Encapsulation Preserves Antioxidant and Antidiabetic Activities of Cactus Acid Fruit Bioactive Compounds under Simulated Digestion Conditions
Abstract
1. Introduction
2. Results
2.1. Composition of the Cactus Acid Pear (Xoconostle) Extract
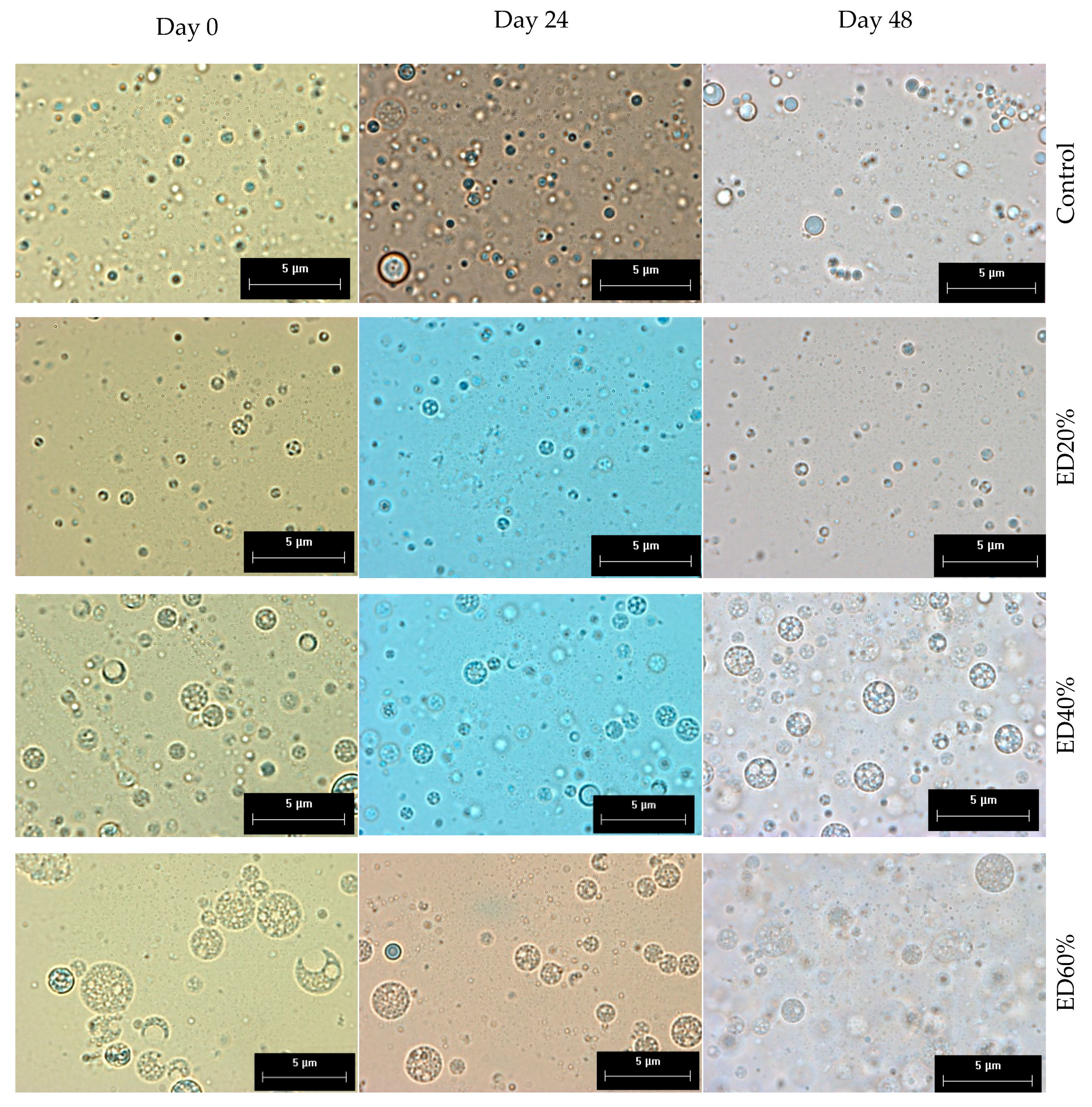
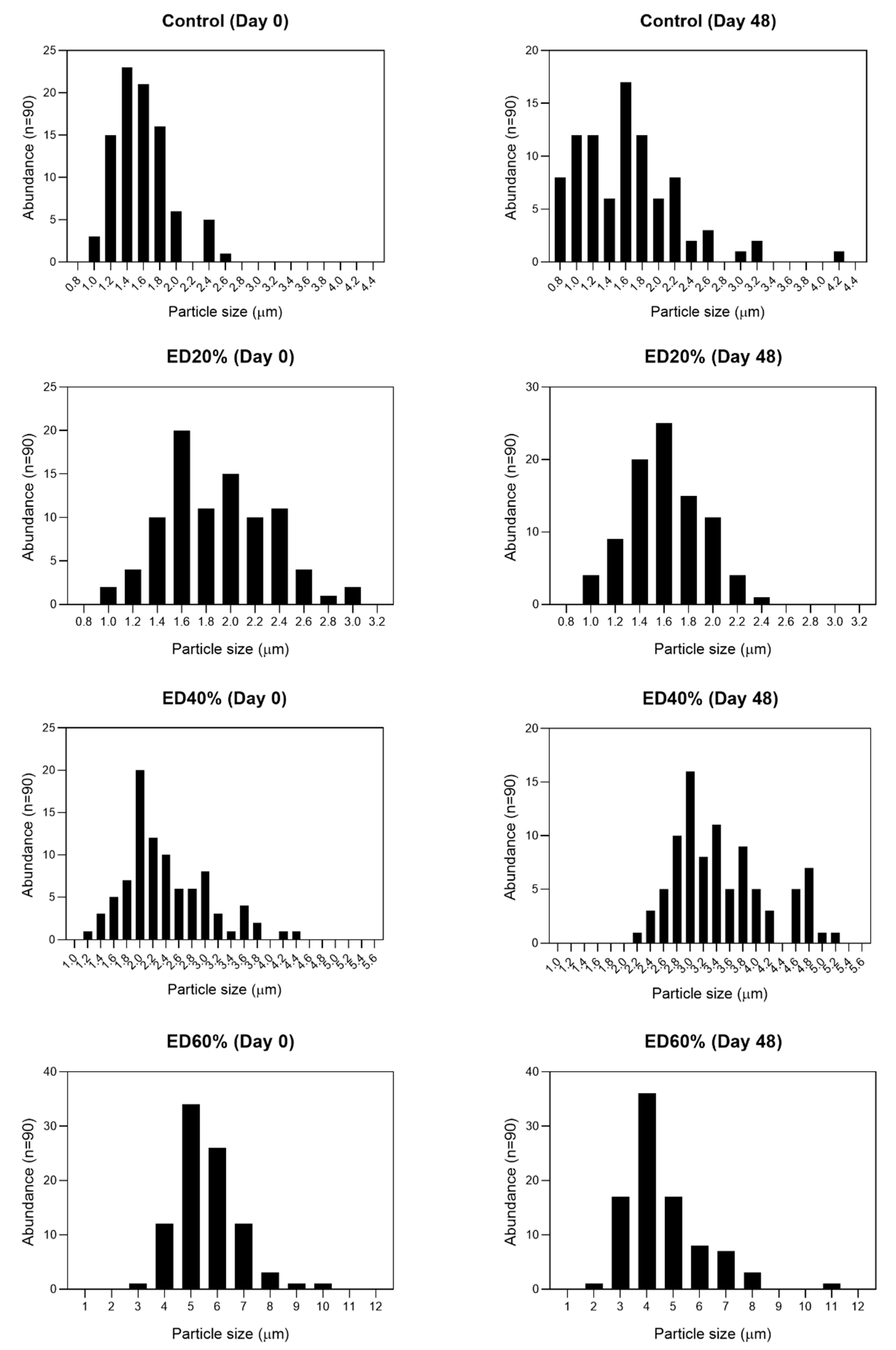
2.2. Droplet Morphology and Encapsulation Efficiency
2.3. Stability of Bioactive Compounds in Multiple Emulsions
2.4. Antioxidant and Antidiabetic Activity of Xoconostle Extract
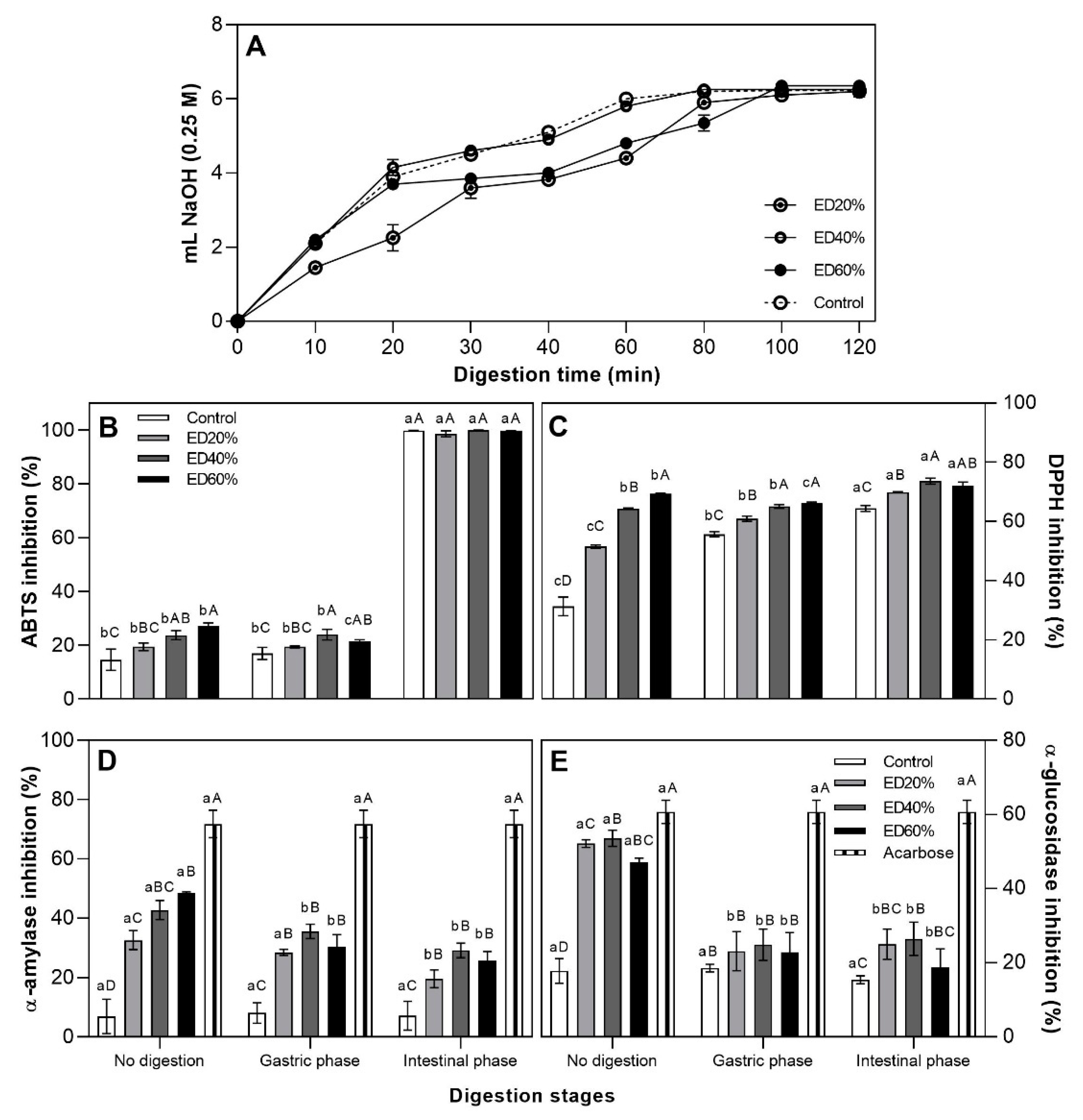
2.5. In Vitro Digestion
2.5.1. Release of Fatty Acids during Digestion
2.5.2. Antioxidant Activity
3. Discussion
3.1. Composition of the Cactus Acid Pear (Xoconostle) Extract
3.2. Droplet Morphology and Encapsulation Efficiency
3.3. Stability of Bioactive Compounds in Multiple Emulsions
3.4. Antioxidant and Antidiabetic Activity of Xoconostle Extract
3.5. In Vitro Assay
3.5.1. Antioxidant Activity
3.5.2. Fatty Acid Release
4. Materials and Methods
4.1. Plant Material and Reagents
4.2. Obtaining the Xoconostle Extract
4.3. Preparation of Double Emulsions
4.3.1. Morphology and Droplet Size
4.3.2. Encapsulation Efficiency
4.4. Bioactive Compounds
4.4.1. Total Phenol Content
4.4.2. Total Flavonoid Content
4.4.3. Total Betalains Determination
4.4.4. Tannin Content
4.5. Antioxidant Activity of Double Emulsions
4.5.1. DPPH Assay
4.5.2. ABTS + Assay
4.6. α-Amylase Inhibition In Vitro Assay
4.7. α-Glucosidase Inhibition In Vitro Assay
4.8. Simulation Intestinal Conditions
4.9. Fatty Acid Release
4.10. Antioxidant and Antidiabetic Activity during Digestion
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Laguna-Hernández, G.; Río-Zamorano, C.A.; Meneses-Ochoa, I.G.; Brechú-Franco, A.E. Histochemistry and immunolocalisation of glucokinin in antidiabetic plants used in traditional Mexican medicine. Eur. J. Histochem. 2017, 61, 135–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soto-Estrada, G.; Moreno Altamirano, L.; García-García, J.J.; Ochoa Moreno, I.; Silberman, M. Trends in frequency of type 2 diabetes in Mexico and its relationship to dietary patterns and contextual factors. Gac. Sanit. 2018, 32, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Chinsembu, K.C. Diabetes mellitus and nature’s pharmacy of putative antidiabetic plants. J. Herb. Med. 2019, 15, 100230. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Soni, L.K.; Dobhal, M.P.; Arya, D.; Bhagour, K.; Parasher, P.; Gupta, R.S. In vitro and in vivo antidiabetic activity of isolated fraction of Prosopis cineraria against streptozotocin-induced experimental diabetes: A mechanistic study. Biomed. Pharmacother. 2018, 108, 1015–1021. [Google Scholar] [CrossRef]
