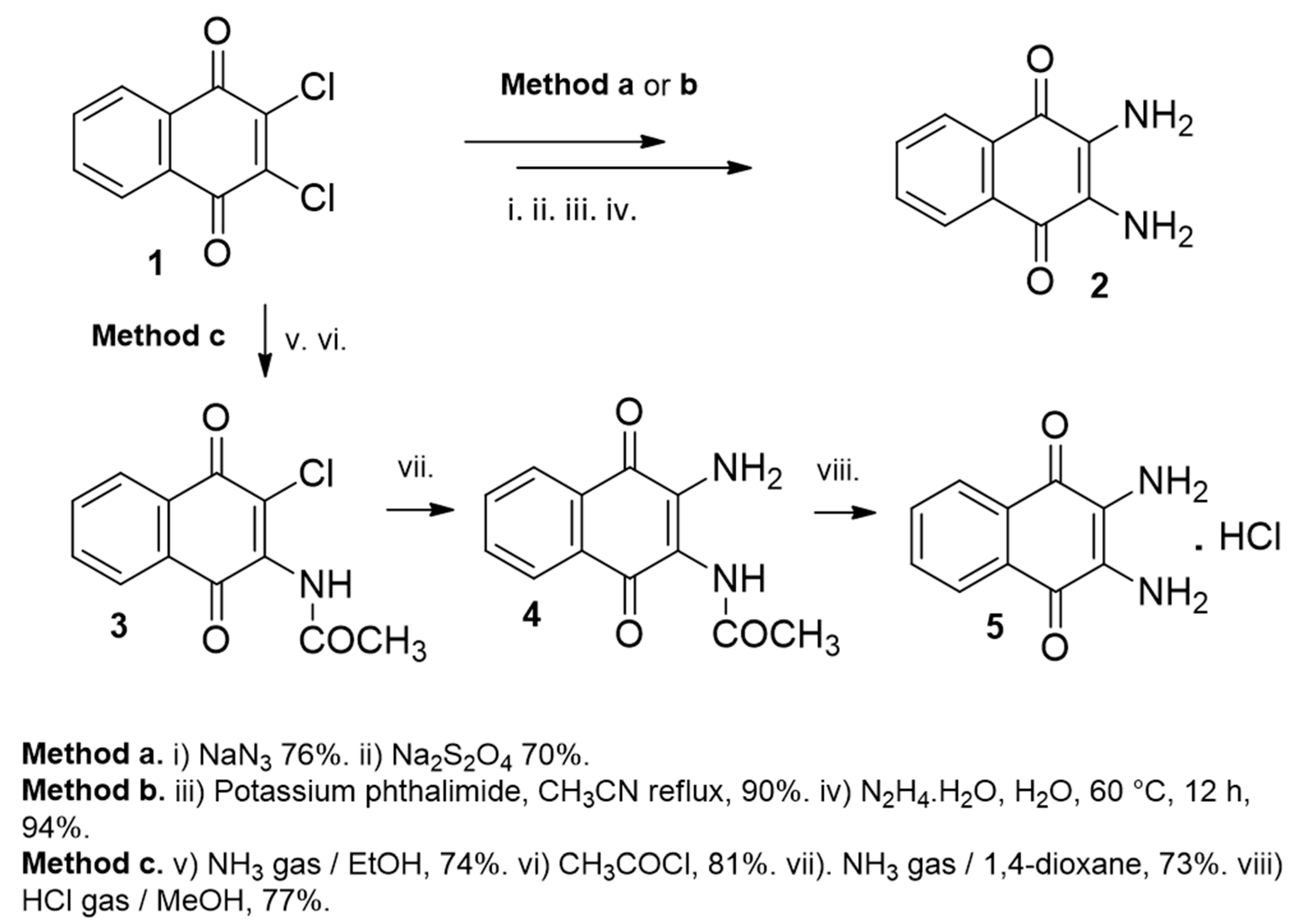
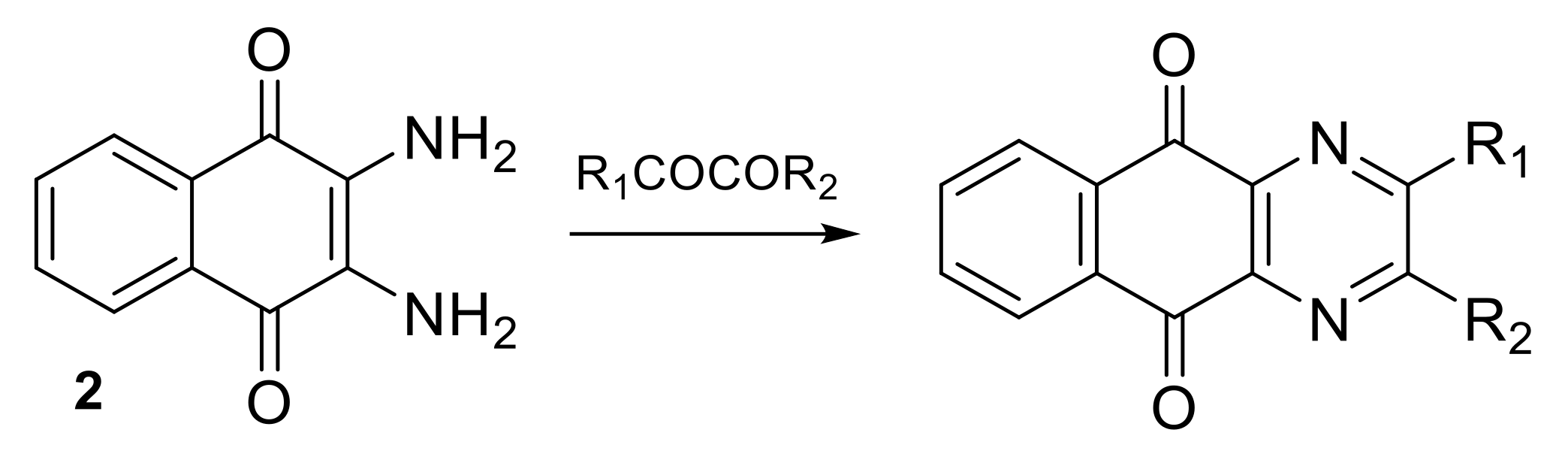
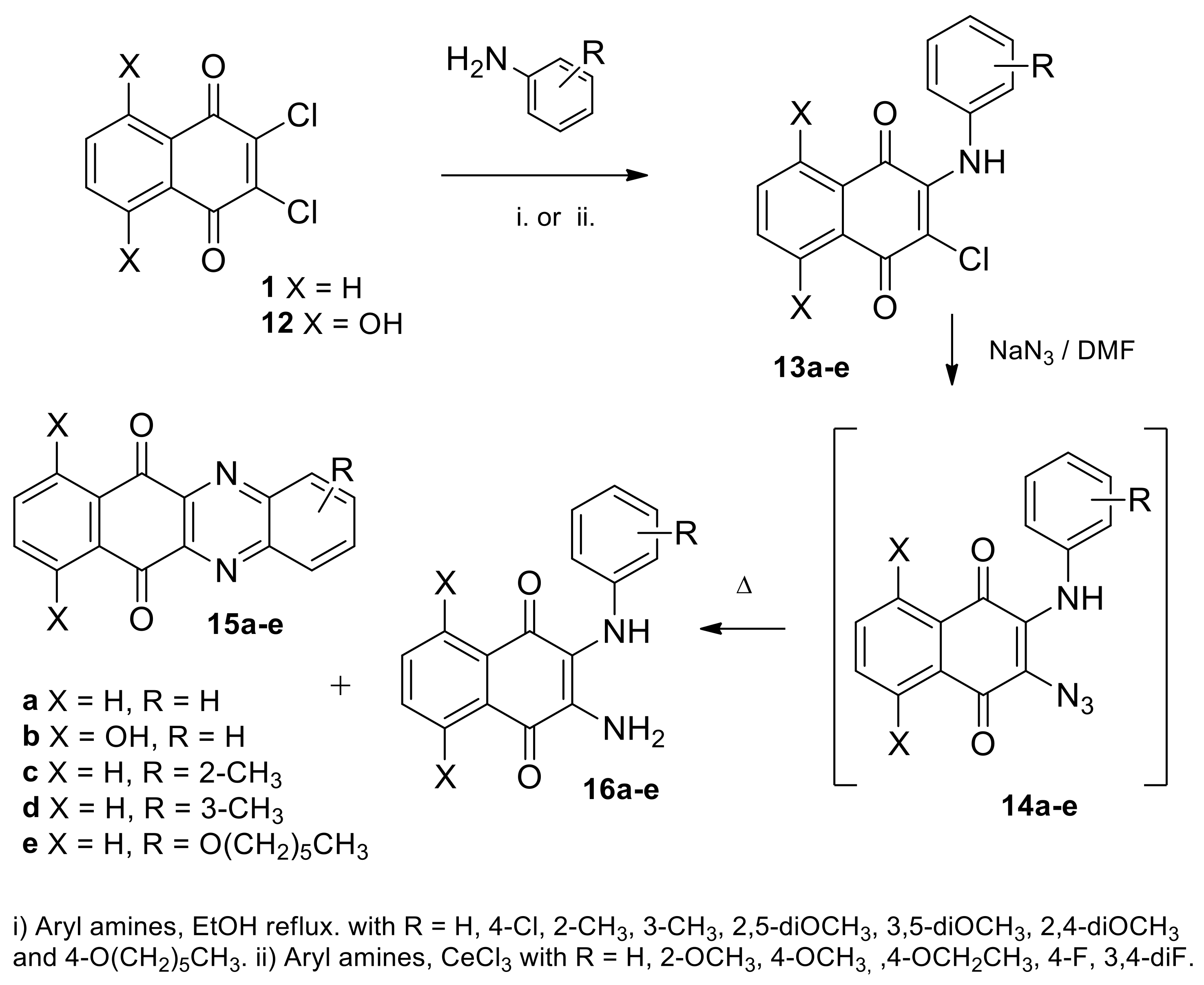
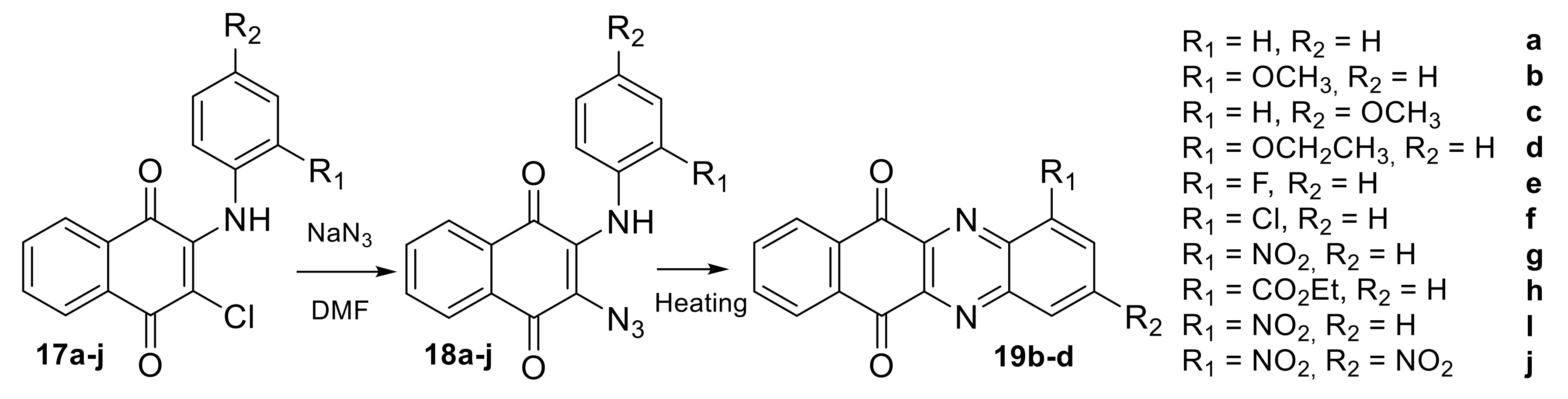
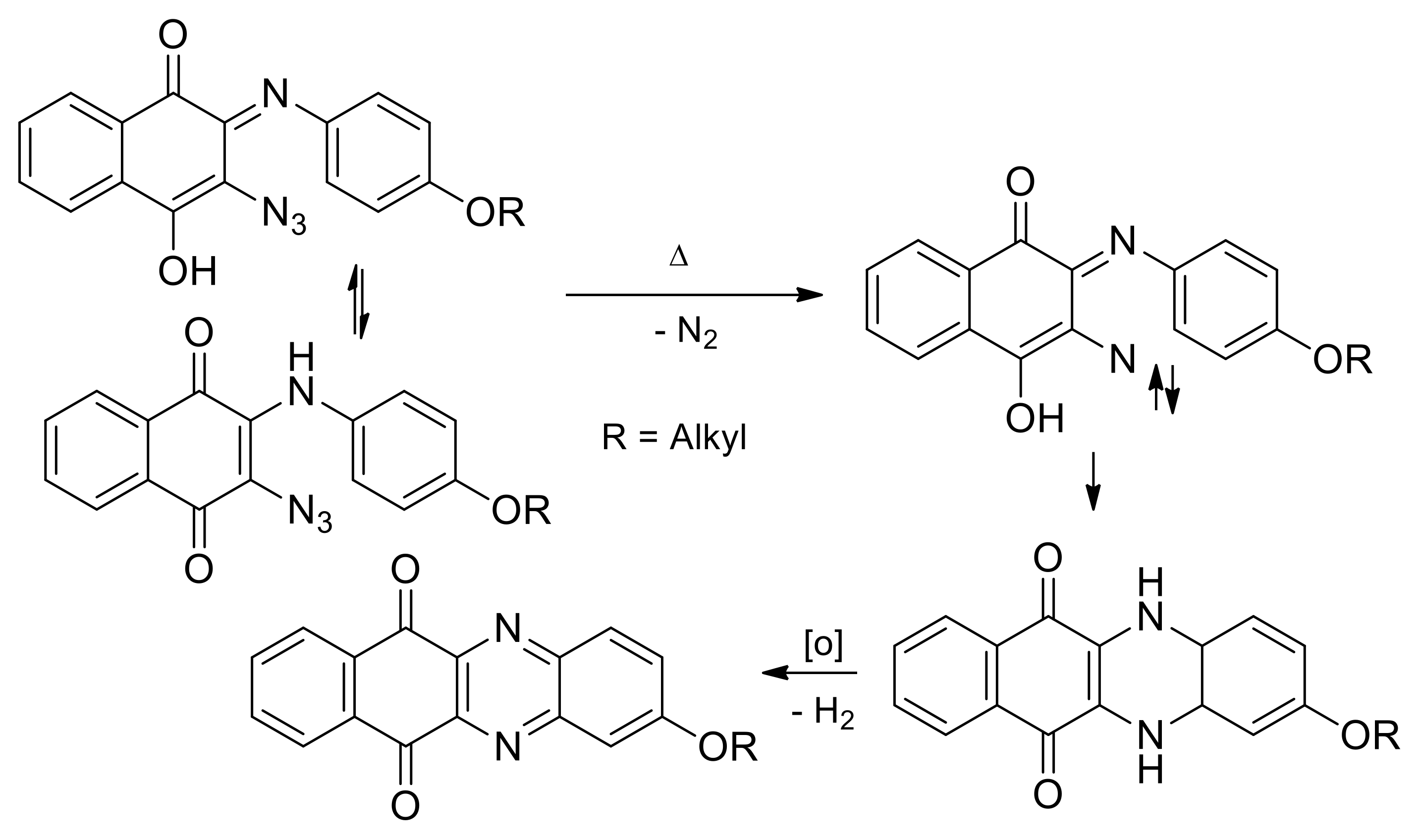
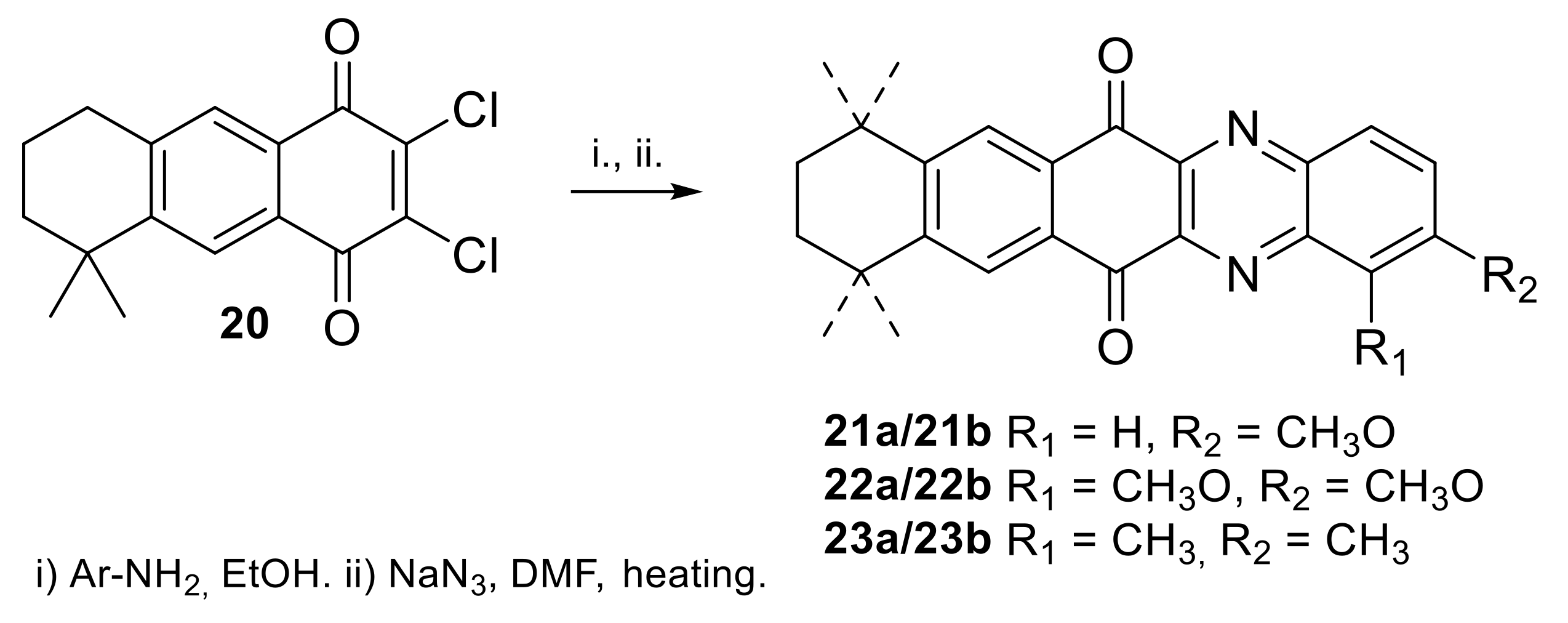
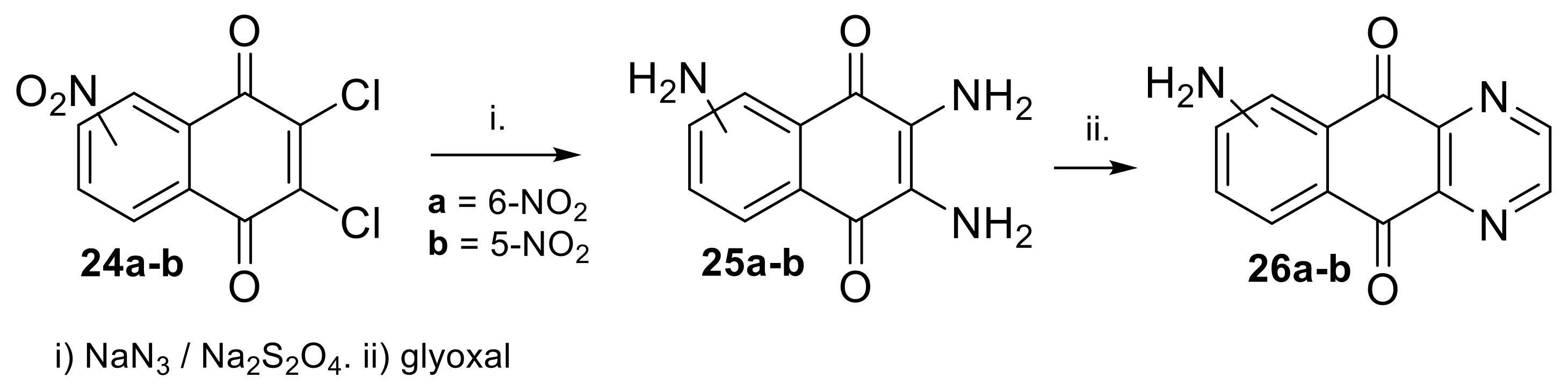
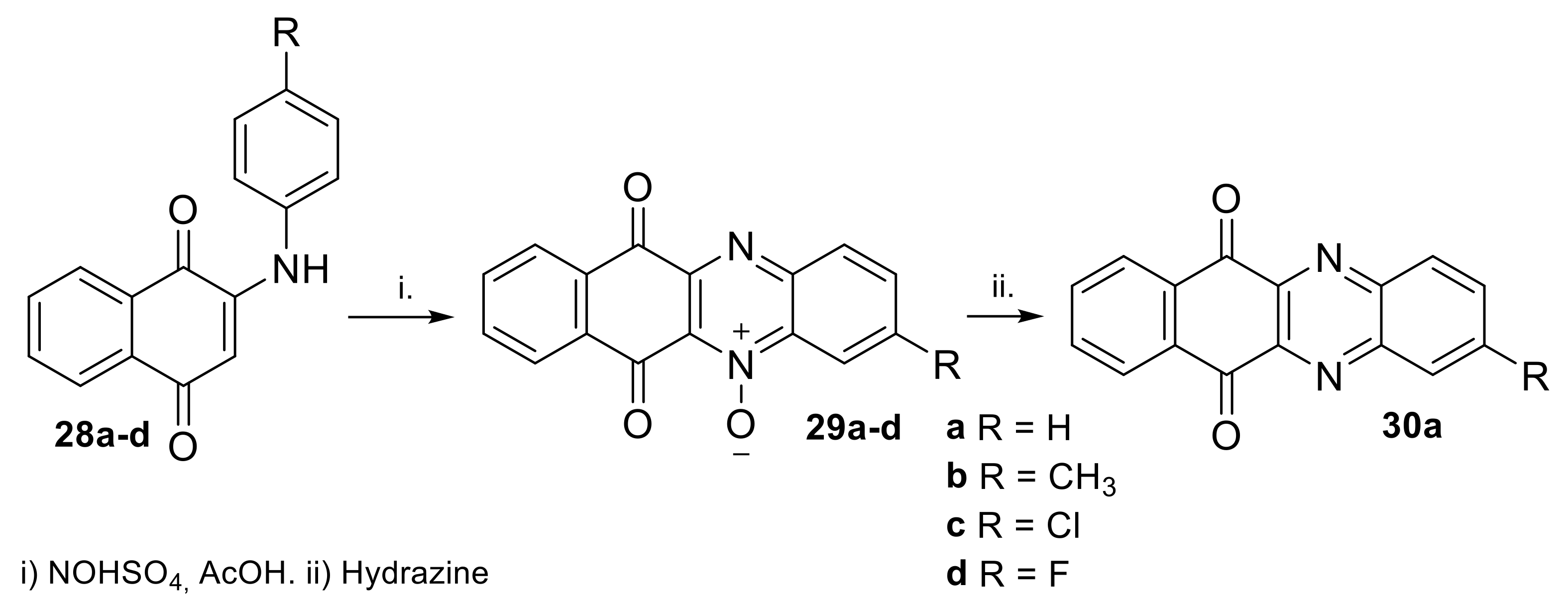
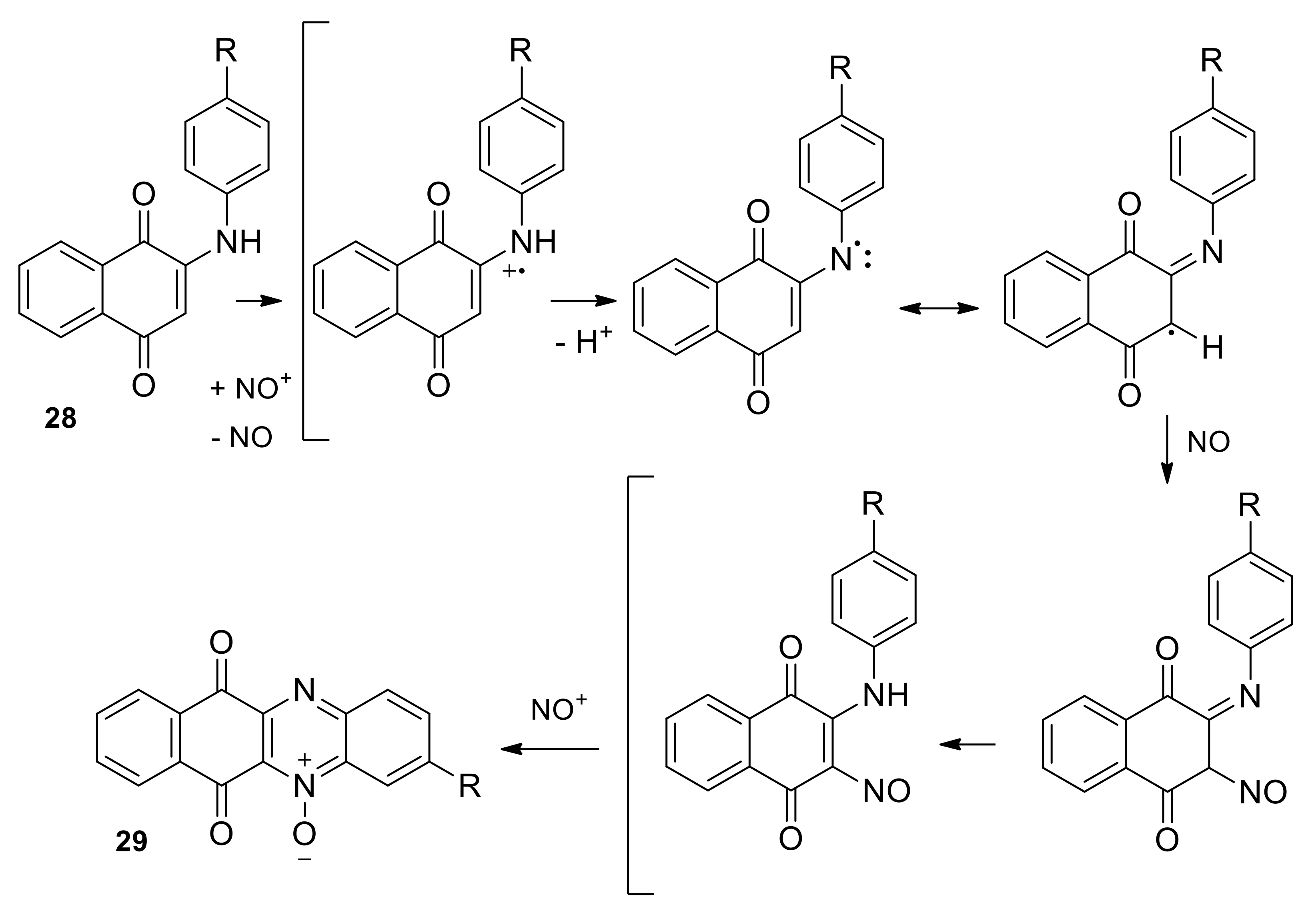
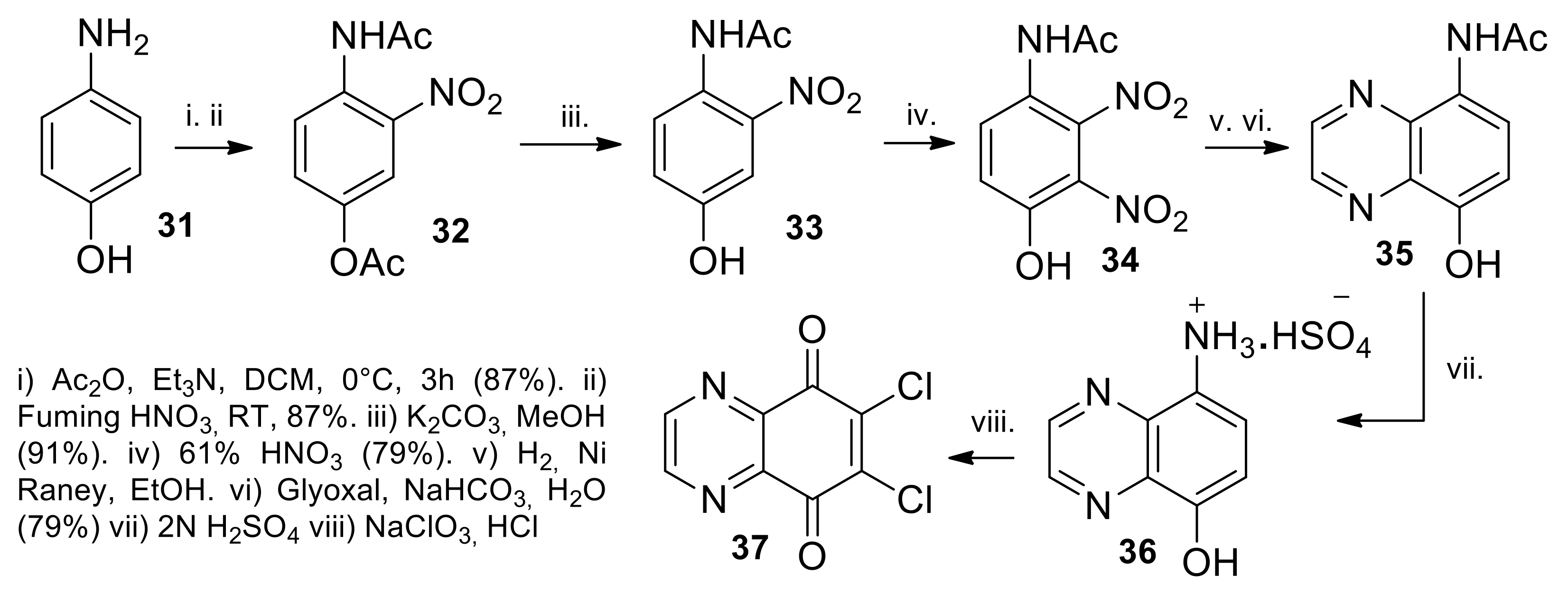
2.1.1. Synthesis from 2,3-Dichloro-1,4-naphthoquinone
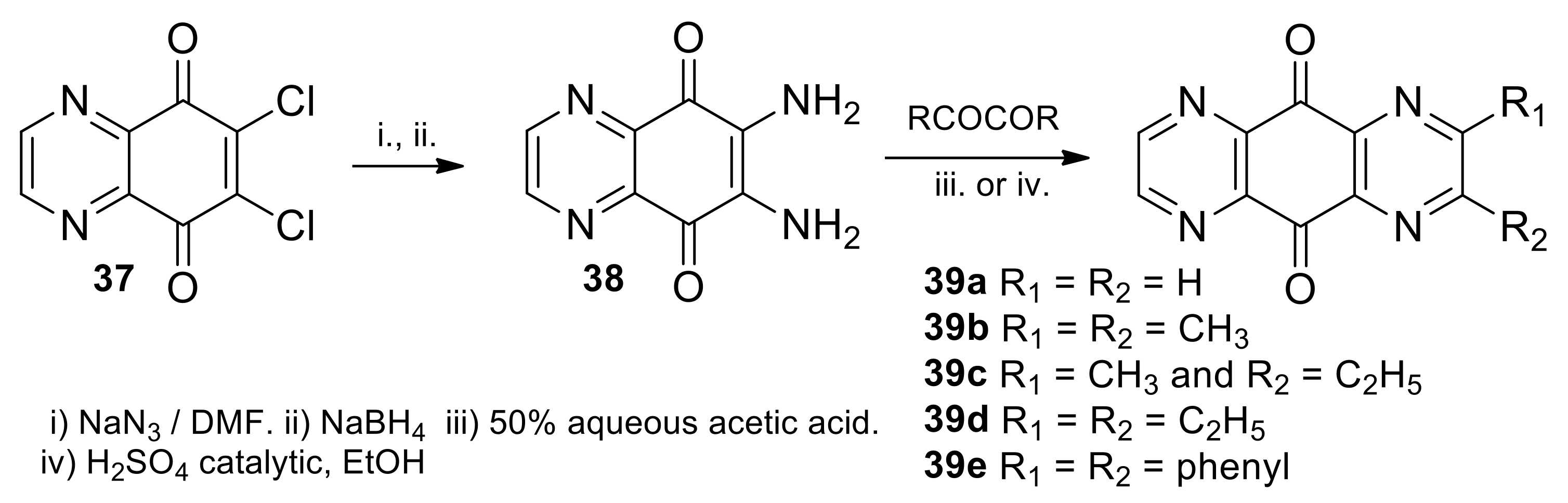
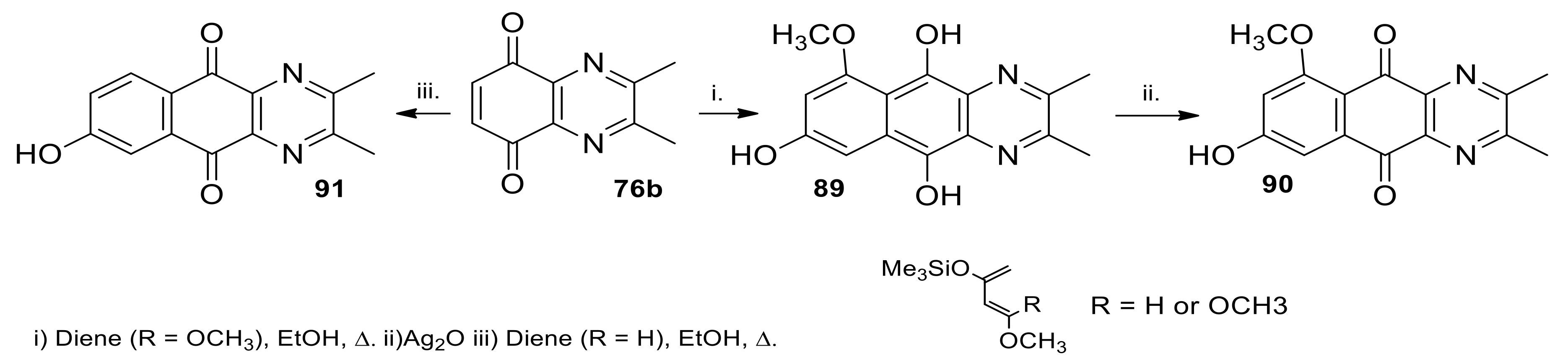
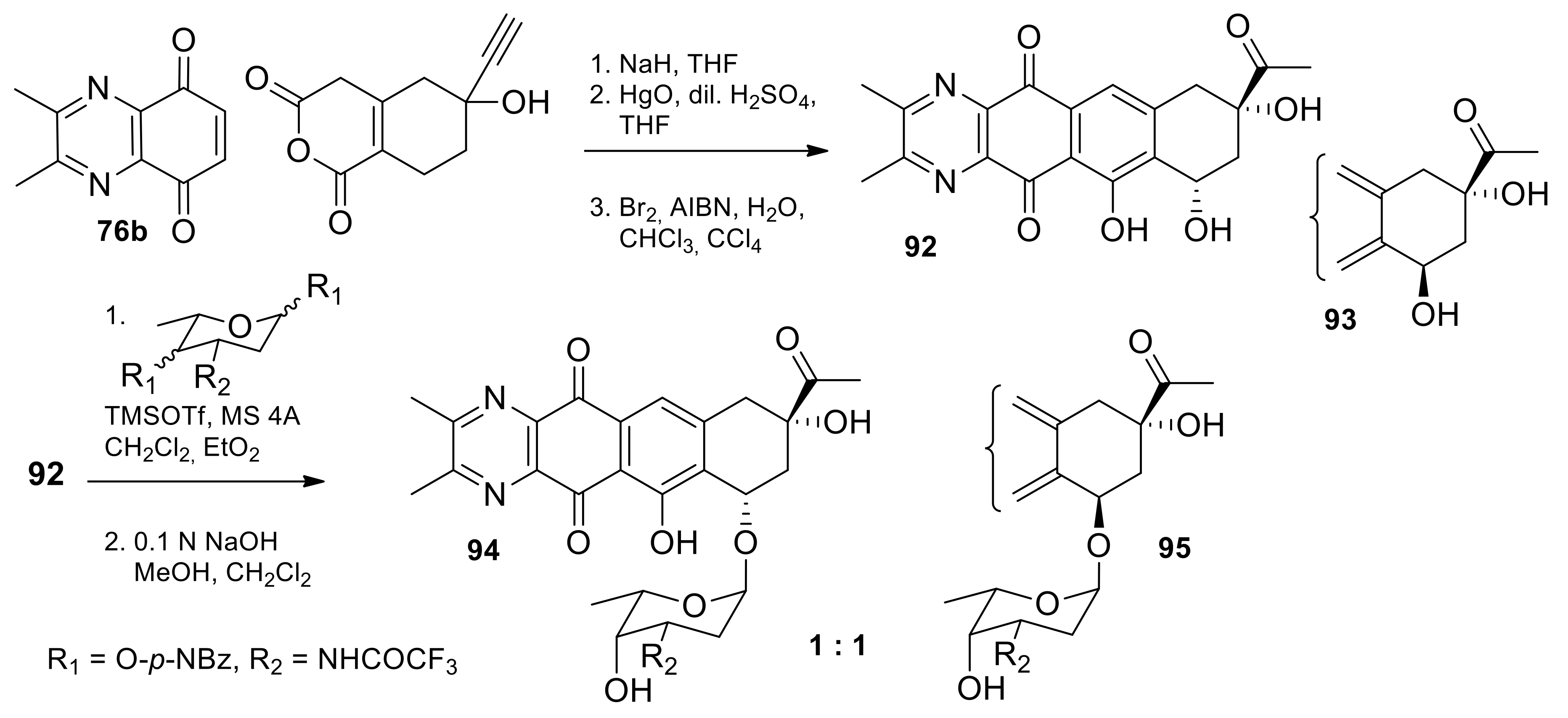
2.1.2. Synthesis from 5,8-quinoxalinedione
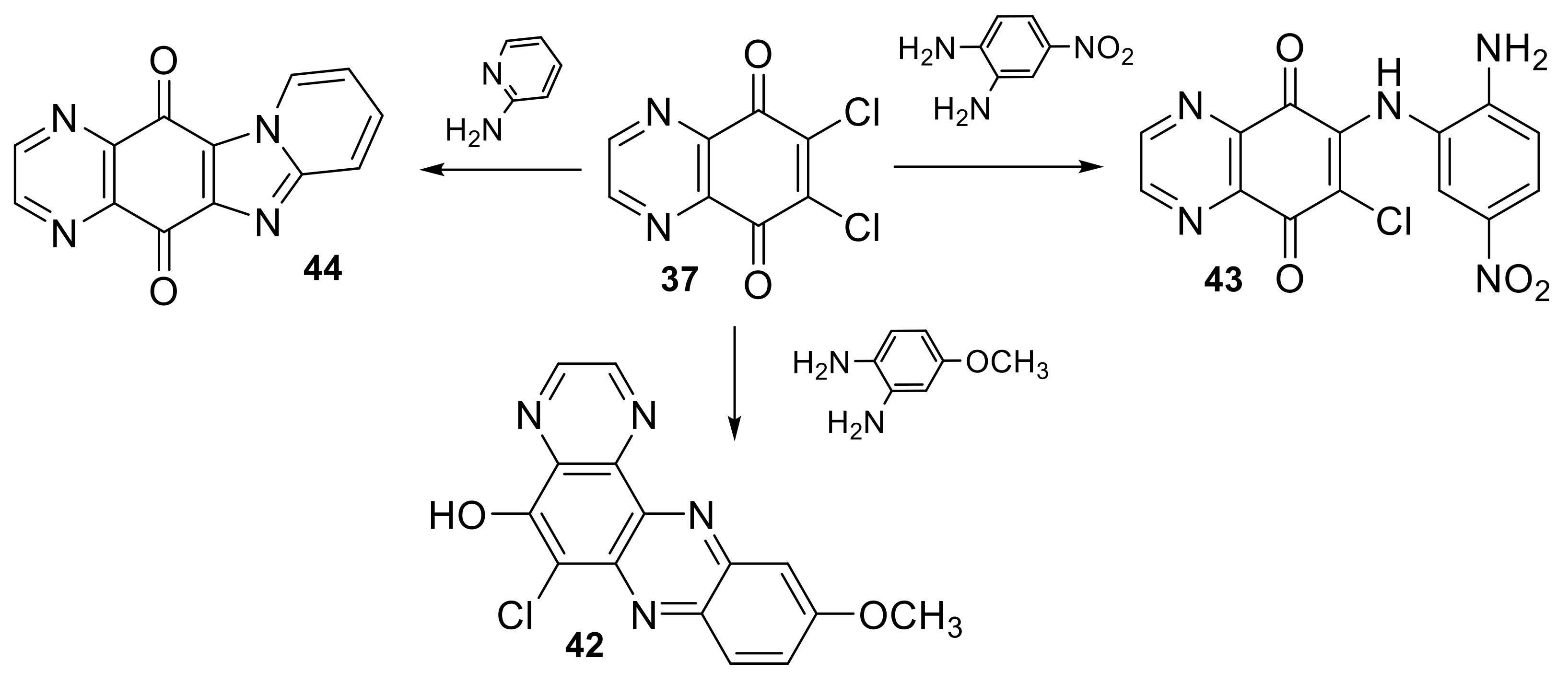
2.1.3. Miscellaneous Reactions
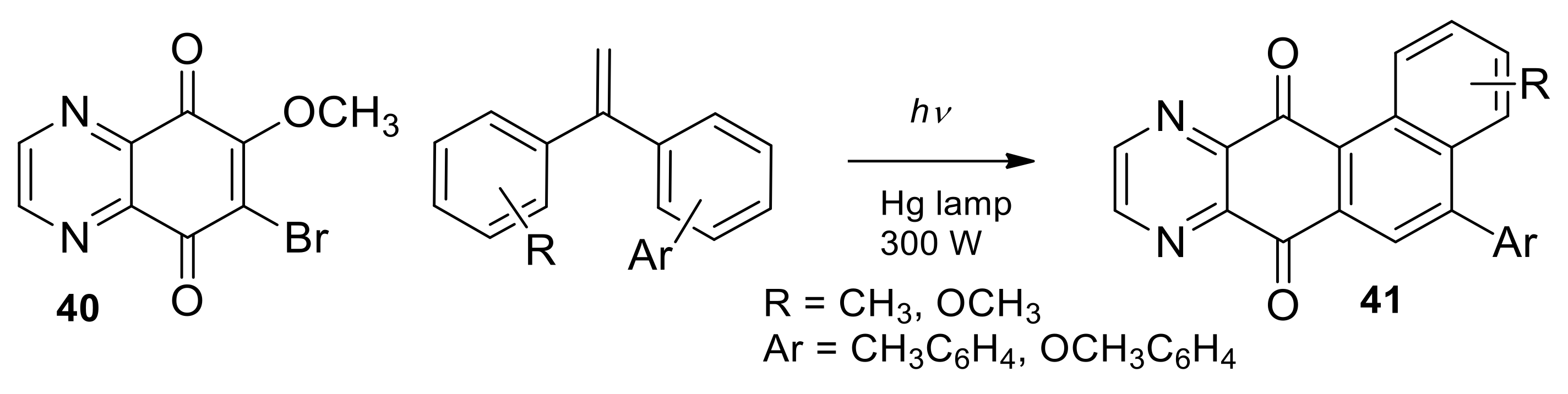
2.1.4. Diels–Alder Reactions
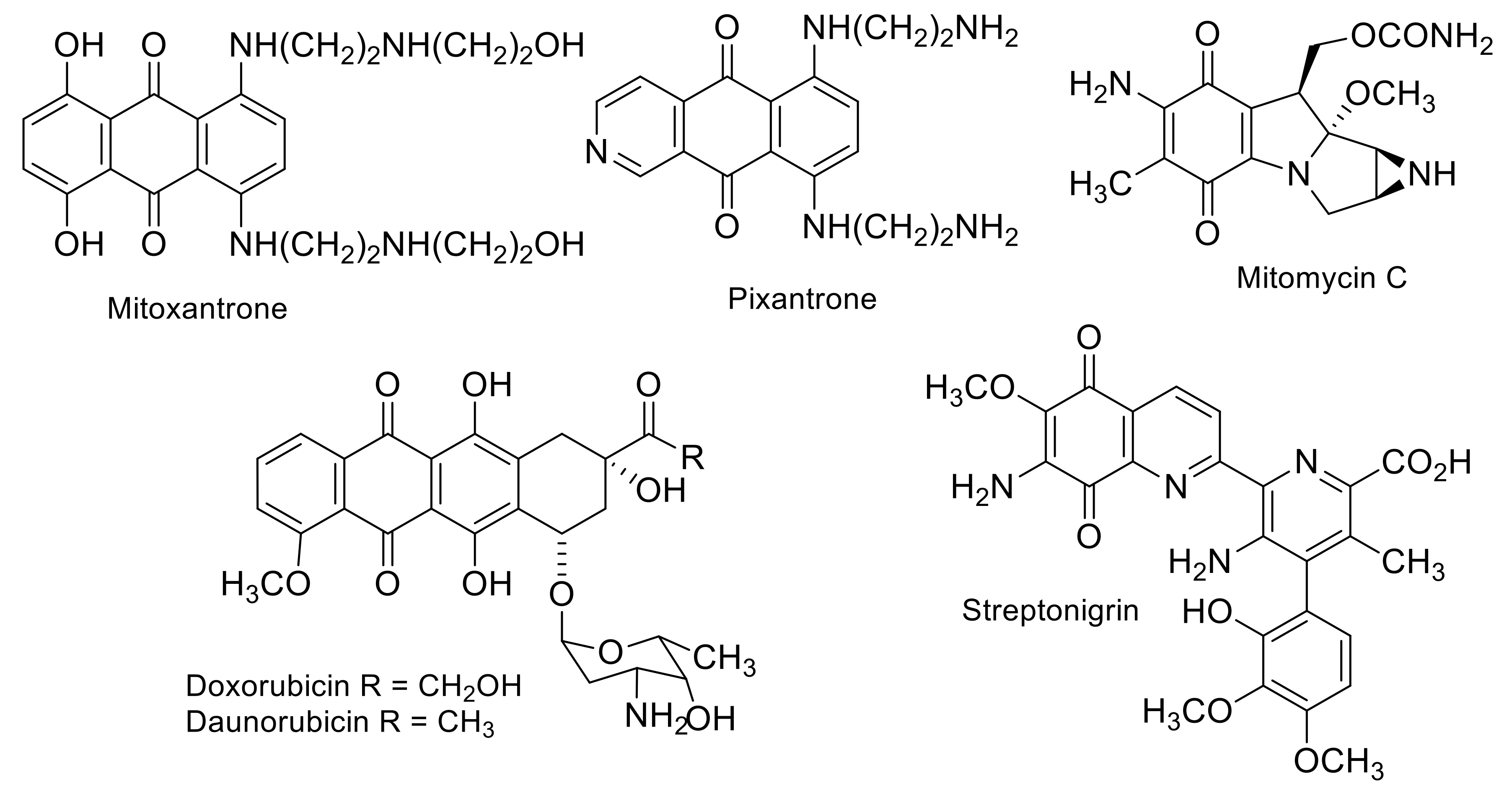
2.2. Antitumor Activities
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kohen, E. Cell Structure and Function by Microspectrofluorometry; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Marshall, R.S.; Mayer, S.A. On Call Neurology: On Call Series; Elsevier Health Sciences: London, UK, 2007. [Google Scholar]
- Vigny, P.; Amirand-Perchard, C. Cell Structure and Function by Microspectrofluorometry; Elsevier: London, UK, 1989; pp. 229–268. [Google Scholar]
- Volpetti, S.; Zaja, F.; Fanin, R. Pixantrone for the treatment of adult patients with relapsed or refractory aggressive non-Hodgkin B-cell lymphomas. OncoTargets Ther. 2014, 7, 865–872. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berthet, N.; Boturyn, D.; Constant, J.-F. DNA repair inhibitors. Exp. Opin. Ther. Pat. 1999, 9, 401–415. [Google Scholar] [CrossRef]
- Inouye, Y.; Take, Y.; Oogose, K.; Kubo, A.; Nakamura, S. The quinoline quinone as the minimum entity for reverse transcriptase inhibitory activity of streptonigrin. J. Antibiot. Res. 1987, 40, 105–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krapcho, A.P.; Petry, M.E.; Getahun, Z.; Landi, J.J.; Stallman, J., Jr.; Polsenberg, J.F.; Gallagher, C.E.; Maresch, M.J.; Hacker, M.P. 6,9-Bis [(aminoalkyl)amino] benzo[g]isoquinoline-5,10-diones. A novel class of chromophore-modified antitumor anthracene-9,10-diones: Synthesis and antitumor evaluations. J. Med. Chem. 1994, 37, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Krapcho, A.P.; Landi, J.J.; Hacker, M.P., Jr.; McCormack, J.J. Synthesis and antineoplastic evaluations of 5, 8-bis[(aminoalkyl)amino]-1-azaanthracene-9,10-diones. J. Med. Chem. 1985, 28, 1124–1126. [Google Scholar] [CrossRef] [PubMed]
- Hazlehurst, L.A.; Krapcho, A.P.; Hacker, M.P. Comparison of aza-anthracenedione-induced DNA damage and cytotoxicity in experimental tumor cells. Biochem. Pharmacol. 1995, 50, 1087–1094. [Google Scholar] [CrossRef]
