Design, Synthesis and Fungicidal Activity of New 1,2,4-Triazole Derivatives Containing Oxime Ether and Phenoxyl Pyridinyl Moiety
Abstract
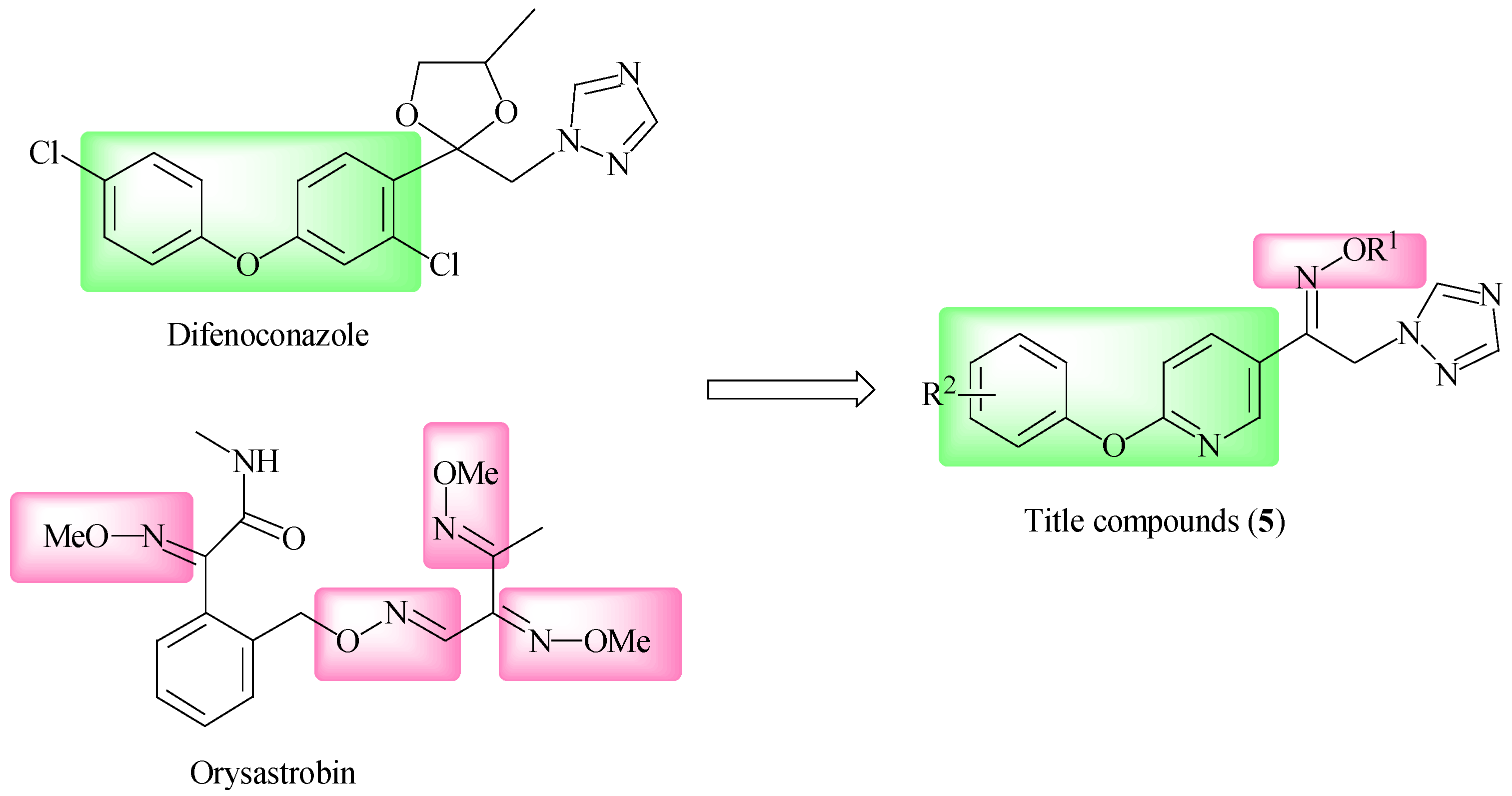
1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Antifungal Activity
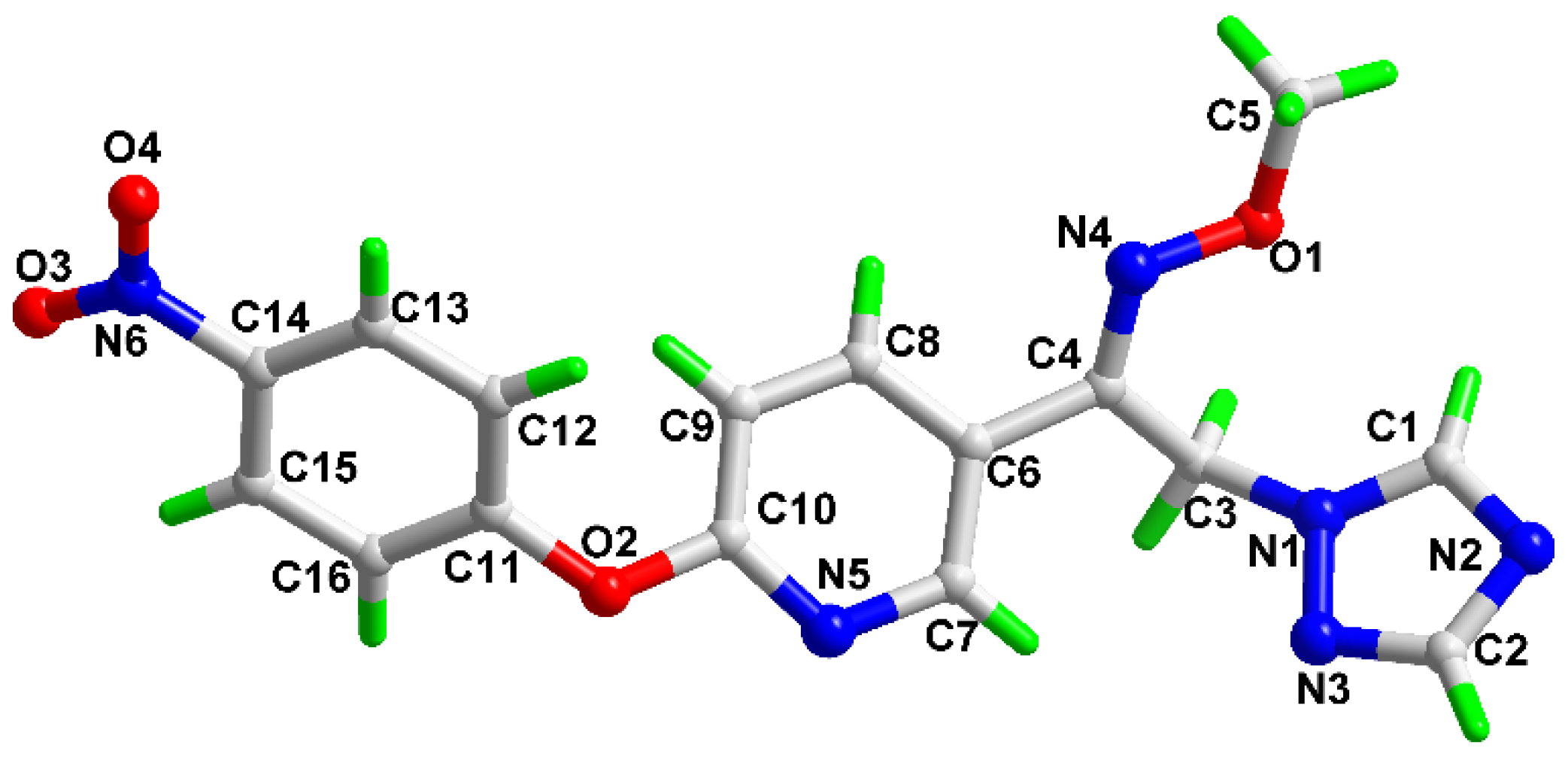
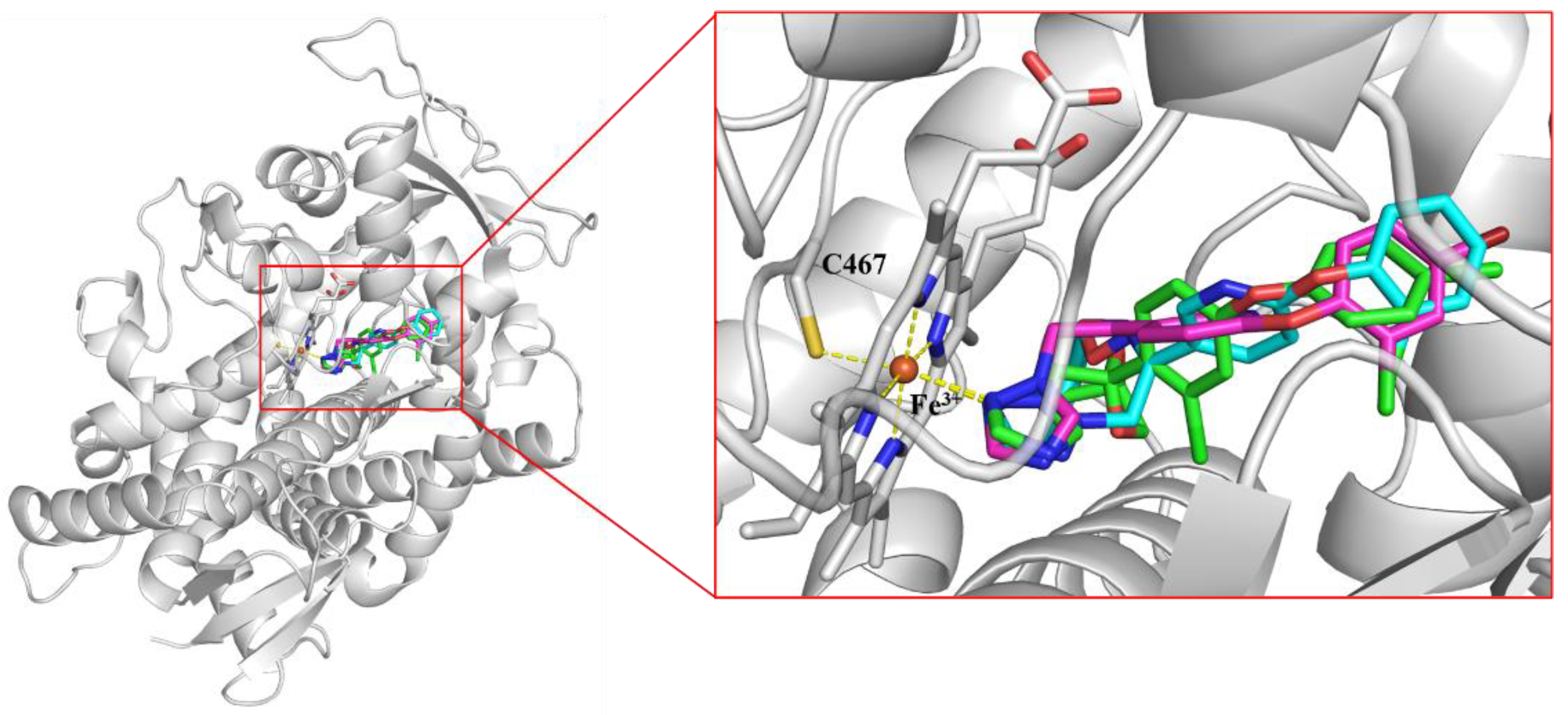
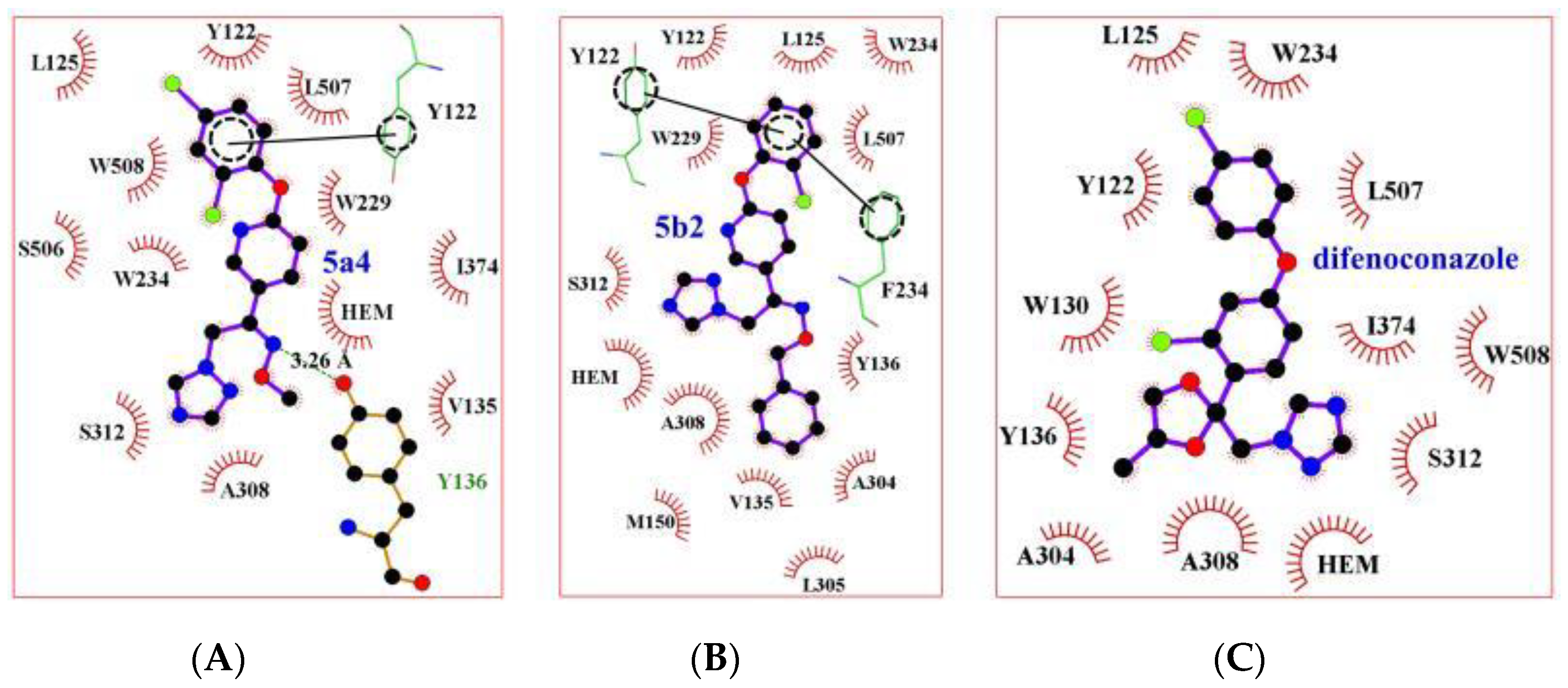
2.3. Homology Modelling and Molecular Docking
3. Materials and Methods
3.1. Instruments and Materials
3.2. Synthesis
3.2.1. Synthetic Procedures and Spectral Data for All Title Compounds and Intermediates
3.2.2. Synthesis of 2-bromo-1-(6-chloropyridin-3-yl)ethan-1-one (2)
3.2.3. Synthesis of 1-(6-chloropyridin-3-yl)-2-(1H-1,2,4-triazol-1-yl)ethan-1-one (3)
3.2.4. Synthesis of (Z)-1-(6-chloropyridin-3-yl)-2-(1H-1,2,4-triazol-1-yl)ethan-1-one O-methyl Oxime (4a) and (Z)-1-(6-chloropyridin-3-yl)-2-(1H-1,2,4-triazol-1-yl)ethan-1-one O-benzyl Oxime (4b)
3.2.5. Synthesis of Title Compounds 5a1–5a18, 5b1–5b9
3.3. Fungicidal Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Oerke, E.C.; Dehne, H.W. Safeguarding production—Losses in major crops and the role of crop protection. Crop Prot. 2004, 23, 275–285. [Google Scholar] [CrossRef]
- Liu, X.H.; Fang, Y.M.; Xie, F.; Zhang, R.R.; Shen, Z.H.; Tan, C.X.; Weng, J.Q.; Xu, T.M.; Huang, H.Y. Synthesis and in vivo fungicidal activity of some new quinoline derivatives against rice blast. Pest Manag. Sci. 2017, 73, 1900–1907. [Google Scholar] [CrossRef] [PubMed]
