Hydrogel Properties and Their Impact on Regenerative Medicine and Tissue Engineering
Abstract
:1. Introduction
2. Physicochemical Properties
2.1. Swelling Ratio
2.2. Porosity
- c.a. 5 µm for neovascularisation;
- c.a. 20 µm for hepatocyte ingrowth;
- between 5 and 15 µm for fibroblast ingrowth;
- between 20 and 125 µm for adult mammalian cells;
- between 40 and 100 µm for osteoid ingrowth;
- between 100 and 350 µm for bone tissue regeneration;
- above 500 µm for fibrovascular tissue development.
2.3. Rheology
- All materials exposed to isotropic stress behave like a perfectly elastic material.
- Each material has all rheological properties, although to different degrees.
- The rheological equation of a simpler object can be derived from that of a more complex object by comparing the relevant parameters to zero.
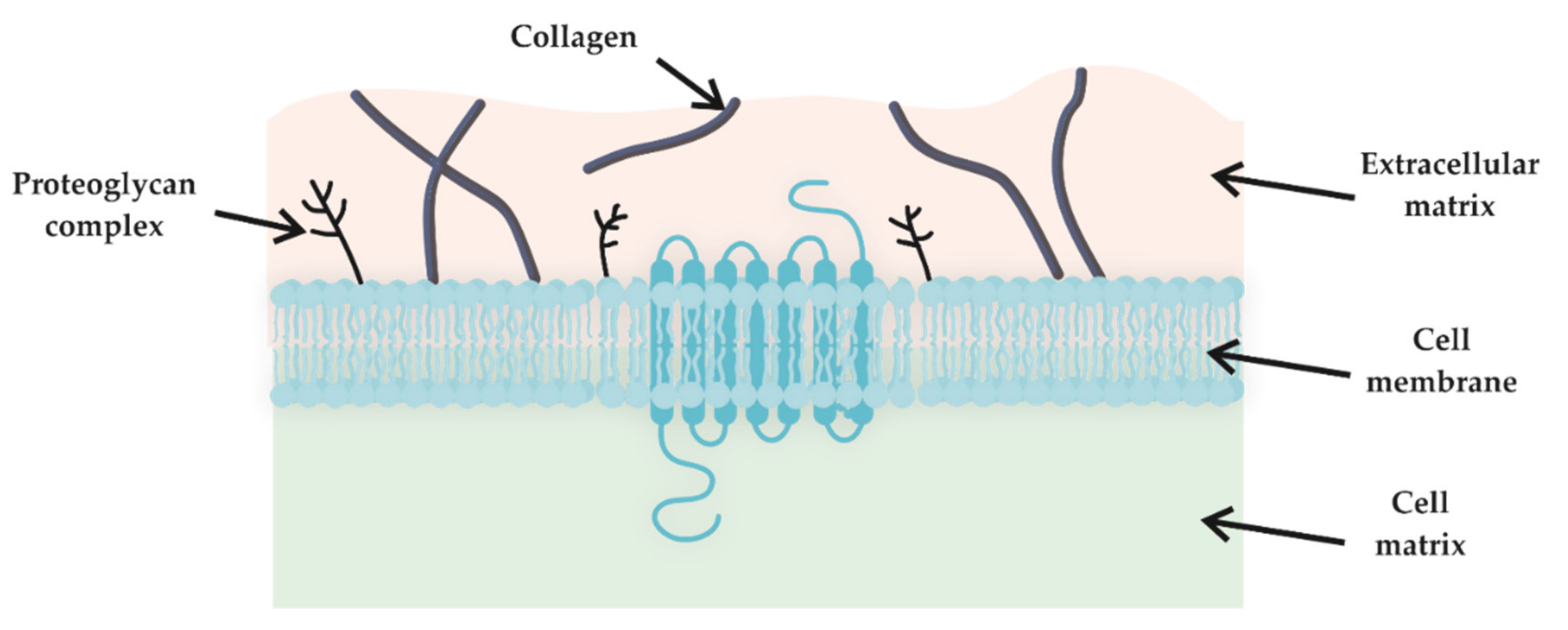
2.4. Biocompatibility
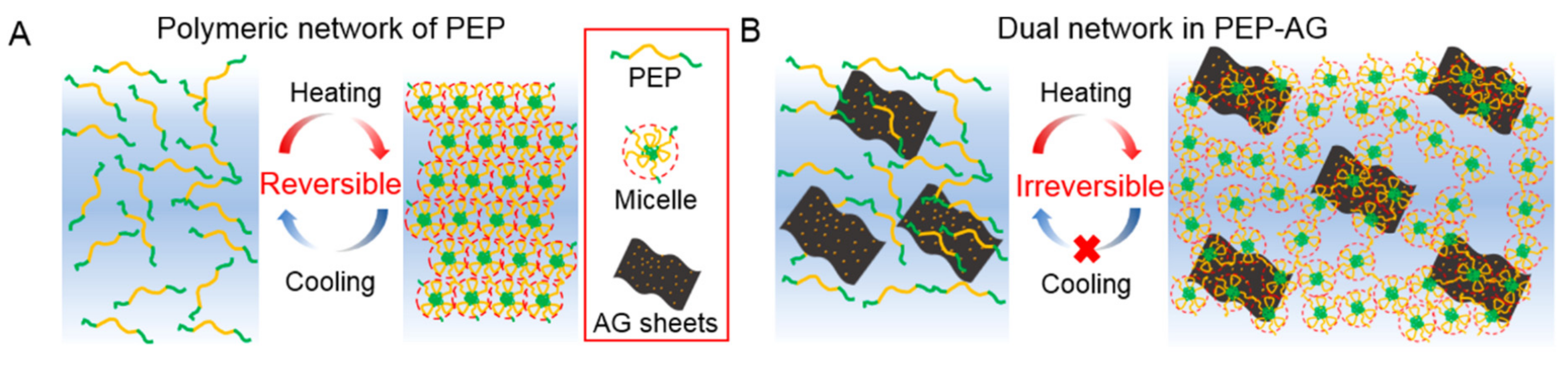
2.5. Self-Healing
3. Chemical Modification of HGs
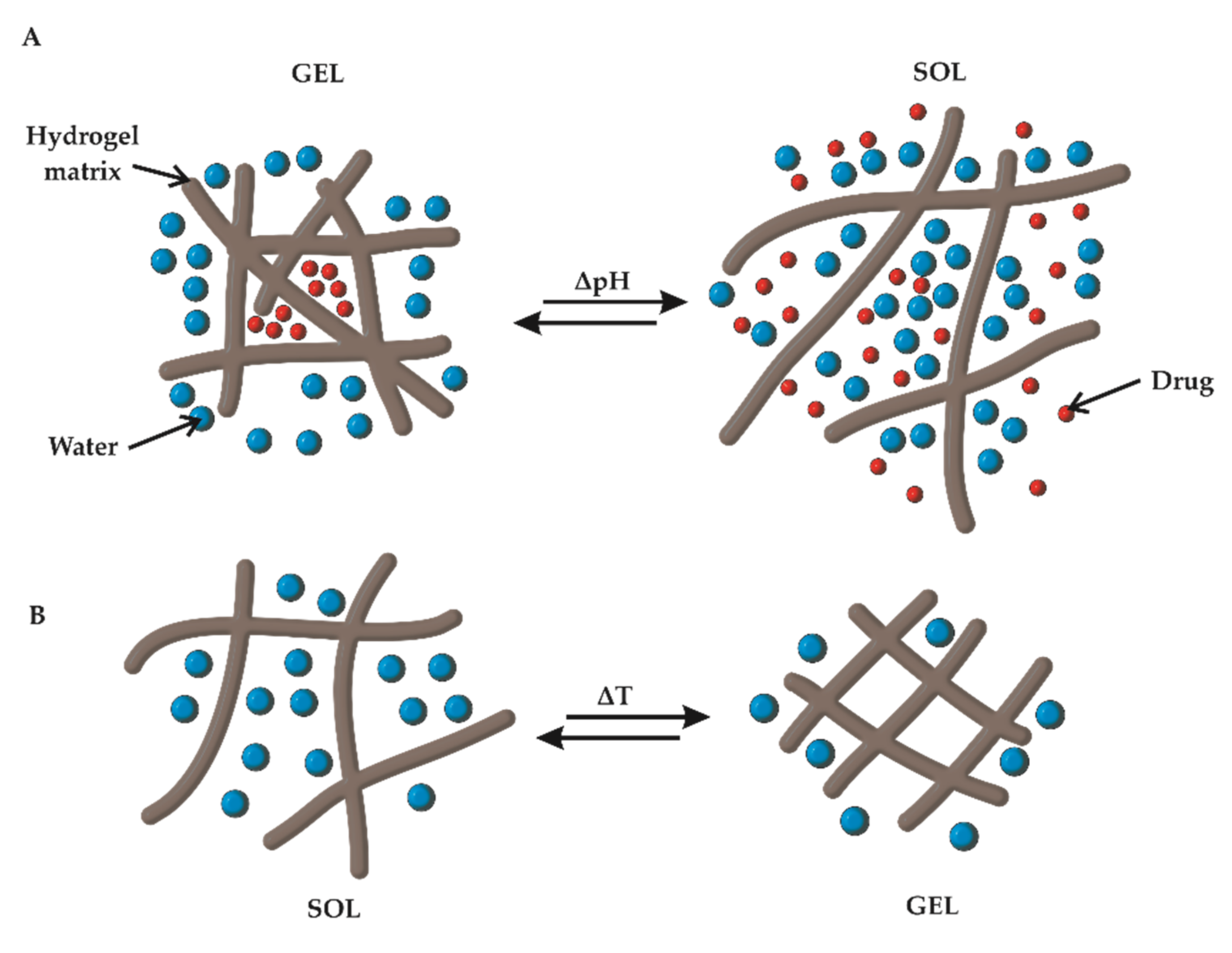
3.1. pH Responsiveness
3.2. Thermoresponsiveness
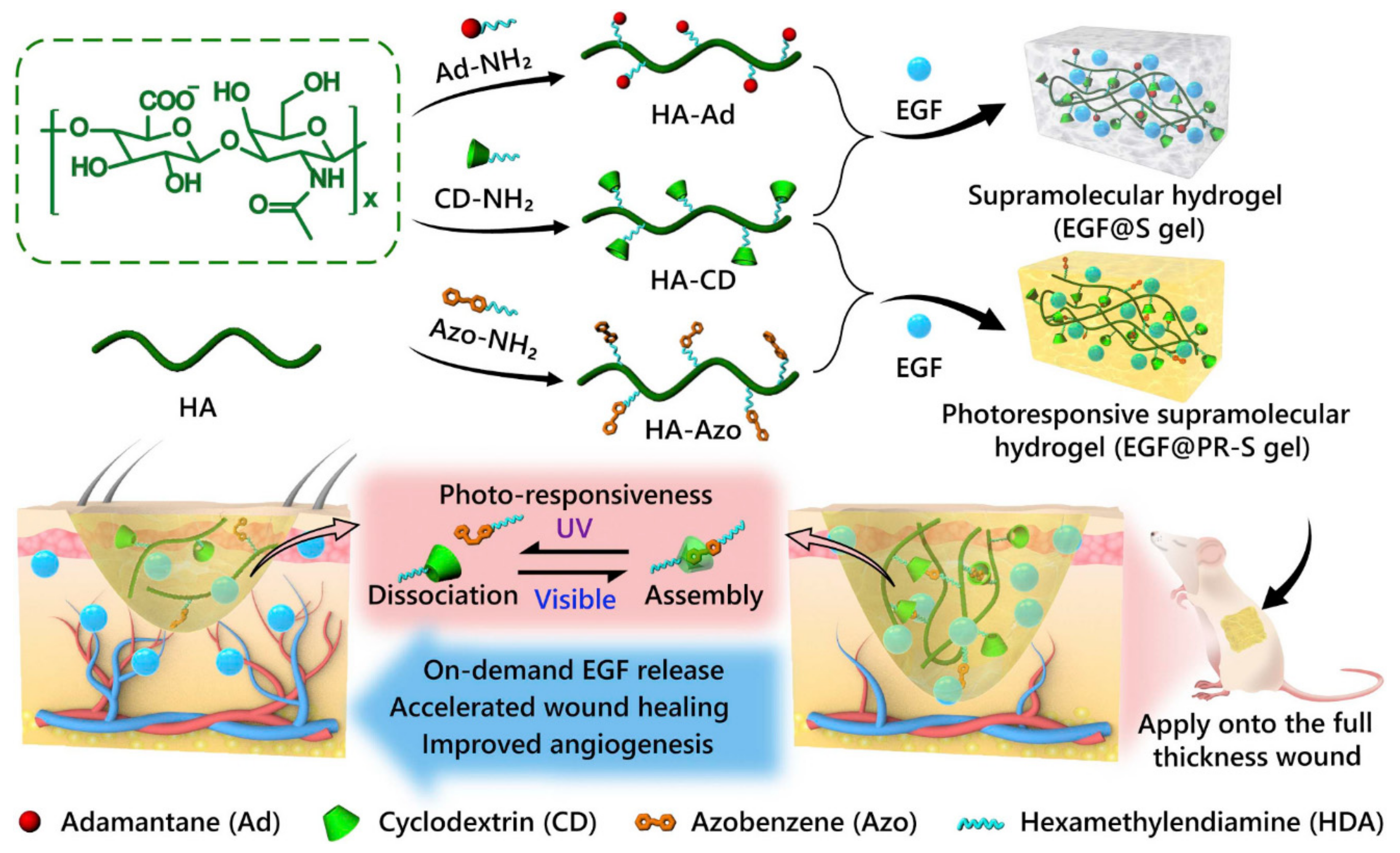
3.3. Photoresponsiveness
3.4. Conductive HGs
3.5. 3D Bioprinting
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, C.; Gao, L.; Liu, X.; Yang, T.; Yin, G.; Chen, J.; Guo, H.; Yu, B.; Cong, H. Injectable Schiff base polysaccharide hydrogels for intraocular drug loading and release. J. Biomed. Mater. Res. A 2019, 107, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Han, S.; Huang, X.; Han, M.; Cao, J.; Liang, Y.; Sun, Y. LDH hybrid thermosensitive hydrogel for intravaginal delivery of anti-HIV drugs. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1234–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Martínez, M.; Rodríguez-Berna, G.; Bermejo, M.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Merino, V. Covalently crosslinked organophosphorous derivatives-chitosan hydrogel as a drug delivery system for oral administration of camptothecin. Eur. J. Pharm. Biopharm. 2019, 136, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Wang, Y.; Cai, R.; Chang, H.; Song, K.; Zuo, H.; Zhao, P.; Xia, Q.; He, H. Design and performance of sericin/poly(vinyl alcohol) hydrogel as a drug delivery carrier for potential wound dressing application. Mater. Sci. Eng. C 2019, 101, 341–351. [Google Scholar] [CrossRef]
- Wu, Z.; Hong, Y. Combination of the Silver-Ethylene Interaction and 3D Printing to Develop Antibacterial Superporous Hydrogels for Wound Management. ACS Appl. Mater. Interfaces 2019, 11, 33734–33747. [Google Scholar] [CrossRef]
- Farghaly Aly, U.; Aboutaleb, H.A.; Abdellatif, A.A.; Sameh Tolba, N. Formulation and evaluation of simvastatin polymeric nanoparticles loaded in hydrogel for optimum wound healing purpose. Drug Des. Dev. Ther. 2019, 13, 1567–1580. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Li, X.; Sun, F. Cyclodextrin-containing hydrogels for contact lenses as a platform for drug incorporation and release. Acta Biomater. 2010, 6, 486–493. [Google Scholar] [CrossRef]
- Hu, X.; Tan, H.; Chen, P.; Wang, X.; Pang, J. Polymer Micelles Laden Hydrogel Contact Lenses for Ophthalmic Drug Delivery. J. Nanosci. Nanotechnol. 2016, 16, 5480–5488. [Google Scholar] [CrossRef]
- Pimenta, A.F.R.; Ascenso, J.; Fernandes, J.C.S.; Colaço, R.; Serro, A.P.; Saramago, B. Controlled drug release from hydrogels for contact lenses: Drug partitioning and diffusion. Int. J. Pharm. 2016, 515, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Liu, F.; Chen, Y.; Liu, J.; Wang, X.; Chen, A.T.; Deng, G.; Zhang, H.; Liu, J.; Hong, Z.; et al. Targeted Delivery of CRISPR/Cas9-Mediated Cancer Gene Therapy via Liposome-Templated Hydrogel Nanoparticles. Adv. Funct. Mater. 2017, 27, 1703036. [Google Scholar] [CrossRef]
- Madry, H.; Gao, L.; Rey-Rico, A.; Venkatesan, J.K.; Müller-Brandt, K.; Cai, X.; Goebel, L.; Schmitt, G.; Speicher-Mentges, S.; Zurakowski, D.; et al. Thermosensitive Hydrogel Based on PEO–PPO–PEO Poloxamers for a Controlled In Situ Release of Recombinant Adeno-Associated Viral Vectors for Effective Gene Therapy of Cartilage Defects. Adv. Mater. 2020, 32, 1906508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Li, Z.; Loh, X.J.; Chen, K.; Li, Z.; Wu, Y.-L. Targeted and Sustained Corelease of Chemotherapeutics and Gene by Injectable Supramolecular Hydrogel for Drug-Resistant Cancer Therapy. Macromol. Rapid Commun. 2019, 40, 1800117. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.D.; Lee, E.A.; An, Y.-H.; Kim, S.L.; Lee, S.S.; Yu, S.J.; Jang, H.L.; Nam, K.T.; Im, S.G.; Hwang, N.S. Chondroitin Sulfate-Based Biomineralizing Surface Hydrogels for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2017, 9, 21639–21650. [Google Scholar] [CrossRef]
- Park, H.; Guo, X.; Temenoff, J.S.; Tabata, Y.; Caplan, A.I.; Kasper, F.K.; Mikos, A.G. Effect of Swelling Ratio of Injectable Hydrogel Composites on Chondrogenic Differentiation of Encapsulated Rabbit Marrow Mesenchymal Stem Cells In Vitro. Biomacromolecules 2009, 10, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Peppas, N. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A. The swollen polymer network hypothesis: Quantitative models of hydrogel swelling, stiffness, and solute transport. Prog. Polym. Sci. 2020, 105, 101243. [Google Scholar] [CrossRef]
