A Selenophene-Incorporated Metal–Organic Framework for Enhanced CO2 Uptake and Adsorption Selectivity
Abstract
1. Introduction
2. Results and Discussion
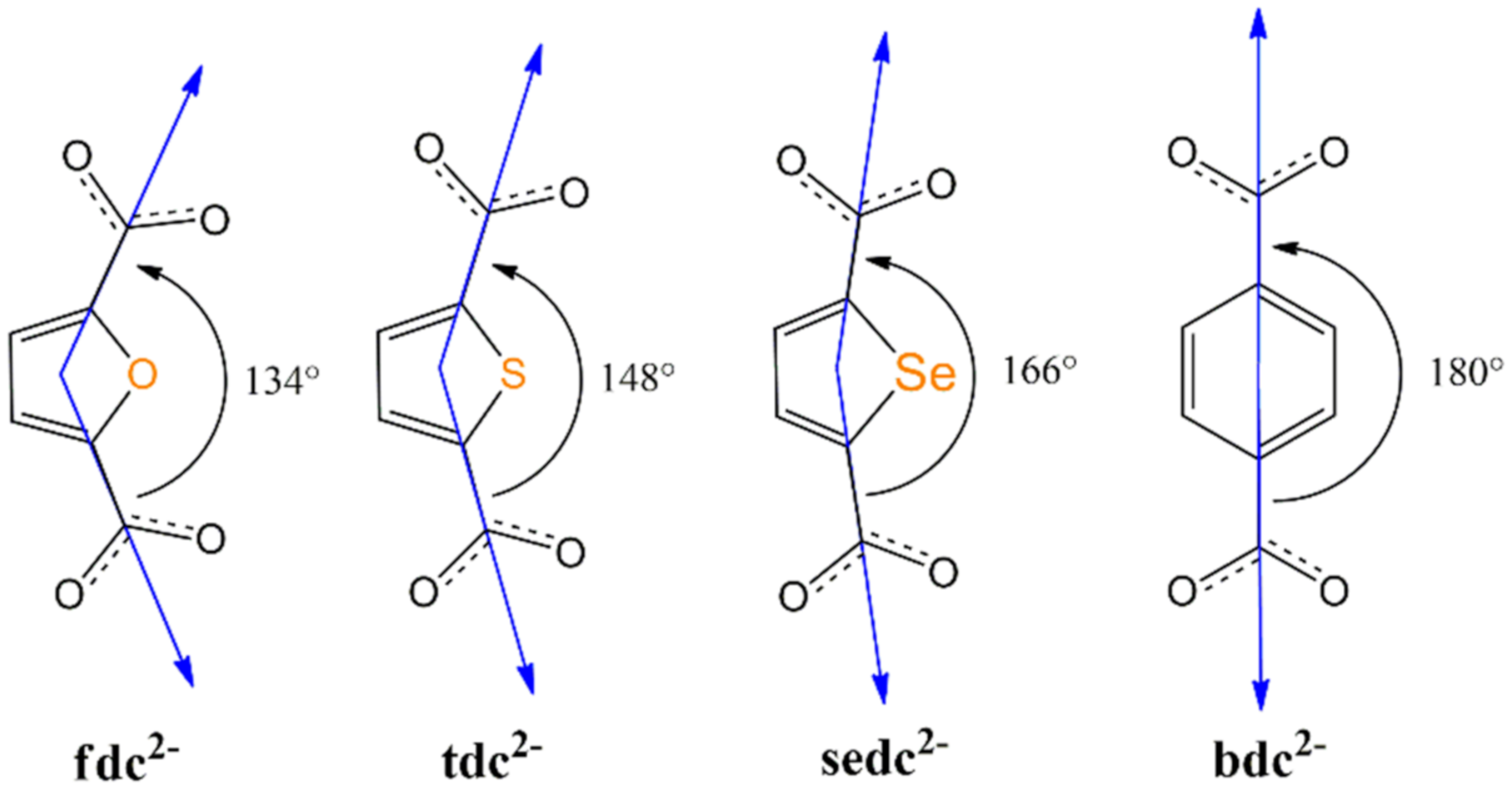
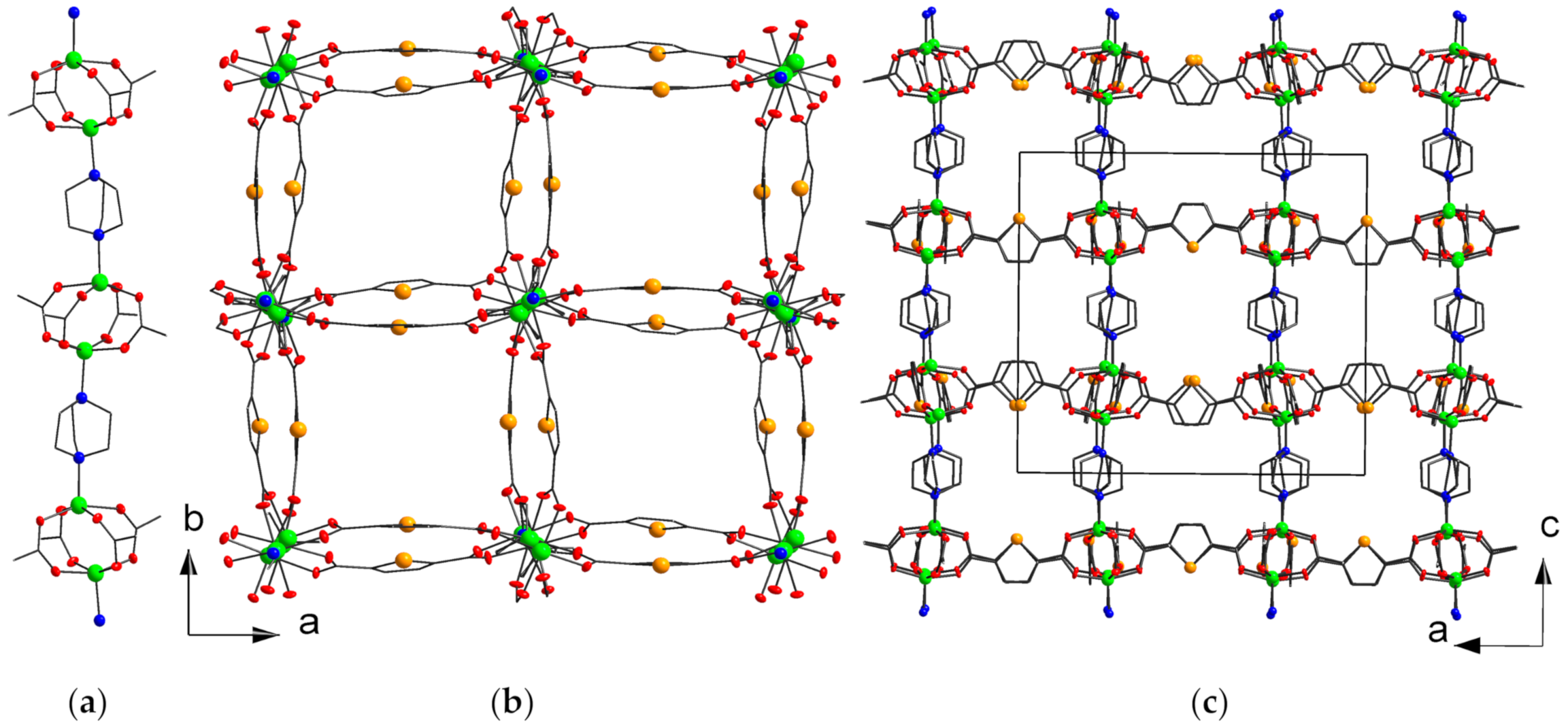
2.1. Structure Description
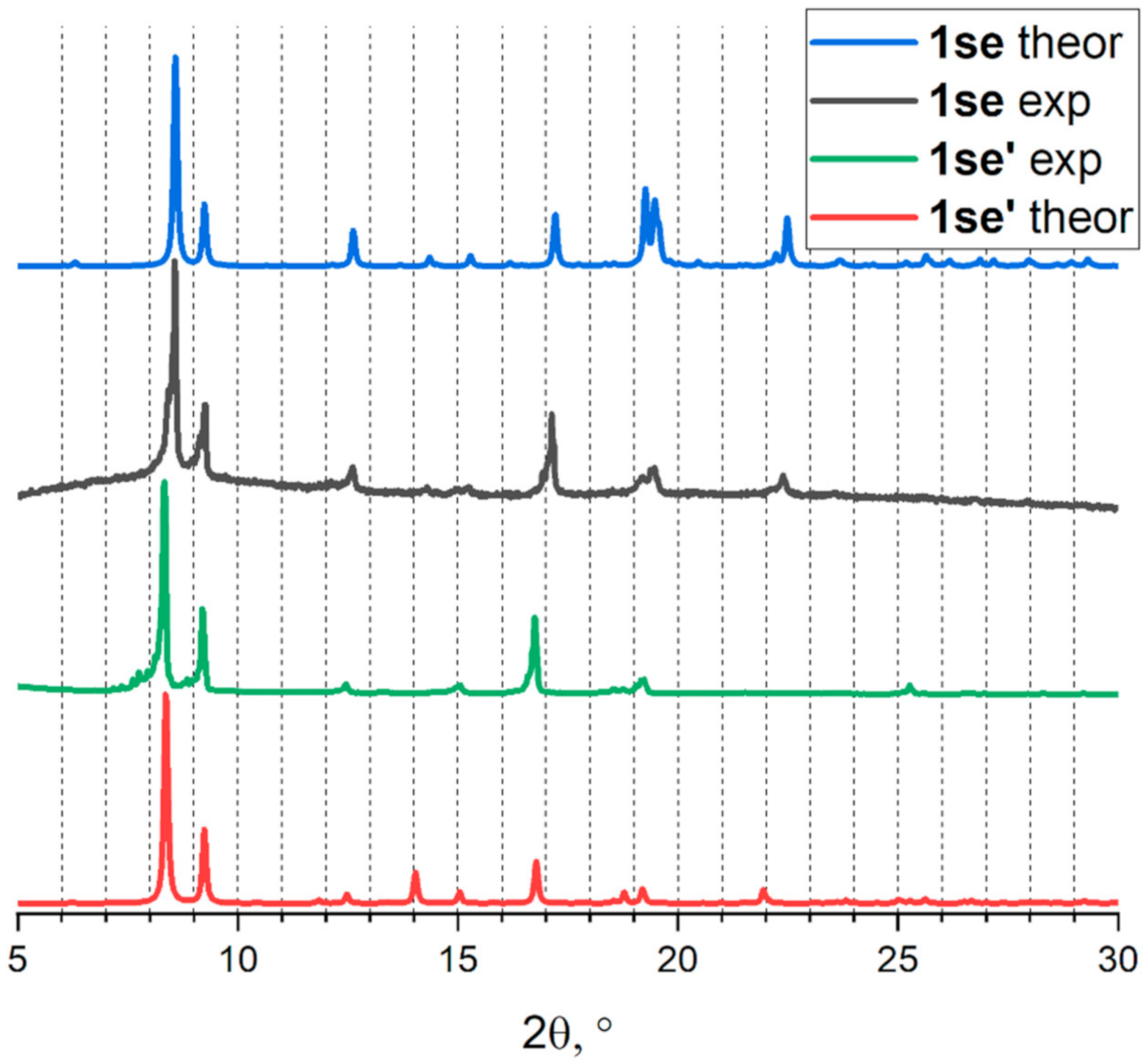
2.2. Characterization and Activation
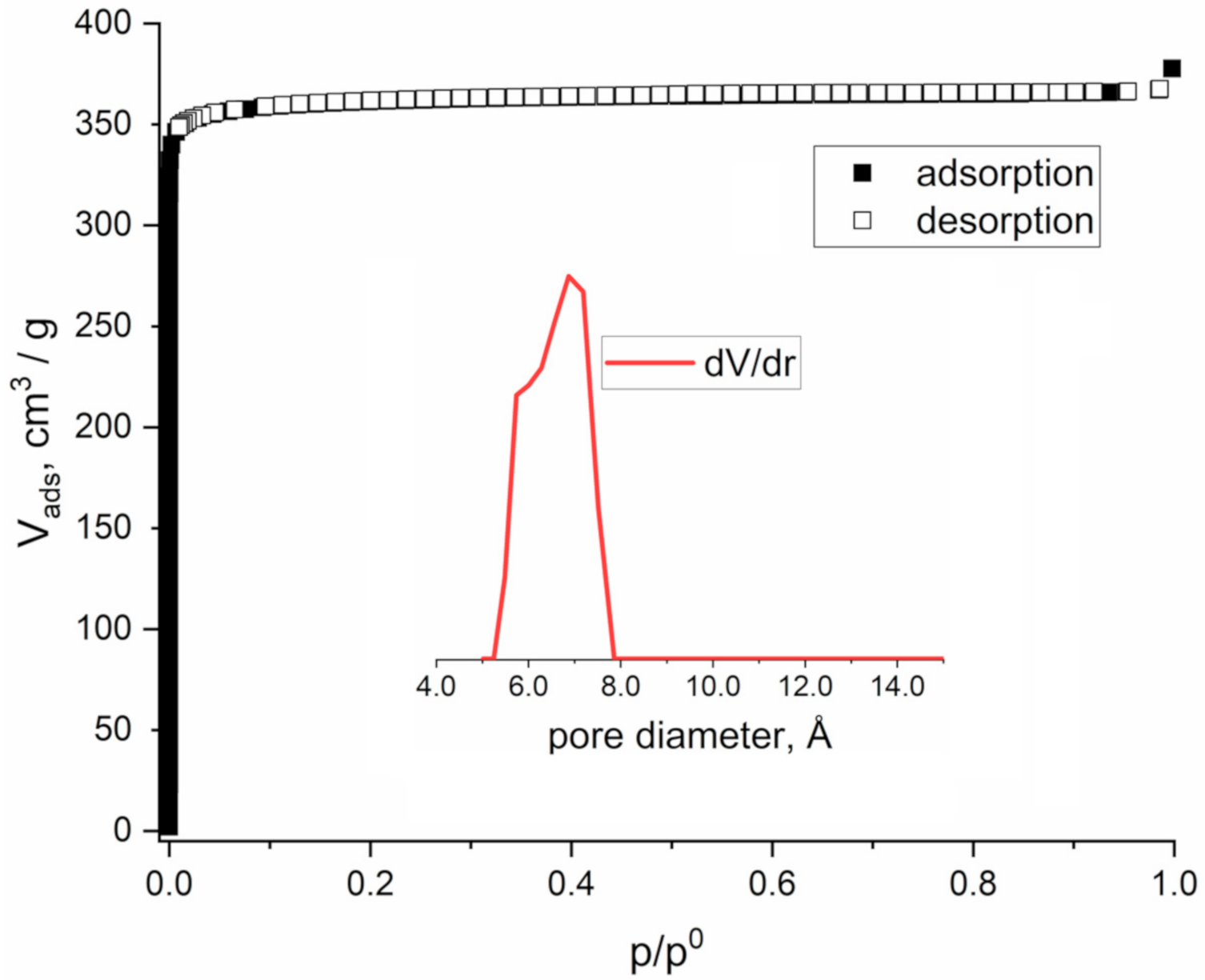
2.3. Adsorption Measurements
3. Materials and Methods
3.1. Reagents
3.2. Instruments
3.3. X-ray Crystallography
3.4. Synthetic Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A. Crystallographic data
| Identification Code | 1se |
|---|---|
| CCDC Number | 2026693 |
| Empirical formula | C30H44N6O12Se2Zn2 |
| M, g/mol | 969.37 |
| Crystal system | Tetragonal |
| Space group | P−421c |
| a, b, Å | 20.59224(17) |
| c, Å | 19.1260(2) |
| V, Å3 | 8110.20(16) |
| Z | 8 |
| D(calc.), g/cm3 | 1.588 |
| μ, mm−1 | 3.040 |
| F(000) | 3920 |
| Crystal size, mm | 0.36 × 0.26 × 0.20 |
| θ range for data collection, deg. | 1.98–28.28 |
| Index ranges | −27 ≤ h ≤ 19, −22 ≤ k ≤ 25, −22 ≤ l ≤ 25 |
| Reflections collected/independent | 24,964 8741 |
| Rint | 0.0225 |
| Reflections with I > 2σ(I) | 8293 |
| Goodness-of-fit on F2 | 1.044 |
| Final R indices [I > 2σ(I)] | R1 = 0.0223, wR2 = 0.0492 |
| R indices (all data) | R1 = 0.0253, wR2 = 0.0502 |
| Largest diff. peak/hole, e/Å3 | 0.397/−0.271 |
| Identification Code | 1se′ |
|---|---|
| Crystal System | Tetragonal |
| a, b, Å | 21.1165 |
| c, Å | 19.1304 |
| V, Å3 | 8530.5 |
| D(calc.), g/cm3 | 1.055 |
References