- Salgueiro, A.C.F.; Folmer, V.; Bassante, F.E.M.; Cardoso, M.H.S.; da Rosa, H.S.; Puntel, G.O. Predictive antidiabetic activities of plants used by persons with Diabetes mellitus. Complement. Ther. Med. 2018, 41, 1–9. [Google Scholar] [CrossRef]
- Ahmad, L.A.; Crandall, J.P. Type 2 diabetes prevention: A review. Clin. Diabetes 2010, 28, 53–59. [Google Scholar] [CrossRef]
- Arulselvan, P.; Ghofar, H.A.A.; Karthivashan, G.; Halim, M.F.A.; Ghafar, M.S.A.; Fakurazi, S. Antidiabetic therapeutics from natural source: A systematic review. Biomed. Prev. Nutr. 2014, 4, 607–617. [Google Scholar] [CrossRef]
- Herrera, T.; del Navarro Hierro, J.; Fornari, T.; Reglero, G.; Martin, D. Inhibitory effect of quinoa and fenugreek extracts on pancreatic lipase and α-amylase under in vitro traditional conditions or intestinal simulated conditions. Food Chem. 2019, 270, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, Y.; Zou, J.; Gao, K. α-Glucosidase inhibition and antihyperglycemic activity of flavonoids from Ampelopsis grossedentata and the flavonoid derivatives. Bioorg. Med. Chem. 2016, 24, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- González-Stuart, A.E.; Rivera, J.O. Nutritional and Therapeutic Applications of Prickly Pear Cacti. In Bioactive Food as Dietary Interventions for Diabetes; Academic Press: Cambridge, MA, USA, 2019; pp. 349–360. [Google Scholar]
- Espinosa-Muñoz, V.; RoldáN-cruz, C.A.; HernáNdez-Fuentes, A.D.; Quintero-Lira, A.; Almaraz-Buendía, I.; Campos-Montiel, R.G. Ultrasonic-Assisted Extraction of Phenols, Flavonoids, and Biocompounds with Inhibitory Effect Against Salmonella Typhimurium and Staphylococcus Aureus from Cactus Pear. J. Food Process Eng. 2017, 40, e12358. [Google Scholar] [CrossRef]
- de Cenobio-Galindo, A.J.; Pimentel-González, D.J.; Del Razo-Rodríguez, O.E.; Medina-Pérez, G.; Carrillo-Inungaray, M.L.; Reyes-Munguía, A.; Campos-Montiel, R.G. Antioxidant and antibacterial activities of a starch film with bioextracts microencapsulated from cactus fruits (Opuntia oligacantha). Food Sci. Biotechnol. 2019, 28, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Medina-Pérez, G.; Zaldívar-Ortega, A.K.; de Cenobio-Galindo, A.J.; Afanador-Barajas, L.N.; Vieyra-Alberto, R.; Estefes-Duarte, J.A.; Campos-Montiel, R.G. Antidiabetic Activity of Cactus Acid Fruit Extracts: Simulated Intestinal Conditions of the Inhibitory Effects on α-amylase and α-glucosidase. Appl. Sci. 2019, 9, 4066. [Google Scholar] [CrossRef]
- Parada, J.; Pérez-Correa, J.R.; Pérez-Jiménez, J. Design of low glycemic response foods using polyphenols from seaweed. J. Funct. Foods 2019, 56, 33–39. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Zhou, H.; Muriel Mundo, J.L.; McClements, D.J. Protection of anthocyanin-rich extract from pH-induced color changes using water-in-oil-in-water emulsions. J. Food Eng. 2019, 254, 1–9. [Google Scholar] [CrossRef]
