Cytotoxic Plant Extracts towards Insect Cells: Bioactivity and Nanoencapsulation Studies for Application as Biopesticides
Abstract
1. Introduction
2. Results and Discussion
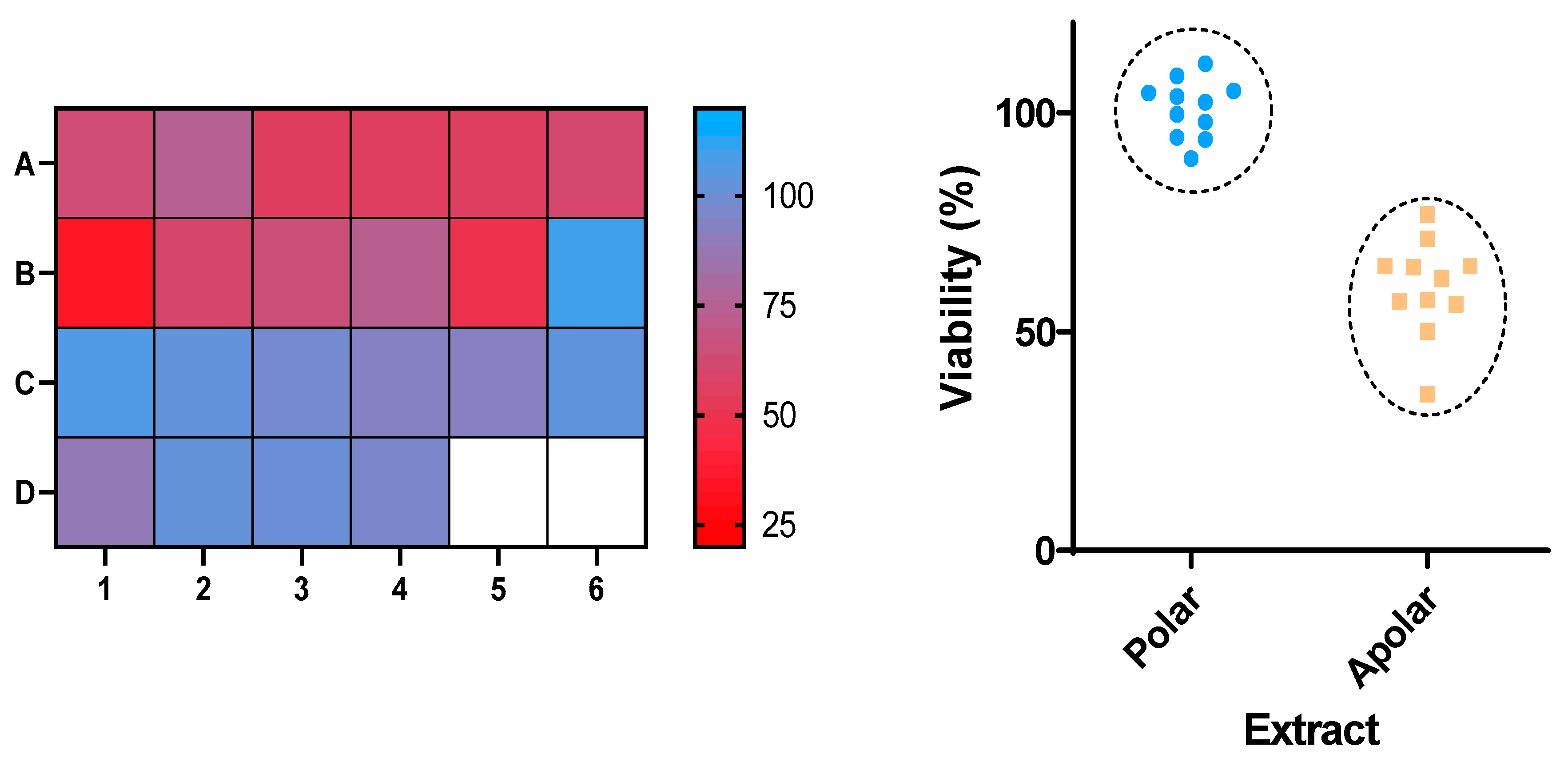
2.1. Plant Extracts
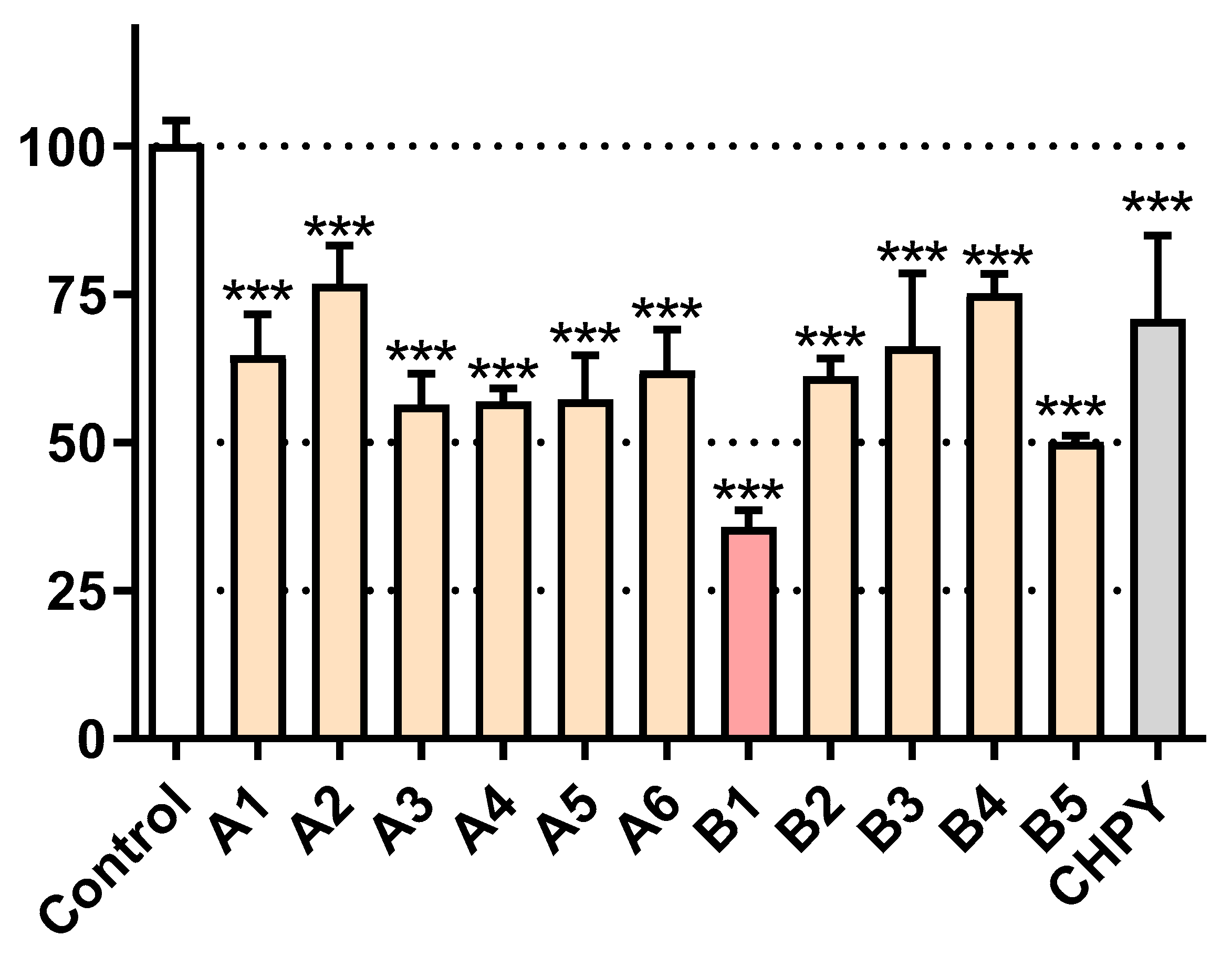
2.2. Evaluation of the Activity Against Sf9 Cells
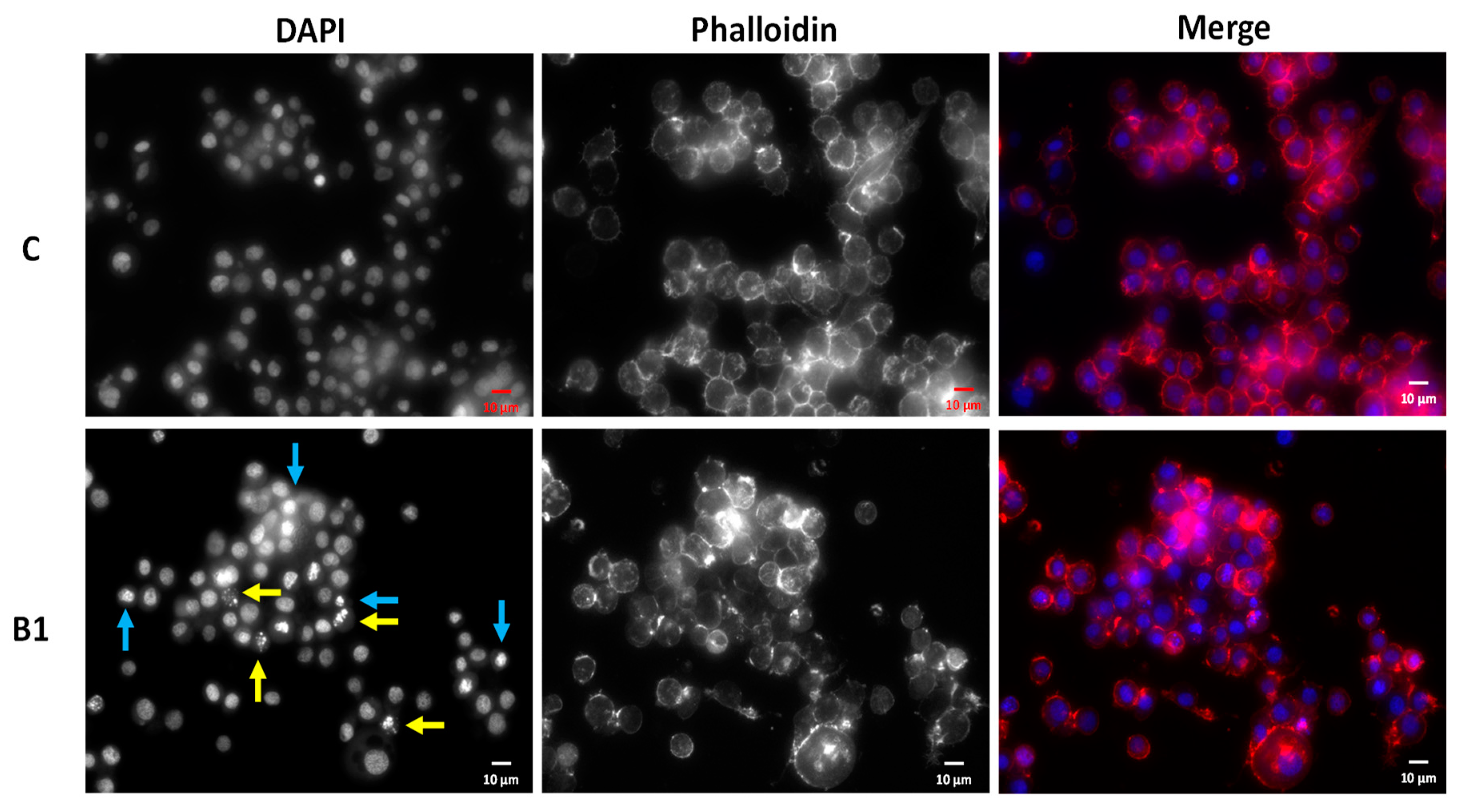
2.3. Morphological Assessment
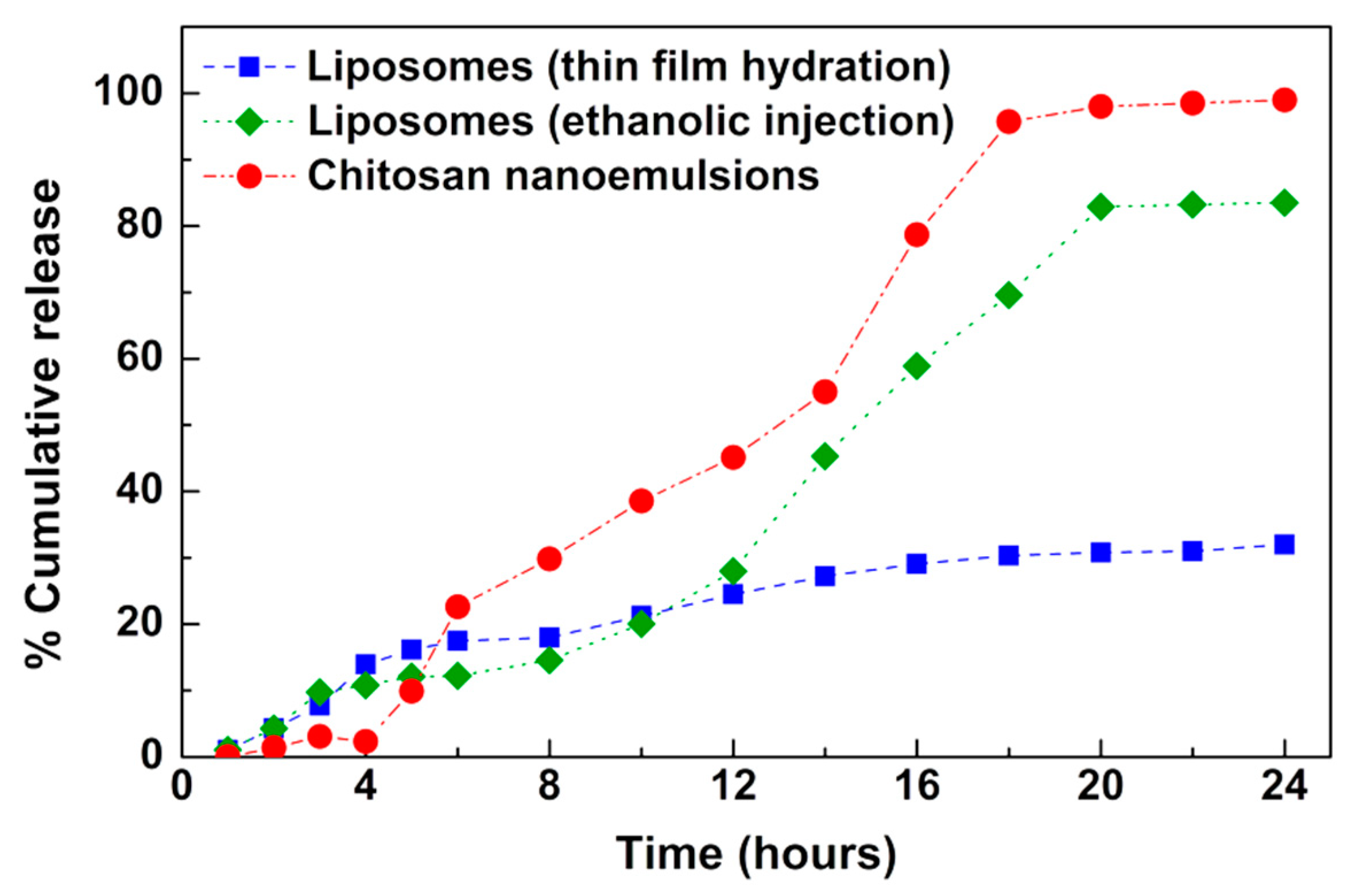
2.4. Nanoencapsulation Studies
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Plant Materials Extraction
3.4. Cell Culture
3.5. Assessment of Viability
3.6. Morphological Assessment
3.7. Nanoencapsulation Studies
3.7.1. Liposomes Preparation
3.7.2. Chitosan Nanocapsules Preparation
3.7.3. Size Measurements
3.7.4. Encapsulation Efficiencies and Release Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Biopesticides: State of the art and future opportunities. J. Agric. Food Chem. 2014, 62, 11613. [Google Scholar] [CrossRef] [PubMed]
