Cyanide Complexes Based on {Mo6I8}4+ and {W6I8}4+ Cluster Cores
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Stability in Aqueous Solutions
2.3. Crystal Structures
2.4. Calculated Geometry and Electronic Structure
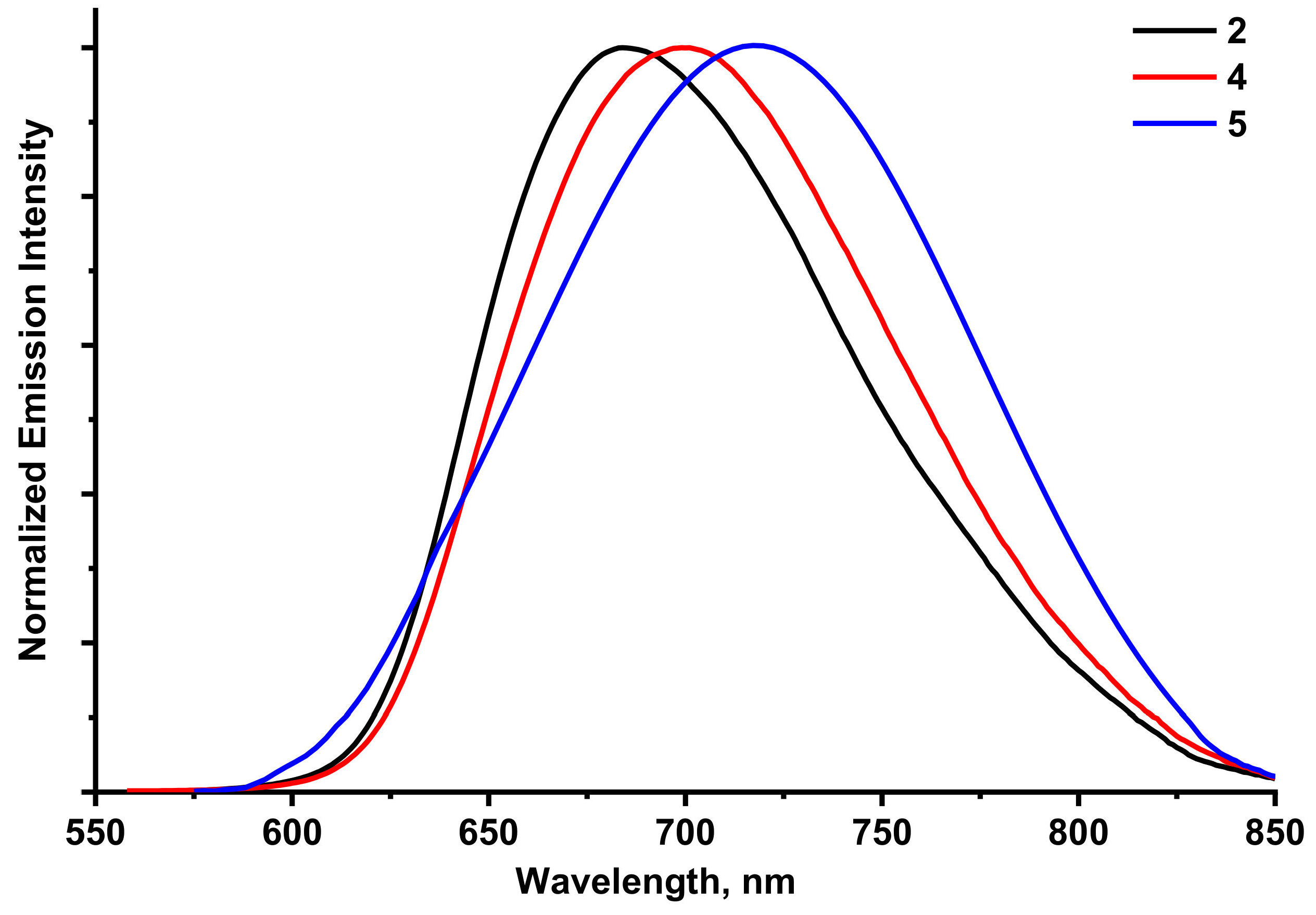
2.5. Luminescence Properties
3. Materials and Methods
3.1. Synthesis of Cs1.3Na0.7[{Mo6I8}(CN)6]·2H2O (1)
3.2. Synthesis of (Bu4N)2[{Mo6I8}(CN)6]·0.7H2O (2)
3.2.1. Metathesis Reaction
3.2.2. Solvothermal Ligand Exchange Reaction
3.3. Synthesis of (Bu4N)2[{Mo6I8}(CN)6]·2DMSO (3)
3.4. Preparation of (Bu4N)2[{Mo6I8}(CN)4(MeO)2] (4)
3.5. Preparation of (Bu4N)2[{W6I8}(CN)2(MeO)4]·5H2O (5)
3.6. Single Crystal Diffraction Studies
3.7. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sokolov, M.N.; Brylev, K.A.; Abramov, P.A.; Gallyamov, M.R.; Novozhilov, I.N.; Kitamura, N.; Mikhaylov, M.A. Complexes of {W6I8}4+ Clusters with Carboxylates: Preparation, Electrochemistry, and Luminescence. Eur. J. Inorg. Chem. 2017, 2017, 4131–4137. [Google Scholar] [CrossRef]
- Seyboldt, A.; Enseling, D.; Jüstel, T.; Ivanović, M.; Peisert, H.; Chassé, T.; Meyer, H.-J. Ligand Influence on the Photophysical Properties and Electronic Structures of Tungsten Iodide Clusters. Eur. J. Inorg. Chem. 2017, 2017, 5387–5394. [Google Scholar] [CrossRef]
- Kirakci, K.; Fejfarová, K.; Kučeráková, M.; Lang, K. Hexamolybdenum Cluster Complexes with Pyrene and Anthracene Carboxylates: Ultrabright Red Emitters with the Antenna Effect. Eur. J. Inorg. Chem. 2014, 2014, 2331–2336. [Google Scholar] [CrossRef]
- Zietlow, T.C.; Nocera, D.G.; Gray, H.B. Photophysics and Electrochemistry of Hexanuclear Tungsten Halide Clusters. Inorg. Chem. 1986, 25, 1351–1353. [Google Scholar] [CrossRef]
- Maverick, A.W.; Najdzionek, J.S.; MacKenzie, D.; Nocera, D.G.; Gray, H.B. Spectroscopic, Electrochemical, and Photochemical Properties of Molybdenum(II) and Tungsten(II) Halide Clusters. J. Am. Chem. Soc. 1983, 105, 1878–1882. [Google Scholar] [CrossRef]
- Akagi, S.; Fujii, S.; Kitamura, N. A study on the redox, spectroscopic, and photophysical characteristics of a series of octahedral hexamolybdenum(ii) clusters: [{Mo6X8}Y6]2− (X, Y = Cl, Br, or I). Dalton Trans. 2018, 47, 1131–1139. [Google Scholar] [CrossRef]
- Mikhaylov, M.A.; Sokolov, M.N. Molybdenum Iodides – from Obscurity to Bright Luminescence. Eur. J. Inorg. Chem. 2019, 2019, 4181–4197. [Google Scholar] [CrossRef]
- Fujii, S.; Horiguchi, T.; Akagi, S.; Kitamura, N. Quasi-One-Step Six-Electron Electrochemical Reduction of an Octahedral Hexanuclear Molybdenum(II) Cluster. Inorg. Chem. 2016, 55, 10259–10266. [Google Scholar] [CrossRef]
- Cordier, S.; Fabre, B.; Molard, Y.; Fadjie-Djomkam, A.-B.; Turban, P.; Tricot, S.; Ababou-Girard, S.; Godet, C. Elaboration, Characterizations, and Energetics of Robust Mo6 Cluster-Terminated Silicon-Bound Molecular Junctions. J. Phys. Chem. C 2016, 120, 2324–2334. [Google Scholar] [CrossRef]
