Recent Advances on Synthetic Methodology Merging C–H Functionalization and C–C Cleavage
Abstract
1. Introduction
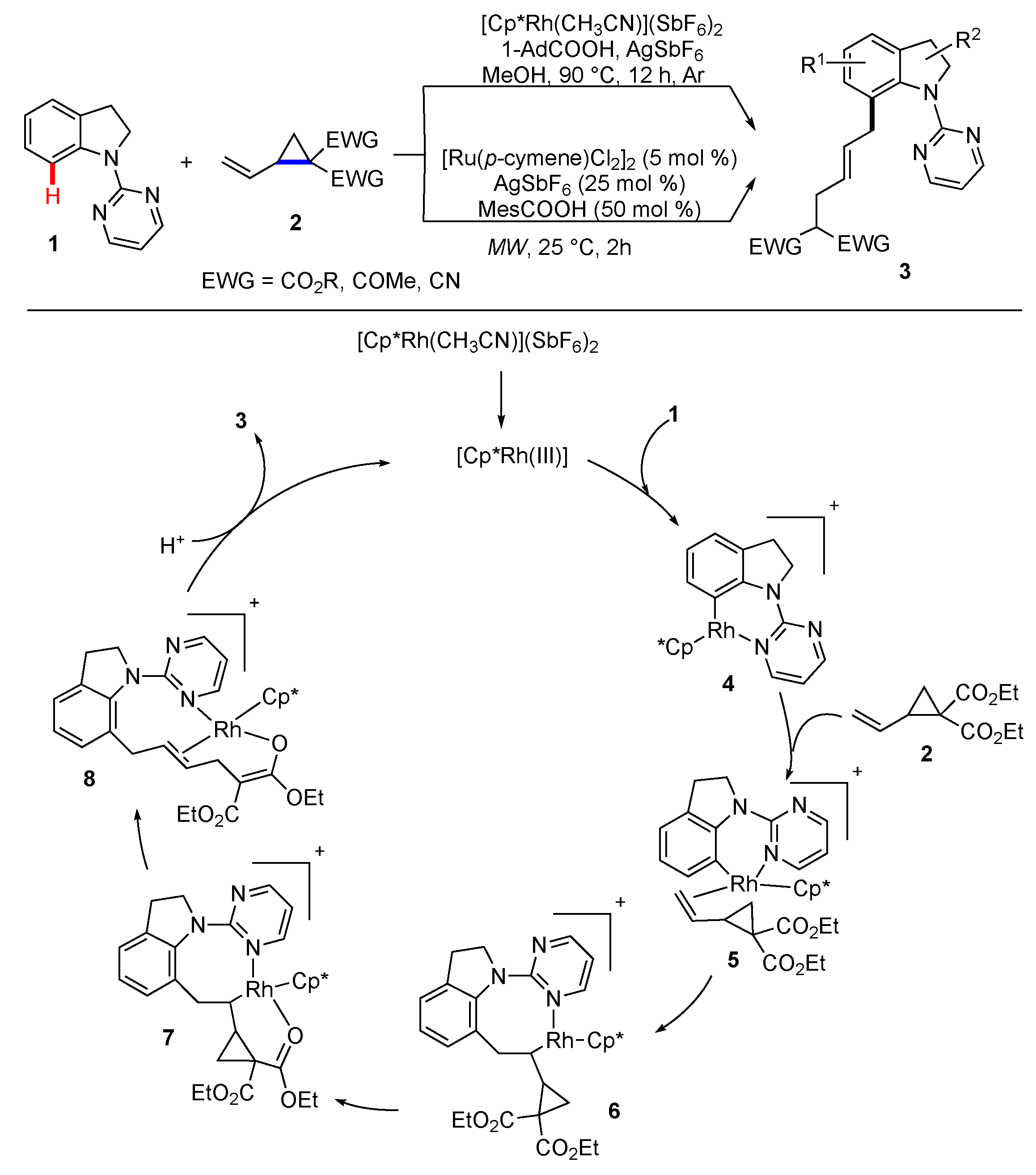
2. Synthetic Methodology Involving C–H Functionalization Along with the Cleavage of Strained C–C Bonds
2.1. Reactions Involving Transition-Metal Catalyzed or Mediated Processes and Strained Substrates
2.2. Reactions Involving Radical Intermediates
2.3. Reactions Promoted by Lewis or Brønsted Acids or Bases
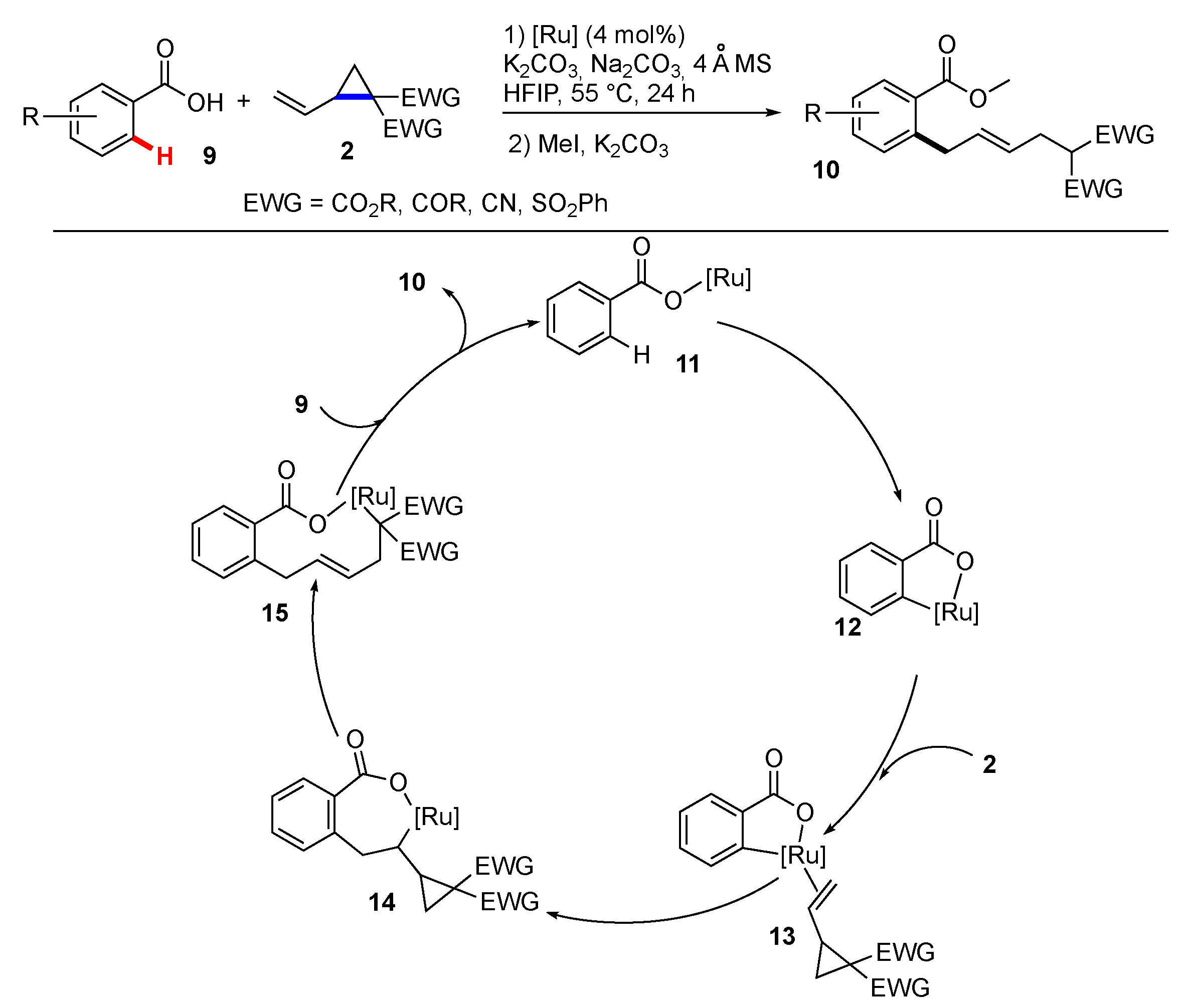
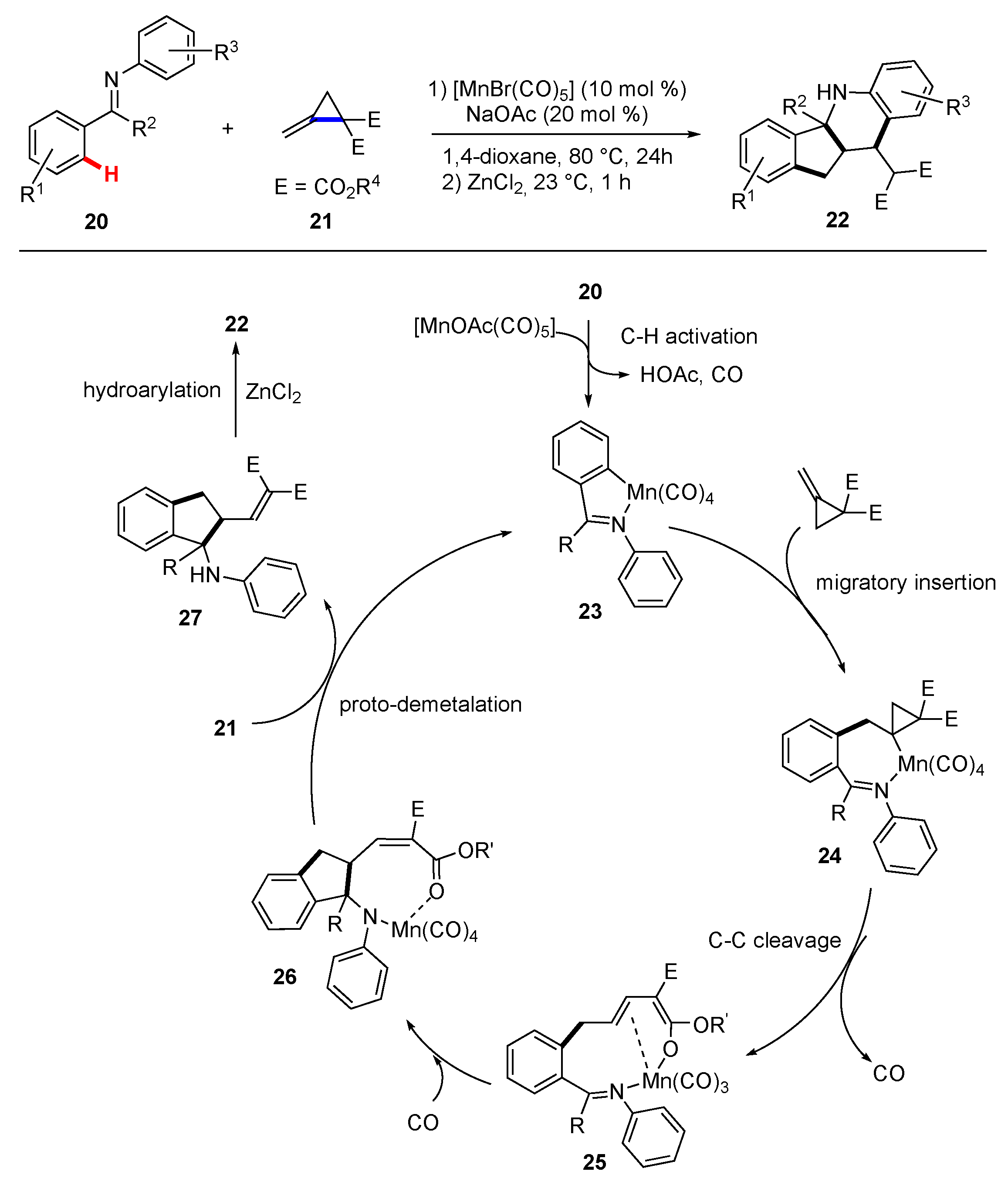
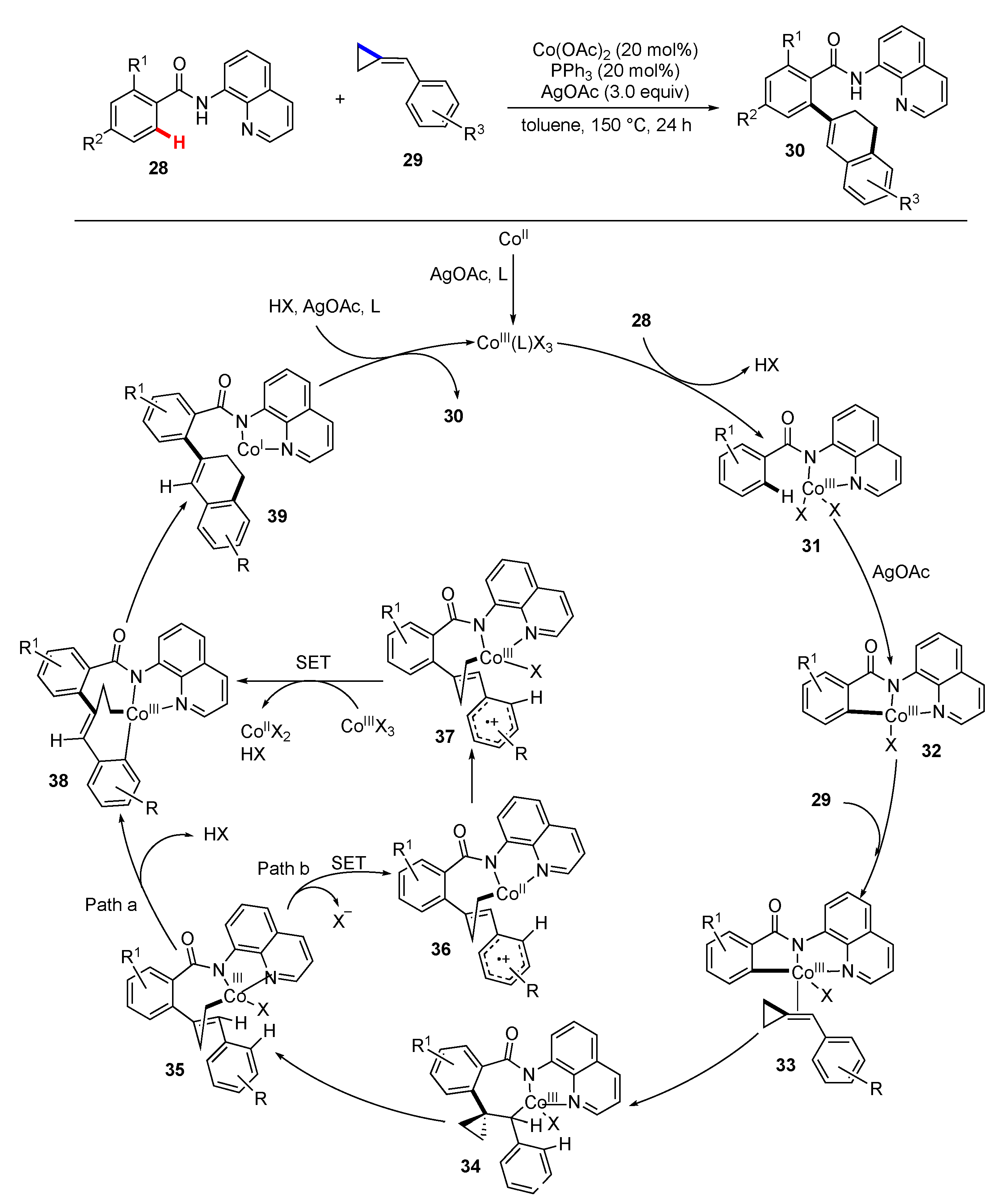
3. Synthetic Methodology Involving C–H Functionalization Along with the Cleavage of Unstrained C–C Bonds
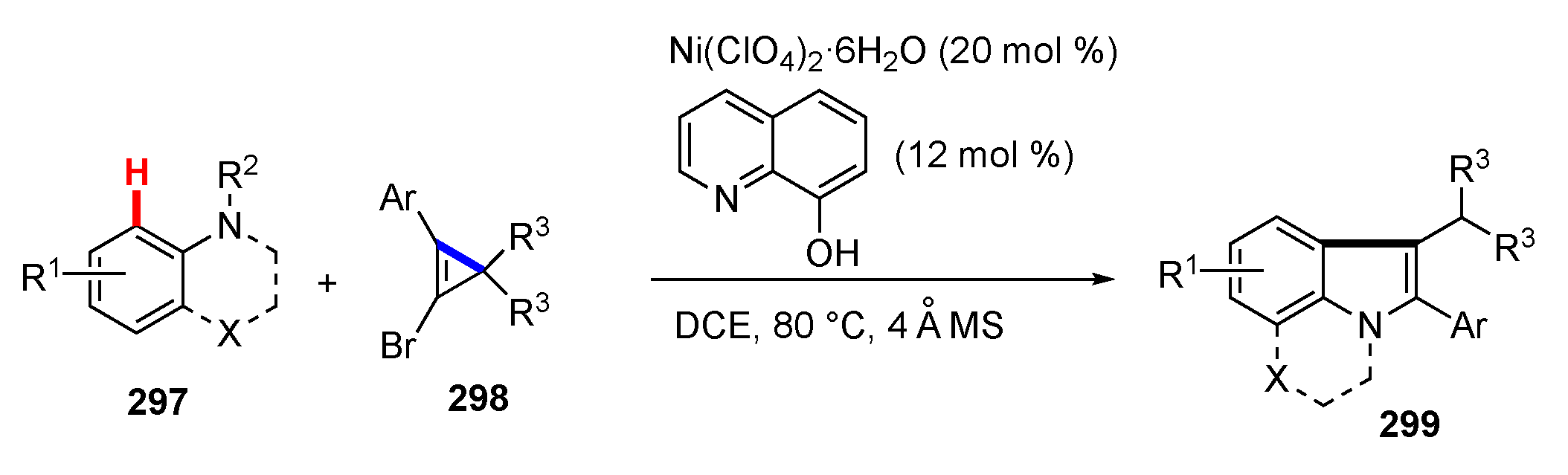
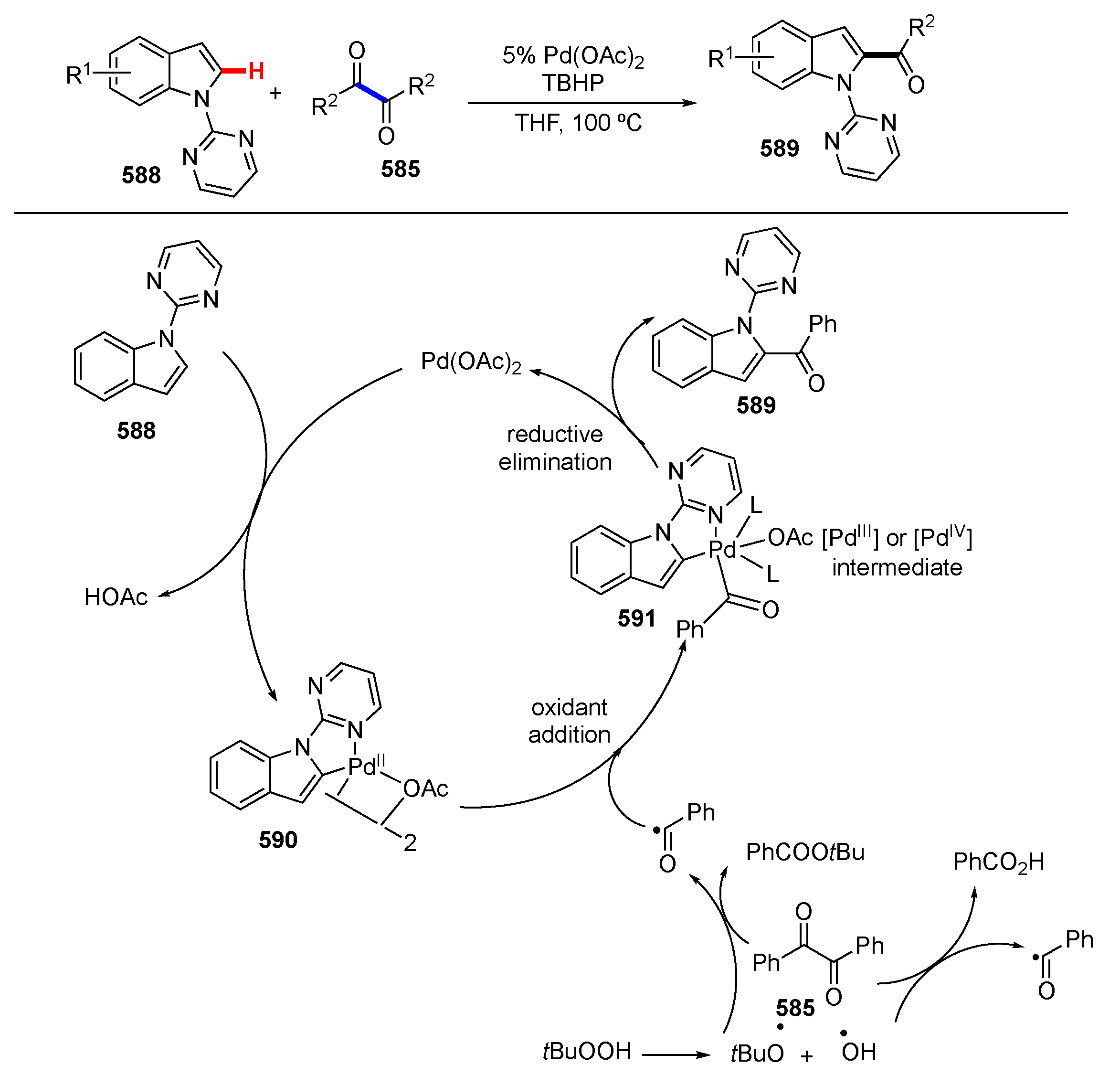
3.1. Reactions Involving Transition-Metal Catalyzed or Mediated Processes and Unstrained Substrates
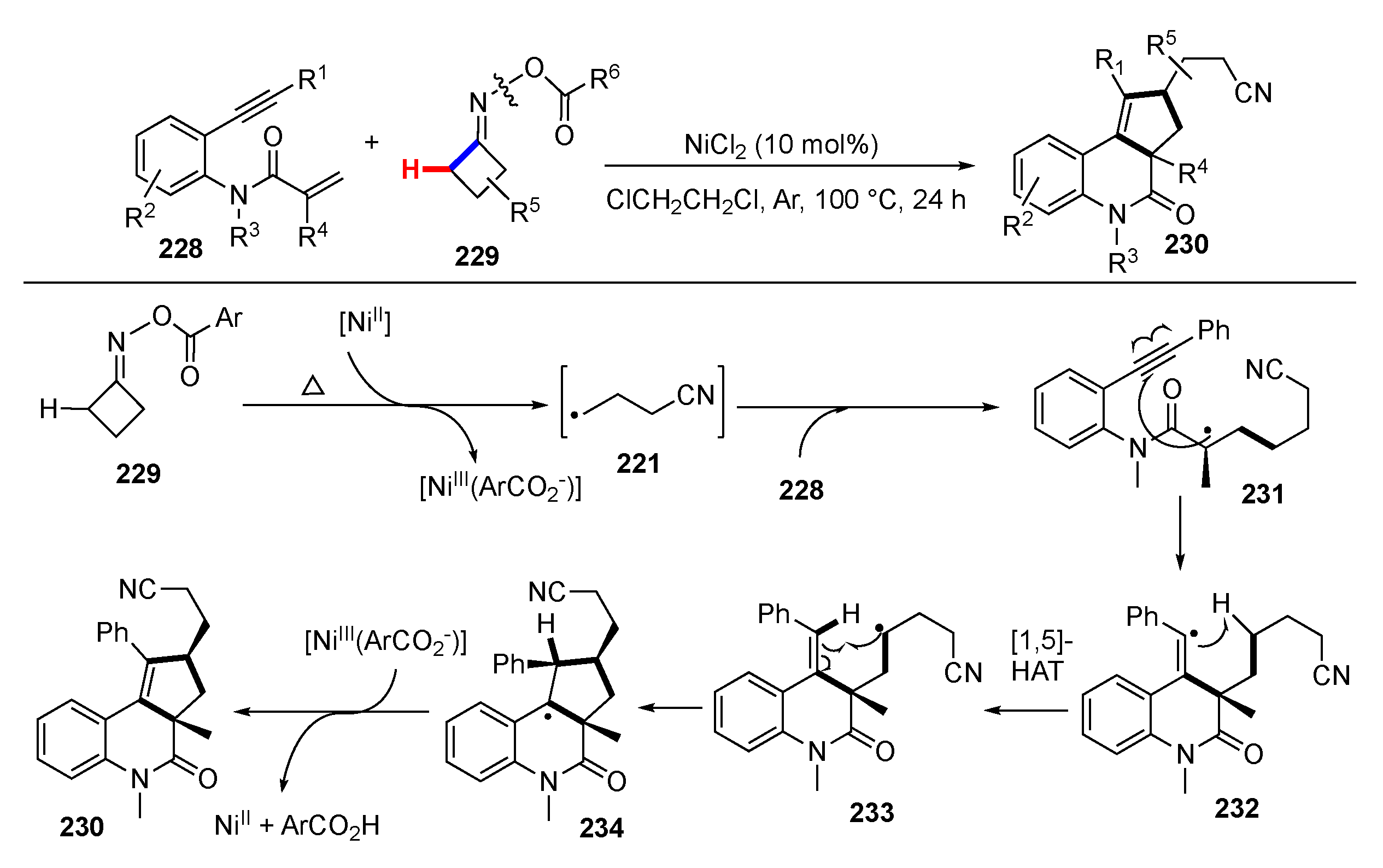
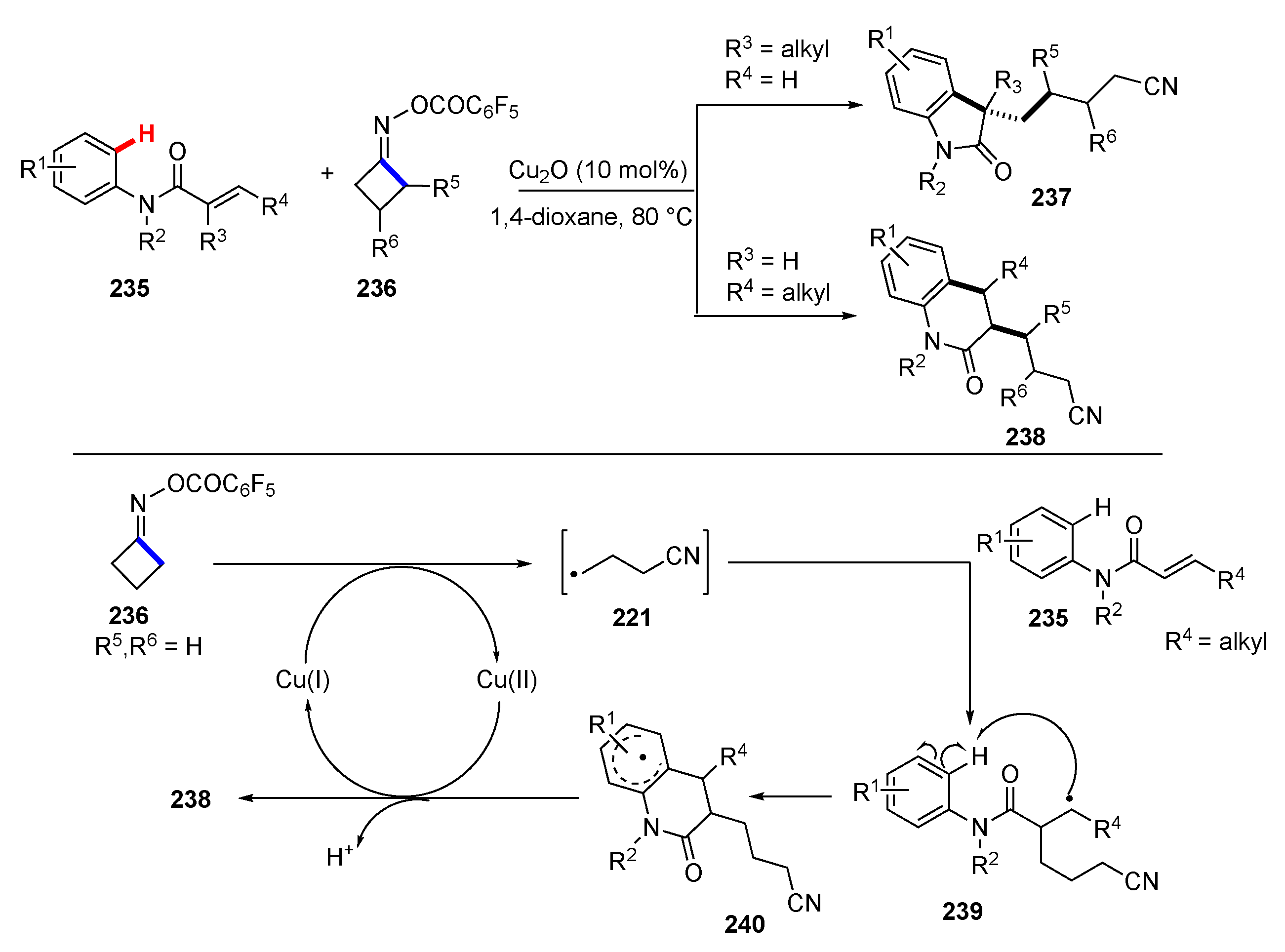
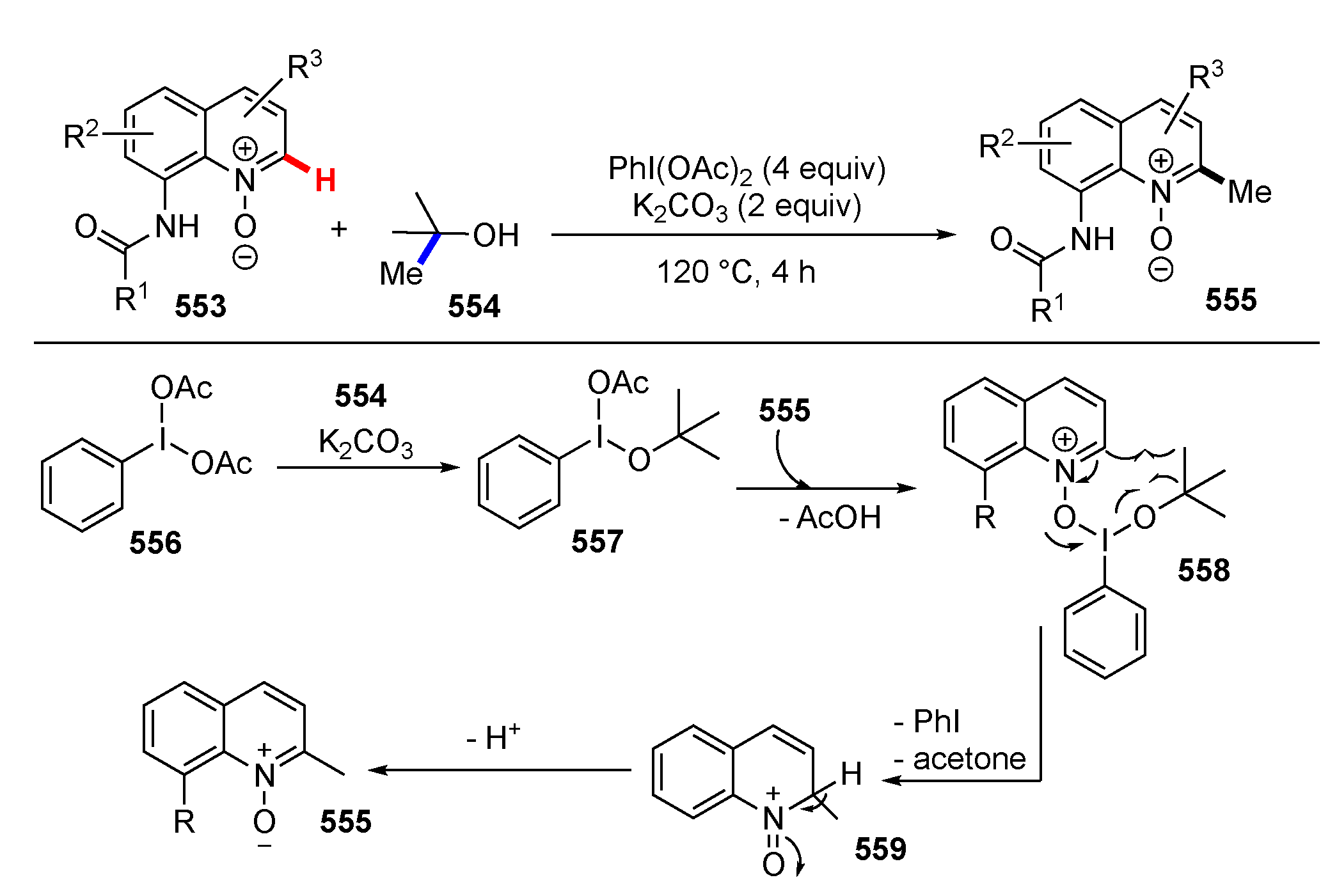
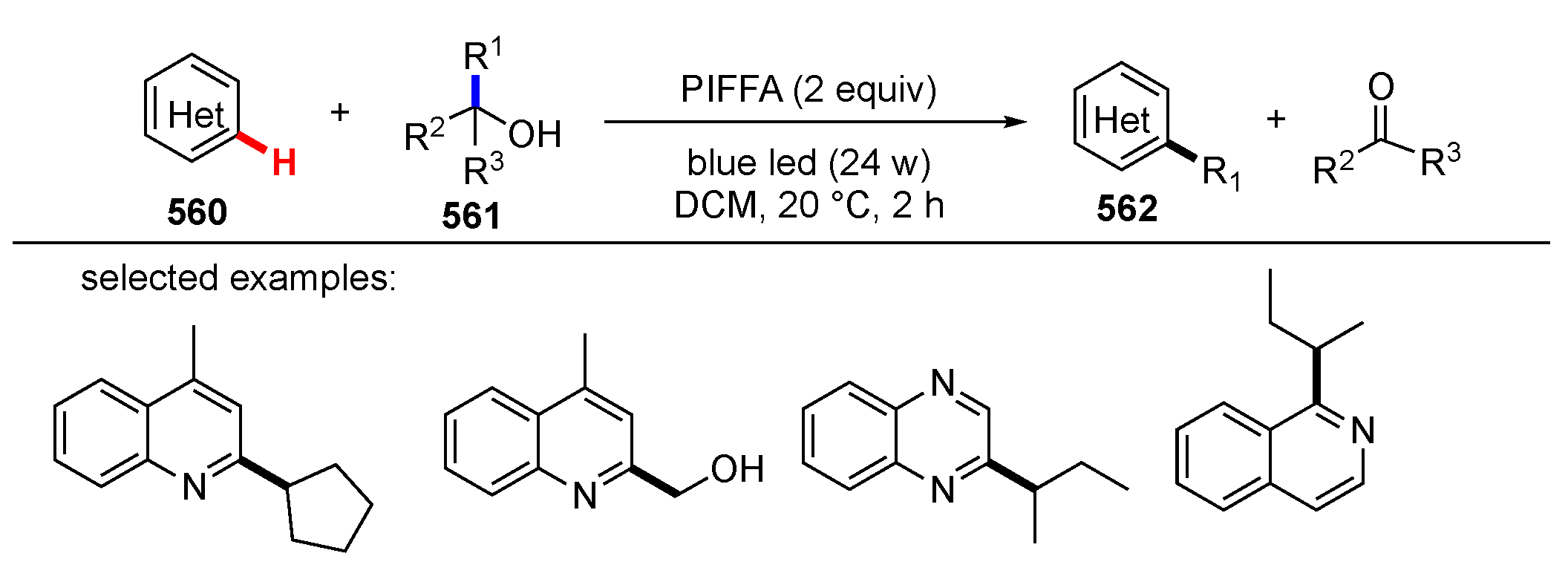
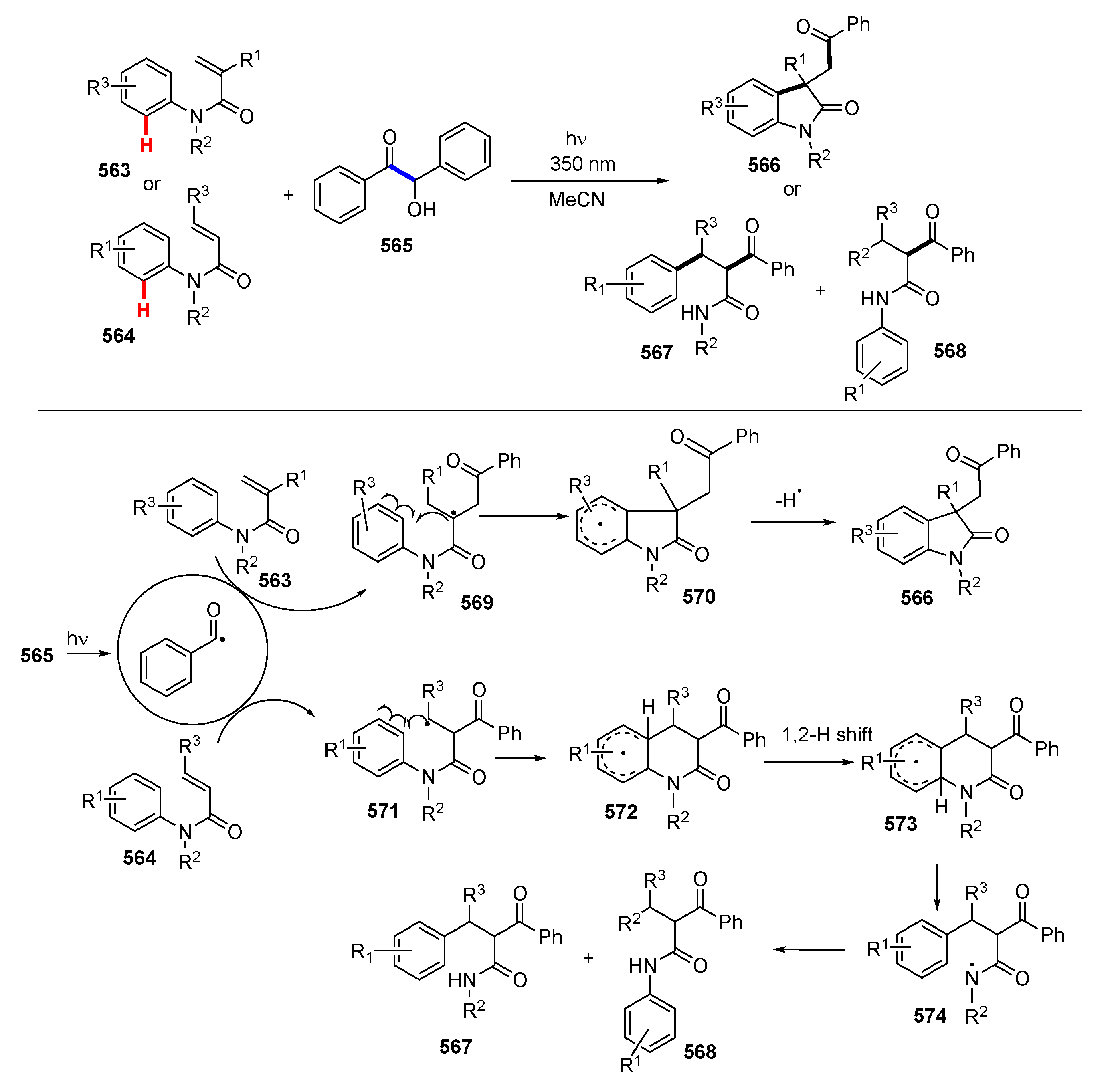
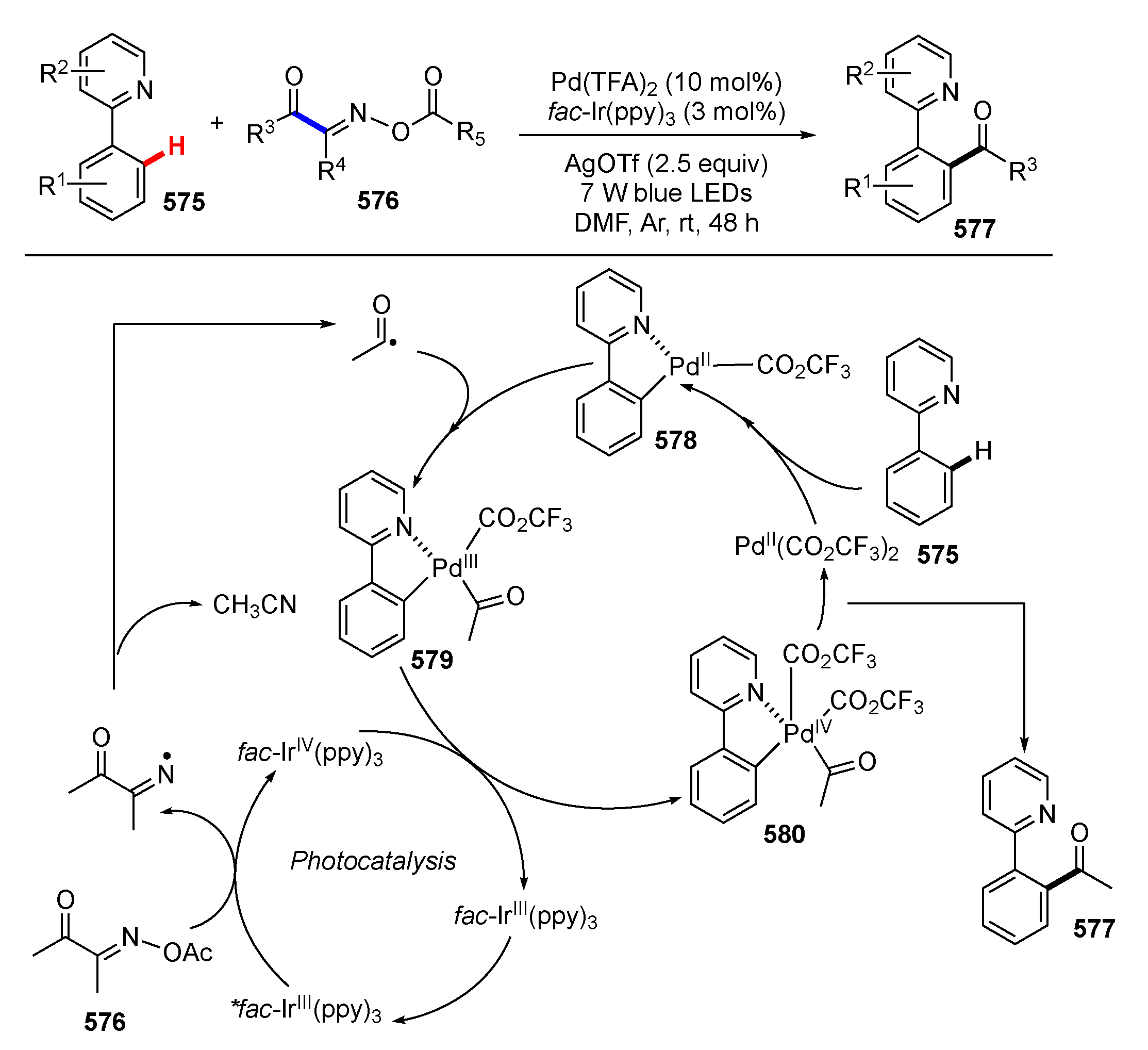
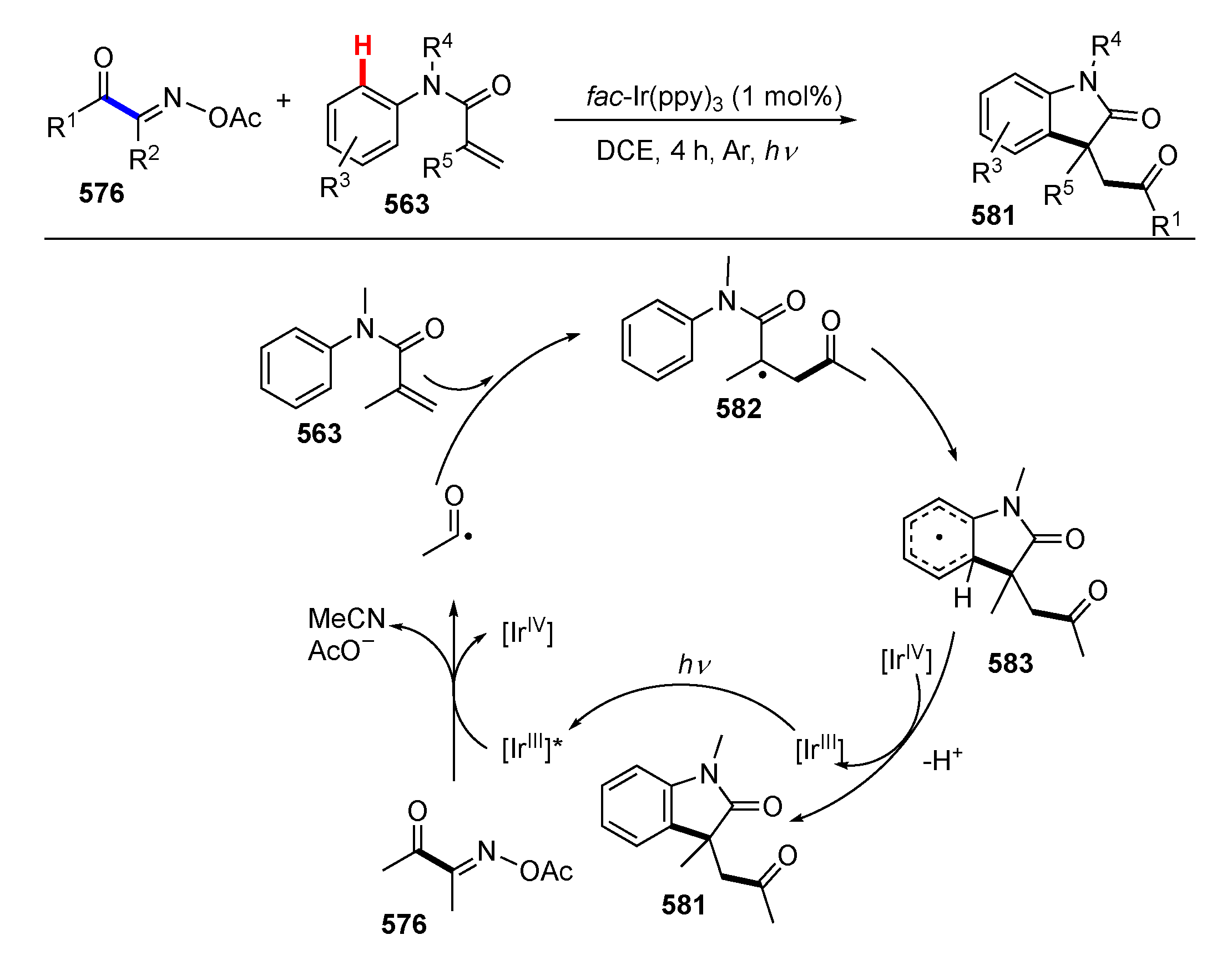
3.2. Reactions Involving Radical Intermediates
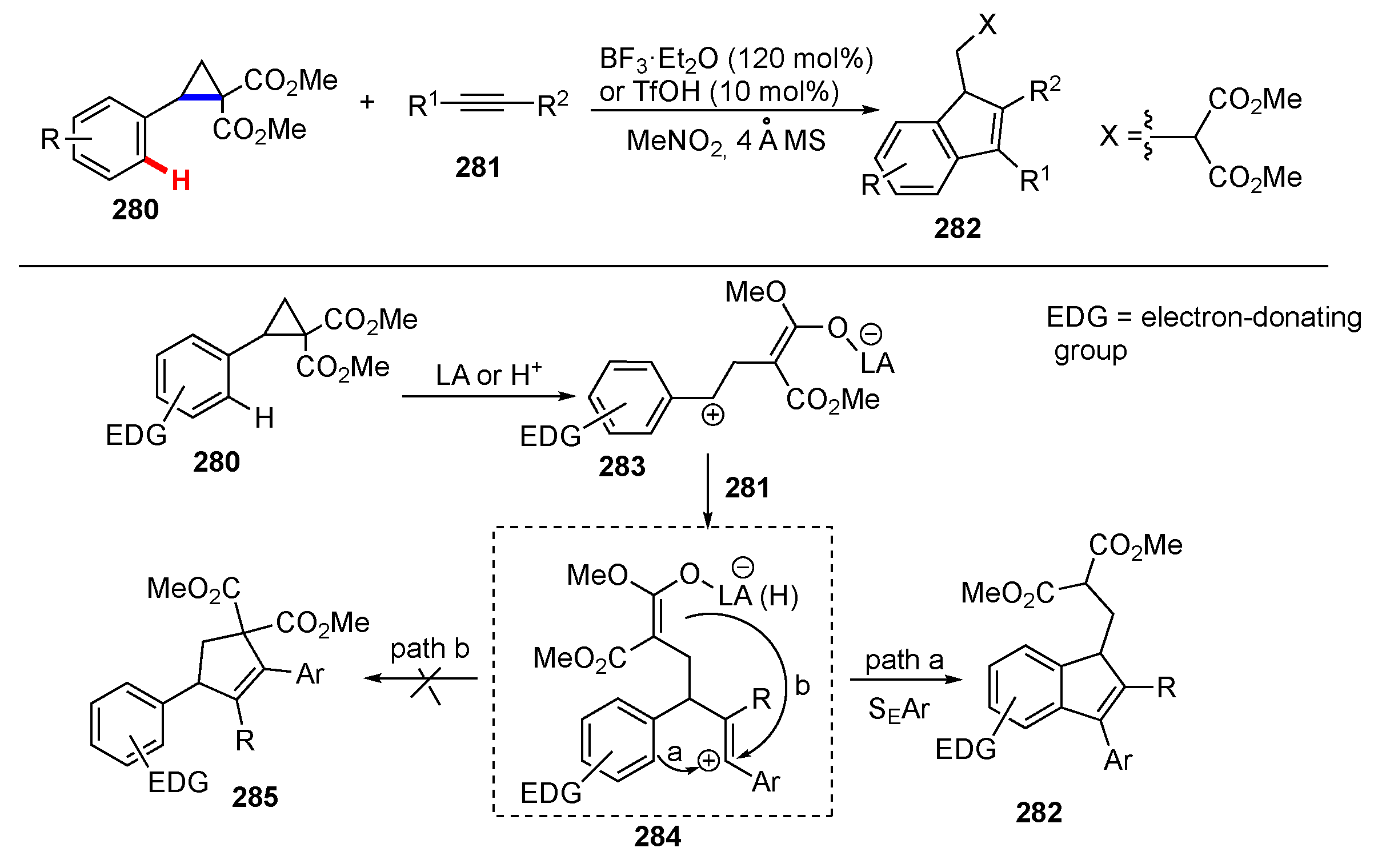
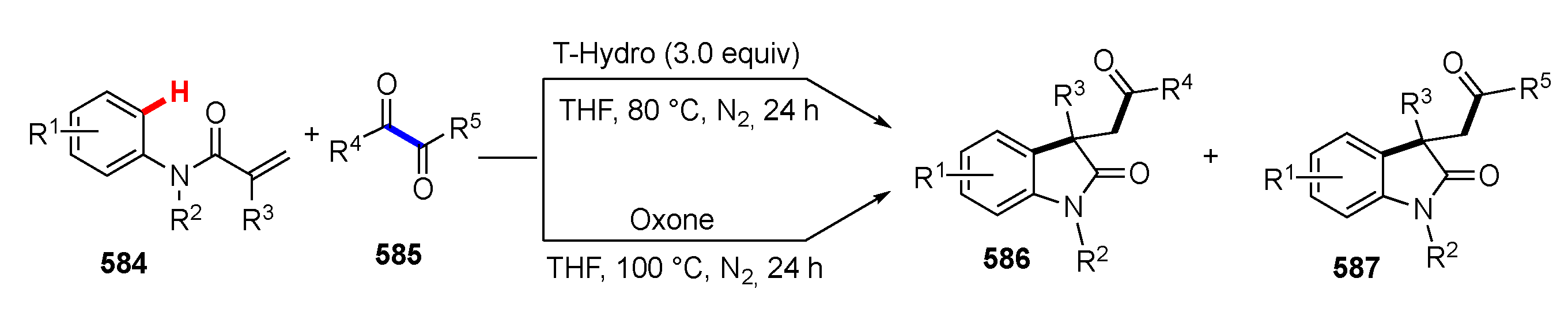
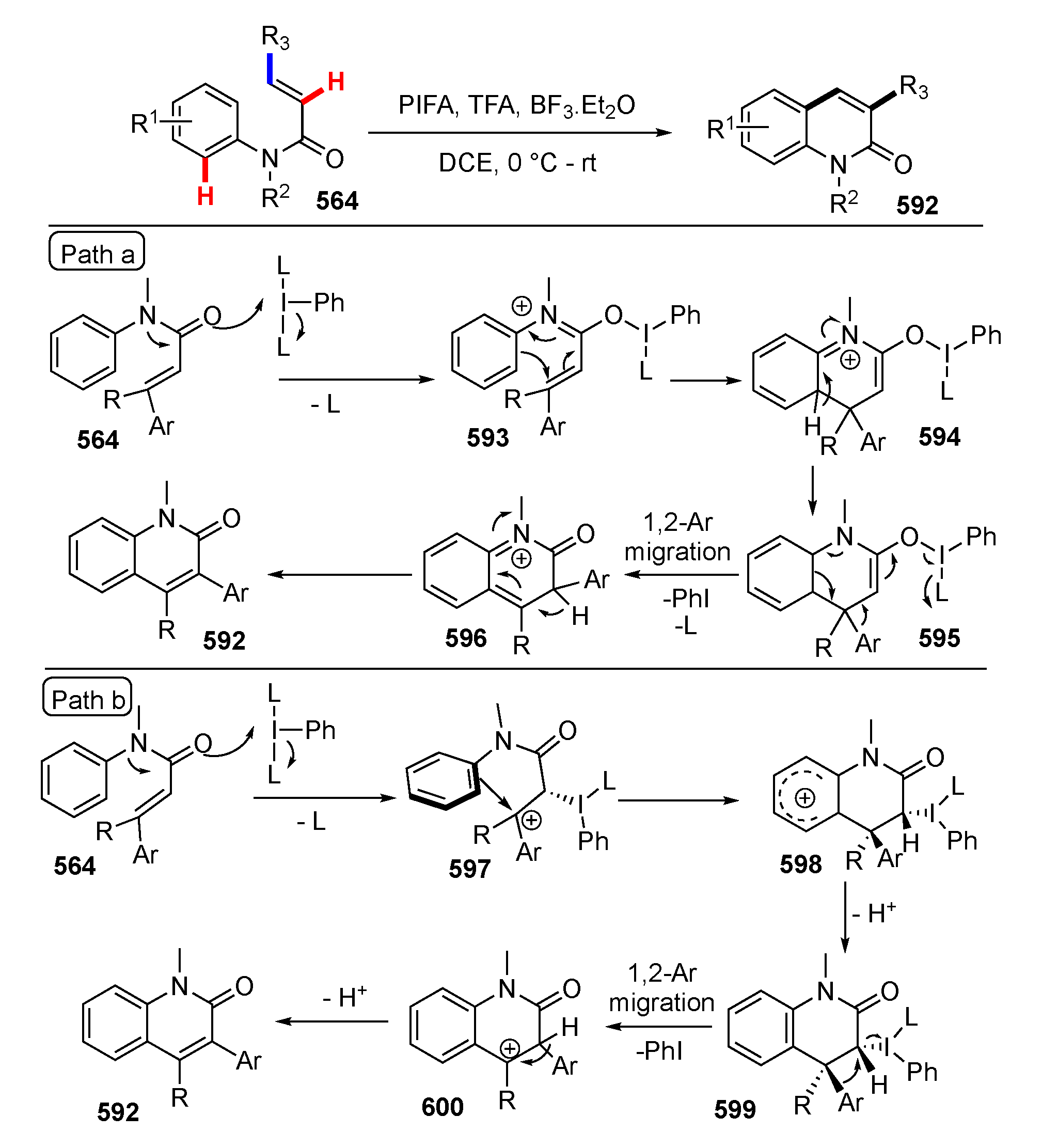
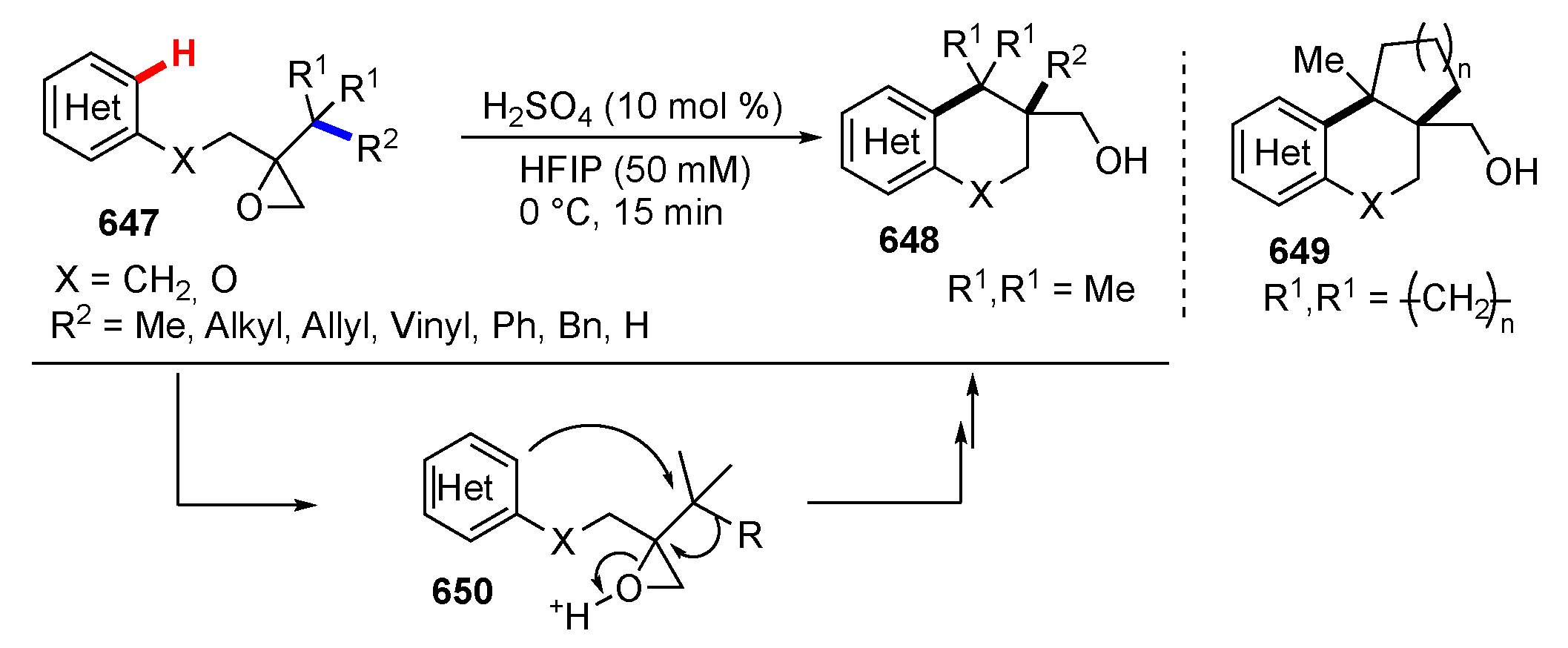
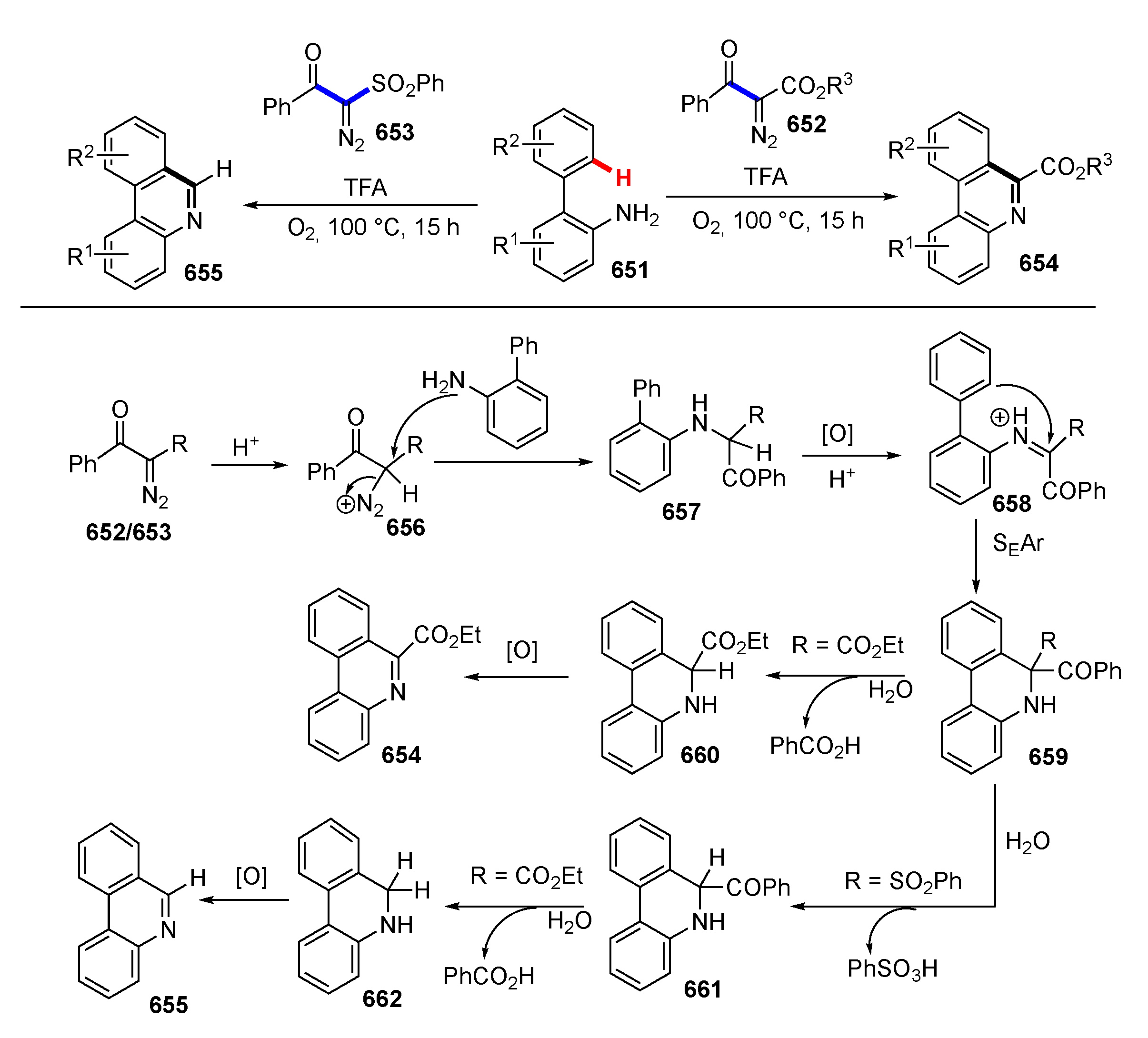
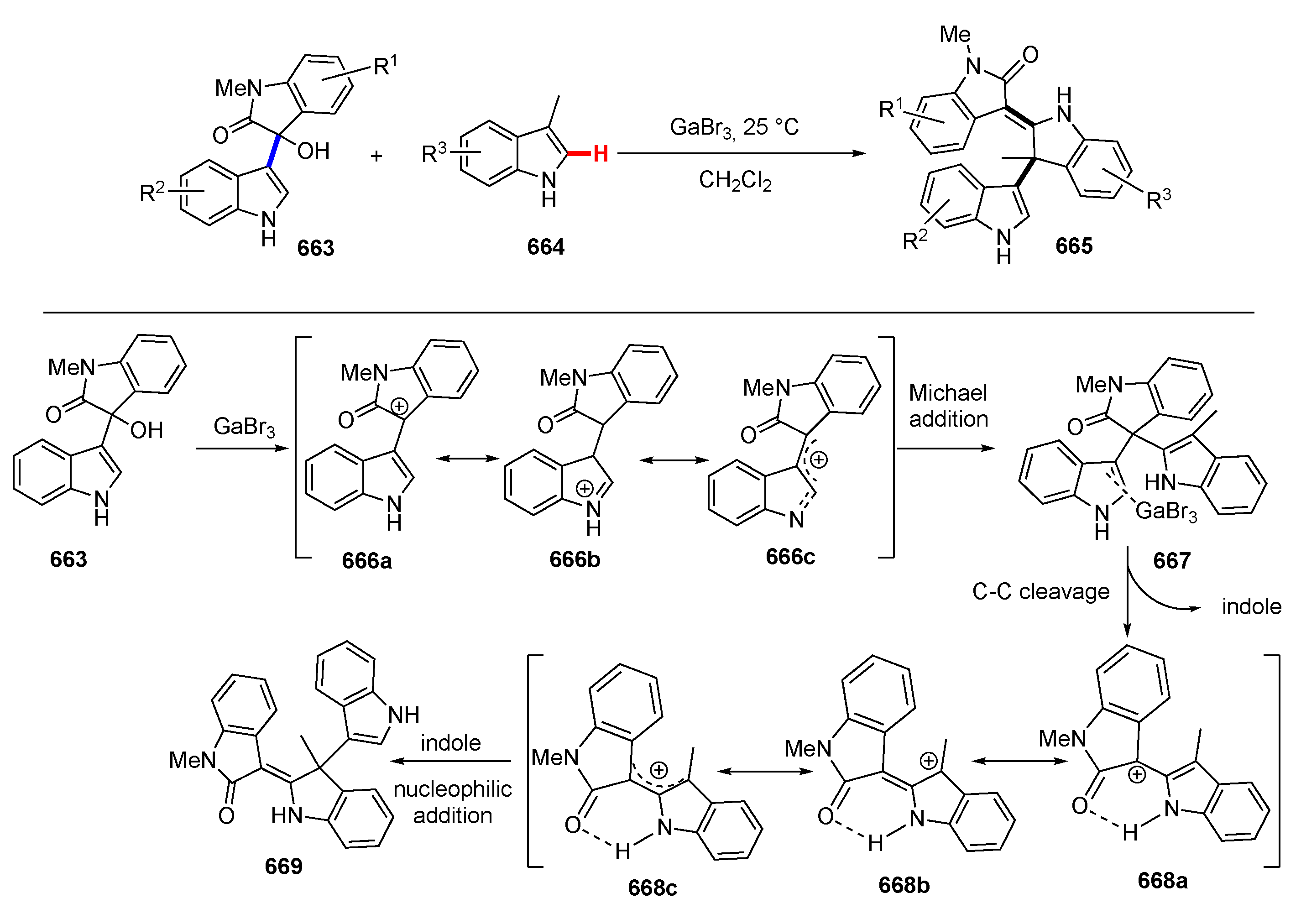
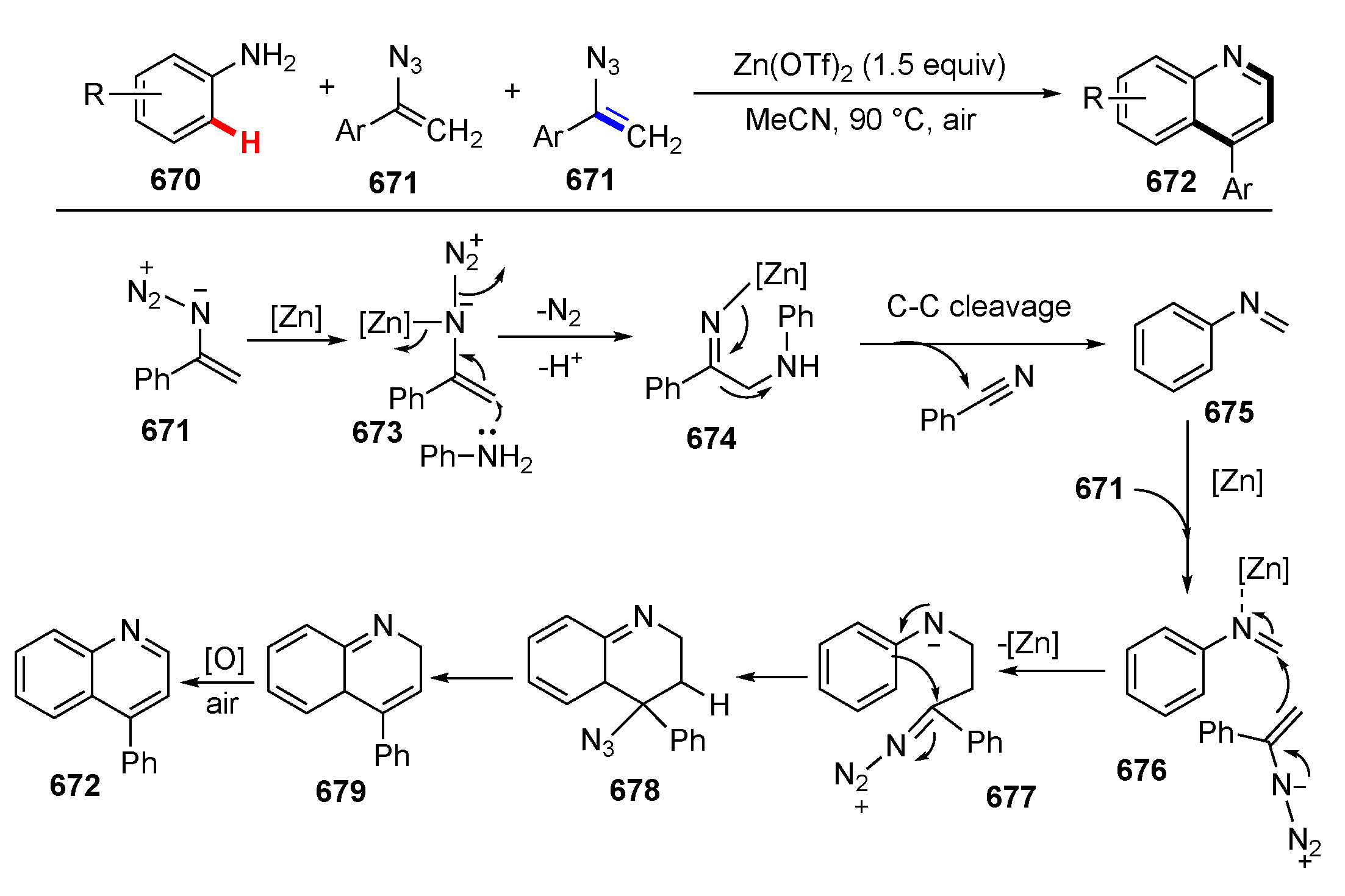
3.3. Reactions Promoted by Lewis or Brønsted Acids or Bases
4. Reactions Involving Triple Bond Scission
5. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Girard, S.A.; Knauber, T.; Li, C.J. The cross-dehydrogenative coupling of Csp3–H bonds: A versatile strategy for C–C bond formations. Angew. Chem. Int. Ed. 2014, 53, 74–100. [Google Scholar] [CrossRef] [PubMed]
- Newton, C.G.; Wang, S.G.; Oliveira, C.C.; Cramer, N. Catalytic Enantioselective Transformations Involving C–H Bond Cleavage by Transition-Metal Complexes. Chem. Rev. 2017, 117, 8908–8976. [Google Scholar] [CrossRef] [PubMed]
- Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M.F.; Wencel-Delord, J.; Besset, T.; et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.; Chung, D.S.; Bouffard, J.; Lee, S.G. Transition metal-catalyzed site- and regio-divergent C–H bond functionalization. Chem. Soc. Rev. 2017, 46, 4299–4328. [Google Scholar] [CrossRef]
- He, J.; Wasa, M.; Chan, K.S.L.; Shao, Q.; Yu, J.Q. Palladium-Catalyzed Transformations of Alkyl C–H Bonds. Chem. Rev. 2017, 117, 8754–8786. [Google Scholar] [CrossRef]
- Mihai, M.T.; Genov, G.R.; Phipps, R.J. Access to the meta position of arenes through transition metal catalysed C–H bond functionalisation: A focus on metals other than palladium. Chem. Soc. Rev. 2018, 47, 149–171. [Google Scholar] [CrossRef]
- Chu, J.C.K.; Rovis, T. Complementary Strategies for Directed C(sp3)−H Functionalization: A Comparison of Transition-Metal-Catalyzed Activation, Hydrogen Atom Transfer, and Carbene/Nitrene Transfer. Angew. Chem. Int. Ed. 2018, 57, 62–101. [Google Scholar] [CrossRef]
- Cernak, T.; Dykstra, K.D.; Tyagarajan, S.; Vachal, P.; Krska, S.W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 2016, 45, 546–576. [Google Scholar] [CrossRef]
- Ma, C.; Fang, P.; Mei, T.S. Recent Advances in C–H Functionalization Using Electrochemical Transition Metal Catalysis. ACS Catal. 2018, 8, 7179–7189. [Google Scholar] [CrossRef]
- Sauermann, N.; Meyer, T.H.; Qiu, Y.; Ackermann, L. Electrocatalytic C–H Activation. ACS Catal. 2018, 8, 7086–7103. [Google Scholar] [CrossRef]
- Dwivedi, V.; Kalsi, D.; Sundararaju, B. Electrochemical-/Photoredox Aspects of Transition Metal-Catalyzed Directed C−H Bond Activation. Chem. Cat. Chem. 2019, 11, 5160–5187. [Google Scholar] [CrossRef]
- Zhou, W.J.; Zhang, Y.H.; Gui, Y.Y.; Sun, L.; Yu, D.G. Merging Transition-Metal Catalysis with Photoredox Catalysis: An Environmentally Friendly Strategy for C–H Functionalization. Synthesis 2018, 50, 3359–3378. [Google Scholar] [CrossRef]
- Siddiqui, R.; Ali, R. Recent developments in photoredox-catalyzed remote ortho and para C–H bond functionalizations. Beilstein J. Org. Chem. 2020, 16, 248–280. [Google Scholar] [CrossRef]
- Mehta, V.P.; García-López, J.A. σ-Alkyl-PdII Species for Remote C−H Functionalization. ChemCatChem 2017, 9, 1149–1156. [Google Scholar] [CrossRef]
- Baccalini, A.; Faita, G.; Zanoni, G.; Maiti, D. Transition Metal Promoted Cascade Heterocycle Synthesis through C−H Functionalization. Chem. Eur. J. 2020, 26, 9749–9783. [Google Scholar] [CrossRef]
- Leow, D.; Li, G.; Mei, T.S.; Yu, J.Q. Activation of remote meta-C–H bonds assisted by an end-on template. Nature 2012, 486, 518–522. [Google Scholar] [CrossRef]
- Schranck, J.; Tlili, A.; Beller, M. Functionalization of remote C–H bonds: Expanding the frontier. Angew. Chem. Int. Ed. 2014, 53, 9426–9428. [Google Scholar] [CrossRef]
- Sommer, H.; Juliá-Hernández, F.; Martin, R.; Marek, I. Walking Metals for Remote Functionalization. ACS Cent. Sci. 2018, 4, 153–165. [Google Scholar] [CrossRef]
- Dey, A.; Sinha, S.K.; Achar, T.K.; Maiti, D. Accessing Remote meta- and para-C(sp2)−H Bonds with Covalently Attached Directing Groups. Angew. Chem. Int. Ed. 2019, 58, 10820–10843. [Google Scholar] [CrossRef]
- Murakami, M.; Ishida, N. Potential of Metal-Catalyzed C–C Single Bond Cleavage for Organic Synthesis. J. Am. Chem. Soc. 2016, 138, 13759–13769. [Google Scholar] [CrossRef]
