pH-Sensitive Biomaterials for Drug Delivery
Abstract
1. Introduction
2. pH-Sensitive Bonds
2.1. Imine Bonds
2.2. Hydrazone Bonds
2.3. Oxime Bonds
2.4. Amide Bonds
2.5. Acetals
2.6. Orthoester
3. pH-Sensitive Nanomaterials
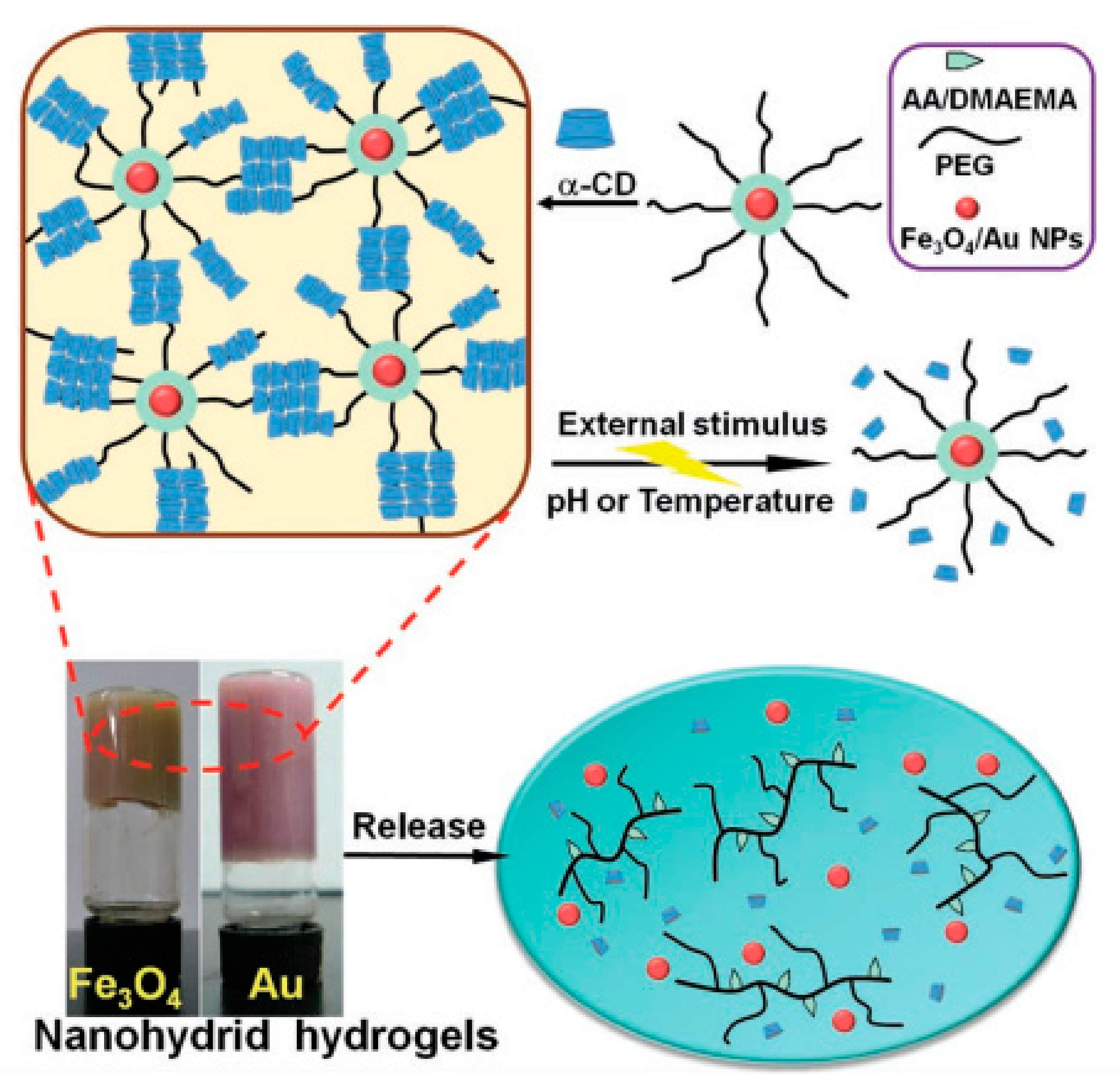
3.1. Hydrogels
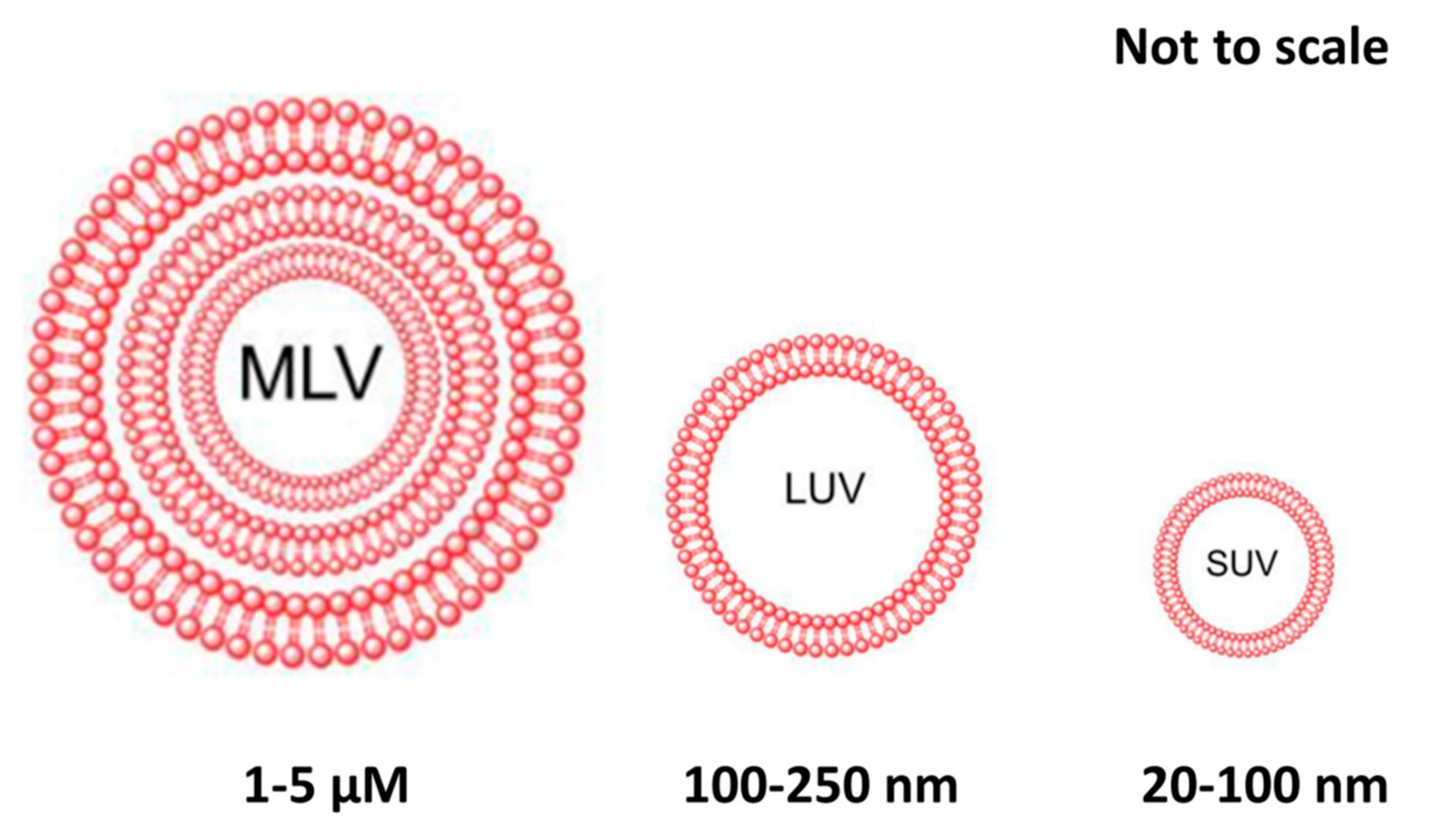
3.2. Liposomes
3.3. Polymer Micelles
- Protonated groups are introduced into the polymer chain through the mechanism of protonation/deprotonation [175,176,177,178,179]. The common functional groups of protonation include the amine group, imidazolyl group, sulfonic acid group, and carboxyl group. When such polymer micelles reach acidic target sites, rapid targeted release of the drug contained in the micelles would be triggered as the unstable micelle structures due to acidic precipitation/aggregation, or depolymerization.