- de Cenobio-Galindo, A.J.; Díaz-Monroy, G.; Medina-Pérez, G.; Franco-Fernández, M.J.; Ludeña-Urquizo, F.E.; Vieyra-Alberto, R.; Campos-Montiel, R.G. Multiple Emulsions with Extracts of Cactus Pear Added in A Yogurt: Antioxidant Activity, In Vitro Simulated Digestion and Shelf Life. Foods 2019, 8, 429. [Google Scholar] [CrossRef]
- Ye, Q.; Georges, N.; Selomulya, C. Microencapsulation of active ingredients in functional foods: From research stage to commercial food products. Trends Food Sci. Technol. 2018, 78, 167–179. [Google Scholar] [CrossRef]
- Pimentel-González, D.J.; Aguilar-García, M.E.; Aguirre-Álvarez, G.; Salcedo-Hernández, R.; Guevara-Arauza, J.C.; Campos-Montiel, R.G. The Process and Maturation Stability of Chihuahua Cheese with Antioxidants in Multiple Emulsions. J. Food Process. Preserv. 2015, 39, 1027–1035. [Google Scholar] [CrossRef]
- Muschiolik, G.; Dickinson, E. Double Emulsions Relevant to Food Systems: Preparation, Stability, and Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 532–555. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Acevedo-Fani, A.; González-Aguilar, G.A.; Martín-Belloso, O. Encapsulation and stability of a phenolic-rich extract from mango peel within water-in-oil-in-water emulsions. J. Funct. Foods 2019, 56, 65–73. [Google Scholar] [CrossRef]
- Morales, P.; Ramírez-Moreno, E.; de Sanchez-Mata, M.C.; Carvalho, A.M.; Ferreira, I.C.F.R. Nutritional and antioxidant properties of pulp and seeds of two xoconostle cultivars (Opuntia joconostle F.A.C. Weber ex Diguet and Opuntia matudae Scheinvar) of high consumption in Mexico. Food Res. Int. 2012, 46, 279–285. [Google Scholar] [CrossRef]
- Osorio-Esquivel, O.; Álvarez, V.B.; Dorantes-Álvarez, L.; Giusti, M.M. Phenolics, betacyanins and antioxidant activity in Opuntia joconostle fruits. Food Res. Int. 2011, 44, 2160–2168. [Google Scholar] [CrossRef]
- Hernández-Fuentes, A.D.; Trapala-Islas, A.; Gallegos-Vásquez, C.; Campos-Montiel, R.G.; Pinedo-Espinoza, J.M.; Guzmán-Maldonado, S.H. Physicochemical variability and nutritional and functional characteristics of xoconostles (Opuntia spp.) accessions from Mexico. Fruits 2015, 70, 109–116. [Google Scholar] [CrossRef]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Revisión Los polifenoles, compuestos de origen natural con efectos saludables sobre el sistema cardiovascular. Nutr. Hosp. 2012, 27, 76–89. [Google Scholar] [CrossRef]
- Kim, J.; Kang, H.; Suh, K.; Oh, S. Stabilization of Water-Soluble Antioxidant in Water-in-Oil-in-Water Double Emulsions. J. Dispers. Sci. Technol. 2003, 24, 833–839. [Google Scholar] [CrossRef]
- Iqbal, S.; Baloch, M.K.; Hameed, G.; McClements, D.J. Controlling W/O/W multiple emulsion microstructure by osmotic swelling and internal protein gelation. Food Res. Int. 2013, 54, 1613–1620. [Google Scholar] [CrossRef]