- Remusat, V.; Terme, T.; Gellis, A.; Rathelot, P.; Vanelle, P. Synthesis of original benzo[g]quinoxaline-5,10-diones by bis-SRN1 methodology. J. Heterocycl. Chem. 2004, 41, 221–225. [Google Scholar] [CrossRef]
- Okada, H.; Shimizu, T. Preparation of Naphthoquinone Derivative for Electrophotographic Photoreceptor; Kyocera Document Solutions: Osaka, Japan, 2017; Volume 31. [Google Scholar]
- Hoover, J.R.E.; Day, A.R. Preparation of some 1-alkyl-1,2-dihydro-3-hydroxybenzo[g]quinoxaline-5,10-diones. J. Am. Chem. Soc. 1955, 77, 35–37. [Google Scholar] [CrossRef]
- Lien, J.-C.; Huang, L.-J.; Teng, C.-M.; Wang, J.-P.; Kuo, S.-C. Synthesis and Aniplatelet, Antiinflammatory and Antiallergic Activities of 2,3-disubstituted 1,4-naphtoquinones. Chem. Pharm. Bull. 1996, 44, 1181–1187. [Google Scholar] [CrossRef]
- Anacona, J.R.; Bastardo, E.; Camus, J. Manganese (II) and palladium (II) complexes containing a new macrocyclic Schiff base ligand: Antibacterial properties. Transit. Met. Chem. 1999, 24, 478–480. [Google Scholar] [CrossRef]
- Winkelmann, E. 2,3,5,6-Tetra-amino-1,4-benzochinon (TABC): Darstellung, eigenschaften und reaktionen. Tetrahedron 1969, 25, 2427–2454. [Google Scholar] [CrossRef]
- Chesneau, B.; Hardouin-Lerouge, M.; Hudhomme, P. A Fused Donor-Acceptor System Based on an Extended Tetrathiafulvalene and a Ruthenium Complex of Dipyridoquinoxaline. Org. Lett. 2010, 12, 4868–4871. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.; Reyes, O.; Francois, A.; Leiva, A.M.; Loeb, B. Synthesis of a new polypyridinic highly conjugated ligand with electron-acceptor properties. Tetrahedron Lett. 2001, 42, 6463–6467. [Google Scholar] [CrossRef][Green Version]
- Giorgi-Renault, S.; Renault, J.; Baron, M.; Servolles, P.; Paoletti, C. Heterocyclic quinones. VI: Synthesis and antitumoral effects of 5,10-benzo[g]quinoxalinediones and aza-analogs. Eur. J. Med. Chem. 1985, 20, 144–148. [Google Scholar] [CrossRef]
- Manivannan, R.; Satheshkumar, A.; El-Mossalamy, E.-S.-H.; Al-Harbi, L.M.; Kosa, S.A.; Elango, K.P. Design, synthesis and characterization of indole based anion sensing receptors. New J. Chem. 2015, 39, 3936–3947. [Google Scholar] [CrossRef]
- Zhu, L.; Chang, H.; Vavallo, C.L.; Jiang, J.; Zeng, Z.; Yang, J.; Smith, M.D.; Miao, S. Synthesis and properties of tetracyanoquinodimethane derivatives. Heterocycl. Commun. 2018, 24, 249–254. [Google Scholar] [CrossRef]
- Anzenbacher, P.; Palacios, M.A., Jr.; Jursikova, K.; Marquez, M. Simple Electrooptical Sensors for Inorganic Anions. Org. Lett. 2005, 7, 5027–5030. [Google Scholar] [CrossRef]