- Chen, P.H.; Billett, B.A.; Tsukamoto, T.; Dong, G. Cut and Sew Transformations via Transition-Metal-Catalyzed Carbon-Carbon Bond Activation. ACS Catal. 2017, 7, 1340–1360. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Gou, T.; Wang, B.Q.; Shi, Z.J. Catalytic activations of unstrained C–C bond involving organometallic intermediates. Chem. Soc. Rev. 2018, 47, 7078–7115. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Perea, M.A.; Sarpong, R. Transition Metal-Mediated C−C Single Bond Cleavage: Making the Cut in Total Synthesis. Angew. Chem. Int. Ed. 2020, 59, 18898–18919. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Chen, J.R.; Xiao, W.J. Visible Light-Driven Radical-Mediated C–C Bond Cleavage/Functionalization in Organic Synthesis. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Shi, S.-H.; Liang, Y.; Jiao, N. Electrochemical Oxidation Induced Selective C–C Bond Cleavage. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Fumagalli, G.; Stanton, S.; Bower, J.F. Recent Methodologies That Exploit C–C Single-Bond Cleavage of Strained Ring Systems by Transition Metal Complexes. Chem. Rev. 2017, 117, 9404–9432. [Google Scholar] [CrossRef]
- Nairoukh, Z.; Cormier, M.; Marek, I. Merging C–H and C–C bond cleavage in organic synthesis. Nat. Rev. Chem. 2017, 1, 1–17. [Google Scholar] [CrossRef]
- Wei, Y.; Hu, P.; Zhang, M.; Su, W. Metal-Catalyzed Decarboxylative C–H Functionalization. Chem. Rev. 2017, 117, 8864–8907. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, N.X.; Xing, Y. Advances in Decarboxylative Oxidative Coupling Reaction. J. Org. Chem. 2018, 83, 7559–7565. [Google Scholar] [CrossRef]
- Lu, H.; Yu, T.Y.; Xu, P.F.; Wei, H. Selective Decarbonylation via Transition-Metal-Catalyzed Carbon-Carbon Bond Cleavage. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Della Ca, N.; Fontana, M.; Motti, E.; Catellani, M. Pd/Norbornene: A Winning Combination for Selective Aromatic Functionalization via C–H Bond Activation. Acc. Chem. Res. 2016, 49, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Maestri, G.; Derat, E. Alkenyl boost for Catellani. Nat. Chem. 2019, 11, 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.G.; Chen, S.; Chen, R.; Zhou, Q. Palladium(II)-Initiated Catellani-Type Reactions. Angew. Chem. Int. Ed. 2019, 58, 5832–5844. [Google Scholar] [CrossRef] [PubMed]
- Seiser, T.; Saget, T.; Tran, D.N.; Cramer, N. Cyclobutanes in catalysis. Angew. Chem. Int. Ed. 2011, 50, 7740–7752. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R. C–C Bond Cleavages of Cyclopropenes: Operating for Selective Ring-Opening Reactions. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Wang, J.; Blaszczyk, S.A.; Li, X.; Tang, W. Transition Metal-Catalyzed Selective Carbon-Carbon Bond Cleavage of Vinylcyclopropanes in Cycloaddition Reactions. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Sokolova, O.O.; Bower, J.F. Selective Carbon-Carbon Bond Cleavage of Cyclopropylamine Derivatives. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Murakami, M.; Ishida, N. Cleavage of Carbon-Carbon σ-Bonds of Four-Membered Rings. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Rubin, M.; Rubina, M.; Gevorgyan, V. Transition metal chemistry of cyclopropenes and cyclopropanes. Chem. Rev. 2007, 107, 3117–3179. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, C. Recent Advances in Ring-Opening Functionalization of Cycloalkanols by C–C σ-Bond Cleavage. Chem. Rec. 2018, 18, 587–598. [Google Scholar] [CrossRef]
- McDonald, T.R.; Mills, L.R.; West, M.S.; Rousseaux, S.A.L. Selective Carbon–Carbon Bond Cleavage of Cyclopropanols. Chem. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Beaumier, E.P.; Pearce, A.J.; See, X.Y.; Tonks, I.A. Modern applications of low-valent early transition metals in synthesis and catalysis. Nat. Rev. Chem. 2019, 3, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Qiu, D.; Wang, J. Transition-Metal-Catalyzed Cross-Couplings through Carbene Migratory Insertion. Chem. Rev. 2017, 117, 13810–13889. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhi, C.L.; Lu, P.P.; Liu, S.; Zhu, X.; Hao, X.Q.; Song, M.P. Rhodium(III)-catalyzed direct C7 allylation of indolines via 4 sequential C–H and C–C activation. Adv. Synth. Catal. 2019, 361, 1253–1258. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, L.; Liu, S.; Zhi, C.; Fu, L.R.; Zhu, X.; Hao, X.Q.; Song, M.P. Solvent-free and room temperature microwave-assisted direct C7 allylation of indolines: Via sequential C–H and C–C activation. RSC Adv. 2020, 10, 10883–10887. [Google Scholar] [CrossRef]
- Hu, Z.; Hu, X.Q.; Zhang, G.; Gooßen, L.J. Ring-Opening Ortho-C-H Allylation of Benzoic Acids with Vinylcyclopropanes: Merging Catalytic C–H and C–C Activation Concepts. Org. Lett. 2019, 21, 6770–6773. [Google Scholar] [CrossRef]
- Gandeepan, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. 3d Transition Metals for C–H Activation. Chem. Rev. 2019, 119, 2192–2452. [Google Scholar] [CrossRef]
- Zell, D.; Bu, Q.; Feldt, M.; Ackermann, L. Mild C−H/C−C Activation by Z-Selective Cobalt Catalysis. Angew. Chem. Int. Ed. 2016, 55, 7408–7412. [Google Scholar] [CrossRef]
- Lu, Q.; Klauck, F.J.R.; Glorius, F. Manganese-catalyzed allylation: Via sequential C–H and C–C/C–Het bond activation. Chem. Sci. 2017, 8, 3379–3383. [Google Scholar] [CrossRef]
- Meyer, T.H.; Liu, W.; Feldt, M.; Wuttke, A.; Mata, R.A.; Ackermann, L. Manganese(I)-Catalyzed Dispersion-Enabled C−H/C−C Activation. Chem. Eur. J. 2017, 23, 5443–5447. [Google Scholar] [CrossRef]
- Liang, Y.F.; Müller, V.; Liu, W.; Münch, A.; Stalke, D.; Ackermann, L. Methylenecyclopropane Annulation by Manganese(I)-Catalyzed Stereoselective C−H/C−C Activation. Angew. Chem. Int. Ed. 2017, 56, 9415–9419. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kwong, F.Y. Cobalt-Catalyzed Tandem C−H Activation/C−C Cleavage/C−H Cyclization of Aromatic Amides with Alkylidenecyclopropanes. Angew. Chem. Int. Ed. 2018, 57, 6512–6516. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, D.; He, X.; Wang, Y.; Tang, Y.; Zhang, J.; Xu, S. Redox-Neutral Annulation of Alkynylcyclopropanes with N-Aryloxyamides via Rhodium(III)-Catalyzed Sequential C−H/C−C Activation. J. Org. Chem. 2019, 84, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.A.M.A.; Suliman, A.M.Y.; Gong, T.J.; Fu, Y. Access to Divergent Fluorinated Enynes and Arenes via Palladium-Catalyzed Ring-Opening Alkynylation of gem-Difluorinated Cyclopropanes. Org. Lett. 2020, 22, 1414–1419. [Google Scholar] [CrossRef]
