The Role of Epigenetic Functionalization of Implants and Biomaterials in Osseointegration and Bone Regeneration—A Review †
Abstract
1. Introduction
1.1. Pitfalls of Current Biomaterials/Implants
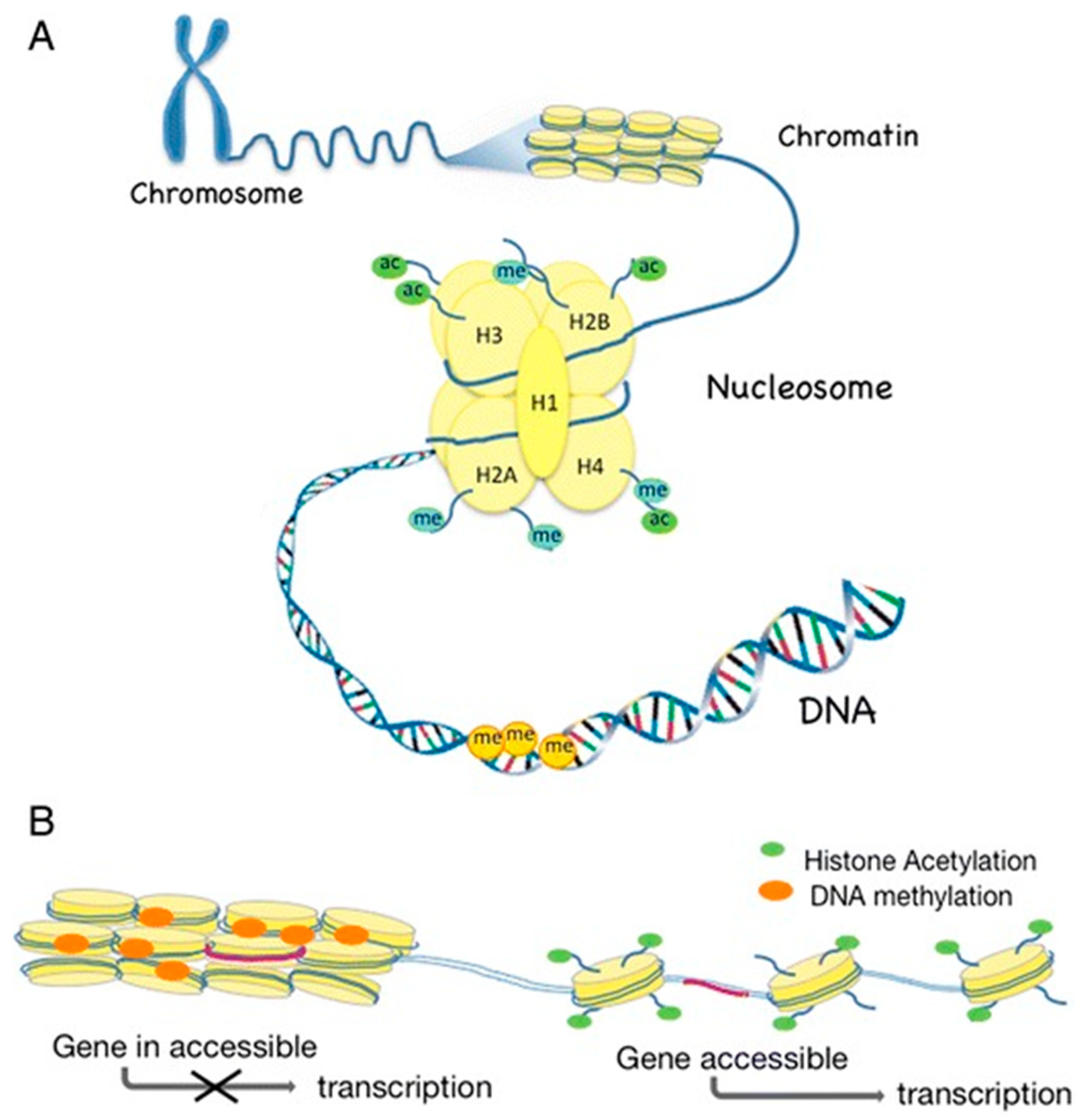
1.2. What Is Epigenetics?
1.2.1. DNA Methylation
1.2.2. Histone Modifications
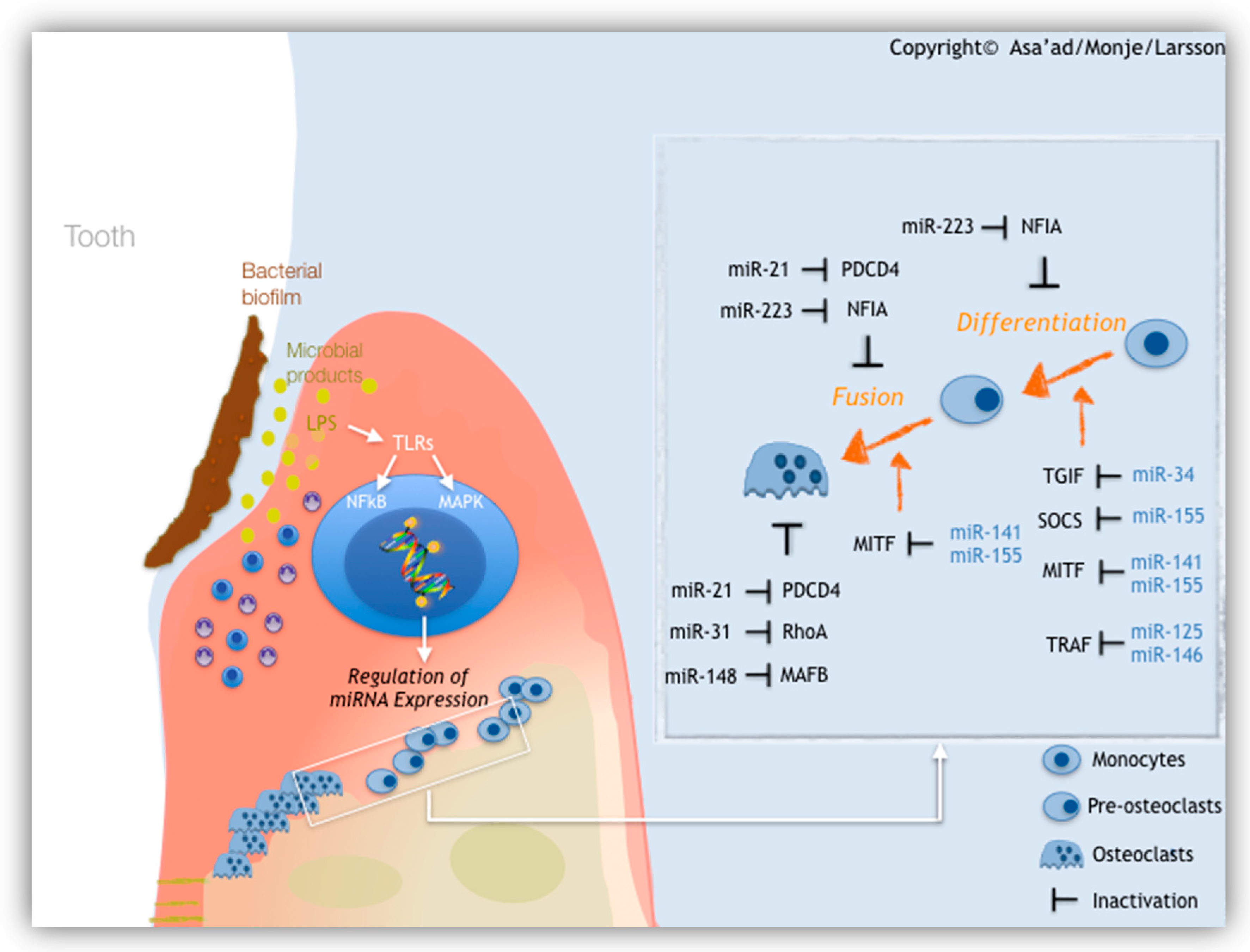
1.2.3. MicroRNAs (miRNAs)
2. Materials and Methods
- (i)
- Which, if any, are the epigenetic mechanisms used to functionalize implant surfaces to achieve better osseointegration?
- (ii)
- Which, if any, are the epigenetic mechanisms used to functionalize biomaterials to achieve better tissue regeneration?