- Fauvarque, M.-O.; Mortier, M.; Pillet, C.; Aguilar, C.; Soleilhac, E.; Barette, C.; Remusat, V.; Terme, T.; Vanelle, P. Heterocyclic Naphthoquinones Derivatives for Use in the Treatment of Cancers Including Cushing Disease. U.S. Patent 16/323,691, 6 June 2018. [Google Scholar]
- Hosokawa, C.; Morishita, H.; Yoshinaga, T.; Kijima, Y. Material for Organic Electroluminescent Device, Organic Electroluminescent Device, and Organic Electroluminescent Display Device. U.S. Patent 7,326,475, 5 February 2008. [Google Scholar]
- Cheesman, G.W.H.; Cookson, R.F. Chemistry of Heterocyclic Compounds; Wiley: Hoboken, NJ, USA, 1979; Volume 35, pp. 730–731. [Google Scholar]
- Yamashita, Y.; Suzuki, T.; Miyashi, T. 2,2′-(5,8-Dihydroquinoxaline-5,8-diylidene)bis(1,3-benzodithiole)s. A new type of electron donor. Chem. Lett. 1989, 1607–1610. [Google Scholar] [CrossRef]
- Efimova, G.A.; Efros, L.S. Heterocyclic derivatives based on substituted 1,4-naphthoquinones. II. Synthesis and properties of 2,3-disubstituted benzoquinoxaline-5,10-diones. Zhurnal Organicheskoi Khimii 1966, 2, 1900. [Google Scholar]
- Diaz, R.; Francois, A.; Barrera, M.; Loeb, B. Synthesis, characterization and theoretical studies of ruthenium(II) complexes with the quinone functionalized polypyridine ligand, Nqphen. Polyhedron 2012, 39, 59–65. [Google Scholar] [CrossRef]
- Foxon, S.P.; Green, C.; Walker, M.G.; Wragg, A.; Adams, H.; Weinstein, J.A.; Parker, S.C.; Meijer, A.J.H.M.; Thomas, J.A. Synthesis, Characterization, and DNA Binding Properties of Ruthenium(II) Complexes Containing the Redox Active Ligand Benzo[i]dipyrido[3,2-a:2’,3’-c]phenazine-11,16-quinone. Inorg. Chem. 2012, 51, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rodríguez, S.; Jimé>nez-Cataño, R.; Leyva, E.; Loredo-Carrillo, S.E. Intramolecular hydrogen bonds in fluorinated, methoxylated, or unsubstituted 2-(anilino)-1,4-naphthoquinones: A theoretical study. J. Fluorine Chem. 2013, 145, 58–62. [Google Scholar]
- Kurban, S.; Deniz, N.G.; Sayil, C. Synthesis and cyclization reactions of novel benzo [a] phenazine-and phenoxazine-5-ones derivatives. Bulg. Chem. Commun. 2016, 48, 43–48. [Google Scholar]
- Tuyun, F.; Bayrak, N.; Yıldırı>m, H.; Onul, N.; Kara, E.M.; Celik, B.O. Synthesis and in vitro biological evaluation of aminonaphthoquinones and Benzo[b]phenazine-6,11-dione derivatives as potential antibacterial and antifungal compounds. J. Chem. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Nakazumi, H.; Kondo, K.; Kitao, T. Synthesis of 7,10-disubstituted benzo[b]phenazine-6,11-quinones. Synthesis 1982, 10, 878–879. [Google Scholar] [CrossRef]