- Becher, R.; Wirsel, S.G.R. Fungal cytochrome P450 sterol 14á-demethylase (CYP51) and azole resistance in plant and human pathogens. Appl. Microbiol. Biotechnol. 2012, 95, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ni, X.; Peng, Q.; Hou, Y.; Fang, Y.; Mu, W.; Liu, C.; Liu, P.; Liu, X. The novel fungicide SYP-14288 acts as an uncoupler against Phytophthora capsici. Pestic. Biochem. Physiol. 2018, 147, 83–89. [Google Scholar] [CrossRef]
- Casida, J.E. Pesticide Interactions: Mechanisms, Benefits, and Risks. J. Agric. Food Chem. 2017, 65, 4553–4561. [Google Scholar] [CrossRef]
- Mair, W.; Lopez-Ruiz, F.; Stammler, G.; Clark, W.; Burnett, F.; Hollomon, D.; Ishii, H.; Thind, T.S.; Brown, J.K.; Fraaije, B.; et al. Proposal for a unified nomenclature for target-site mutations associated with resistance to fungicides. Pest Manag. Sci. 2016, 72, 1449–1459. [Google Scholar] [CrossRef]
- Qian, H.; Duan, M.; Sun, X.; Chi, M.; Zhao, Y.; Liang, W.; Du, J.; Huang, J.; Li, B. The binding mechanism between azoles and FgCYP51B, sterol 14á−demethylase of Fusarium graminearum. Pest Manag. Sci. 2018, 74, 126–134. [Google Scholar] [CrossRef]