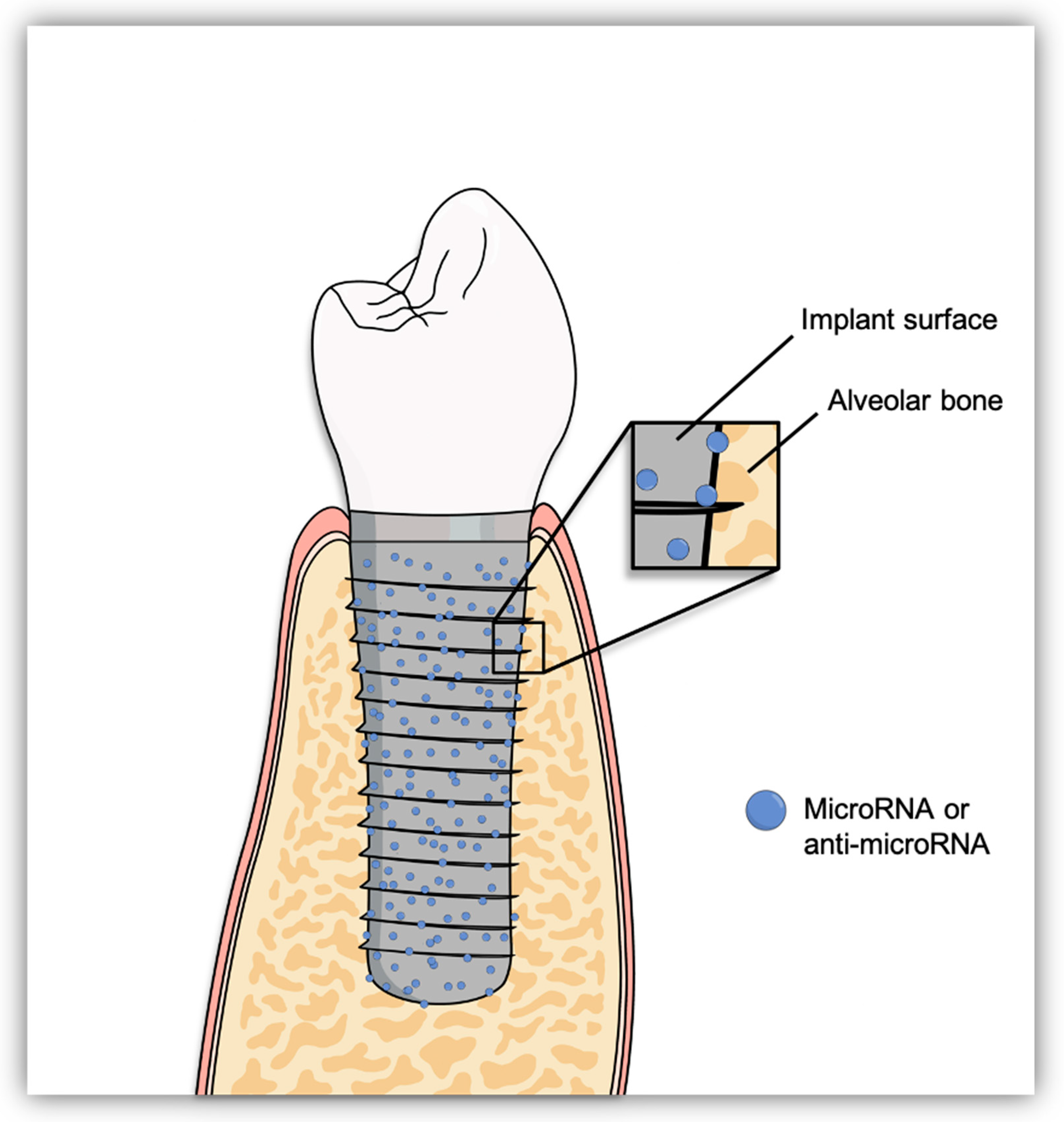
3. Epigenetic Functionalization of Implant Surfaces to Enhance Osseointegration
4. Epigenetic Functionalization of Biomaterials to Enhance Bone Regeneration
4.1. Biomaterials and Scaffolds
4.1.1. Natural Polymers
4.1.2. Synthetic Polymers
4.1.3. Bioceramics
5. Conclusions and Future Directions
- Functionalization of implant surfaces have been done with miRNAs or anti-miRNAs, directly coated on the surface or coated on a biomaterial then attached to the implant surface or by seeding miRNA/anti-miRNA-transfected cells on the implant.