- VanAllan, J.A.; Reynolds, G.A.; Adel, R.E. Polynuclear heterocycles. III. The chlorination and nitration of benzo[b]phenazine. J. Org. Chem. 1963, 28, 520–524. [Google Scholar] [CrossRef]
- Kurban, S.; Deniz, N.G.; Sayil, C.; Ozyurek, M.; Guclu, K.; Stasevych, M.; Zvarych, V.; Komarovska-Porokhnyavet, O.; Novikov, V. Synthesis, antimicrobial properties, and inhibition of catalase activity of 1,4-naphtho- and benzoquinone derivatives containing N.-, S.-, O-substituted. Heteroat. Chem. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Loredo-Carrillo, S.E.; Leyva, E.; Platz, M.S.; Cárdenas-Chaparro, A.; Martínez-Richa, A. Thermolysis of 2-azido-3-(R-anilino)-1,4-naphthoquinones. Nitrene insertion versus hydrogen abstraction. Tetrahedron Lett. 2020, 61, 151731–151735. [Google Scholar] [CrossRef]
- Win, T.; Yerushalmi, S.; Bittner, S. Direct nitration of 3-arylamino-2-chloro-1, 4-naphthoquinones. Synthesis 2005, 2005, 1631–1634. [Google Scholar] [CrossRef]
- Wasserman, E. Electron spin resonance of nitrenes. Prog. Phys. Org. Chem. 1971, 8, 319–336. [Google Scholar]
- Castro, M.A.; Gamito, A.M.; Tangarife-Castano, V.; Roa-Linares, V.; Miguel del Corral, J.M.; Mesa-Arango, A.C.; Betancur-Galvis, L.; Francesh, A.M.; San Feliciano, A. New 1,4-anthracenedione derivatives with fused heterocyclic rings: Synhesis and biological evaluation. RSC Adv. 2015, 5, 1244–1261. [Google Scholar] [CrossRef]
- Nakazumi, H.; Agawa, T.; Kitao, T. Synthesis and absorption spectra of 1,4-diazaanthraquinone derivatives. Bull. Chem. Soc. Jpn. 1979, 52, 2445–2446. [Google Scholar] [CrossRef]
- Gornostaev, L.; Khalyavina, Y.G.; Lavrikova, T.; Stashina, G.; Firgang, S.; Chernyshev, V. Cyclization of 2-arylamino-1, 4-naphthoquinones to benzo[b]phenazine-6, 11-dione 5-oxides. Russ. Chem. Bull. 2014, 63, 739–743. [Google Scholar] [CrossRef]
- Titova, S.; Arinin, A.; Gorelik, M. Ortho-nitrosodiphenylamines in the Fisher-Hepp rearrangement. Zhurnal Organicheskoi Khimii 1986, 22, 1562–1564. [Google Scholar]
- Shaikh, I.A.; Johnson, F.; Grollman, A.P. Streptonigrin. 1. Structure-activity relationships among simple bicyclic analogs. Rate dependence of DNA degradation on quinone reduction potential. J. Med. Chem. 1986, 29, 1329–1340. [Google Scholar] [CrossRef]
- Han, G.; Shin, K.J.; Kim, D.C.; Yoo, K.H.; Kim, D.J.; Park, S.W. A new synthetic route to 6, 7-dichloro-5, 8-quinoxaline-dione and synthesis of its derivatives. Heterocycles 1996, 43, 2496–2502. [Google Scholar]
- Yoo, H.-W.; Suh, M.-E.; Park, S.W. Synthesis and Cytotoxicity of 2-Methyl-4, 9-dihydro-1-substituted-1 H-imidazo [4, 5-g] quinoxaline-4, 9-diones and 2, 3-Disubstituted-5, 10-pyrazino [2, 3-g] quinoxalinediones. J. Med. Chem. 1998, 41, 4716–4722. [Google Scholar] [CrossRef]