- Rob, M.M.; Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Nogushi, H. Phytotoxic activity and identification of phytotoxic substances from Schumanniathus dichotomus. Plants 2020, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Maggi, F.; Iannareli, R.; Benelli, G. Plant extracts for developing mosquito larvicides: From laboratory to the field, with insights on the modes of action. Acta Trop. 2019, 193, 236–271. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-Z.; Zhang, J.; Peng, J.-W.; Zhang, Z.-J.; Zhao, W.-B.; Wang, R.-X.; Ma, K.-Y.; Li, J.-C.; Liu, Y.-Q.; Zhao, Z.-M.; et al. Discovery of luotonin A analogues as potent fungicides and insecticides: Design, synthesis and biological evaluation inspired by natural alkaloid. Eur. J. Med. Chem. 2020, 194, 112253. [Google Scholar] [CrossRef] [PubMed]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential oils extracted from different species of the Lamiaceae plant family as prospective bioagents against several detrimental pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef]
- Magierowicz, K.; Górska-Dabrik, E.; Golan, K. Effects of plant extracts and essential oils on the behavior of Acrobasis advenella (Zinck.) caterpillars and females. J. Plant Dis. Prot. 2020, 127, 63–71. [Google Scholar] [CrossRef]
- Walia, S.; Saha, S.; Tripathi, V.; Sharma, K.K. Phytochemical biopesticides: Some recent developments. Phytochem. Rev. 2017, 16, 989–1007. [Google Scholar] [CrossRef]
- Sparks, T.C.; Lorsbach, B.A. Agrochemical discovery—Building the next generation of insect control agents. In Advances in Agrochemicals: Ion Channels and G Protein-Coupled Receptors (GPCRs) as Targets for Pest Control; Gross, A.D., Ozoe, Y., Coats, J.R., Eds.; ACS Symposium Series; ACS: Washington, DC, USA, 2017; Volume 1264, pp. 1–17. [Google Scholar]
- Singh, R.P.; Chidambara Murthy, K.N.; Jayaprakasha, G.K. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef]
- Yoon, I.S.; Park, D.H.; Kim, J.E.; Yoo, J.C.; Bae, M.S.; Oh, D.S.; Cho, S.S. Identification of the biologically active constituents of Camellia japonica leaf and anti-hyperuricemic effect in vitro and in vivo. Int. J. Mol. Med. 2017, 39, 1613–1620. [Google Scholar] [CrossRef]