- Unlike cancer studies, which heavily reported on DNA methylation and histone modifications, functionalization of biomaterials and scaffolds for bone regeneration have been done with miRNAs or anti-miRNAs, except for one study that reported on the use HDACis.
- For bone regeneration, the functionalized scaffolds of different biomaterials were either cell free, or were loaded with miRNA or anti-miRNA-transfected stem cells.
- Modulating the inflammatory and immune reaction with these functionalized scaffolds to enhance bone regeneration is possible, either by influencing macrophage polarization or the recruitment of Tregs.
- Microspheres, nanoparticles, and PEI-based nanoparticles are heavily applied for miRNA or anti-miRNA delivery.
Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palmquist, A.; Omar, O.M.; Esposito, M.; Lausmaa, J.; Thomsen, P. Titanium oral implants: Surface characteristics, interface biology and clinical outcome. J. R. Soc. Interface 2010, 7, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Meagher, P.; O’Cearbhaill, E.D.; Byrne, J.H.; Browne, D.J. Bulk Metallic Glasses for Implantable Medical Devices and Surgical Tools. Adv. Mater. 2016, 28, 5755–5762. [Google Scholar] [CrossRef] [PubMed]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 2019, 84, 1–15. [Google Scholar] [CrossRef]
- Shah, F.A.; Thomsen, P.; Palmquist, A. A Review of the Impact of Implant Biomaterials on Osteocytes. J. Dent. Res. 2018, 97, 977–986. [Google Scholar] [CrossRef]
- Shah, F.A.; Stenlund, P.; Martinelli, A.; Thomsen, P.; Palmquist, A. Direct communication between osteocytes and acid-etched titanium implants with a sub-micron topography. J. Mater. Sci. Mater. Med. 2016, 27, 167. [Google Scholar] [CrossRef]
- Shah, F.A.; Nilson, B.; Brånemark, R.; Thomsen, P.; Palmquist, A. The bone-implant interface—Nanoscale analysis of clinically retrieved dental implants. Nanomedicine 2014, 10, 1729–1737. [Google Scholar] [CrossRef]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Dallas, S.L.; Prideaux, M.; Bonewald, L.F. The osteocyte: An endocrine cell. and more. Endocr. Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef]
- Brånemark, R.; Emanuelsson, L.; Palmquist, A.; Thomsen, P. Bone response to laser-induced micro- and nano-size titanium surface features. Nanomedicine 2011, 7, 220–227. [Google Scholar] [CrossRef]
- Palmquist, A.; Grandfield, K.; Norlindh, B.; Mattsson, T.; Brånemark, R.; Thomsen, P. Bone-titanium oxide interface in humans revealed by transmission electron microscopy and electron tomography. J. R. Soc. Interface 2012, 9, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Grandfield, K.; Gustafsson, S.; Palmquist, A. Where bone meets implant: The characterization of nano-osseointegration. Nanoscale 2013, 5, 4302–4308. [Google Scholar] [CrossRef] [PubMed]
- Grandfield, K.; Palmquist, A.; Engqvist, H. Three-dimensional structure of laser-modified Ti6Al4V and bone interface revealed with STEM tomography. Ultramicroscopy 2013, 127, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Trtica, M.; Gakovic, B.; Batani, D.; Desai, T.; Panjan, P.; Radak, B. Surface modifications of a titanium implant by a picosecond Nd:YAG laser operating at 1064 and 532 nm. Appl. Surf. Sci. 2006, 253, 2551–2556. [Google Scholar] [CrossRef]
- Pobloth, A.M.; Checa, S.; Razi, H.; Petersen, A.; Weaver, J.C.; Schmidt-Bleek, K.; Windolf, M.; Tatai, A.Á.; Roth, C.P.; Schaser, K.D.; et al. Mechanobiologically optimized 3D titanium-mesh scaffolds enhance bone regeneration in critical segmental defects in sheep. Sci. Transl. Med. 2018, 10, eaam8828. [Google Scholar] [CrossRef] [PubMed]
- Murr, L.E.; Amato, K.N.; Li, S.J.; Tian, Y.X.; Cheng, X.Y.; Gaytan, S.M.; Martinez, E.; Shindo, P.W.; Medina, F.; Wicker, R.B. Microstructure and mechanical properties of open-cellular biomaterials prototypes for total knee replacement implants fabricated by electron beam melting. J. Mech. Behav. Biomed. Mater. 2011, 4, 1396–1411. [Google Scholar] [CrossRef] [PubMed]
- Amin Yavari, S.; Ahmadi, S.M.; Wauthle, R.; Pouran, B.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Relationship between unit cell type and porosity and the fatigue behavior of selective laser melted meta-biomaterials. J. Mech. Behav. Biomed. Mater. 2015, 43, 91–100. [Google Scholar] [CrossRef]
- Palmquist, A.; Snis, A.; Emanuelsson, L.; Browne, M.; Thomsen, P. Long-term biocompatibility and osseointegration of electron beam melted, free-form-fabricated solid and porous titanium alloy: Experimental studies in sheep. J. Biomater. Appl. 2013, 27, 1003–1016. [Google Scholar] [CrossRef]
- Shah, F.A.; Snis, A.; Matic, A.; Thomsen, P.; Palmquist, A. 3D printed Ti6Al4V implant surface promotes bone maturation and retains a higher density of less aged osteocytes at the bone-implant interface. Acta Biomater. 2016, 30, 357–367. [Google Scholar] [CrossRef]
- Palmquist, A.; Shah, F.A.; Emanuelsson, L.; Omar, O.; Suska, F. A technique for evaluating bone ingrowth into 3D printed, porous Ti6Al4V implants accurately using X-ray micro-computed tomography and histomorphometry. Micron 2017, 94, 1–8. [Google Scholar] [CrossRef]