- Bock, H.; Dickmann, P.; Herrmann, H.F. Electron transfer and ion pairing. 18. Radical anions and radical ion pairs of aza-substituted naphtho- and anthraquinones. Zeitschrift für Naturforschung B Chem. Sci. 1991, 46, 326–338. [Google Scholar] [CrossRef]
- Yoo, H.W.; Shin, K.J.; Suh, M.E.; Park, S.W. The Reaction of 6, 7-Dichloro-5, 8-quinoxalinedione with Aromatic and Aliphatic Dinucleophiles and Molecular Modeling Study of Their Intercalation Complexes. Bull. Korean Chem. Soc. 1997, 18, 484–488. [Google Scholar] [CrossRef]
- Krapcho, A.P.; Maresch, M.J.; Helgason, A.L.; Rosner, K.E.; Hacker, M.P.; Spinelli, S.; Menta, E.; Oliva, A. The synthesis of 6, 9-bis[(aminoalkyl)amino] substituted benzo[g]quinoxaline-, benzo[g]quinazoline and benzo[g]phthalazine-5, 10-diones via regiospecific displacements. J. Heterocycl. Chem. 1993, 30, 1597–1606. [Google Scholar] [CrossRef]
- Krapcho, A.P.; Gallagher, C.E.; Hammach, A.; Ellis, M.; Menta, E.; Oliva, A. Synthesis of regioisomeric 6,9-(chlorofluoro)-substituted benzo[g]quinoline-5,10-diones, benzo[g]isoquinoline-5,10-diones and 6-chloro-9-fluorobenzo[g]quinoxaline-5,10-dione. J. Heterocycl. Chem. 1997, 34, 27–32. [Google Scholar] [CrossRef]
- Potts, K.T.; Bhattacharjee, D. Synthesis of 1, 4-Diaminoazaanthraquinone Derivatives. Synthesis 1983, 31–32. [Google Scholar] [CrossRef]
- Warren, J.D.; Lee, V.J.; Angier, R.B. Synthesis of 5,8-dihydroxynaphtho[2,3-c][1,2,5]thiadiazole-4,9-dione and 6,9-dihydroxybenzo[g]quinoxaline-5,10-dione. J. Heterocycl. Chem. 1979, 16, 1617–1624. [Google Scholar] [CrossRef]
- Potts, K.T.; Bhattacharjee, D.; Walsh, E.B. Cycloaddition routes to azaanthraquinone derivatives. 1. Use of azadienophiles. J. Org. Chem. 1986, 51, 2011–2021. [Google Scholar] [CrossRef]
- Newman, M.S.; Ihrman, K.G. The Behavior of o-Aroylbenzoic Acid Types in Acidic Media. J. Am. Chem. Soc. 1958, 80, 3652–3656. [Google Scholar] [CrossRef]
- VanAllan, J.A.; Reynolds, G.A.; Adel, R.E. Polynuclear heterocycles. II. Additions reactions of benzophenazines. J. Org. Chem. 1962, 27, 2873–2878. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, N.; Drabu, S. Synthesis of benzo[g]quinoxaline-5,10-dione based pyridine derivatives and their antimycobacterial activity. Orient. J. Chem. 2017, 33, 821–828. [Google Scholar] [CrossRef]
- Quiroga, J.; Sanchez, N.E.; Acosta, P.; Insuasty, B.; Abonia, R. Microwave-assisted synthesis of fused pyrazolo[3,4-b]pyrazines by the reaction of ortho-aminonitrosopyrazoles and cyclic β-diketones. Tetrahedron Lett. 2012, 53, 3181–3187. [Google Scholar] [CrossRef]
