From 1D to 2D Cd(II) and Zn(II) Coordination Networks by Replacing Monocarboxylate with Dicarboxylates in Partnership with Azine Ligands: Synthesis, Crystal Structures, Inclusion, and Emission Properties
Abstract
1. Introduction
2. Results and Discussion
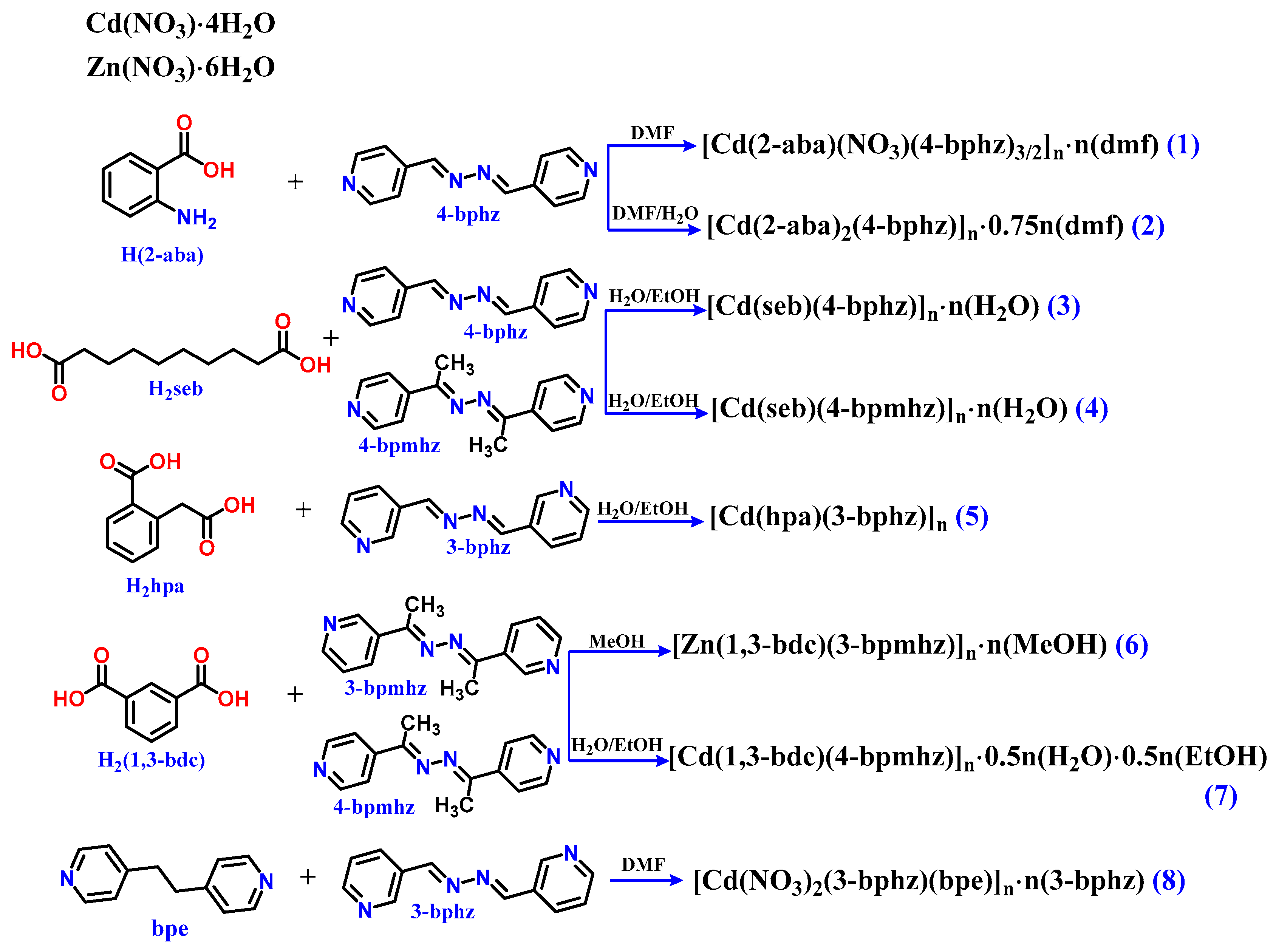
2.1. Generals
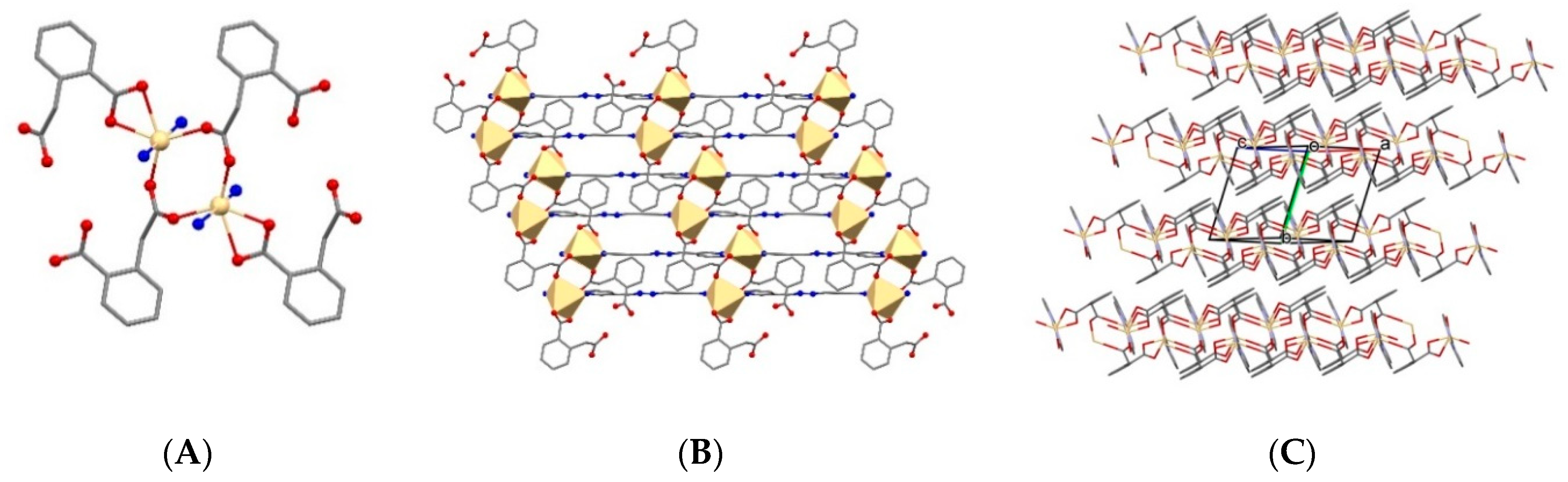
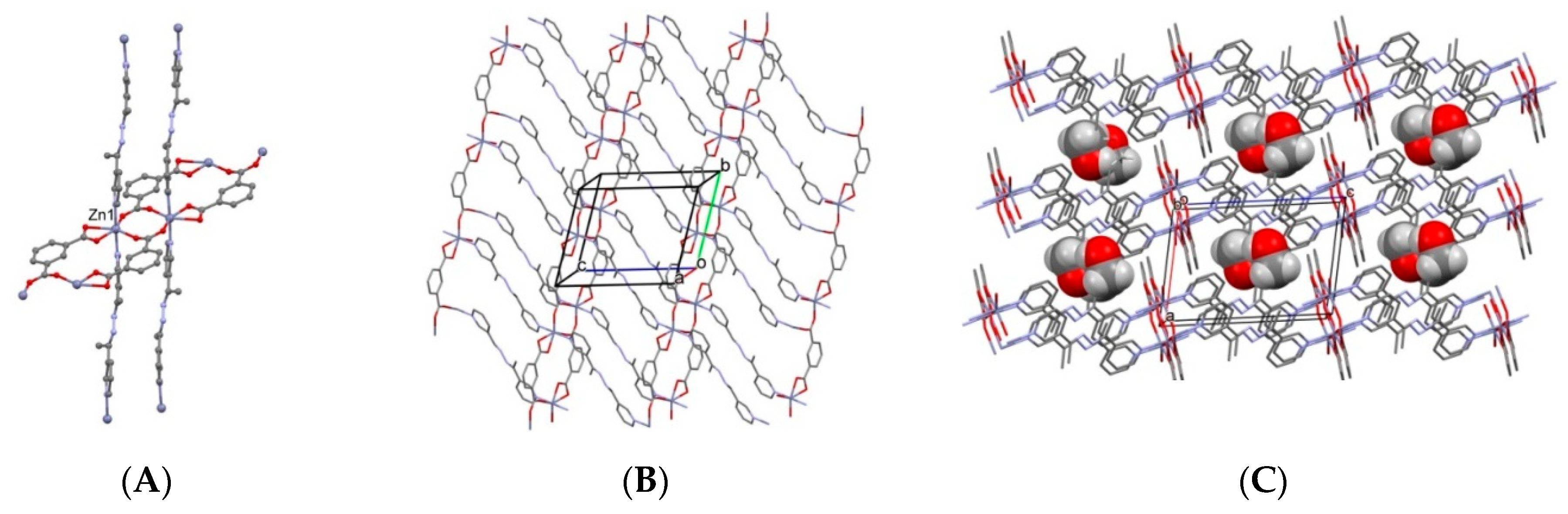
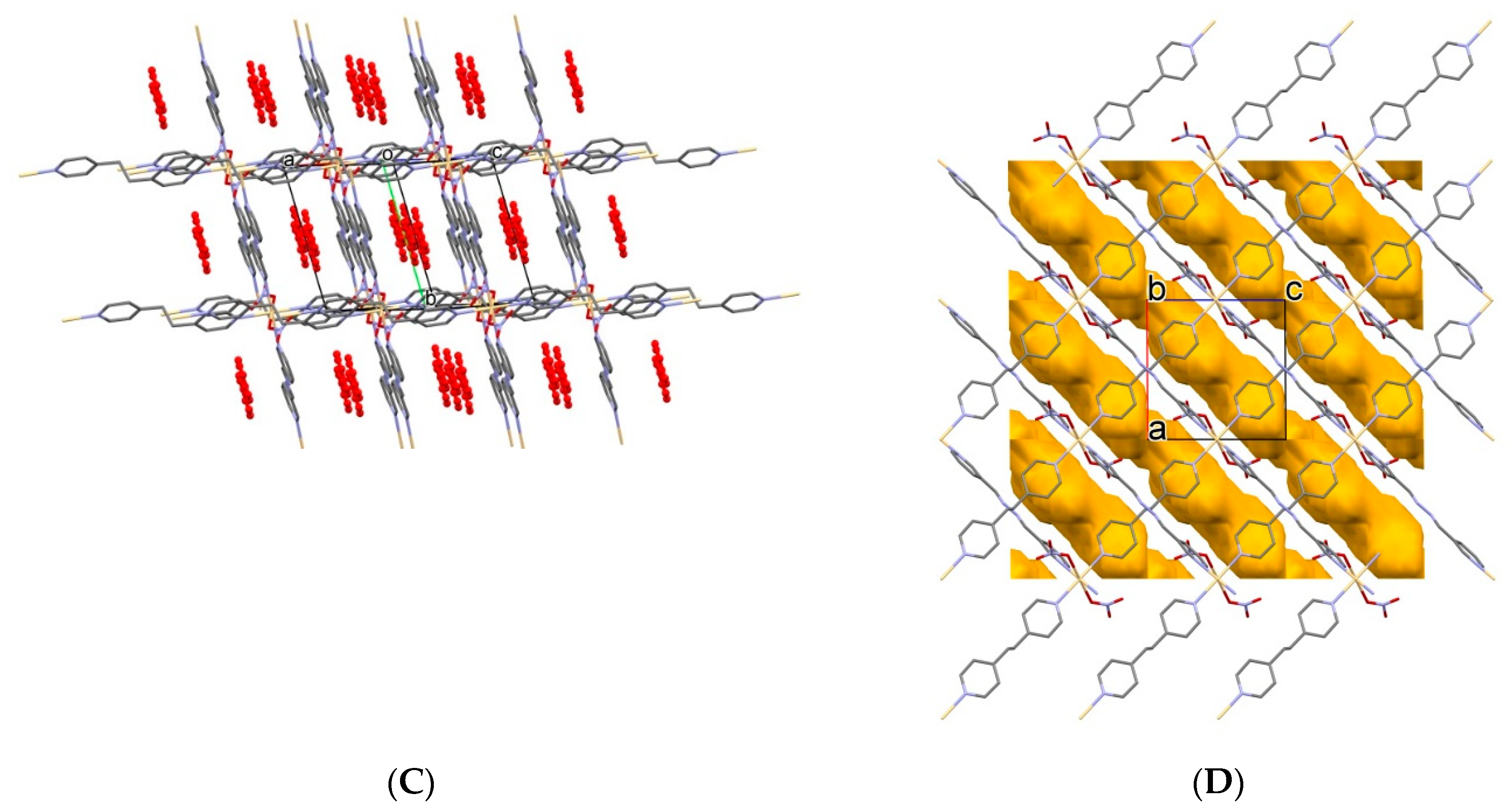
2.2. Description of Crystal Structures
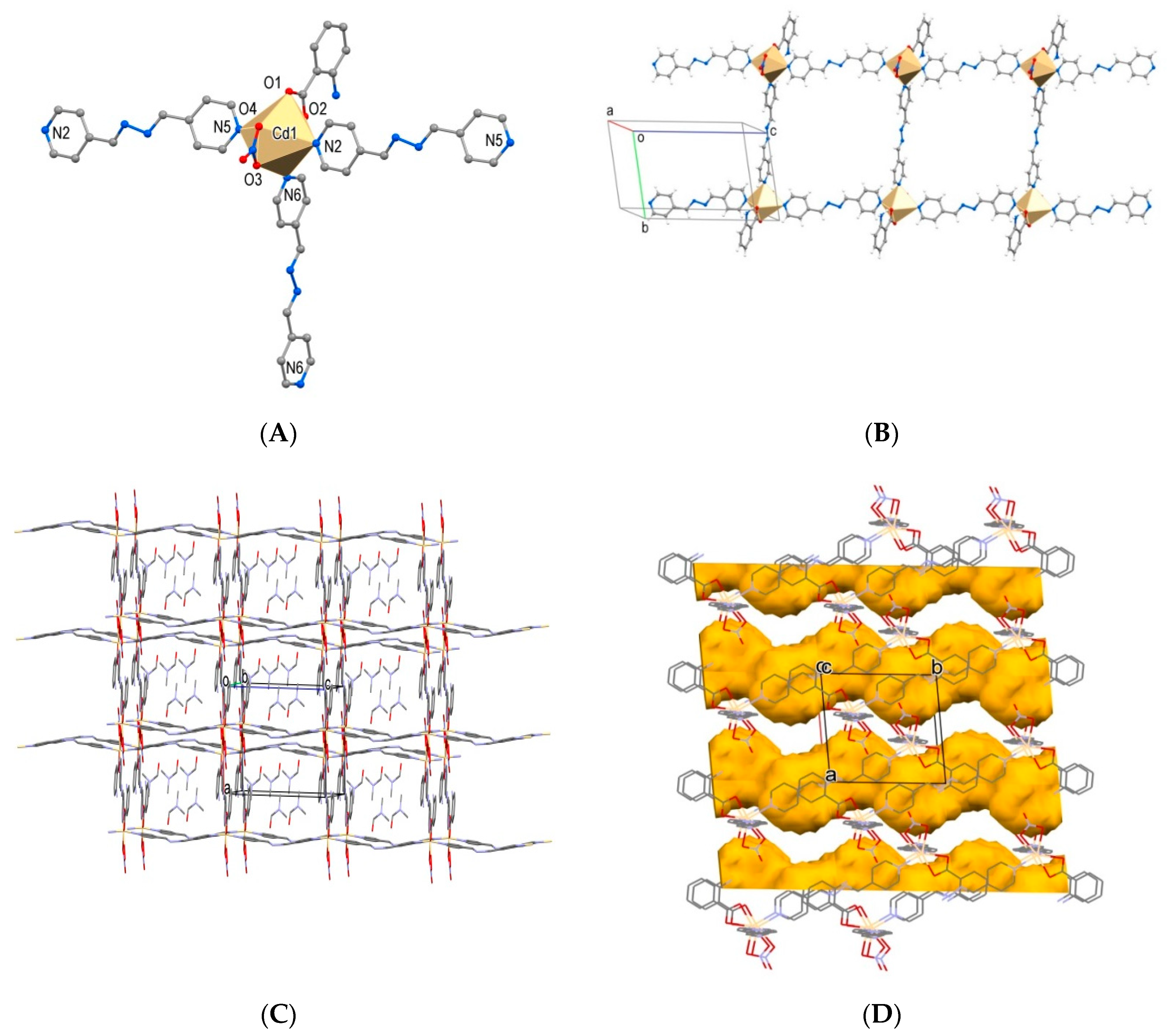
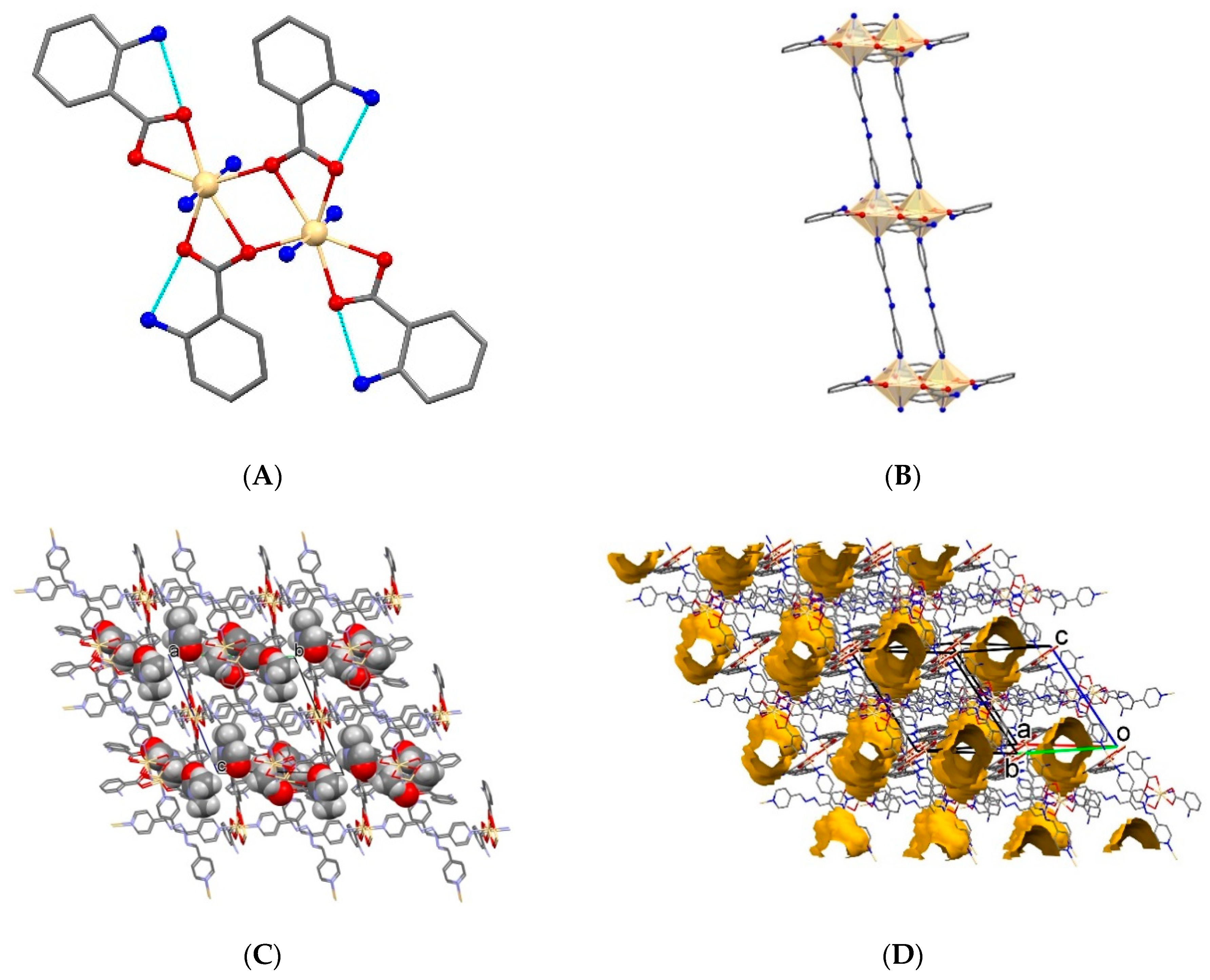
2.2.1. 1D Coordination Polymers
2.2.2. 2D Coordination Polymers
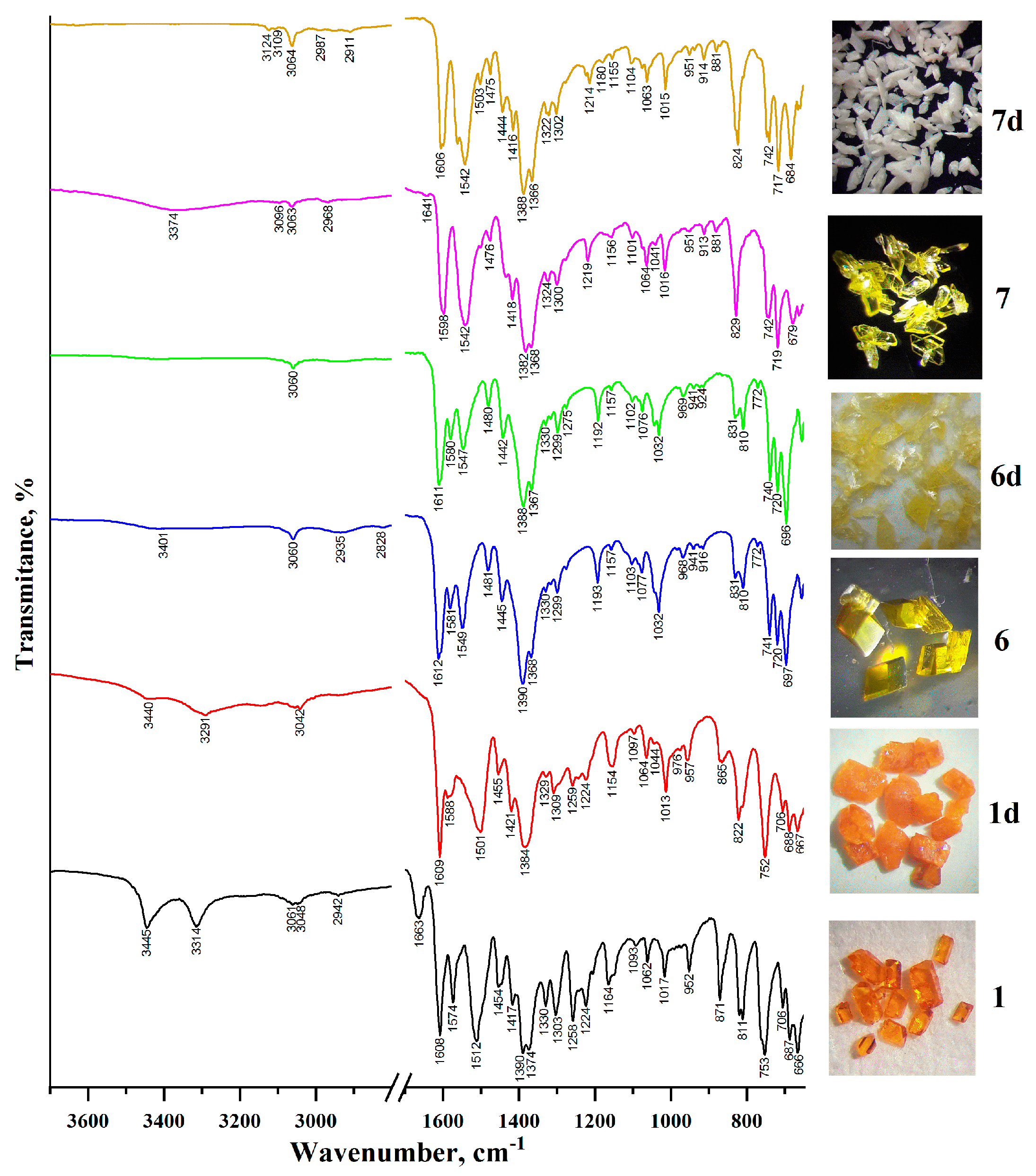
2.3. IR Spectroscopic Characterization
2.4. Desolvation, Solvent-Exchange, and Adsorption Properties
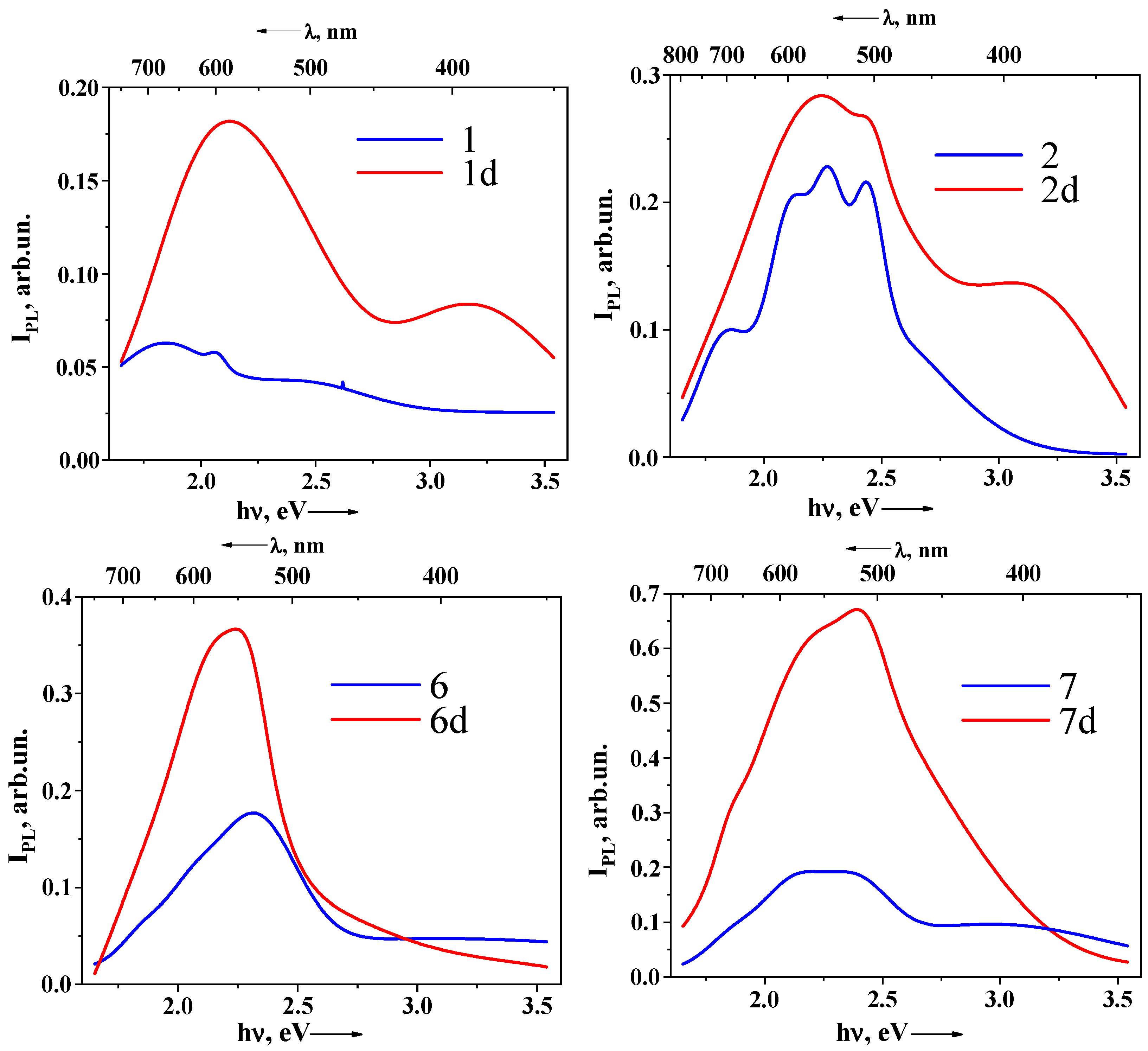
2.5. Photoluminescence Properties
3. Materials and Methods
4. X-ray Crystallography
5. Synthetic Procedures
Synthesis of the Schiff-Base Ligands
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal-organic framework. Nat. Cell Biol. 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Morris, R.E.; Wheatley, P.S. Gas Storage in Nanoporous Materials. Angew. Chem. Int. Ed. 2008, 47, 4966–4981. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Liu, M.-L.; Zhang, J.; Shi, W.; Cheng, P.; Liao, D.-Z.; Yan, S.-P. 1D, 2D and 3D luminescent zinc(II) coordination polymers assembled from varying flexible thioether ligands. Dalton Trans. 2008, 35, 4711–4713. [Google Scholar] [CrossRef] [PubMed]