- Audic, B.; Cramer, N. Rhodium(III)-Catalyzed Cyclopropane C–H/C–C Activation Sequence Provides Diastereoselective Access to α-Alkoxylated γ-Lactams. Org. Lett. 2020, 22, 5030–5034. [Google Scholar] [CrossRef]
- Masarwa, A.; Didier, D.; Zabrodski, T.; Schinkel, M.; Ackermann, L.; Marek, I. Merging allylic carbon–hydrogen and selective carbon–carbon bond activation. Nature 2014, 505, 199–203. [Google Scholar] [CrossRef]
- Vasseur, A.; Marek, I. Merging allylic C–H bond activation and C–C bond cleavage en route to the formation of a quaternary carbon stereocenter in acyclic systems. Nat. Protoc. 2017, 12, 74–87. [Google Scholar] [CrossRef]
- Oskar, L.; Perrin, L.; Eisenstein, O.; Marek, I. Zirconocene-Mediated Selective C−C Bond Cleavage of Strained Carbocycles: Scope and Mechanism. J. Org. Chem. 2018, 83, 3497–3515. [Google Scholar] [CrossRef]
- Nakano, T.; Endo, K.; Ukaji, Y. Silver-Catalyzed Allylation of Ketones and Intramolecular Cyclization through Carbene Intermediates from Cyclopropenes under Ambient Conditions. Chem. Asian J. 2016, 11, 713–721. [Google Scholar] [CrossRef]
- Drew, M.A.; Arndt, S.; Richardson, C.; Rudolph, M.; Hashmi, A.S.K.; Hyland, C.J.T. Divergent gold-catalysed reactions of cyclopropenylmethyl sulfonamides with tethered heteroaromatics. Chem. Commun. 2019, 55, 13971–13974. [Google Scholar] [CrossRef]
- Wang, X.; Lerchen, A.; Daniliuc, C.G.; Glorius, F. Efficient Synthesis of Arylated Furans by a Sequential Rh-Catalyzed Arylation and Cycloisomerization of Cyclopropenes. Angew. Chem. Int. Ed. 2018, 57, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, Y.; Su, K.; Wang, P.; Guo, X.; Chen, B. Rhodium(III)-catalyzed [3 + 3] annulation reactions of: N-nitrosoanilines and cyclopropenones: An approach to functionalized 4-quinolones. Org. Chem. Front. 2019, 6, 3973–3977. [Google Scholar] [CrossRef]
- Li, H.S.; Lu, S.C.; Chang, Z.X.; Hao, L.; Li, F.R.; Xia, C. Rhodium-Catalyzed Ring-Opening Hydroacylation of Alkylidenecyclopropanes with Chelating Aldehydes for the Synthesis of γ,δ-Unsaturated Ketones. Org. Lett. 2020, 22, 5145–5150. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shi, P.; Liu, R.H.; Hu, X.H.; Loh, T.P. Cobalt-Catalyzed N–O and C–C Bond Cleavage in 1,2-Oxazetidines: Solvent-Controlled C–H Aminomethylation and Hydroxymethylation of Heteroarenes. Org. Lett. 2019, 21, 1602–1606. [Google Scholar] [CrossRef]
- Sun, F.N.; Yang, W.C.; Chen, X.B.; Sun, Y.L.; Cao, J.; Xu, Z.; Xu, L.W. Enantioselective palladium/copper-catalyzed C-C σ-bond activation synergized with Sonogashira-type C(sp3)–C(sp) cross-coupling alkynylation. Chem. Sci. 2019, 10, 7579–7583. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, T.; Bai, J.; Ye, D.; Xu, P.; Wei, H. Divergent Coupling of Benzocyclobutenones with Indoles via C–H and C–C Activations. Angew. Chem. Int. Ed. 2020, 59. [Google Scholar] [CrossRef]
- Zhou, X.; Qi, Z.; Yu, S.; Kong, L.; Li, Y.; Tian, W.F.; Li, X. Synthesis of 2-Substituted Quinolines via Rhodium(III)-Catalyzed C–H Activation of Imidamides and Coupling with Cyclopropanols. Adv. Synth. Catal. 2017, 359, 1620–1625. [Google Scholar] [CrossRef]
- Meng, R.; Bi, S.; Jiang, Y.Y.; Liu, Y. C–H activation versus ring opening and inner-versus outer-sphere concerted metalation-deprotonation in Rh(III)-catalyzed oxidative coupling of oxime ether and cyclopropanol: A density functional theory study. J. Org. Chem. 2019, 84, 11150–11160. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, S.; Kong, L.; Li, X. Rhodium(III)-Catalyzed Coupling of Arenes with Cyclopropanols via C–H Activation and Ring Opening. ACS Catal. 2016, 6, 647–651. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, S.; Qi, Z.; Kong, L.; Li, X. Rhodium(III)-Catalyzed Mild Alkylation of (Hetero)Arenes with Cyclopropanols via C–H Activation and Ring Opening. J. Org. Chem. 2016, 81, 4869–4875. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, G. Expedient Synthesis of 1,5-Diketones by Rhodium-Catalyzed Hydroacylation Enabled by C–C Bond Cleavage. J. Am. Chem. Soc. 2017, 139, 12891–12894. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Gu, Z. 1,4-Migration of rhodium and palladium in catalytic organometallic reactions. Angew. Chem. Int. Ed. 2005, 44, 7512–7517. [Google Scholar] [CrossRef] [PubMed]
- Dhungana, R.K.; Sapkota, R.R.; Niroula, D.; Giri, R. Walking metals: Catalytic difunctionalization of alkenes at nonclassical sites. Chem. Sci. 2020, 11, 9757–9774. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, R.; Lou, J.; Zhang, D.H.; Zhou, Y.G.; Yu, Z. Highly Regioselective C–H Alkylation of Alkenes through an Aryl to Vinyl 1,4-Palladium Migration/C–C Cleavage Cascade. ACS Catal. 2019, 9, 11669–11675. [Google Scholar] [CrossRef]
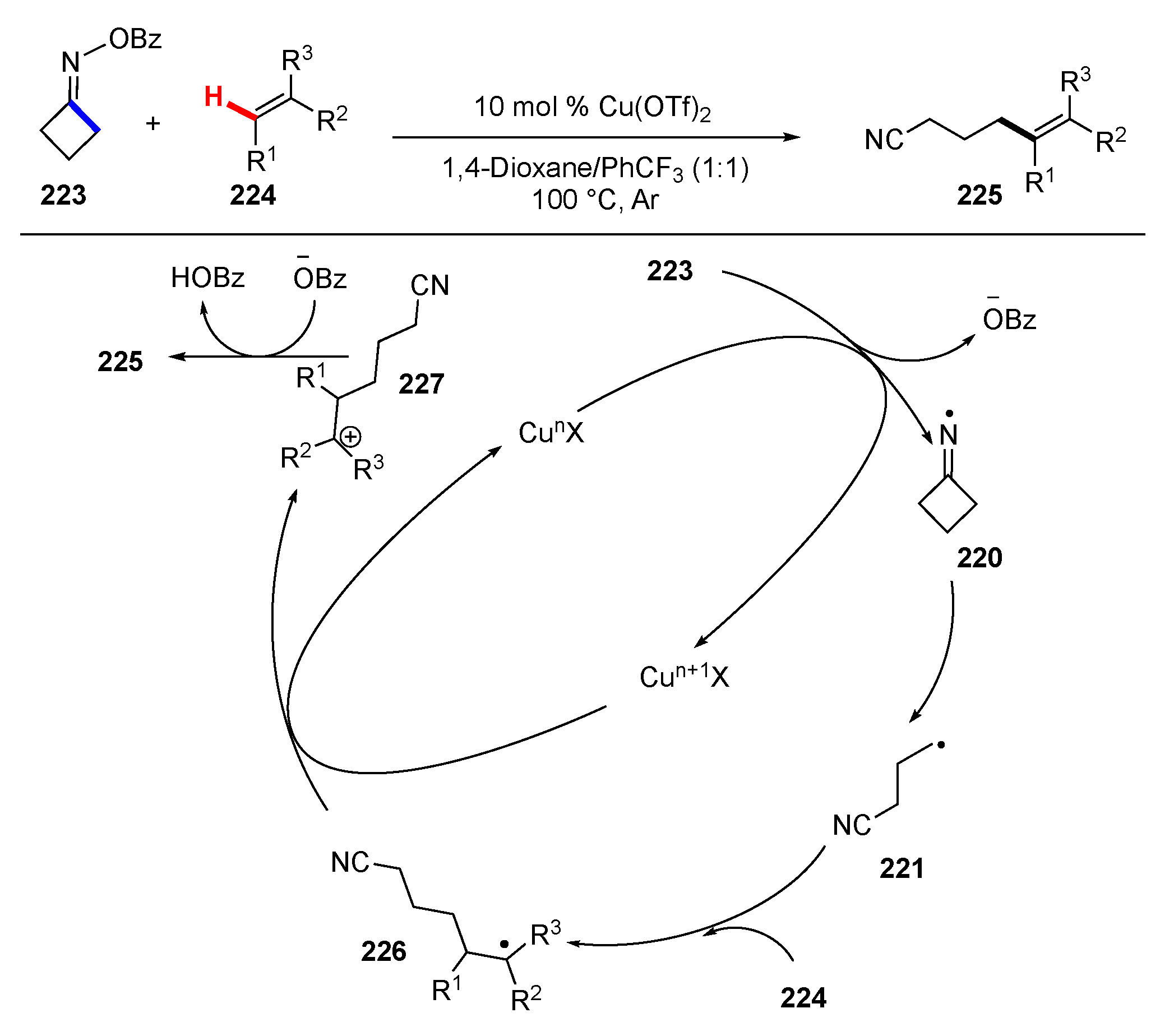
- Xiao, T.; Huang, H.; Anand, D.; Zhou, L. Iminyl-Radical-Triggered C–C Bond Cleavage of Cycloketone Oxime Derivatives: Generation of Distal Cyano-Substituted Alkyl Radicals and Their Functionalization. Synthesis 2020, 52, 1585–1601. [Google Scholar] [CrossRef]
- Xiao, W.; Wu, J. Recent advances for the photoinduced C–C bond cleavage of cycloketone oximes. Chin. Chem. Lett. 2020. [Google Scholar] [CrossRef]
- Nishimura, T.; Uemura, S. Palladium-Catalyzed Arylation of tert-Cyclobutanols with Aryl Bromide via C−C Bond Cleavage: New Approach for the γ-Arylated Ketones. J. Am. Chem. Soc. 1999, 121, 11010–11011. [Google Scholar] [CrossRef]