- Hammershøj, P.; Reenberg, T.K.; Pittelkow, M.; Nielsen, C.B.; Hammerich, O.; Christensen, J.B. Synthesis and Properties of 2, 3-Dialkynyl-1, 4-benzoquinones. Eur. J. Org. Chem. 2006, 2006, 2786–2794. [Google Scholar] [CrossRef]
- Kolmer-Anderl, N.; Kolmer, A.; Thiele, C.M.; Rehahn, M. Exploration of the Photodegradation of Naphtho [2, 3-g] quinoxalines and Pyrazino [2, 3-b] phenazines. Chem. Eur. J. 2016, 22, 5277–5287. [Google Scholar] [CrossRef]
- Morin, C.; Besset, T.; Moutet, J.-C.; Fayolle, M.; Brückner, M.; Limosin, D.; Becker, K.; Davioud-Charvet, E. The aza-analogues of 1,4-naphthoquinones are potent substrates and inhibitors of plasmodial thioredoxin and glutathione reductases and of human erythrocyte glutathione reductase. Org. Biomol. Chem. 2008, 6, 2731–2742. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yan, M.; Chen, J.; Yuan, B.; Chen, G.; Cheng, S.; Huang, D.; Gao, Z.; Cao, C. Identification of ortho-naphthoquinones as anti-AML agents by highly efficient oxidation of phenols. Bioorg. Chem. 2019, 86, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Taleb, A.; Alvarez, F.; Nebois, P.; Walchshofer, N. An improved methodology for the preparation of 4, 7-dimethoxy-1H-benzimidazole, a key intermediate in the synthesis of 1-alkyl-1H-benzimidazole-4, 7-diones. Heterocycl. Commun. 2006, 12, 111–114. [Google Scholar] [CrossRef]
- Nose, M.; Suzuki, H. A mild one-pot procedure for the polynitration of activated arenes. Convenient preparation of dinitro-and trinitrodialkoxybenzenes. Synthesis 2000, 2000, 1539–1542. [Google Scholar] [CrossRef]
- Weinberger, L.; Day, A.R. Syntheses of dimethoxybenzimidazoles, dihydroxybenzimidazoles and imidazo-p-benzoquinones. J. Org. Chem. 1959, 24, 1451–1455. [Google Scholar] [CrossRef]
- Dwyer, C.L.; Holzapfel, C.W. The nitration of electron-rich aromatics. Tetrahedron 1998, 54, 7843–7848. [Google Scholar] [CrossRef]
- Kitahara, Y.; Nakahara, S.; Tanaka, Y.; Kubo, A. Synthesis of 5, 8-quinoxalinediones and 5, 8-quinazolinediones. Heterocycles 1992, 34, 1623–1630. [Google Scholar]
- Lee, H.; Cho, S.; Namgoong, K.; Jung, J.-K.; Cho, J.; Yang, S.-I. Synthesis and in vitro evaluation of 7-dialkylaminomethylbenzo[g]quinoxaline-5,10-diones. Bioorg. Med. Chem. Lett. 2004, 14, 1235–1237. [Google Scholar] [CrossRef]
- Kwak, J.-H.; Namgoong, K.; Jung, J.-K.; Cho, J.; Kim, H.-M.; Park, S.-G.; Yoo, Y.-A.; Kwon, J.-H.; Lee, H. Synthesis and cytotoxic activities of 2-alkyl-2,3-dihydro-1H-2,6,9-triazacyclopenta[b]anthracene-5,10-diones. Arch. Pharmacal Res. 2008, 31, 995–998. [Google Scholar] [CrossRef]
- Lee, H.; Cho, S.; Choi, B.; Namgoong, K.; Jung, J.-K. Synthesis of 2,3,8-trisubstituted 7H-isoindolo[5,6-g]quinoxaline-5,7,9,11(8H)-tetraones. Heterocycles 2004, 63, 819–826. [Google Scholar] [CrossRef]