- Navabi, S.M.; Ebrahimzadeh, M.A.; Navabi, S.F.; Bahramian, F. In vitro antioxidant activity of Phytolacca americana berries. Pharmacologyonline 2009, 1, 81–88. [Google Scholar]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Etnopharmacol. 2012, 143, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate Peel and Fruit Extracts: A Review of Potential Plants for A Future. Tagetes Patula French Marigold, Dwarf French Marigold PFAF Plant Database. Available online: https://pfaf.org/user/Plant.aspx?LatinName=Tagetes+patula (accessed on 16 October 2020).
- Pardo-Muras, M.; Puig, C.G.; Lopez-Nogueira, A.; Cavaleiro, C.; Pedrol, N. On the bioherbicide potential of Ulex europaeus and Cytisus scoparius: Profiles of volatile organic compounds and their phytotoxic effects. PLoS ONE 2018, 13, e0205997. [Google Scholar] [CrossRef] [PubMed]
- Fabrick, J.A.; Yool, A.J.; Spurgeon, D.W. Insecticidal activity of marigold Tagetes patula plants and foliar extracts against the hemipteran pests, Lygus hesperus and Bemisia tabaci. PLoS ONE 2020, 15, e0233511. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, S.B.; Hamdi, S.H.; Mahjoubi, K.; Jemâa, J.M.B. Composition and insecticidal activity of essential oil from Ruta graveolens, Mentha pulegium and Ocimum basilicum against Ectomyelois ceratoniae Zeller and Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). J. Plant Dis. Prot. 2019, 126, 237–246. [Google Scholar] [CrossRef]
- Semerdjieva, I.B.; Burducea, M.; Astatkie, T.; Zheljazkov, V.D.; Dincheva, I. Essential oil composition of Ruta graveolens L. fruits and Hyssopus officinalis Subsp. aristatus (Godr.) Nyman biomass as a function of hydrodistillation time. Molecules 2019, 24, 4047. [Google Scholar] [CrossRef]
- Plants for a Future. Ruta graveolens Rue, Common rue, Herb of Grace, Garden Rue PFAF Plant Database. Available online: https://pfaf.org/user/Plant.aspx?LatinName=Ruta+graveolens (accessed on 16 October 2020).
