Bergenia Genus: Traditional Uses, Phytochemistry and Pharmacology
Abstract
1. Introduction
2. Review Methodology
3. Distribution
4. Botanical Description
5. Traditional Medicinal Uses
6. Phytochemistry
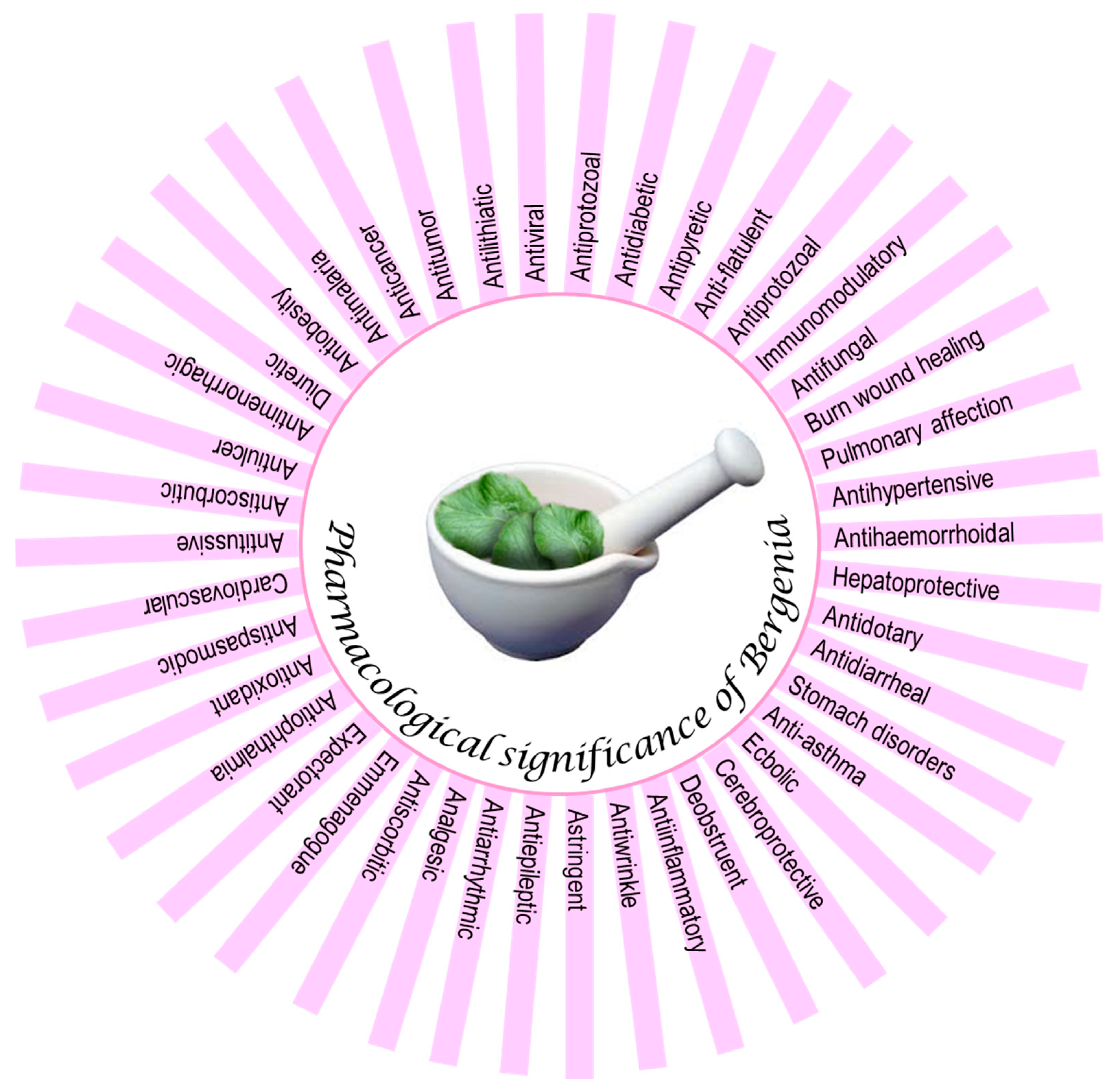
7. Pharmacological Activities
7.1. Antilithiatic Activity
7.2. Diuretic Activity
7.3. Antidiabetic Activity
7.4. Antitussive Activity
7.5. Insecticidal Activity
7.6. Anti-Inflammatory Activity
7.7. Antipyretic Activity
7.8. Anti-Bradykinin Activity
7.9. Antiviral Activity
7.10. Antibacterial Activity
7.11. Antimalarial Activity
7.12. Hepatoprotective Activity
7.13. Antiulcer Activity
7.14. Anticancer Activity
7.15. Antioxidant Activity
7.16. Antiobesity Activity
7.17. Adaptogenic Activity
8. Other Benefits of Bergenia Species
9. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koul, B. Herbs for Cancer Treatment, 1st ed.; Springer: New York, NY, USA, 2020. [Google Scholar]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Pandey, M.; Rastogi, S.; Rawat, A. Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid. Based Complement. Alternat. Med. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R. Toward the integration and advancement of herbal medicine: A focus on traditional Indian medicine. Bot. Target Ther. 2015, 5, 33–44. [Google Scholar] [CrossRef]
- Kasote, D.M.; Jagtap, S.D.; Thapa, D.; Khyade, M.S.; Russell, W.R. Herbal remedies for urinary stones used in India and China: A review. J. Ethnopharmacol. 2017, 203, 55–68. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Liao, B.; Luo, D.; Wang, K.; Li, H.; Zeng, G. Epidemiology of urolithiasis in Asia. Asian J. Urol. 2018, 5, 205–214. [Google Scholar] [CrossRef]
- Vitale, C.; Croppi, E.; Marangella, M. Biochemical evaluation in renal stone disease. Clin. Cases Miner. Bone Metab. 2008, 5, 127. [Google Scholar]
- Ramello, A.; Vitale, C.; Marangella, M. Epidemiology of nephrolithiasis. J. Nephrol. 2000, 13 (Suppl. S3), S45–S50. [Google Scholar]
- Sharma, I.; Khan, W.; Parveen, R.; Alam, M.; Ahmad, I.; Ansari, M.H.R.; Ahmad, S. Antiurolithiasis activity of bioactivity guided fraction of Bergenia ligulata against ethylene glycol induced renal calculi in rat. Biomed. Res. Int. 2017, 2017, 1–11. [Google Scholar]
- Wadkar, K.A.; Kondawar, M.S.; Lokapure, S.G. Standardization of marketed cystone tablet: A herbal formulation. J. Pharmacogn. Phytochem. 2017, 6, 10–16. [Google Scholar]
- Ahmad, M.; Butt, M.A.; Zhang, G.; Sultana, S.; Tariq, A.; Zafar, M. Bergenia ciliata: A comprehensive review of its traditional uses, phytochemistry, pharmacology and safety. Biomed. Pharmacother. 2018, 97, 708–721. [Google Scholar] [CrossRef]