- Santos, M.G.; Bozza, F.T.; Thomazini, M.; Favaro-Trindade, C.S. Microencapsulation of xylitol by double emulsion followed by complex coacervation. Food Chem. 2015, 171, 32–39. [Google Scholar] [CrossRef]
- Ding, S.; Serra, C.A.; Vandamme, T.F.; Yu, W.; Anton, N. Double emulsions prepared by two–step emulsification: History, state-of-the-art and perspective. J. Control. Release 2019, 295, 31–49. [Google Scholar] [CrossRef]
- Ilyasoglu Buyukkestelli, H.; El, S.N. Development and characterization of double emulsion to encapsulate iron. J. Food Eng. 2019, 263, 446–453. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Sagis, L.; Schroën, K. Formation, Structure, and Functionality of Interfacial Layers in Food Emulsions. Annu. Rev. Food Sci. Technol. 2018, 9, 551–587. [Google Scholar] [CrossRef] [PubMed]
- Garti, N. Progress in stabilization and transport phenomena of double emulsions in food applications. LWT-Food Sci. Technol. 1997, 30, 222–235. [Google Scholar] [CrossRef]
- Lekshmi, R.G.K.; Rahima, M.; Chatterjee, N.S.; Tejpal, C.S.; Anas, K.K.; Vishnu, K.V.; Sarika, K.; Asha, K.K.; Anandan, R.; Suseela, M. Chitosan–Whey protein as efficient delivery system for squalene: Characterization and functional food application. Int. J. Biol. Macromol. 2019, 135, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Chouaibi, M.; Mejri, J.; Rezig, L.; Abdelli, K.; Hamdi, S. Experimental study of quercetin microencapsulation using water-in-oil-in-water (W1/O/W2) double emulsion. J. Mol. Liq. 2019, 273, 183–191. [Google Scholar] [CrossRef]
- Shaddel, R.; Hesari, J.; Azadmard-Damirchi, S.; Hamishehkar, H.; Fathi-Achachlouei, B.; Huang, Q. Double emulsion followed by complex coacervation as a promising method for protection of black raspberry anthocyanins. Food Hydrocoll. 2018, 77, 803–816. [Google Scholar] [CrossRef]
- Silva, W.; Torres-Gatica, M.F.; Oyarzun-Ampuero, F.; Silva-Weiss, A.; Robert, P.; Cofrades, S.; Giménez, B. Double emulsions as potential fat replacers with gallic acid and quercetin nanoemulsions in the aqueous phases. Food Chem. 2018, 253, 71–78. [Google Scholar] [CrossRef]
- de Almeida Paula, D.; Mota Ramos, A.; de Basílio Oliveira, E.; Maurício Furtado Martins, E.; de Augusto Ribeiro Barros, F.; Cristina Teixeira Ribeiro Vidigal, M.; de Almeida Costa, N.; da Tatagiba Rocha, C. Increased thermal stability of anthocyanins at pH 4.0 by guar gum in aqueous dispersions and in double emulsions W/O/W. Int. J. Biol. Macromol. 2018, 117, 665–672. [Google Scholar] [CrossRef]
