Development of Gas-Chromatographic Method for Simultaneous Determination of Cannabinoids and Terpenes in Hemp
Abstract
1. Introduction
2. Results and Discussion
2.1. Sample Preparation
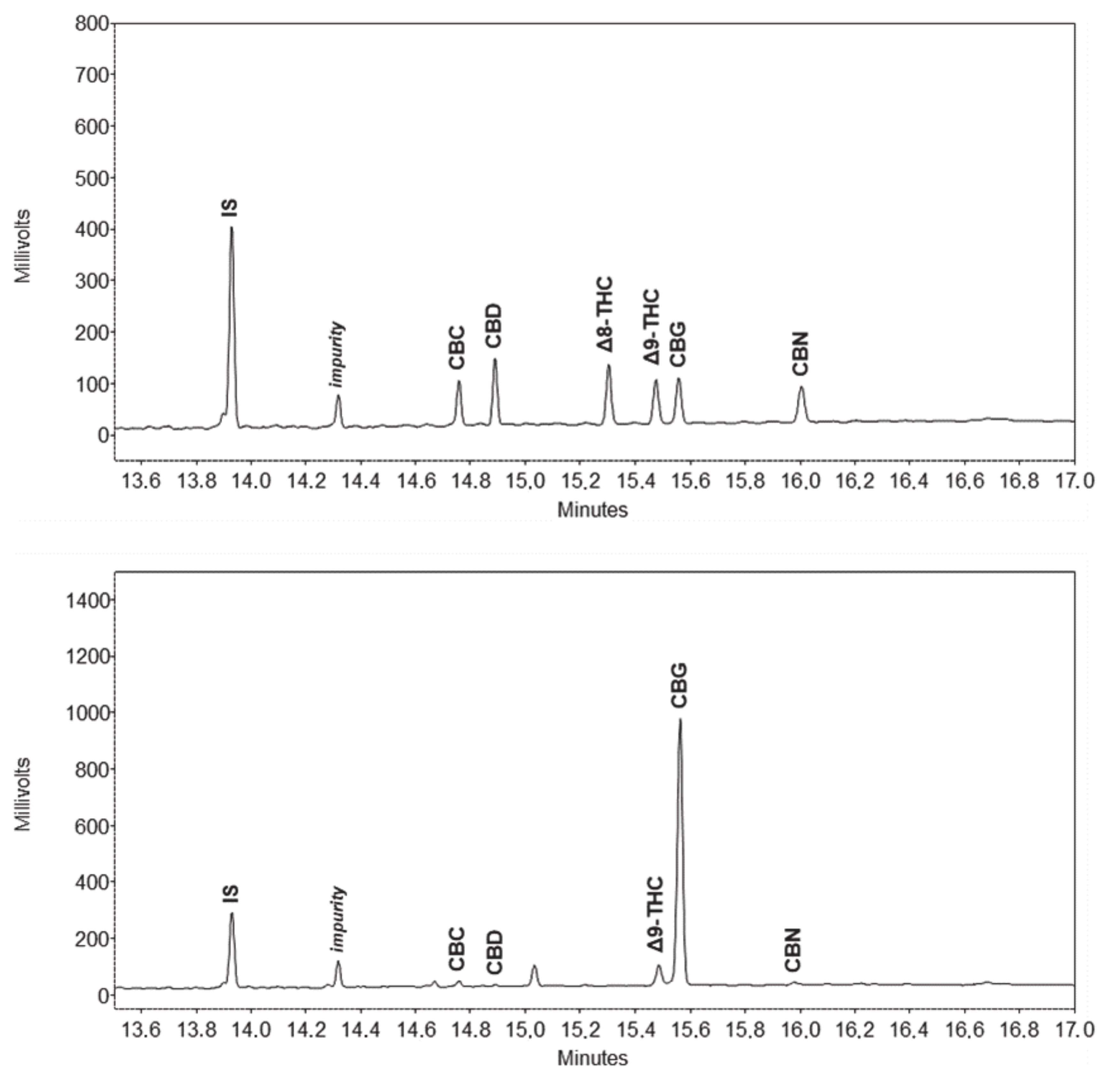
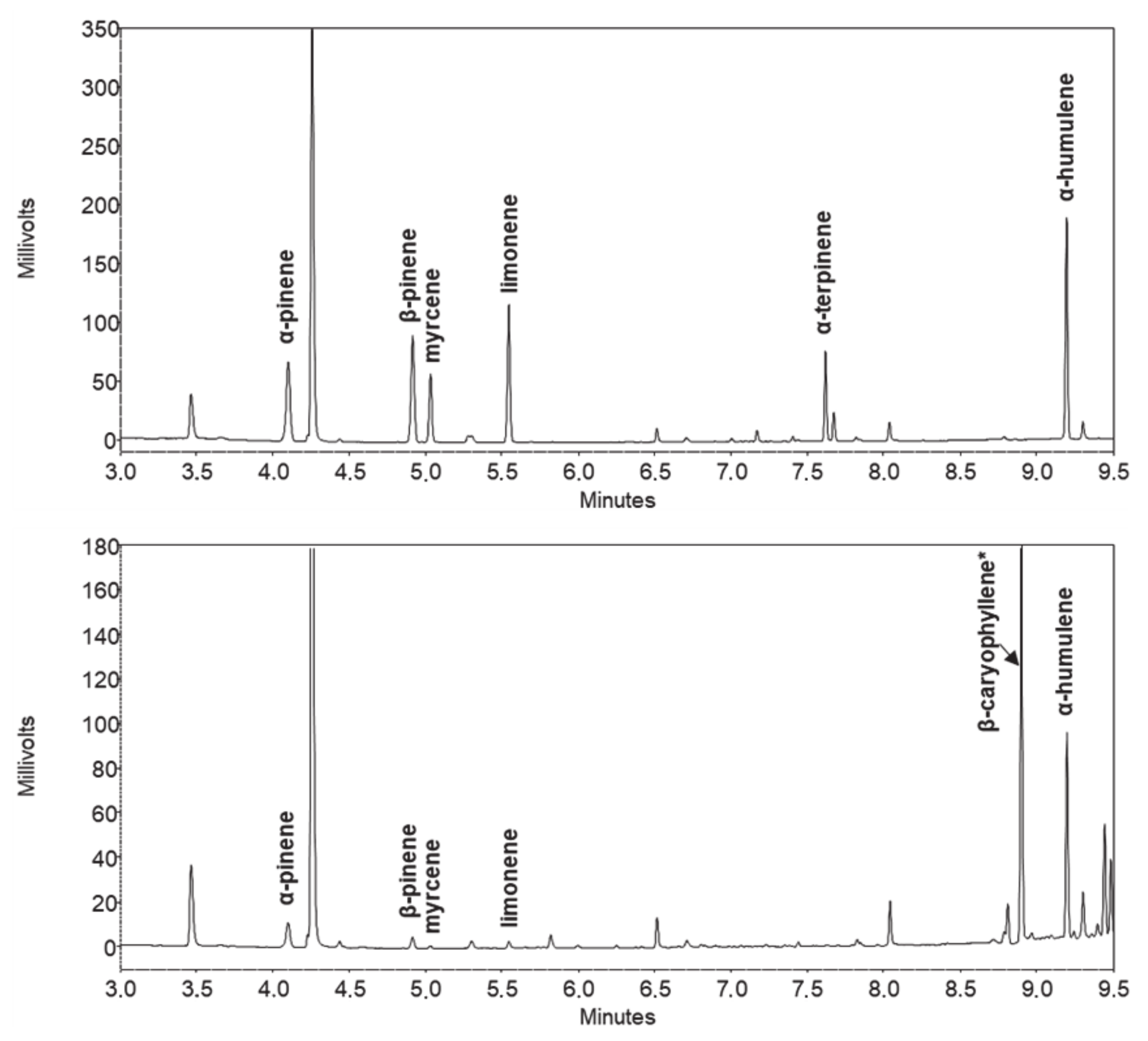
2.2. Gas Chromatographic Separation
2.3. Method Performance and Validation
2.4. Comparison with HPLC
3. Materials and Methods
3.1. Chemicals, Reagents and Samples
3.2. GC Analysis
3.3. HPLC Analysis
3.4. Standard Solutions Preparation
3.5. Samples and Preparation of Extracts
3.6. Quantitation, Method Precision, Accuracy, Sensitivity, Linearity, and Stability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abuhasira, R.; Shbiro, L.; Landschaft, Y. Medical use of cannabis and cannabinoids containing products—Regulations in Europe and North America. Eur. J. Intern. Med. 2018, 49, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, K.E.; Bidwell, L.C.; Ellingson, J.M.; Bryan, A.D. Cannabis and Health Research: Rapid Progress Requires Innovative Research Designs. Value Health 2019, 22, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O. Medical cannabis use in older patients: Update on medical knowledge. Maturitas 2018, 118, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Ebbert, J.O.; Scharf, E.L.; Hurt, R.T. Medical Cannabis. Mayo Clin. Proc. 2018, 93, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Benito, S.; Seijo-Vila, M.; Caro-Villalobos, M.; Tundidor, I.; Andradas, C.; García-Taboada, E.; Wade, J.; Smith, S.; Guzmán, M.; Pérez-Gómez, E.; et al. Appraising the “entourage effect”: Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem. Pharmacol. 