- Dutcher, B.; Fan, M.; Russell, A.G. Amine-Based CO2 Capture Technology Development from the Beginning of 2013—A Review. ACS Appl. Mater. Interfaces 2015, 7, 2137–2148. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hareb, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Miller, D.C. Post-combustion CO2 capture technologies—A review of processes for solvent-based and sorbent-based CO2 capture. Curr. Opin. Chem. Eng. 2017, 17, 78–92. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Mercedes Maroto-Valer, M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Lin, R.B.; Li, L.; Alsalme, A.; Chen, B. An Ultramicroporous Metal-Organic Framework for Sieving Separation of Carbon Dioxide from Methane. Small Struct. 2020. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon capture and conversion using metal–organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yao, Z.-Z.; Xiang, S.; Chen, B. Perspective of microporous metal–organic frameworks for CO2 capture and separation. Energy Environ. Sci. 2014, 7, 2868–2899. [Google Scholar] [CrossRef]
- Siegelman, R.L.; Milner, P.J.; Forse, A.C.; Lee, J.-H.; Colwell, K.A.; Neaton, J.B.; Reimer, J.A.; Weston, S.C.; Long, J.R. Water Enables Efficient CO2 Capture from Natural Gas Flue Emissions in an Oxidation-Resistant Diamine-Appended Metal–Organic Framework. J. Am. Chem. Soc. 2019, 141, 13171–13186. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, C.; Zhang, Q.; Chen, L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Guillerm, V.; Eddaoudi, M. Low concentration CO2 capture using physical adsorbents: Are metal-organic frameworks becoming the new benchmark materials? Chem. Eng. J. 2016, 296, 386–397. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef]