- Henschel, A.; Gedrich, K.; Kraehnert, R.; Kaskel, S. Catalytic properties of MIL-101. Chem. Commun. 2008, 35, 4192–4194. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.-Q.; Zou, R.; Du, M.; Jiang, L.; Yamada, T.; Maruta, G.; Takeda, S.; Xu, Q. Metal–organic coordination architectures with 3-pyridin-3-yl-benzoate: Crystal structures, fluorescent emission and magnetic properties. CrystEngComm 2008, 10, 605–613. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Y.-Y.; Li, Z.-J.; Lin, Q.-P.; Cheng, J.-K.; Zhang, J.; Yao, Y.-G. Topology Analysis and Nonlinear-Optical-Active Properties of Luminescent Metal-Organic Framework Materials Based on Zinc/Lead Isophthalates. Inorg. Chem. 2008, 47, 8286–8293. [Google Scholar] [CrossRef]
- Alvaro, M.; Carbonell, E.; Ferrer, B.; I Xamena, F.X.L.; García, H. Semiconductor Behavior of a Metal-Organic Framework (MOF). Chem. Eur. J. 2007, 13, 5106–5112. [Google Scholar] [CrossRef]
- Seo, J.; Sakamoto, H.; Matsuda, R.; Kitagawa, S. Chemistry of porous coordination polymers having multimodal nanospace and their multimodal functionality. J. Nanosci. Nanotechnol. 2010, 10, 3–20. [Google Scholar] [CrossRef]
- Guo, X.; Geng, S.; Zhuo, M.; Chen, Y.; Zaworotko, M.J.; Cheng, P.; Zhang, Z. The utility of the template effect in metal-organic frameworks. Coord. Chem. Rev. 2019, 391, 44–68. [Google Scholar] [CrossRef]
- Li, C.; Wang, K.; Li, J.; Zhang, Q. Recent Progress in Stimulus-Responsive Two-Dimensional Metal-Organic Frameworks. ACS Mater. Lett. 2020, 2, 779–797. [Google Scholar] [CrossRef]
- Kumar, N.; Wang, S.-Q.; Mukherjee, S.; Bezrukov, A.A.; Patyk-Kaźmierczak, E.; O′Nolan, D.; Kumar, A.; Yu, M.-H.; Chang, Z.; Bu, X.-H.; et al. Crystal engineering of a rectangular sql coordination network to enable xylenes selectivity over ethylbenzene. Chem. Sci. 2020, 11, 6889–6895. [Google Scholar] [CrossRef] [PubMed]