- Suska, F.; Kjeller, G.; Tarnow, P.; Hryha, E.; Nyborg, L.; Snis, A.; Palmquist, A. Electron Beam Melting Manufacturing Technology for Individually Manufactured Jaw Prosthesis: A Case Report. J. Oral Maxillofac. Surg. 2016, 74, 1706.e1–1706.e15. [Google Scholar] [CrossRef] [PubMed]
- Thor, A.; Palmquist, A.; Hirsch, J.M.; Rännar, L.E.; Dérand, P.; Omar, O. Clinical, Morphological, and Molecular Evaluations of Bone Regeneration With an Additive Manufactured Osteosynthesis Plate. J. Craniofac. Surg. 2016, 27, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Omar, O.; Suska, F.; Snis, A.; Matic, A.; Emanuelsson, L.; Norlindh, B.; Lausmaa, J.; Thomsen, P.; Palmquist, A. Long-term osseointegration of 3D printed CoCr constructs with an interconnected open-pore architecture prepared by electron beam melting. Acta Biomater. 2016, 36, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Jergéus, E.; Chiba, A.; Palmquist, A. Osseointegration of 3D printed microalloyed CoCr implants—Addition of 0.04% Zr to CoCr does not alter bone material properties. J. Biomed. Mater. Res. A 2018, 106, 1655–1663. [Google Scholar] [CrossRef]
- Stenlund, P.; Kurosu, S.; Koizumi, Y.; Suska, F.; Matsumoto, H.; Chiba, A.; Plamquist, A. Osseointegration Enhancement by Zr doping of Co-Cr-Mo Implants Fabricated by Electron Beam Melting. Addit. Manuf. 2015, 6, 6–15. [Google Scholar] [CrossRef]
- Puleo, D.A.; Nanci, A. Understanding and controlling the bone-implant interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Shah, N.J.; Hyder, M.N.; Moskowitz, J.S.; Quadir, M.A.; Morton, S.W.; Seeherman, H.J.; Padera, R.F.; Spector, M.; Hammond, P.T. Surface-mediated bone tissue morphogenesis from tunable nanolayered implant coatings. Sci. Transl. Med. 2013, 5, 191ra83. [Google Scholar] [CrossRef]
- Thorfve, A.; Lindahl, C.; Xia, W.; Igawa, K.; Lindahl, A.; Thomsen, P.; Palmquist, A.; Tengvall, P. Hydroxyapatite coating affects the Wnt signaling pathway during peri-implant healing in vivo. Acta Biomater. 2014, 10, 1451–1462. [Google Scholar] [CrossRef]
- Harmankaya, N.; Karlsson, J.; Palmquist, A.; Halvarsson, M.; Igawa, K.; Andersson, M.; Tengvall, P. Raloxifene and alendronate containing thin mesoporous titanium oxide films improve implant fixation to bone. Acta Biomater. 2013, 9, 7064–7073. [Google Scholar] [CrossRef] [PubMed]
- Sela, M.N.; Kohavi, D.; Krausz, E.; Steinberg, D.; Rosen, G. Enzymatic degradation of collagen-guided tissue regeneration membranes by periodontal bacteria. Clin. Oral Implants Res. 2003, 14, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yang, W.Z.; Shi, D.; Wu, M.; Xiong, X.L.; Chen, Z.G.; Wei, S.C. Bioinspired and osteopromotive polydopamine nanoparticle-incorporated fibrous membranes for robust bone regeneration. NPG Asia Mater. 2019, 11, 39. [Google Scholar] [CrossRef]
- Waddington, C.H. The epigenotype. Int. J. Epidemiol. 2012, 41, 10–13. [Google Scholar] [CrossRef]
- Allis, C.D.; Jenuwein, T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016, 17, 487–500. [Google Scholar] [CrossRef]
- Sun, S.; Barreiro, L.B. The epigenetically-encoded memory of the innate immune system. Curr. Opin. Immunol. 2020, 65, 7–13. [Google Scholar] [CrossRef]
- Barros, S.P.; Offenbacher, S. Epigenetics: Connecting environment and genotype to phenotype and disease. J. Dent. Res. 2009, 88, 400–408. [Google Scholar] [CrossRef]
- Larsson, L.; Castilho, R.M.; Giannobile, W.V. Review Epigenetics and Its Role in Periodontal Diseases: A State-of-the-Art Review. J. Periodontol. 2015, 86, 556–568. [Google Scholar] [CrossRef]
- Ivanov, M.; Barragani, I.; Ingelman-Sundberg, M. Epigenetic mechanisms of importance for drug treatment. Trends Pharmacol. Sci. 2014, 35, 384–396. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Song, J.; Liu, Y.; Song, C.X.; Yi, C. Mapping the epigenetic modifications of DNA and RNA. Protein Cell 2020, 22. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.V.C.; Bourc’his, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell. Biol. 2019, 20, 590–607. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Kriaucionis, S.; Heintz, N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 2009, 324, 929–930. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Shen, L.; Dai, Q.; Wu, S.C.; Collins, L.B.; Swenberg, J.A.; He, C.; Zhang, Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011, 333, 1300–1303. [Google Scholar] [CrossRef]
- He, Y.F.; Li, B.Z.; Li, Z.; Liu, P.; Wang, Y.; Tang, Q.; Ding, J.; Jia, Y.; Chen, Z.; Li, L.; et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 2011, 333, 1303–1307. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, G.Z.; Chen, K.; Deng, X.; Yu, M.; Han, D.; Hao, Z.; Liu, J.; Lu, X.; Dore, L.C.; et al. N6-methyldeoxyadenosine marks active transcription start sites in Chlamydomonas. Cell 2015, 161, 879–892. [Google Scholar] [CrossRef]
- Greer, E.L.; Blanco, M.A.; Gu, L.; Sendinc, E.; Liu, J.; Aristizábal-Corrales, D.; Hsu, C.H.; Aravind, L.; He, C.; Shi, Y. DNA Methylation on N6-Adenine in, C. elegans. Cell 2015, 161, 868–878. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, H.; Liu, D.; Cheng, Y.; Liu, X.; Zhang, W.; Yin, R.; Zhang, D.; Zhang, P.; Liu, J.; et al. N6-methyladenine DNA modification in Drosophila. Cell 2015, 161, 893–906. [Google Scholar] [CrossRef]