- Barras, A.; Das, M.R.; Devarapalli, R.R.; Shelke, M.V.; Cordier, S.; Szunerits, S.; Boukherroub, R. One-pot synthesis of gold nanoparticle/molybdenum cluster/graphene oxide nanocomposite and its photocatalytic activity. Appl. Catal. B Environ. 2013, 130-131, 270–276. [Google Scholar] [CrossRef]
- Feliz, M.; Puche, M.; Atienzar, P.; Concepción, P.; Cordier, S.; Molard, Y. In Situ Generation of Active Molybdenum Octahedral Clusters for Photocatalytic Hydrogen Production from Water. ChemSusChem 2016, 9, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mungse, H.P.; Cordier, S.; Boukherroub, R.; Khatri, O.P.; Jain, S.L. Hexamolybdenum clusters supported on graphene oxide: Visible-light induced photocatalytic reduction of carbon dioxide into methanol. Carbon 2015, 94, 91–100. [Google Scholar] [CrossRef]
- Feliz, M.; Atienzar, P.; Amela-Cortés, M.; Dumait, N.; Lemoine, P.; Molard, Y.; Cordier, S. Supramolecular Anchoring of Octahedral Molybdenum Clusters onto Graphene and Their Synergies in Photocatalytic Water Reduction. Inorg. Chem. 2019, 58, 15443–15454. [Google Scholar] [CrossRef] [PubMed]
- Cîrcu, V.; Molard, Y.; Amela-Cortes, M.; Bentaleb, A.; Barois, P.; Dorcet, V.; Cordier, S. From Mesomorphic Phosphine Oxide to Clustomesogens Containing Molybdenum and Tungsten Octahedral Cluster Cores. Angew. Chem. Int. Ed. 2015, 54, 10921–10925. [Google Scholar] [CrossRef] [PubMed]
- Efremova, O.A.; Shestopalov, M.A.; Chirtsova, N.A.; Smolentsev, A.I.; Mironov, Y.V.; Kitamura, N.; Brylev, K.A.; Sutherland, A.J. A highly emissive inorganic hexamolybdenum cluster complex as a handy precursor for the preparation of new luminescent materials. Dalton Trans. 2014, 43, 6021–6025. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubát, P.; Langmaier, J.; Polívka, T.; Fuciman, M.; Fejfarová, K.; Lang, K. A comparative study of the redox and excited state properties of (nBu4N)2[Mo6X14] and (nBu4N)2[Mo6X8(CF3COO)6] (X. = Cl, Br, or I). Dalton Trans. 2013, 42, 7224–7232. [Google Scholar] [CrossRef]
- Jackson, J.A.; Turro, C.; Newsham, M.D.; Nocera, D.G. Oxygen quenching of electronically excited hexanuclear molybdenum and tungsten halide clusters. J. Phys. Chem. 1990, 94, 4500–4507. [Google Scholar] [CrossRef]
- Solovieva, A.O.; Vorotnikov, Y.A.; Trifonova, K.E.; Efremova, O.A.; Krasilnikova, A.A.; Brylev, K.A.; Vorontsova, E.V.; Avrorov, P.A.; Shestopalova, L.V.; Poveshchenko, A.F.; et al. Cellular internalisation, bioimaging and dark and photodynamic cytotoxicity of silica nanoparticles doped by {Mo6I8}4+ metal clusters. J. Mater. Chem. B 2016, 4, 4839–4846. [Google Scholar] [CrossRef]
- Svezhentseva, E.V.; Solovieva, A.O.; Vorotnikov, Y.A.; Kurskaya, O.G.; Brylev, K.A.; Tsygankova, A.R.; Edeleva, M.V.; Gyrylova, S.N.; Kitamura, N.; Efremova, O.A.; et al. Water-soluble hybrid materials based on {Mo6X8}4+ (X = Cl, Br, I) cluster complexes and sodium polystyrene sulfonate. New J. Chem. 2017, 41, 1670–1676. [Google Scholar] [CrossRef]