- Guo, P.; Dutta, P.; Wong-Foy, A.G.; Gidley, D.W.; Matzger, A.J. Water Sensitivity in Zn4O-Based MOFs is Structure and History Dependent. J. Am. Chem. Soc. 2015, 137, 2651–2657. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-F.; Liu, B.; Hou, L.; Zhang, W.-Y.; Wang, Y.-Y. Rational construction of a stable Zn4O-based MOF for highly efficient CO2 capture and conversion. Chem. Commun. 2018, 54, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Férey, G. A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration. Chem. Eur. J. 2004, 10, 1373–1382. [Google Scholar] [CrossRef]
- Senkovska, I.; Hoffmann, F.; Fröba, M.; Getzschmann, J.; Böhlmann, W.; Kaskel, S. New highly porous aluminium based metal-organic frameworks: Al(OH)(ndc) (ndc = 2,6-naphthalene dicarboxylate) and Al(OH)(bpdc) (bpdc = 4,4′-biphenyl dicarboxylate). Microporous Mesoporous Mater. 2009, 122, 93–98. [Google Scholar] [CrossRef]
- Rabe, T.; Pewe, H.; Reinsch, H.; Willhammar, T.; Svensson Grape, E.; Stock, N. Influence of the substitution pattern of four naphthalenedicarboxylic acids on the structures and properties of group 13 metal-organic frameworks and coordination polymers. Dalton Trans. 2020, 49, 4861–4868. [Google Scholar] [CrossRef] [PubMed]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Lammert, M.; Wharmby, M.; Smolders, S.; Bueken, B.; Lieb, A.; Lomachenko, K.A.; De Vos, D.; Stock, N. Cerium-based metal organic frameworks with UiO-66 architecture: Synthesis, properties and redox catalytic activity. Chem. Commun. 2015, 51, 12578–12581. [Google Scholar] [CrossRef]
- Waitschat, S.; Fröhlich, D.; Reinsch, H.; Terraschke, H.; Lomachenko, K.A.; Lamberti, C.; Kummer, H.; Helling, T.; Baumgartner, M.; Henninger, S.; et al. Synthesis of M-UiO-66 (M = Zr, Ce or Hf) employing 2,5-pyridinedicarboxylic acid as a linker: Defect chemistry, framework hydrophilisation and sorption properties. Dalton Trans. 2018, 47, 1062–1070. [Google Scholar] [CrossRef]