- Weintraub, H.; Palter, K.; Van Lente, F. Histones H2a, H2b, H3, and H4 form a tetrameric complex in solutions of high salt. Cell 1975, 6, 85–110. [Google Scholar] [CrossRef]
- Szerlong, H.J.; Hansen, J.C. Nucleosome distribution and linker DNA: Connecting nuclear function to dynamic chromatin structure. Biochem. Cell Biol. 2011, 89, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.M. Cellular memory and the histone code. Cell 2002, 111, 285–291. [Google Scholar] [CrossRef]
- Jenuwein, T.; Allis, C.D. Translating the histone code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Javaid, N.; Choi, S. Acetylation- and Methylation-Related Epigenetic Proteins in the Context of Their Targets. Genes 2017, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Shi, Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Black, J.C.; Van Rechem, C.; Whetstine, J.R. Histone lysine methylation dynamics: Establishment, regulation, and biological impact. Mol. Cell 2012, 48, 491–507. [Google Scholar] [CrossRef]
- Robertson, K.D.; Wolffe, A.P. DNA methylation in health and disease. Nat. Rev. Genet. 2000, 1, 11–19. [Google Scholar] [CrossRef]
- Larsson, L. Current Concepts of Epigenetics and Its Role in Periodontitis. Curr. Oral Health Rep. 2017, 4, 286–293. [Google Scholar] [CrossRef]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef]
- Selbach, M.; Schwanhäusser, B.; Thierfelder, N.; Fang, Z.; Khanin, R.; Rajewsky, N. Widespread changes in protein synthesis induced by microRNAs. Nature 2008, 455, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Sonkoly, E.; Pivarcsi, A. Advances in microRNAs: Implications for immunity and inflammatory diseases. J. Cell Mol. Med. 2009, 13, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Asa’ad, F.; Garaicoa-Pazmiño, C.; Dahlin, C.; Larsson, L. Expression of micrornas in periodontal and peri-implant diseases: A systematic review and meta-analysis. Int. J. Mol. Sci. 2020, 21, 4147. [Google Scholar] [CrossRef] [PubMed]
- Asa’ad, F.; Monje, A.; Larsson, L. Role of epigenetics in alveolar bone resorption and regeneration around periodontal and peri-implant tissues. Eur. J. Oral Sci. 2019, 127, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kitagawa, N.; Isobe, A. Implant treatment in ultra-aged society. Jpn. Dent. Sci. Rev. 2018, 54, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.; Soldatos, N.; Tran, D.; Stylianou, P.; Angelov, N.; Weltman, R. Survival of Dental Implants Replacing Previously Failed Implants: A Retrospective Study in a University Setting. Int. J. Oral Maxillofac. Implants 2018, 33, 1312–1319. [Google Scholar] [CrossRef]
- Pamies, P. Long-lived biomaterials. Nat. Biomed. Eng. 2017, 1, 95. [Google Scholar]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol 2000 2017, 73, 7–21. [Google Scholar] [CrossRef]
- Bai, L.; Du, Z.; Du, J.; Yao, W.; Zhang, J.; Weng, Z.; Liu, S.; Zhao, Y.; Liu, Y.; Zhang, X.; et al. A multifaceted coating on titanium dictates osteoimmunomodulation and osteo/angio-genesis towards ameliorative osseointegration. Biomaterials 2018, 162, 154–169. [Google Scholar] [CrossRef]
- Meng, Y.; Li, X.; Li, Z.; Liu, C.; Zhao, J.; Wang, J.; Liu, Y.; Yuan, X.; Cui, Z.; Yang, X. Surface Functionalization of Titanium Alloy with miR-29b Nanocapsules To Enhance Bone Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 5783–5793. [Google Scholar] [CrossRef]
- Singh, A.; Ali, S.; Mahdi, A.A.; Srivastava, R.N. MicroRNAs and Their Role in Bone Remodeling and Pathogenesis. Br. J. Med. Med. Res. 2012, 2, 727–749. [Google Scholar] [CrossRef][Green Version]
- Suh, J.S.; Lee, J.Y.; Choi, Y.S.; Chung, C.P.; Park, Y.J. Peptide-mediated intracellular delivery of miRNA-29b for osteogenic stem cell differentiation. Biomaterials 2013, 34, 4347–4359. [Google Scholar] [CrossRef]
- Fang, S.; Deng, Y.; Gu, P.; Fan, X. MicroRNAs Regulate Bone Development and Regeneration. Int. J. Mol. Sci. 2015, 16, 8227–8253. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Pitari, M.R.; Amodio, N.; Di Martino, M.T.; Conforti, F.; Leone, E.; Botta, C.; Paolino, F.M.; Del Giudice, T.; Iuliano, E.; et al. miR-29b negatively regulates human osteoclastic cell differentiation and function: Implications for the treatment of multiple myeloma-related bone disease. J. Cell Physiol. 2013, 228, 1506–1515. [Google Scholar] [CrossRef]
- Bushati, N.; Cohen, S.M. MicroRNA functions. Annu. Rev. Cell Dev. Biol. 2007, 23, 175–205. [Google Scholar] [CrossRef] [PubMed]
- Gámez, B.; Rodriguez-Carballo, E.; Ventura, F. MicroRNAs and post-transcriptional regulation of skeletal development. J. Mol. Endocrinol. 2014, 52, R179–R197. [Google Scholar] [CrossRef] [PubMed]
- Krichevsky, A.M.; Gabriely, G. miR-21: A small multi-faceted RNA. J. Cell Mol. Med. 2009, 13, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, T.D.; Zhou, L.; Sokol, L.; Kessel, R.; Caceres, G.; Gundabolu, K.; Tamari, R.; Gordon, S.; Mantzaris, I.; Jodlowski, T.; et al. miR-21 mediates hematopoietic suppression in MDS by activating TGF-β signaling. Blood 2013, 121, 2875–2881. [Google Scholar] [CrossRef]