2018, 157, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis sativa—From plant genome to humans. Plant. Sci. 2019, 284, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Namdar, D.; Mazuz, M.; Ion, A.; Koltai, H. Variation in the compositions of cannabinoid and terpenoids in Cannabis sativa derived from inflorescence position along the stem and extraction methods. Ind. Crops Prod. 2018, 113, 376–382. [Google Scholar] [CrossRef]
- Delgado-Povedano, M.M.; Sánchez-Carnerero Callado, C.; Priego-Capote, F.; Ferreiro-Vera, C. Untargeted characterization of extracts from Cannabis sativa L. cultivars by gas and liquid chromatography coupled to mass spectrometry in high resolution mode. Talanta 2020, 208, 120384. [Google Scholar] [CrossRef]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef]
- Citti, C.; Linciano, P.; Russo, F.; Luongo, L.; Iannotta, M.; Maione, S.; Laganà, A.; Capriotti, A.L.; Forni, F.; Vandelli, M.A.; et al. A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Sci. Rep. 2019, 9, 20335. [Google Scholar] [CrossRef]
- Gambaro, V.E.; Froldi, R.; Saligari, E.; Dell’Acqua, L.; Salomone, E.; Roses, O.E. A simple method for analyzing cannabis by gas chromatography. Acta Toxicol. Argent. 1995, 3, 11–13. [Google Scholar]