- Martí-Rujas, J.; Bonafede, S.; Tushi, D.; Cametti, M. Multiple single-crystal-to-single-crystal guest exchange in a dynamic 1D coordination polymer. Chem. Commun. 2015, 51, 12357–12360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Application; RSC Publishing: Cambridge, UK, 2008. [Google Scholar]
- Li, X.; Cao, R.; Sun, Y.; Bi, W.; Li, X.; Wang, Y. Syntheses and Characterizations of Multidimensional Metal-Organic Frameworks Based on Rings and 1D Chains. Eur. J. Inorg. Chem. 2005, 2005, 321–329. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Su, Z.-M.; Wang, Y.; Fu, Y.-M.; Liu, S.-D.; Li, P. Two novel lead-carboxylate complexes based on nicotinic acid N-oxide: Synthesis, crystal structures and luminescent properties. Inorg. Chem. Commun. 2007, 10, 410–414. [Google Scholar] [CrossRef]
- Wei, Y.; Hou, H.; Li, L.; Fan, Y.; Zhu, Y. From Dicarboxylic Acid to Tetranuclear Metallamacrocyclic Complex and 1D and 2D Polymers. Cryst. Growth Des. 2005, 5, 1405–1413. [Google Scholar] [CrossRef]
- Stavila, V.; Davidovich, R.L.; Gulea, A.; Whitmire, K.H. Bismuth(III) complexes with aminopolycarboxylate and polyaminopolycarboxylate ligands: Chemistry and structure. Coord. Chem. Rev. 2006, 250, 2782–2810. [Google Scholar] [CrossRef]
- Uemura, K.; Yamasaki, Y.; Onishi, F.; Kita, H.; Ebihara, M. Two-Step Adsorption on Jungle-Gym-Type Porous Coordination Polymers: Dependence on Hydrogen-Bonding Capability of Adsorbates, Ligand-Substituent Effect, and Temperature. Inorg. Chem. 2010, 49, 10133–10143. [Google Scholar] [CrossRef]
- Andruh, M. Compartmental Schiff-base ligands—A rich library of tectons in designing magnetic and luminescent materials. Chem. Commun. 2011, 47, 3025–3042. [Google Scholar] [CrossRef]
- Ciurtin, D.M.; Dong, Y.-B.; Smith, M.D.; Barclay, A.T.; Loye, H.-C.Z. Two Versatile N,N′-Bipyridine-Type Ligands for Preparing Organic-Inorganic Coordination Polymers: New Cobalt- and Nickel-Containing Framework Materials. Inorg. Chem. 2001, 40, 2825–2834. [Google Scholar] [CrossRef]