- Perera, A.; Karunaratne, M.; Chinthaka, S. Biological activity and secondary metabolite profile of Ruta graveolens leaves against maize weevil infestations. J. Entomol. Zool. Stud. 2017, 5, 233–241. [Google Scholar]
- Jeon, J.-H.; Lee, S.-G.; Lee, H.-S. Isolation of insecticidal constituent from Ruta graveolens and structure-activity relationship studies against stored-food pests (Coleoptera). J. Food Prot. 2015, 78, 1536–1540. [Google Scholar] [CrossRef]
- Perera, A.G.W.U.; Karunaratne, M.M.S.C.; Chinthaka, S.D.M. Qualitative determination, quantitative evaluation and comparative insecticidal potential of Ruta Graveolens essential oil and its major constituents in the management of two stored pests Sitophilus Zeamais (Coleoptera: Curculionidae) and Corcya Cephalonica (Lepidoptera: Pyralidae). Sustain. Dev. Res. 2019, 1, 55–66. [Google Scholar]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Shi, F.; Zhao, J.-H.; Liu, Y.; Wang, Z.; Zhang, Y.-T.; Feng, N.-P. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int. J. Nanomed. 2012, 7, 2033–2043. [Google Scholar]
- Varona, S.; Marti’n, A.; Cocero, M.J. Liposomal incorporation of lavandin essential oil by a thin-film hydration method and by particles from gas-saturated solutions. Ind. Eng. Chem. Res. 2011, 50, 2088–2097. [Google Scholar] [CrossRef]
- Liolios, C.C.; Gortzi, O.; Lalas, S.; Tsaknis, J.; Chinou, I. Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem. 2009, 112, 77–83. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, nano-guard for pesticides: A new window for safe application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; Mortazavian, A.M.; Mohammadi, A.; Khosravi-Darani, K.; Shojaee-Aliabadi, S.; Dehghan, S.; Khaksar, R. Incorporation of essential oil in alginate microparticles by multiple emulsion/ionic gelation process. Int. J. Biol. Macromol. 2003, 62, 582–588. [Google Scholar] [CrossRef]
- López, A.; Castro, S.; Andina, M.J.; Ures, X.; Munguía, B.; Llabot, J.M.; Elder, H.; Dellacassa, E.; Palma, S.; Domínguez, L. Insecticidal activity of microencapsulated Schinus molle essential oil. Ind. Crops. Prod. 2014, 53, 209–216. [Google Scholar] [CrossRef]
- Sutaphanit, P.; Chitprasert, P. Optimisation of microencapsulation of holy basil essential oil in gelatin by response surface methodology. Food Chem. 2014, 150, 313–320. [Google Scholar] [CrossRef]
- Leimann, F.V.; Gonçalves, O.H.; Machado, R.A.F.; Bolzan, A. Antimicrobial activity of microencapsulated lemongrass essential oil and the effect of experimental parameters on microcapsules size and morphology. Mater. Sci. Eng. C 2009, 29, 430–436. [Google Scholar] [CrossRef]
- Iannitelli, A.; Grande, R.; Di Stefano, A.; Di Giulio, M.; Sozio, P.; Bessa, L.J.; Laserra, S.; Paolini, C.; Protasi, F.; Cellini, L. Potential antibacterial activity of carvacrol-loaded poly(DL-lactide-co-glycolide) (PLGA) nanoparticles against microbial biofilm. Int. J. Mol. Sci. 2011, 12, 5039–5051. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.; Ihsan, A.; Javed, S.; Bokhari, H.; Imran, M. Encapsulation of cardamom essential oil in chitosan nano-composites: In-vitro efficacy on antibiotic-resistant bacterial pathogens and cytotoxicity studies. Front. Microbiol. 2016, 7, 1580. [Google Scholar] [CrossRef] [PubMed]
- Ulubelen, A.; Ozturk, M. Alkaloids and coumariuns from Ruta Species. Nat. Prod. Commun. 2006, 1, 851–857. [Google Scholar]
- Oliva, A.; Meepagala, K.M.; Wedge, D.E.; Harries, D.; Hale, A.L.; Aliotta, G.; Duke, S.O. Natural fungicides from Ruta graveolens L. leaves, including a new quinolone alkaloid. J. Agric. Food Chem. 2003, 51, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Hale, A.L.; Meepagala, K.M.; Oliva, A.; Aliotta, G.; Duke, S.O. Phytotoxins from the leaves of Ruta graveolens. J. Agric. Food Chem. 2004, 52, 3345–3349. [Google Scholar] [CrossRef]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef]