- Gibson, C.R.; Williams, R.D.; Browder, R.O. Analysis of Hempen Ale for cannabinoids. J. Anal. Toxicol. 1998, 22, 179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ross, S.A.; Mehmedic, Z.; Murphy, T.P.; Elsohly, M.A. GC-MS analysis of the total delta9-THC content of both drug- and fiber-type cannabis seeds. J. Anal. Toxicol. 2000, 24, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Raharjo, T.J.; Verpoorte, R. Methods for the analysis of cannabinoids in biological materials: A review. Phytochem. Anal. 2004, 15, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Marchei, E.; Pacifici, R.; Pichini, S. A rapid and simple procedure for the determination of cannabinoids in hemp food products by gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2005, 36, 939–946. [Google Scholar] [CrossRef]
- Gambaro, V.; Dell’Acqua, L.; Fare, F.; Froldi, R.; Saligari, E.; Tassoni, G. Determination of primary active constituents in Cannabis preparations by high-resolution gas chromatography/flame ionization detection and high-performance liquid chromatography/UV detection. Anal. Chim. Acta 2002, 468, 245–254. [Google Scholar] [CrossRef]
- Cardenia, V.; Toschi, T.G.; Scappini, S.; Rubino, R.C.; Rodriguez-Estrada, M.T. Development and validation of a Fast gas chromatography/mass spectrometry method for the determination of cannabinoids in Cannabis sativa L. J. Food Drug Anal. 2018, 26, 1283–1292. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. Recommended Methods for the Identification and Analysis of Cannabis and Cannabis Products; United Nations Publications: New York, NY, USA, 2009; Available online: https://www.unodc.org/documents/scientific/ST-NAR-40-Ebook_1.pdf (accessed on 3 April 2020).