- Patil, S.R.; Asrondkar, A.; Patil, V.; Sangshetti, J.N.; Khan, F.A.K.; Damale, M.G.; Patil, R.H.; Bobade, A.S.; Shinde, D.B. Antileishmanial potential of fused 5-(pyrazin-2-yl)-4H-1,2,4-triazole-3-thiols: Synthesis, biological evaluations and computational studies. Bioorg. Med. Chem. Lett. 2017, 27, 3845–3850. [Google Scholar] [CrossRef]
- Sathyanarayana, R.; Poojary, B. Exploring recent developments on 1,2,4-triazole: Synthesis and biological applications. J. Chin. Chem. Soc. 2019, 67, 459–477. [Google Scholar] [CrossRef]
- Monk, B.C.; Sagatova, A.A.; Hosseini, P.; Ruma, Y.N.; Wilson, R.K.; Keniya, M.V. Fungal Lanosterol 14á-demethylase: A target for next-generation antifungal design. Biochim. Biophys Acta Proteins Proteom. 2020, 1868. [Google Scholar] [CrossRef]
- Choi, J.Y.; Podust, L.M.; Roush, W.R. Drug Strategies Targeting CYP51 in Neglected Tropical Diseases. Chem. Rev. 2014, 114, 11242–11271. [Google Scholar] [CrossRef] [PubMed]
- Guan, A.Y.; Liu, C.L.; Sun, X.F.; Xie, Y.; Wang, M.A. Discovery of pyridine-based agrochemicals by using Intermediate Derivatization Methods. Bioorg. Med. Chem. 2015, 24, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Lü, M.; Chen, L.; Li, Q.; Song, H.; Bi, F.; Huang, R.; Wang, Q. Design, Synthesis, Bioactivity, and Structure−Activity Relationship (SAR) Studies of Novel Benzoylphenylureas Containing Oxime Ether Group. J. Agric. Food Chem. 2008, 56, 11376–11391. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, C.; Zheng, Y.; Wei, X.; Ma, Q.; Wei, P.; Liu, Y.; Qin, Y.; Yang, N.; Sun, Y.; et al. Design, Synthesis, Acaricidal Activity, and Mechanism of Oxazoline Derivatives Containing an Oxime Ether Moiety. J. Agric. Food Chem. 2014, 62, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liu, C.; Zhang, H.; Wang, Q. Benzoylurea Chitin Synthesis Inhibitors. J. Agric. Food Chem. 2015, 63, 6847–6865. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Yang, D. High-Activity Imino Phenylacetate Compounds and Preparation Method and Application. CN 201,710,160,407.4, 14 July 2017. [Google Scholar]
- Guha, S.K.; Wu, B.; Kim, B.S.; Baik, W.; Koo, S. TMS OTf-Catalyzed α-bromination of carbonyl compounds by N-bromosuccinimide. Tetrahedron Lett. 2006, 47, 291–293. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Wang, B.L.; Zhan, Y.Z.; Zhang, Y.; Zhang, X.; Li, Z.M. Synthesis and biological activities of some fluorine- and piperazine-containing 1,2,4-triazole thione derivatives. Chin. Chem. Lett. 2016, 27, 163–167. [Google Scholar] [CrossRef]
- El-Sherief, H.A.; Youssif, B.G.; Abdelazeem, A.H.; Abdel-Aziz, M.; Abdel-Rahman, H.M. Design, Synthesis and Antiproliferative Evaluation of Novel 1,2,4-Triazole/Schiff Base Hybrids with EGFR and B-RAF Inhibitory Activities. Anti Cancer Agents Med. Chem. 2019, 19, 697–706. [Google Scholar] [CrossRef]