- Pereira, D.S.M.; Cardoso, B.D.; Rodrigues, A.R.O.; Amorim, C.O.; Amaral, V.S.; Almeida, B.G.; Queiroz, M.J.R.P.; Martinho, O.; Baltazar, F.; Calhelha, R.C.; et al. Magnetoliposomes containing calcium ferrite nanoparticles for applications in breast cancer therapy. Pharmaceutics 2019, 11, 477. [Google Scholar] [CrossRef]
- Zhang, H. Thin-film hydration followed by extrusion method for liposome preparation. Methods Mol. Biol. 2017, 1522, 17–22. [Google Scholar]
- Pradhan, P.; Giri, J.; Banerjee, R.; Bellare, J.; Bahadur, D. Preparation and characterization of manganese ferrite-based magnetic liposomes for hyperthermia treatment of cancer. J. Magn. Magn. Mater. 2007, 311, 208–215. [Google Scholar] [CrossRef]
- Chauhan, N.; Dilbaghi, N.; Gopal, M.; Kumar, R.; Kim, K.H.; Kumar, S. Development of chitosan nanocapsules for the controlled release of hexaconazole. Int. J. Biol. Macromol. 2017, 97, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Esquerdo, V.M.; Dotto, G.L.; Pinto, L.A.A. Preparation of nanoemulsions containing unsaturated fatty acid concentrate-chitosan capsules. J. Coll. Interface Sci. 2015, 445, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Lin, B.; Lai, F.; Xu, C.; Zou, H. A green modified microsphere of chitosan encapsulating dimethyl fumarate and cross-linked by vanillin and its application for litchi preservation. Ind. Eng. Chem. Res. 2016, 55, 4490–4498. [Google Scholar] [CrossRef]
- Xiao, Z.; Xu, Z.; Zhu, G. Production and characterization of nanocapsules encapsulated linalool by ionic gelation method using chitosan as wall material. Food Sci. Technol. 2017, 37, 613–619. [Google Scholar] [CrossRef]
- Bruschi, M. (Ed.) Strategies to Modify the Drug Release from Pharmaceutical Systems, 1st ed.; Elsevier/Woodhead Publishing: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modelling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Fernandes, M.J.G.; Castanheira, E.M.S.; Fortes, A.G.; Pereira, R.B.; Pereira, D.M.; Gonçalves, M.S.T. New eugenol derivatives and their selective toxicity towards insect cells: Synthesis and biological assessment. Int. J. Mol. Sci. 2020, 21, 9257. [Google Scholar] [CrossRef]
- Ribeiro, V.; Andrade, P.B.; Valentão, P.; Pereira, D.M. Benzoquinones from Cyperus spp. trigger IRE1α-independent and PERK-dependent ER stress in human stomach cancer cells and are novel proteasome inhibitors. Phytomedicine 2019, 63, 153017. [Google Scholar] [CrossRef]
- Chouiter, I.; Boulebd, H.; Pereira, D.M.; Valentão, P.; Andrade, P.; Belfaitah, A.; Silva, A. Chalcone-type compounds and 2-pyrazoline derivatives bearing an imidazole moiety: Synthesis and caspase-dependent anticancer activity. Future Med. Chem. 2020, 12, 493–509. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
| Entry | Specie | Part of the Plant | Solvent | Code |
|---|---|---|---|---|
| 1 | Phytolacca americana | Leaves | DCM | A1 |
| 2 | Phytolacca americana | Berries | DCM | A2 |
| 3 | Phytolacca americana | Berries | Water/ EtOH (1:1) | D2 |
| 4 | Phytolacca americana | Leaves | Water/ EtOH (1:1) | D3 |
| 5 | Tagetes patula (red flowers) | Leaves | DCM (F.1-3) | A3 |
| 6 | Tagetes patula (yellow flowers) | Leaves | DCM (F.1-4) | A4 |
| 7 | Tagetes patula (orange flowers) | Leaves | DCM (F.1-3) | A5 |
| 8 | Tagetes patula (red flowers) | Flowers | DCM | A6 |
| 9 | Tagetes patula (red flowers) | Leaves | Water/ EtOH (1:1) | B6 |
| 10 | Tagetes patula | Flowers (red) | Water/ EtOH (1:1) | C1 |
| 11 | Tagetes patula (yellow flowers) | Leaves | Water/ EtOH (1:1) | C2 |
| 12 | Tagetes patula (orange flowers) | Leaves | Water/ EtOH (1:1) | C3 |