- De Backer, B.; Debrus, B.; Lebrun, P.; Theunis, L.; Dubois, N.; Decock, L.; Verstraete, A.; Hubert, P.; Charlier, C. Innovative development and validation of an HPLC/DAD method for the qualitative and quantitative determination of major cannabinoids in cannabis plant material. J. Chromatogr. B 2009, 877, 4115–4124. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Omar, J.; Navarro, P.; Olivares, M.; Etxebarria, N.; Usobiaga, A. Identification and quantification of cannabinoids in Cannabis sativa L. plants by high performance liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 7549–7560. [Google Scholar] [CrossRef]
- Zgair, A.; Wong, J.C.M.; Sabri, A.; Fischer, P.M.; Barrett, D.A.; Constantinescu, C.S.; Gershkovich, P. Development of a simple and sensitive HPLC-UV method for the simultaneous determination of cannabidiol and Δ(9)-tetrahydrocannabinol in rat plasma. J. Pharm. Biomed. Anal. 2015, 114, 145–151. [Google Scholar] [CrossRef]
- Gul, W.; Gul, S.W.; Radwan, M.M.; Wanas, A.S.; Mehmedic, Z.; Khan, I.I.; Sharaf, M.H.; ElSohly, M.A. Determination of 11 Cannabinoids in Biomass and Extracts of Different Varieties of Cannabis Using High-Performance Liquid Chromatography. J. AOAC Int. 2015, 98, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Giese, M.W.; Lewis, M.A.; Giese, L.; Smith, K.M. Development and Validation of a Reliable and Robust Method for the Analysis of Cannabinoids and Terpenes in Cannabis. J. AOAC Int. 2015, 98, 1503–1522. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Ciccarella, G.; Braghiroli, D.; Parenti, C.; Vandelli, M.A.; Cannazza, G. Medicinal cannabis: Principal cannabinoids concentration and their stability evaluated by a high performance liquid chromatography coupled to diode array and quadrupole time of flight mass spectrometry method. J. Pharm. Biomed. Anal. 2016, 128, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Wene, D.; Fan, Z. Qualitative and quantitative measurement of cannabinoids in cannabis using modified HPLC/DAD method. J. Pharm. Biomed. Anal. 2017, 146, 15–23. [Google Scholar] [CrossRef]
- Ciolino, L.A.; Ranieri, T.L.; Taylor, A.M. Commercial cannabis consumer products part 2: HPLC-DAD quantitative analysis of cannabis cannabinoids. Forensic Sci. Int. 2018, 289, 438–447. [Google Scholar] [CrossRef]
- Mudge, E.M.; Murch, S.J.; Brown, P.N. Leaner and greener analysis of cannabinoids. Anal. Bioanal. Chem. 2017, 409, 3153–3163. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.-H.; Avula, B.; Radwan, M.M.; Wanas, A.S.; Van Antwerp, J.; Parcher, J.F.; ElSohly, M.A.; Khan, I.A. Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry. Cannabis Cannabinoid Res. 2016, 1, 262–271. [Google Scholar] [CrossRef]
- Dussy, F.E.; Hamberg, C.; Luginbühl, M.; Schwerzmann, T.; Briellmann, T.A. Isolation of Delta9-THCA-A from hemp and analytical aspects concerning the determination of Delta9-THC in cannabis products. Forensic Sci. Int. 2005, 149, 3–10. [Google Scholar] [CrossRef]