- Roque, J.B.; Kuroda, Y.; Jurczyk, J.; Xu, L.P.; Ham, J.S.; Göttemann, L.T.; Roberts, C.A.; Adpressa, D.; Saurí, J.; Joyce, L.A.; et al. C-C Cleavage Approach to C-H Functionalization of Saturated Aza-Cycles. ACS Catal. 2020, 10, 2929–2941. [Google Scholar] [CrossRef]
- Ham, J.S.; Park, B.; Son, M.; Roque, J.B.; Jurczyk, J.; Yeung, C.S.; Baik, M.H.; Sarpong, R. C–H/C–C Functionalization Approach to N-Fused Heterocycles from Saturated Azacycles. J. Am. Chem. Soc. 2020, 142, 13041–13050. [Google Scholar] [CrossRef]
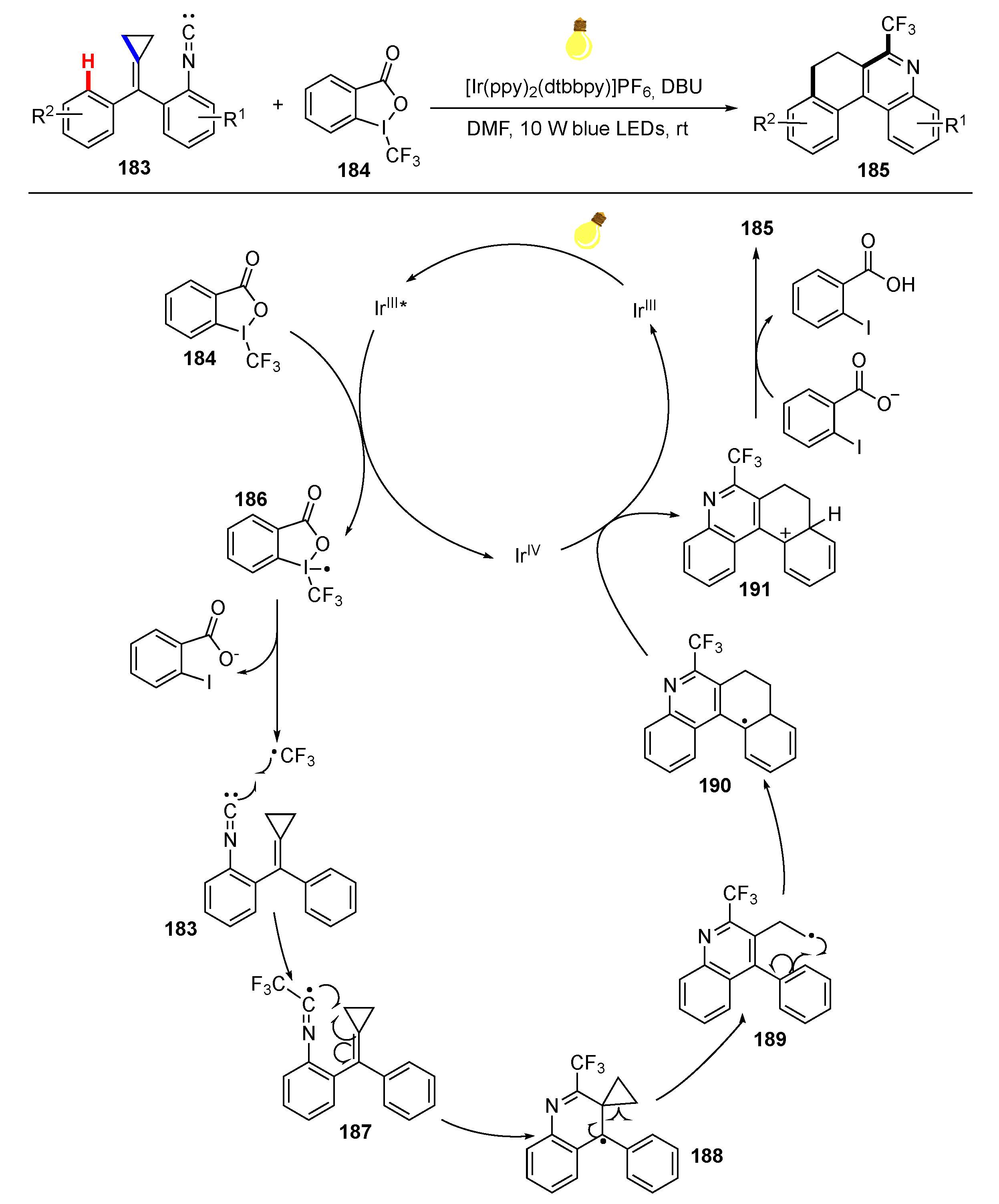
- Yuan, Y.C.; Liu, H.L.; Hu, X.B.; Wei, Y.; Shi, M. Visible-Light-Induced Trifluoromethylation of Isonitrile-Substituted Methylenecyclopropanes: Facile Access to 6-(Trifluoromethyl)-7,8-Dihydrobenzo[k]phenanthridine Derivatives. Chem. Eur. J. 2016, 22, 13059–13063. [Google Scholar] [CrossRef]
- Dange, N.S.; Hussain Jatoi, A.; Robert, F.; Landais, Y. Visible-Light-Mediated Addition of Phenacyl Bromides onto Cyclopropenes. Org. Lett. 2017, 19, 3652–3655. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Studer, A. Chemistry With N-Centered Radicals Generated by Single-Electron Transfer-Oxidation Using Photoredox Catalysis. CCS Chem. 2019, 38–49. [Google Scholar] [CrossRef]
- Xiong, P.; Xu, H.-C. Chemistry with Electrochemically Generated N-Centered Radicals. Acc. Chem. Res. 2019, 52, 3339–3350. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Chen, J.R.; Wei, Q.; Liu, F.L.; Deng, Q.H.; Beauchemin, A.M.; Xiao, W.J. Photocatalytic Generation of N-Centered Hydrazonyl Radicals: A Strategy for Hydroamination of β,γ-Unsaturated Hydrazones. Angew. Chem. Int. Ed. 2014, 53, 12163–12167. [Google Scholar] [CrossRef]
- Yu, X.Y.; Zhao, Q.Q.; Chen, J.; Xiao, W.J.; Chen, J.R. When Light Meets Nitrogen-Centered Radicals: From Reagents to Catalysts. Acc. Chem. Res. 2020, 53, 1066–1083. [Google Scholar] [CrossRef]
- Proctor, R.S.J.; Phipps, R.J. Recent Advances in Minisci-Type Reactions. Angew. Chem. Int. Ed. 2019, 58, 13666–13699. [Google Scholar] [CrossRef]
- Gu, Y.R.; Duan, X.H.; Yang, L.; Guo, L.N. Direct C–H Cyanoalkylation of Heteroaromatic N-Oxides and Quinones via C–C Bond Cleavage of Cyclobutanone Oximes. Org. Lett. 2017, 19, 5908–5911. [Google Scholar] [CrossRef]
- Yang, L.; Gao, P.; Duan, X.H.; Gu, Y.R.; Guo, L.N. Direct C–H Cyanoalkylation of Quinoxalin-2(1H)-ones via Radical C–C Bond Cleavage. Org. Lett. 2018, 20, 1034–1037. [Google Scholar] [CrossRef]
- Gao, P.; Cheng, Y.B.; Yang, F.; Guo, L.N.; Duan, X.H. Iron(II)-catalyzed direct C–H cyanoalkylation of 2H-indazoles and coumarins via radical C–C bond cleavage. Tetrahedron Lett. 2019, 60, 150967–150970. [Google Scholar] [CrossRef]
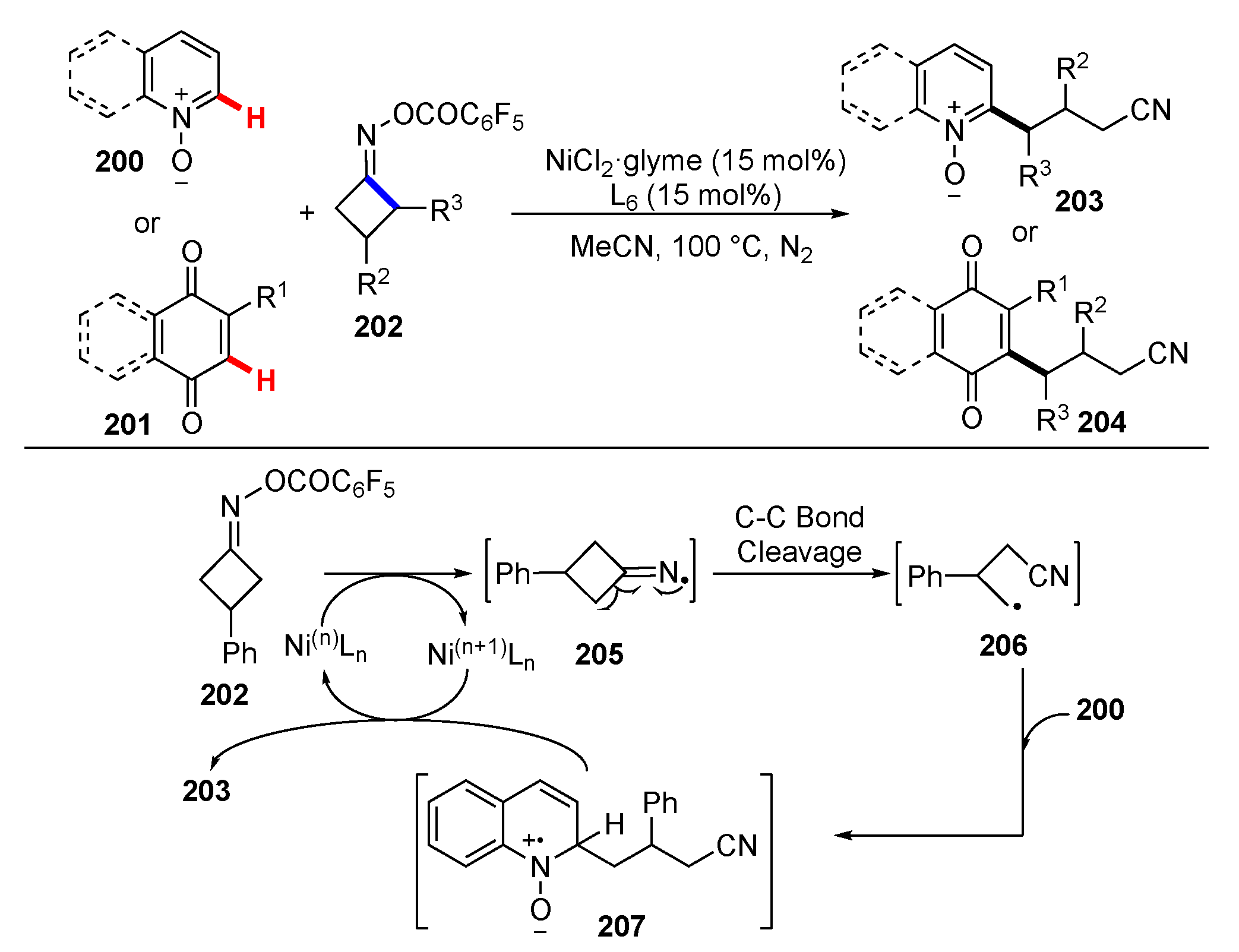
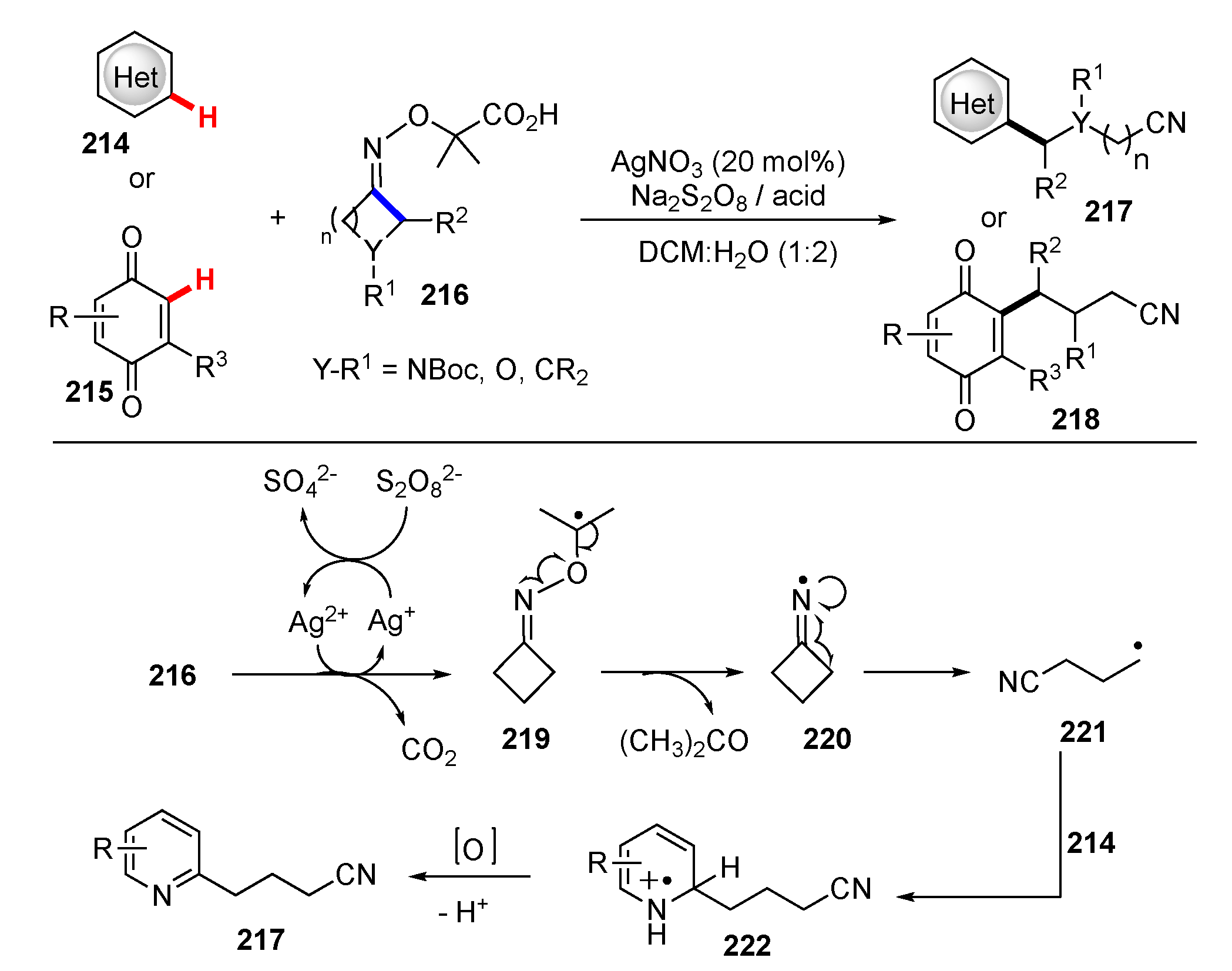
- Li, X.; Yan, X.; Wang, Z.; He, X.; Dai, Y.; Yan, X.; Zhao, D.; Xu, X. Complementary oxidative generation of iminyl radicals from α-imino-oxy acids: Silver-catalyzed C–H cyanoalkylation of heterocycles and quinones. J. Org. Chem. 2020, 85, 2504–2511. [Google Scholar] [CrossRef]
- Zhao, B.; Shi, Z. Copper-Catalyzed Intermolecular Heck-Like Coupling of Cyclobutanone Oximes Initiated by Selective C−C Bond Cleavage. Angew. Chem. Int. Ed. 2017, 56, 12727–12731. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.X.; Teng, F.; Xiang, J.N.; Deng, W.; Li, J.H. One-Carbon Incorporation Using Cyclobutanone Oxime Ester Enabled [2 + 2 + 1] Carboannulation of 1,7-Enynes by C–C/N–O Bond Cleavage and C–H Functionalization. Org. Lett. 2019, 21, 9434–9437. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, J.; Gao, P.; Xu, S.; Guo, L. Copper-Catalyzed Redox-Neutral Cyanoalkylarylation of Activated Alkenes with Cyclobutanone Oxime Esters. J. Org. Chem. 2018, 83, 1046–1055. [Google Scholar] [CrossRef]
- Davies, J.; Booth, S.G.; Essafi, S.; Dryfe, R.A.W.; Leonori, D. Visible-Light-Mediated Generation of Nitrogen-Centered Radicals: Metal-Free Hydroimination and Iminohydroxylation Cyclization Reactions. Angew. Chem. Int. Ed. 2015, 54, 14017–14021. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; An, X.; Tong, K.; Zheng, T.; Zhang, Y.; Yu, S. Visible-Light-Promoted Iminyl-Radical Formation from Acyl Oximes: A Unified Approach to Pyridines, Quinolines, and Phenanthridines. Angew. Chem. Int. Ed. 2015, 54, 4055–4059. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Studer, A. Iminyl-Radicals by Oxidation of α-Imino-oxy Acids: Photoredox-Neutral Alkene Carboimination for the Synthesis of Pyrrolines. Angew. Chem. Int. Ed. 2017, 56, 12273–12276. [Google Scholar] [CrossRef]
- Chen, J.R.; Hu, X.Q.; Lu, L.Q.; Xiao, W.J. Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev. 2016, 45, 2044–2056. [Google Scholar] [CrossRef]
- Jian, Y.; Chen, M.; Yang, C.; Xia, W.-J. Minisci-Type C–H Cyanoalkylation of Heteroarenes Through N–O/C–C Bonds Cleavage. Eur. J. Org. Chem. 2020, 2020, 1439–1442. [Google Scholar] [CrossRef]
- Yu, X.; Chen, J.; Wang, P.; Yang, M.; Liang, D.; Xiao, W. Photoredox Catalysis A Visible-Light-Driven Iminyl Radical-Mediated C–C Single Bond Cleavage/Radical Addition Cascade of Oxime Esters. Angew. Chem. Int. Ed. 2018, 57, 738–743. [Google Scholar] [CrossRef]
- Yuan, Y.; Dong, W.H.; Gao, X.S.; Xie, X.M.; Zhang, Z.G. Visible-light-induced radical cascade cyclization of oxime esters and aryl isonitriles: Synthesis of cyclopenta[b]quinoxalines. Chem. Commun. 2019, 55, 11900–11903. [Google Scholar] [CrossRef]