- Brandhonneur, N.; Hatahet, T.; Amela-Cortes, M.; Molard, Y.; Cordier, S.; Dollo, G. Molybdenum cluster loaded PLGA nanoparticles: An innovative theranostic approach for the treatment of ovarian cancer. Eur. J. Pharm. Biopharm. 2018, 125, 95–105. [Google Scholar] [CrossRef]
- Cheplakova, A.M.; Solovieva, A.O.; Pozmogova, T.N.; Vorotnikov, Y.A.; Brylev, K.A.; Vorotnikova, N.A.; Vorontsova, E.V.; Mironov, Y.V.; Poveshchenko, A.F.; Kovalenko, K.A.; et al. Nanosized mesoporous metal–organic framework MIL-101 as a nanocarrier for photoactive hexamolybdenum cluster compounds. J. Inorg. Biochem. 2017, 166, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Elistratova, J.; Mukhametshina, A.; Kholin, K.; Nizameev, I.; Mikhailov, M.; Sokolov, M.; Khairullin, R.; Miftakhova, R.; Shammas, G.; Kadirov, M.; et al. Interfacial uploading ofluminescent hexamolybdenum cluster units onto amino-decorated silica nanoparticles as new design of nanomaterial for cellular imaging and photodynamic therapy. J. Colloid Interf. Sci. 2019, 538, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, A.; Mikhailov, M.; Sokolov, M.N.; Pérez-Laguna, V.; Rezusta, A.; Revillo, M.J.; Galindo, F. A photobleaching resistant polymer supported hexanuclear molybdenum iodide cluster for photocatalytic oxygenations and photodynamic inactivation of Staphylococcus aureus. J. Mater. Chem. B 2016, 4, 5975–5979. [Google Scholar] [CrossRef] [PubMed]
- Felip-León, C.; Arnau del Valle, C.; Pérez-Laguna, V.; Isabel Millán-Lou, M.; Miravet, J.F.; Mikhailov, M.; Sokolov, M.N.; Rezusta-López, A.; Galindo, F. Superior performance of macroporous over gel type polystyrene as a support for the development of photo-bactericidal materials. J. Mater. Chem. B 2017, 5, 6058–6064. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Mancilla, E.; Oyarce, A.; Verdugo, V.; Morales-Verdejo, C.; Echeverria, C.; Velásquez, F.; Chnaiderman, J.; Valiente-Echeverría, F.; Ramirez-Tagle, R. The [Mo6Cl14]2− Cluster is Biologically Secure and Has Anti-Rotavirus Activity In Vitro. Molecules 2017, 22, 1108. [Google Scholar] [CrossRef] [PubMed]
- Neaime, C.; Amela-Cortes, M.; Grasset, F.; Molard, Y.; Cordier, S.; Dierre, B.; Mortier, M.; Takei, T.; Takahashi, K.; Haneda, H.; et al. Time-gated luminescence bioimaging with new luminescent nanocolloids based on [Mo6I8(C2F5COO)6]2− metal atom clusters. Phys. Chem. Chem. Phys. 2016, 18, 30166–30173. [Google Scholar] [CrossRef] [PubMed]
- Vorotnikova, N.A.; Edeleva, M.V.; Kurskaya, O.G.; Brylev, K.A.; Shestopalov, A.M.; Mironov, Y.V.; Sutherland, A.J.; Efremova, O.A.; Shestopalov, M.A. One-pot synthesis of {Mo6I8}4+-doped polystyrene microspheres via a free radical dispersion copolymerisation reaction. Polym. Int. 2017, 66, 1906–1912. [Google Scholar] [CrossRef]