- pH-sensitive chemical bonding arms are introduced between polymer chain segments or between polymers and drugs [180,181,182]. The acid sensitive connector arm refers to a molecule or group that can exist stably under neutral conditions but can hydrolyze quickly under weak acidic conditions, including imine bond [183], hydrazine bond, hydrazone bond [56,184,185,186], cisaconitamide, dimethyl maleamide [94,187], ether bond [56,188], orthate ester [70,188,189], polyacylaldehyde (ketone) [190], etc.
4. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Fenton, O.S.; Katy, N.O.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Allen, T.M. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging Frontiers in Drug Delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Mooney, D.J. Inspiration and application in the evolution of biomaterials. Nature 2009, 462, 426–432. [Google Scholar]
- Lu, Y.A.; Alex, A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 1, 16075. [Google Scholar] [CrossRef]
- Yun, H.Y.; Lee, B.K.; Park, K. Controlled Drug Delivery: Historical perspective for the next generation. J. Control. Release 2015, 219, 2–7. [Google Scholar] [CrossRef]
- Kanamala, M.R.; Wilson, W.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X. pH-Sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef]
- Cao, Y.; Ronald, A.D.; Matthias, E.; Vousden, K. Cancer research: Past, present and future. Nat. Rev. Cancer 2011, 11, 749–754. [Google Scholar] [CrossRef]
- Patel, N.R.P.; Bhushan, S.; Abouzeid, H.A.; Torchilin, P.V. Nanopreparations to overcome multidrug resistance in cancer. Adv. Drug Deliv. Rev. 2013, 65, 1748–1762. [Google Scholar] [CrossRef]
- Shen, M.; Huang, Y.-Z.; Han, L.; Qin, J.; Fang, X.; Wang, J.; Yang, V.C. Multifunctional drug delivery system for targeting tumor and its acidic microenvironment. J. Control. Release 2012, 161, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Trédan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer I. 2007, 99, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; McDonald, P.C.; Oloumi, A.; Chia, S.; Ostlund, C.; Ahmadi, A.; Kyle, A.; Keller, U.A.D.; Leung, S.; Huntsman, D.; et al. Targeting tumor hypoxia: Suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011, 71, 3364–3376. [Google Scholar] [CrossRef]
- Milosevic, M.; Warde, P.; Menard, C.; Chung, P.; Toi, A.; Ishkanian, A.; McLean, M.; Pintilie, M.; Sykes, J.; Gospodarowicz, M.; et al. Tumor hypoxia predicts biochemical failure following radiotherapy for clinically localized prostate cancer. Clin. Cancer Res. 2012, 18, 2108–2114. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Ji, R.-C. Hypoxia and lymphangiogenesis in tumor microenvironment and metastasis. Cancer Lett. 2014, 346, 6–16. [Google Scholar] [CrossRef]
- Fukumura, D.; Duda, D.G.; Munn, L.L.; Jain, R.K. Tumor Microvasculature and Microenvironment: Novel Insights Through Intravital Imaging in Pre-Clinical Models. Microcirculation 2010, 17, 206–225. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; He, D.; Rödl, W.; Preiß, T.; Rädler, J.O.; Wagner, E.; Lächelt, U. pH-Reversible Cationic RNase A Conjugates for Enhanced Cellular Delivery and Tumor Cell Killing. Biomacromolecules 2016, 17, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, A.; Pranali, D.; Torchilin, V. Stimuli-sensitive nanopreparations for combination cancer therapy. J. Control. Release 2014, 190, 352–370. [Google Scholar] [CrossRef] [PubMed]
- Pathania, D.; Millard, M.; Neamati, N. Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Adv. Drug Deliv. Rev. 2009, 61, 1250–1275. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Dominik, W.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Ulrich, S. Growing Prospects of Dynamic Covalent Chemistry in Delivery Applications. Acc. Chem. Res. 2019, 52, 510–519. [Google Scholar] [CrossRef]
- Leriche, G.; Chisholm, L.; Wagner, A. Cleavable linkers in chemical biology. Bioorganic Med. Chem. 2012, 20, 571–582. [Google Scholar] [CrossRef]
- Bernardes, G.J.; Boutureira, O.L. Advances in Chemical Protein Modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar]
- Herrmann, A. Dynamic combinatorial/covalent chemistry: A tool to read, generate and modulate the bioactivity of compounds and compound mixtures. Chem. Soc. Rev. 2014, 43, 1899–1933. [Google Scholar] [CrossRef]
- West, K.; Sijbren, O. Reversible Covalent Chemistry in Drug Delivery. Curr. Drug Discov. Technol. 2005, 2, 123–160. [Google Scholar] [CrossRef]
- Webber, M.J.; Robert, L. Drug delivery by supramolecular design. Chem. Soc. Rev. 2017, 46, 6600–6620. [Google Scholar] [CrossRef]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef]
- Drozdz, W.; Camille, B.; Kotras, C.; Richeter, S.; Barboiu, M.; Clement, S.; Stefankiewicz, A.R.; Ulrich, S. Generation of Multicomponent Molecular Cages using Simultaneous Dynamic Covalent Reactions. Chem. A Eur. J. 2017, 23, 18010–18018. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, N.; Bolandparvaz, A.; Lewis, J.S. Stimuli-esponsive Biomaterials for Vaccines and Immunotherapeutic Applications. Adv. Ther. 2020, 3, 2000129. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.N.; Tare, S.M.; Mishra, V.; Tripathi, K.P. The development, characterization and in vivo anti-ovarian cancer activity of poly(propylene imine) (PPI)-antibody conjugates containing encapsulated paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, G. Chemoselective ligation reactions with proteins, oligosaccharides and cells. Trends Biotechnol. 1998, 16, 506–513. [Google Scholar] [CrossRef]
- Zeng, X.; Gan, L.; Tao, W.; Ma, Y.; Zhang, X.; He, F.; Pan, J.; Mei, L.; Pan, G. A Drug-Self-Gated Mesoporous Antitumor Nanoplatform Based on pH-Sensitive Dynamic Covalent Bond. Adv. Funct. Mater. 2017, 27, 27. [Google Scholar] [CrossRef]