- Kemp, P.M.; Abukhalaf, I.K.; Manno, J.E.; Manno, B.R.; Alford, D.D.; Abusada, G.A. Cannabinoids in Humans. I. Analysis of Δ9-Tetrahydrocannabinol and Six Metabolites in Plasma and Urine Using GC-MS. J. Anal. Toxicol. 1995, 19, 285–291. [Google Scholar] [CrossRef]
- Steinmeyer, S.; Bregel, D.; Warth, S.; Kraemer, T.; Moeller, M.R. Improved and validated method for the determination of Delta(9)-tetrahydrocannabinol (THC), 11-hydroxy-THC and 11-nor-9-carboxyTHC in serum, and in human liver microsomal preparations using gas chromatography-mass spectrometry. J. Chromatogr. B 2002, 772, 239–248. [Google Scholar] [CrossRef]
- Leghissa, A.; Hildenbrand, Z.L.; Foss, F.W., Jr.; Schug, K.A. Determination of cannabinoids from a surrogate hops matrix using multiple reaction monitoring gas chromatography with triple quadrupole mass spectrometry. J. Sep. Sci. 2017, 41, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Križman, M. A simplified approach for isocratic HPLC analysis of cannabinoids by fine tuning chromatographic selectivity. Eur. Food Res. Technol. 2020, 246, 315–322. [Google Scholar] [CrossRef]
- Phenomenex, Terpenes and Cannabinoids in Marijuana by GC/FID on ZB-35HT. Available online: https://www.phenomenex.com/Application/Detail/22828 (accessed on 18 March 2020).
- Restek, Medical Cannabis Cannabinoids and Internal Standards on Rxi-35Sil MS. Available online: https://www.restek.com/chromatogram/view/GC_FS0549/cannabinoids (accessed on 18 March 2020).
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Thorpe, J.W.; Caddy, B. Comparison of Cannabis Samples from Different Origins by the Headspace Technique and an Assessment of Chromatographic Traces Using the r-Matrix. J. Forensic Sci. Soc. 1985, 25, 427–433. [Google Scholar] [CrossRef]
- Franchina, F.A.; Dubois, L.M.; Focant, J.-F. In-Depth Cannabis Multiclass Metabolite Profiling Using Sorptive Extraction and Multidimensional Gas Chromatography with Low- and High-Resolution Mass Spectrometry. Anal. Chem. 2020, 92, 10512–10520. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J.; Dyson, P.J.; Tavener, S.J. Chemistry in Alternative Reaction Media; John Wiley & Sons: Chichester, UK, 2004; p. 4. [Google Scholar]
- Restek, Terpenes in Blueberry Jack Medical Cannabis on Rxi-624Sil MS. Available online: https://www.restek.com/chromatogram/view/GC_FS0515 (accessed on 7 April 2020).
- Restek, Cannabis Terpenes Standard #1 & #2 on Rxi-624Sil MS. Available online: https://www.restek.com/chromatogram/view/GC_GN1200 (accessed on 7 April 2020).
- Agilent, Analysis of Terpene and Terpenoid Content in Cannabis sativa Using Headspace with GC/MSD. Available online: https://www.agilent.com/cs/library/applications/application-terpenes-8890-gcms-headspace-5994-1497en-agilent.pdf (accessed on 7 April 2020).