- Kaur, P.; Gondil, V.S.; Chhibber, S. A novel wound dressing consisting of PVA-SA hybrid hydrogel membrane for topical delivery of bacteriophages and antibiotics. Int. J. Pharm. 2019, 572, 118779. [Google Scholar] [CrossRef]
- Suhaeri, M.; Noh, M.H.; Moon, J.-H.; Kim, I.G.; Oh, S.J.; Ha, S.S.; Lee, J.H.; Park, K. Novel skin patch combining human fibroblast-derived matrix and ciprofloxacin for infected wound healing. Theranostics 2018, 8, 5025–5038. [Google Scholar] [CrossRef]
- Wu, D.-Q.; Zhu, J.; Han, H.; Zhang, J.-Z.; Wu, F.-F.; Qin, X.-H.; Yu, J.-Y. Synthesis and characterization of arginine-NIPAAm hybrid hydrogel as wound dressing: In vitro and in vivo study. Acta Biomater. 2018, 65, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, Q.; Zhang, J.; Zhao, W.; Zhao, C. Substrate-Independent Ag-Nanoparticle-Loaded Hydrogel Coating with Regenerable Bactericidal and Thermoresponsive Antibacterial Properties. ACS Appl. Mater. Interfaces 2017, 9, 44782–44791. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liao, X.; Zhang, J.; Yang, F.; Fan, Z. Novel chitosan hydrogels reinforced by silver nanoparticles with ultrahigh mechanical and high antibacterial properties for accelerating wound healing. Int. J. Biol. Macromol. 2018, 119, 402–412. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, R.; Hua, X.; Chen, H.; Xu, J.; Wu, R.; Cen, L. Highly stretchable HA/SA hydrogels for tissue engineering. J. Biomater. Sci. Polym. Ed. 2018, 29, 543–561. [Google Scholar] [CrossRef]
- Kim, S.W.; Bae, Y.H.; Okano, T. Hydrogels: Swelling, drug loading, and release. Pharm. Res. 1992, 9, 283–290. [Google Scholar] [CrossRef]
- Elbert, D.L. Liquid–liquid two-phase systems for the production of porous hydrogels and hydrogel microspheres for biomedical applications: A tutorial review. Acta Biomater. 2011, 7, 31–56. [Google Scholar] [CrossRef] [Green Version]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Staruch, R.M.T.; Glass, G.E.; Rickard, R.; Hettiaratchy, S.P.; Butler, P.E.M. Injectable Pore-Forming Hydrogel Scaffolds for Complex Wound Tissue Engineering: Designing and Controlling Their Porosity and Mechanical Properties. Tissue Eng. Part B Rev. 2017, 23, 183–198. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Braschler, T.M.; Renaud, P. Advances in the design of macroporous polymer scaffolds for potential applications in dentistry. J. Periodontal Implant. Sci. 2013, 43, 251. [Google Scholar] [CrossRef] [Green Version]
- De France, K.J.; Xu, F.; Hoare, T. Structured Macroporous Hydrogels: Progress, Challenges, and Opportunities. Adv. Healthc. Mater. 2018, 7, 1700927. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Shim, K.Y.; Kim, B.; Sung, J.H. Hydrogel-based three-dimensional cell culture for organ-on-a-chip applications. Biotechnol. Prog. 2017, 33, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hideyoshi, S.; Jiang, H.; Matsumura, Y.; Dziki, J.L.; LoPresti, S.T.; Huleihel, L.; Faria, G.N.F.; Fuhrman, L.C.; Lodono, R.; et al. Injectable, porous, biohybrid hydrogels incorporating decellularized tissue components for soft tissue applications. Acta Biomater. 2018, 73, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lin, S.; Yin, S.; Jiang, F.; Zhou, M.; Yang, G.; Sun, N.; Zhang, W.; Jiang, X. In situ gas foaming based on magnesium particle degradation: A novel approach to fabricate injectable macroporous hydrogels. Biomaterials 2020, 232, 119727. [Google Scholar] [CrossRef] [PubMed]
- Nalawade, A.C.; Ghorpade, R.V.; Shadbar, S.; Qureshi, M.S.; Chavan, N.N.; Khan, A.A.; Ponrathnam, S. Inverse high internal phase emulsion polymerization (i-HIPE) of GMMA, HEMA and GDMA for the preparation of superporous hydrogels as a tissue engineering scaffold. J. Mater. Chem. B 2016, 4, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Inci, I.; Kirsebom, H.; Galaev, I.Y.; Mattiasson, B.; Piskin, E. Gelatin cryogels crosslinked with oxidized dextran and containing freshly formed hydroxyapatite as potential bone tissue-engineering scaffolds: Potential gelatin-dextran-hydroxyapatite bone TE scaffold. J. Tissue Eng. Regen. Med. 2013, 7, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Mathew, A.P. 3D Printed Porous Cellulose Nanocomposite Hydrogel Scaffolds. J. Vis. Exp. 2019, 59401. [Google Scholar] [CrossRef]
- Jin, G.; Lee, S.; Kim, S.-H.; Kim, M.; Jang, J.-H. Bicomponent electrospinning to fabricate three-dimensional hydrogel-hybrid nanofibrous scaffolds with spatial fiber tortuosity. Biomed. Microdevices 2014, 16, 793–804. [Google Scholar] [CrossRef]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying. Acta Biomater. 2019, 94, 195–203. [Google Scholar] [CrossRef]
- Wang, H.; Gu, H.; Chen, Z.; Shang, L.; Zhao, Z.; Gu, Z.; Zhao, Y. Enzymatic Inverse Opal Hydrogel Particles for Biocatalyst. ACS Appl. Mater. Interfaces 2017, 9, 12914–12918. [Google Scholar] [CrossRef]