- Halder, A.; Bhattacharya, B.; Haque, F.; Ghoshal, D. Structural Diversity in Six Mixed Ligand Zn(II) Metal-Organic Frameworks Constructed by Rigid and Flexible Dicarboxylates and Different N,N′ Donor Ligands. Cryst. Growth Des. 2017, 17, 6613–6624. [Google Scholar] [CrossRef]
- Hijikata, Y.; Horike, S.; Sugimoto, M.; Sato, H.; Matsuda, R.; Kitagawa, S. Relationship between Channel and Sorption Properties in Coordination Polymers with Interdigitated Structures. Chem. Eur. J. 2011, 17, 5138–5144. [Google Scholar] [CrossRef] [PubMed]
- Horike, S.; Tanaka, D.; Nakagawa, K.; Kitagawa, S. Selective guest sorption in an interdigitated porous framework with hydrophobic pore surfaces. Chem. Commun. 2007, 3395–3397. [Google Scholar] [CrossRef] [PubMed]
- Kanoo, P.; Mostafa, G.; Matsuda, R.; Kitagawa, S.; Maji, T.K. A pillared-bilayer porous coordination polymer with a 1D channel and a 2D interlayer space, showing unique gas and vapor sorption. Chem. Commun. 2011, 47, 8106. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Chand, S.; Senthilkumar, S.; Neogi, S.; Das, M.C. Structural variation of transition metal coordination polymers based on bent carboxylate and flexible spacer ligand: Polymorphism, gas adsorption and SC-SC transmetallation. CrystEngComm 2016, 18, 4323–4335. [Google Scholar] [CrossRef]
- Lozovan, V.; Kravtsov, V.C.; Coropceanu, E.B.; Siminel, N.; Kulikova, O.V.; Costriucova, N.V.; Fonari, M.S. Seven Zn(II) and Cd(II) 1D coordination polymers based on azine donor linkers and decorated with 2-thiophenecarboxylate: Syntheses, structural parallels, Hirshfeld surface analysis, and spectroscopic and inclusion properties. Polyhedron 2020, 188, 114702. [Google Scholar] [CrossRef]