| Analyte | Injection Precision (% RSD, n = 5) | Accuracy (%) | Extraction Efficiency (%) | Repeatability (% RSD, n = 3) | Intermediate Precision (% RSD, n = 9) | LOD b (µg/mL) | LOQ b (µg/mL) | Regression Coefficient (R) | Stability 48 h (%) |
|---|---|---|---|---|---|---|---|---|---|
| CBD | 0.17 (1.27) | 100.3 (97.3) | 92.0 | 0.08 (0.78) | 1.59 (1.27) | 0.662 (0.093) | 2.207 (0.310) | 0.9999 (1.0000) | 99.9 (102.7) |
| CBG | 1.60 (0.52) | 97.6 (96.9) | 92.1 | 0.96 (2.00) | 1.77 (2.48) | 0.697 (0.94) | 2.322 (0.313) | 0.9999 (0.9999) | 98.2 (101.2) |
| Δ8-THC | 0.62 (1.51) | 99.1 (93.7) | - a | 0.89 (0.94) | 2.21 (2.53) | 0.817 (0.205) | 2.724 (0.684) | 0.9999 (0.9999) | 99.4 (95.6) |
| Δ9-THC | 0.54 (0.65) | 98.8 (93.4) | 93.1 | 0.42 (0.85) | 2.35 (2.34) | 0.822 (0.196) | 2.739 (0.654) | 1.0000 (0.9999) | 99.6 (94.0) |
| CBC | 0.79 (0.18) | 100.1 (113.3) | 92.0 | 1.34 (6.53) | 1.67 (9.02) | 0.815 (0.024) | 2.716 (0.082) | 1.0000 (0.9986) | 98.0 (103.9) |
| CBN | 1.27 (0.20) | 98.9 (88.4) | - a | 1.25 (3.38) | 1.88 (3.86) | 0.857 (0.007) | 2.858 (0.023) | 0.9995 (0.9999) | 100.7 (103.8) |
| α-pinene | 1.34 | 99.6 | 95.5 | 0.54 | 1.52 | 0.259 | 0.862 | 0.9999 | 99.8 |
| β-pinene | 0.65 | 100.1 | 95.2 | 1.39 | 1.33 | 0.175 | 0.585 | 0.9999 | 97.7 |
| mircene | 1.79 | 100.2 | - a | 0.65 | 3.15 | 0.183 | 0.609 | 0.9998 | 100.7 |
| limonene | 1.05 | 99.9 | 95.9 | 0.64 | 2.89 | 0.124 | 0.412 | 0.9999 | 100.3 |
| α-terpinene | 0.62 | 100.2 | - a | 1.54 | 2.83 | 0.193 | 0.642 | 1.0000 | 97.0 |
| α-humulene | 0.75 | 100.8 | 93.2 | 0.75 | 1.60 | 0.185 | 0.616 | 0.9999 | 99.6 |
| Hemp Sample Cultivar | Fedora 17 | Carmagnola | Futura 75 | Santhica |
|---|---|---|---|---|
| Sample Solution | 1 | 2 | 3 | 4 |
| CBDA (µg/mL)—HPLC | 298 | 1110 | - | - |
| CBD (µg/mL)—HPLC | 485 | 468 | - | - |
| CBGA (µg/mL)—HPLC | - | - | 428 | 510 |
| CBG (µg/mL)—HPLC | - | - | 118 | 106 |
| HPLC—total CBD * | 783 | 1578 | - | - |
| HPLC—total CBG * | - | - | 546 | 616 |
| GC—total CBD | 839 | 1635 | - | - |
| GC—total CBG | - | - | 605 | 572 |
| CBD—relative difference (%) | +7.1 | +3.6 | - | - |
| CBG—relative difference (%) | - | - | +10.8 | −7.1 |
Sample Availability: Samples of the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zekič, J.; Križman, M. Development of Gas-Chromatographic Method for Simultaneous Determination of Cannabinoids and Terpenes in Hemp. Molecules 2020, 25, 5872. https://doi.org/10.3390/molecules25245872
Zekič J, Križman M. Development of Gas-Chromatographic Method for Simultaneous Determination of Cannabinoids and Terpenes in Hemp. Molecules. 2020; 25(24):5872. https://doi.org/10.3390/molecules25245872
Chicago/Turabian StyleZekič, Jure, and Mitja Križman. 2020. "Development of Gas-Chromatographic Method for Simultaneous Determination of Cannabinoids and Terpenes in Hemp" Molecules 25, no. 24: 5872. https://doi.org/10.3390/molecules25245872
APA StyleZekič, J., & Križman, M. (2020). Development of Gas-Chromatographic Method for Simultaneous Determination of Cannabinoids and Terpenes in Hemp. Molecules, 25(24), 5872. https://doi.org/10.3390/molecules25245872