- Mansour, F.R.; Waheed, S.; Paull, B.; Maya, F. Porogens and porogen selection in the preparation of porous polymer monoliths. J. Sep. Sci. 2020, 43, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Tran, R.T.; Naseri, E.; Kolasnikov, A.; Bai, X.; Yang, J. A new generation of sodium chloride porogen for tissue engineering. Biotechnol. Appl. Biochem. 2011, 58, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Du, K.-F.; Yang, D.; Sun, Y. Fabrication of high-permeability and high-capacity monolith for protein chromatography. J. Chromatogr. A 2007, 1163, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.C.; Bellair, R.; Manitiu, M.; Herkowitz, H.N.; Kannan, R.M. Structure and mechanical properties of supercritical carbon dioxide processed porous resorbable polymer constructs. J. Mech. Behav. Biomed. Mater. 2009, 2, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Hammer, J.; Han, L.-H.; Tong, X.; Yang, F. A Facile Method to Fabricate Hydrogels with Microchannel-Like Porosity for Tissue Engineering. Tissue Eng. Part C Methods 2014, 20, 169–176. [Google Scholar] [CrossRef]
- Pivinskii, Y.E. Rheology in ceramic and refractory technology. Principal concepts and rheological models. Refractories 1994, 35, 87–96. [Google Scholar] [CrossRef]
- Yang, X.; Bakaic, E.; Hoare, T.; Cranston, E.D. Injectable Polysaccharide Hydrogels Reinforced with Cellulose Nanocrystals: Morphology, Rheology, Degradation, and Cytotoxicity. Biomacromolecules 2013, 14, 4447–4455. [Google Scholar] [CrossRef]
- Patil, S.; Nune, K.; Misra, R. Alginate/poly(amidoamine) injectable hybrid hydrogel for cell delivery. J. Biomater. Appl. 2018, 33, 295–314. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, T.; Li, T.; Ma, Y.; Shen, S.; He, B.; Mo, R. Enhanced Transdermal Drug Delivery by Transfersome-Embedded Oligopeptide Hydrogel for Topical Chemotherapy of Melanoma. ACS Nano 2018, 12, 9693–9701. [Google Scholar] [CrossRef]
- De Groot, C. In vitro biocompatibility of biodegradable dextran-based hydrogels tested with human fibroblasts. Biomaterials 2001, 22, 1197–1203. [Google Scholar] [CrossRef]
- Ravishankar, K.; Venkatesan, M.; Desingh, R.P.; Mahalingam, A.; Sadhasivam, B.; Subramaniyam, R.; Dhamodharan, R. Biocompatible hydrogels of chitosan-alkali lignin for potential wound healing applications. Mater. Sci. Eng. C 2019, 102, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Stubbe, B.; Mignon, A.; Declercq, H.; Vlierberghe, S.; Dubruel, P. Development of Gelatin-Alginate Hydrogels for Burn Wound Treatment. Macromol. Biosci. 2019, 19, 1900123. [Google Scholar] [CrossRef] [PubMed]
- Ailincai, D.; Tartau Mititelu, L.; Marin, L. Drug delivery systems based on biocompatible imino-chitosan hydrogels for local anticancer therapy. Drug Deliv. 2018, 25, 1080–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Gong, X. A new route to fabricate biocompatible hydrogels with controlled drug delivery behavior. J. Colloid Interface Sci. 2016, 470, 62–70. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Xie, Y.; Jiang, Z. Injectable and biocompatible chitosan-alginic acid hydrogels. Biomed. Mater. 2019, 14, 025010. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.-K.; Kim, S.; Baljon, J.J.; Wu, B.M.; Aghaloo, T.; Lee, M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 2019, 10, 3523. [Google Scholar] [CrossRef] [Green Version]
- Sheikhpour, M.; Barani, L.; Kasaeian, A. Biomimetics in drug delivery systems: A critical review. J. Control. Release 2017, 253, 97–109. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [Green Version]
- De Moraes Porto, I.C.C. Polymer Biocompatibility. In Polymerization; De Souza Gomes, A., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0745-3. [Google Scholar]
- Taylor, D.L.; in het Panhuis, M. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef]
- Canestrari, F.; Virgili, A.; Graziani, A.; Stimilli, A. Modeling and assessment of self-healing and thixotropy properties for modified binders. Int. J. Fatigue 2015, 70, 351–360. [Google Scholar] [CrossRef]
- Wang, Y.; Adokoh, C.K.; Narain, R. Recent development and biomedical applications of self-healing hydrogels. Expert Opin. Drug Deliv. 2018, 15, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Chen, N.; Li, C.; Liu, H.; Zhu, R.; Chen, S.; Xiao, Q.; Liu, J.; Ramakrishna, S.; He, L. Advances in injectable self-healing biomedical hydrogels. Acta Biomater. 2019, 90, 1–20. [Google Scholar] [CrossRef]
- Fan, L.; Ge, X.; Qian, Y.; Wei, M.; Zhang, Z.; Yuan, W.-E.; Ouyang, Y. Advances in Synthesis and Applications of Self-Healing Hydrogels. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Macdougall, L.J.; Pérez-Madrigal, M.M.; Shaw, J.E.; Inam, M.; Hoyland, J.A.; O’Reilly, R.; Richardson, S.M.; Dove, A.P. Self-healing, stretchable and robust interpenetrating network hydrogels. Biomater. Sci. 2018, 6, 2932–2937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, W.; Jiang, F.; Chen, P.; Wei, B. A POSS based hydrogel with mechanical robustness, cohesiveness and a rapid self-healing ability by electrostatic interaction. Soft Matter 2017, 13, 5645–5648. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, Y.; Zhang, H.; Chen, L.; Fu, S. Facile strategy to construct a self-healing and biocompatible cellulose nanocomposite hydrogel via reversible acylhydrazone. Carbohydr. Polym. 2019, 218, 68–77. [Google Scholar] [CrossRef]