- Carlucci, L.; Ciani, G.; Proserpio, D.M.; Mitina, T.G.; Blatov, V.A. Entangled Two-Dimensional Coordination Networks: A General Survey. Chem. Rev. 2014, 114, 7557–7580. [Google Scholar] [CrossRef] [PubMed]
- Croitor, L.; Coropceanu, E.B.; Duca, G.; Siminel, A.V.; Fonari, M.S. Nine Mn(II), Zn(II) and Cd(II) mixed-ligand coordination networks with rigid dicarboxylate and pyridine-n-aldoxime ligands: Impact of the second ligand in the structures′ dimensionality and solvent capacity. Polyhedron 2017, 129, 9–21. [Google Scholar] [CrossRef]
- Lozovan, V.; Kravtsov, V.C.; Gorincioi, E.; Rotaru, A.; Coropceanu, E.B.; Siminel, N.; Fonari, M.S. Chromism, positional, conformational and structural isomerism in a series of Zn(II) and Cd(II) coordination polymers based on methylated azine N,N′-donor linkers. Polyhedron 2020, 180, 114411. [Google Scholar] [CrossRef]
- Lozovan, V.; Kravtsov, V.C.; Coropceanu, E.; Siminel, A.V.; Kulikova, O.V.; Costriucova, N.V.; Fonari, M.S. Water-sulfate anion interplay in the evolution of solid state architectures and emission properties of Zn and Cd coordination networks with four azine ligands. J. Solid State Chem. 2020, 286, 121312. [Google Scholar] [CrossRef]
- Lozovan, V.; Coropceanu, E.; Bourosh, P.N.; Micu, A.; Fonari, M.S. Coordination Polymers of Zn and Cd Based on Two Isomeric Azine Ligands: Synthesis, Crystal Structures, and Luminescence Properties. Russ. J. Coord. Chem. 2019, 45, 11–21. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Maity, D.K.; Mondal, R.; Colacio, E.; Ghoshal, D. Two Series of Isostructural Coordination Polymers with Isomeric Benzenedicarboxylates and Different Azine Based N,N′-Donor Ligands: Syntheses, Characterization and Magnetic Properties. Cryst. Growth Des. 2015, 15, 4427–4437. [Google Scholar] [CrossRef]