- Liu, J.B.; Tao, W.F.; Dai, H.; Jin, Z.; Fang, J.X. Synthesis, structures, and biological activities of new 1H-1,2,4-triazole derivatives containing pyridine unit. Heteroatom Chem. 2007, 18, 376–380. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, D.; Wang, X.; Ding, K. (2-Pyridyl) acetone-Promoted Cu-Catalyzed O-Arylation of Phenols with Aryl Iodides, Bromides, and Chlorides. J. Org. Chem. 2009, 74, 7187–7191. [Google Scholar] [CrossRef]
- Jeschke, P. The unique role of halogen substituents in the design of modern agrochemicals. Pest Manag. Sci. 2010, 66, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P. Latest generation of halogen–Containing pesticides. Pest Manag. Sci. 2017, 73, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xia, S.; Zhang, P.; Zhang, T.; Wang, W.; Tian, Y.; Meng, G.; Jiang, S.; Liu, K. Discovery of Hydrocarbon-Stapled Short á-Helical Peptides as Promising Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Fusion Inhibitors. J. Med. Chem. 2018, 61, 5679–5691. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Guide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. A New Approach for Rapid, Accurate Docking and Scoring. 2. Enrichment Factors in Database Screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Räth, C.; Schiffmann, F.; Binz, A.; Räth, C. Über Derivate Des 3-Cyanpyridins. XIV. Mitteilung über Derivate des Pyridins. Justus Liebigs Ann. Chem. 1931, 487, 127–134. [Google Scholar] [CrossRef]