- Lü, S.; Bai, X.; Liu, H.; Ning, P.; Wang, Z.; Gao, C.; Ni, B.; Liu, M. An injectable and self-healing hydrogel with covalent cross-linking in vivo for cranial bone repair. J. Mater. Chem. B 2017, 5, 3739–3748. [Google Scholar] [CrossRef]
- Rao, Z.; Liu, S.; Wu, R.; Wang, G.; Sun, Z.; Bai, L.; Wang, W.; Chen, H.; Yang, H.; Wei, D.; et al. Fabrication of dual network self-healing alginate/guar gum hydrogels based on polydopamine-type microcapsules from mesoporous silica nanoparticles. Int. J. Biol. Macromol. 2019, 129, 916–926. [Google Scholar] [CrossRef]
- Liu, S.; Rao, Z.; Wu, R.; Sun, Z.; Yuan, Z.; Bai, L.; Wang, W.; Yang, H.; Chen, H. Fabrication of Microcapsules by the Combination of Biomass Porous Carbon and Polydopamine for Dual Self-Healing Hydrogels. J. Agric. Food Chem. 2019, 67, 1061–1071. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Chen, X.J.; Hong, Z.P.; Zhang, L.Y.; Zhang, L.; Guo, J.W.; Rong, M.Z.; Zhang, M.Q. Repeatedly Intrinsic Self-Healing of Millimeter-Scale Wounds in Polymer through Rapid Volume Expansion Aided Host–Guest Interaction. ACS Appl. Mater. Interfaces 2020, 12, 22534–22542. [Google Scholar] [CrossRef]
- Liu, L.; Han, Y.; Lv, S. Design of Self-Healing and Electrically Conductive Silk Fibroin-Based Hydrogels. ACS Appl. Mater. Interfaces 2019, 11, 20394–20403. [Google Scholar] [CrossRef]
- Kim, Y.; Jeong, D.; Shinde, V.V.; Hu, Y.; Kim, C.; Jung, S. Azobenzene-grafted carboxymethyl cellulose hydrogels with photo-switchable, reduction-responsive and self-healing properties for a controlled drug release system. Int. J. Biol. Macromol. 2020, 163, 824–832. [Google Scholar] [CrossRef]
- Shi, W.; Hass, B.; Kuss, M.A.; Zhang, H.; Ryu, S.; Zhang, D.; Li, T.; Li, Y.; Duan, B. Fabrication of versatile dynamic hyaluronic acid-based hydrogels. Carbohydr. Polym. 2020, 233, 115803. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Wulf, V.; Vázquez-González, M.; Fadeev, M.; Willner, I. DNA-Based Hydrogels Loaded with Au Nanoparticles or Au Nanorods: Thermoresponsive Plasmonic Matrices for Shape-Memory, Self-Healing, Controlled Release, and Mechanical Applications. ACS Nano 2019, 13, 3424–3433. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, S.-H. Synthesis and Biomedical Applications of Self-healing Hydrogels. Front. Chem. 2018, 6, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Kumar, A.D.; Kumar, R.; Rana, N.K.; Koch, B. Development of a novel chitosan based biocompatible and self-healing hydrogel for controlled release of hydrophilic drug. Int. J. Biol. Macromol. 2018, 116, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Zheng, J.; Liu, Y.; Tan, Y.; Yang, Z.; Song, X.; Yang, S.; Fan, R.; Zhang, Y.; Wang, Y. A natural cordycepin/chitosan complex hydrogel with outstanding self-healable and wound healing properties. Int. J. Biol. Macromol. 2019, 134, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.L.; Singha, N.K. A new class of dual responsive self-healable hydrogels based on a core crosslinked ionic block copolymer micelle prepared via RAFT polymerization and Diels–Alder “click” chemistry. Soft Matter 2017, 13, 9024–9035. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Yu, X.; Wang, C.; Wang, Z. Synthesis and characterization of a novel double cross-linked hydrogel based on Diels-Alder click reaction and coordination bonding. Mater. Sci. Eng. C 2018, 82, 299–309. [Google Scholar] [CrossRef]
- Ghanian, M.H.; Mirzadeh, H.; Baharvand, H. In Situ Forming, Cytocompatible, and Self-Recoverable Tough Hydrogels Based on Dual Ionic and Click Cross-Linked Alginate. Biomacromolecules 2018, 19, 1646–1662. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Tang, Q.; Zhou, Q.; Peng, K.; Yang, H.; Zhang, X. A photo-degradable injectable self-healing hydrogel based on star poly(ethylene glycol)-b-polypeptide as a potential pharmaceuticals delivery carrier. Soft Matter 2018, 14, 7420–7428. [Google Scholar] [CrossRef]
- Voorhaar, L.; De Meyer, B.; Du Prez, F.; Hoogenboom, R. One-Pot Automated Synthesis of Quasi Triblock Copolymers for Self-Healing Physically Crosslinked Hydrogels. Macromol. Rapid Commun. 2016, 37, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, S.; Yan, T.; Fan, X.; Li, F.; Yang, X.; Ren, B.; Xu, J.; Liu, J. Injectable and fast self-healing protein hydrogels. Soft Matter 2019, 15, 7583–7589. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, B.; Gao, G.; Xiao, C.; Li, G. Single component Pluronic F127-lipoic acid hydrogels with self-healing and multi-responsive properties. Eur. Polym. J. 2019, 115, 346–355. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef] [PubMed]
- Gavel, P.K.; Dev, D.; Parmar, H.S.; Bhasin, S.; Das, A.K. Investigations of Peptide-Based Biocompatible Injectable Shape-Memory Hydrogels: Differential Biological Effects on Bacterial and Human Blood Cells. ACS Appl. Mater. Interfaces 2018, 10, 10729–10740. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef]
- Du, X.; Liu, Y.; Wang, X.; Yan, H.; Wang, L.; Qu, L.; Kong, D.; Qiao, M.; Wang, L. Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Mater. Sci. Eng. C 2019, 104, 109930. [Google Scholar] [CrossRef]