- Cheng, C.; Yabin, M.; Zhang, Z.; Chen, J.; Zhang, Q. Imine Bond- and Coordinate Bond-Linked pH-Sensitive Cisplatin Complex Nanoparticles for Active Targeting to Tumor Cells. J. Nanosci. Nanotechnol. 2019, 19, 3277–3287. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhao, L.; Qu, X.; Yang, Z. pH-Sensitive Polymeric Vesicles from Coassembly of Amphiphilic Cholate Grafted Poly(L-lysine) and Acid-Cleavable Polymer-Drug Conjugate. Langmuir Acs J. Surf. Colloids 2012, 28, 11988–11996. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Kuang, Y.; Li, S.; Zhang, M.; Liu, J.; Sun, Z.; Jiang, B.; Chen, X.; Li, C. Stepwise-acid-active organic/inorganic hybrid drug delivery system for cancer therapy. Colloids Surf. B Biointerfaces 2018, 167, 407–414. [Google Scholar] [CrossRef]
- Gu, J.; Cheng, W.-P.; Liu, J.; Lo, S.-Y.; Smith, D.; Qu, X.; Yang, Z. pH-Triggered Reversible “stealth”Polycationic Micelles. Biomacromolecules 2008, 9, 255–262. [Google Scholar] [CrossRef]
- Suri, S.S.; Fenniri, H.; Singh, B. Nanotechnology-based Drug Delivery Systems. J. Occup. Med. Toxicol. 2007. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Liu, S.; Zhang, Y.; Chi, Z.; Xu, J. A pH-responsive polymer based on dynamic imine bonds as a drug delivery material with pseudo target release behavior. Polym. Chem. 2018, 9, 878–884. [Google Scholar] [CrossRef]
- Xu, J.; Benkai, Q.; Luan, S.; Qi, P.; Wang, Y.; Wang, K.; Song, S. Acid-labile poly(ethylene glycol) shell of hydrazone-containing biodegradable polymeric micelles facilitating anticancer drug delivery. J. Bioact. Compat. Polym. 2018, 33, 119–133. [Google Scholar] [CrossRef]
- Liang, Y.; Zhihui, S.; Yao, Y.; Zhang, N. Preparation of pH Sensitive Pluronic-Docetaxel Conjugate Micelles to Balance the Stability and Controlled Release Issues. Materials 2015, 8, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Rahoui, N.; Bo, J.; Taloub, N.; Hegazy, M.; Huang, Y.D. Synthesis and evaluation of water soluble pH sensitive poly (vinyl alcohol)-doxorubicin conjugates. J. Biomater. Sci. Polym. Ed. 2018, 29, 1482–1497. [Google Scholar] [CrossRef]
- Su, Z.; Liang, Y.; Yao, Y.; Wang, T.; Zhang, N. Polymeric complex micelles based on the double-hydrazone linkage and dual drug-loading strategy for pH-sensitive docetaxel delivery. J. Mater. Chem. B 2016, 4, 1122–1133. [Google Scholar] [CrossRef]
- Wang, L.; Ren, K.-F.; Wang, H.-B.; Wang, Y.; Ji, J. pH-sensitive controlled release of doxorubicin from polyelectrolyte multilayers. Colloids Surf. B Biointerfaces 2015, 125, 127–133. [Google Scholar] [CrossRef]
- Sun, T.-M.; Wang, Y.-C.; Wang, F.; Du, J.-Z.; Mao, C.-Q.; Sun, C.-Y.; Tang, R.-Z.; Zhu, Y.; Zhu, Y.-H.; Yang, X.-Z.; et al. Cancer stem cell therapy using doxorubicin conjugated to gold nanoparticles via hydrazone bonds. Biomaterials 2014, 35, 836–845. [Google Scholar] [CrossRef]
- Jiang, T.; You-Mei, L.; Lv, Y.; Cheng, Y.; He, F.; Zhuo, R. Amphiphilic polycarbonate conjugates of doxorubicin with pH-sensitive hydrazone linker for controlled release. Colloids Surf. B Biointerfaces 2013, 111, 542–548. [Google Scholar] [CrossRef]
- Sirova, M.; Mrkvan, T.; Etrych, T.; Chytil, P.; Rossmann, P.; Ibrahimova, M.; Kovář, L.; Ulbrich, K.; Říhová, B. Preclinical Evaluation of Linear HPMA-Doxorubicin Conjugates with pH-Sensitive Drug Release: Efficacy, Safety, and Immunomodulating Activity in Murine Model. Pharm. Res. 2009, 27, 200. [Google Scholar] [CrossRef]
- Qi, P.; Wu, X.; Liu, L.; Yu, H.; Song, S. Hydrazone-Containing Triblock Copolymeric Micelles for pH-Controlled Drug Delivery. Front. Pharmacol. 2018, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Szabó, I.; Manea, M.; Orbán, E.; Csámpai, A.; Bosze, S.; Szabó, R.; Tejeda, M.; Gaál, D.; Kapuvári, B.; Przybylski, M.; et al. Development of an Oxime Bond Containing Daunorubicin-Gonadotropin-Releasing Hormone-III Conjugate as a Potential Anticancer Drug. Bioconjug. Chem. 2009, 20, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Song, L.; Su, Y.; Zhu, L.; Pang, Y.; Qiu, F.; Tong, G.; Yan, D.; Zhu, B.; Zhu, X. Oxime lnkage: A robust tool for the design of pH-sensitive polymeric drug carriers. Biomacromolecules 2011, 12, 3460–3468. [Google Scholar] [CrossRef] [PubMed]
- Kölmel, D.K.; Kool, E.T. Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis. Chem. Rev. 2017, 117, 10358–10376. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, H.; Li, X.; Zhao, C.; Liu, Y.; Zhu, L.; Deng, H.; Li, J.; Li, G.; Guo, F.; et al. pH-responsive flower-like micelles constructed via oxime linkage for anticancer drug delivery. Rsc Adv. 2014, 4, 48943–48951. [Google Scholar] [CrossRef]
- Deng, H.; Liu, J.; Zhao, X.; Zhang, Y.; Liu, J.; Xu, S.; Deng, L.; Dong, A.; Zhang, J. PEG-b-PCL CopolymerMicelles with the Ability of pH-Controlled Negative-to-Positive ChargeReversal for Intracellular Delivery of Doxorubicin. Biomacromolecules 2014, 15, 4281–4292. [Google Scholar] [CrossRef] [PubMed]
- Zloh, M.; Dinand, E.; Brocchini, S. Aconityl-derived polymers for biomedical applications. Modeling study of cis–trans isomerisation. Theor. Chem. Acc. 2003, 109, 206–212. [Google Scholar] [CrossRef]
- Liu, G.-Y.; Li, M.; Zhu, C.-S.; Jin, Q.; Zhang, Z.; Ji, J. Charge-conversional and pH-sensitive PEGylated polymeric micelles as efficient nanocarriers for drug delivery. Macromol. Bioence 2015, 14, 1280–1290. [Google Scholar] [CrossRef]
- Chen, J.; Ding, J.; Zhang, Y.; Xiao, C.; Zhuang, X.; Chen, X. Polyion complex micelles with gradient pH-sensitivity for adjustable intracellular drug delivery. Polym. Chem. 2014, 6, 397–405. [Google Scholar] [CrossRef]
- Shao, W.; Liu, C.; Ma, H.; Hong, Z.; Xie, Q.; Lu, Y. Fabrication of pH-sensitive thin-film nanocomposite nanofiltration membranes with enhanced performance by incorporating amine-functionalized graphene oxide. Appl. Surface Sci. 2019, 487, 1209–1221. [Google Scholar] [CrossRef]