- Fedorov, V.E.; Naumov, N.G.; Mironov, Y.V.; Virovets, A.V.; Artemkina, S.B.; Brylev, K.A.; Yarovoi, S.S.; Efremova, O.A.; Peak, U.H. Inorganic Coordination Polymers Based on Chalcocyanide Cluster Complexes. J. Struct. Chem. 2002, 43, 669–684. [Google Scholar] [CrossRef]
- Alexandrov, E.V.; Virovets, A.V.; Blatov, V.A.; Peresypkina, E.V. Topological Motifs in Cyanometallates: From Building Units to Three-Periodic Frameworks. Chem. Rev. 2015, 115, 12286–12319. [Google Scholar] [CrossRef]
- Efremova, O.A.; Mironov, Y.V.; Fedorov, V.E. Design of Cyano-Bridged Coordination Polymers Based on Tetrahedral Rhenium Cluster Cyanide Complexes and 3d Transition Metals. Eur. J. Inorg. Chem. 2006, 2006, 2533–2549. [Google Scholar] [CrossRef]
- Amela-Cortes, M.; Cordier, S.; Naumov, N.G.; Mériadec, C.; Artzner, F.; Molard, Y. Hexacyano octahedral metallic clusters as versatile building blocks in the design of extended polymeric framework and clustomesogens. J. Mater. Chem. C 2014, 2, 9813–9823. [Google Scholar] [CrossRef]
- Daigre, G.; Lemoine, P.; Pham, T.D.; Demange, V.; Gautier, R.; Naumov, N.G.; Ledneva, A.; Amela-Cortes, M.; Dumait, N.; Audebrand, N.; et al. Low dimensional solids based on Mo6 cluster cyanides and Mn2+, Mn3+ or Cd2+ metal ions: Crystal chemistry, magnetic and optical properties. CrystEngComm 2018, 20, 3396–3408. [Google Scholar] [CrossRef]
- Fedin, V.P.; Sokolov, M.N.; Myakishev, K.G.; Geras’ko, O.A.; Fedorov, V.Y.; Maciček, J. Mechanochemical synthesis of soluble complexes containing M3S74+ and M3Se74+ fragments from polymeric M3Y7Br4 (M. = Mo, W; Y = S, Se). The crystal structure of (PPN)2W3S7Cl6. Polyhedron 1991, 10, 1311–1317. [Google Scholar] [CrossRef]
- Szczepura, L.F.; Ketcham, K.A.; Ooro, B.A.; Edwards, J.A.; Templeton, J.N.; Cedeño, D.L.; Jircitano, A.J. Synthesis and Study of Hexanuclear Molybdenum Clusters Containing Thiolate Ligands. Inorg. Chem. 2008, 47, 7271–7278. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Kubát, P.; Kučeráková, M.; Šícha, V.; Gbelcová, H.; Lovecká, P.; Grznárová, P.; Ruml, T.; Lang, K. Water-soluble octahedral molybdenum cluster compounds Na2[Mo6I8(N3)6] and Na2[Mo6I8(NCS)6]: Syntheses, luminescence, and in vitro studies. Inorg. Chim. Acta 2016, 441, 42–49. [Google Scholar] [CrossRef]
- Vorotnikov, Y.A.; Efremova, O.A.; Novozhilov, I.N.; Yanshole, V.V.; Kuratieva, N.V.; Brylev, K.A.; Kitamura, N.; Mironov, Y.V.; Shestopalov, M.A. Hexaazide octahedral molybdenum cluster complexes: Synthesis, properties and the evidence of hydrolysis. J. Mol. Struct. 2017, 1134, 237–243. [Google Scholar] [CrossRef]
- Kirakci, K.; Demel, J.; Hynek, J.; Zelenka, J.; Rumlová, M.; Ruml, T.; Lang, K. Phosphinate Apical Ligands: A Route to a Water-Stable Octahedral Molybdenum Cluster Complex. Inorg. Chem. 2019, 58, 16546–16552. [Google Scholar] [CrossRef] [PubMed]