- Wang, H.; Wen, R.-M.; Hu, T.-L. Two Series of Lanthanide Metal-Organic Frameworks Constructed from Crown-Ether-Like Secondary Building Units. Eur. J. Inorg. Chem. 2014, 2014, 1185–1191. [Google Scholar] [CrossRef]
- Sapchenko, S.A.; Demakov, P.A.; Samsonenko, D.G.; Dybtsev, D.N.; Schröder, M.; Fedin, V.P. A Cryptand Metal–Organic Framework as a Platform for the Selective Uptake and Detection of Group I Metal Cations. Chem. Eur. J. 2017, 23, 2286–2289. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Feng, F.; Li, S.; Li, X.-X.; Shu, L. Metal-organic framework DUT-67 (Zr) for adsorptive removal of trace Hg2+ and CH3Hg+ in water. Chem. Speciat. Bioavailab. 2018, 30, 99–106. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, P.-C.; Zhao, S.-P.; Liu, J.-L.; Ren, X.-M. A rhombus channel metal–organic framework comprised of Sr2+ and thiophene-2,5-dicarboxylic acid exhibiting novel dielectric bistability. CrystEngComm 2013, 15, 1264–1270. [Google Scholar] [CrossRef]
- Dreischarf, A.C.; Lammert, M.; Stock, N.; Reinsch, H. Green Synthesis of Zr-CAU-28: Structure and Properties of the First Zr-MOF Based on 2,5-Furandicarboxylic Acid. Inorg. Chem. 2017, 56, 2270–2277. [Google Scholar] [CrossRef]
- Lysova, A.A.; Samsonenko, D.G.; Dorovatovskii, P.V.; Lazarenko, V.A.; Khrustalev, V.N.; Kovalenko, K.A.; Dybtsev, D.N.; Fedin, V.P. Tuning the Molecular and Cationic Affinity in a Series of Multifunctional Metal–Organic Frameworks Based on Dodecanuclear Zn(II) Carboxylate Wheels. J. Am. Chem. Soc. 2019, 141, 17260–17269. [Google Scholar] [CrossRef]