- Ruby, K.; Chauhan, R.; Dwivedi, J. Himalayan bergenia a comprehensive review. Int. J. Pharm. Sci. 2012, 14, 139–141. [Google Scholar]
- Srivastava, S.; Rawat, A.K.S. Botanical and phytochemical comparison of three bergenia species. J. Sci. Ind. Res. 2008, 67, 65–72. [Google Scholar]
- Árok, R.; Végh, K.; Alberti, Á.; Kéry, Á. Phytochemical comparison and analysis of Bergenia crassifolia l.(fritsch.) and Bergenia cordifolia sternb. Eur. Chem. Bull. 2012, 1, 31–34. [Google Scholar]
- de Oliveira, C.M.; Nonato, F.R.; de Lima, F.O.; Couto, R.D.; David, J.P.; David, J.M.; Soares, M.B.P.; Villarreal, C.F. Antinociceptive properties of bergenin. J. Nat. Prod. 2011, 74, 2062–2068. [Google Scholar] [CrossRef]
- Dhalwal, K.; Shinde, V.; Biradar, Y.; Mahadik, K. Simultaneous quantification of bergenin, catechin, and gallic acid from Bergenia ciliata and Bergenia ligulata by using thin-layer chromatography. J. Food Compos. Anal. 2008, 21, 496–500. [Google Scholar] [CrossRef]
- Li, F.; Zhou, D.; Qin, X.; Zhang, Z.-R.; Huang, Y. Studies on the physicochemical properties of bergenin. Chin. Pharm. J. 2009, 44, 92–95. [Google Scholar]
- Rastogi, S.; Rawat, A. A comprehensive review on bergenin, a potential hepatoprotective and antioxidative phytoconstituent. Herba Polonica 2008, 54, 66–79. [Google Scholar]
- Singh, D.P.; Srivastava, S.K.; Govindarajan, R.; Rawat, A.K.S. High-performance liquid chromatographic determination of bergenin in different bergenia species. Acta Chromatogr. 2007, 19, 246–252. [Google Scholar]
- Nazir, N.; Koul, S.; Qurishi, M.A.; Najar, M.H.; Zargar, M.I. Evaluation of antioxidant and antimicrobial activities of bergenin and its derivatives obtained by chemoenzymatic synthesis. Eur. J. Med. Chem. 2011, 46, 2415–2420. [Google Scholar] [CrossRef]
- Rousseau, C.; Martin, O.R. Synthesis of bergenin-related natural products by way of an intramolecular c-glycosylation reaction. Tetrahedron: Asymmetry 2000, 11, 409–412. [Google Scholar] [CrossRef]
- Suh, K.S.; Chon, S.; Jung, W.W.; Choi, E.M. Effect of bergenin on rankl-induced osteoclast differentiation in the presence of methylglyoxal. Toxicol. In Vitro 2019, 61, 104613. [Google Scholar] [CrossRef]
- Gurav, S.; Gurav, N. A comprehensive review: Bergenia ligulata wall-a controversial clinical candidate. Int. J. Pharm. Sci. Rev. Res. 2014, 5, 1630–1642. [Google Scholar]
- Singh, N.; Gupta, A.; Juyal, V. A review on Bergenia ligulata wall. IJCAS 2010, 1, 71–73. [Google Scholar]
- Chitme, H.R.; Alok, S.; Jain, S.; Sabharwal, M. Herbal treatment for urinary stones. Int. J. Pharm. Sci. Res. 2010, 1, 24–31. [Google Scholar]