- Banks, S.R.; Enck, K.; Wright, M.; Opara, E.C.; Welker, M.E. Chemical Modification of Alginate for Controlled Oral Drug Delivery. J. Agric. Food Chem. 2019, 67, 10481–10488. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Sun, C.; Yan, L. Galactose Targeted pH-Responsive Copolymer Conjugated with Near Infrared Fluorescence Probe for Imaging of Intelligent Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Su, T.; Zhang, L.; Liu, R.; Wang, G.; He, B.; Gu, Z. Polymeric micelles with citraconic amide as pH-sensitive bond in backbone for anticancer drug delivery. Int. J. Pharm. 2014, 471, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Thati, S.; Bagby, T.R.; Diab, H.-M.; Davies, N.M.; Cohen, M.S.; Forrest, M.L. Localized doxorubicin chemotherapy with a biopolymeric nanocarrier improves survival and reduces toxicity in xenografts of human breast cancer. J. Control. Release 2010, 146, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhou, X.; Jia, L.; Ma, C.; Song, R.; Deng, Y.; Hu, X.; Sun, W. Acetal-Linked Paclitaxel Polymeric Prodrug Based on Functionalized mPEG-PCL Diblock Polymer for pH-Triggered Drug Delivery. Polymers 2017, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Dimde, M.; Steinhilber, D.; Neumann, F.; Li, Y.; Paulus, F.; Ma, N.; Haag, R. Synthesis of pH-Cleavable dPG-Amines for Gene Delivery Application. Macromol. Biosci. 2017, 17, 1600190. [Google Scholar] [CrossRef]
- Gillies, E.R.; Goodwin, A.P.; Fréchet, J.M.J. Acetals as pH-sensitive linkages for drug delivery. Bioconjug. Chem. 2004, 15, 1254–1263. [Google Scholar] [CrossRef]
- Huang, F.; Cheng, R.; Meng, F.; Deng, C.; Zhong, Z. Micelles Based on Acid Degradable Poly(acetal urethane): Preparation, pH-Sensitivity, and Triggered Intracellular Drug Release. Biomacromolecules 2015, 16, 2228–2236. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Zhao, M.; Wu, L.; Luo, K.; Pu, Y.; He, B. Tumor-pH-Sensitive PLLA-Based Microsphere with Acid Cleavable Acetal Bonds on the Backbone for Efficient Localized Chemotherapy. Biomacromolecules 2018, 19, 3140–3148. [Google Scholar] [CrossRef]
- Masson, C.; Garinot, M.; Mignet, N.; Wetzer, B.; Mailhe, P.; Scherman, D.; Bessodes, M. pH-sensitive PEG lipids containing orthoester linkers: New potential tools for nonviral gene delivery. J Control Release 2004, 99, 423–434. [Google Scholar] [CrossRef]
- Thambi, T.; Deepagan, V.; Yoo, C.K.; Park, J.H. Synthesis and physicochemical characterization of amphiphilic block copolymers bearing acid-sensitive orthoester linkage as the drug carrier. Polymer 2011, 52, 4753–4759. [Google Scholar] [CrossRef]
- Du, H.; Liu, M.; Yang, X.; Zhai, G. The design of pH-sensitive chitosan-based formulations for gastrointestinal delivery. Drug Discov. Today 2015, 20, 1004–1011. [Google Scholar] [CrossRef]
- Xin-long, Z.; Xiu-feng, Z.; Da-wei, C.; Xiu-li, Z. Recent progress of design targeted vector pH responsive to tumor microenvironment. J. Shenyang Pharm. Univ. 2014, 31, 575–583. [Google Scholar]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef] [PubMed]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W.; Yang, J.; Zhou, C.; Sun, J. pH-sensitive polymeric micelles triggered drug release for extracellular and intracellular drug targeting delivery. Asian J. Pharm. Sci. 2013, 8, 159–167. [Google Scholar] [CrossRef]
- Torchilin, V.P. Nanotechnology for Intracellular Delivery and Targeting. Nanotechnol. Drug Deliv. 2009, 313–346. [Google Scholar] [CrossRef]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A Diagnostic Biomarker. J. Indian Soc. Periodontol. 2013, 17, 461–465. [Google Scholar] [CrossRef]
- Yin, Q.; Shen, J.; Zhang, Z.; Yu, H.; Li, Y. Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv. Drug Deliv. Rev. 2013, 65, 1699–1715. [Google Scholar] [CrossRef]
- Yasuhiro, M. The Drug Discovery by NanoMedicine and its Clinical Experience. Jpn. J. Clin. Oncol. 2014, 44, 515–525. [Google Scholar]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lane, L.A.; Nie, S. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J. Control. Release 2015, 219, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J.C.L. Gold Nanoparticles for Drug Delivery and Cancer Therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Mody, N.; Agrawal, U.; Vyas, S.P.; Sharma, R. Theranostic Nanomedicine; A Next Generation Platform for Cancer Diagnosis and Therapy. Mini Reviews Med. Chem. 2017, 17, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Oh, Y.-O.; Seo, H.; Oh, J. Marine Biopolymer-Based Nanomaterials as a Novel Platform for Theranostic Applications. Polym. Rev. 2017, 57, 631–667. [Google Scholar] [CrossRef]
- Popović, Z.; Liu, W.; Chauhan, V.P.; Lee, J.; Wong, C.; Greytak, A.B.; Insin, N.; Nocera, D.G.; Fukumura, D.; Jain, R.K.; et al. A nanoparticle size series for in vivo fluorescence imaging. Angew. Chem. 2010, 122, 8831–8834. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Zhang, M.; Tao, K.; Wang, B.; Lin, M.; Zhang, X.; Liu, Y.; Hou, Y.; Zhang, H.; et al. Tumor Microenvironment-Responsive Nanoshuttles with Sodium Citrate Modification for Hierarchical Targeting and Improved Tumor Theranostics. ACS Appl. Mater. Interfaces 2019, 11, 25730–25739. [Google Scholar] [CrossRef]
- Chan, C.-F.; Zhou, Y.; Guo, H.; Zhang, J.; Jiang, L.; Chen, W.; Shiu, K.-K.; Kwong, D.W.J.; Wong, K.-L. pH-Dependent Cancer-Directed Photodynamic Therapy by a Water-Soluble Graphitic-Phase Carbon Nitride–Porphyrin Nanoprobe. ChemPlusChem 2016, 81, 535–540. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Kohestanian, M.; Shirzad, M. Synthesis and characterization of magnetic hybrid nanomaterials via RAFT polymerization: A pH sensitive drug delivery system. Colloids Surf. B Biointerfaces 2019, 174, 153–160. [Google Scholar] [CrossRef]