- Meng, Y.B.; Li, X.; Li, Z.Y.; Zhao, J.; Yuan, X.B.; Ren, Y.; Cui, Z.D.; Liu, Y.D.; Yang, X.J. microRNA-21 promotes osteogenic differentiation of mesenchymal stem cells by the PI3K/β-catenin pathway. J. Orthop. Res. 2015, 33, 957–964. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, X.; Zhao, J.; Li, Z.; Ma, L.; Zhu, S.; Liang, Y.; Cui, Z.; He, H.; Yang, X. The synergistic effect of strontium-substituted hydroxyapatite and microRNA-21 on improving bone remodeling and osseointegration. Biomater. Sci. 2018, 6, 2694–2703. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, G.; Feng, Z.; Bai, S.; Dong, Y.; Wu, G.; Zhao, Y. Microarc-oxidized titanium surfaces functionalized with microRNA-21-loaded chitosan/hyaluronic acid nanoparticles promote the osteogenic differentiation of human bone marrow mesenchymal stem cells. Int. J. Nanomed. 2015, 10, 6675–6687. [Google Scholar]
- Shao, D.; Wang, C.; Sun, Y.; Cui, L. Effects of oral implants with miR-122-modified cell sheets on rat bone marrow mesenchymal stem cells. Mol. Med. Rep. 2018, 17, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Yang, C.; Svend Le, D.Q.; Zhang, Y.; Kjems, J. Calcium-MicroRNA Complex-Functionalized Nanotubular Implant Surface for Highly Efficient Transfection and Enhanced Osteogenesis of Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 7756–7764. [Google Scholar] [CrossRef] [PubMed]
- Eskildsen, T.; Taipaleenmäki, H.; Stenvang, J.; Abdallah, B.M.; Ditzel, N.; Nossent, A.Y.; Bak, M.; Kauppinen, S.; Kassem, M. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 6139–6144. [Google Scholar] [CrossRef] [PubMed]
- Young, S.R.L.; Gerard-O’Riley, R.; Kim, J.B.; Pavalko, F.M. Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. J. Bone Miner. Res. 2009, 24, 411–424. [Google Scholar] [CrossRef]
- Yan, J.; Chang, B.; Hu, X.; Cao, C.; Zhao, L.; Zhang, Y. Titanium implant functionalized with antimiR-138 delivered cell sheet for enhanced peri-implant bone formation and vascularization. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 52–64. [Google Scholar] [CrossRef]
- Wu, K.; Song, W.; Zhao, L.; Liu, M.; Yan, J.; Andersen, M.Ø.; Kjems, J.; Gao, S.; Zhang, Y. MicroRNA functionalized microporous titanium oxide surface by lyophilization with enhanced osteogenic activity. ACS Appl. Mater. Interfaces 2013, 5, 2733–2744. [Google Scholar] [CrossRef]
- Liu, X.; Tan, N.; Zhou, Y.; Wei, H.; Ren, S.; Yu, F.; Chen, H.; Jia, C.; Yang, G.; Song, Y. Delivery of antagomiR204-conjugated gold nanoparticles from PLGA sheets and its implication in promoting osseointegration of titanium implant in type 2 diabetes mellitus. Int. J. Nanomed. 2017, 12, 7089–7101. [Google Scholar] [CrossRef]
- Asa’ad, F.; Pagni, G.; Pilipchuk, S.P.; Giannì, A.B.; Giannobile, W.V.; Rasperini, G. 3D-Printed Scaffolds and Biomaterials: Review of Alveolar Bone Augmentation and Periodontal Regeneration Applications. Int. J. Dent. 2016, 2016, 1239842. [Google Scholar] [CrossRef]
- Pilipchuk, S.P.; Plonka, A.B.; Monje, A.; Taut, A.D.; Lanis, A.; Kang, B.; Giannobile, W.V. Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent. Mater. 2015, 31, 317–338. [Google Scholar] [CrossRef]
- Larsson, L.; Decker, A.M.; Nibali, L.; Pilipchuk, S.P.; Berglundh, T.; Giannobile, W.V. Regenerative Medicine for Periodontal and Peri-implant Diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Chen, H.; Jin, R.; Weng, T.; Ho, J.K.; You, C.; Zhang, L.; Wang, X.; Han, C. Non-viral gene delivery systems for tissue repair and regeneration. J. Transl. Med. 2018, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E.; Hasturk, H.; Kantarci, A.; Freire, M.O.; Nguyen, D.; Dalli, J.; Serhan, C.N. Proresolving nanomedicines activate bone regeneration in periodontitis. J. Dent. Res. 2015, 94, 148–156. [Google Scholar] [CrossRef]
- Larsson, L.; Pilipchuk, S.P.; Giannobile, W.V.; Castilho, R.M. When epigenetics meets bioengineering-A material characteristics and surface topography perspective. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2065–2071. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Chandran, S.V.; Arumugam, B.; Saravanan, S.; Devanand Venkatasubbu, G.; Selvamurugan, N. Chitosan/nano-hydroxyapatite/nano-zirconium dioxide scaffolds with miR-590-5p for bone regeneration. Int. J. Biol. Macromol. 2018, 111, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, G.; Wei, M.; Liu, Q.; Zhou, J.; Qin, T.; Feng, X.; Liu, H.; Feng, Z.; Zhao, Y. Improving the osteogenesis of human bone marrow mesenchymal stem cell sheets by microRNA-21-loaded chitosan/hyaluronic acid nanoparticles via reverse transfection. Int. J. Nanomed. 2016, 11, 2091–2105. [Google Scholar]
- Wu, G.; Feng, C.; Hui, G.; Wang, Z.; Tan, J.; Luo, L.; Xue, P.; Wang, Q.; Chen, X. Improving the osteogenesis of rat mesenchymal stem cells by chitosan-based-microRNA nanoparticles. Carbohydr. Polym. 2016, 138, 49–58. [Google Scholar] [CrossRef]
- Moncal, K.K.; Aydin, R.S.T.; Abu-Laban, M.; Heo, D.N.; Rizk, E.; Tucker, S.M.; Lewis, G.S.; Hayes, D.; Ozbolat, I.T. Collagen-infilled 3D printed scaffolds loaded with miR-148b-transfected bone marrow stem cells improve calvarial bone regeneration in rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110128. [Google Scholar] [CrossRef]
- Lee, S.U.; Kwak, H.B.; Pi, S.H.; You, H.K.; Byeon, S.R.; Ying, Y.; Luesch, H.; Hong, J.; Kim, S.H. In Vitro and In Vivo Osteogenic Activity of Largazole. ACS Med. Chem. Lett. 2011, 2, 248–251. [Google Scholar] [CrossRef]