- Petkov, P.S.; Bon, V.; Hobday, C.L.; Kuc, A.B.; Melix, P.; Kaskel, S.; Düren, T.; Heine, T. Conformational isomerism controls collective flexibility in metal–organic framework DUT-8(Ni). Phys. Chem. Chem. Phys. 2019, 21, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.; Dybtsev, D.N.; Kim, H.; Kim, K. Synthesis, X-ray crystal structures, and gas sorption properties of pillared square grid nets based on paddle-wheel motifs: Implications for hydrogen storage in porous materials. Chem. Eur. J. 2005, 11, 3521–3529. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Yutkin, M.P.; Peresypkina, E.V.; Virovets, A.V.; Serre, C.; Férey, G.; Fedin, V.P. Isoreticular homochiral porous metal-organic structures with tunable pore sizes. Inorg. Chem. 2007, 46, 6843–6845. [Google Scholar] [CrossRef]
- Khan, I.S.; Samsonenko, D.G.; Irgashev, R.A.; Kazin, N.A.; Rusinov, G.L.; Charushin, V.N.; Zavakhina, M.S.; Fedin, V.P. Synthesis, crystal structure and fluorescent properties of indolo[3,2-b]carbazole-based metal–organic coordination polymers. Polyhedron 2017, 141, 337–342. [Google Scholar] [CrossRef]
- Cheplakova, A.M.; Kovalenko, K.A.; Samsonenko, D.G.; Lazarenko, V.A.; Khrustalev, V.N.; Vinogradov, A.S.; Karpov, V.M.; Platonov, V.E.; Fedin, V.P. Metal-organic frameworks based on octafluorobiphenyl-4,4′-dicarboxylate: Synthesis, crystal structure, and surface functionality. Dalton Trans. 2018, 47, 3283–3297. [Google Scholar] [CrossRef]
- Bolotov, V.A.; Kovalenko, K.A.; Samsonenko, D.G.; Han, X.; Zhang, X.; Smith, G.L.; McCormick, L.J.; Teat, S.J.; Yang, S.; Lennox, M.J.; et al. Enhancement of CO2 Uptake and Selectivity in a Metal–Organic Framework by the Incorporation of Thiophene Functionality. Inorg. Chem. 2018, 57, 5074–5082. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; D’Alessandro, D.M. Systematic Tuning of Zn(II) frameworks with Furan, Thiophene and Selenophene Dipyridyl and Dicarboxylate Ligands. Cryst. Growth Des. 2017, 17, 6262–6272. [Google Scholar] [CrossRef]