- Lunts, L.H.C.; Hartley, D.; Toon, P. 1-(Pyridyl)-2-Aminoaethanole, Ihre Aromatischen N-Oxyde, Ihre Salze mit Saeuren und Verfahren Zuihrer Herstellung. DE. Patent 1,811,833 A1, 29 November 1968. [Google Scholar]
- Cao, X.; Li, F.; Hu, M.; Lu, W.; Yu, G.A.; Liu, S.H. Chiral γ-Aryl-1H-1,2,4-triazole Derivatives as Highly Potential Antifungal Agents: Design, Synthesis, Structure, and in Vitro Fungicidal Activities. J. Agric. Food Chem. 2008, 56, 11367–11375. [Google Scholar] [CrossRef]
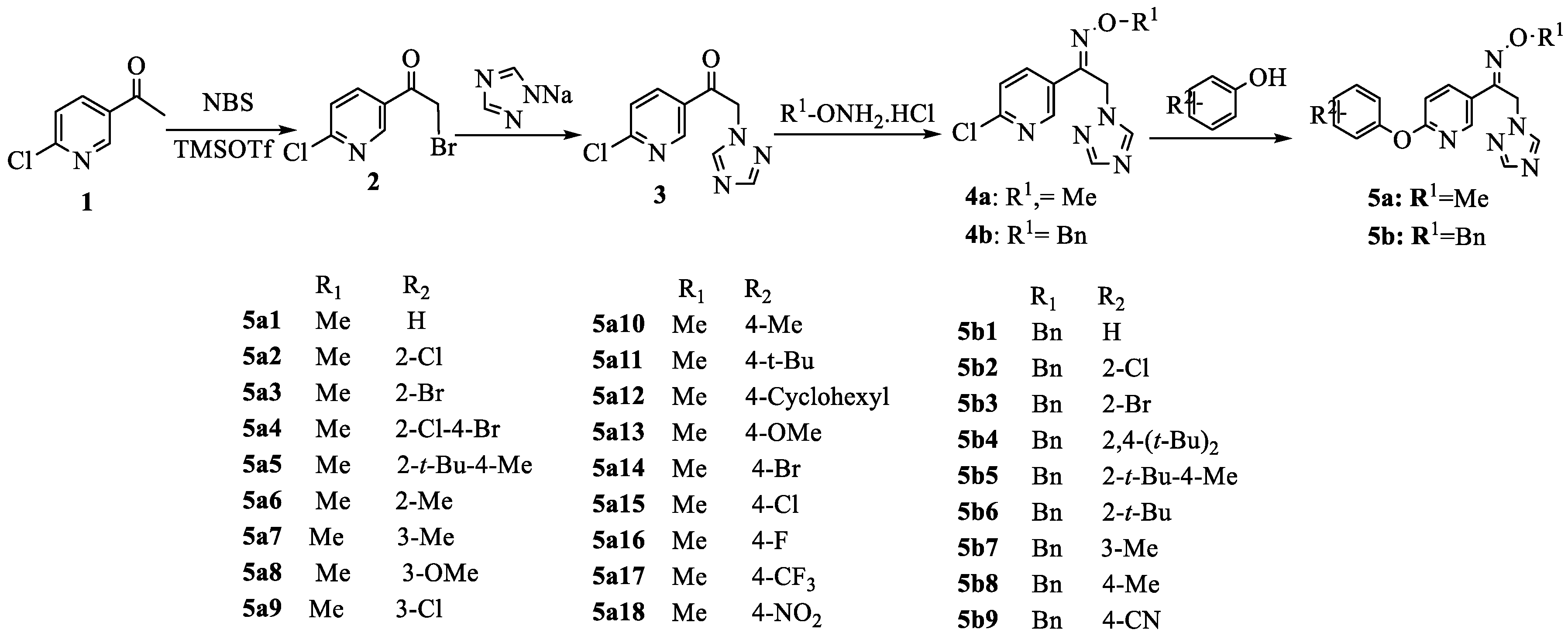
| Compds. | Mycelium Growth Inhibitory Rate (%) at 50 mg/L b | |||||||
|---|---|---|---|---|---|---|---|---|
| FG | SS | BC | PI | PG | RS | FO | AA | |
| 5a1 | 44.8 | 50.2 | 22.9 | 69.0 | 72.7 | 62.7 | 49.1 | 29.2 |
| 5a2 | 59.0 | 89.0 | 44.8 | 61.7 | 46.6 | 71.3 | 51.8 | 29.6 |
| 5a3 | 55.7 | 85.4 | 32.6 | 64.2 | 48.6 | 74.5 | 52.9 | 24.0 |
| 5a4 | 51.5 | 92.3 | 75.6 | 75.2 | 42.7 | 77.9 | 58.5 | 41.9 |
| 5a5 | 27.3 | 88.0 | 81.0 | 65.0 | 52.8 | 70.7 | 49.8 | 52.5 |
| 5a6 | 30.1 | 24.0 | 20.7 | 36.6 | 32.2 | 47.8 | 61.1 | 25.6 |
| 5a7 | 44.8 | 90.7 | 72.5 | 60.6 | 43.9 | 64.9 | 45.6 | 38.3 |
| 5a8 | 38.8 | 44.0 | 62.8 | 60.5 | 37.7 | 71.3 | 56.5 | 52.1 |
| 5a9 | 66.7 | 83.1 | 49.6 | 59.7 | 67.8 | 68.2 | 63.8 | 55.1 |
| 5a10 | 45.1 | 62.2 | 30.9 | 74.5 | 62.6 | 68.2 | 64.2 | 31.4 |
| 5a11 | 39.9 | 88.7 | 70.5 | 58.7 | 47.0 | 75.9 | 47.9 | 37.6 |