- Guzmán-Díaz, D.A.; Treviño-Garza, M.Z.; Rodríguez-Romero, B.A.; Gallardo-Rivera, C.T.; Amaya-Guerra, C.A.; Báez-González, J.G. Development and Characterization of Gelled Double Emulsions Based on Chia (Salvia hispanica L.) Mucilage Mixed with Different Biopolymers and Loaded with Green Tea Extract (Camellia sinensis). Foods 2019, 8, 677. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Q.; Chen, X.Y.; Li, X.; Wang, Y.; Zhang, J.L. Comparison and screening of bioactive phenolic compounds in different blueberry cultivars: Evaluation of anti-oxidation and α-glucosidase inhibition effect. Food Res. Int. 2017, 100, 312–324. [Google Scholar] [CrossRef]
- Chávez-Silva, F.; Cerón-Romero, L.; Arias-Durán, L.; Navarrete-Vázquez, G.; Almanza-Pérez, J.; Román-Ramos, R.; Ramírez-Ávila, G.; Perea-Arango, I.; Villalobos-Molina, R.; Estrada-Soto, S. Antidiabetic effect of Achillea millefollium through multitarget interactions: α-glucosidases inhibition, insulin sensitization and insulin secretagogue activities. J. Ethnopharmacol. 2018, 212, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Alongi, M.; Verardo, G.; Gorassini, A.; Anese, M. Effect of pasteurization on in vitro α-glucosidase inhibitory activity of apple juice. LWT 2018, 98, 366–371. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Tanzeela, N.; Sun, L.; Fang, Z.; Yan, Y.; Li, D.; Xie, H.; Wang, H.; Guo, Y. Effect of in vitro gastrointestinal digestion on the composition and bioactivity of anthocyanins in the fruits of cultivated Lycium ruthenicum Murray. CyTA J. Food 2019, 17, 552–562. [Google Scholar] [CrossRef]
- Pinto, M.D.S.; Ranilla, L.G.; Apostolidis, E.; Lajolo, F.M.; Genovese, M.I.; Shetty, K. Evaluation of Antihyperglycemia and Antihypertension Potential of Native Peruvian Fruits Using In Vitro Models. J. Med. Food 2009, 12, 278–291. [Google Scholar] [CrossRef]
- Liu, Z.; Dang, J.; Wang, Q.; Yu, M.; Jiang, L.; Mei, L.; Shao, Y.; Tao, Y. Optimization of polysaccharides from Lycium ruthenicum fruit using RSM and its anti-oxidant activity. Int. J. Biol. Macromol. 2013, 61, 127–134. [Google Scholar] [CrossRef]
- Mendoza Meza, D.L.; Valdés, R. Inhibición in vitro de las enzimas alfa-amilasa y lipasa pancreática por fracciones fenólicas de extractos etanólicos de hojas de Yacón (Smallanthus sonchifolius Poepp. & Endl). Av. Química 2015, 10, 33–44. [Google Scholar]
- Ghosh, S.; Ahire, M.; Patil, S.; Jabgunde, A.; Bhat Dusane, M.; Joshi, B.N.; Pardesi, K.; Jachak, S.; Dhavale, D.D.; Chopade, B.A. Antidiabetic activity of gnidia glauca and dioscorea bulbifera: Potent amylase and glucosidase inhibitors. Evid.-Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- López-Martínez, L.X.; Cisneros, L.M.A.; Dublán-García, O. Actividad antioxidante e inhibidora de glucosidasa y amilasa de tres variedades de cebolla (Allium cepa L.). Nov. Sci. 2014, 6, 234–247. [Google Scholar] [CrossRef]