- Ding, B.; Hua, C.; Kepert, C.J.; D’Alessandro, D.M. Influence of structure–activity relationships on through-space intervalence charge transfer in metal–organic frameworks with cofacial redox-active units. Chem. Sci. 2019, 10, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Lysova, A.; Samsonenko, D.; Dybtsev, D.; Fedin, V. Synthesis and luminescence properties of new metal-organic frameworks based on zinc(II) ions and 2,5-thiophendicarboxylate ligands. Crystals 2018, 8, 7. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Chun, H.; Kim, K. Rigid and Flexible: A Highly Porous Metal–Organic Framework with Unusual Guest-Dependent Dynamic Behavior. Angew. Chem. Int. Ed. 2004, 43, 5033–5036. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. POWDER CELL–A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Antonio Zárate, J.; Sánchez-González, E.; Jurado-Vázquez, T.; Gutiérrez-Alejandre, A.; González-Zamora, E.; Castillo, I.; Maurin, G.; Ibarra, I.A. Outstanding reversible H2S capture by an Al(III)-based MOF. Chem. Commun. 2019, 55, 3049–3052. [Google Scholar] [CrossRef]
- Georgiadis, A.G.; Charisiou, N.; Yentekakis, I.V.; Goula, M.A. Hydrogen Sulfide (H2S) Removal via MOFs. Materials 2020, 13, 3640. [Google Scholar] [CrossRef]
- Dong, Q.; Guo, Y.; Cao, H.; Wang, S.; Matsuda, R.; Duan, J. Accelerated C2H2/CO2 Separation by a Se-Functionalized Porous Coordination Polymer with Low Binding Energy. ACS Appl. Mater. Interfaces 2020, 12, 3764–3772. [Google Scholar] [CrossRef]