- Svezhentseva, E.V.; Vorotnikov, Y.A.; Solovieva, A.O.; Pozmogova, T.N.; Eltsov, I.V.; Ivanov, A.A.; Evtushok, D.V.; Miroshnichenko, S.M.; Yanshole, V.V.; Eling, C.J.; et al. From Photoinduced to Dark Cytotoxicity through an Octahedral Cluster Hydrolysis. Chem. Eur. J. 2018, 24, 17915–17920. [Google Scholar] [CrossRef] [PubMed]
- Aubert, T.; Burel, A.; Esnault, M.-A.; Cordier, S.; Grasset, F.; Cabello-Hurtado, F. Root uptake and phytotoxicity of nanosized molybdenum octahedral clusters. J. Hazard. Mater. 2012, 219–220, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Efremova, O.A.; Vorotnikov, Y.A.; Brylev, K.A.; Vorotnikova, N.A.; Novozhilov, I.N.; Kuratieva, N.V.; Edeleva, M.V.; Benoit, D.M.; Kitamura, N.; Mironov, Y.V.; et al. Octahedral molybdenum cluster complexes with aromatic sulfonate ligands. Dalton Trans. 2016, 45, 15427–15435. [Google Scholar] [CrossRef] [PubMed]
- Brückner, P.; Preetz, W.; Pünjer, M. Darstellung, Kristallstrukturen, NMR-, Schwingungsspektren und Normalkoordinatenanalyse der Clusteranionen [(Mo6I)Y]2−, Ya = F, Cl, Br, I.Z. Anorg. Allg. Chem. 1997, 623, 8–17. [Google Scholar] [CrossRef]
- Mikhailov, M.A.; Brylev, K.A.; Abramov, P.A.; Sakuda, E.; Akagi, S.; Ito, A.; Kitamura, N.; Sokolov, M.N. Synthetic Tuning of Redox, Spectroscopic, and Photophysical Properties of {Mo6I8}4+ Core Cluster Complexes by Terminal Carboxylate Ligands. Inorg. Chem. 2016, 55, 8437–8445. [Google Scholar] [CrossRef] [PubMed]
- Simsek, M.K.; Bublitz, D.; Preetz, W. Darstellung, Kristallstrukturen, Schwingungsspektren und Normalkoordinatenanalyse von [(Mo6Br)Y]2−; Ya = CN, NCS. Z. Anorg. Allg. Chem. 1997, 623, 1885–1891. [Google Scholar] [CrossRef]
- Cordier, S.; Naumov, N.G.; Salloum, D.; Paul, F.; Perrin, C. Synthesis and Characterization of Mo6 Chalcobromides and Cyano-Substituted Compounds Built from a Novel [(Mo6Bri6Yi2)La6]n- Discrete Cluster Unit (Yi = S or Se and La = Br or CN). Inorg. Chem. 2004, 43, 219–226. [Google Scholar] [CrossRef]
- Petunin, A.A.; Evtushok, D.V.; Vorotnikova, N.A.; Kuratieva, N.V.; Vorotnikov, Y.A.; Shestopalov, M.A. Hexasubstituted Iodide Tungsten Cluster Complexes with Azide and Isothiocyanate Ligands. Eur. J. Inorg. Chem. 2020, 2020, 2177–2181. [Google Scholar] [CrossRef]
- Riehl, L.; Seyboldt, A.; Ströbele, M.; Enseling, D.; Jüstel, T.; Westberg, M.; Ogilby, P.R.; Meyer, H.J. A ligand substituted tungsten iodide cluster: Luminescence vs. singlet oxygen production. Dalton Trans. 2016, 45, 15500–15506. [Google Scholar] [CrossRef]
- Mikhailov, M.A.; Abramov, P.A.; Mironova, A.D.; Gallyamov, M.R.; Sheven’, D.G.; Pervukhin, V.V.; Sokolov, M.N. Methyl Propiolate Cluster Complex (Ph4P)2[W6I8(C≡C–C(O)OCH3)6]. Russ. J. Coord. Chem. 2019, 45, 56–61. [Google Scholar] [CrossRef]