- Li., Q.-L.; Sun, Y.; Sun, Y.L.; Wen, J.; Zhou, Y.; Bing, Q.M.; Isaacs, L.D.; Jin, Y.; Zhou, Y.; Gao, H.; et al. Mesoporous Silica Nanoparticles Coated by Layer-by-Layer Self-assembly Using Cucurbit[7]uril for in Vitro and in Vivo Anticancer Drug Release. Chem. Mater. 2014, 26, 6418–6431. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Zhao, X.; Ma, P.X.; Guo, B.; Du, Y.; Han, X. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 2019, 536, 224–234. [Google Scholar] [CrossRef]
- Pham, T.-N.; Su, C.-F.; Huang, C.-C.; Jan, J.-S. Biomimetic hydrogels based on L-Dopa conjugated gelatin as pH-responsive drug carriers and antimicrobial agents. Colloids Surf. B Biointerfaces 2020, 196, 111316. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, H.; Song, T.; Liu, X.; Gao, Y.; Zhou, J.; Li, Y. Sustainable Dual Release of Antibiotic and Growth Factor from pH-Responsive Uniform Alginate Composite Microparticles to Enhance Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 22730–22744. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Qazvini, N.T.; Sadati, M.; Zeng, Z.; Huang, S.; De La Lastra, A.L.; Zhang, L.; Feng, Y.; Liu, W.; Huang, B.; et al. A pH-Triggered, Self-Assembled, and Bioprintable Hybrid Hydrogel Scaffold for Mesenchymal Stem Cell Based Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 8749–8762. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hui, P.; Kan, C. Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef] [Green Version]
- Maeda, T. Structures and Applications of Thermoresponsive Hydrogels and Nanocomposite-Hydrogels Based on Copolymers with Poly (Ethylene Glycol) and Poly (Lactide-Co-Glycolide) Blocks. Bioengineering 2019, 6, 107. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Wang, Y.; Hu, X.; Gong, H.; Li, R.; Cox, H.; Zhang, J.; Waigh, T.A.; Xu, H.; Lu, J.R. Reversible Thermoresponsive Peptide–PNIPAM Hydrogels for Controlled Drug Delivery. Biomacromolecules 2019, 20, 3601–3610. [Google Scholar] [CrossRef]
- Varma, D.; Lin, H.; Long, R.; Gold, G.; Hecht, A.; Iatridis, J.; Nicoll, S. Thermoresponsive, redox-polymerized cellulosic hydrogels undergo in situ gelation and restore intervertebral disc biomechanics post discectomy. Eur. Cell. Mater. 2018, 35, 300–317. [Google Scholar] [CrossRef]
- Zimoch, J.; Padial, J.S.; Klar, A.S.; Vallmajo-Martin, Q.; Meuli, M.; Biedermann, T.; Wilson, C.J.; Rowan, A.; Reichmann, E. Polyisocyanopeptide hydrogels: A novel thermo-responsive hydrogel supporting pre-vascularization and the development of organotypic structures. Acta Biomater. 2018, 70, 129–139. [Google Scholar] [CrossRef]
- Zhao, C.; Zeng, Z.; Qazvini, N.T.; Yu, X.; Zhang, R.; Yan, S.; Shu, Y.; Zhu, Y.; Duan, C.; Bishop, E.; et al. Thermoresponsive Citrate-Based Graphene Oxide Scaffold Enhances Bone Regeneration from BMP9-Stimulated Adipose-Derived Mesenchymal Stem Cells. ACS Biomater. Sci. Eng. 2018, 4, 2943–2955. [Google Scholar] [CrossRef]
- Yan, X.; Fang, W.-W.; Xue, J.; Sun, T.-C.; Dong, L.; Zha, Z.; Qian, H.; Song, Y.-H.; Zhang, M.; Gong, X.; et al. Thermoresponsive in Situ Forming Hydrogel with Sol–Gel Irreversibility for Effective Methicillin-Resistant Staphylococcus aureus Infected Wound Healing. ACS Nano 2019, 13, 10074–10084. [Google Scholar] [CrossRef]
- Li, X.; Su, X. Multifunctional smart hydrogels: Potential in tissue engineering and cancer therapy. J. Mater. Chem. B 2018, 6, 4714–4730. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Jin, G.; Hong, Y.; Zhu, H.; Lu, T.J.; Xu, F.; Bai, D.; Lin, M. Engineering the Cell Microenvironment Using Novel Photoresponsive Hydrogels. ACS Appl. Mater. Interfaces 2018, 10, 12374–12389. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, I.; Peng, K.; Kros, A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2011, 63, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Nowak, B.P.; Ravoo, B.J. Photoresponsive hybrid hydrogel with a dual network of agarose and a self-assembling peptide. Soft Matter 2020, 16, 7299–7304. [Google Scholar] [CrossRef]
- Roth-Konforti, M.E.; Comune, M.; Halperin-Sternfeld, M.; Grigoriants, I.; Shabat, D.; Adler-Abramovich, L. UV Light-Responsive Peptide-Based Supramolecular Hydrogel for Controlled Drug Delivery. Macromol. Rapid Commun. 2018, 39, 1800588. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Zhang, X.; Zhang, R.; Hu, Y.; Boyer, C.; Xu, F.-J. Photo-responsive supramolecular hyaluronic acid hydrogels for accelerated wound healing. J. Control. Release 2020, 323, 24–35. [Google Scholar] [CrossRef]
- Yang, N.; Zhu, M.; Xu, G.; Liu, N.; Yu, C. A near-infrared light-responsive multifunctional nanocomposite hydrogel for efficient and synergistic antibacterial wound therapy and healing promotion. J. Mater. Chem. B 2020, 8, 3908–3917. [Google Scholar] [CrossRef]
- Ko, S.; Park, J.Y.; Oh, Y.-K. A Microbial Siderophore-Inspired Self-Gelling Hydrogel for Noninvasive Anticancer Phototherapy. Cancer Res. 2019, 79, 6178–6189. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Wang, Y.; Liu, Z.; Ma, C.; Yan, H.; Xu, N.; Gang, F.; Wang, X.; Zhao, L.; Sun, X. Three-Dimensional Printing and Injectable Conductive Hydrogels for Tissue Engineering Application. Tissue Eng. Part B Rev. 2019, 25, 398–411. [Google Scholar] [CrossRef]