- Kita, Y.; Kirihara, M.; Fujii, Y.; Okunaka, R.; Akai, S.; Maeda, H.; Tamura, Y.; Shimooka, K.; Ohishi, H.; Ishida, T. Synthetic anthracyclines: Total synthesis of D-ring pyridine and pyrazine analogs of 11-deoxydaunomycin. Chem. Pharm. Bull. 1991, 39, 857–864. [Google Scholar] [CrossRef][Green Version]
- Tanno, N.; Terashima, S. Asymmetric synthesis of optically active anthracyclinone intermediate and 4-demethoxyanthracyclinones by the use of a novel chiral reducing agent. Chem. Pharm. Bull. 1983, 31, 821–836. [Google Scholar] [CrossRef][Green Version]
- Acton, E.M.; Tong, G.L. Approaches to phenazine-derived N-isosteres of anthracyclinones. J. Heterocycl. Chem. 1981, 18, 1141–1147. [Google Scholar] [CrossRef]
- Tišler, M.; Stanovnik, B. Advances in Heterocyclic Chemistry; Elsevier: London, UK, 1968; Volume 9, pp. 211–320. [Google Scholar]
- Snyder, C.D.; Rapoport, H. Oxidative cleavage of hydroquinone ethers with argentic oxide. J. Am. Chem. Soc. 1972, 94, 227–231. [Google Scholar] [CrossRef]
- Danishefsky, S.; Kitahara, T. Useful diene for the Diels-Alder reaction. J. Am. Chem. Soc. 1974, 96, 7807–7808. [Google Scholar] [CrossRef]
- Saenger, W. Principles of Nucleic Acid Structure; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Yamashita, Y.; Tsubata, Y.; Suzuki, T.; Miyashi, T.; Mukai, T.; Tanaka, S. Benzo[g][1,2,5]thiadiazolo[3,4-b]quinoxaline-5,10-dione and its selenium analog. An unusual type of quinones. Chem. Lett. 1990, 19, 445–448. [Google Scholar] [CrossRef]
- Koyama, J.; Morita, I.; Yamori, T. Correlation between cytotoxic activities and reduction potentials of heterocyclic quinones. Molecules 2010, 15, 6559–6569. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Park, S.-Y.; Lee, H.-J.; Suh, M.-E.; Schollmeyer, D.; Lee, C.O. Synthesis and cytotoxicity of 6,11-Dihydro-pyrido- and 6,11-Dihydro-benzo[2,3-b]phenazine-6,11-dione derivatives. Bioorg. Med. Chem. 2003, 11, 1709–1714. [Google Scholar] [CrossRef]
- Choudhary, A.; Zachek, B.; Lera, R.F.; Zasadil, L.M.; Lasek, A.; Denu, R.A.; Kim, H.; Kanugh, C.; Laffin, J.J.; Harter, J.M.; et al. Identification of Selective Lead Compounds for Treatment of High-Ploidy Breast Cancer. Mol. Cancer Ther. 2016, 15, 48–59. [Google Scholar] [CrossRef]
- Yoo, H.-W.; Lee, Y.-S.; Eun Suh, M.; Kim, D.J.; Park, S.W. Cytotoxic effects of quinoxaline derivatives on human cancer cell lines. Arch. Pharm. 1998, 331, 331–333. [Google Scholar] [CrossRef]
- Tudor, G.; Gutierrez, P.; Aguilera-Gutierrez, A.; Sausville, E.A. Cytotoxicity and apoptosis of benzoquinones: Redox cycling, cytochrome C release, and BAD protein expression. Biochem. Pharmacol. 2003, 65, 1061–1075. [Google Scholar] [CrossRef]