| 5a12 | 37.8 | 73.7 | 66.7 | 42.9 | 24.1 | 45.3 | 40.9 | 35.2 |
| 5a13 | 55.2 | 27.9 | 12.8 | 76.4 | 27.6 | 51.1 | 59.6 | 26.2 |
| 5a14 | 48.1 | 87.8 | 47.0 | 74.6 | 61.4 | 79.7 | 63.1 | 32.6 |
| 5a15 | 42.1 | 88.7 | 32.1 | 72.0 | 53.3 | 75.9 | 59.6 | 25.9 |
| 5a16 | 37.6 | 79.8 | 0.0 | 57.2 | 56.7 | 71.8 | 58.0 | 23.2 |
| 5a17 | 40.3 | 83.7 | 78.3 | 52.5 | 57.6 | 78.6 | 73.1 | 26.6 |
| 5a18 | 37.6 | 84.3 | 43.5 | 74.6 | 19.8 | 82.7 | 48.4 | 21.4 |
| 5b1 | 45.1 | 65.1 | 47.2 | 48.4 | 57.2 | 42.4 | 40.6 | 30.4 |
| 5b2 | 49.0 | 90.0 | 47.2 | 36.1 | 43.9 | 36.9 | 43.7 | 18.7 |
| 5b3 | 45.2 | 76.8 | 39.9 | 39.5 | 43.1 | 36.6 | 38.3 | 15.1 |
| 5b4 | 0.0 | 43.8 | 16.1 | 0.0 | 0.0 | 19.1 | 7.9 | 1.5 |
| 5b5 | 26.6 | 45.2 | 30.2 | 20.6 | 7.3 | 22.1 | 18.0 | 7.2 |
| 5b6 | 18.7 | 27.0 | 35.3 | 11.5 | 0.0 | 27.2 | 16.0 | 0.0 |
| 5b7 | 30.3 | 73.4 | 33.6 | 27.4 | 35.4 | 34.4 | 26.4 | 17.2 |
| 5b8 | 28.1 | 70.4 | 14.3 | 21.8 | 12.8 | 26.4 | 18.8 | 16.2 |
| 5b9 | 39.1 | 82.3 | 31.7 | 46.2 | 33.3 | 35.6 | 32.8 | 26.3 |
| Difenoconazole | 93.7 | 99.3 | 90.2 | 86.0 | 99.5 | 89.3 | 94.2 | 84.3 |
| Compds. | EC50 (mg/L) | |||
|---|---|---|---|---|
| SS | PI | RS | BC | |
| 5a2 | 4.40 | 29.87 | 10.43 | 58.90 |
| 5a3 | 10.07 | 18.64 | 9.80 | 71.56 |
| 5a4 | 1.59 | 0.46 | 0.27 | 11.39 |
| 5a5 | 4.71 | 26.78 | 34.25 | 19.68 |
| 5a7 | 4.30 | 19.09 | 11.95 | 46.25 |
| 5a14 | 17.47 | 5.92 | 19.33 | 37.04 |