- Calahorro, A.J.; Sebastián, E.S.; Salinas-Castillo, A.; Seco, J.M.; Mendicute-Fierro, C.; Fernández, B.; Rodríguez-Diéguez, A. Effect of π–π stacking interactions on the emission properties of cadmium metal–organic frameworks based on 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene. CrystEngComm 2015, 17, 3659–3666. [Google Scholar] [CrossRef]
- Rad-Yousefnia, N.; Shaabani, B.; Zahedi, M.; Kubicki, M.; Aygün, M.; Lloret, F.; Julve, M.; Grześkiewicz, A.M.; Zakerhamidi, M.; Kazak, C. Cd(II) and Cu(II) coordination polymers constructed from the expanded 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene ligand: Conventional and ultrasound-assisted synthesis, crystal structure, luminescence and magnetic properties. New J. Chem. 2018, 42, 15860–15870. [Google Scholar] [CrossRef]
- Su, S.; Qin, C.; Guo, Z.; Guo, H.; Song, S.; Deng, R.; Cao, F.; Wang, S.; Li, G.; Zhang, H. Five three/two-fold interpenetrating architectures from self-assembly of fluorene-2,7-dicarboxylic acid derivatives and d10 metals. CrystEngComm 2011, 13, 2935–2941. [Google Scholar] [CrossRef]
- Nagaraja, C.M.; Ugale, B.; Chanthapally, A. Construction of 2D interwoven and 3D interpenetrated metal–organic frameworks of Zn(II) by varying N,N′-donor spacers. CrystEngComm 2014, 16, 4805–4815. [Google Scholar] [CrossRef]
- Zhou, J.; Du, L.; Qiao, Y.-F.; Hu, Y.; Li, B.; Li, L.; Wang, X.-Y.; Yang, J.; Xie, M.-J.; Zhao, Q.-H. Intriguing Architectures Generated from 1,4-Bis(3- or 4-pyridyl)-2,3-diaza-1,3-butadiene with Aromatic Dicarboxylates: Syntheses, Crystal Structures, and Properties. Cryst. Growth Des. 2014, 14, 1175–1183. [Google Scholar] [CrossRef]