- Chandrareddy, U.D.; Chawla, A.S.; Mundkinajeddu, D.; Maurya, R.; Handa, S.S. Paashaanolactone from Bergenia ligulata. Phytochemistry 1998, 47, 907–909. [Google Scholar] [CrossRef]
- Khan, M.Y.; Vimal, K.V. Phytopharmacological and chemical profile of Bergenia ciliate. Int. J. Phytopharm. 2016, 6, 90–98. [Google Scholar]
- Hendrychová, H.; Tůmová, L. Bergenia genus-content matters and biological activity. Ceska a Slovenska farmacie Casopis Ceske farmaceuticke spolecnosti a Slovenske farmaceuticke spolecnosti 2012, 61, 203–209. [Google Scholar]
- Liu, S.J.; Yu, B.; Hu, C.H. In The variation of pod activities in Bergenla tianquanensis in tissue culture progress. In Advanced Materials Research; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2011; pp. 196–200. [Google Scholar]
- Wu, Z.-Y.; Raven, P.H. Flora of China; Science Press (Beijing) & Missouri Botanical Garden Press: St. Louis, MO, USA, 2001; Volume 8. [Google Scholar]
- Zhang, Y.; Liao, C.; Liu, X.; Li, J.; Fang, S.; Li, Y.; He, D. Biological advances in bergenia genus plant. Afr. J. Biotechnol. 2011, 10, 8166–8169. [Google Scholar]
- Jin-tang, P. New taxa of the genus bergenia from Hengduan mountains. Acta Phytotax. Sin. 1994, 32, 571–573. [Google Scholar]
- Jin-tang, P.; Soltis, D.E. Flora China. Bergenia 2001, 8, 278–280. [Google Scholar]
- Zhou, G.Y.; Li, W.C.; Guo, F.G. Resource investigation and observation of biological characteristics of Bergenia purpurascens (Hook. f. et. Thoms.). Engl. Chin. Agric. Sci. Bull. 2007, 23, 390–392. [Google Scholar]
- Alok, S.; Jain, S.K.; Verma, A.; Kumar, M.; Sabharwal, M. Pathophysiology of kidney, gallbladder and urinary stones treatment with herbal and allopathic medicine: A review. Asian Pac. J. Trop. Dis. 2013, 3, 496–504. [Google Scholar] [CrossRef]
- Chowdhary, S.; Verma, D.; Kumar, H. Biodiversity and traditional knowledge of Bergenia spp. in kumaun himalaya. Sci. J. 2009, 2, 105–108. [Google Scholar]
- Rajbhandari, M.; Mentel, R.; Jha, P.; Chaudhary, R.; Bhattarai, S.; Gewali, M.; Karmacharya, N.; Hipper, M.; Lindequist, U. Antiviral activity of some plants used in nepalese traditional medicine. Evid. Based Complement. Alternat. Med. 2009, 6, 517–522. [Google Scholar] [CrossRef]
- Kumar, V.; Tyagi, D. Review on phytochemical, ethnomedical and biological studies of medically useful genus bergenia. Int. J. Curr. Microbiol. App. Sci 2013, 2, 328–334. [Google Scholar]
- Patel, A.M.; Kurbetti, S.; Savadi, R.; Thorat, V.; Takale, V.; Horkeri, S. Preparation and evaluation of wound healing activity of new polyherbal formulations in rats. Am. J. Phytomed. Clin. Ther. 2013, 1, 498–506. [Google Scholar]