- He, H.; Chen, S.; Zhou, J.; Dou, Y.; Song, L.; Che, L.; Zhou, X.; Chen, X.; Jia, Y.; Zhang, J.; et al. Cyclodextrin-derived pH-responsive nanoparticles for delivery of paclitaxel. Biomaterials 2013, 34, 5344–5358. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Li, Q.; Mei, L. pH-Sensitive nanoscale materials as robust drug delivery systems for cancer therapy. Chin. Chem. Lett. 2020, 31, 1345–1356. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, D.; Liu, J.; Kuang, Y.; Li, Q.; Zhang, M.; Ye, H.; Qin, H.; Xu, Y.; Li, C.; et al. pH-Sensitive drug delivery system based on modified dextrin coated mesoporous silica nanoparticles. Int. J. Biol. Macromol. 2016, 85, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.-X.; Li, D.; Zhuang, X.; Chen, X. Self-assemblies of pH-activatable PEGylated multiarm poly(lactic acid-co-glycolic acid)-doxorubicin prodrugs with improved long-term antitumor efficacies. Macromol. Biosci. 2013, 13, 1300–1307. [Google Scholar] [CrossRef]
- YYar, M.; Shahzad, S.; Siddiqi, S.A.; Mahmood, N.; Rauf, A.; Anwar, M.S.; Chaudhry, A.A.; Rehman, I.U. Triethyl orthoformate mediated a novel crosslinking method for the preparation of hydrogels for tissue engineering applications: Characterization and in vitro cytocompatibility analysis. Mater. Sci. Eng. C 2015, 56, 154–164. [Google Scholar] [CrossRef]
- Samanta, H.S.; Ray, S.K. Controlled Release of Tinidazole and Theophylline from Chitosan Based Composite Hydrogels. Carbohydr. Polym. 2014, 106, 109–120. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Hendi, A.; Hassan, M.U.; Elsherif, M.; Alqattan, B.; Park, S.; Yetisen, A.K.; Butt, H. Healthcare Applications of pH-Sensitive Hydrogel-Based Devices: A Review. Int. J. Nanomed. 2020, 15, 3887–3901. [Google Scholar] [CrossRef]
- Ana, H.B.; Ibrahim, F.C.; Joana, S.-C.; Rui, A.S.; Joaquim, M.O.; Rui, L.R. “Smart” Hydrogels in Tissue Engineering and Regenerative Medicine Applications. Handb. Intell. Scaffolds Regen. 2017, 333–367. [Google Scholar]
- Zhao, Y.; Lei, M.; Liu, S.-X.; Zhao, Q. Smart hydrogel-based optical fiber SPR sensor for pH measurements. Sens. Actuators B Chem. 2018, 261, 226–232. [Google Scholar] [CrossRef]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Moreddu, R.; Elsherif, M.; Butt, H.; Vigolo, D.; Yetisen, A.K. Contact lenses for continuous corneal temperature monitoring. RSC Adv. 2019, 9, 11433–11442. [Google Scholar] [CrossRef]
- Hunt, J.; Chen, R.; Van Veen, T.; Bryan, N. Hydrogels for tissue engineering and regenerative medicine. J. Mater. Chem. B 2014, 2, 5319–5338. [Google Scholar] [CrossRef] [PubMed]
- Elsherif, M.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Hydrogel optical fibers for continuous glucose monitoring. Biosens. Bioelectron. 2019, 137, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Etrych, T.; Jelınková, M.; ́Řı́hová, B.; Ulbrich, K. New HPMA copolymers containing doxorubicin bound via pH-sensitive linkage: Synthesis and preliminary in vitro and in vivo biological properties. J. Control. Release 2001, 73, 89–102. [Google Scholar] [CrossRef]
- Vashist, A.; Kaushik, A.; Alexis, K.; Jayant, R.D.; Sagar, V.; Vashist, A.; Nair, M. Bioresponsive Injectable Hydrogels for On-demand Drug Release and Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3595–3602. [Google Scholar] [CrossRef]
- Onaciu, A.; Munteanu, R.A.; Moldovan, C.S.; Berindan-Neagoe, I. Hydrogels Based Drug Delivery Synthesis, Characterization and Administration. Pharmaceutics 2019, 11, 432. [Google Scholar] [CrossRef]
- Li, X.; Su, X. Multifunctional smart hydrogels: Potential in tissue engineering and cancer therapy. J. Mater. Chem. B Mater. Biol. 2018, 6, 4714–4730. [Google Scholar] [CrossRef]
- Vemula, P.K.; Wiradharma, N.; Ankrum, J.A.; Miranda, O.R.; John, G.; Karp, J.M. Prodrugs as self-assembled hydrogels: A new paradigm for biomaterials. Curr. Opin. Biotechnol. 2013, 24, 1174–1182. [Google Scholar] [CrossRef]
- Wang, Y.; Cheetham, A.G.; Angacian, G.; Su, H.; Xie, L.; Cui, H. Peptide–drug conjugates as effective prodrug strategies for targeted delivery. Adv. Drug Deliv. Rev. 2017, 110, 112–126. [Google Scholar] [CrossRef]
- Oliva, N.; Conde, J.; Wang, K.; Artzi, N. Designing Hydrogels for On-Demand Therapy. Acc. Chem. Res. 2017, 50, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Tomar, L.K.; Tyagi, C.; Choonara, Y.E.; Kumar, P.; Pillay, V. Rheological and Swelling Behavior of pH Sensitive Hydrogel Particles. APCBEE Procedia 2014, 9, 192–196. [Google Scholar] [CrossRef]
- Yadav, H.K.S.; Shivakumar, H.G. In Vitro and In Vivo Evaluation of pH-Sensitive Hydrogels of Carboxymethyl Chitosan for Intestinal Delivery of Theophylline. Isrn Pharm. 2012, 2012, 1–9. [Google Scholar]
- Wu, R.-S.; Lin, J.; Xing, Y.-M.; Dai, Z.; Wang, L.-W.; Zhang, X.-P. PH-sensitive Black Phosphorous Incorporated Hydrogel as Novel Implant for Cancer Treatment. J. Pharm. Sci. 2019, 108, 2542–2551. [Google Scholar] [CrossRef]
- Qi, X.; Wei, W.; Li, J.; Zuo, G.; Pan, X.; Su, T.; Zhang, J.; Dong, W. Salecan-Based pH-Sensitive Hydrogels for Insulin Delivery. Mol. Pharm. 2017, 14, 431–440. [Google Scholar] [CrossRef]
- Qi, X.; Wei, W.; Li, J.; Zuo, G.; Pan, X.; Su, T.; Zhang, J.; Dong, W. Metabolic Study of Cancer Cells Using a pH Sensitive Hydrogel Nanofiber Light Addressable Potentiometric Sensor. ACS Sens. 2017, 2, 151–156. [Google Scholar]
- Pal, K.; Singh, V.K.; Anis, A.; Thakur, G.; Bhattacharya, M.K. Hydrogel-Based Controlled Release Formulations: Designing Considerations, Characterization Techniques and Applications. J. Macromol. Sci. Part D 2013, 52, 1391–1422. [Google Scholar] [CrossRef]
- Chitra, G.; Selvi, M.S.; Franklin, D.S.; Sudarsan, S.; Sakthivel, M.; Guhanathan, S. pH-sensitive biopolymeric hydrogel-based on indole-3-acetic acid for wound healing and anti-cancer applications. SN Appl. Ences 2019, 1, 1641. [Google Scholar] [CrossRef]
- Shim, J.K.; Lee, Y.B.; Lee, Y.M. pH-Dependent permeation through polysulfone ultrafiltration membranes prepared by ultraviolet polymerization technique. J. Appl. Polym. Sci. 1999, 74, 75–82. [Google Scholar] [CrossRef]