- Díaz-Ramírez, M.L.; Vargas, B.; Raziel Álvarez, J.; Landeros-Rivera, B.; Rivera-Almazo, M.; Ramos, C.; Flores, G.; Morales, E.; Vargas, R.; Garza, J.; et al. Fluorometric detection of iodine by MIL-53(Al)-TDC. Dalton Trans. 2020, 49, 6572–6577. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.; Yim, H.; Ko, N.; Choi, S.B.; Oh, Y.; Park, H.J.; Park, S.Y.; Kim, J. Gas adsorption properties of highly porous metal–organic frameworks containing functionalized naphthalene dicarboxylate linkers. Dalton Trans. 2014, 43, 18017–18024. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Wang, D.; Cao, Y.; Li, G.; Huo, Q.; Liu, Y. Two stable 3D porous metal–organic frameworks with high performance for gas adsorption and separation. J. Mater. Chem. A 2015, 3, 16627–16632. [Google Scholar] [CrossRef]
- Ding, Q.-R.; Wang, F. A pillared-layer framework with high uptake and selective sorption of light hydrocarbons. Dalton Trans. 2016, 45, 7004–7007. [Google Scholar] [CrossRef]
- Lin, R.B.; Xiang, S.; Zhou, W.; Chen, B. Microporous Metal-Organic Framework Materials for Gas Separation. Chem 2020, 6, 337–363. [Google Scholar] [CrossRef]
- Sapchenko, S.A.; Barsukova, M.O.; Belosludov, R.V.; Kovalenko, K.A.; Samsonenko, D.G.; Poryvaev, A.S.; Sheveleva, A.M.; Fedin, M.V.; Bogomyakov, A.S.; Dybtsev, D.N.; et al. Understanding Hysteresis in Carbon Dioxide Sorption in Porous Metal–Organic Frameworks. Inorg. Chem. 2019, 58, 6811–6820. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro 1.171.38.46; Rigaku Oxford Diffraction: The Woodlands, TX, USA, 2015. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Pishchur, D.P.; Kompankov, N.B.; Lysova, A.A.; Kozlova, S.G. Order-disorder phase transitions in Zn2(C8H4O4)2·C6H12N2 in atmospheres of noble gases. J. Chem. Thermodyn. 2019, 130, 147–153. [Google Scholar] [CrossRef]