- Kim, Y.S.; Cho, K.; Lee, H.J.; Chang, S.; Lee, H.; Kim, J.H.; Koh, W.-G. Highly conductive and hydrated PEG-based hydrogels for the potential application of a tissue engineering scaffold. React. Funct. Polym. 2016, 109, 15–22. [Google Scholar] [CrossRef]
- Heo, D.N.; Lee, S.-J.; Timsina, R.; Qiu, X.; Castro, N.J.; Zhang, L.G. Development of 3D printable conductive hydrogel with crystallized PEDOT:PSS for neural tissue engineering. Mater. Sci. Eng. C 2019, 99, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.Y.; Park, J.; Kim, W.; Kim, J.; Lee, J.Y.; Koh, W.-G. A Novel Conductive and Micropatterned PEG-Based Hydrogel Enabling the Topographical and Electrical Stimulation of Myoblasts. ACS Appl. Mater. Interfaces 2019, 11, 47695–47706. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Liu, Y.; Darabi, M.A.; Tu, G.; Huang, L.; Ying, L.; Xiao, B.; Wu, Y.; Xing, M.; Zhang, L.; et al. An injectable conductive Gelatin-PANI hydrogel system serves as a promising carrier to deliver BMSCs for Parkinson’s disease treatment. Mater. Sci. Eng. C 2019, 100, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, B.; Zarrintaj, P.; Surwase, S.S.; Baheiraei, N.; Saeb, M.R.; Mozafari, M.; Kim, Y.C.; Park, O.O. Self-gelling electroactive hydrogels based on chitosan–aniline oligomers/agarose for neural tissue engineering with on-demand drug release. Colloids Surf. B Biointerfaces 2019, 184, 110549. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef]
- Cui, Z.; Ni, N.C.; Wu, J.; Du, G.-Q.; He, S.; Yau, T.M.; Weisel, R.D.; Sung, H.-W.; Li, R.-K. Polypyrrole-chitosan conductive biomaterial synchronizes cardiomyocyte contraction and improves myocardial electrical impulse propagation. Theranostics 2018, 8, 2752–2764. [Google Scholar] [CrossRef]
- Ren, K.; Cheng, Y.; Huang, C.; Chen, R.; Wang, Z.; Wei, J. Self-healing conductive hydrogels based on alginate, gelatin and polypyrrole serve as a repairable circuit and a mechanical sensor. J. Mater. Chem. B 2019, 7, 5704–5712. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, L.; Yi, X.; Zhou, Z.; Liu, C.; Fu, R.; Dai, C.; Wang, Z.; Chen, X.; Yu, P.; et al. Soft Conducting Polymer Hydrogels Cross-Linked and Doped by Tannic Acid for Spinal Cord Injury Repair. ACS Nano 2018, 12, 10957–10967. [Google Scholar] [CrossRef]
- Park, J.; Choi, J.H.; Kim, S.; Jang, I.; Jeong, S.; Lee, J.Y. Micropatterned conductive hydrogels as multifunctional muscle-mimicking biomaterials: Graphene-incorporated hydrogels directly patterned with femtosecond laser ablation. Acta Biomater. 2019, 97, 141–153. [Google Scholar] [CrossRef]
- Mottet, L.; Le Cornec, D.; Noël, J.-M.; Kanoufi, F.; Delord, B.; Poulin, P.; Bibette, J.; Bremond, N. A conductive hydrogel based on alginate and carbon nanotubes for probing microbial electroactivity. Soft Matter 2018, 14, 1434–1441. [Google Scholar] [CrossRef]
- Min, J.; Patel, M.; Koh, W.-G. Incorporation of Conductive Materials into Hydrogels for Tissue Engineering Applications. Polymers 2018, 10, 1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Zhang, N.; Ma, M. Electroconductive hydrogels for biomedical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1568. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Chen, G.; Tian, J.; He, M. Highly Stretchable, Strain-Sensitive, and Ionic-Conductive Cellulose-Based Hydrogels for Wearable Sensors. Polymers 2019, 11, 2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Xia, X.-X.; Huang, S.-C.; Qian, Z.-G. Development of Adhesive and Conductive Resilin-Based Hydrogels for Wearable Sensors. Biomacromolecules 2019, 20, 3283–3293. [Google Scholar] [CrossRef]
- Alizadeh, R.; Zarrintaj, P.; Kamrava, S.K.; Bagher, Z.; Farhadi, M.; Heidari, F.; Komeili, A.; Gutiérrez, T.J.; Saeb, M.R. Conductive hydrogels based on agarose/alginate/chitosan for neural disorder therapy. Carbohydr. Polym. 2019, 224, 115161. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Gao, G.; Chen, J.; Hou, R.; Wang, Q.; Liu, L.; Fu, J. Conductive graphene oxide hydrogels reduced and bridged by l -cysteine to support cell adhesion and growth. J. Mater. Chem. B 2017, 5, 511–516. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Rezayat, S.M.; Vashegani-Farahani, E.; Mahmoudifard, M.; Zamanlui, S.; Soleimani, M. Nanofibrous hydrogel with stable electrical conductivity for biological applications. Polymer 2016, 97, 205–216. [Google Scholar] [CrossRef]
- Qu, J.; Liang, Y.; Shi, M.; Guo, B.; Gao, Y.; Yin, Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int. J. Biol. Macromol. 2019, 140, 255–264. [Google Scholar] [CrossRef]
- Guo, B.; Qu, J.; Zhao, X.; Zhang, M. Degradable conductive self-healing hydrogels based on dextran-graft-tetraaniline and N-carboxyethyl chitosan as injectable carriers for myoblast cell therapy and muscle regeneration. Acta Biomater. 2019, 84, 180–193. [Google Scholar] [CrossRef]