- James, E.N.; Van Doren, E.; Li, C.; Kaplan, D.L. Silk Biomaterials-Mediated miRNA Functionalized Orthopedic Devices. Tissue Eng. Part A 2019, 25, 12–23. [Google Scholar] [CrossRef]
- Kim, H.K.; Chung, H.J.; Park, T.G. Biodegradable polymeric microspheres with “open/closed” pores for sustained release of human growth hormone. J. Control. Release 2006, 112, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.E.; Son, J.S.; Park, K.; Han, D.K. Fabrication of covered porous PLGA microspheres using hydrogen peroxide for controlled drug delivery and regenerative medicine. J. Control. Release 2009, 133, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, X.; Sun, X.; Long, C.; Sun, F.; Liu, J.; Li, X.; Lee, R.J.; Liu, N.; Li, Y.; et al. Ketoprofen and MicroRNA-124 Co-loaded poly (lactic-co-glycolic acid) microspheres inhibit progression of Adjuvant-induced arthritis in rats. Int. J. Pharm. 2018, 552, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Chen, Y.E.; Chen, J.; Ma, P.X. Cell-free 3D scaffold with two-stage delivery of miRNA-26a to regenerate critical-sized bone defects. Nat. Commun. 2016, 7, 10376. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X.; Zhang, Z.; Zhang, X.; Saunders, L.; Zhou, Y.; Ma, P.X. Nanofibrous Spongy Microspheres To Distinctly Release miRNA and Growth Factors To Enrich Regulatory T Cells and Rescue Periodontal Bone Loss. ACS Nano 2018, 12, 9785–9799. [Google Scholar] [CrossRef]
- Xiong, A.; He, Y.; Gao, L.; Li, G.; Weng, J.; Kang, B.; Wang, D.; Zeng, H. Smurf1-targeting miR-19b-3p-modified BMSCs combined PLLA composite scaffold to enhance osteogenic activity and treat critical-sized bone defects. Biomater. Sci. 2020, 8, 6069–6081. [Google Scholar] [CrossRef]
- Lin, J.; Mohamed, I.; Lin, P.H.; Shirahama, H.; Milbreta, U.; Sieow, J.L.; Peng, Y.; Bugiani, M.; Wong, S.C.; Levinson, H.; et al. Modulating Macrophage Phenotype by Sustained MicroRNA Delivery Improves Host-Implant Integration. Adv. Healthc Mater. 2020, 9, 1901257. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Enderami, S.E.; Saburi, E.; Islami, M.; Yaslianifard, S.; Mahabadi, J.A.; Ardeshirylajimi, A.; Soleimanifar, F.; Moghadam, A.S. Micro-RNA-incorporated electrospun nanofibers improve osteogenic differentiation of human-induced pluripotent stem cells. J. Biomed. Mater. Res. A 2020, 108, 377–386. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Jeon, O.; Dang, P.N.; Huynh, C.T.; Varghai, D.; Riazi, H.; McMillan, A.; Herberg, S.; Alsberg, E. RNA interfering molecule delivery from in situ forming biodegradable hydrogels for enhancement of bone formation in rat calvarial bone defects. Acta Biomater. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Pan, T.; Song, W.; Gao, H.; Li, T.; Cao, X.; Zhong, S.; Wang, Y. miR-29b-Loaded Gold Nanoparticles Targeting to the Endoplasmic Reticulum for Synergistic Promotion of Osteogenic Differentiation. ACS Appl. Mater. Interfaces 2016, 8, 19217–19227. [Google Scholar] [CrossRef]
- Menciá Castanõ, I.; Curtin, C.M.; Shaw, G.; Mary Murphy, J.; Duffy, G.P.; O’Brien, F.J. A novel collagen-nanohydroxyapatite microRNA-activated scaffold for tissue engineering applications capable of efficient delivery of both miR-mimics and antagomiRs to human mesenchymal stem cells. J. Control. Release 2015, 200, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Menciá Castanõ, I.; Curtin, C.M.; Duffy, G.P.; O’Brien, F.J. Next generation bone tissue engineering: Non-viral miR-133a inhibition using collagen-nanohydroxyapatite scaffolds rapidly enhances osteogenesis. Sci. Rep. 2016, 6, 27941. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Bakhshandeh, B.; Dehghan, M.M.; Mehrnia, M.R.; Khojasteh, A. Functional synergy of anti-mir221 and nanohydroxyapatite scaffold in bone tissue engineering of rat skull. J. Mater. Sci. Mater. Med. 2016, 27, 132. [Google Scholar] [CrossRef] [PubMed]
- Kesse, S.; Boakye-Yiadom, K.O.; Ochete, B.O.; Opoku-Damoah, Y.; Akhtar, F.; Filli, M.S.; Asim Farooq, M.; Aquib, M.; Maviah Mily, B.J.; Murtaza, G.; et al. Mesoporous Silica Nanomaterials: Versatile Nanocarriers for Cancer Theranostics and Drug and Gene Delivery. Pharmaceutics 2019, 11, 77. [Google Scholar] [CrossRef]
- Yan, J.; Lu, X.; Zhu, X.; Hu, X.; Wang, L.; Qian, J.; Zhang, F.; Liu, M. Effects of miR-26a on Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells by a Mesoporous Silica Nanoparticle-PEI-Peptide System. Int. J. Nanomed. 2020, 15, 497–511. [Google Scholar] [CrossRef]
- Lei, L.; Liu, Z.; Yuan, P.; Jin, R.; Wang, X.; Jiang, T.; Chen, X. Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. J. Mater. Chem. B 2019, 7, 2722–2735. [Google Scholar] [CrossRef]
- Yuan, X.; Han, L.; Lin, H.; Guo, Z.; Huang, Y.; Li, S.; Long, T.; Tang, W.; Tian, W.; Long, J. The role of antimiR-26a-5p/biphasic calcium phosphate in repairing rat femoral defects. Int. J. Mol. Med. 2019, 44, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Lan, Y.; Feng, Z.; Feng, L.; Yang, J.; Liu, Y.; Bian, L.; Tan, J.; Lai, R.; Guo, R. Functionalization of SF/HAP Scaffold with GO-PEI-miRNA inhibitor Complexes to Enhance Bone Regeneration through Activating Transcription Factor 4. Theranostics 2019, 9, 4525–4541. [Google Scholar] [CrossRef]