- Costuas, K.; Garreau, A.; Bulou, A.; Fontaine, B.; Cuny, J.; Gautier, R.; Mortier, M.; Molard, Y.; Duvail, J.L.; Faulques, E.; et al. Combined theoretical and time-resolved photoluminescence investigations of [Mo6Bri8Bra6]2− metal cluster units: Evidence of dual emission. Phys. Chem. Chem. Phys. 2015, 17, 28574–28585. [Google Scholar] [CrossRef]
- Gray, T.G. Divergent Electronic Structures of Isoelectronic Metalloclusters: Tungsten(II) Halides and Rhenium(III) Chalcogenide Halides. Chem. Eur. J. 2009, 15, 2581–2593. [Google Scholar] [CrossRef]
- Ramirez-Tagle, R.; Arratia-Pérez, R. Electronic structure and molecular properties of the [Mo6X8L6]2−; X=Cl, Br, I.; L=F, Cl, Br, I clusters. Chem. Phys. Lett. 2008, 460, 438–441. [Google Scholar] [CrossRef]
- Robinson, L.M.; Bain, R.L.; Shriver, D.F.; Ellis, D.E. Effect of Coordination Environment on the Electronic Structure and Properties of Mo6-Based Systems: A Density Functional Treatment. Inorg. Chem. 1995, 34, 5588–5596. [Google Scholar] [CrossRef]
- Sokolov, M.N.; Mihailov, M.A.; Peresypkina, E.V.; Brylev, K.A.; Kitamura, N.; Fedin, V.P. Highly luminescent complexes [Mo6X8(n-C3F7COO)6]2– (X = Br, I). Dalton Trans. 2011, 40, 6375–6377. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, M.N.; Mikhailov, M.A.; Brylev, K.A.; Virovets, A.V.; Vicent, C.; Kompankov, N.B.; Kitamura, N.; Fedin, V.P. Alkynyl complexes of high-valence clusters. Synthesis and luminescence properties of [Mo6I8(C≡CC(O)OMe)6]2–, the first complex with exclusively organometallic outer ligands in the family of octahedral {M6X8} clusters. Inorg. Chem. 2013, 52, 12477–12481. [Google Scholar] [CrossRef] [PubMed]
- Evtushok, D.V.; Melnikov, A.R.; Vorotnikova, N.A.; Vorotnikov, Y.A.; Ryadun, A.A.; Kuratieva, N.V.; Kozyr, K.V.; Obedinskaya, N.R.; Kretov, E.I.; Novozhilov, I.N.; et al. A comparative study of optical properties and X-ray induced luminescence of octahedral molybdenum and tungsten cluster complexes. Dalton Trans. 2017, 46, 11738–11747. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Cordier, S.; Perrin, C. Synthesis and Characterization of Cs2Mo6X14 (X = Br or I) Hexamolybdenum Cluster Halides: Efficient Mo6 Cluster Precursors for Solution Chemistry Syntheses. Z. Anorg. Allg. Chem. 2005, 631, 411–416. [Google Scholar] [CrossRef]
- Vorotnikov, Y.A.; Mikhailov, M.A.; Brylev, K.A.; Piryazev, D.A.; Kuratieva, N.V.; Sokolov, M.N.; Mironov, Y.V.; Shestopalov, M.A. Synthesis, crystal structure, and luminescence properties of complexes (4-ViBnNMe3)2[{M6(µ3-I)8}I6] (M = Mo, W; (4-ViBnNMe3)+ is trimethyl(4-vinylbenzyl)ammonium). Russ. Chem. Bull. 2015, 64, 2591–2596. [Google Scholar] [CrossRef]
- Hogue, R.D.; McCarley, R.E. Chemistry of polynuclear metal halides. V. Reactions and characterization of compounds containing tungsten halide cluster species. Inorg. Chem. 1970, 9, 1354–1360. [Google Scholar] [CrossRef]
- Ishida, H.; Tobita, S.; Hasegawa, Y.; Katoh, R.; Nozaki, K. Recent advances in instrumentation for absolute emission quantum yield measurements. Coord. Chem. Rev. 2010, 254, 2449–2458. [Google Scholar] [CrossRef]