- Xu, C.; Guan, S.; Wang, S.; Gong, W.; Liu, T.; Ma, X.; Sun, C. Biodegradable and electroconductive poly(3,4-ethylenedioxythiophene)/carboxymethyl chitosan hydrogels for neural tissue engineering. Mater. Sci. Eng. C 2018, 84, 32–43. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, Y.; Wang, H.; Xu, Z.; Chen, J.; Bao, R.; Tan, B.; Cui, Y.; Fan, G.; Wang, W.; et al. Paintable and Rapidly Bondable Conductive Hydrogels as Therapeutic Cardiac Patches. Adv. Mater. 2018, 30, 1704235. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.D.; Olof, S.N.; Burke, M.J.; Armstrong, J.P.K.; Mikhailova, E.A.; Nicholson, J.G.; Box, S.J.; Szele, F.G.; Perriman, A.W.; Bayley, H. High-Resolution Patterned Cellular Constructs by Droplet-Based 3D Printing. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Aubin-Tam, M.-E.; Meyer, A.S. 3D Printing for the Fabrication of Biofilm-Based Functional Living Materials. ACS Synth. Biol. 2019, 8, 1564–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negro, A.; Cherbuin, T.; Lutolf, M.P. 3D Inkjet Printing of Complex, Cell-Laden Hydrogel Structures. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Yan, W.-C.; Lu, W.F.; Wang, C.-H.; Fuh, J.Y.H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef] [PubMed]
- Athirasala, A.; Tahayeri, A.; Thrivikraman, G.; França, C.M.; Monteiro, N.; Tran, V.; Ferracane, J.; Bertassoni, L.E. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication 2018, 10, 024101. [Google Scholar] [CrossRef]
- Jeon, O.; Lee, Y.B.; Hinton, T.J.; Feinberg, A.W.; Alsberg, E. Cryopreserved cell-laden alginate microgel bioink for 3D bioprinting of living tissues. Mater. Today Chem. 2019, 12, 61–70. [Google Scholar] [CrossRef]
- Anada, T.; Pan, C.-C.; Stahl, A.; Mori, S.; Fukuda, J.; Suzuki, O.; Yang, Y. Vascularized Bone-Mimetic Hydrogel Constructs by 3D Bioprinting to Promote Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2019, 20, 1096. [Google Scholar] [CrossRef] [Green Version]
- Stratesteffen, H.; Köpf, M.; Kreimendahl, F.; Blaeser, A.; Jockenhoevel, S.; Fischer, H. GelMA-collagen blends enable drop-on-demand 3D printablility and promote angiogenesis. Biofabrication 2017, 9, 045002. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, L.; Du, X.; Fan, Z.; Wang, L.; Sun, W.; Cheng, Y.; Zhu, Y.; Chen, C. Reversible physical crosslinking strategy with optimal temperature for 3D bioprinting of human chondrocyte-laden gelatin methacryloyl bioink. J. Biomater. Appl. 2018, 33, 609–618. [Google Scholar] [CrossRef]
- Möller, T.; Amoroso, M.; Hägg, D.; Brantsing, C.; Rotter, N.; Apelgren, P.; Lindahl, A.; Kölby, L.; Gatenholm, P. In Vivo Chondrogenesis in 3D Bioprinted Human Cell-laden Hydrogel Constructs. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1227. [Google Scholar] [CrossRef] [PubMed]
- Huber, T.; Najaf Zadeh, H.; Feast, S.; Roughan, T.; Fee, C. 3D Printing of Gelled and Cross-Linked Cellulose Solutions; an Exploration of Printing Parameters and Gel Behaviour. Bioengineering 2020, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.W.; Kim, D.Y.; Roh, H.H.; Kim, H.S.; Lee, J.W.; Lee, K.Y. Three-Dimensional Bioprinting of Cell-Laden Constructs Using Polysaccharide-Based Self-Healing Hydrogels. Biomacromolecules 2019, 20, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, K.; Zhou, X.; Li, T.; Xu, Y.; Qiang, L.; Peng, M.; Xu, Y.; Xie, L.; He, C.; et al. Controllable fabrication of hydroxybutyl chitosan/oxidized chondroitin sulfate hydrogels by 3D bioprinting technique for cartilage tissue engineering. Biomed. Mater. 2019, 14, 025006. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.F.; Bártolo, P.J. 3D bioprinting of photocrosslinkable hydrogel constructs. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef] [Green Version]
- Solis, L.H.; Ayala, Y.; Portillo, S.; Varela-Ramirez, A.; Aguilera, R.; Boland, T. Thermal inkjet bioprinting triggers the activation of the VEGF pathway in human microvascular endothelial cells in vitro. Biofabrication 2019, 11, 045005. [Google Scholar] [CrossRef]
- Gao, G.; Hubbell, K.; Schilling, A.F.; Dai, G.; Cui, X. Bioprinting Cartilage Tissue from Mesenchymal Stem Cells and PEG Hydrogel. In 3D Cell Culture; Methods in Molecular Biology; Koledova, Z., Ed.; Springer: New York, NY, USA, 2017; Volume 1612, pp. 391–398. ISBN 978-1-4939-7019-3. [Google Scholar]
- Nishiyama, Y.; Nakamura, M.; Henmi, C.; Yamaguchi, K.; Mochizuki, S.; Nakagawa, H.; Takiura, K. Development of a Three-Dimensional Bioprinter: Construction of Cell Supporting Structures Using Hydrogel and State-Of-The-Art Inkjet Technology. J. Biomech. Eng. 2009, 131, 035001. [Google Scholar] [CrossRef]
- Yang, S.; Tang, H.; Feng, C.; Shi, J.; Yang, J. The Research on Multi-Material 3D Vascularized Network Integrated Printing Technology. Micromachines 2020, 11, 237. [Google Scholar] [CrossRef] [Green Version]
- Hiller, T.; Berg, J.; Elomaa, L.; Röhrs, V.; Ullah, I.; Schaar, K.; Dietrich, A.-C.; Al-Zeer, M.; Kurtz, A.; Hocke, A.; et al. Generation of a 3D Liver Model Comprising Human Extracellular Matrix in an Alginate/Gelatin-Based Bioink by Extrusion Bioprinting for Infection and Transduction Studies. Int. J. Mol. Sci. 2018, 19, 3129. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhong, Z.; Hu, N.; Zhou, Y.; Maggio, L.; Miri, A.K.; Fragasso, A.; Jin, X.; Khademhosseini, A.; Zhang, Y.S. Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments. Biofabrication 2018, 10, 024102. [Google Scholar] [CrossRef] [PubMed]