- Wang, P.Z.; Yu, X.Y.; Li, C.Y.; He, B.Q.; Chen, J.R.; Xiao, W.J. A photocatalytic iminyl radical-mediated C–C bond cleavage/addition/cyclization cascade for the synthesis of 1,2,3,4-tetrahydrophenanthrenes. Chem. Commun. 2018, 54, 9925–9928. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chen, K.; Yu, L.; Tang, X.; Shi, M. Copper(I)-Catalyzed Intramolecular Trifluoromethylation of Methylenecyclopropanes. Org. Lett. 2015, 17, 5994–5997. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.T.; Tang, X.Y.; Shi, M. A facile approach for the trifluoromethylthiolation of methylenecyclopropanes. Org. Chem. Front. 2017, 4, 86–90. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Zhou, C.; Xiong, B.; Zhang, P.; Yang, C. Metal-Free Oxidative C−C Bond Functionalization of Methylenecyclopropanes with Ethers Leading to 2-Substituted 3,4-Dihydronaphthalenes. J. Org. Chem. 2017, 82, 7394–7401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Q.L.; Chen, Z.; Zhou, Q.; Li, H.; Xu, W.Y.; Xiong, B.Q.; Tang, K.W. Oxone-Mediated Radical C–C Bond Acetmethylation/Arylation of Methylenecyclopropanes and Vinylcyclopropanes with α-Alkyl Ketones: Facile Access to Oxoalkyl-Substituted 3,4-Dihydronaphthalenes. J. Org. Chem. 2019, 84, 5413–5424. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Q.; Zhou, C.; Xiong, B.; Zhang, P.; Yang, C.; Tang, K. Oxidative C−C Bond Functionalization of Methylenecyclopropanes with Aldehydes for the Formation of 2-Acyl-3,4-dihydronaphthalenes. J. Org. Chem. 2018, 83, 4657–4664. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Chen, Z.; Zhou, Q.; Zhou, C.; Xiong, B.; Zhang, P.; Yang, C.; Tang, K. Biomolecular Chemistry sodium sulfinates: Facile access to 3-sulfonyl-1,2-dihydronaphthalenes. Org. Biomol. Chem. 2019, 17, 1365–1369. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.L.; Chen, Z.; Zhou, Q.; Li, H.; Zhou, C.S.; Xiong, B.Q.; Zhang, P.L.; Tang, K.W. Visible-Light-Catalyzed C–C Bond Difunctionalization of Methylenecyclopropanes with Sulfonyl Chlorides for the Synthesis of 3-Sulfonyl-1,2-dihydronaphthalenes. J. Org. Chem. 2019, 84, 2829–2839. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.L.; Chen, Z.; Li, H.; Xiong, B.Q.; Zhang, P.L.; Tang, K.W. Visible-light photoredox-catalyzed dual C–C bond cleavage: Synthesis of 2-cyanoalkylsulfonylated 3,4-dihydronaphthalenes through the insertion of sulfur dioxide. Chem. Commun. 2020, 56, 3011–3014. [Google Scholar] [CrossRef]
- Shao, L.-X.; Shi, M. Lewis and Bronsted Acid Mediated Ring-Opening Reactions of Methylenecyclopropanes and Further Transformation of the Ring-Opened Products. Curr. Org. Chem. 2007, 11, 1135–1153. [Google Scholar] [CrossRef]
- Rakhmankulov, E.R.; Ivanov, K.L.; Budynina, E.M.; Ivanova, O.A.; Chagarovskiy, A.O.; Skvortsov, D.A.; Latyshev, G.V.; Trushkov, I.V.; Melnikov, M.Y. Lewis and Brønsted acid induced (3 + 2)-annulation of donor-acceptor cyclopropanes to alkynes: Indene assembly. Org. Lett. 2015, 17, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Varshnaya, R.K.; Banerjee, P. Lewis Acid-Catalyzed [3 + 3] Annulation of Donor−Acceptor Cyclopropanes and Indonyl Alcohols: One Step Synthesis of Substituted Carbazoles with Promising Photophysical Properties. J. Org. Chem. 2019, 84, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yan, W.; Wang, L.; Zhang, X.P.; Tang, Y. One-Pot Catalytic Asymmetric Synthesis of Tetrahydrocarbazoles. Org. Lett. 2015, 17, 4014–4017. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.L.; Tang, X.Y.; Shi, M. Intramolecular cyclizations of cyclopropenes with indole. Chem. Commun. 2016, 52, 7245–7248. [Google Scholar] [CrossRef]
- Cao, Z.; Zhu, J.B.; Wang, L.; Liao, S.; Tang, Y. A Synthesis of Multifunctionalized Indoles from [3 + 2] Annulation of 2-Bromocyclopropenes with Anilines. Org. Lett. 2019, 21, 4097–4100. [Google Scholar] [CrossRef]
- Gerosa, G.G.; Schwengers, S.A.; Maji, R.; De, C.K.; List, B. Homologation of the Fischer Indolization: A Quinoline Synthesis via Homo-Diaza-Cope Rearrangement. Angew. Chem. Int. Ed. 2020, 59, 20485–20488. [Google Scholar] [CrossRef]
- He, T.; Wang, G.; Long, P.-W.; Kemper, S.; Irran, E.; Klare, H.F.T.; Oestreich, M. Intramolecular Friedel–Crafts alkylation with a silylium-ion-activated cyclopropyl group: Formation of tricyclic ring systems from benzyl-substituted vinylcyclopropanes and hydrosilanes. Chem. Sci. 2020. [Google Scholar] [CrossRef]
- Lutz, M.D.R.; Morandi, B. Metal-Catalyzed Carbon-Carbon Bond Cleavage of Unstrained Alcohols. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Nogi, K.; Yorimitsu, H. Carbon-Carbon Bond Cleavage at Allylic Positions: Retro-allylation and Deallylation. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Xia, Y.; Dong, G. Temporary or removable directing groups enable activation of unstrained C–C bonds. Nat. Rev. Chem. 2020, 4, 600–614. [Google Scholar] [CrossRef]
- Iwasaki, M.; Araki, Y.; Nishihara, Y. Phenanthrene Synthesis by Palladium-Catalyzed Benzannulation with o-Bromobenzyl Alcohols through Multiple Carbon-Carbon Bond Formations. J. Org. Chem. 2017, 82, 6242–6258. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.Y.; Zheng, Z.J.; Sun, W.; Qiao, Z.H. Direct C2-Heteroarylation of Indoles by Rhodium-Catalyzed C−C Bond Cleavage of Secondary Alcohols. Asian J. Org. Chem. 2019, 8, 466–469. [Google Scholar] [CrossRef]
- Qiu, Y.; Scheremetjew, A.; Ackermann, L. Electro-Oxidative C–C Alkenylation by Rhodium(III) Catalysis. J. Am. Chem. Soc. 2019, 141, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Caspers, L.D.; Nachtsheim, B.J. Directing-Group-mediated C−H-Alkynylations. Chem. Asian J. 2018, 13, 1231–1247. [Google Scholar] [CrossRef] [PubMed]
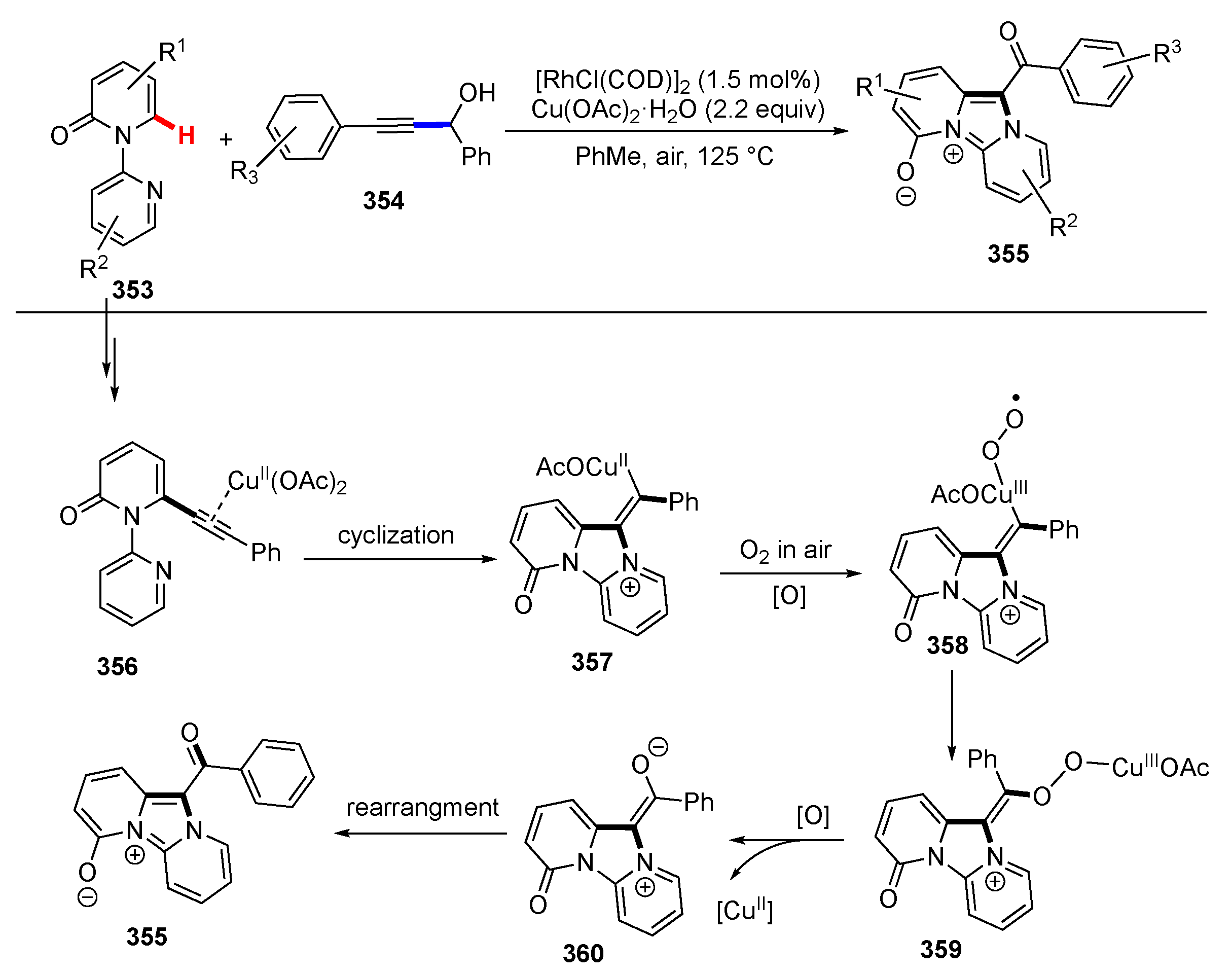
- Li, T.; Wang, Z.; Zhang, M.; Zhang, H.J.; Wen, T. Bin Rh/Cu-catalyzed multiple C–H, C–C, and C–N bond cleavage: Facile synthesis of pyrido[2,1-a]indoles from 1-(pyridin-2-yl)-1H-indoles and γ-substituted propargyl alcohols. Chem. Commun. 2015, 51, 6777–6780. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Z.; Qin, W.B.; Wen, T. Bin Rhodium-Catalyzed/Copper-Mediated Selective C2 Alkynylation of Indoles and C1 Alkynylation of Carbazoles with γ-Substituted tert-Propargyl Alcohols. ChemCatChem 2016, 8, 2146–2154. [Google Scholar] [CrossRef]