- Peng, T.; Cheng, Y.L. PNIPAAm and PMAA co-g rafted porous PE membranes:living radical co-grafting mechanism and multi-stimuli responsive permeability. Polymer 2001, 42, 2091–2100. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Md. Akil, H. Classification, Processing and Application of Hydrogels: A Review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From Controlled Release to pH-Responsive Drug Delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Sarkar, K.; Bhattacharya, S.; Bhattacharyya, A.; Mishra, R.; Kundu, P.P. pH sensitive N-succinyl chitosan grafted polyacrylamide hydrogel for oral insulin delivery. Carbohyd. Polym. 2014, 112, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Huang, W.; Jiang, L.; Lei, Z.; Li, X.; Deng, H. KGM and PMAA based pH-sensitive interpenetrating polymer network hydrogel for controlled drug release. Carbohydr. Polym. 2013, 97, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Lee, E.S.; Bae, Y.H. Self-Organized Nanogels Responding to Tumor Extracellular pH: pH-Dependent Drug Release and in Vitro Cytotoxicity against MCF-7 Cells. Bioconjug. Chem. 2007, 18, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Yuan, X.; Zhao, Y.; Zhang, W.; Ren, L. Temperature and pH Dual-Responsive Supramolecular Polymer Hydrogels Hybridized with Functional Inorganic Nanoparticles. Macromol. Chem. Phys. 2017, 218, 1600540. [Google Scholar] [CrossRef]
- Way, A.E.; Hsu, L.; Shanmuganathan, K.; Weder, C.; Rowan, S.J. pH-Responsive Cellulose Nanocrystal Gels and Nanocomposites. ACS Macro Lett. 2012, 1, 1001–1006. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Weissmann, G. The action of steroids and streptolysin S on the permeability of phospholipid structures to cations. J. Mol. Biol. 1965, 13, 253–259. [Google Scholar] [CrossRef]
- Sessa, G.; Weissmann, G. Phospholipid spherules (liposomes) as a model for biological membranes. J. Lipid Res. 1968, 9, 310–318. [Google Scholar]
- Gabizon, A.; Papahadjooulos, D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc. Natl. Acad. Sci. USA 1988, 85, 6949–6953. [Google Scholar] [CrossRef]
- Xing, H.; Hwang, K.; Lu, Y. Recent Developments of Liposomes as Nanocarriers for Theranostic Applications. Theranostics 2016, 6, 1336–1352. [Google Scholar] [CrossRef] [PubMed]
- Abu Lila, A.S.; Ishida, T. Liposomal Delivery Systems: Design Optimization and Current Applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Goyal, P.; Goyal, K.; Kumar, S.G.V.; Singh, A.; Katare, O.P.; Mishra, D.N. Liposomal drug delivery systems--Clinical applications. Acta Pharm. 2005, 55, 1–25. [Google Scholar] [PubMed]
- Al-Jamal, W.T.; Kostarelos, K. Liposomes: From a Clinically Established Drug Delivery System to a Nanoparticle Platform for Theranostic Nanomedicine. Acc. Chem. Res. 2011, 44, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- López-Sagaseta, J.; Malito, E.; Rappuoli, R.; Bottomley, M.J. Self-assembling protein nanoparticles in the design of vaccines. Comput. Struct. Biotechnol. J. 2016, 14, 58–68. [Google Scholar] [CrossRef]
- Basha, G.; I Novobrantseva, T.; Rosin, N.; Tam, Y.Y.C.; Hafez, I.M.; Wong, M.K.; Sugo, T.; Ruda, V.M.; Qin, J.; Klebanov, B.; et al. Influence of Cationic Lipid Composition on Gene Silencing Properties of Lipid Nanoparticle Formulations of siRNA in Antigen-Presenting Cells. Mol. Ther. 2011, 19, 2186–2200. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, X.; Zhang, X.; Liu, B.; Huang, L. Nanoparticles Modified With Tumor-targeting scFv Deliver siRNA and miRNA for Cancer Therapy. Mol. Ther. 2010, 18, 1650–1656. [Google Scholar] [CrossRef]
- Ma, Y.; Zhuang, Y.; Xie, X.; Wang, C.; Wang, F.; Zhou, D.; Zeng, J.; Cai, L. The role of surface charge density in cationic liposome-promoted dendritic cell maturation and vaccine-induced immune responses. Nanoscale 2011, 3, 2307–2314. [Google Scholar] [CrossRef]
- Tagami, T.; Foltz, W.D.; Ernsting, M.J.; Lee, C.M.; Tannock, I.F.; May, J.; Li, S.-D. MRI monitoring of intratumoral drug delivery and prediction of the therapeutic effect with a multifunctional thermosensitive liposome. Biomaterials 2011, 32, 6570–6578. [Google Scholar] [CrossRef]
- Ren, L.; Chen, S.; Li, H.; Zhang, Z.; Ye, C.; Liu, M.; Zhou, X. MRI-visible liposome nanovehicles for potential tumor-targeted delivery of multimodal therapies. Nanoscale 2015, 7, 12843–12850. [Google Scholar] [CrossRef] [PubMed]
- Kaasgaard, T.A.; Thomas, L. Liposomal cancer therapy: Exploiting tumor characteristics. Expert Opin. Drug Deliv. 2010, 7, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Kierstead, P.H.; Okochi, H.; Venditto, V.J.; Chuong, T.C.; Kivimae, S.; Fréchet, J.M.; Szoka, F.C. The effect of polymer backbone chemistry on the induction of the accelerated blood clearance in polymer modified liposomes. J. Control. Release 2015, 213, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Akita, H.; Harashima, H. The Polyethyleneglycol Dilemma: Advantage and Disadvantage of PEGylation of Liposomes for Systemic Genes and Nucleic Acids Delivery to Tumors. Biol. Pharm. Bull. 2013, 36, 892–899. [Google Scholar] [CrossRef]
- Kang, H.; O’Donoghue, M.B.; Liu, H.; Tan, W. A liposome-based nanostructure for aptamer directed delivery. Chem. Commun. 2009, 46, 249–251. [Google Scholar] [CrossRef]
- Sonali, L.J.; Singh, R.P.; Singh, N.; Sharma, G.; Vijayakumar, M.R.; Koch, B.; Singh, S.; Singh, U.; Dash, D.; Pandey, B.L.; et al. Transferrin liposomes of docetaxel for brain-targeted cancer applications: Formulation and brain theranostics. Drug Deliv. 2016, 23, 1261–1271. [Google Scholar] [CrossRef]
- Xu, H.; Paxton, J.; Wu, Z. Enhanced pH-Responsiveness, Cellular Trafficking, Cytotoxicity and Long-circulation of PEGylated Liposomes with Post-insertion Technique Using Gemcitabine as a Model Drug. Pharm. Res. 2015, 32, 2428–2438. [Google Scholar] [CrossRef]