- Hopp, B.; Smausz, T.; Kresz, N.; Barna, N.; Bor, Z.; Kolozsvári, L.; Chrisey, D.B.; Szabó, A.; Nógrádi, A. Survival and Proliferative Ability of Various Living Cell Types after Laser-Induced Forward Transfer. Tissue Eng. 2005, 11, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Barron, J.A.; Ringeisen, B.R.; Kim, H.; Spargo, B.J.; Chrisey, D.B. Application of laser printing to mammalian cells. Thin Solid Films 2004, 453–454, 383–387. [Google Scholar] [CrossRef]
- Mahdavi, S.S.; Abdekhodaie, M.J.; Kumar, H.; Mashayekhan, S.; Baradaran-Rafii, A.; Kim, K. Stereolithography 3D Bioprinting Method for Fabrication of Human Corneal Stroma Equivalent. Ann. Biomed. Eng. 2020, 48, 1955–1970. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kumar, H.; Tian, Z.; Jin, X.; Holzman, J.F.; Menard, F.; Kim, K. Visible Light Photoinitiation of Cell-Adhesive Gelatin Methacryloyl Hydrogels for Stereolithography 3D Bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 26859–26869. [Google Scholar] [CrossRef]
- Yan, W.-C.; Davoodi, P.; Vijayavenkataraman, S.; Tian, Y.; Ng, W.C.; Fuh, J.Y.H.; Robinson, K.S.; Wang, C.-H. 3D bioprinting of skin tissue: From pre-processing to final product evaluation. Adv. Drug Deliv. Rev. 2018, 132, 270–295. [Google Scholar] [CrossRef] [PubMed]
- Zidarič, T.; Milojević, M.; Gradišnik, L.; Stana Kleinschek, K.; Maver, U.; Maver, T. Polysaccharide-Based Bioink Formulation for 3D Bioprinting of an In Vitro Model of the Human Dermis. Nanomaterials 2020, 10, 733. [Google Scholar] [CrossRef] [Green Version]
- Ning, E.; Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Vendrell, M.; Graham, D.; Wark, A.W.; Faulds, K.; Shu, W. 3D bioprinting of mature bacterial biofilms for antimicrobial resistance drug testing. Biofabrication 2019, 11, 045018. [Google Scholar] [CrossRef]
- Long, J.; Etxeberria, A.E.; Nand, A.V.; Bunt, C.R.; Ray, S.; Seyfoddin, A. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater. Sci. Eng. C 2019, 104, 109873. [Google Scholar] [CrossRef]
- Si, H.; Xing, T.; Ding, Y.; Zhang, H.; Yin, R.; Zhang, W. 3D Bioprinting of the Sustained Drug Release Wound Dressing with Double-Crosslinked Hyaluronic-Acid-Based Hydrogels. Polymers 2019, 11, 1584. [Google Scholar] [CrossRef] [Green Version]



| Method | Mechanism | Advantages | Limitations |
|---|---|---|---|
| Porogen templating | The usage of porogens during the gelation process, that are removed after HGs formation. | Simple process; good pore size control | Difficulties with porogen removal |
| Gas foaming | High-speed stirring of gel, which generates bubbles, or addition of substance, that produces gas particles through a chemical reaction. | Simple process; inexpensive method | Insufficient possibility of pore size control |
| Bicontinuous emulsion templating | Preparation of emulsion, where the aqueous phase is a mixture of monomers for polymerization. | Simple process | Various pores size; water soluble polymers |
| Cryogelation | Polymerization at very low temperatures (formation of crystals). During controlled heating, crystals are melt and pores are formed. | More interconnective porogen structure | Use of sub-zero temperatures |
| 3D printing | 3D printing of HG matrix with specially planned and strictly defined pore sizes. | Controllable pores size | Insufficient resolution of 3D printers |
| Electrospinning | Usage of electric charge to obtain porous structure of polymeric HG. | Microscale and macroscale process | Relatively slow process |
| Freeze-drying | Preparation of an oil-in-water emulsion from which water phase is removed during freeze-drying (formation of pores). | Good pore size control | Water insoluble polymers |
| Inverse opal hydrogelation | Preparation of a 3D pattern from colloidal particles, between which polymer solution is poured in, followed by removal of the template after polymerization | Interconnected pores | Selection of colloidal particles |
| Type of Self-Healing Mechanism | ||
|---|---|---|
| Intrinsic | Extrinsic | |
| Physical (Non-Covalent) | Chemical (Covalent) | |
| Electrostatic interactions [66,67] | Acylhydrazone bonds [68,69] | Microcapsule [70,71] |
| Host–guest interactions [72,73,74] | Boronate-diol complexation [75,76] | |
| Hydrogen bond [77,78,79] | Diels–Alder click chemistry [80,81,82] | |
| Hydrophobic interactions [83,84] | Disulfide bonds [85,86] | |
| π–π stacking [87,88] | Imine bonds (Schiff base) [89,90] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chyzy, A.; Plonska-Brzezinska, M.E. Hydrogel Properties and Their Impact on Regenerative Medicine and Tissue Engineering. Molecules 2020, 25, 5795. https://doi.org/10.3390/molecules25245795
Chyzy A, Plonska-Brzezinska ME. Hydrogel Properties and Their Impact on Regenerative Medicine and Tissue Engineering. Molecules. 2020; 25(24):5795. https://doi.org/10.3390/molecules25245795
Chicago/Turabian StyleChyzy, Adam, and Marta E. Plonska-Brzezinska. 2020. "Hydrogel Properties and Their Impact on Regenerative Medicine and Tissue Engineering" Molecules 25, no. 24: 5795. https://doi.org/10.3390/molecules25245795
APA StyleChyzy, A., & Plonska-Brzezinska, M. E. (2020). Hydrogel Properties and Their Impact on Regenerative Medicine and Tissue Engineering. Molecules, 25(24), 5795. https://doi.org/10.3390/molecules25245795