- Li, T.; Wang, Z.; Xu, K.; Liu, W.; Zhang, X.; Mao, W.; Guo, Y.; Ge, X.; Pan, F. Rhodium-Catalyzed/Copper-Mediated Tandem C(sp2)-H Alkynylation and Annulation: Synthesis of 11-Acylated Imidazo[1,2-a:3,4-a’]dipyridin-5-ium-4-olates from 2H-[1,2′-Bipyridin]-2-ones and Propargyl Alcohols. Org. Lett. 2016, 18, 1064–1067. [Google Scholar] [CrossRef]
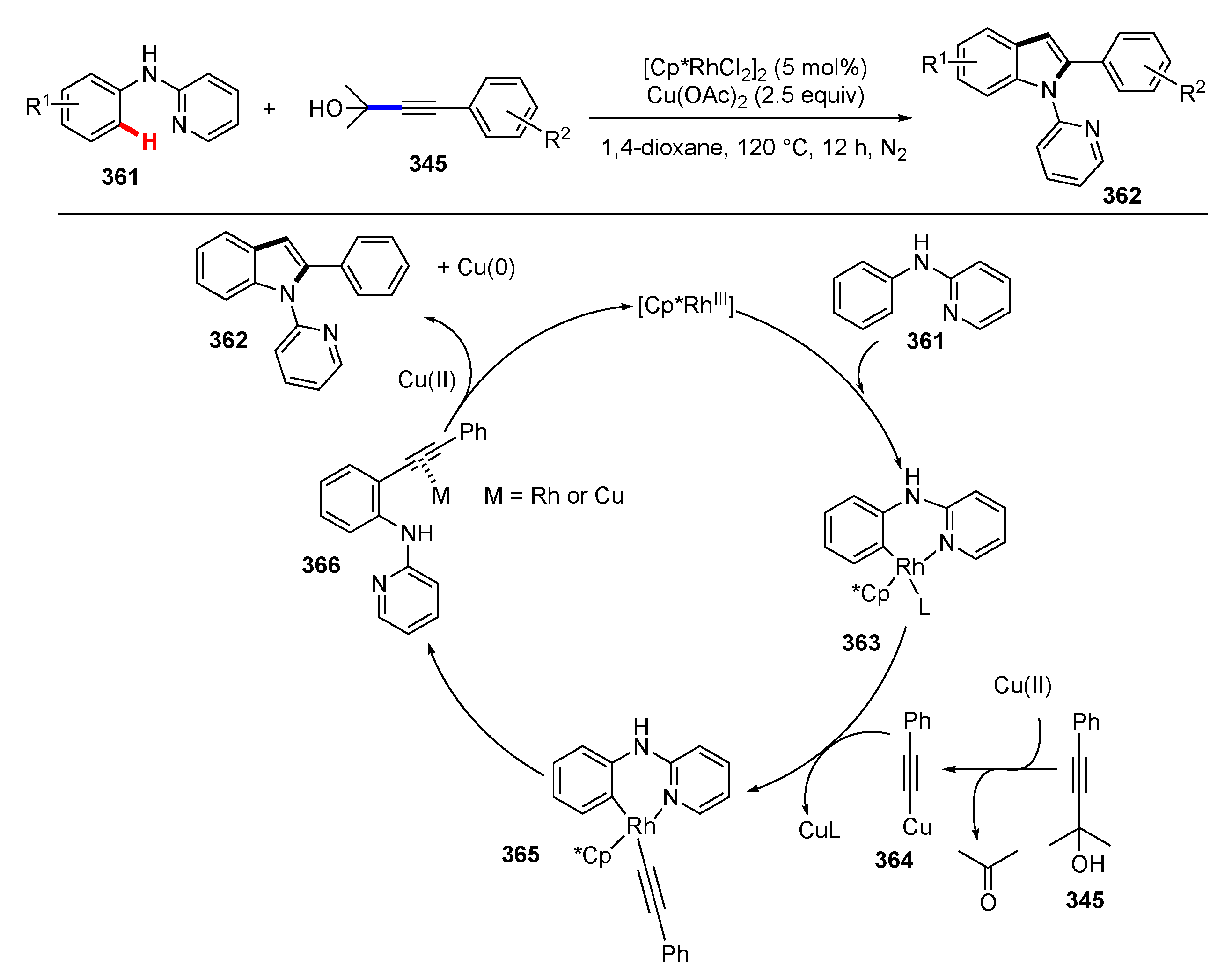
- Yan, X.; Ye, R.; Sun, H.; Zhong, J.; Xiang, H.; Zhou, X. Synthesis of 2-Arylindoles by Rhodium-Catalyzed/Copper-Mediated Annulative Coupling of N-Aryl-2-aminopyridines and Propargyl Alcohols via Selective C–H/C–C Activation. Org. Lett. 2019, 21, 7455–7459. [Google Scholar] [CrossRef]
- He, S.; Yan, X.; Lei, Y.; Xiang, H.; Zhou, X. Rhodium-catalyzed annulative coupling of N-aryl-2-aminopyridine and propargylic amine via selective C–C and C–H bond activation. Chem. Commun. 2020, 56, 2284–2287. [Google Scholar] [CrossRef]
- Nakao, Y. Metal-mediated C–CN Bond Activation in Organic Synthesis. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Hanson, M.G.; Olson, N.M.; Yi, Z.; Wilson, G.; Kalyani, D. Nickel-Catalyzed Coupling of Azoles with Aromatic Nitriles. Org. Lett. 2017, 19, 4271–4274. [Google Scholar] [CrossRef] [PubMed]
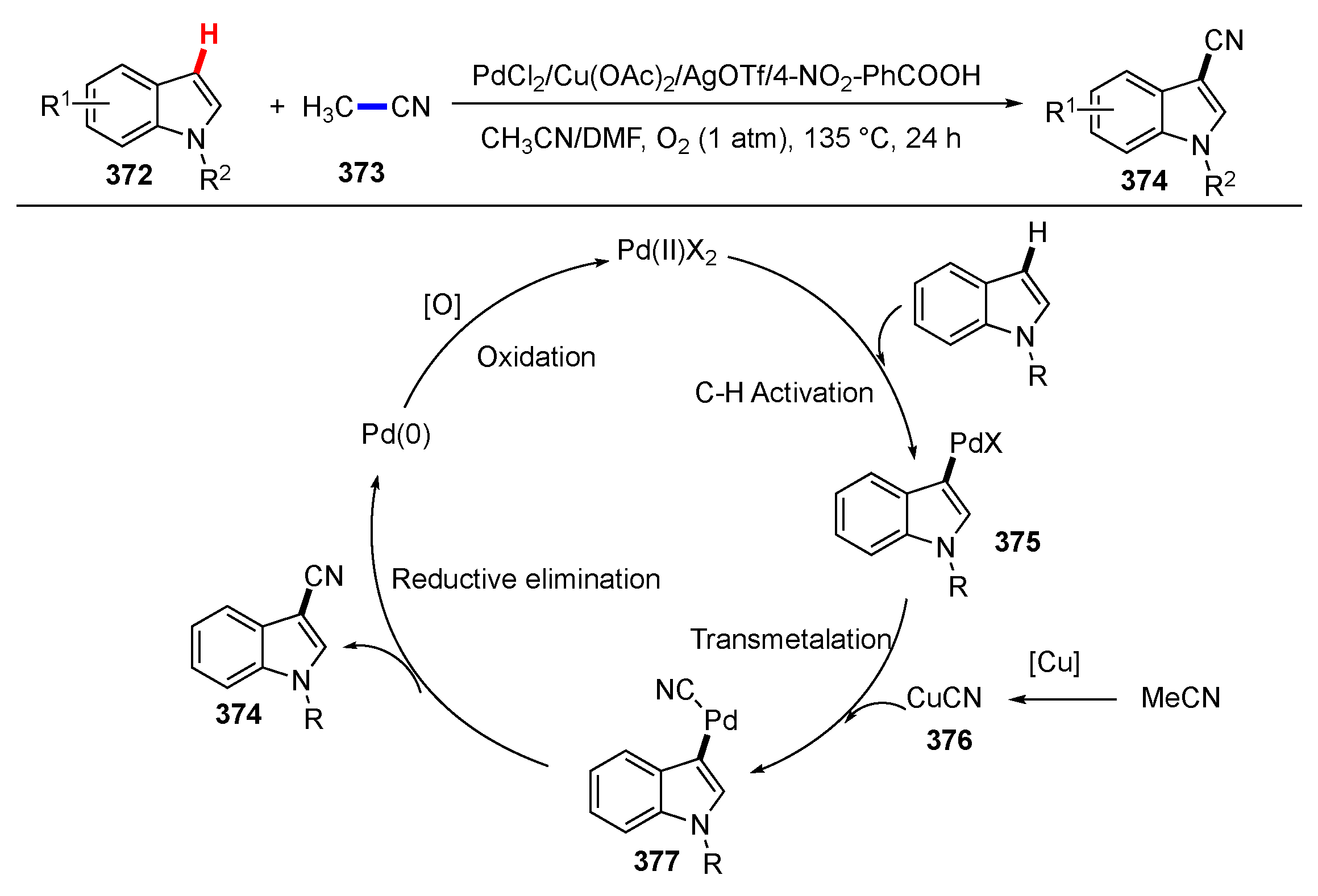
- Liu, B.; Liu, M.; Li, Q.; Li, Y.; Feng, K.; Zhou, Y. The palladium-catalyzed direct C3-cyanation of indoles using acetonitrile as the cyanide source. Org. Biomol. Chem. 2020, 18, 6108–6114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, W.; Shen, Z. Cu-Catalyzed Cyanation of Indoles with Acetonitrile as a Cyano Source. J. Org. Chem. 2015, 80, 8868–8873. [Google Scholar] [CrossRef] [PubMed]
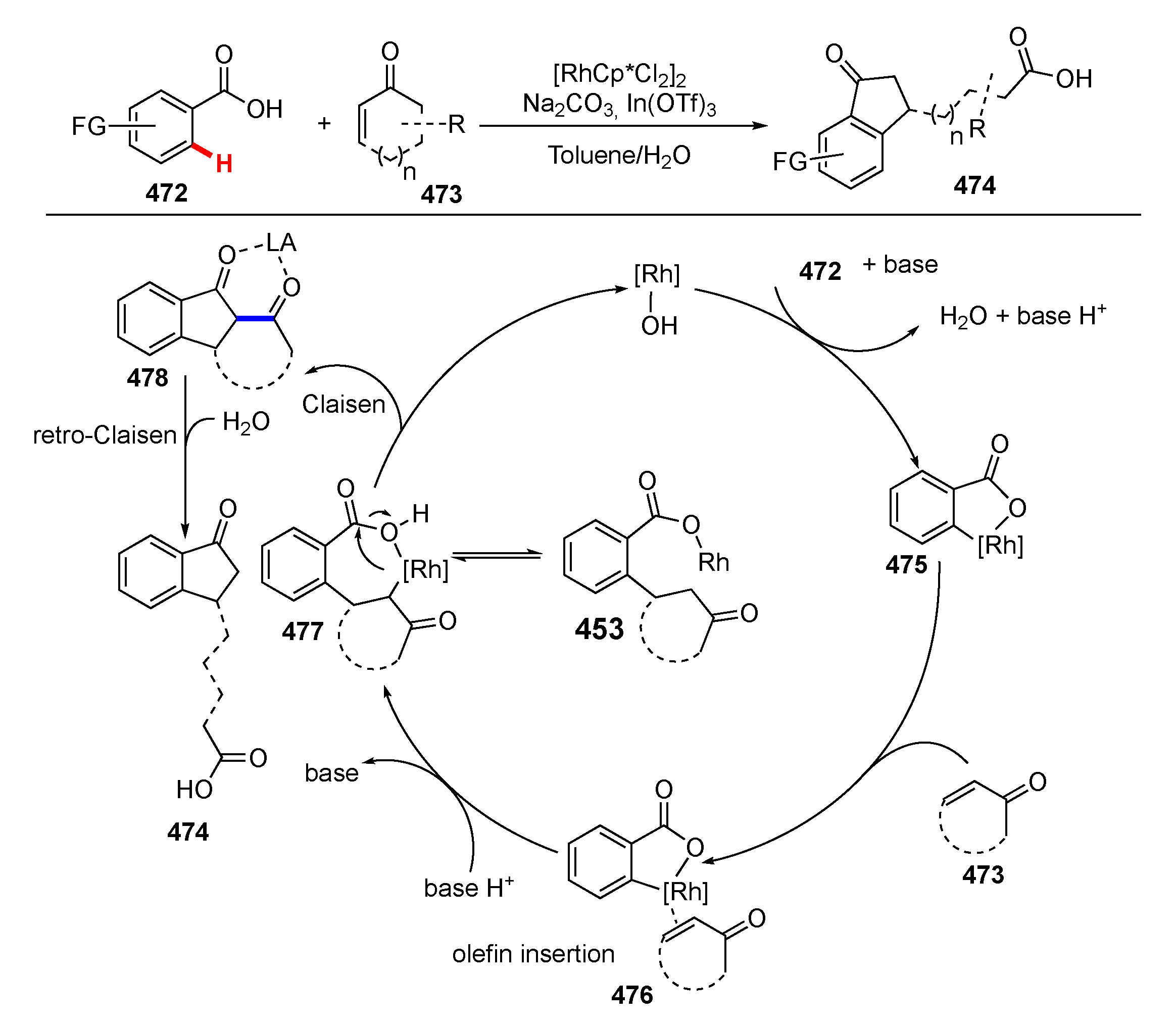
- Xia, Y.; Lu, G.; Liu, P.; Dong, G. Catalytic activation of carbon–carbon bonds in cyclopentanones. Nature 2016, 539, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, J.; Dong, G. Distal-Bond-Selective C−C Activation of Ring-Fused Cyclopentanones: An Efficient Access to Spiroindanones. Angew. Chem. Int. Ed. 2017, 56, 2376–2380. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Gurak, J.A.; Yang, K.S.; Engle, K.M. Activation of diverse carbon–heteroatom and carbon–carbon bonds via palladium(II)-catalysed β-X elimination. Nat. Chem. 2018, 10, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gómez, M.; Azizollahi, H.; Franzoni, I.; Larin, E.M.; Lautens, M.; García-López, J.A. Tandem Remote Csp3–H Activation/Csp3–Csp3 Cleavage in Unstrained Aliphatic Chains Assisted by Palladium(II). Organometallics 2019, 38, 973–980. [Google Scholar] [CrossRef]
- Onodera, S.; Ishikawa, S.; Kochi, T.; Kakiuchi, F. Direct Alkenylation of Allylbenzenes via Chelation-Assisted C–C Bond Cleavage. J. Am. Chem. Soc. 2018, 140, 9788–9792. [Google Scholar] [CrossRef]
- Onodera, S.; Togashi, R.; Ishikawa, S.; Kochi, T.; Kakiuchi, F. Catalytic, Directed C–C Bond Functionalization of Styrenes. J. Am. Chem. Soc. 2020, 142, 7345–7349. [Google Scholar] [CrossRef]
- Yu, S.; Lv, N.; Liu, Z.; Zhang, Y. Cu(II)-Mediated C−C/C−O Bond Formation via C−H/C−C Bond Cleavage: Access to Benzofurans Using Amide as a Traceless Directing Group. Adv. Synth. Catal. 2020, 362, 118–125. [Google Scholar] [CrossRef]
- Pannilawithana, N.; Yi, C.S. Catalytic Carbon-Carbon Bond Activation of Saturated and Unsaturated Carbonyl Compounds via Chelate-Assisted Coupling Reaction with Indoles. ACS Catal. 2020, 10, 5852–5861. [Google Scholar] [CrossRef]
- Yang, Z.; Yue, Q.; Yang, M.; Zhang, H.; Cui, X. Ru(II)-Catalyzed Tunable Cascade Reaction via C–H/C–C Bond Cleavage. J. Org. Chem. 2020, 85, 12960–12970. [Google Scholar] [CrossRef] [PubMed]
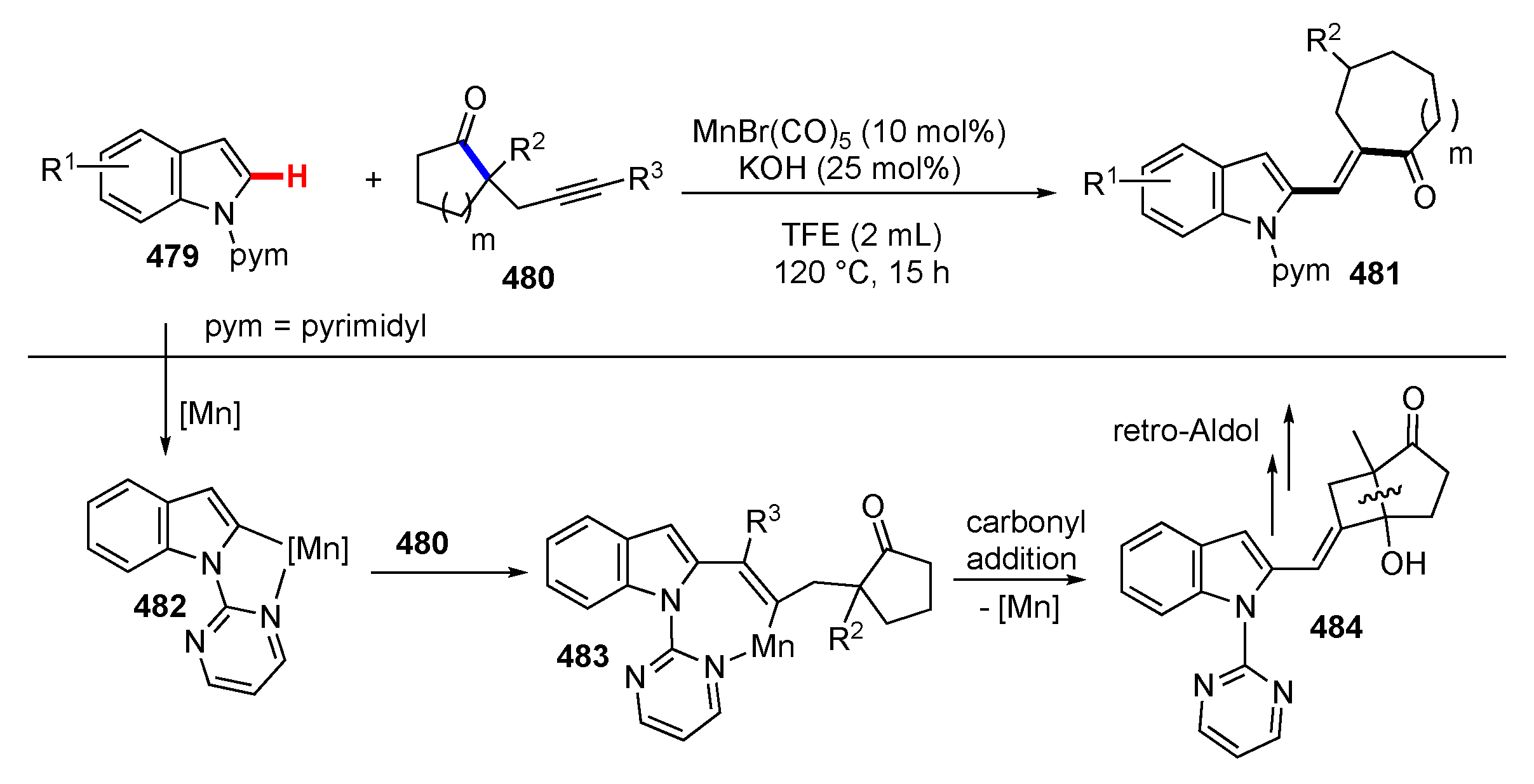
- Zheng, G.; Sun, J.; Xu, Y.; Zhai, S.; Li, X. Mn-Catalyzed Dehydrocyanative Transannulation of Heteroarenes and Propargyl Carbonates through C–H Activation: Beyond the Permanent Directing Effects of Pyridines /Pyrimidines. Angew. Chem. Int. Ed. 2019, 58, 5090–5094. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Kuniyil, R.; Ackermann, L. Manganese(I)-Catalyzed C–H Activation/Diels–Alder/retro-Diels–Alder Domino Alkyne Annulation featuring Transformable Pyridines. Angew. Chem. Int. Ed. 2019, 58, 5338–5342. [Google Scholar] [CrossRef]
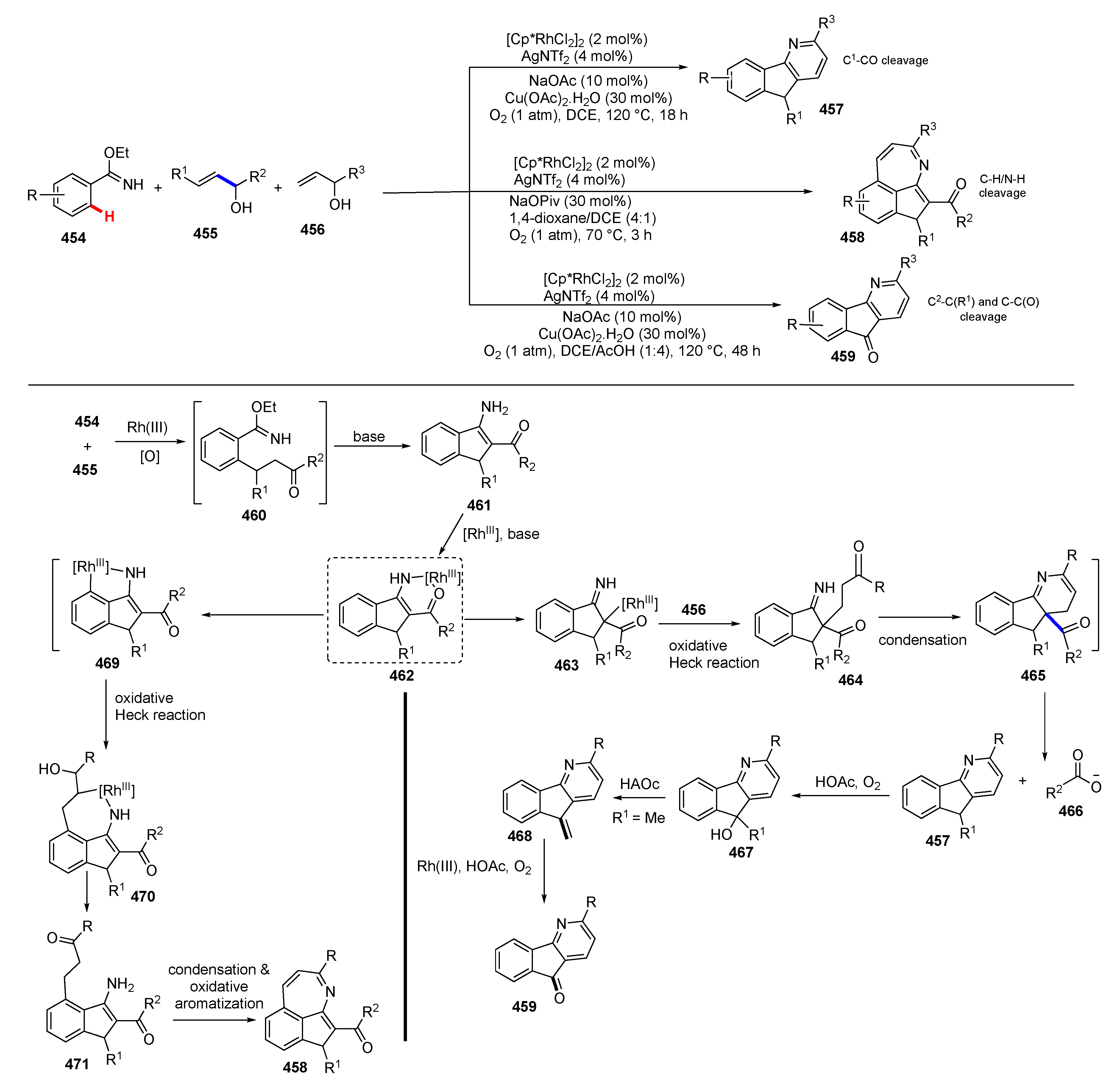
- Li, X.; Rao, J.; Ouyang, W.; Chen, Q.; Cai, N.; Lu, Y.; Huo, Y. Sequential C−H and C−C Bond Cleavage: Divergent Constructions of Fused N-Heterocycles via Tunable Cascade. ACS Catal. 2019, 9, 8749–8756. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, Z.; Belitz, F.; Ou, Y.; Pirkl, N.; Gooßen, L.J. Rhodium-Catalyzed Annelation of Benzoic Acids with α,β-Unsaturated Ketones with Cleavage of C−H, CO−OH, and C−C Bonds. Angew. Chem. Int. Ed. 2019, 58, 6435–6439. [Google Scholar] [CrossRef]
- Liu, B.; Yuan, Y.; Hu, P.; Zheng, G.; Bai, D.; Chang, J.; Li, X. Mn(I)-Catalyzed nucleophilic addition/ring expansion: Via C–H activation and C–C cleavage. Chem. Commun. 2019, 55, 10764–10767. [Google Scholar] [CrossRef]
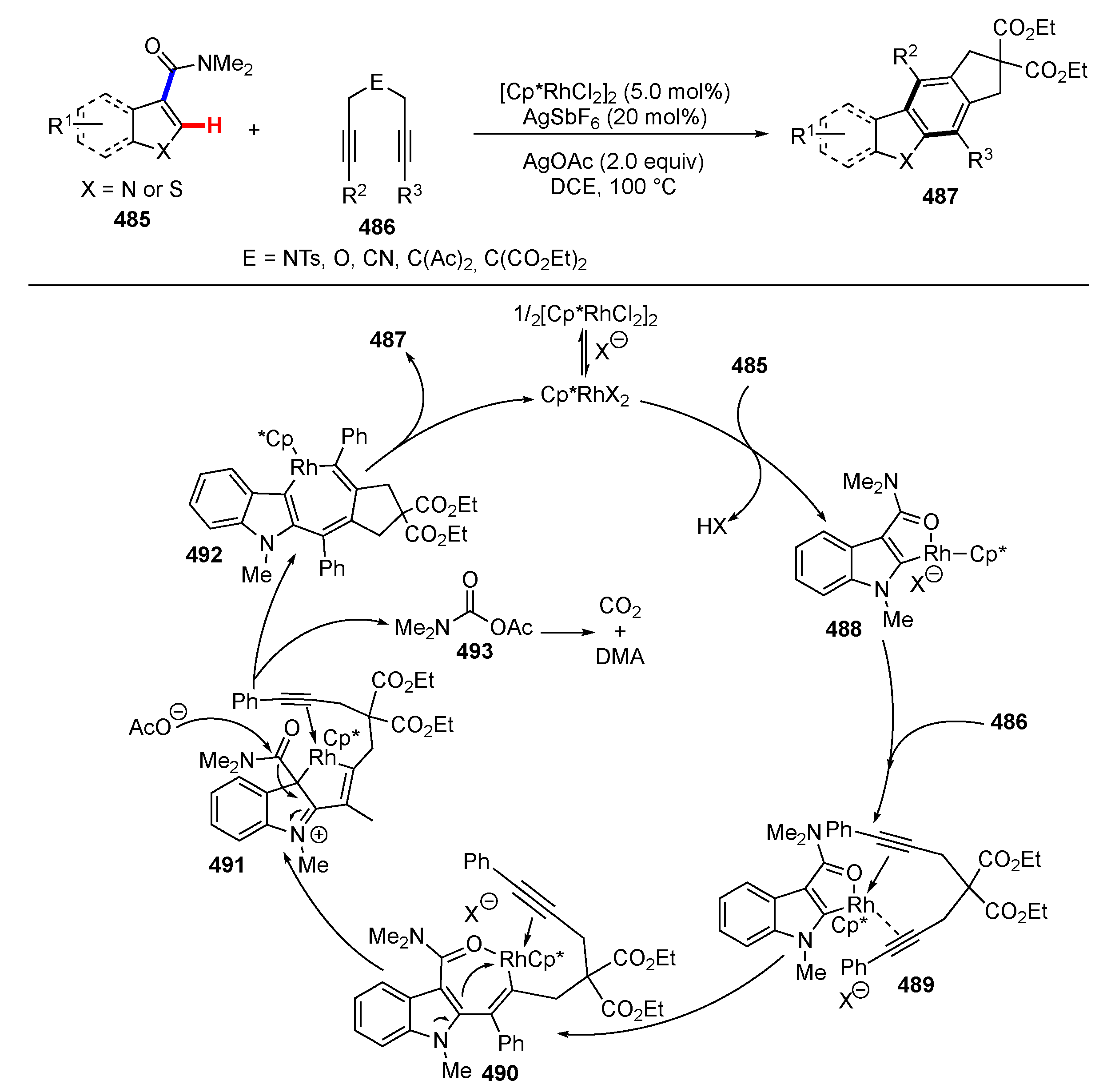
- Wang, Y.; Li, B.; Wang, B. RhIII-Catalyzed Synthesis of Cyclopenta[b]carbazoles via Cascade C–H/C–C Bond Cleavage and Cyclization Reactions: Using Amide as a Traceless Directing Group. Org. Lett. 2020, 22, 83–87. [Google Scholar] [CrossRef]
- Borah, A.J.; Shi, Z. Palladium-catalyzed regioselective C–H fluoroalkylation of indoles at the C4-position. Chem. Commun. 2017, 53, 3945–3948. [Google Scholar] [CrossRef]
- Chowdhury, D.; Dana, S.; Mandal, A.; Baidya, M. A ruthenium-catalyzed free amine directed (5 + 1) annulation of anilines with olefins: Diverse synthesis of phenanthridine derivatives. Chem. Commun. 2019, 55, 11908–11911. [Google Scholar] [CrossRef]