- CrysAlisPro 1.171.39.46; Rigaku Oxford Diffraction: The Woodlands, TX, USA, 2018.
- APEX3; SAINT; SADABS. Bruker Advanced X-ray Solutions; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Sheldrick, G. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Fonseca Guerra, C.; Snijders, J.G.; te Velde, G.; Baerends, E.J. Towards an order-N DFT method. Theor. Chem. Acc. 1998, 99, 391–403. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef] [PubMed]
- Swart, M. A new family of hybrid density functionals. Chem. Phys. Lett. 2013, 580, 166–171. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J. Optimized Slater-type basis sets for the elements 1–118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef]
- van Lenthe, E.; Ehlers, A.; Baerends, E.-J. Geometry optimizations in the zero order regular approximation for relativistic effects. J. Chem. Phys. 1999, 110, 8943–8953. [Google Scholar] [CrossRef]
- Pye, C.C.; Ziegler, T. An implementation of the conductor-like screening model of solvation within the Amsterdam density functional package. Theor. Chem. Acc. 1999, 101, 396–408. [Google Scholar] [CrossRef]
Sample Availability: Samples of compounds 1–5 are available from the authors. |




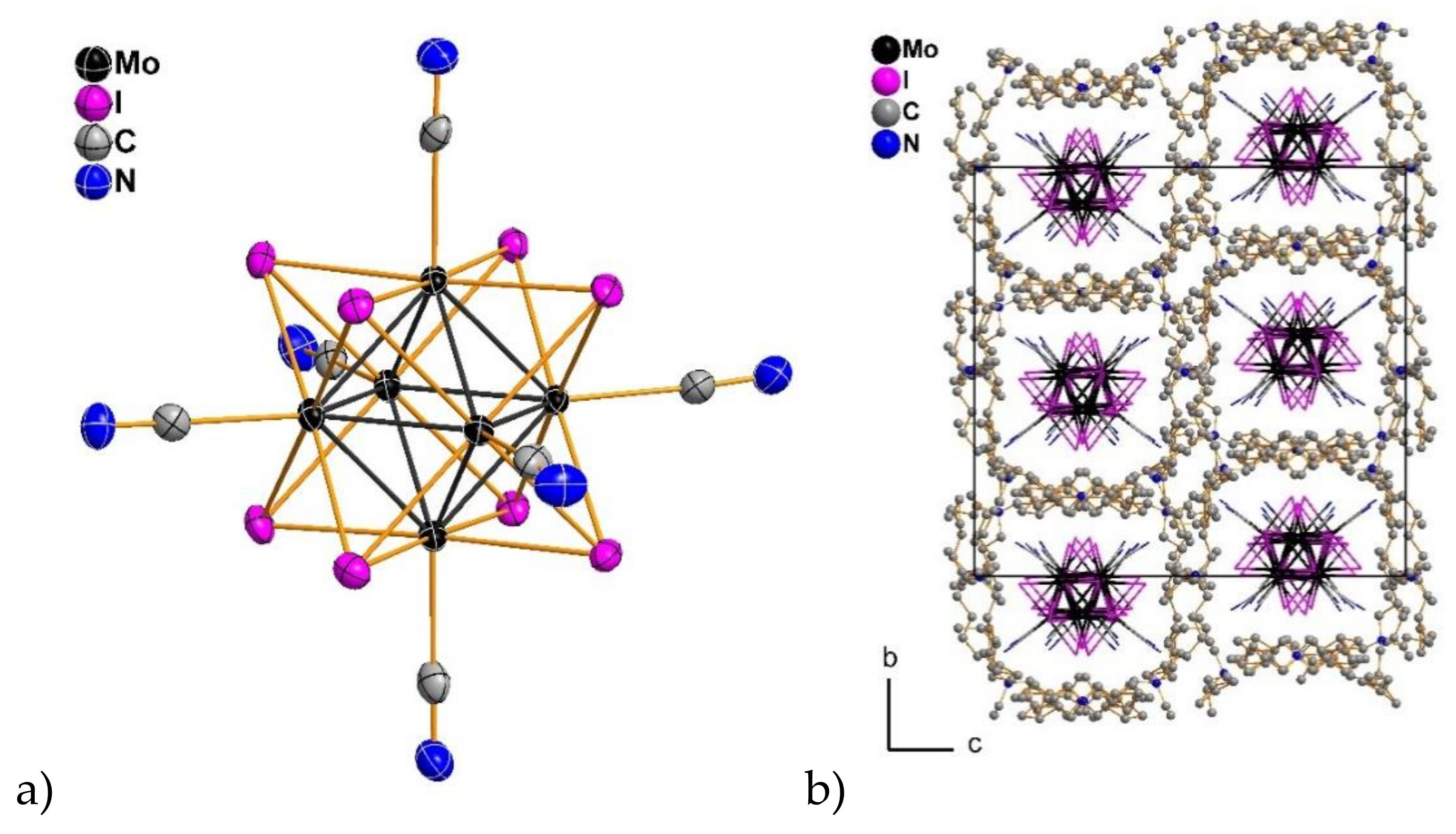


| (Bu4N)2[Mo6I8(CN)6]·H2O (2) | (Bu4N)2[Mo6I8(CN)6]·2DMSO (3) | (Bu4N)2[Mo6I8(CN)4(MeO)2] (4) | (Bu4N)2[W6I8(MeO)4(CN)2]·5H2O (5) | |
|---|---|---|---|---|
| M–M | 2.6738(8)–2.6904(8)/2.680(5) | 2.6832(4)–2.6990(3)/2.689(6) | 2.6799(7)–2.6829(8)/2.681(1) | 2.6558(4)–2.6772(4)/2.6703(8) |
| M–I | 2.7581(8)–2.7903(7)/2.773(8) | 2.7679(4)–2.7907(3)/2.778(6) | 2.7714(3)–2.7974(3)/2.78(1) | 2.7998(5)–2.8388(5)/2.82(1) |
| M–C | 2.177(8)–2.200(8)/2.188(8) | 2.19(3)3–2.201(3)/2.196(4) | 2.190(7) | 2.195(7) |
| M–O | - | - | 2.049(8) | 2.049(5)–2.051(5)/2.050(1) |
| [Mo6I8(CN)6]2− | [Mo6I8(CN)4(MeO)2]2− | [W6I8(CN)2(MeO)4]2− | |
|---|---|---|---|
| M–M | 2.711 | 2.704–2.722/2.712(8) | 2.692–2.707/2.699(4) |
| M–I | 2.862 | 2.872–2.934/2.89(2) | 2.912–2.992/2.95(3) |
| M–C | 2.207 | 2.218 | 2.214 |
| M–O | – | 2.018 | 2.020 |
| Compound/Anion | Deaerated MeCN Solution | ||
|---|---|---|---|
| λmax, nm | Φem | τem, µs | |
| 2/[{Mo6I8}(CN)6]2− | 685 | 0.02 | 162 |
| 4/[{Mo6I8}(CN)4(MeO)2]2− | 700 | 0.14 | 151 |
| 5/[{W6I8}(CN)2(MeO)4]2− | 720 | 0.01 | 92 |
| (Bu4N)2[{Mo6I8}I6]/[{Mo6I8}I6]2− [54] | 730 | 0.12 | 90 |
| (Bu4N)2[W6I8}I6]/[{W6I8}I6]2− [54] | 685 | 0.23 | 35 |
| Compound | 2 | 3 | 4 | 5 |
|---|---|---|---|---|
| Chemical Formula | (Bu4N)2[Mo6I8(CN)6]·0.7H2O | (Bu4N)2[Mo6I8(CN)6]·2DMSO | (Bu4N)2[Mo6I8(CN)4(MeO)2] | (Bu4N)2[W6I8(MeO)4(CN)2]·5H2O |
| Empirical Formula | C38H73.4I8Mo6N8O0.7 | C42H84I8Mo6N8O2S2 | C38H78I8Mo6N6O2 | C38H94I8N4O9W6 |
| Formula Weight | 2244.48 | 2388.13 | 2241.90 | 2859.39 |