- Xue, Y.; Guo, Y.; Yu, M.; Wang, M.; Ma, P.X.; Lei, B. Monodispersed Bioactive Glass Nanoclusters with Ultralarge Pores and Intrinsic Exceptionally High miRNA Loading for Efficiently Enhancing Bone Regeneration. Adv. Healthc. Mater. 2017, 6, 1700630. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Wennerberg, A. Current concepts for the biological basis of dental implants: Foreign body equilibrium and osseointegration dynamics. Oral Maxillofac. Surg. Clin. N. Am. 2015, 27, 175–183. [Google Scholar] [CrossRef]
- Lin, Y.H.; Huang, P.; Lu, X.; Guan, D.H.; Man, Y.; Wei, N.; Wang, Y.Y.; Gong, P. The relationship between IL-1 gene polymorphism and marginal bone loss around dental implants. J. Oral Maxillofac. Surg. 2007, 65, 2340–2344. [Google Scholar] [CrossRef] [PubMed]
- Shimpuku, H.; Nosaka, Y.; Kawamura, T.; Tachi, Y.; Shinohara, M.; Ohura, K. Genetic polymorphisms of the interleukin-1 gene and early marginal bone loss around endosseous dental implants. Clin. Oral Implants Res. 2003, 14, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin. Implant Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Dahlin, C.; Jemt, T.; Sennerby, L.; Turri, A.; Wennerberg, A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin. Implant Dent. Relat. Res. 2014, 16, 155–165. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Study Type | MicroRNA and/or Anti-miRNA | Implant Surface Type | Functionalized Coating Method | Results of the Functionalized Coating (FC) Group |
|---|---|---|---|---|---|
| Wu et al. (2013) [87] | In vitro | MiRNA 29b and anti-miRNA 138 | Microarc oxidation (MAO)-treated titanium surfaces | Lyophilizing miRNA lipoplexes | - FC with miR-29b: Increased expression of ALP. Increased expression of COL1 at day 7. Increased mineralization. - FC with anti-miRNA-138: Increased expression of BMP, OCN, OSX, and RUNX2. Increased expression of COL1 at day 14. Increased mineralization. |
| Wang et al. (2015) [81] | In vitro | MiRNA-21 | Microarc oxidation (MAO)-treated titanium surfaces | Cross-linking of chitosan, hyaluronic acid, and miRNA-21 nano-particles | - Increased expression of COLI, COL3, RUNX2, OPN, and OCN. |
| Meng et al. (2016) [70] | In vitro and in vivo (rat tibial defect model) | MiRNA-29b | Machined titanium | MiRNA-29b nanocapsules encapsulated in O-carboxymethyl chitosan coating | - Increased expression of OCN and RUNX2 in vitro. New bone formation was evident in vivo. |
| Liu et al. (2017) [88] | In vitro and in vivo (diabetic rats) | Anti-miRNA-204 | Microarc oxidation (MAO)-treated titanium surfaces | MiRNA-204 conjugated with gold nanoparticles (AuNP-antago-miRNA-204) and dispersed in a poly(lactic-co-glycolic) acid (PLGA) solution | - Increased expression of BMP, OPG, ALP, RUNX2, and COL1 in vitro. High removal torque in vivo. |
| Geng et al. (2018) [80] | In vitro and in vivo (rabbits) | MiRNA-21 | Acid-treated titanium surface | MiRNA-21 nanocapsules encapsulated in strontium/hydroxyapatite coating | - Osteoblast proliferation, differentiation, and mineralization were evident in vitro. - Increased expression of CD31, COL-I, RUNX2, OCN, OPN, and OPG in vivo. - Decreased expression of RANKL in vivo. |
| Shao et al. (2018) [82] | In vitro | MiRNA-122 | Microarc oxidation (MAO)-treated titanium surfaces | MiRNA-122-modified cell sheets complexed | - Increased expression of RUNX2, OSX, OCN, COL1, ALP, and BMP-2. |
| Song et al. (2018) [83] | In vitro | Anti-miRNA-138 | Anodized titanium surfaces | Premixed CaCl2 and siRNA to form Ca/siRNA coating | - Enhanced osteogenic differentiation of hMSCs on and around the implant surface |
| Yan et al. (2018) [86] | In vitro and in vivo (mice) | Anti-miRNA-138 | Microarc oxidation (MAO)-treated titanium surfaces | Anti-miRNA-138 delivered MSC sheet to the titanium surface, forming MSC sheet–implant complex (MSIC) | - Increased expression of endogenous osteogenesis and angiogenesis-related genes and proteins, alkaline phosphatase activity, extracellular matrix mineralization, and collagen in vitro. - Robust vascularized bone formation in vivo. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asa’ad, F.; Pelanyte, G.; Philip, J.; Dahlin, C.; Larsson, L. The Role of Epigenetic Functionalization of Implants and Biomaterials in Osseointegration and Bone Regeneration—A Review. Molecules 2020, 25, 5879. https://doi.org/10.3390/molecules25245879
Asa’ad F, Pelanyte G, Philip J, Dahlin C, Larsson L. The Role of Epigenetic Functionalization of Implants and Biomaterials in Osseointegration and Bone Regeneration—A Review. Molecules. 2020; 25(24):5879. https://doi.org/10.3390/molecules25245879
Chicago/Turabian StyleAsa’ad, Farah, Goda Pelanyte, Jincy Philip, Christer Dahlin, and Lena Larsson. 2020. "The Role of Epigenetic Functionalization of Implants and Biomaterials in Osseointegration and Bone Regeneration—A Review" Molecules 25, no. 24: 5879. https://doi.org/10.3390/molecules25245879
APA StyleAsa’ad, F., Pelanyte, G., Philip, J., Dahlin, C., & Larsson, L. (2020). The Role of Epigenetic Functionalization of Implants and Biomaterials in Osseointegration and Bone Regeneration—A Review. Molecules, 25(24), 5879. https://doi.org/10.3390/molecules25245879