- Wen, S.; Chen, Y.; Zhao, Z.; Ba, D.; Lv, W.; Cheng, G. Ruthenium(II)-Catalyzed Construction of Isocoumarins via Dual C−H/C−C Activation of Sulfoxonium Ylides. J. Org. Chem. 2020, 85, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Peng, Z.; Wang, H.; Wang, Z.; Hao, D. Ruthenium(II)-Catalyzed Homocoupling of Weakly Coordinating Sulfoxonium Ylides via C–H Activation/Annulations: Synthesis of Functionalized Isocoumarins. Adv. Synth. Catal. 2019, 361, 5191–5197. [Google Scholar] [CrossRef]
- Bao, H.; Xu, Z.; Wu, D.; Zhang, H.; Jin, H.; Liu, Y. Copper(0)/Selectfluor System-Promoted Oxidative Carbon-Carbon Bond Cleavage/Annulation of o-Aryl Chalcones: An Unexpected Synthesis of 9,10-Phenanthraquinone Derivatives. J. Org. Chem. 2017, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
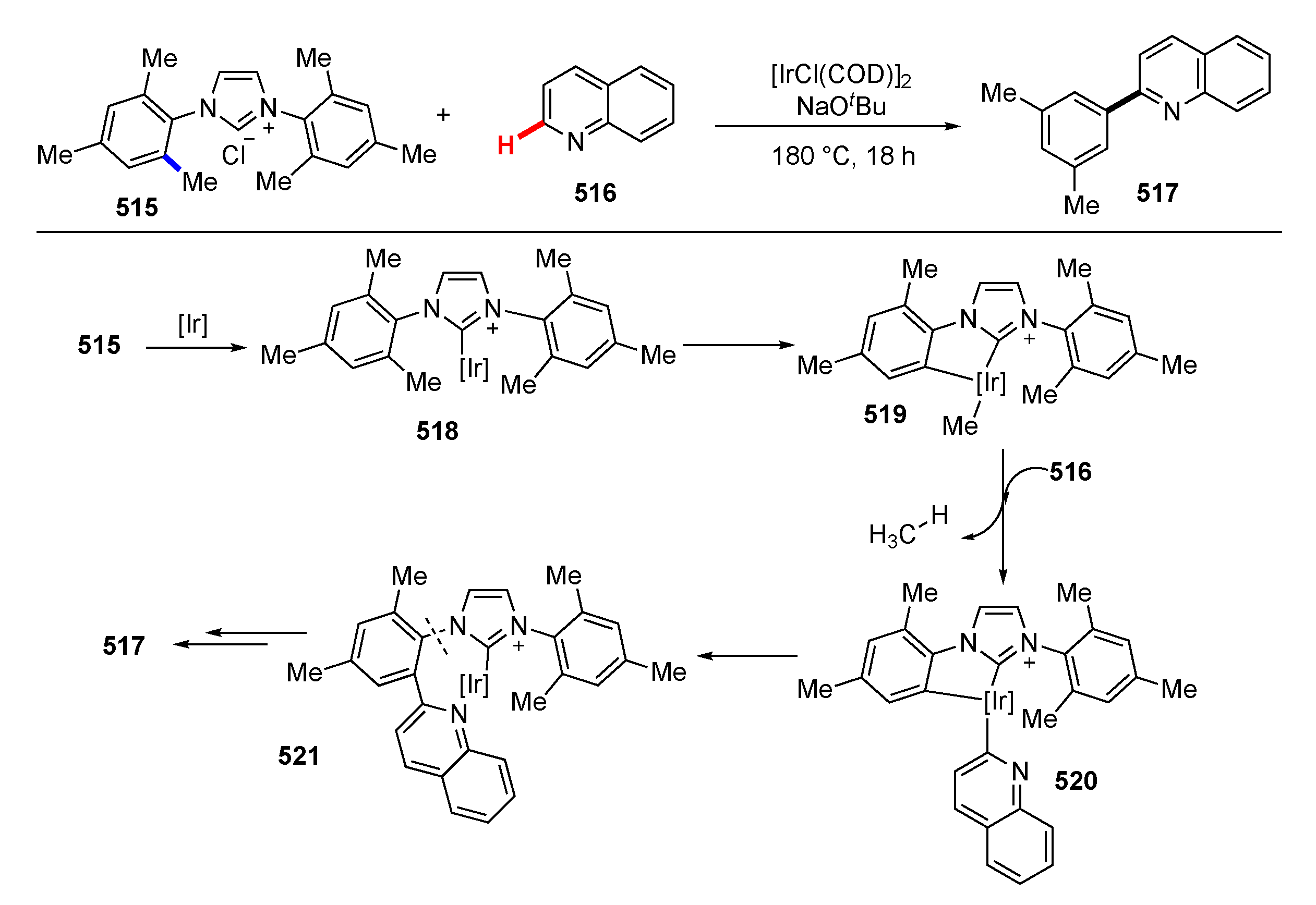
- Sakurai, S.; Tobisu, M. Iridium-Mediated Arylation of Quinoline via the Cleavage of Carbon-Carbon and Carbon-Nitrogen Bonds of 1,3-Dimesitylimidazol-2-ylidene. Organometallics 2019, 38, 2834–2838. [Google Scholar] [CrossRef]
- Li, W.; Xu, W.; Xie, J.; Yu, S.; Zhu, C. Distal radical migration strategy: An emerging synthetic means. Chem. Soc. Rev. 2018, 47, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Sivaguru, P.; Wang, Z.; Zanoni, G.; Bi, X. Cleavage of carbon-carbon bonds by radical reactions. Chem. Soc. Rev. 2019, 48, 2615–2656. [Google Scholar] [CrossRef]
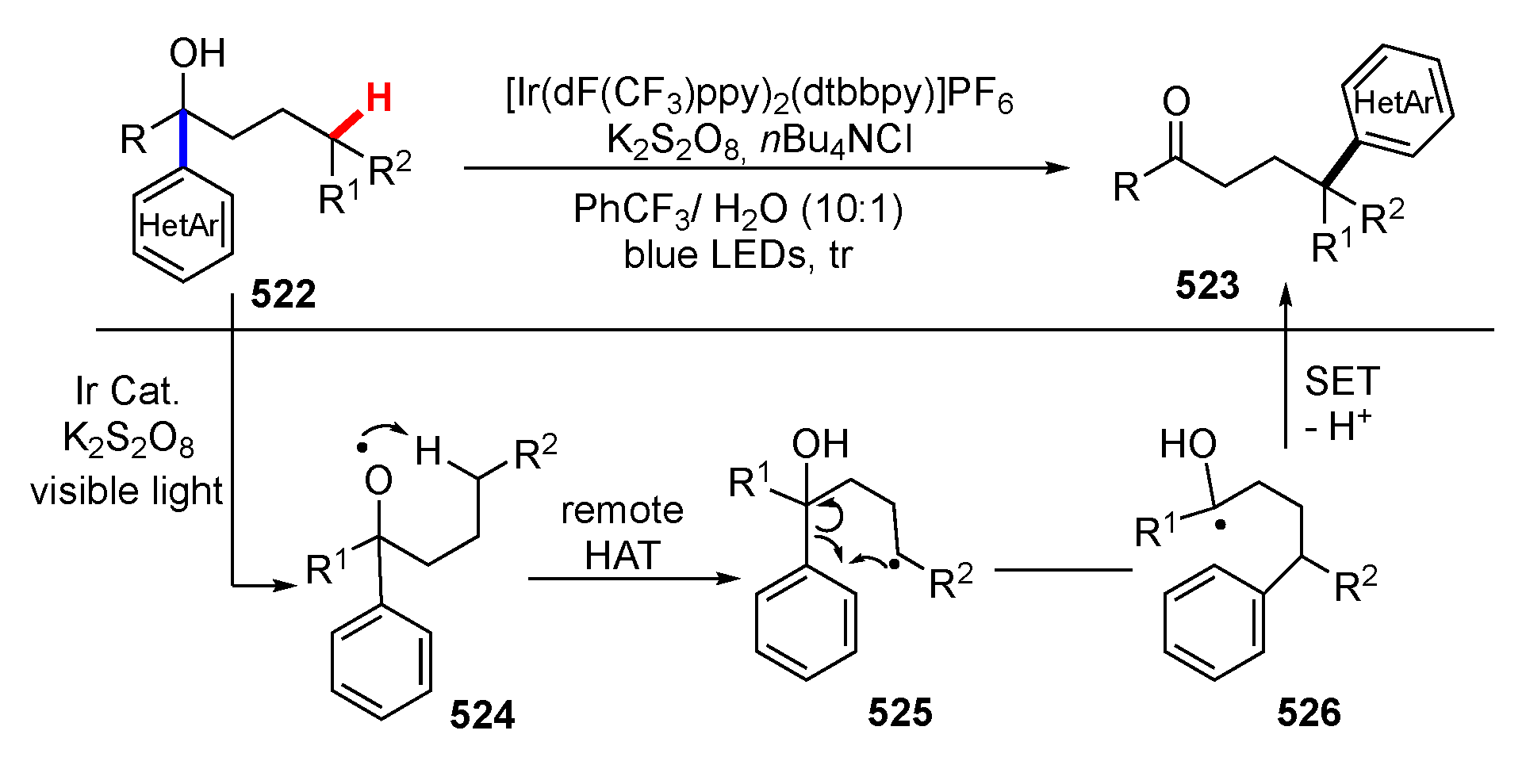
- Chen, H.; Yu, S. Remote C-C bond formation: Via visible light photoredox-catalyzed intramolecular hydrogen atom transfer. Org. Biomol. Chem. 2020, 18, 4519–4532. [Google Scholar] [CrossRef]
- Wu, X.; Wang, M.; Huan, L.; Wang, D.; Wang, J.; Zhu, C. Tertiary-Alcohol-Directed Functionalization of Remote C(sp3)−H Bonds by Sequential Hydrogen Atom and Heteroaryl Migrations. Angew. Chem. Int. Ed. 2018, 57, 1640–1644. [Google Scholar] [CrossRef]
- Wu, S.; Wu, X.; Wang, D.; Zhu, C. Regioselective Vinylation of Remote Unactivated C(sp3)−H Bonds: Access to Complex Fluoroalkylated Alkenes. Angew. Chem. Int. Ed. 2019, 58, 1499–1503. [Google Scholar] [CrossRef]
- Yang, S.; Wu, X.; Wu, S.; Zhu, C. Regioselective Sulfonylvinylation of the Unactivated C(sp3)−H Bond via a C–Centered Radical-Mediated Hydrogen Atom Transfer (HAT) Process. Org. Lett. 2019, 21, 4837–4841. [Google Scholar] [CrossRef]
- He, F.S.; Yao, Y.; Xie, W.; Wu, J. Metal-Free Synthesis of (E)-Vinyl Sulfones via An Insertion of Sulfur Dioxide/1,5-Hydrogen Atom Transfer Sequence. Adv. Synth. Catal. 2020, 362, 4744–4748. [Google Scholar] [CrossRef]
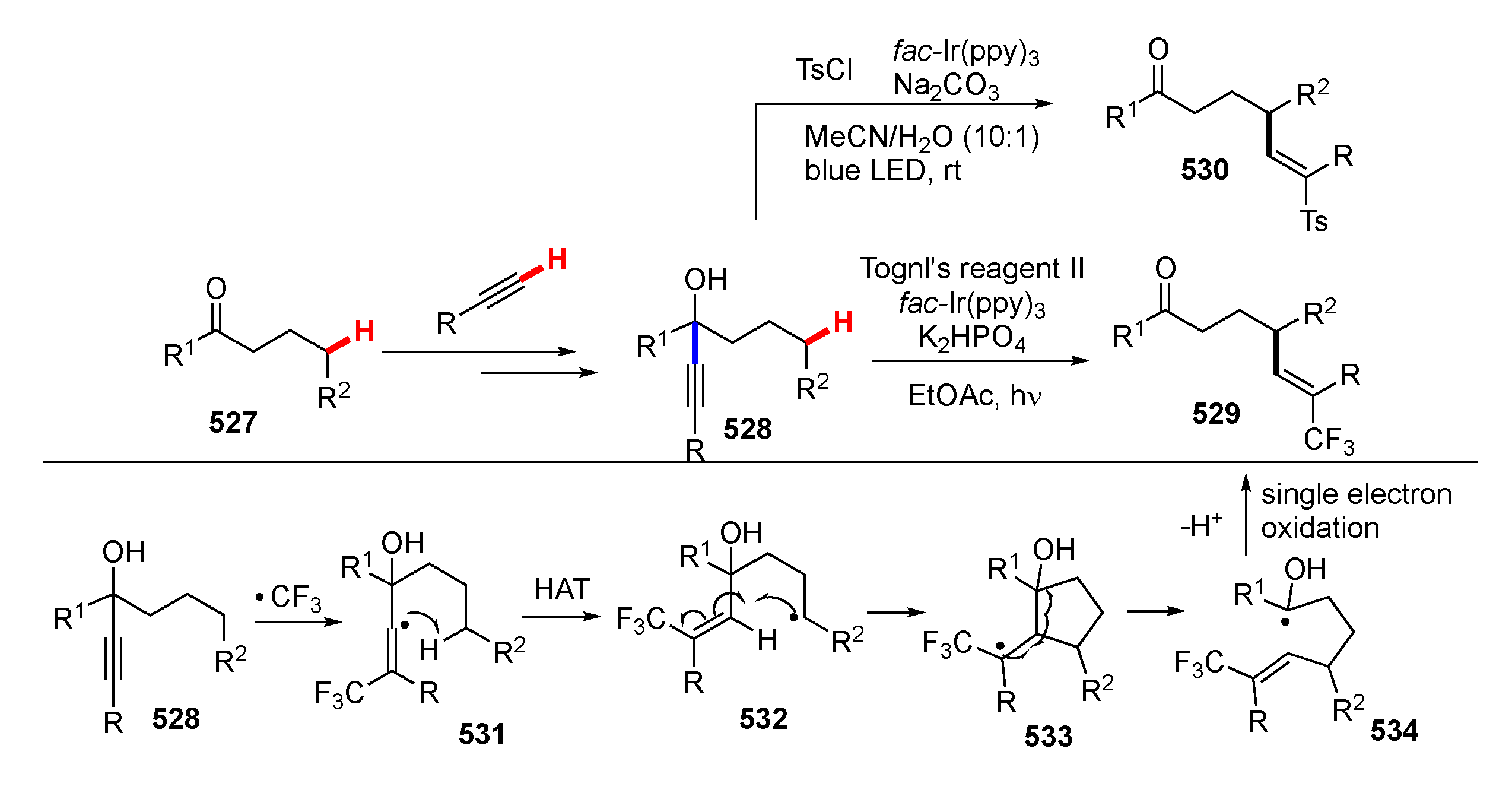
- Zhao, Q.; Ji, X.S.; Gao, Y.Y.; Hao, W.J.; Zhang, K.Y.; Tu, S.J.; Jiang, B. Merging “Anti-Baldwin” 3-Exo-Dig Cyclization with 1,2-Alkynyl Migration for Radical Alkylalkynylation of Unactivated Olefins. Org. Lett. 2018, 20, 3596–3600. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, Z.; Jiang, J.; Ke, Z.; Zhu, C. Merging Distal Alkynyl Migration and Photoredox Catalysis for Radical Trifluoromethylative Alkynylation of Unactivated Olefins. Angew. Chem. Int. Ed. 2017, 56, 4545–4548. [Google Scholar] [CrossRef] [PubMed]
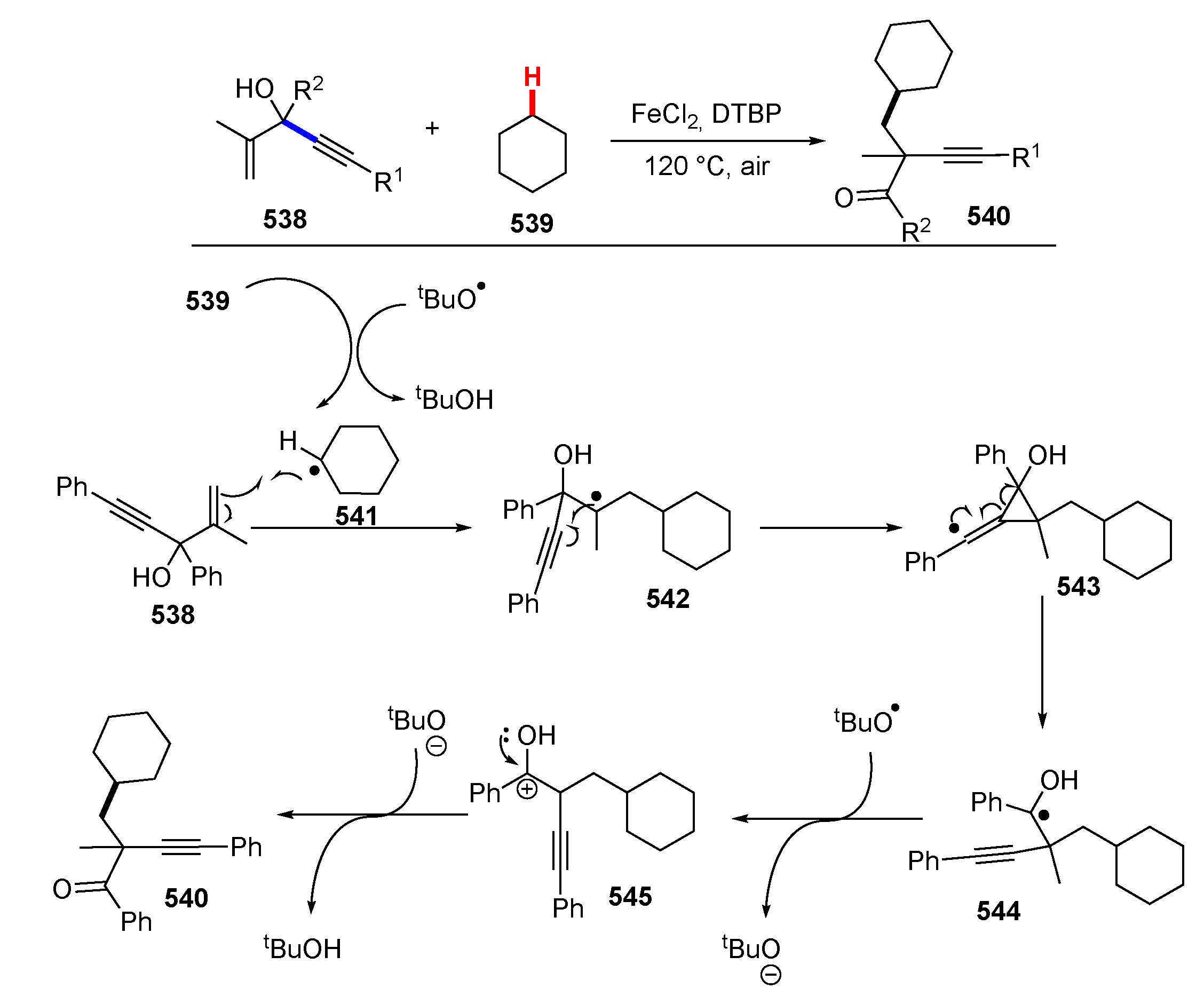
- Tian, T.; Wang, X.; Lv, L.; Li, Z. Iron-catalyzed acylation-functionalization of unactivated alkenes with aldehydes. Chem. Commun. 2020, 56, 14637–14640. [Google Scholar] [CrossRef]
- Sen, C.; Ghosh, S.C. Transition-Metal-Free Regioselective Alkylation of Quinoline N-Oxides via Oxidative Alkyl Migration and C−C Bond Cleavage of tert-/sec-Alcohols. Adv. Synth. Catal. 2018, 360, 905–910. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Liu, S.; Huang, L.; Liu, Z.Q. Surgical Cleavage of Unstrained C(sp3)−C(sp3) Bonds in General Alcohols for Heteroaryl C−H Alkylation and Acylation. Adv. Synth. Catal. 2019, 361, 4568–4574. [Google Scholar] [CrossRef]
- Zheng, L.; Huang, H.; Yang, C.; Xia, W. UV light-mediated difunctionalization of alkenes through aroyl radical addition/1,4-/1,2-Aryl shift cascade reactions. Org. Lett. 2015, 17, 1034–1037. [Google Scholar] [CrossRef]
- He, B.Q.; Gao, Y.; Wang, P.Z.; Wu, H.; Zhou, H.B.; Liu, X.P.; Chen, J.R. Dual photoredox/palladium-catalyzed C–H acylation of 2-arylpyridines with oxime esters. Synlett 2020, 31. [Google Scholar] [CrossRef]
- Fan, X.; Lei, T.; Chen, B.; Tung, C.H.; Wu, L.Z. Photocatalytic C–C Bond Activation of Oxime Ester for Acyl Radical Generation and Application. Org. Lett. 2019, 21, 4153–4158. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Ji, P.Y.; Liu, Y.F.; Guo, C.C. Transition-Metal-Free Synthesis of Carbonyl-Containing Oxindoles from N-Arylacrylamides and α-Diketones via TBHP- or Oxone-Mediated Oxidative Cleavage of C(sp2)–C(sp2) Bonds. J. Org. Chem. 2015, 80, 10777–10786. [Google Scholar] [CrossRef]
- Li, C.; Zhu, W.; Shu, S.; Wu, X.; Liu, H. Palladium-catalyzed C2-acylation of indoles with α-diketones assisted by the removable N-(2-pyrimidyl) group. Eur. J. Org. Chem. 2015, 2015, 3743–3750. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, T.; Yang, Y.; Zhang-negrerie, D.; Zhang, X.; Du, Y.; Wu, Y.; Zhao, K. Metal-Free Synthesis of 3-Arylquinolin-2-ones from Acrylic Amides via a Highly Regioselective 1,2-Aryl Migration: An Experimental and Computational Study. J. Org. Chem. 2016, 81, 4058–4065. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lu, H.; Wang, H.; Yang, C.; Zhang, X.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. PhI(OCOCF3)2-Mediated C–C Bond Formation Concomitant with a 1,2-Aryl Shift in a Metal-Free Synthesis of 3-Arylquinolin-2-ones. Org. Lett. 2013, 15, 2906–2909. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Han, Y.P.; Li, M.; Li, X.S.; Liang, Y.M. Copper-Catalyzed Radical Sulfonylation of N-Propargylindoles with Concomitant 1,2-Aryl Migration. Adv. Synth. Catal. 2018, 360, 3460–3465. [Google Scholar] [CrossRef]