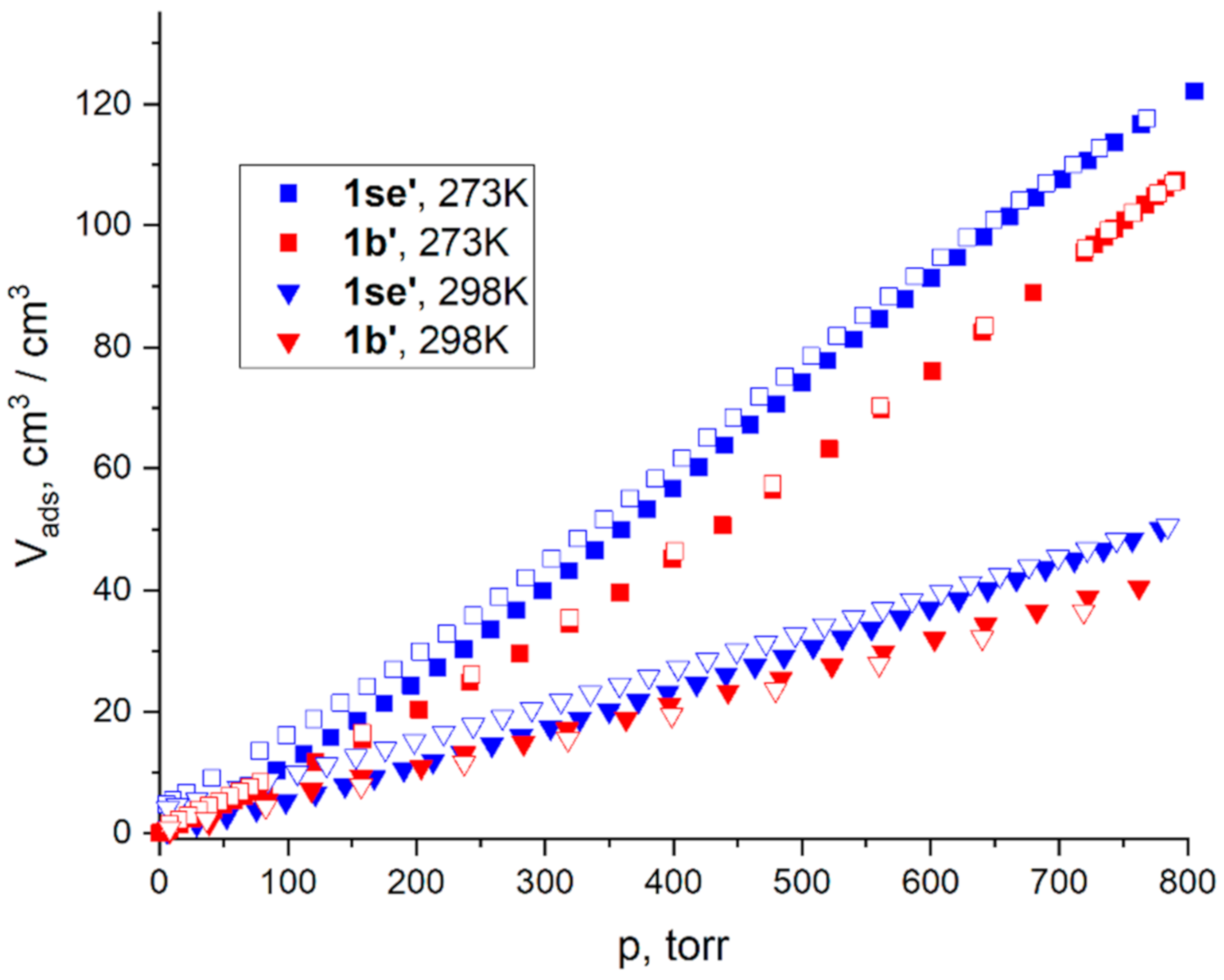
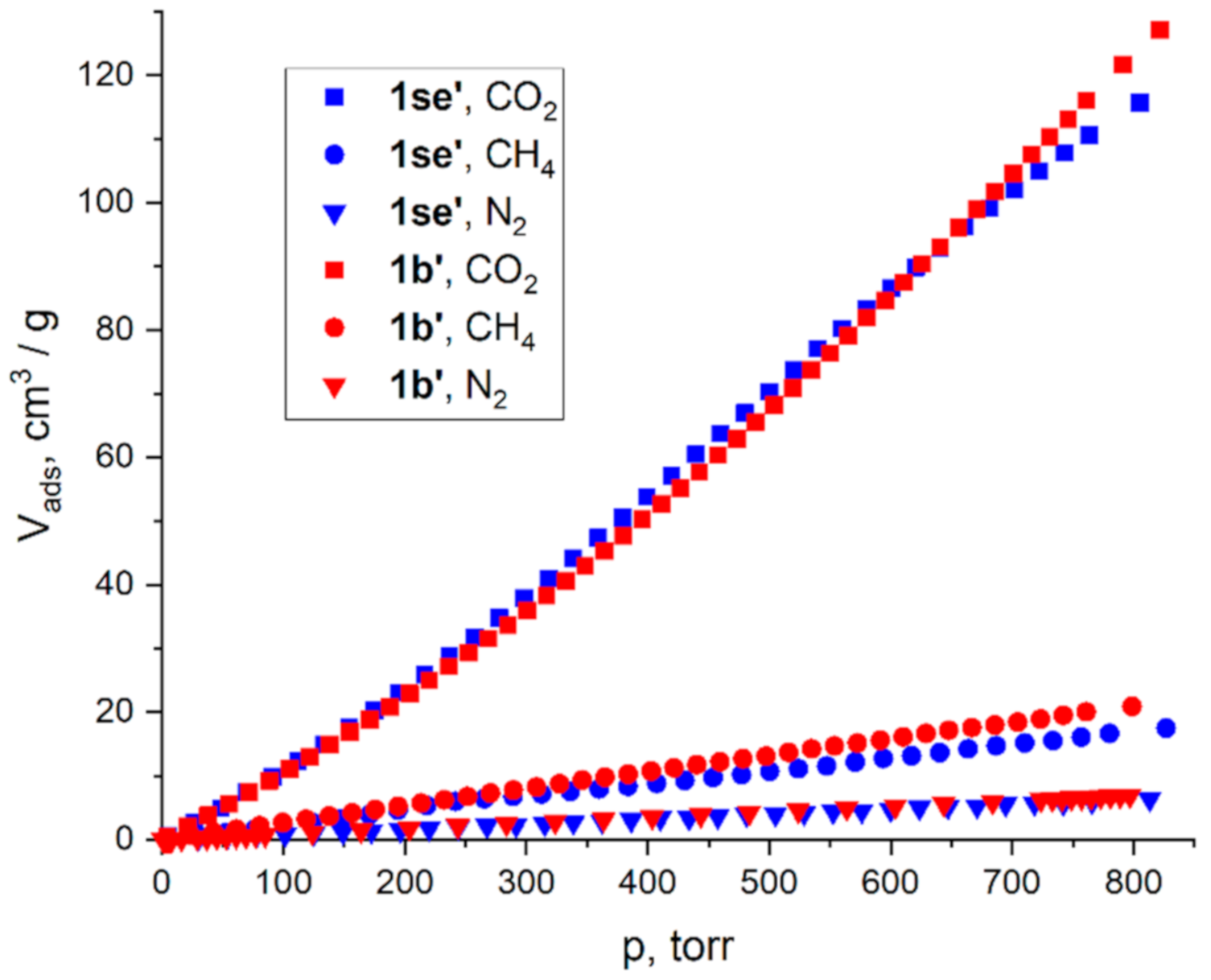
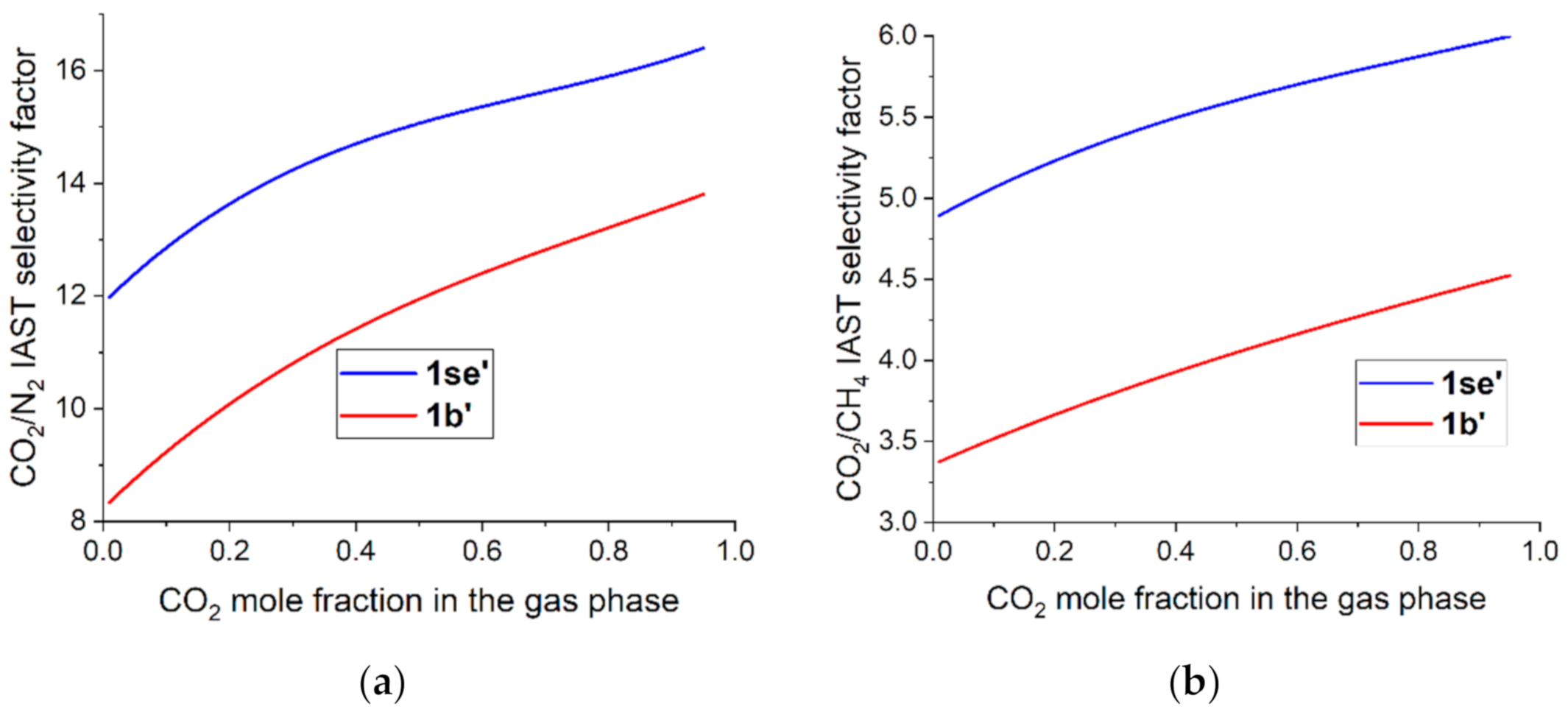
| V1/V2 (273 K, 1 Bar) | Kh1/Kh2 (273 K) | IAST (273 K, 50:50 Ratio) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gas Mixture | 1se | 1t | 1b | 1se | 1t | 1b | 1se | 1t | 1b |
| CO2/N2 (in this work) | 18.6 | -- | 17.7 | 12.9 | -- | 11.8 | 15.1 | -- | 11.9 |
| CO2/N2 (from Ref. [29]) | -- | 10.3 | 18.8 | -- | 12.5 | 8.9 | -- | 11.2 | 9.2 |
| CO2/CH4 (in this work) | 6.8 | -- | 5.8 | 4.8 | -- | 3.9 | 5.6 | -- | 4.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demakov, P.A.; Volynkin, S.S.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. A Selenophene-Incorporated Metal–Organic Framework for Enhanced CO2 Uptake and Adsorption Selectivity. Molecules 2020, 25, 4396. https://doi.org/10.3390/molecules25194396
Demakov PA, Volynkin SS, Samsonenko DG, Fedin VP, Dybtsev DN. A Selenophene-Incorporated Metal–Organic Framework for Enhanced CO2 Uptake and Adsorption Selectivity. Molecules. 2020; 25(19):4396. https://doi.org/10.3390/molecules25194396
Chicago/Turabian StyleDemakov, Pavel A., Sergey S. Volynkin, Denis G. Samsonenko, Vladimir P. Fedin, and Danil N. Dybtsev. 2020. "A Selenophene-Incorporated Metal–Organic Framework for Enhanced CO2 Uptake and Adsorption Selectivity" Molecules 25, no. 19: 4396. https://doi.org/10.3390/molecules25194396
APA StyleDemakov, P. A., Volynkin, S. S., Samsonenko, D. G., Fedin, V. P., & Dybtsev, D. N. (2020). A Selenophene-Incorporated Metal–Organic Framework for Enhanced CO2 Uptake and Adsorption Selectivity. Molecules, 25(19), 4396. https://doi.org/10.3390/molecules25194396