| 5a15 | 2.64 | 17.93 | 15.43 | 67.78 |
| 5a17 | 3.65 | 41.46 | 65.41 | 7.48 |
| 5a18 | 15.09 | 3.02 | 5.25 | 14.50 |
| 5b2 | 0.12 | >100 | >100 | 89.05 |
| Difenoconazole | 0.02 | 0.26 | 0.09 | 8.86 |
Sample Availability: Samples of the all test compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, H.; Liu, X.; Chenzhang, P.; Xiao, Y.; Fu, B.; Qin, Z. Design, Synthesis and Fungicidal Activity of New 1,2,4-Triazole Derivatives Containing Oxime Ether and Phenoxyl Pyridinyl Moiety. Molecules 2020, 25, 5852. https://doi.org/10.3390/molecules25245852
Bai H, Liu X, Chenzhang P, Xiao Y, Fu B, Qin Z. Design, Synthesis and Fungicidal Activity of New 1,2,4-Triazole Derivatives Containing Oxime Ether and Phenoxyl Pyridinyl Moiety. Molecules. 2020; 25(24):5852. https://doi.org/10.3390/molecules25245852
Chicago/Turabian StyleBai, Hui, Xuelian Liu, Pengfei Chenzhang, Yumei Xiao, Bin Fu, and Zhaohai Qin. 2020. "Design, Synthesis and Fungicidal Activity of New 1,2,4-Triazole Derivatives Containing Oxime Ether and Phenoxyl Pyridinyl Moiety" Molecules 25, no. 24: 5852. https://doi.org/10.3390/molecules25245852
APA StyleBai, H., Liu, X., Chenzhang, P., Xiao, Y., Fu, B., & Qin, Z. (2020). Design, Synthesis and Fungicidal Activity of New 1,2,4-Triazole Derivatives Containing Oxime Ether and Phenoxyl Pyridinyl Moiety. Molecules, 25(24), 5852. https://doi.org/10.3390/molecules25245852