- Pasukamonset, P.; Kwon, O.; Adisakwattana, S. Alginate-based encapsulation of polyphenols from Clitoria ternatea petal flower extract enhances stability and biological activity under simulated gastrointestinal conditions. Food Hydrocoll. 2016, 61, 772–779. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Quirós, A.; Amigo, L.; Recio, I. Identification of bioactive peptides after digestion of human milk and infant formula with pepsin and pancreatin. Int. Dairy J. 2007, 17, 42–49. [Google Scholar] [CrossRef]
- Links, M.R.; Taylor, J.; Kruger, M.C.; Taylor, J.R.N. Sorghum condensed tannins encapsulated in kafirin microparticles as a nutraceutical for inhibition of amylases during digestion to attenuate hyperglycaemia. J. Funct. Foods 2015, 12, 55–63. [Google Scholar] [CrossRef]
- Qin, D.; Yang, X.; Gao, S.; Yao, J.; McClements, D.J. Influence of dietary fibers on lipid digestion: Comparison of single-stage and multiple-stage gastrointestinal models. Food Hydrocoll. 2017, 69, 382–392. [Google Scholar] [CrossRef]
- Gasa-Falcon, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Influence of mandarin fiber addition on physico-chemical properties of nanoemulsions containing β-carotene under simulated gastrointestinal digestion conditions. LWT Food Sci. Technol. 2017, 84, 331–337. [Google Scholar] [CrossRef]
- Quintero-Lira, A.; Ángeles Santos, A.; Aguirre-Álvarez, G.; Reyes-Munguía, A.; Almaraz-Buendía, I.; Campos-Montiel, R.G. Effects of liquefying crystallized honey by ultrasound on crystal size, 5-hydroxymethylfurfural, colour, phenolic compounds and antioxidant activity. Eur. Food Res. Technol. 2017, 243, 619–626. [Google Scholar] [CrossRef]
- Hernández-Fuentes, A.; López-Vargas, E.; Pinedo-Espinoza, J.; Campos-Montiel, R.; Valdés-Reyna, J.; Juárez-Maldonado, A. Postharvest Behavior of Bioactive Compounds in Tomato Fruits Treated with Cu Nanoparticles and NaCl Stress. Appl. Sci. 2017, 7, 980. [Google Scholar] [CrossRef]
- Price, M.L.; Butler, L.G. Rapid Visual Estimation and Spectrophotometric Determination of Tannin Content of Sorghum Grain. J. Agric. Food Chem. 1977, 26, 1266–1273. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Leb. u.-Technology 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound are available from the authors. |
| Days | 0 | 3 | 6 | 12 | 24 | 48 |
|---|---|---|---|---|---|---|
| Total Phenols (mgEAG/100 g ED) | ||||||
| Control | 8.7 ± 0.27 aC | 5.9 ± 0.05 bC | 5.0 ± 1.48 bcD | 4.9 ± 0.35 abD | 4.5 ± 0.20 abD | 4.9 ± 0.57 aC |
| ED20% | 10.5 ± 0.11 aB | 10.4 ± 0.21 aB | 8.8 ± 0.07 bC | 8.7 ± 0.14 bC | 8.1 ± 0.25 bC | 6.7 ± 0.08 cD |
| ED40% | 18.0 ± 0.31 aA | 16.9 ± 0.26 bA | 16.7 ± 0.57 bA | 16.2 ± 0.18 bA | 16.0 ± 0.26 bA | 13.9 ± 0.11 cA |
| ED60% | 18.7 ± 0.38 aA | 16.6 ± 0.53 bA | 14.7 ± 0.13 bcB | 13.1 ± 0.21 cB | 12.2 ± 0.06 cB | 9.7 ± 0.08 eB |
| Total Flavonoids (mgEQ/100 g ED) | ||||||
| Control | 0.27 ± 0.03 aC | 0.26 ± 0.04 aC | 0.27 ± 0.04 aB | 0.24 ± 0.01 aD | 0.22 ± 0.01 aD | 0.22 ± 0.07 aD |