- Li, Y.; Liu, R.; Yang, J.; Shi, Y.; Ma, G.; Zhang, Z.; Zhang, X. Enhanced retention and anti-tumor efficacy of liposomes by changing their cellular uptake and pharmacokinetics behavior. Biomaterials 2015, 41, 1–14. [Google Scholar] [CrossRef]
- Andresen, T.L.; Jensen, S.S.; Jørgensen, K. Advanced Strategies in Liposomal Cancer Therapy: Problems and Prospects of Active and Tumor Specific Drug Release. Prog. Lipid Res. 2005, 44, 68–97. [Google Scholar] [CrossRef]
- Yu, B.; Tai, H.C.; Xue, W.; Lee, L.J.; Lee, R.J. Receptor-Targeted Nanocarriers for Therapeutic Delivery to Cancer. Mol. Membr. Biol. 2010, 27, 286–298. [Google Scholar] [CrossRef]
- Zhu, L.; Kate, P.; Torchilin, V.P. Matrix Metalloprotease 2-Responsive Multifunctional Liposomal Nanocarrier for Enhanced Tumor Targeting. Acs Nano 2012, 6, 3491–3498. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv. Drug Deliv. Rev. 2011, 63, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, S.R.; Paliwal, R.; Vyas, S.P. A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery. Drug Deliv. 2015, 22, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.S.R.; Karanth, H. pH-sensitive liposomes--principle and application in cancer therapy. J. Pharm. Pharmacol. 2007, 59, 469–483. [Google Scholar]
- Xu, H.; Hu, M.; Yu, X.; Li, Y.; Fu, Y.; Zhou, X.; Zhang, D.; Li, J. Design and evaluation of pH-sensitive liposomes constructed by poly(2-ethyl-2-oxazoline)-cholesterol hemisuccinate for doxorubicin delivery. Eur. J. Pharm. Biopharm. 2015, 91, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Sun, Q.; Xue, J.; Li, N.; Li, W.; Zhang, C.; Ping, Q. Drug Delivery: Multistage pH-Responsive Liposomes for Mitochondrial-Targeted Anticancer Drug Delivery (Adv. Mater. 27/2012). Adv. Mater. 2012, 24, 3659–3665. [Google Scholar] [CrossRef]
- Himanshu, P.; Radha, R.; Vishnu, A. Liposome and Their Applications in Cancer Therapy. Braz. Arch. Biol. Technol. 2016, 59, e16150477. [Google Scholar]
- Hong, M.-S.; Lim, S.-J.; Oh, Y.-K.; Kim, C.-K. pH-Sensitive, SerumStable and Long-circulating Liposomes as a New Drug Delivery System. J. Pharm. Pharmacol. 2002, 54, 51–58. [Google Scholar] [CrossRef]
- Ferreira, D.D.S.; Lopes, S.C.; Franco, M.S.; Oliveira, M.C. pH-sensitive liposomes for drug delivery in cancer treatment. Ther. Deliv. 2013, 4, 1099–1123. [Google Scholar] [CrossRef]
- Ramishetti, S.; Leaf, H. Intelligent design of multifunctional lipid-coated nanoparticle platforms for cancer therapy. Ther. Deliv. 2012, 3, 1429–1445. [Google Scholar] [CrossRef]
- Kaul, A.; Chaturvedi, S.; Attri, A.; Kalra, M.; Mishra, A.K. Targeted theranostic liposomes: Rifampicin and ofloxacin loaded pegylated liposomes for theranostic application in mycobacterial infections. Rsc Adv. 2016, 6, 28919–28926. [Google Scholar] [CrossRef]
- Lozano, N.; Al-Ahmady, Z.S.; Beziere, N.S.; Ntziachristos, V.; Kostarelos, K. Monoclonal antibody-targeted PEGylated liposome-ICG encapsulating doxorubicin as a potential theranostic agent. Int. J. Pharm. 2015, 482, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-S.; Choi, M.-J.; Cheong, H.-S.; Kim, K. Development of Th1-Mediated CD8+ Effector T Cells by Vaccination with Epitope Peptides Encapsulated in pH-Sensitive Liposomes. Vaccine 2001, 19, 3608–3614. [Google Scholar] [CrossRef]
- Sethuraman, V.A.; Lee, M.C.; Bae, Y.H. A Biodegradable pH-sensitive Micelle System for Targeting Acidic Solid Tumors. Pharm. Res. 2008, 25, 657–666. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, L.; Liu, T.; Zhang, L.; Yao, Y.; Yu, D.; Wang, L.; Zhang, N. Multifunctional pH-sensitive polymeric nanoparticles for theranostics evaluated experimentally in cancer. Nanoscale 2014, 6, 3231–3242. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qiu, L.; Chen, Q.; Hao, T.; Qiao, M.; Zhao, H.; Zhang, J.; Hu, H.; Zhao, X.; Chen, D.; et al. pH-sensitive nanoparticles of poly(l-histidine)–poly(lactide-co-glycolide)–tocopheryl polyethylene glycol succinate for anti-tumor drug delivery. Acta Biomater. 2015, 11, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Kanapathipillai, M.; Brock, A.; Ingber, D.E. Nanoparticle Targeting of Anti-Cancer Drugs that Alter Intracellular Signaling or Influence the Tumor Microenvironment. Dvanced Drug Deliv. Rev. 2014, 79, 107–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Pan, H.; Liu, L.; Ji, M.; Sheng, N.; Wang, C.; Cai, L.; Ma, Y. Retinal-Conjugated pHSensitive Micelles Induce Tumor Senescence for Boosting Breast Cancer Chemotherapy. Biomaterials 2016, 83, 219–232. [Google Scholar] [CrossRef]
- Nishiyama, N.; Kataoka, K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006, 112, 630–648. [Google Scholar] [CrossRef]
- Wang, J.; Mao, W.; Lock, L.L.; Tang, J.; Sui, M.; Sun, W.; Cui, H.; Xu, D.; Shen, Y. The role of micelle size in tumor accumulation, penetration, and treatment. ACS Nano 2015, 9, 7195–7206. [Google Scholar] [CrossRef]
- Cheng, J.; Ji, R.; Gao, S.-J.; Du, F.-S.; Li, Z.-C. Facile Synthesis of Acid-Labile Polymers with Pendent Ortho Esters. Biomacromolecules 2012, 13, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, P.; Song, Q.; Gong, N.; Yang, L.; Wu, W.D. Surface charge-reversible polyelectrolyte complex nanoparticles for hepatoma-targeting delivery of doxorubicin. J. Mater. Chem. B 2015, 3, 6185–6193. [Google Scholar] [CrossRef] [PubMed]
- Harashima, H.; Ito, E.; Akita, H.; Oishi, M.; Nagasaki, Y.; Futaki, S.; Harashima, H. A pH-sensitive fusogenic peptide facilitates endosomal escape and greatly enhances the gene silencing of siRNA-containing nanoparticles in vitro and in vivo. J. Control. Release 2009, 139, 127–132. [Google Scholar]
- Fei, L.; Yap, L.-P.; Conti, P.S.; Shen, W.-C.; Zaro, J.L. Tumor targeting of a cell penetrating peptide by fusing with a pH-sensitive histidine-glutamate co-oligopeptide. Biomaterials 2014, 35, 4082–4087. [Google Scholar] [CrossRef]