| Temperature (K) | 140(2) | 150(2) | 150(2) | 150(2) |
| Crystal Size (mm3) | 0.18 × 0.10 × 0.09 | 0.15 × 0.14 × 0.07 | 0.16 × 0.15 × 0.08 | 0.12 × 0.05 × 0.05 |
| Crystal System | Monoclinic | Triclinic | Tetragonal | Monoclinic |
| Space Group | C2/c | P͞1 | I4/mmm | P21/n |
| Z | 16 | 1 | 2 | 2 |
| a (Å) | 37.2726(6) | 10.7807(4) | 14.6656(3) | 9.8189(3) |
| b (Å) | 24.8482(5) | 11.4600(5) | 14.6656(3) | 15.4901(5) |
| c (Å) | 28.2845(5) | 15.2964(7) | 14.5746(4) | 21.8314(8) |
| Α (°) | 90 | 92.784(4) | 90 | 90 |
| β (°) | 111.997(2) | 91.598(3) | 90 | 97.512(1) |
| γ (°) | 90 | 113.849(4) | 90 | 90 |
| V (Å3) | 24288.9(8) | 1724.1(1) | 3134.7(2) | 3292.0(2) |
| Dcalcd. (g cm−3) | 2.455 | 2.300 | 2.375 | 2.895 |
| μ (Mo Kα) (mm−1) | 5.303 | 4.737 | 5.137 | 14.235 |
| θ range (°) | 1.95 to 25.35 | 1.947 to 29.066 | 1.964 to 29.580 | 1.882 to 27.482 |
| h, k, l index ranges | −43 ≤ h ≤ 44 −29 ≤ k ≤ 24 −34 ≤ l ≤ 22 | −14 ≤ h ≤ 14 −15 ≤ k ≤ 14 −20 ≤ l ≤ 11 | −20 ≤ h ≤ 15 −19 ≤ k ≤ 19 −14 ≤ l ≤ 19 | −12 ≤ h ≤ 12 −20 ≤ k ≤ 20 −25 ≤ l ≤ 28 |
| F(000) | 16624 | 1116 | 2080 | 2560 |
| Reflections Collected | 57967 | 12899 | 8980 | 50844 |
| Independent Reflections | 22209 (Rint = 0.0314) | 7839 (Rint = 0.0177) | 1223 (Rint = 0.0139) | 7549 (Rint = 0.0547) |
| Observed Reflections [I > 2σ(I)] | 16412 | 6675 | 1101 | 6533 |
| R Indices [I > 2σ(I)] | R1 = 0.0389 wR2 = 0.0864 | R1 = 0.0215 wR2 = 0.0457 | R1 = 0.0199 wR2 = 0.0553 | R1 = 0.0306 wR2 = 0.0560 |
| R Indices (all data) | R1 = 0.0643 wR2 = 0.0952 | R1 = 0.0289 wR2 = 0.0479 | R1 = 0.0237 wR2 = 0.0565 | R1 = 0.0377 wR2 = 0.0591 |
| GOOF on F2 | 1.053 | 0.992 | 1.091 | 1.042 |
| Largest Diff. Peak and Hole (e Å−3) | 1.745, −1.050 | 1.305, −0.568 | 2.815, −0.899 | 2.042, −1.951 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pronin, A.S.; Yarovoy, S.S.; Gayfulin, Y.M.; Ryadun, A.A.; Brylev, K.A.; Samsonenko, D.G.; Eltsov, I.V.; Mironov, Y.V. Cyanide Complexes Based on {Mo6I8}4+ and {W6I8}4+ Cluster Cores. Molecules 2020, 25, 5796. https://doi.org/10.3390/molecules25245796
Pronin AS, Yarovoy SS, Gayfulin YM, Ryadun AA, Brylev KA, Samsonenko DG, Eltsov IV, Mironov YV. Cyanide Complexes Based on {Mo6I8}4+ and {W6I8}4+ Cluster Cores. Molecules. 2020; 25(24):5796. https://doi.org/10.3390/molecules25245796
Chicago/Turabian StylePronin, Aleksei S., Spartak S. Yarovoy, Yakov M. Gayfulin, Aleksey A. Ryadun, Konstantin A. Brylev, Denis G. Samsonenko, Ilia V. Eltsov, and Yuri V. Mironov. 2020. "Cyanide Complexes Based on {Mo6I8}4+ and {W6I8}4+ Cluster Cores" Molecules 25, no. 24: 5796. https://doi.org/10.3390/molecules25245796
APA StylePronin, A. S., Yarovoy, S. S., Gayfulin, Y. M., Ryadun, A. A., Brylev, K. A., Samsonenko, D. G., Eltsov, I. V., & Mironov, Y. V. (2020). Cyanide Complexes Based on {Mo6I8}4+ and {W6I8}4+ Cluster Cores. Molecules, 25(24), 5796. https://doi.org/10.3390/molecules25245796