| ED20% | 0.79 ± 0.26 aB | 0.54 ± 0.06 bB | 0.47 ± 0.09 bB | 0.48 ± 0.05 bC | 0.44 ± 0.06 bC | 0.39 ± 0.05 bC |
| ED40% | 1.45 ± 0.08 aA | 1.35 ± 0.05 bA | 1.26 ± 0.10 bA | 1.11 ± 0.05 cB | 0.91 ± 0.04 cA | 0.82 ± 0.15 dA |
| ED60% | 1.51 ± 0.08 aA | 1.22 ± 0.08 abA | 1.12 ± 0.06 bcA | 0.88 ± 0.05 cA | 0.82 ± 0.03 bB | 0.61 ± 0.04 dB |
| Betacyanins (mg betacyanin/100 g ED) | ||||||
| Control | 0.00 ± 0.00 aD | 0.00 ± 0.00 aD | 0.00 ± 0.00 aD | 0.00 ± 0.00 aD | 0.00 ± 0.00 aD | 0.00 ± 0.00 aD |
| ED20% | 0.15 ± 0.02 aC | 0.12 ± 0.02 bC | 0.11 ± 0.01 bC | 0.11 ± 0.00 bC | 0.10 ± 0.02 bC | 0.06 ± 0.01 dC |
| ED40% | 0.32 ± 0.01 aB | 0.29 ± 0.01 bB | 0.27 ± 0.01 bB | 0.26 ± 0.02 cB | 0.23 ± 0.01 dB | 0.22 ± 0.02 eA |
| ED60% | 0.41 ± 0.03 aA | 0.42 ± 0.02 aA | 0.35 ± 0.01 bA | 0.30 ± 0.03 cA | 0.26 ± 0.02 cA | 0.21 ± 0.01 dB |
| Betaxanthins (mg betaxanthin/ 100 g ED) | ||||||
| Control | 0.00 ± 0.00 bD | 0.00 ± 0.00 bD | 0.00 ± 0.00 bD | 0.00 ± 0.00 bD | 0.00 ± 0.00 bD | 0.02 ± 0.00 aD |
| ED20% | 0.05 ± 0.00 aC | 0.04 ± 0.00 abcC | 0.04 ± 0.00 bcdC | 0.03 ± 0.00 cdC | 0.03 ± 0.00 dC | 0.04 ± 0.00 abC |
| ED40% | 0.13 ± 0.01 aA | 0.12 ± 0.01 aB | 0.12 ± 0.01 aB | 0.09 ± 0.01 bB | 0.09 ± 0.02 bB | 0.07 ± 0.01 bB |
| ED60% | 0.16 ± 0.03 aB | 0.12 ± 0.02 bA | 0.09 ± 0.02 cA | 0.08 ± 0.01 cdA | 0.07 ± 0.01 cdA | 0.06 ± 0.01 dA |
| Tannins (mgEC/100 g ED) | ||||||
| Control | 1.57 ± 0.16 aD | 1.52 ± 0.19 abD | 1.33 ± 0.18 abD | 1.30 ± 0.08 abD | 0.95 ± 0.08 bcD | 0.68 ± 0.43 cD |
| ED20% | 9.59 ± 0.94 aC | 8.92 ± 0.50 abC | 7.91 ± 0.40 bcC | 7.69 ± 0.19 bcC | 7.16 ± 0.21 cdC | 5.93 ± 0.26 dC |
| ED40% | 13.25 ± 0.58 aB | 11.58 ± 0.63 bB | 10.83 ± 0.79 bcB | 10.03 ± 0.59 cdB | 10.04 ± 0.30 cdB | 9.15 ± 0.20 dB |
| ED60% | 17.91 ± 0.34 aA | 17.07 ± 1.09 abA | 15.93 ± 1.01 bA | 13.83 ± 0.52 cA | 11.82 ± 0.50 cA | 9.31 ± 0.51 dB |
| Antioxidant Activity | Day | Control | ED20% | ED40% | ED60% |
| DPPH (%) | 0 | 20.64 ± 1.05 cD | 61.16 ± 0.24 aC | 71.95 ± 2.58 bB | 86.41 ± 0.21 aA |
| 3 | 23.79 ± 1.59 abD | 56.38 ± 0.29 bC | 77.00 ± 0.41 aA | 70.48 ± 0.32 bB | |
| 6 | 21.21 ± 0.35 bcC | 41.78 ± 0.69 dC | 59.28 ± 0.48 dA | 50.91 ± 1.15 cB | |
| 12 | 21.03 ± 0.20 cC | 40.27 ± 0.71 deC | 59.18 ± 0.29 dA | 52.80 ± 0.44 cB | |
| 24 | 15.93 ± 1.12 aB | 37.79 ± 0.35 eC | 54.83 ± 0.21 cA | 50.70 ± 0.97 dB | |
| 48 | 15.01 ± 0.73 aC | 38.73 ± 1.93 cB | 53.30 ± 1.67 dA | 49.03 ± 0.99 cB | |
| ABTS (%) | 0 | 29.98 ± 0.79 aD | 53.93 ± 1.62 aC | 62.34 ± 0.44 aB | 76.46 ± 2.30 aA |
| 3 | 28.33 ± 1.15 abD | 41.11 ± 0.45 bcC | 52.75 ± 1.01 cB | 59.73 ± 1.39 bcA | |
| 6 | 25.79 ± 2.83 bC | 45.06 ± 2.26 bB | 55.82 ± 0.80 bA | 53.38 ± 0.40 dA | |
| 12 | 25.55 ± 2.33 abD | 38.08 ± 0.45 cC | 51.62 ± 0.72 cB | 55.65 ± 1.34 cdA | |
| 24 | 25.79 ± 1.88 abD | 41.23 ± 3.62 bcC | 51.16 ± 0.84 cB | 60.99 ± 0.44 bA | |