- Li, M.; Lv, S.; Tang, Z.; Song, W.; Yu, H.; Sun, H.; Liu, H.; Chen, X. Polypeptide/doxorubicin hydrochloride polymersomes prepared through organic solvent-free technique as a smart drug delivery platform. Macromol. Bioence 2013, 13, 1150–1162. [Google Scholar] [CrossRef]
- Elena, R.; Stefano, S.; Francesca, M.; Sara, B.; Elena, G.; Paolo, C. pH-responsive lipid core micelles for tumour targeting. Eur. J. Pharm. Biopharm. 2013, 83, 346–357. [Google Scholar]
- Taghizadeh, B.; Taranejoo, S.; Monemian, S.A.; Moghaddam, Z.S.; Daliri, K.; Derakhshankhah, H.; Derakhshani, Z. Classification of stimuli–responsive polymers as anticancer drug delivery systems. Drug Deliv. 2015, 22, 145–155. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Zhao, B.; Li, Z.D.; Lin, W.J.; Zhang, C.Y.; Guo, X.D.; Wang, J.F.; Zhang, L. pH-sensitive micelles self-assembled from multi-arm star triblock co-polymers poly(ε-caprolactone)-b-poly(2-(diethylamino)ethyl methacrylate)-b-poly(poly(ethylene glycol) methyl ether methacrylate) for controlled anticancer drug delivery. Acta Biomater. 2013, 9, 7679–7690. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Li, Y.; Yuan, L.; Zhou, Y.; Li, J.; Zhao, L.; Zhang, C.; Li, X.; Liu, Y. PSMA-mediated endosome escape-accelerating polymeric micelles for targeted therapy of prostate cancer and the real time tracing of their intracellular trafficking. Nanoscale 2015, 7, 597–612. [Google Scholar] [CrossRef]
- Gillies, E.R.; Fréchet, J.M.J. A new approach towards acid sensitive copolymer micelles for drug delivery. Chem. Commun. 2003, 14, 1640–1641. [Google Scholar] [CrossRef]
- Song, N.; Zhou, L.; Li, J.; Pan, Z.; He, X.; Tan, H.; Wan, X.; Li, J.; Ran, R.; Fu, Q. Inspired by nonenveloped viruses escaping from endo-lysosomes: A pH-sensitive polyurethane micelle for effective intracellular trafficking. Nanoscale 2016, 8, 7711–7722. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Park, S.Y.; Mok, H.; Park, T.G. Synthesis, characterization, antitumor activity of pluronic mimicking copolymer micelles conjugated with doxorubicin via acid-cleavable linkage. Bioconjugate Chem. 2008, 19, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Gu, J.; Qu, X.; Yang, Z. Preparation of Multifunctional Drug Carrier for Tumor-Specific Uptake and Enhanced Intracellular Delivery through the Conjugation of Weak Acid Labile Linker. Bioconjugate Chem. 2009, 20, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Nishiyama, N.; Fukushima, S.; Koyama, H.; Yasuhiro, M.; Kataoka, K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: Tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjug. Chem. 2005, 16, 122–130. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, L.; Yang, Y.; Xu, X.; Huang, Y. Tumor targeting by pH-sensitive, biodegradable, cross-linked N-(2-hydroxypropyl) methacrylamide copolymer micelles. Biomaterials 2014, 35, 6622–6635. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Li, C.; Wang, D.; Gao, Y.; Zhang, C.; Zhao, L.; Li, Y.; Liu, Y.; Li, X. Poly(2-ethyl-2-oxazoline)–Doxorubicin Conjugate-Based Dual Endosomal pH-Sensitive Micelles with Enhanced Antitumor Efficacy. Bioconjug. Chem. 2014, 26, 110–119. [Google Scholar] [CrossRef]
- Varshosaz, J.; Hassanzadeh, F.; Sadeghi-Aliabadi, H.; Larian, Z.; Rostami, M. Synthesis of Pluronic® F127-poly (methyl vinyl ether-alt-maleic acid) copolymer and production of its micelles for doxorubicin delivery in breast cancer. Chem. Eng. J. 2014, 240, 133–146. [Google Scholar] [CrossRef]
- Tang, R.; Ji, W.; Wang, C. Amphiphilic block copolymers bearing ortho ester side-chains: pH-dependent hydrolysis and self-assembly in water. Macromol. Bioence 2010, 10, 192–201. [Google Scholar] [CrossRef]
- Gregory, G. Incorporation of Poly(Ethylene Glycol) Lipid into Lipoplexes: On-Line Incorporation Assessment and Pharmacokinetics Advantages. Liposome Technol. 2006, 273–292. [Google Scholar]
- Wu, Y.; Chen, W.; Meng, F.; Wang, Z.; Cheng, R.; Deng, C.; Liu, H.; Zhong, Z. Core-crosslinked pH-sensitive degradable micelles: A promising approach to resolve the extracellular stability versus intracellular drug release dilemma. J. Control. Release 2012, 164, 338–345. [Google Scholar] [CrossRef]
- Peeler, D.J.; Thai, S.N.; Cheng, Y.; Horner, P.J.; Sellers, D.L.; Pun, S.H. pH-sensitive polymer micelles provide selective and potentiated lytic capacity to venom peptides for effective intracellular delivery. Biomaterials 2019, 192, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Meng, F.; Wang, C.; Cheng, R.; Liu, Z.; Zhong, Z. Folate-conjugated crosslinked biodegradable micelles for receptor-mediated delivery of paclitaxel. J. Mater. Chem. 2011, 21, 5786–5794. [Google Scholar] [CrossRef]






| pH-Sensitive Bonds | Chemical Mechanisms | Applications in Ref. |
|---|---|---|
| Imine |  | [34,35,36,37,38,39,40,41,42] |
| Hydrazone |  | [43,44,45,46,47,48,49,50,51] |
| Oxime |  | [52,53,54,55,56] |
| Amide |  | [57,58,59,60,61,62,63,64,65] |
| Acetals |  | [66,67,68,69,70] |
| Orthoester |  | [71,72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuo, S.; Zhang, F.; Yu, J.; Zhang, X.; Yang, G.; Liu, X. pH-Sensitive Biomaterials for Drug Delivery. Molecules 2020, 25, 5649. https://doi.org/10.3390/molecules25235649
Zhuo S, Zhang F, Yu J, Zhang X, Yang G, Liu X. pH-Sensitive Biomaterials for Drug Delivery. Molecules. 2020; 25(23):5649. https://doi.org/10.3390/molecules25235649
Chicago/Turabian StyleZhuo, Shijie, Feng Zhang, Junyu Yu, Xican Zhang, Guangbao Yang, and Xiaowen Liu. 2020. "pH-Sensitive Biomaterials for Drug Delivery" Molecules 25, no. 23: 5649. https://doi.org/10.3390/molecules25235649
APA StyleZhuo, S., Zhang, F., Yu, J., Zhang, X., Yang, G., & Liu, X. (2020). pH-Sensitive Biomaterials for Drug Delivery. Molecules, 25(23), 5649. https://doi.org/10.3390/molecules25235649