| 13 | Ruta graveolens | Leaves | DCM | B1 |
| 14 | Ruta graveolens | Leaves | Water/ EtOH (1:1) | D4 |
| 15 | Ulex europaeus | Leaves and flowers | DCM | B2 |
| 16 | Ulex europaeus | Leaves and flowers | Water/ EtOH (1:1) | C5 |
| 17 | Glandora prostrata | Leaves and flowers | DCM | B3 |
| 18 | Glandora prostrata | Leaves and flowers | Water/ EtOH (1:1) | D1 |
| 19 | Punica granatum | Fruit peel | DCM | B4 |
| 20 | Punica granatum | Fruit peel | Water/ EtOH (1:1) | C4 |
| 21 | Camellia japonica | Leaves | DCM | B5 |
| 22 | Camellia japonica | Leaves | Water/ EtOH (1:1) | C6 |
| Peak | RT | M+ | Compound | Reference |
|---|---|---|---|---|
| 10 | 19.477 | 279 |  Graveoline | [37] |
| 11 | 20.455 | 259 | 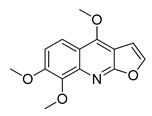 Skimmianin | [37] |
| 11 | 20.455 | 261 |  4,6,7-Trimethoxy-furo[2,3-b]quinoline | [38] |
| 16 | 23.932 | 216 | 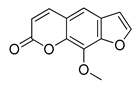  8-Methoxypsoralen 5-Methoxypsoralen | [39] |
| 17 | 24.082 | 229 | 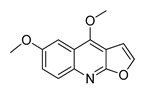 Pteleine | [37] |
| 18 | 33.006 | 314 |  Chalepin | [37] |
| 21 | 38.681 | 356 | 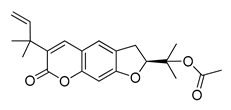 Rutamarin | [37] |
| 20 | 37.305 | 271 | 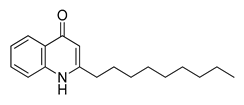 2-N-Nonyl-4-quinolone | [38] |
| 21 | 38.681 | 357 |  Gravacridonetriol | [37] |
| System | Size ± SD (nm) | PDI ± SD |
|---|---|---|
| Liposomes (ethanolic injection) | 121 ± 28 | 0.256 ± 0.08 |
| Liposomes (thin film hydration) | 203 ± 17 | 0.185 ± 0.05 |
| Chitosan nanostructures | 118 ± 23 | 0.203 ± 0.02 |
| System | Sample B1 |
|---|---|
| Liposomes (ethanolic injection) | 93.3 ± 6 |
| Liposomes (thin film hydration) | 73.0 ± 9 |
| Chitosan nanoemulsions | 94.0 ± 2 |
| Nanosystem | Ks | n | l | |
|---|---|---|---|---|
| Liposomes (thin film hydration) | 0.07 | 0.50 | 0.9 | 0.98 |
| Liposomes (ethanolic injection) Liposomes (ethanolic injection) * | 0.01 | 1.39 | 0 | 0.98 |
| 0.0045 | 1.74 | 0 | 0.99 | |
| Chitosan nanostructures Chitosan nanostructures * | 0.04 | 1.07 | 2.13 | 0.98 |
| 0.0314 | 1.21 | 2.11 | 0.99 |
| Nanosystem | a | B | ||
|---|---|---|---|---|
| Liposomes (thin film hydration) | 49.38 | 3.61 | 1.55 | 0.99 |
| Liposomes (ethanolic injection) | 100 | 680.48 | 5.99 | 0.99 |
| Chitosan nanostructures | 100 | 163.23 | 5.34 | 0.97 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.I.F.; Monteiro, M.; Araújo, A.R.L.; Rodrigues, A.R.O.; Castanheira, E.M.S.; Pereira, D.M.; Olim, P.; Fortes, A.G.; Gonçalves, M.S.T. Cytotoxic Plant Extracts towards Insect Cells: Bioactivity and Nanoencapsulation Studies for Application as Biopesticides. Molecules 2020, 25, 5855. https://doi.org/10.3390/molecules25245855
Lopes AIF, Monteiro M, Araújo ARL, Rodrigues ARO, Castanheira EMS, Pereira DM, Olim P, Fortes AG, Gonçalves MST. Cytotoxic Plant Extracts towards Insect Cells: Bioactivity and Nanoencapsulation Studies for Application as Biopesticides. Molecules. 2020; 25(24):5855. https://doi.org/10.3390/molecules25245855
Chicago/Turabian StyleLopes, Ana I. F., Mariana Monteiro, Ana R. L. Araújo, Ana Rita O. Rodrigues, Elisabete M. S. Castanheira, David M. Pereira, Pedro Olim, A. Gil Fortes, and M. Sameiro T. Gonçalves. 2020. "Cytotoxic Plant Extracts towards Insect Cells: Bioactivity and Nanoencapsulation Studies for Application as Biopesticides" Molecules 25, no. 24: 5855. https://doi.org/10.3390/molecules25245855
APA StyleLopes, A. I. F., Monteiro, M., Araújo, A. R. L., Rodrigues, A. R. O., Castanheira, E. M. S., Pereira, D. M., Olim, P., Fortes, A. G., & Gonçalves, M. S. T. (2020). Cytotoxic Plant Extracts towards Insect Cells: Bioactivity and Nanoencapsulation Studies for Application as Biopesticides. Molecules, 25(24), 5855. https://doi.org/10.3390/molecules25245855