| 48 | 24.85 ± 0.59 bC | 36.11 ± 0.57 cB | 45.35 ± 0.26 dA | 35.30 ± 2.40 eB | |
| Antidiabetic Activity | Day | Control | ED20% | ED40% | ED60% |
| Inhibition α-Amylase (%) | 0 | 07.40 ± 4.22 aC | 33.52 ± 2.54 aB | 39.92 ± 2.68 aB | 40.19 ± 2.52 aB |
| 3 | 02.50 ± 2.33 aC | 33.80 ± 5.52 aB | 39.50 ± 2.50 aB | 35.47 ± 1.46 abB | |
| 6 | 5.94 ± 12.36 aC | 30.18 ± 4.01 abB | 34.49 ± 2.84 abB | 26.42 ± 3.98 cB | |
| 12 | 00.75 ± 8.09 aC | 29.49 ± 3.01 abcB | 33.38 ± 2.51 abB | 29.76 ± 4.51 bcB | |
| 24 | 00.90 ± 5.52 aD | 24.20 ± 1.25 bcBC | 32.27 ± 2.52 bB | 17.94 ± 2.21 dC | |
| 48 | 02.65 ± 1.10 aE | 22.25 ± 2.14 cC | 30.46 ± 1.27 bB | 13.91 ± 1.88 dD | |
| Inhibition α-Glucosidase (%) | 0 | 03.78 ± 2.87 aE | 33.88 ± 3.99 aD | 58.77 ± 1.59 aC | 78.84 ± 1.17 aB |
| 3 | 07.25 ± 2.20 aD | 36.09 ± 0.49 aC | 54.61 ± 2.19 aB | 54.16 ± 3.13 bB | |
| 6 | 07.87 ± 3.82 aD | 25.39 ± 3.77 bC | 40.08 ± 9.53 bB | 41.09 ± 2.83 cB | |
| 12 | 05.57 ± 2.60 aD | 25.53 ± 1.77 bC | 33.64 ± 2.99 bB | 38.71 ± 5.95 cB | |
| 24 | 01.05 ± 1.89 aE | 33.62 ± 0.39 aD | 38.33 ± 0.14 bC | 43.50 ± 2.91 cB | |
| 48 | 05.99 ± 2.32 aE | 20.20 ± 1.37 bC | 30.19 ± 0.67 bB | 12.11 ± 1.67 dD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Pérez, G.; Estefes-Duarte, J.A.; Afanador-Barajas, L.N.; Fernández-Luqueño, F.; Zepeda-Velásquez, A.P.; Franco-Fernández, M.J.; Peláez-Acero, A.; Campos-Montiel, R.G. Encapsulation Preserves Antioxidant and Antidiabetic Activities of Cactus Acid Fruit Bioactive Compounds under Simulated Digestion Conditions. Molecules 2020, 25, 5736. https://doi.org/10.3390/molecules25235736
Medina-Pérez G, Estefes-Duarte JA, Afanador-Barajas LN, Fernández-Luqueño F, Zepeda-Velásquez AP, Franco-Fernández MJ, Peláez-Acero A, Campos-Montiel RG. Encapsulation Preserves Antioxidant and Antidiabetic Activities of Cactus Acid Fruit Bioactive Compounds under Simulated Digestion Conditions. Molecules. 2020; 25(23):5736. https://doi.org/10.3390/molecules25235736
Chicago/Turabian StyleMedina-Pérez, Gabriela, José Antonio Estefes-Duarte, Laura N. Afanador-Barajas, Fabián Fernández-Luqueño, Andrea Paloma Zepeda-Velásquez, Melitón Jesús Franco-Fernández, Armando Peláez-Acero, and Rafael Germán Campos-Montiel. 2020. "Encapsulation Preserves Antioxidant and Antidiabetic Activities of Cactus Acid Fruit Bioactive Compounds under Simulated Digestion Conditions" Molecules 25, no. 23: 5736. https://doi.org/10.3390/molecules25235736
APA StyleMedina-Pérez, G., Estefes-Duarte, J. A., Afanador-Barajas, L. N., Fernández-Luqueño, F., Zepeda-Velásquez, A. P., Franco-Fernández, M. J., Peláez-Acero, A., & Campos-Montiel, R. G. (2020). Encapsulation Preserves Antioxidant and Antidiabetic Activities of Cactus Acid Fruit Bioactive Compounds under Simulated Digestion Conditions. Molecules, 25(23), 5736. https://doi.org/10.3390/molecules25235736