- Maity, D.K.; Bhattacharya, B.; Mondal, R.; Ghoshal, D. Five diverse bivalent metal coordination polymers based on benzene dicarboxylate and bent dipyridyl ligands: Syntheses, structures, and photoluminescent properties. CrystEngComm 2014, 16, 8896–8909. [Google Scholar] [CrossRef]
- Manna, B.; Singh, S.; Karmakar, A.; Desai, A.V.; Ghosh, S.K. Selective Anion Exchange and Tunable Luminescent Behaviors of Metal–Organic Framework Based Supramolecular Isomers. Inorg. Chem. 2014, 54, 110–116. [Google Scholar] [CrossRef]
- Vetokhina, V.; Kijak, M.; Wiosna-Salyga, G.; Thummel, R.P.; Herbich, J.; Waluk, J. On the origin of fluorescence quenching of pyridylindoles by hydroxylic solvents. Photochem. Photobiol. Sci. 2010, 9, 923–930. [Google Scholar] [CrossRef]
- Cariati, E.; Forni, A.; Lucenti, E.; Marinotto, D.; Previtali, A.; Righetto, S.; Botta, C.; Bold, V.; Kravtsov, V.C.; Fonari, M.S. Extrinsic Heavy Metal Atom Effect on the Solid-State Room Temperature Phosphorescence of Cyclic Triimidazole. Chem. Asian J. 2019, 14, 853–858. [Google Scholar] [CrossRef]
- Qi, Y.; Xu, H.; Li, X.; Tu, B.; Pang, Q.; Lin, X.; Ning, E.; Li, Q. Structure Transformation of a Luminescent Pillared-Layer Metal–Organic Framework Caused by Point Defects Accumulation. Chem. Mater. 2018, 30, 5478–5484. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2007, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.J.; Van De Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. D 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Brown, K.G.; Graham, D.; Kirkhouse, J.B.; Kittner, M.; Major, C.; McHugh, C.J.; Murdoch, P.; Smith, W.E. Chromophore containing bipyridyl ligands. Part 1: Supramolecular solid-state structure of Ag(I) complexes. New J. Chem. 2005, 29, 826–832. [Google Scholar] [CrossRef]






Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kravtsov, V.C.; Lozovan, V.; Siminel, N.; Coropceanu, E.B.; Kulikova, O.V.; Costriucova, N.V.; Fonari, M.S. From 1D to 2D Cd(II) and Zn(II) Coordination Networks by Replacing Monocarboxylate with Dicarboxylates in Partnership with Azine Ligands: Synthesis, Crystal Structures, Inclusion, and Emission Properties. Molecules 2020, 25, 5616. https://doi.org/10.3390/molecules25235616
Kravtsov VC, Lozovan V, Siminel N, Coropceanu EB, Kulikova OV, Costriucova NV, Fonari MS. From 1D to 2D Cd(II) and Zn(II) Coordination Networks by Replacing Monocarboxylate with Dicarboxylates in Partnership with Azine Ligands: Synthesis, Crystal Structures, Inclusion, and Emission Properties. Molecules. 2020; 25(23):5616. https://doi.org/10.3390/molecules25235616
Chicago/Turabian StyleKravtsov, Victor Ch., Vasile Lozovan, Nikita Siminel, Eduard B. Coropceanu, Olga V. Kulikova, Natalia V. Costriucova, and Marina S. Fonari. 2020. "From 1D to 2D Cd(II) and Zn(II) Coordination Networks by Replacing Monocarboxylate with Dicarboxylates in Partnership with Azine Ligands: Synthesis, Crystal Structures, Inclusion, and Emission Properties" Molecules 25, no. 23: 5616. https://doi.org/10.3390/molecules25235616
APA StyleKravtsov, V. C., Lozovan, V., Siminel, N., Coropceanu, E. B., Kulikova, O. V., Costriucova, N. V., & Fonari, M. S. (2020). From 1D to 2D Cd(II) and Zn(II) Coordination Networks by Replacing Monocarboxylate with Dicarboxylates in Partnership with Azine Ligands: Synthesis, Crystal Structures, Inclusion, and Emission Properties. Molecules, 25(23), 5616. https://doi.org/10.3390/molecules25235616