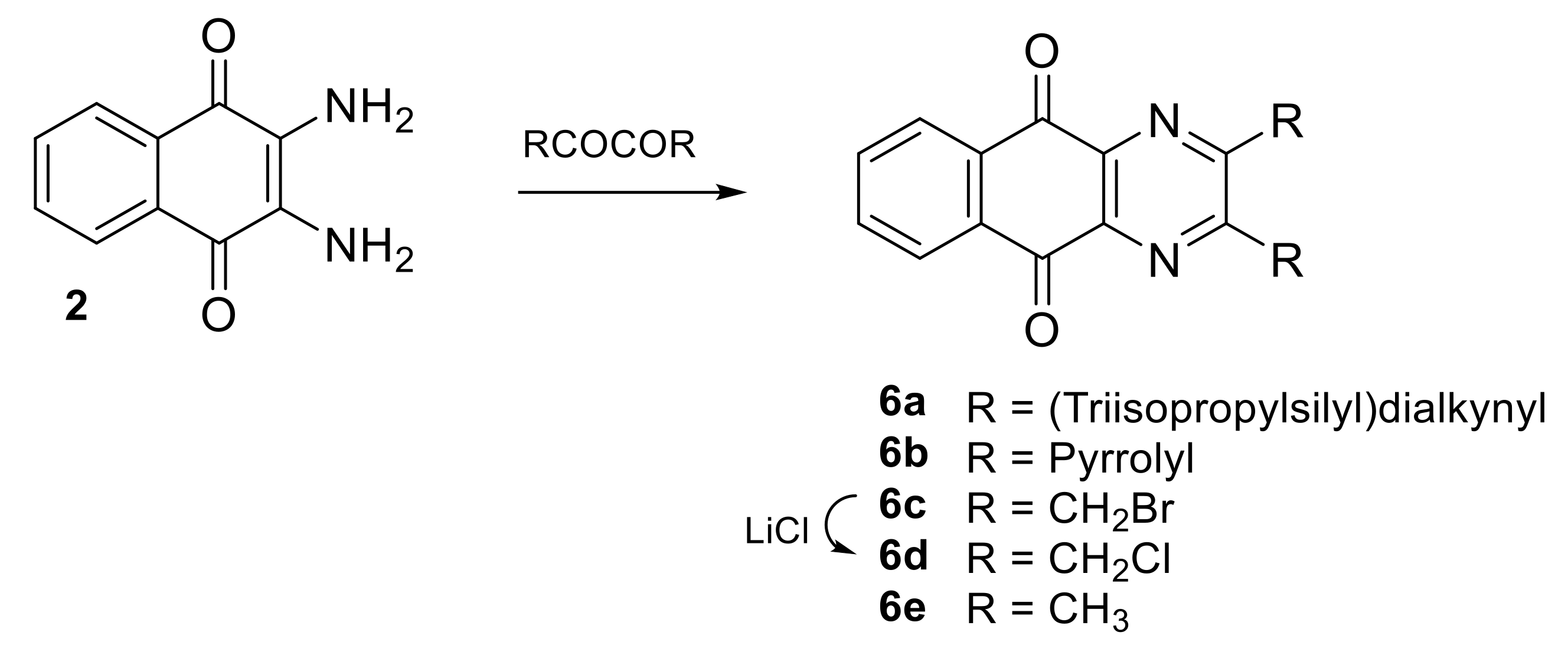







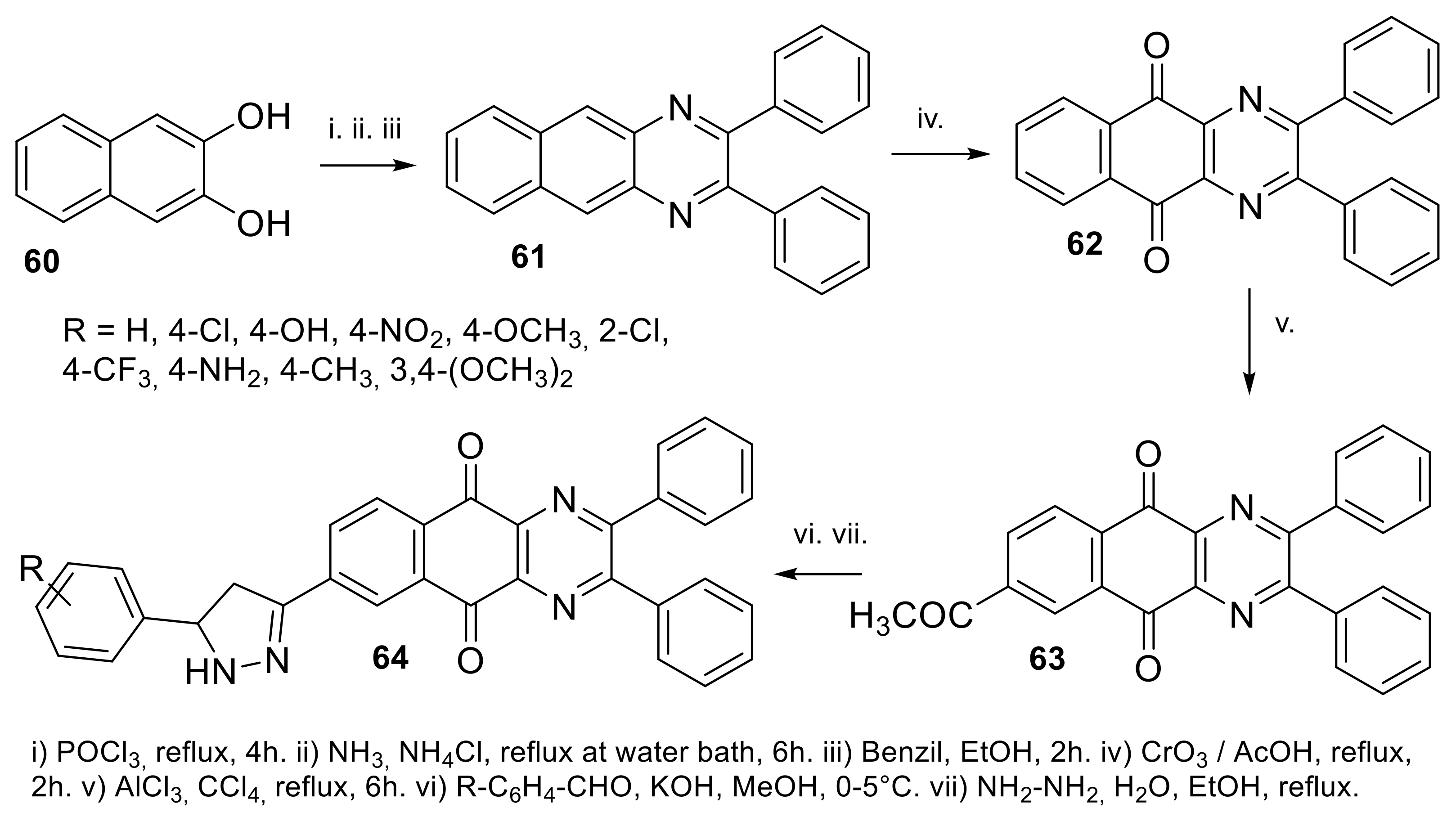

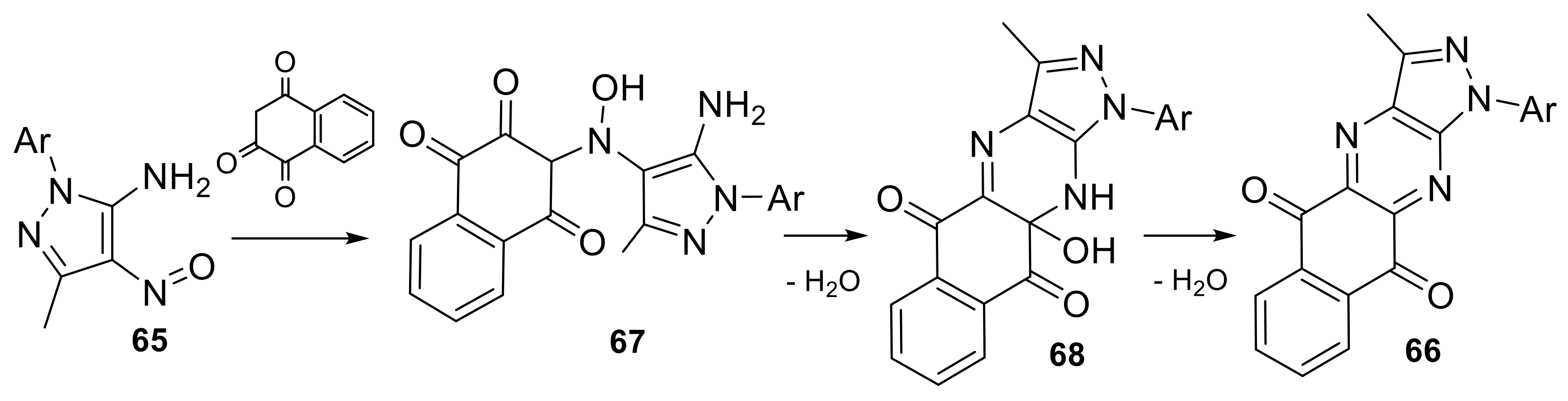


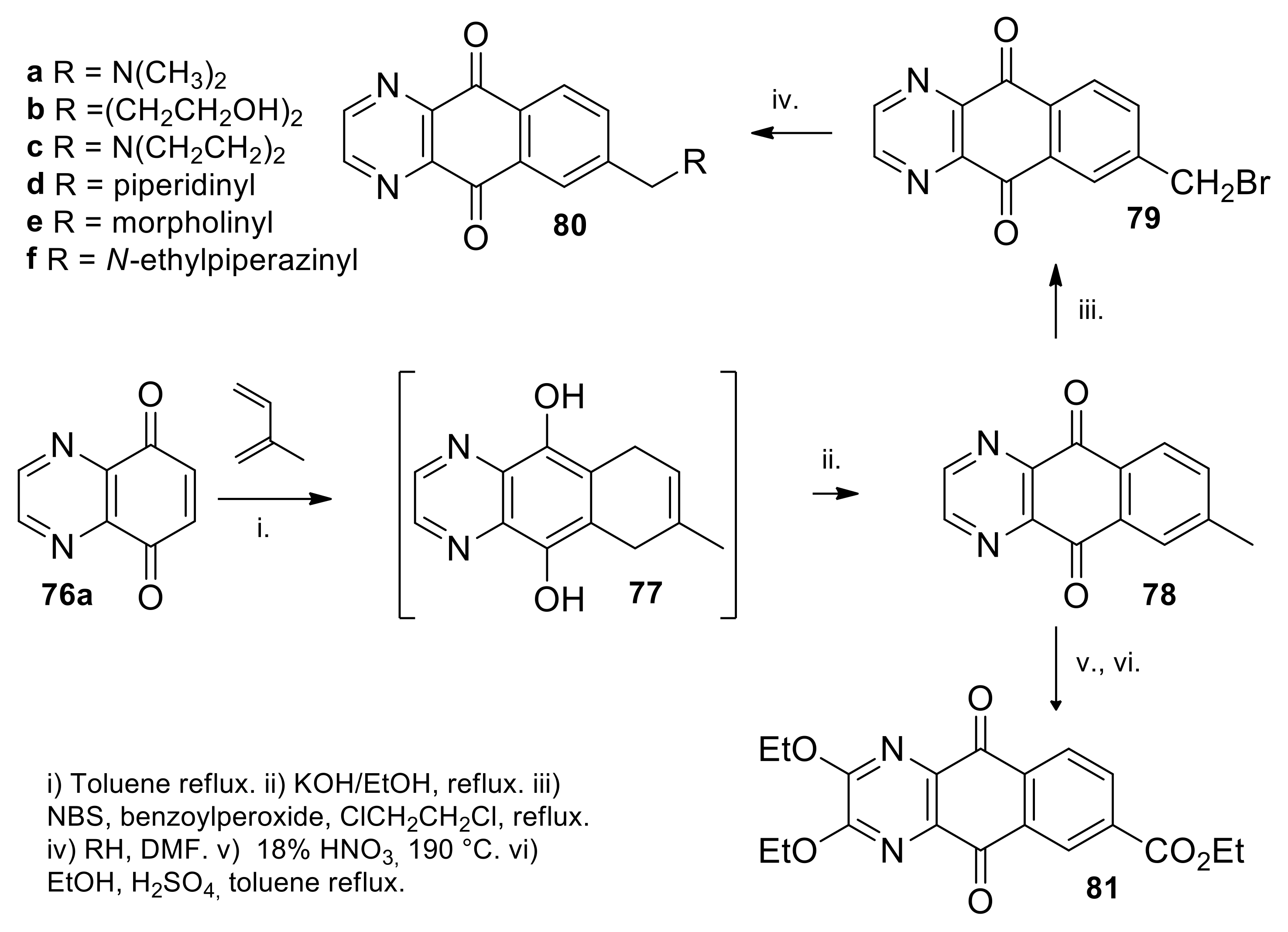


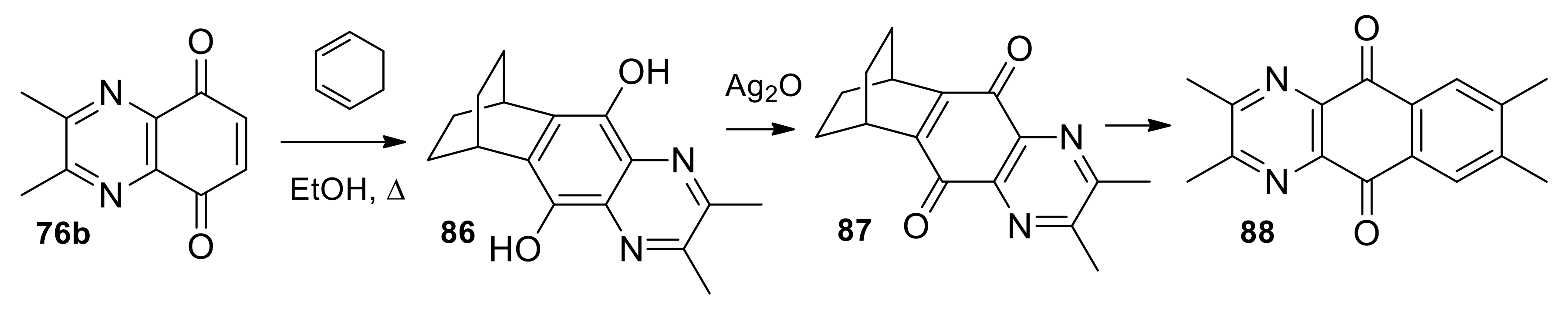

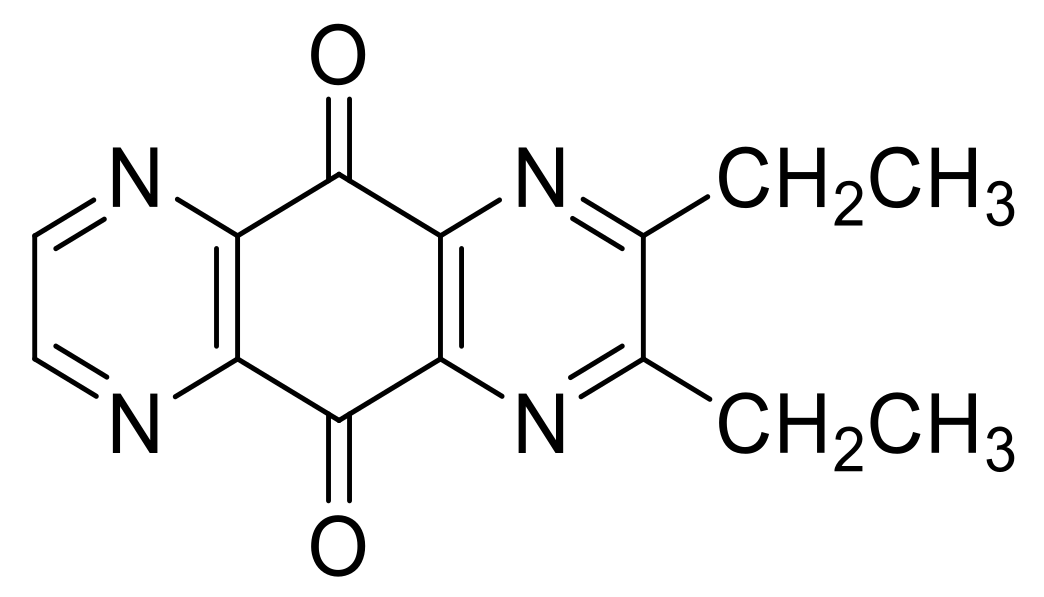


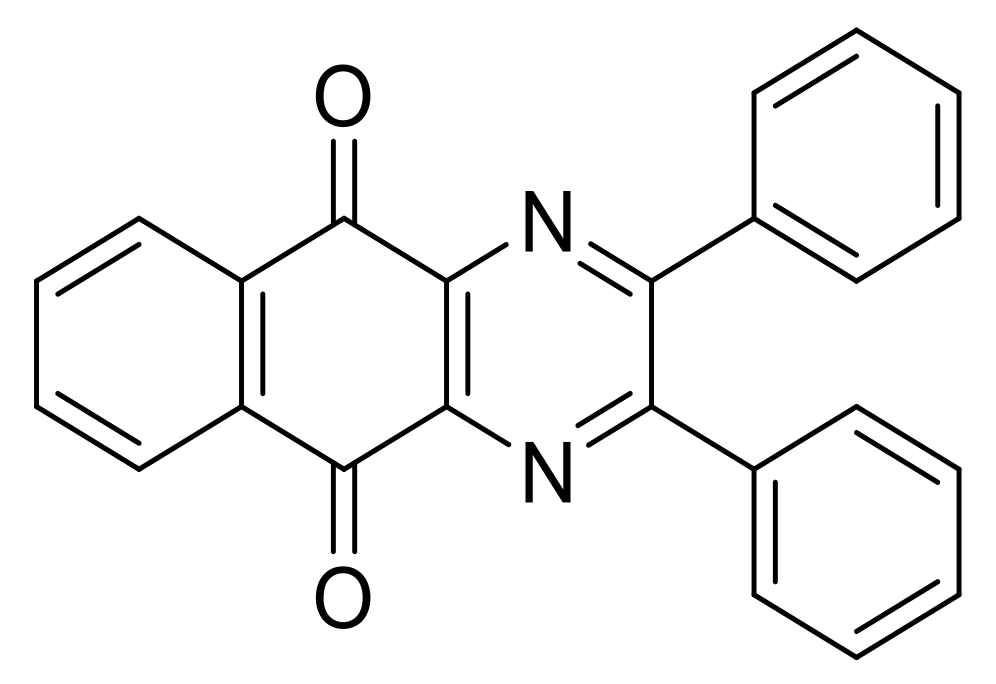
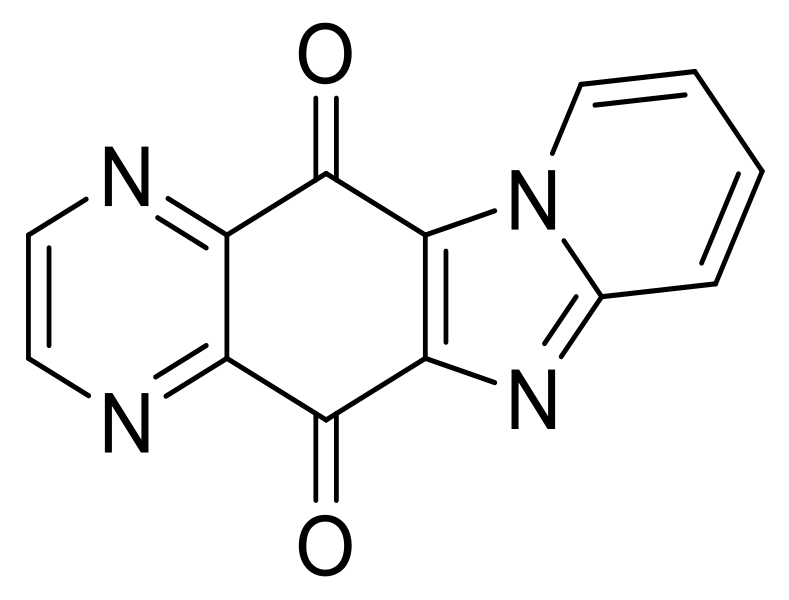
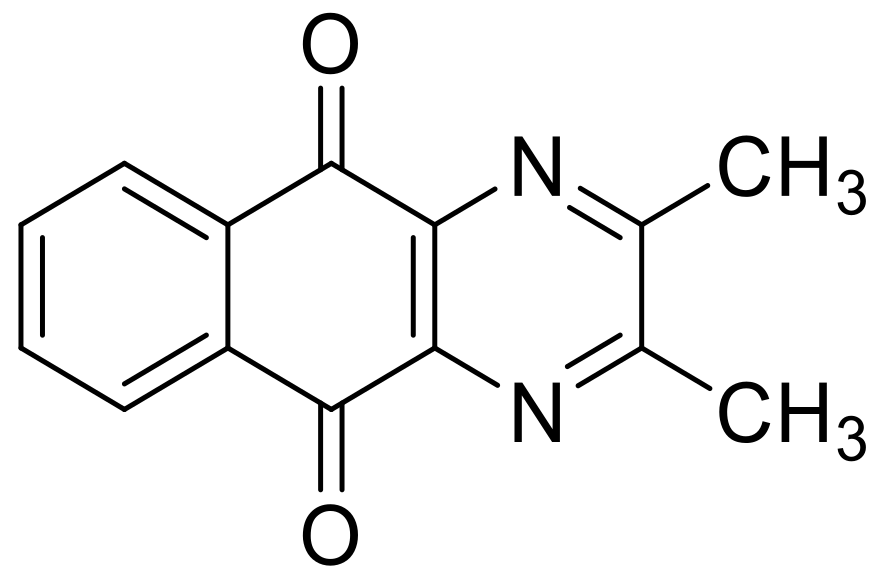
| N° | R1 | R2 | Ref. |
|---|---|---|---|
| 6a | (Triisopropylsilyl)dialkynyl | (Triisopropylsilyl)dialkynyl | [20] |
| 6b | Pyrrolyl | Pyrrolyl | [21] |
| 6c | CH2Br | CH2Br | [18,22] |
| 6d | CH2Cl | CH2Cl | [22] |
| 6e | CH3 | CH3 | [24] |
| 6f | Indolyl | Indolyl | [19] |
| 6f | 5-Bromo-1H-indolyl | 5-Bromo-1H-indolyl | [19] |
| 6h | Phenyl | Phenyl | [24] |
| 6i | Styryl | Styryl | [24] |
| 6j | CH3 | Phenyl | [24] |
| 6k | CH3 | H | [24] |
| 6l | 4-CF3C6H4 | CF3 | [23] |
| 6m | CH3 | 3,5-diClC6H4 | [23] |
| 6n | CH2Cl | CH2CH(CH3)2 | [23] |
| 6o | 4-CH3C6H4 | 4-FC6H4 | [23] |
| 6p | 4-ClC6H4 | C6H5 | [23] |
| 6q | 4-CH3C6H4 | 4-ClC6H4 | [23] |
 | ||||
|---|---|---|---|---|
| N° | R | R1 | R2 | Ref. |
| 57a | Me | H | OMe | [49] |
| 57b | Me | NHCO2Et | NHCO2Et | [49] |
| 57c | Me | NH2 | NH2 | [49] |
| 57d | H | OCH3 | H | [50] |
| 57e | H | NH2 | H | [49,51] |
| 57f | H | F | NH(CH2)2N(Me)2 | [47] |
| 57g | H | F | NH(CH2)2NHCO2C(Me)3 | [47] |
| 57h | H | OH | OH | [50] |
| 48a | H | NH(CH2)2N(Me)2 | NH(CH2)2N(Me)2 | [47] |
| 48b | H | NH(CH2)2NHCO2C(Me)3 | NH(CH2)2NHCO2C(Me)3 | [47] |
| 48c | H | NH(CH2)2NH2 | NH(CH2)2NH2 | [47] |
| 57i | H | F | F | [47] |
| 57j | H | F | Cl | [48] |
| 57k | Me | H | OCH3 | [49] |
| 57l | Me | H | OMe | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuglio-Tonolo, A.G.; Curti, C.; Terme, T.; Vanelle, P. A Survey of Synthetic Routes and Antitumor Activities for Benzo[g]quinoxaline-5,10-diones. Molecules 2020, 25, 5922. https://doi.org/10.3390/molecules25245922
Giuglio-Tonolo AG, Curti C, Terme T, Vanelle P. A Survey of Synthetic Routes and Antitumor Activities for Benzo[g]quinoxaline-5,10-diones. Molecules. 2020; 25(24):5922. https://doi.org/10.3390/molecules25245922
Chicago/Turabian StyleGiuglio-Tonolo, Alain G., Christophe Curti, Thierry Terme, and Patrice Vanelle. 2020. "A Survey of Synthetic Routes and Antitumor Activities for Benzo[g]quinoxaline-5,10-diones" Molecules 25, no. 24: 5922. https://doi.org/10.3390/molecules25245922
APA StyleGiuglio-Tonolo, A. G., Curti, C., Terme, T., & Vanelle, P. (2020). A Survey of Synthetic Routes and Antitumor Activities for Benzo[g]quinoxaline-5,10-diones. Molecules, 25(24), 5922. https://doi.org/10.3390/molecules25245922