- Wen, X.; Li, X.; Luo, X.; Wang, W.; Song, S.; Jiao, N. Intramolecular Csp3–H/C–C bond amination of alkyl azides for the selective synthesis of cyclic imines and tertiary amines. Chem. Sci. 2020, 11, 4482–4487. [Google Scholar] [CrossRef]
- Xu, H.; Yu, F.; Huang, R.; Weng, M.; Chen, H.; Zhang, Z. Synthesis of polysubstituted quinolines through promoter-regulated selective annulation and C–C bond cleavage from 2-styrylanilines and β-keto esters. Org. Chem. Front. 2020, 7, 3368–3373. [Google Scholar] [CrossRef]
- Zhao, P.; Yu, X.X.; Zhou, Y.; Geng, X.; Wang, C.; Huang, C.; Wu, Y.D.; Zhu, Y.P.; Wu, A.X. Splitting Methyl Ketones into Two Parts: Synthesis of 4(3H)-Quinazolinones via Consecutive Cyclization/Ring-Opening Reaction. Org. Lett. 2020, 22, 7103–7107. [Google Scholar] [CrossRef]
- Challa, C.; Varughese, S.; Suresh, C.H.; Lankalapalli, R.S. Metal-Free Multiple Carbon-Carbon and Carbon-Hydrogen Bond Activations via Charge-Switching Mechanism in Unstrained Diindolylmethanes. Org. Lett. 2017, 19, 4219–4222. [Google Scholar] [CrossRef]
- Schmid, M.; Sokol, K.R.; Wein, L.A.; Torres Venegas, S.; Meisenbichler, C.; Wurst, K.; Podewitz, M.; Magauer, T. Synthesis of Vicinal Quaternary All-Carbon Centers via Acid-catalyzed Cycloisomerization of Neopentylic Epoxides. Org. Lett. 2020, 22, 6526–6531. [Google Scholar] [CrossRef]
- Chen, P.; Nan, J.; Hu, Y.; Kang, Y.; Wang, B.; Ma, Y.; Szostak, M. Metal-free tandem carbene N–H insertions and C–C bond cleavages. Chem. Sci. 2020. [Google Scholar] [CrossRef]
- Wang, C.S.; Fan, T.; Zhang, H.H.; Li, C.; Shen, Y.; Mei, G.J.; Shi, F. Gallium Bromide-Promoted Dearomative Indole Insertion in 3-Indolylmethanols: Chemoselective and (Z/E)-Selective Synthesis of 3,3’-Bisindole Derivatives. J. Org. Chem. 2016, 81, 11734–11742. [Google Scholar] [CrossRef] [PubMed]
- Cen, J.; Li, J.; Zhang, Y.; Zhu, Z.; Yang, S.; Jiang, H. Direct Assembly of 4-Substituted Quinolines with Vinyl Azides as a Dual Synthon via C=C and C–N Bond Cleavage. Org. Lett. 2018, 20, 4434–4438. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wang, M.; Wang, Y.; Song, B.; Yang, Y.; Yao, Q.; Li, Y. Merging base-promoted C–C bond cleavage and iron-catalyzed skeletal rearrangement involving C–C/C–H bond activation: Synthesis of highly functionalized carbazoles. Chem. Commun. 2018, 54, 11009–11012. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, J.; Tan, H.; Kong, L.; Wang, M.; Yuan, Y.; Li, Y. Synthesis of cyano-substituted carbazoles via successive C–C/C–H cleavage. Org. Biomol. Chem. 2019, 17, 958–965. [Google Scholar] [CrossRef]
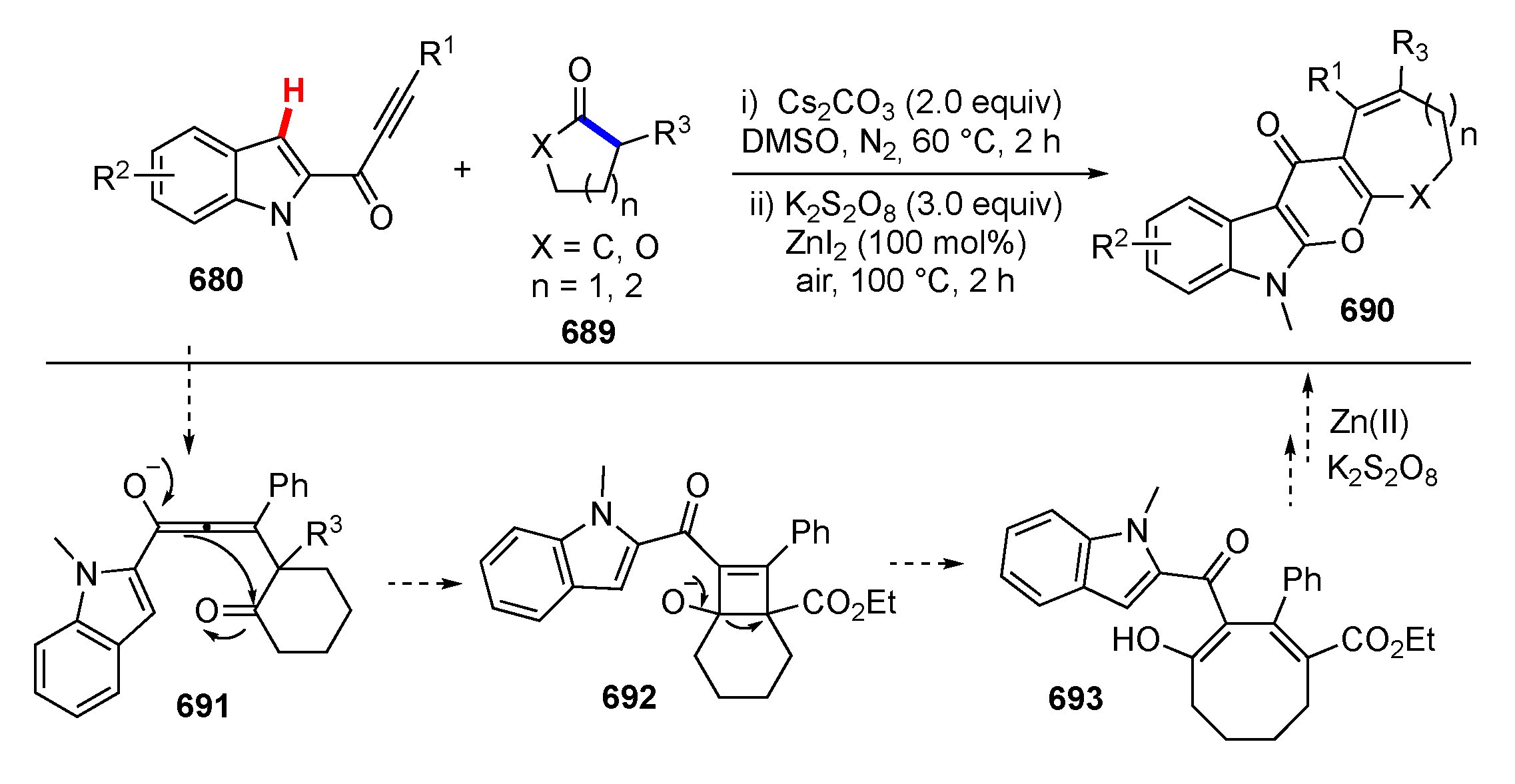
- Wang, M.; Kong, L.; Wang, Y.; Song, B.; Sun, Y.; Tang, R.; Li, Y. Sequential C–C σ-Bond Cleavage/(sp2) C–O Bond Formation via C–H Functionalization toward Pyranoindolones Fused with Medium-Sized Rings. Org. Lett. 2018, 20, 6130–6134. [Google Scholar] [CrossRef]
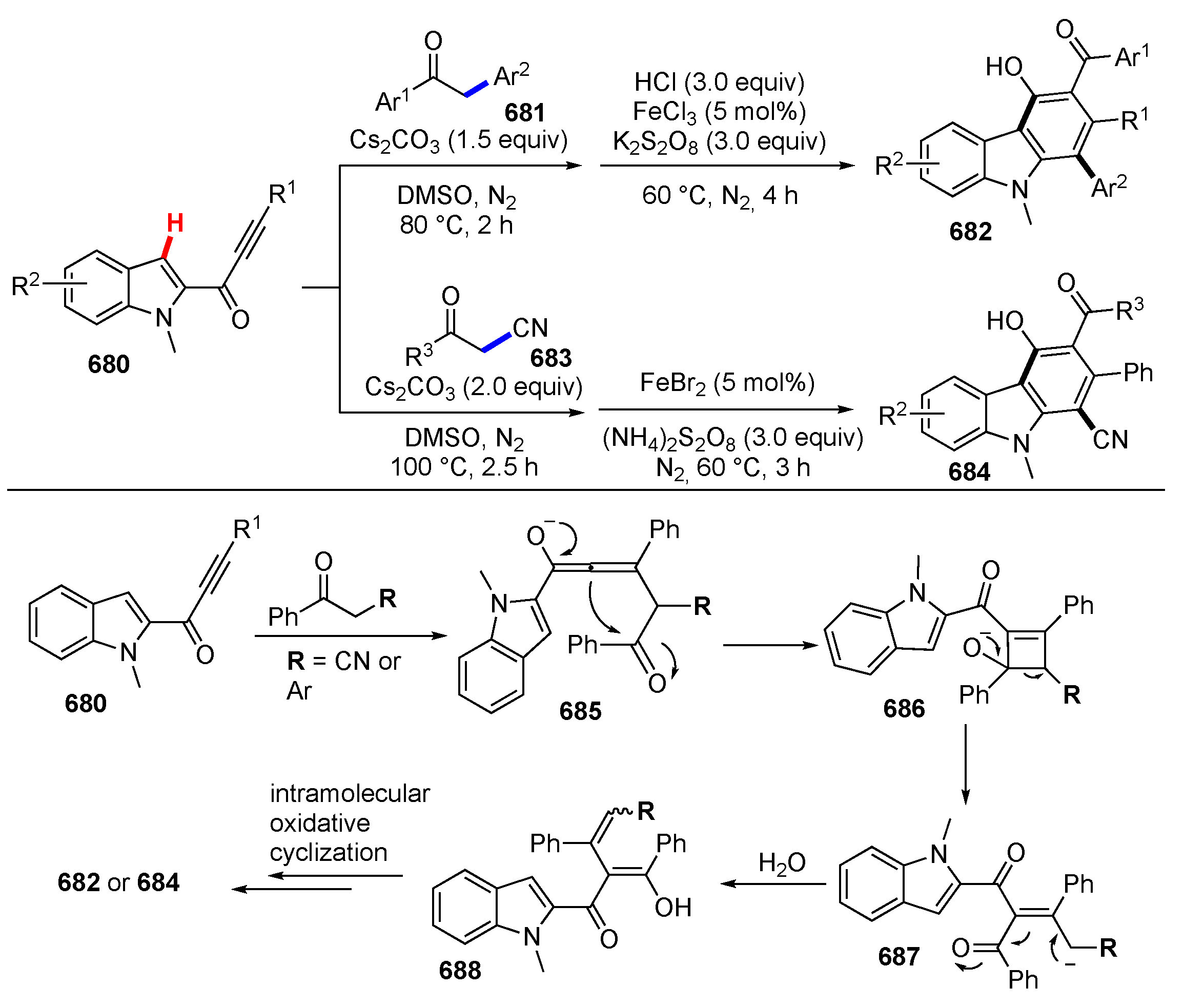
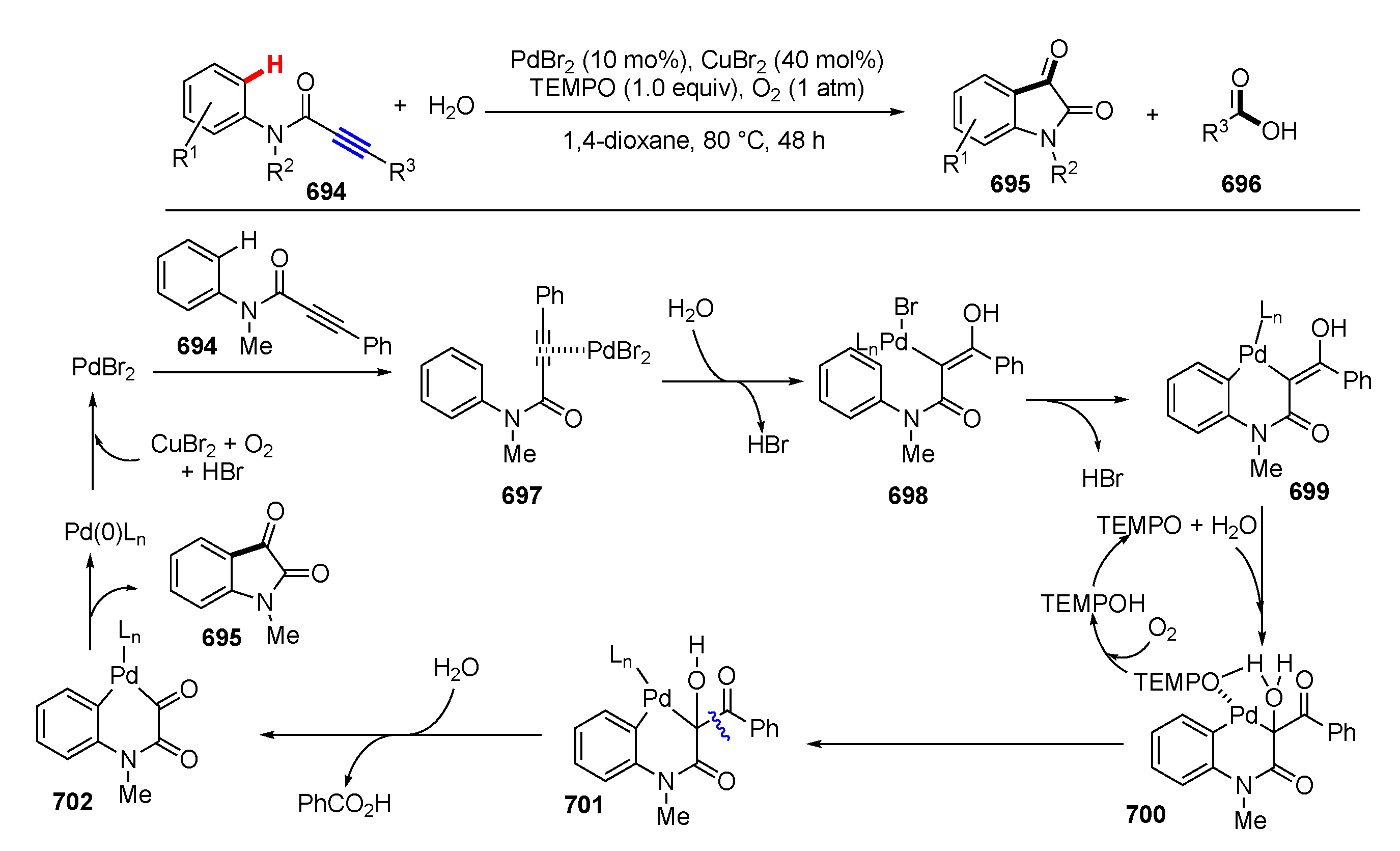
- Zhou, M.B.; Li, Y.; Ouyang, X.H.; Li, J.H. Transformations of N-arylpropiolamides to indoline-2,3-diones and acids via C≡C triple bond oxidative cleavage and C(sp2)–H functionalization. Sci. China Chem. 2020, 63, 222–227. [Google Scholar] [CrossRef]
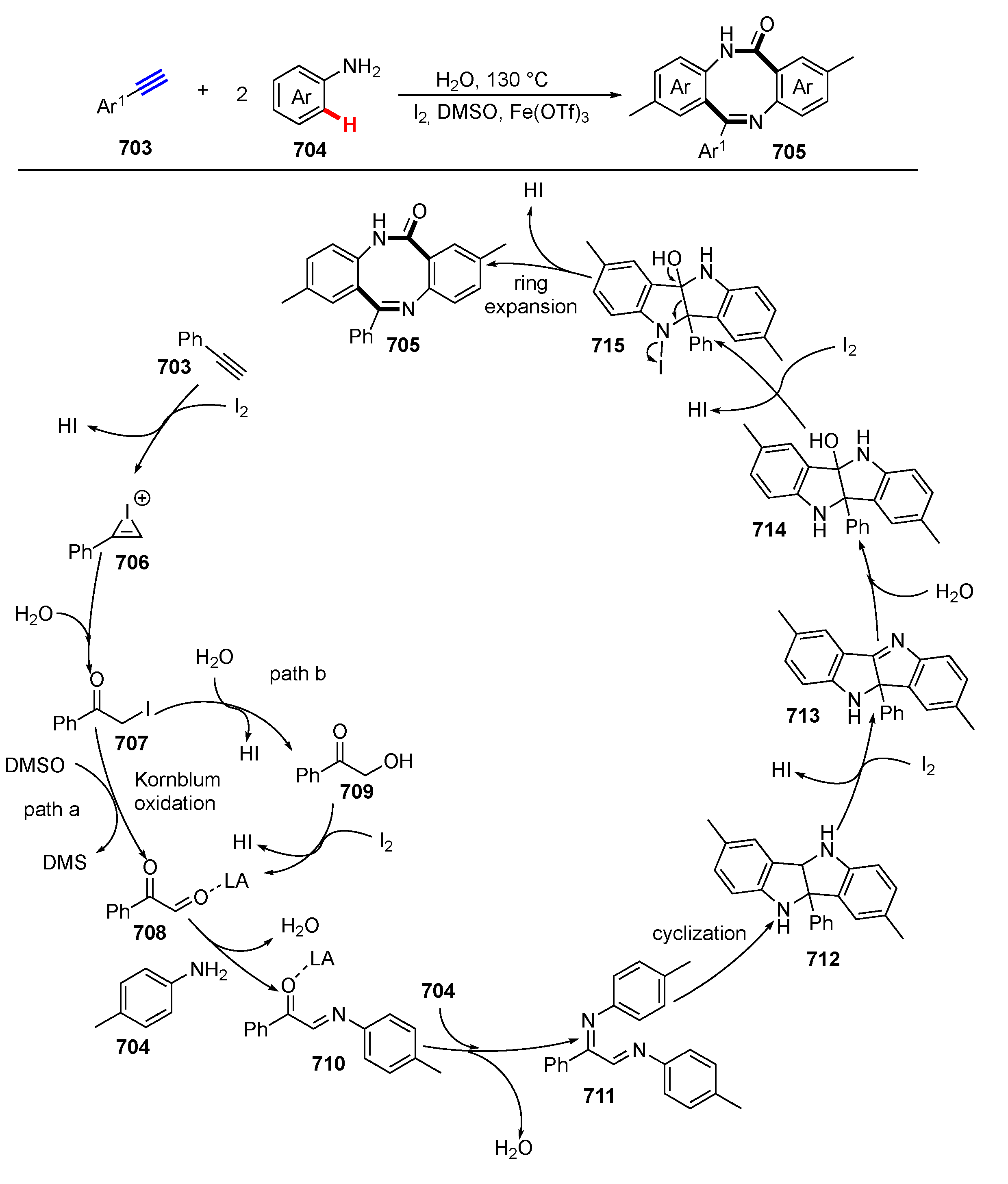
- Zhao, P.; Yu, X.-X.; Zhou, Y.; Huang, C.; Wu, Y.-D.; Zhu, Y.-P.; Wu, A.-X. Arylacetylenes as two-carbon synthons: Synthesis of eight-membered rings via C–C bond cleavage. Chem. Commun. 2020, 56, 12554–12557. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, M.; Vishwakarma, R.A.; Verma, M.K.; Singh, P.P. Room Temperature Metal-Catalyzed Oxidative Acylation of Electron-Deficient Heteroarenes with Alkynes, Its Mechanism, and Application Studies. J. Org. Chem. 2018, 83, 12420–12431. [Google Scholar] [CrossRef]
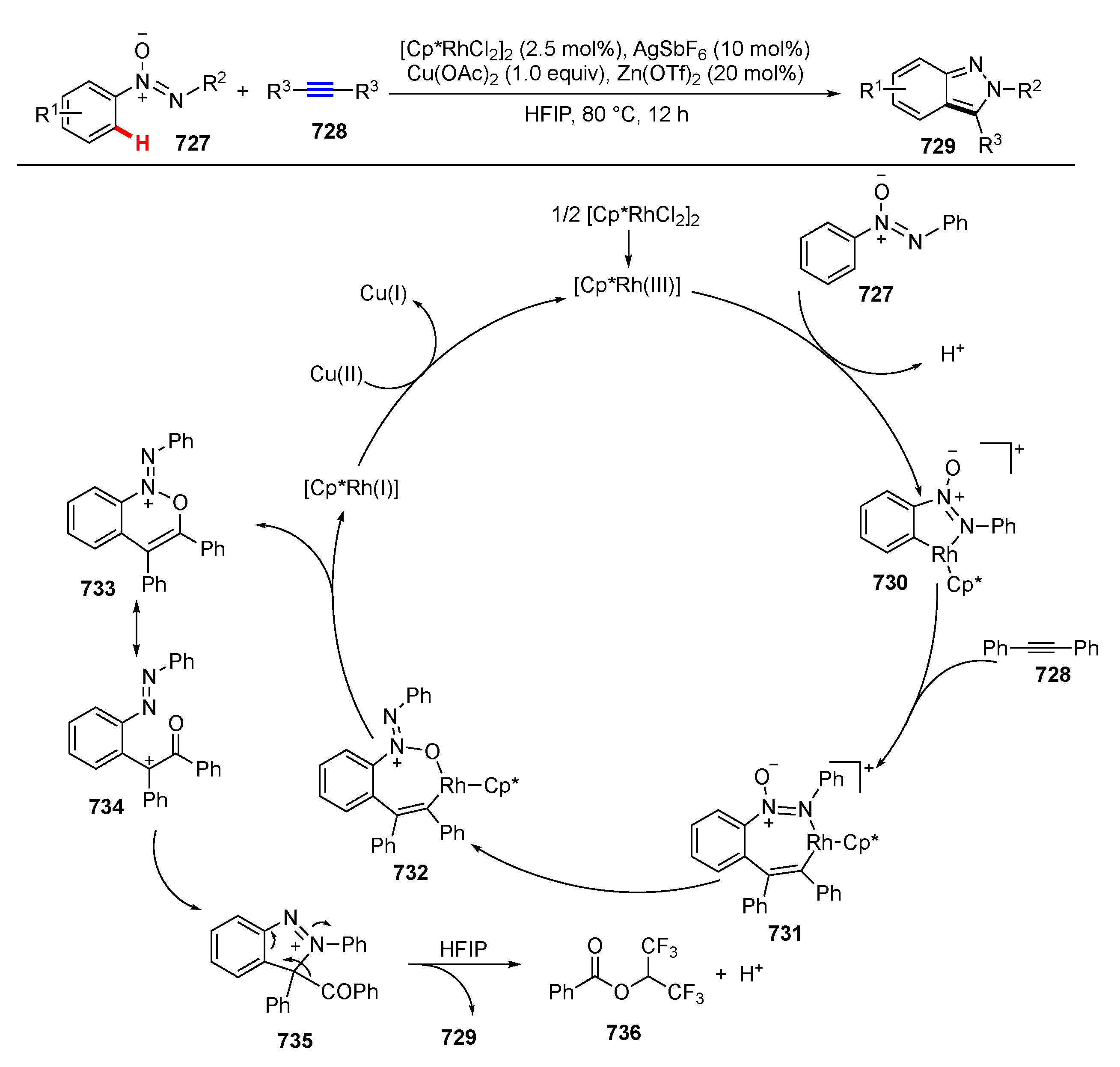
- Long, Z.; Yang, Y.; You, J. Rh(III)-Catalyzed [4 + 1]-Annulation of Azoxy Compounds with Alkynes: A Regioselective Approach to 2H-Indazoles. Org. Lett. 2017, 19, 2781–2784. [Google Scholar] [CrossRef]

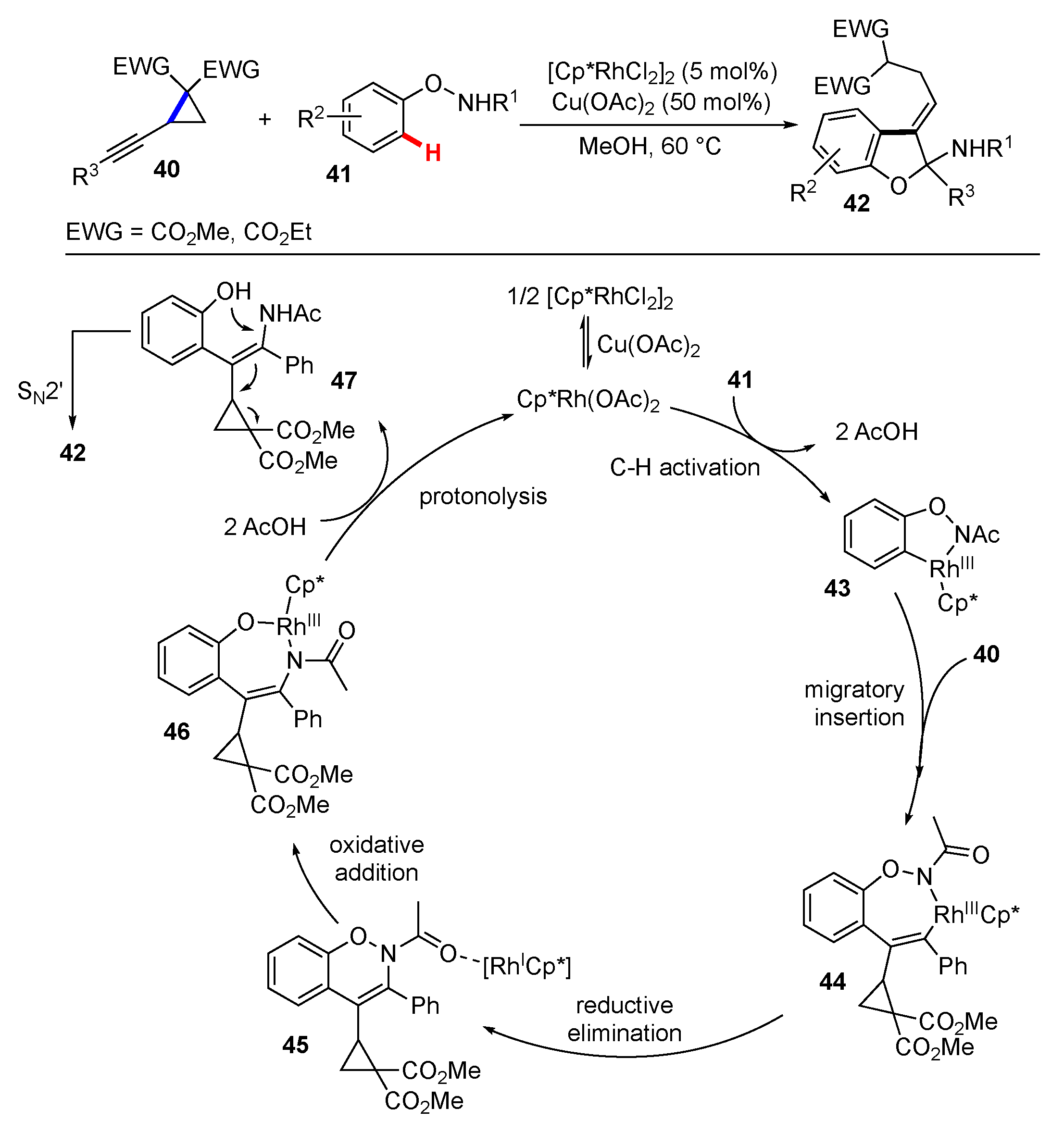

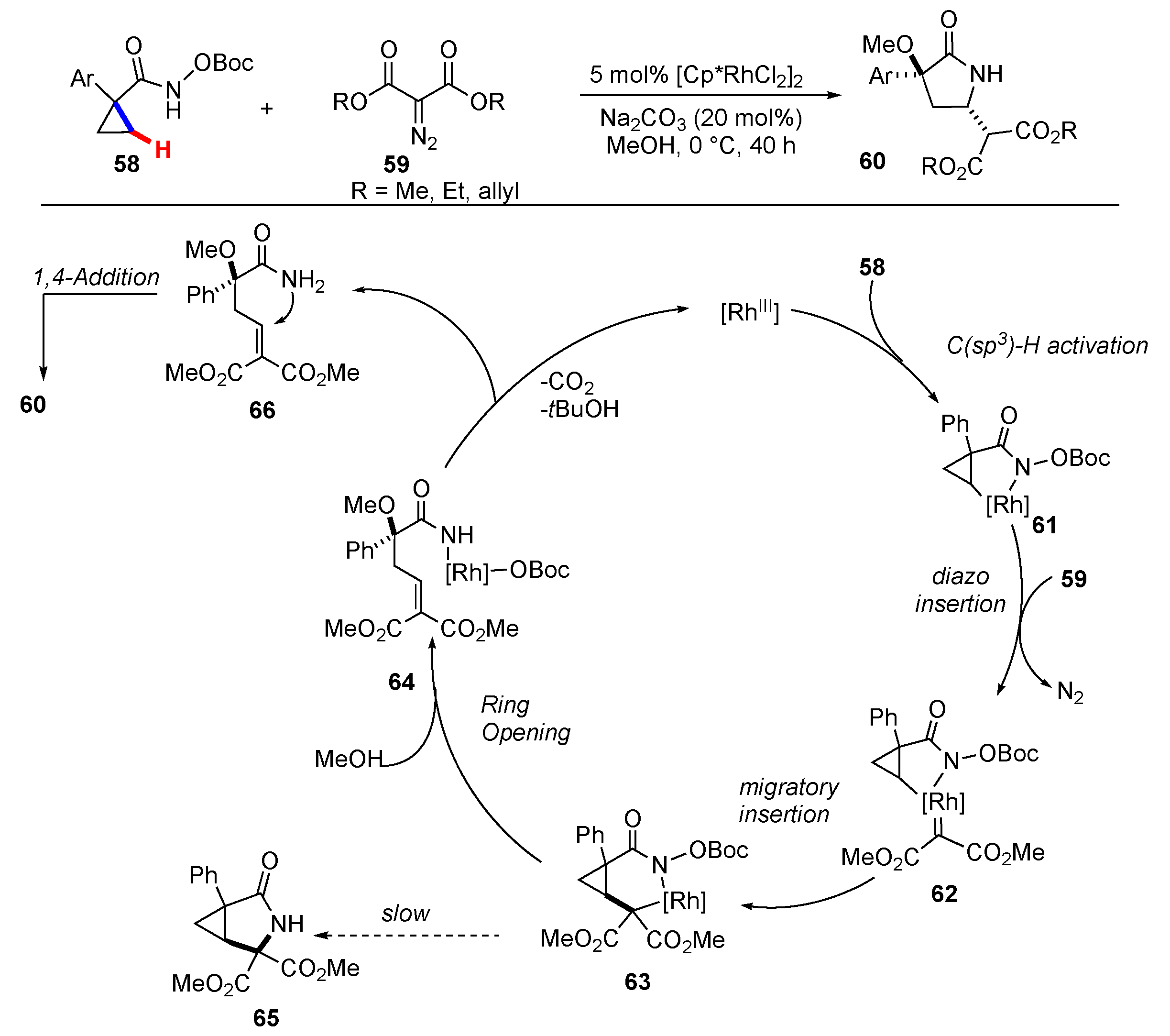
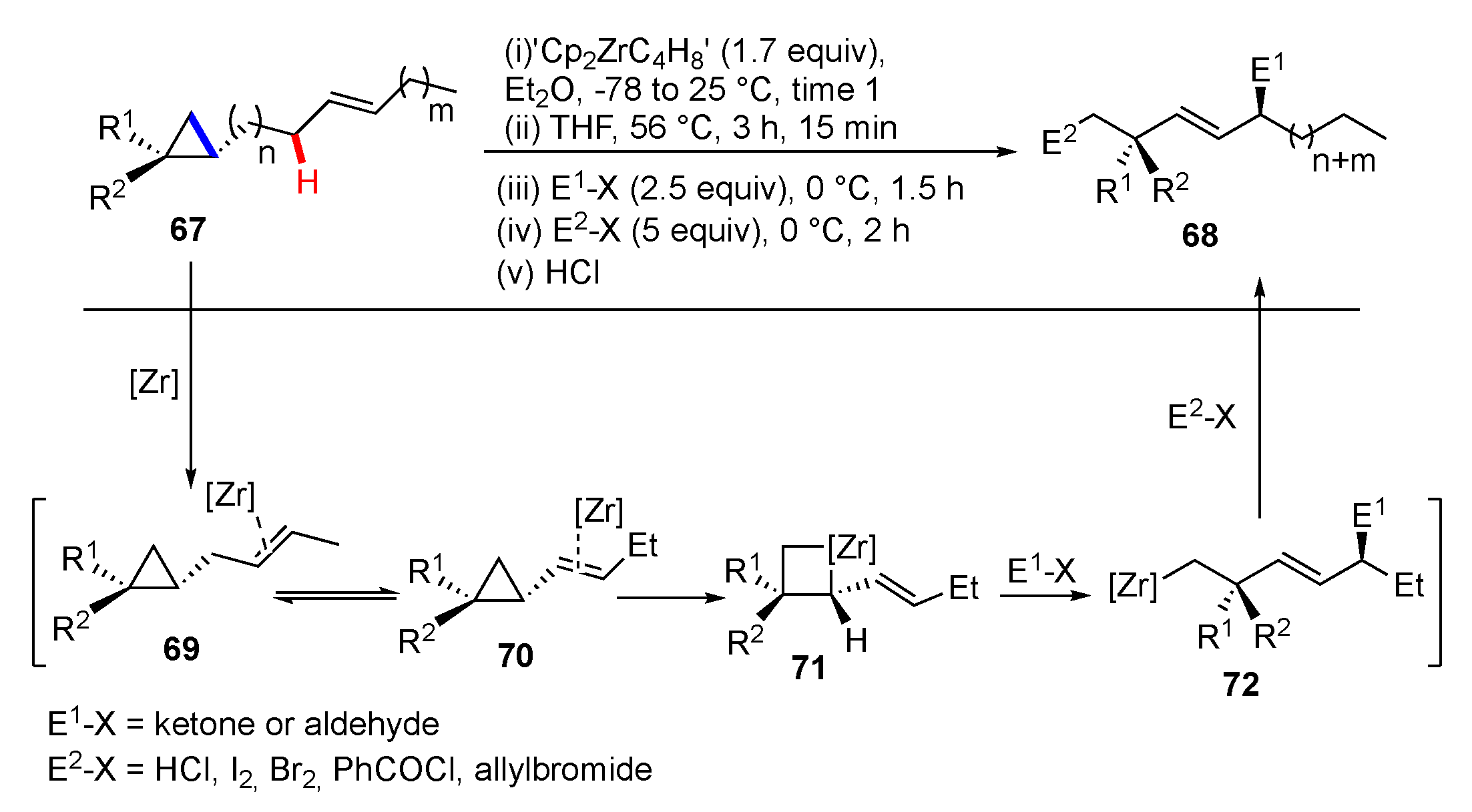
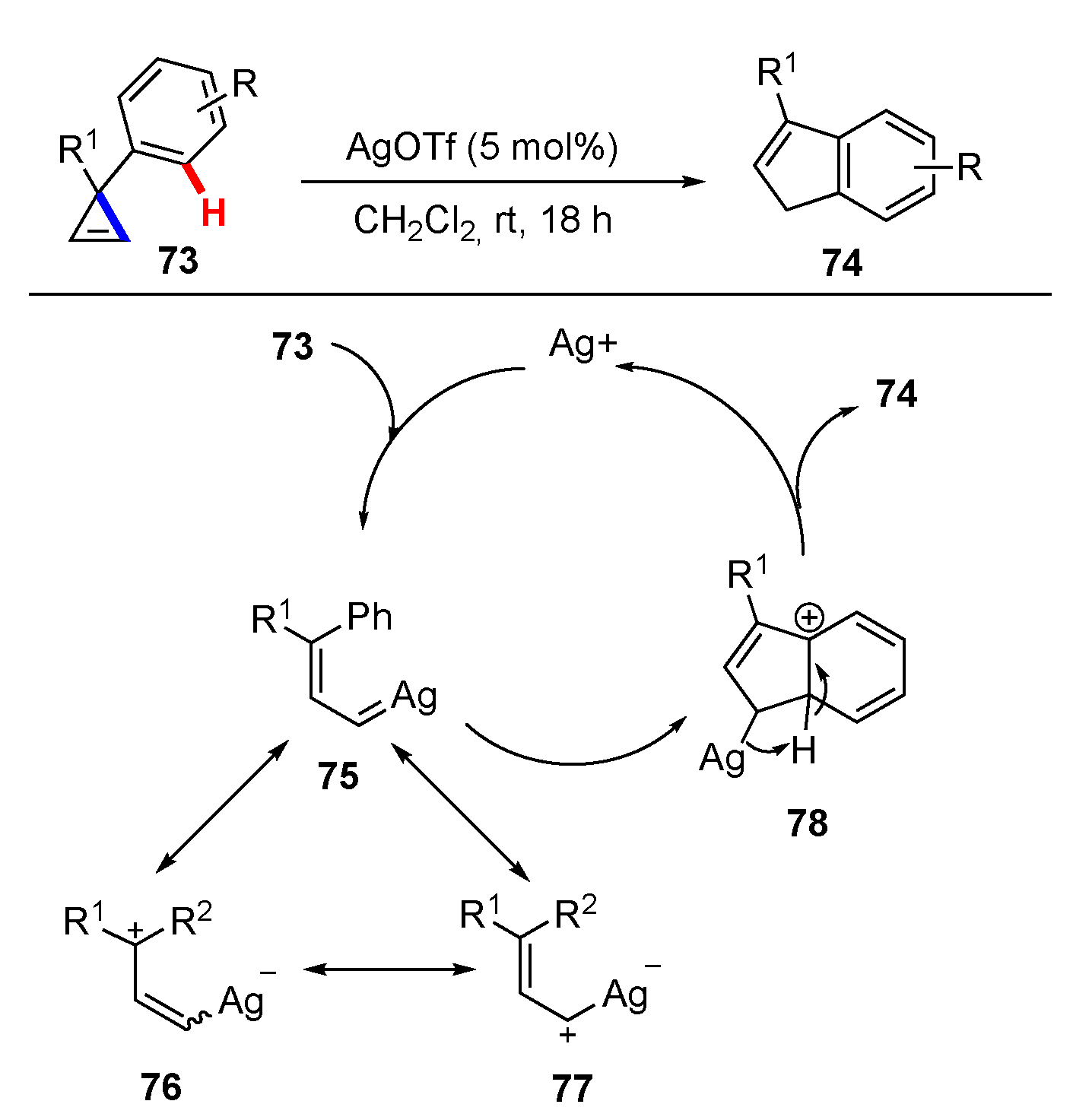
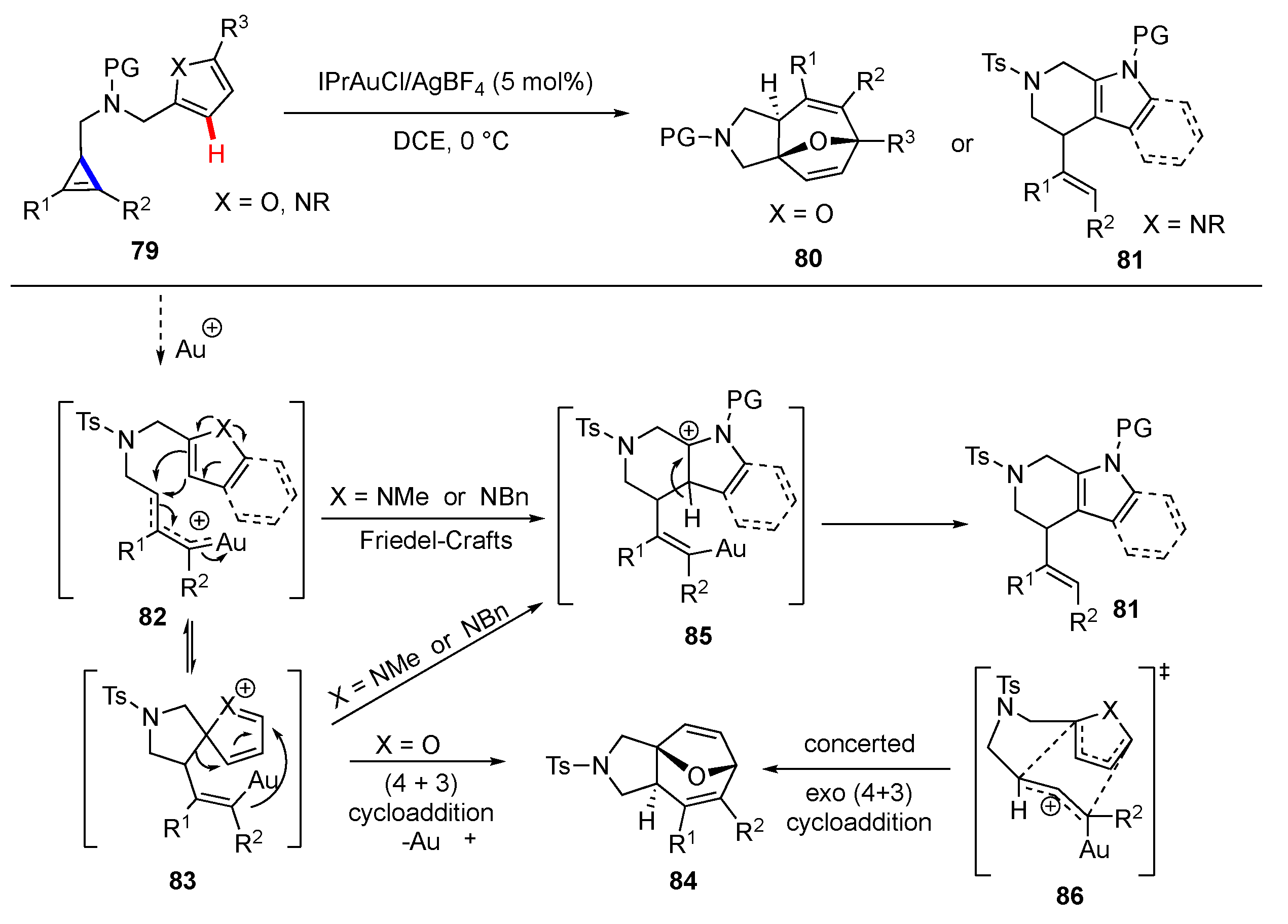
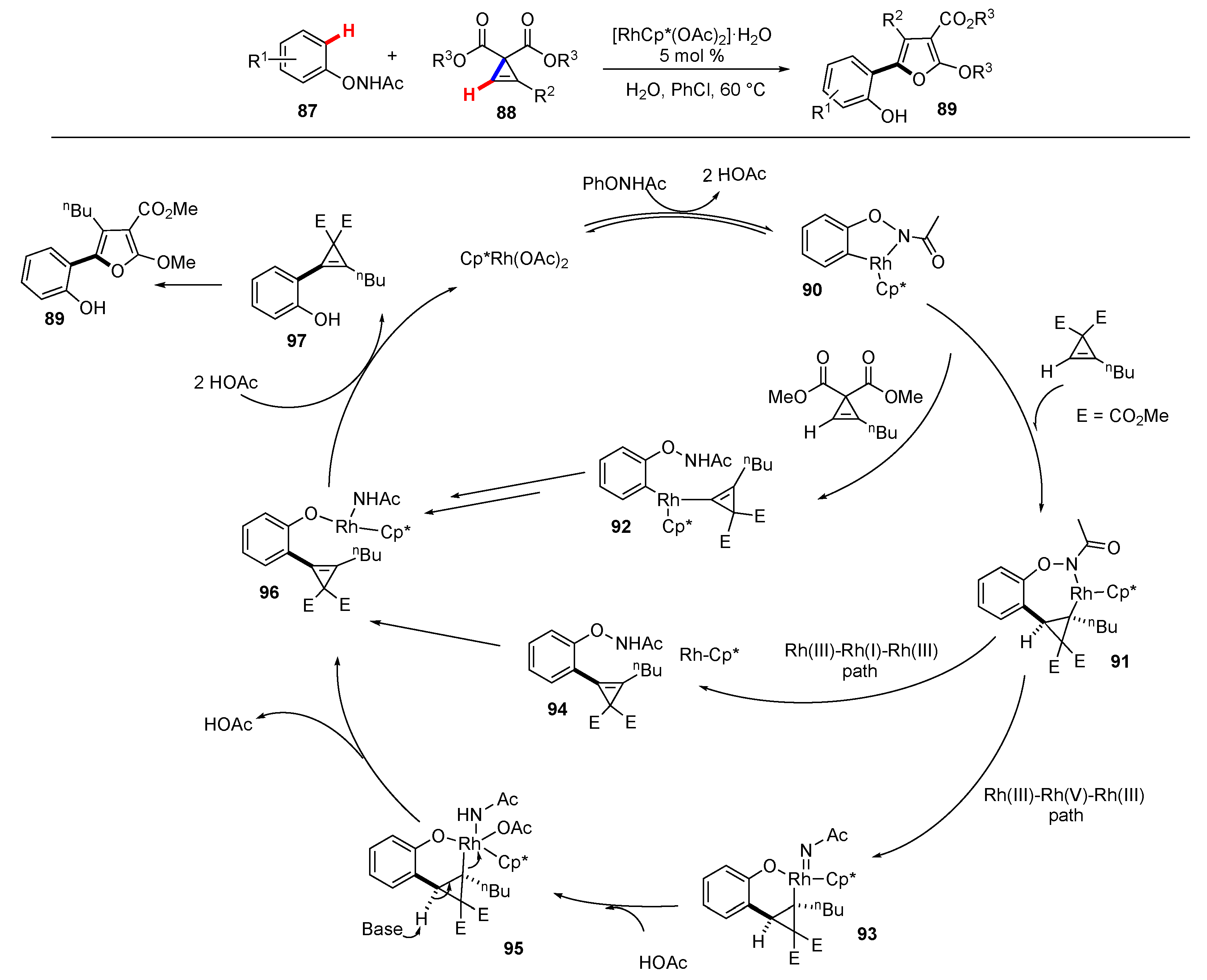
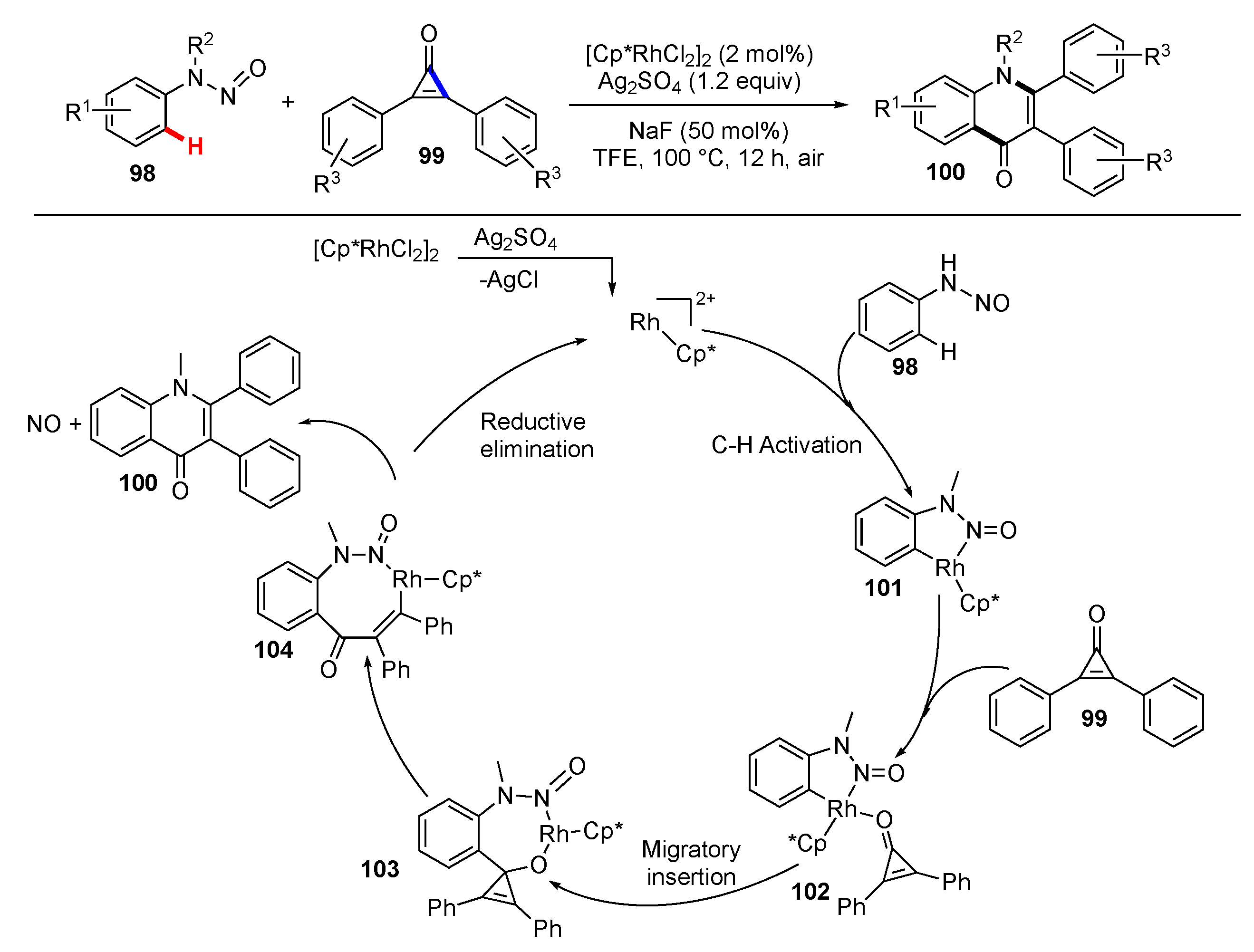




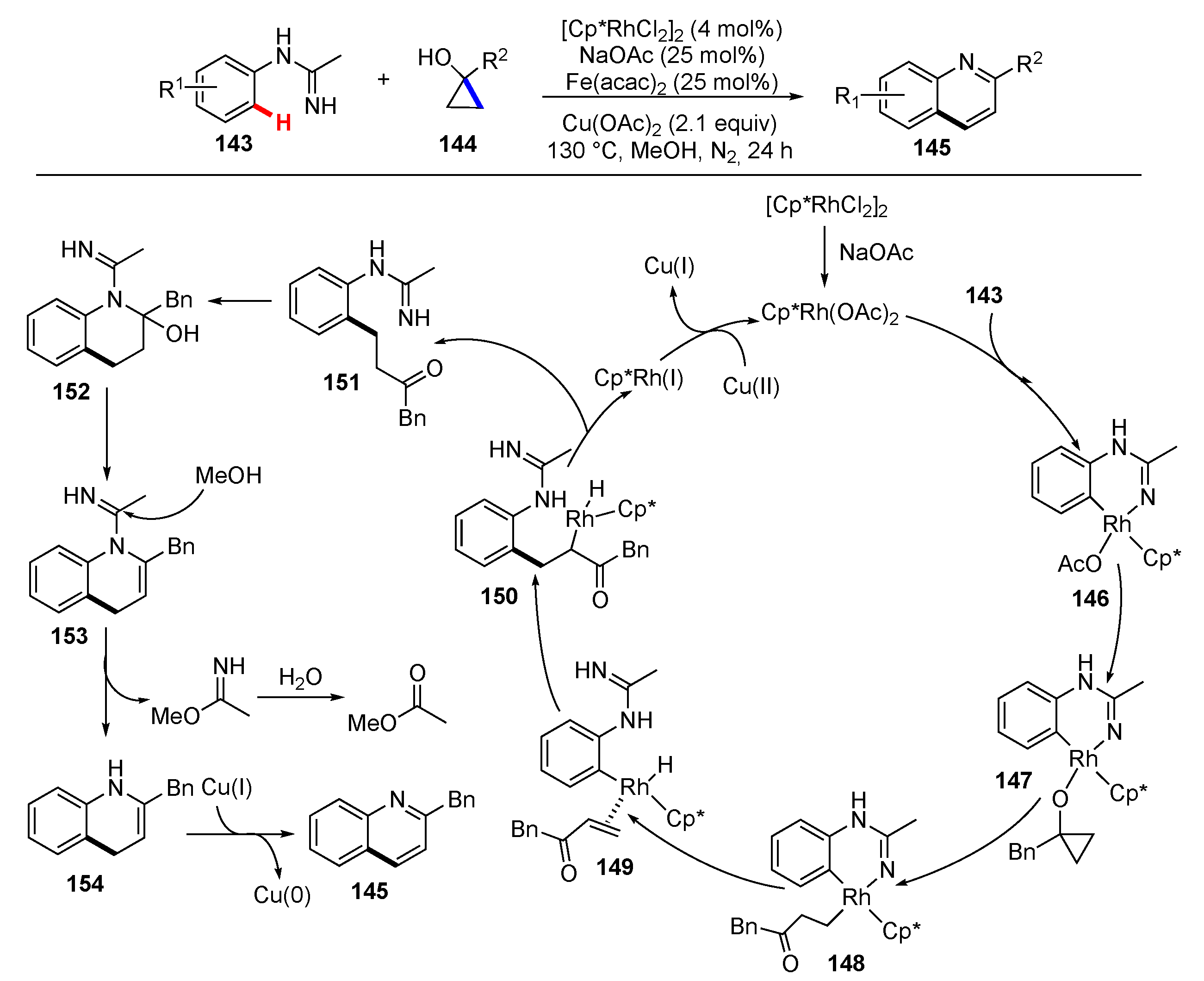








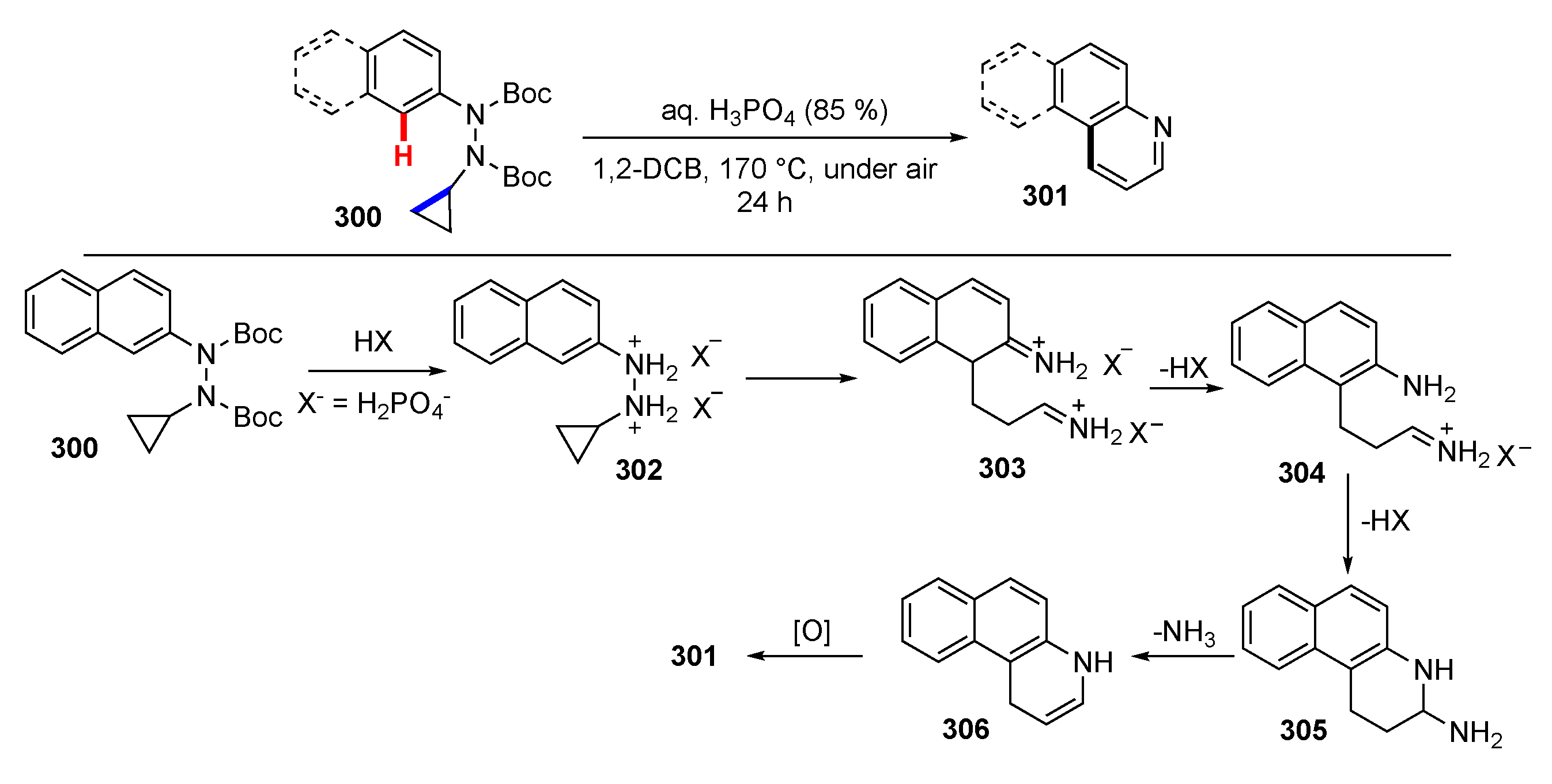
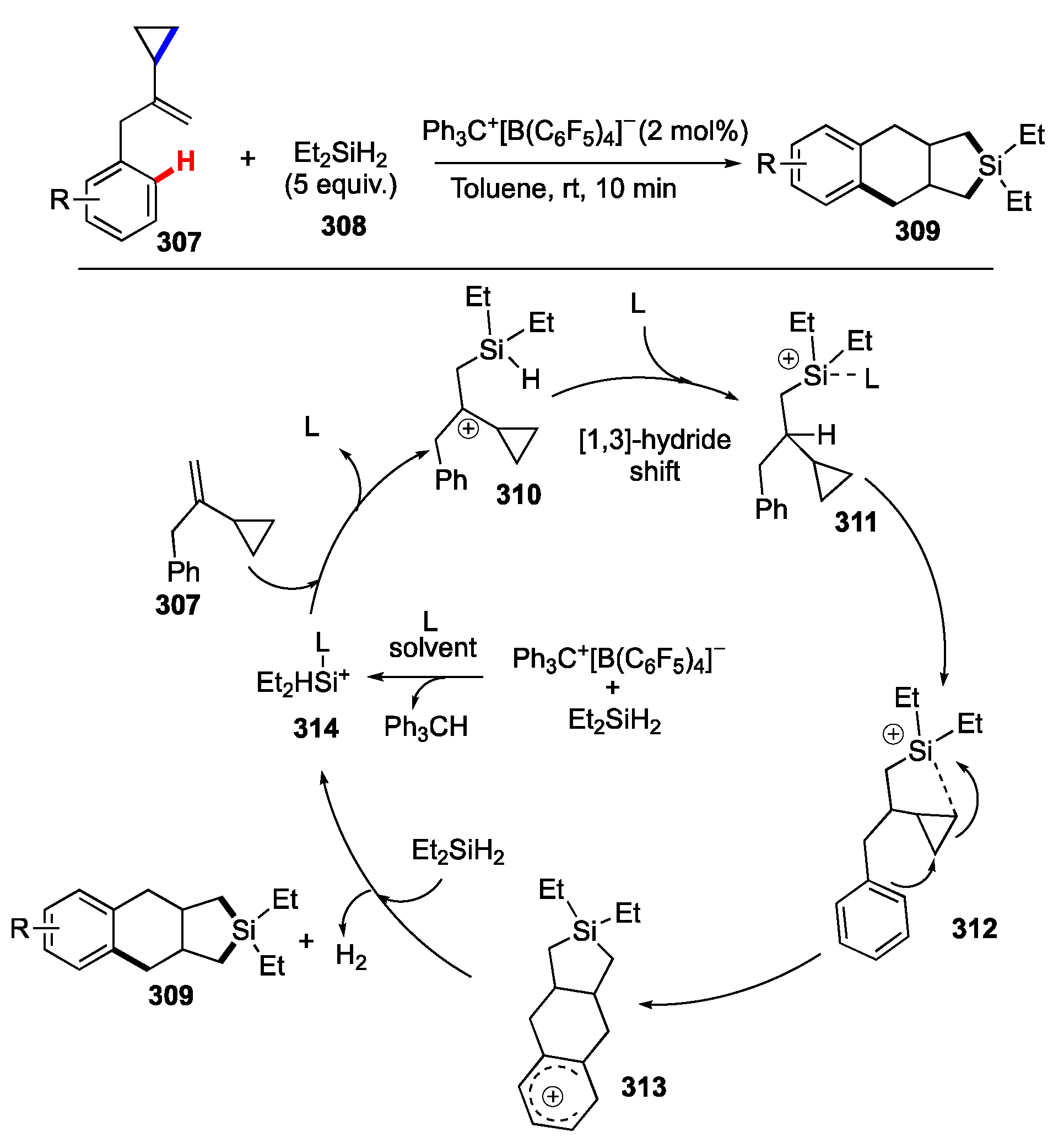
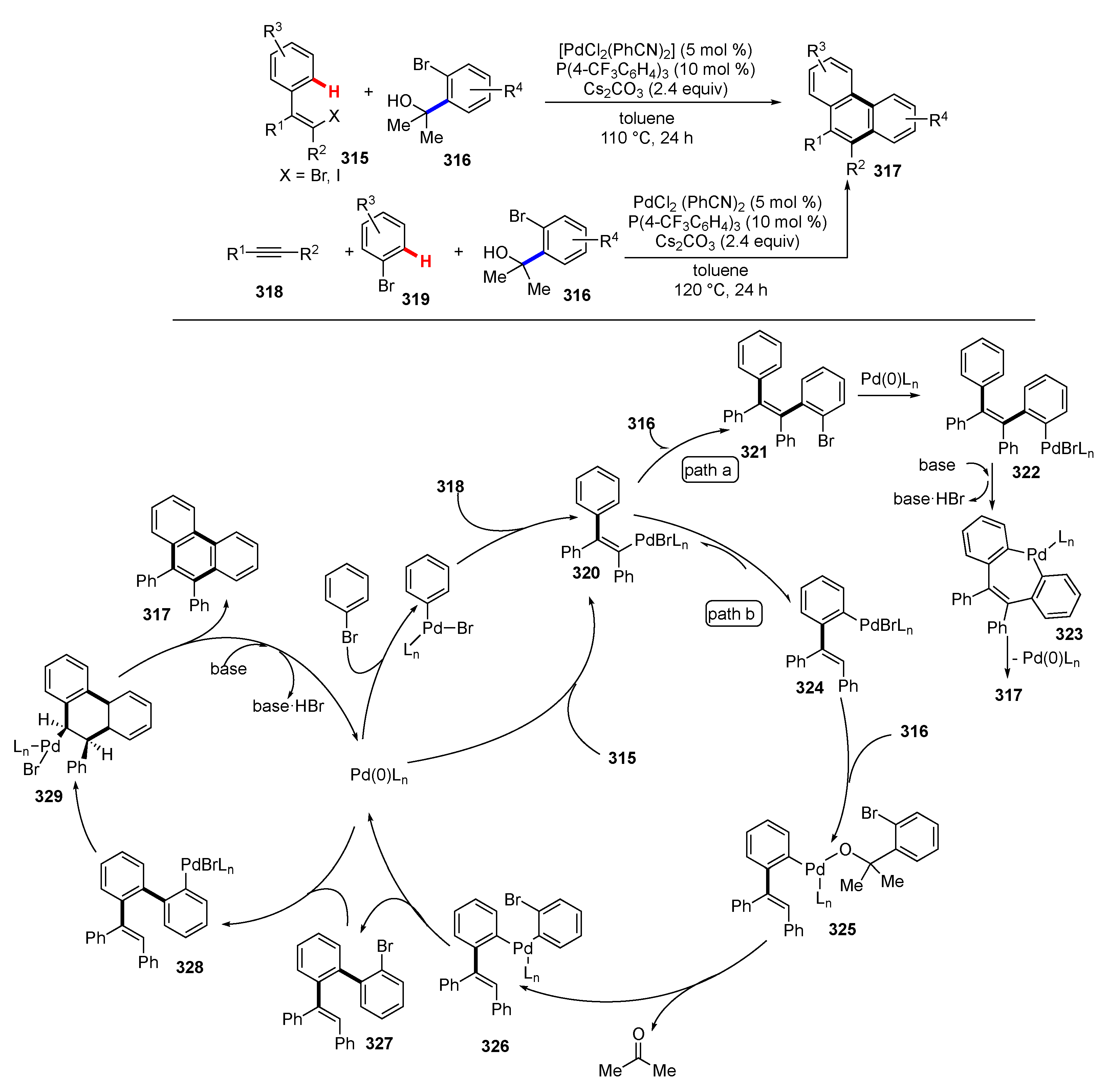


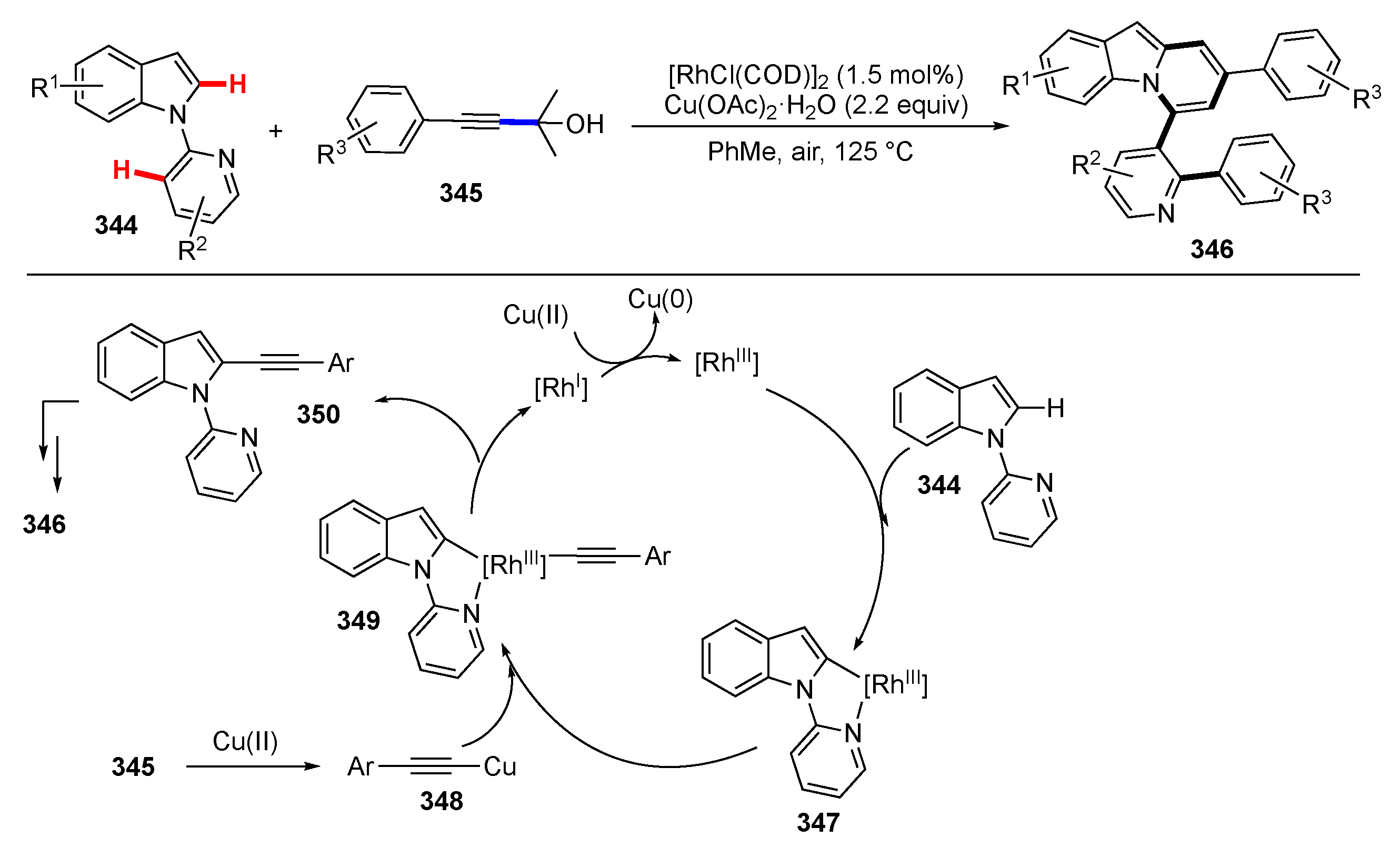
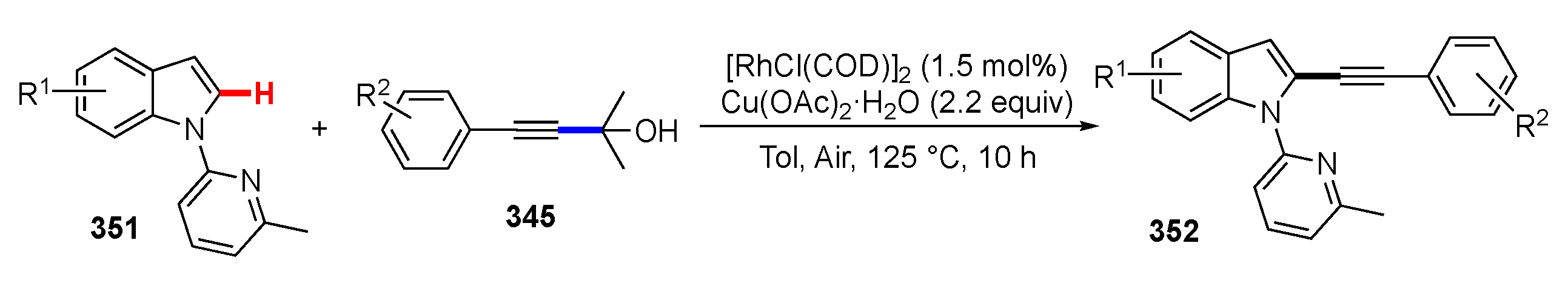

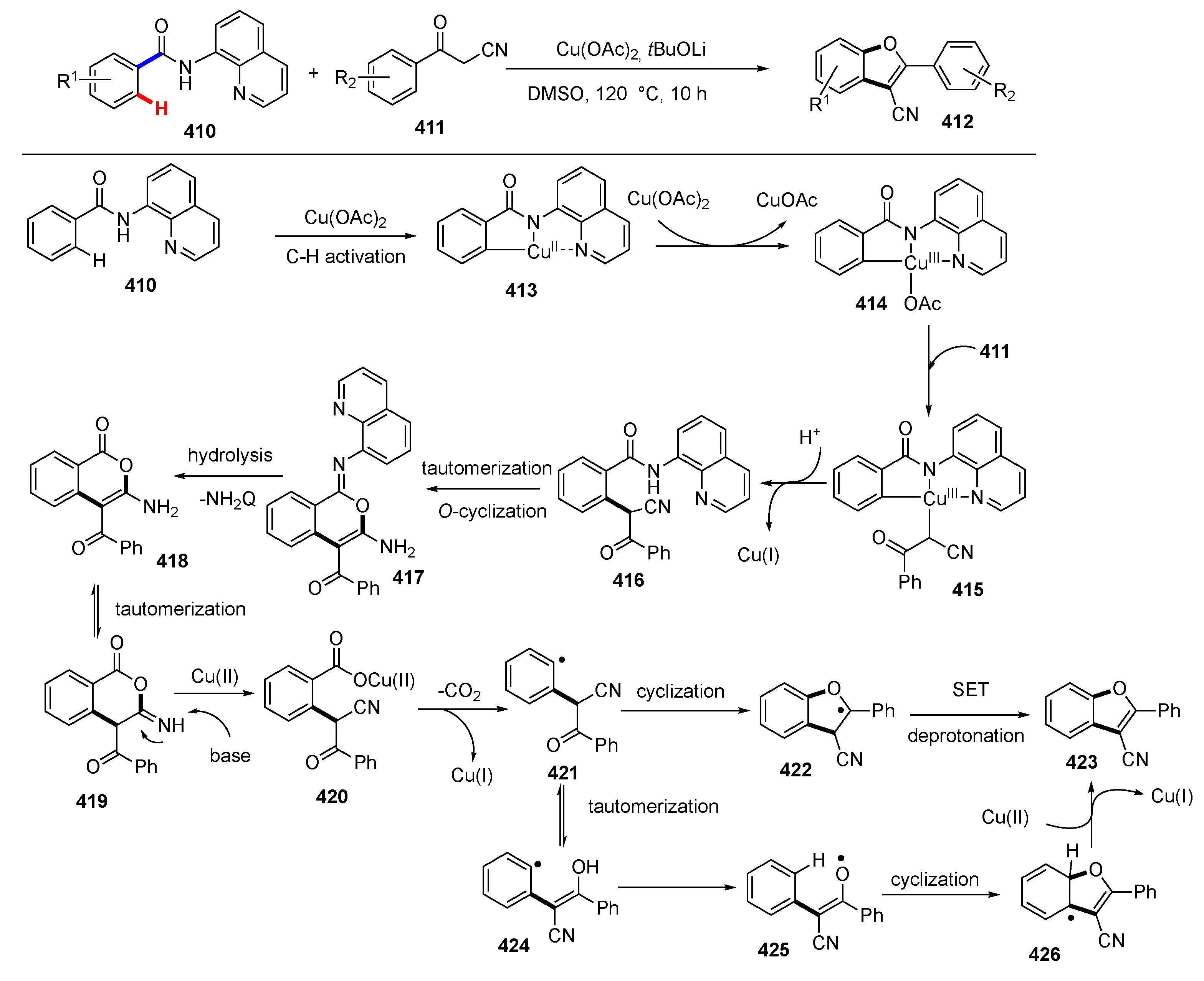
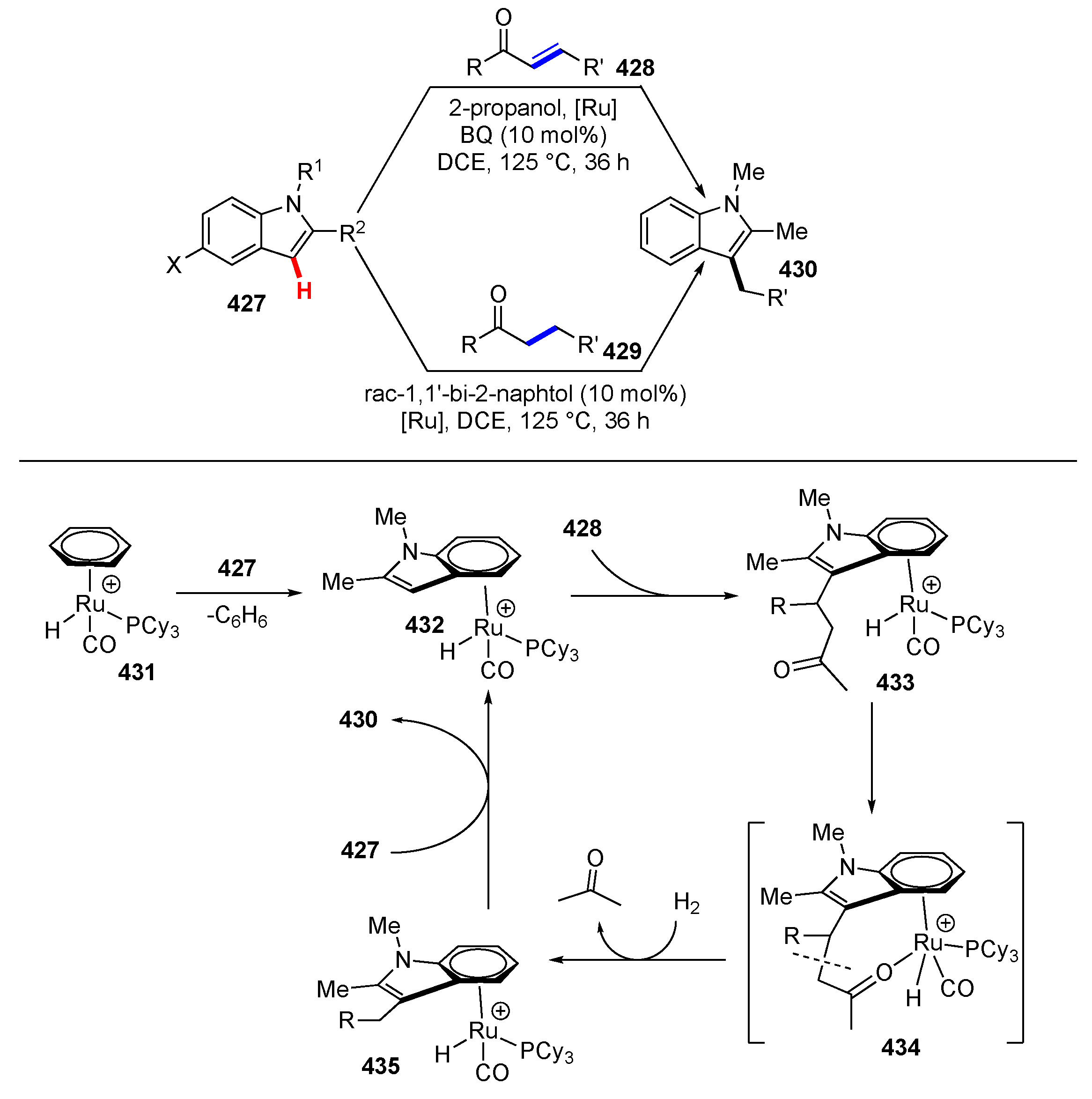
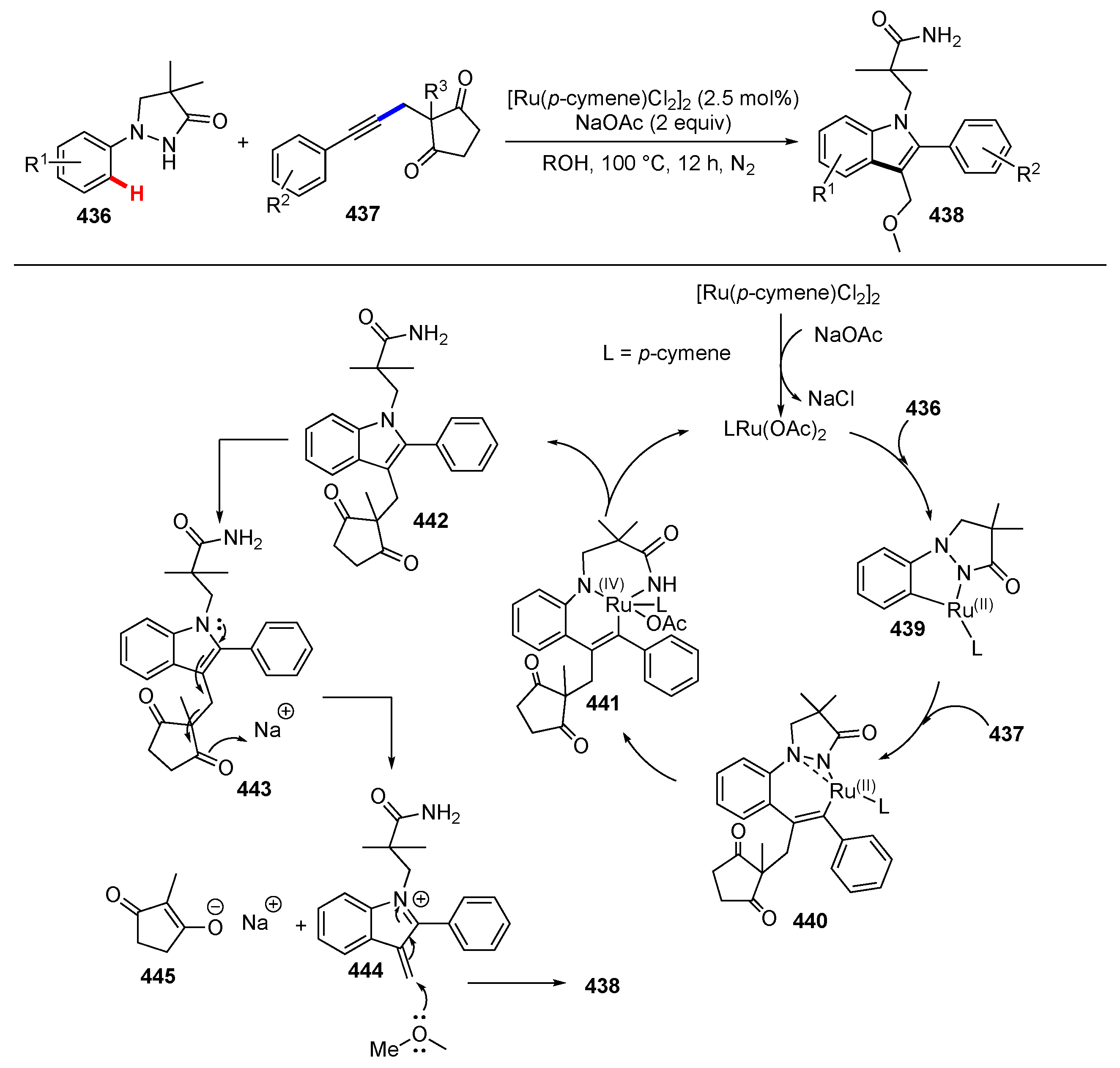
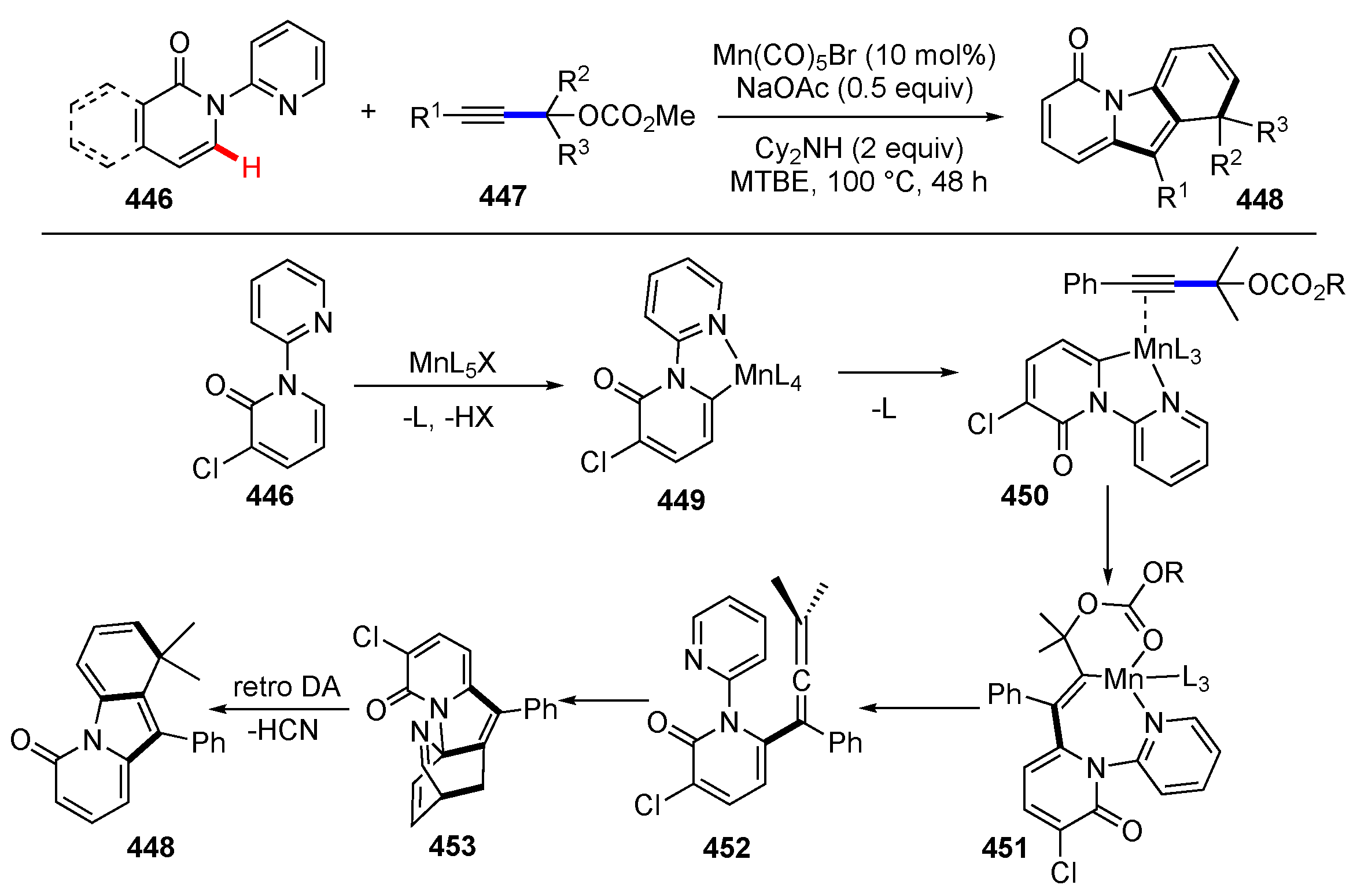





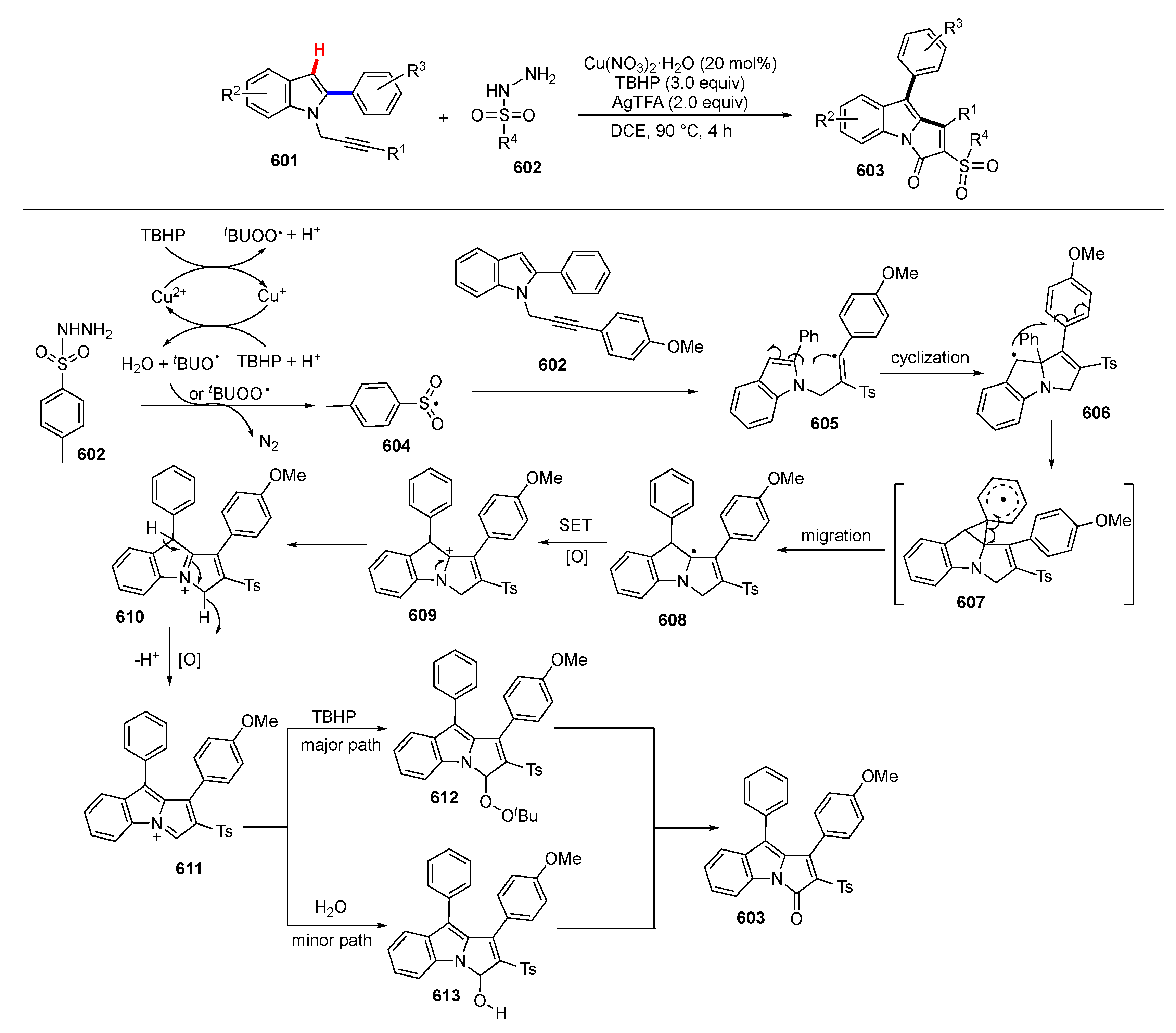
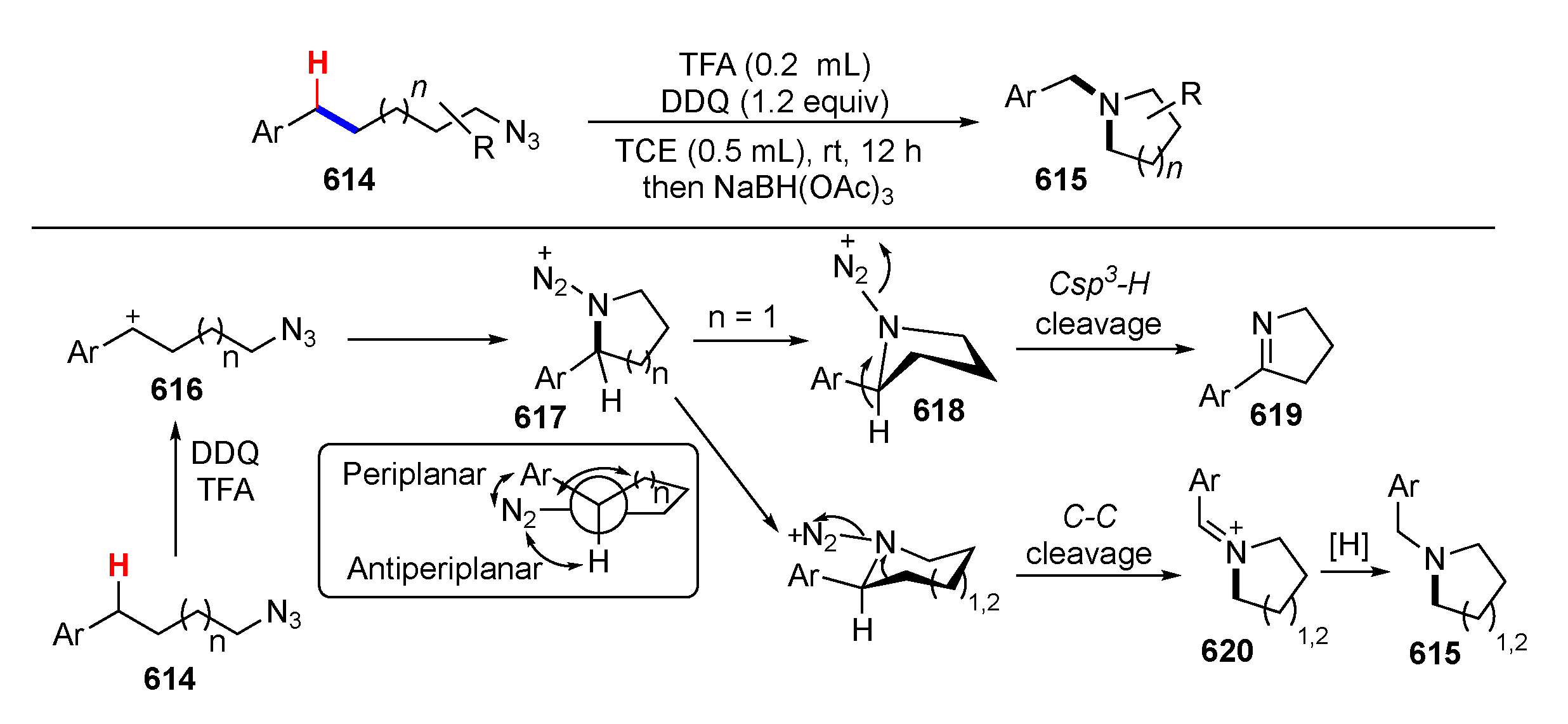
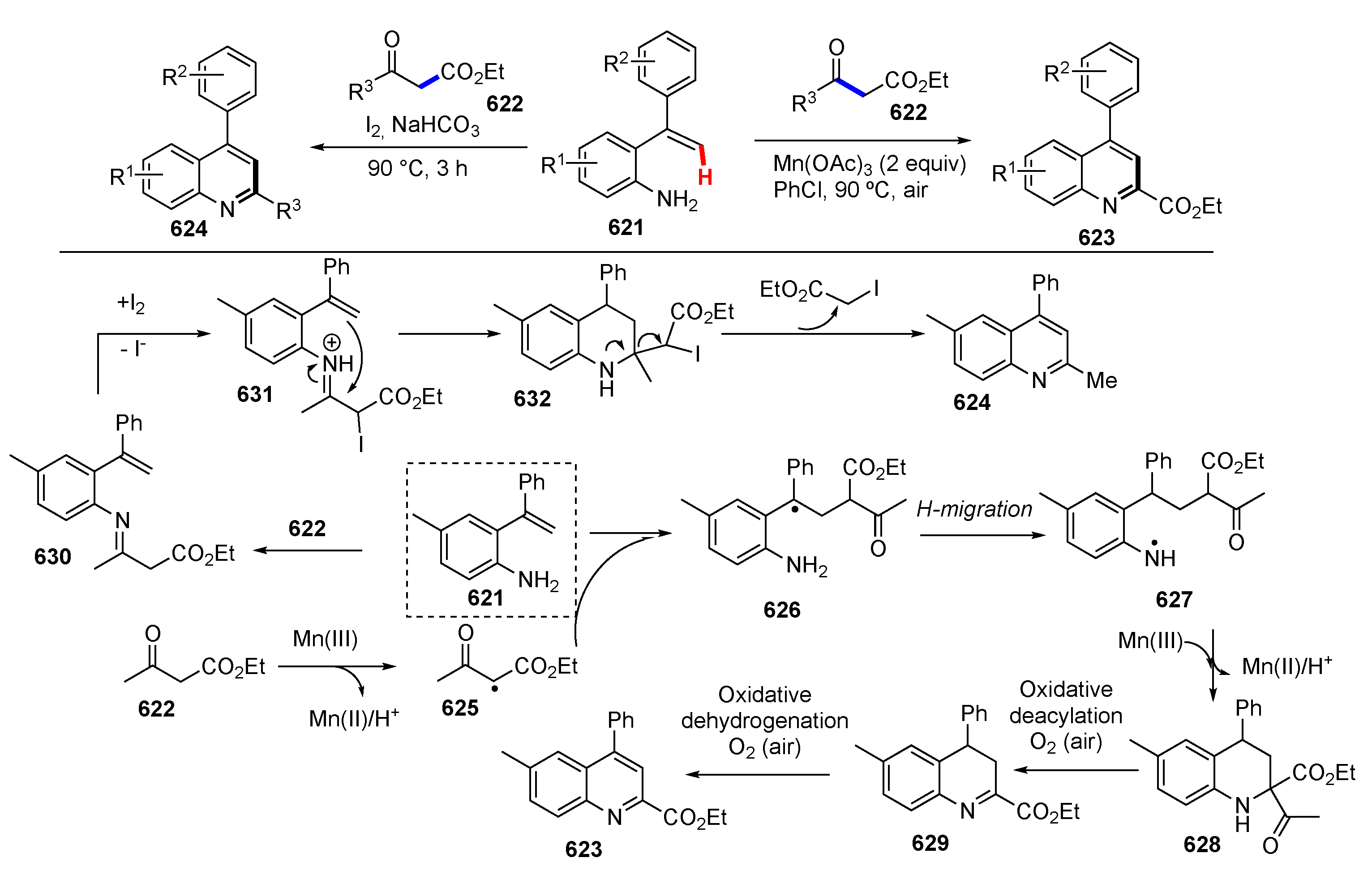

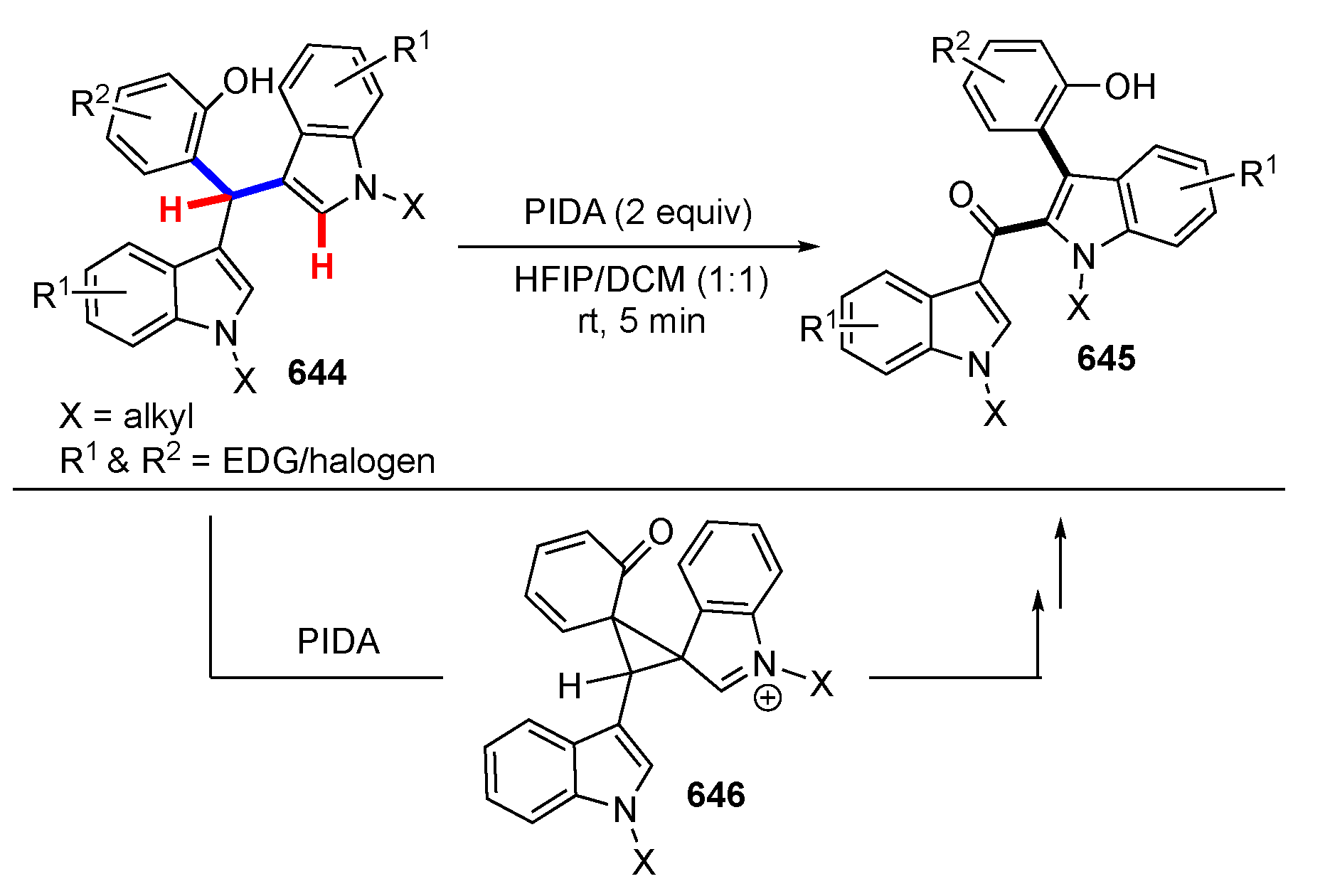

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azizollahi, H.; García-López, J.-A. Recent Advances on Synthetic Methodology Merging C–H Functionalization and C–C Cleavage. Molecules 2020, 25, 5900. https://doi.org/10.3390/molecules25245900
Azizollahi H, García-López J-A. Recent Advances on Synthetic Methodology Merging C–H Functionalization and C–C Cleavage. Molecules. 2020; 25(24):5900. https://doi.org/10.3390/molecules25245900
Chicago/Turabian StyleAzizollahi, Hamid, and José-Antonio García-López. 2020. "Recent Advances on Synthetic Methodology Merging C–H Functionalization and C–C Cleavage" Molecules 25, no. 24: 5900. https://doi.org/10.3390/molecules25245900
APA StyleAzizollahi, H., & García-López, J.-A. (2020). Recent Advances on Synthetic Methodology Merging C–H Functionalization and C–C Cleavage. Molecules, 25(24), 5900. https://doi.org/10.3390/molecules25245900