- Raina, R.; Prawez, S.; Verma, P.; Pankaj, N. Medicinal plants and their role in wound healing. Vet. Scan. 2008, 3, 1–7. [Google Scholar]
- Shakya, A.K. Medicinal plants: Future source of new drugs. Int. J. Herb. Med. 2016, 4, 59–64. [Google Scholar]
- Singh, K.J.; Thakur, A.K. Medicinal plants of the shimla hills, himachal pradesh: A survey. Int. J. Herbal Med. 2014, 2, 118–127. [Google Scholar]
- Walter, N.S.; Bagai, U.; Kalia, S. Antimalarial activity of Bergenia ciliata (haw.) sternb. against Plasmodium berghei. Parasitol. Res. 2013, 112, 3123–3128. [Google Scholar] [CrossRef]
- Bahu, C.P.; Seshadri, R.T. Advances in Research in “Indian Medicine; “Pashanbedi” Drugs for Urinary Calculus; Udupa, K.N., Ed.; Banaras Hindu University: Varanasi, India, 1970; pp. 77–98. [Google Scholar]
- Manandhar, N.P. A survey of medicinal plants of jajarkot district, Nepal. J. Ethnopharmacol. 1995, 48, 1–6. [Google Scholar] [CrossRef]
- Kapur, S. Ethno-medico plants of kangra valley (Himachal Pradesh). J. Econ. Taxon. Bot. 1993, 17, 395–408. [Google Scholar]
- Mukerjee, T.; Bhalla, N.; Singh, A.; Jain, H. Herbal drugs for urinary stones. Indian Drugs 1984, 21, 224–228. [Google Scholar]
- Shah, N.; Jain, S. Ethnomedico-botany of the kumaon himalaya, india. Soc. Pharmacol. 1988, 2, 359–380. [Google Scholar]
- Rani, S.; Rana, J.C. Ethnobotanical uses of some plants of bhattiyat block in district chamba, Himachal Pradesh (Western Himalaya). Ethnobot. Res. Appl. 2014, 12, 407–414. [Google Scholar] [CrossRef]
- Koelz, W.N. Notes on the ethnobotany of lahul, a province of the Punjab. Q. J. Crude Drug Res. 1979, 17, 1–56. [Google Scholar] [CrossRef]
- Vereschagin, V.; Sobolevskaya, K.; Yakubova, A. Useful Plants of West Siberia; Publishing of Academy of Science of USSR: Moscow-Leningrad, Russia, 1959. [Google Scholar]
- Gammerman, A.; Kadaev, G.; Yacenko-Khmelevsky, A. Medicinal Plants (Herbs-Healers); High School: Moscow, Russia, 1984. [Google Scholar]
- Panossian, A.G. Adaptogens: Tonic herbs for fatigue and stress. Altern. Complement. Ther. 2003, 9, 327–331. [Google Scholar] [CrossRef]
- Sokolov, S.Y. Phytotherapy and Phytopharmacology: The Manual for Doctors; Medical News Agency: Moscow, Russia, 2000; pp. 197–199. [Google Scholar]
- Suslov, N.; Churin, A.; Skurikhin, E.; Provalova, N.; Stal’bovskiĭ, A.; Litvinenko, V.; Dygaĭ, A. Effect of natural nootropic and adaptogen preparations on the cortex bioelectrical activity in rats. Eksp. Klin. Farmakol. 2002, 65, 7–10. [Google Scholar]
- Li, W.-C.; Gou, F.-G.; Zhang, L.-M.; Yu, H.-M.; Li, X.; Lin, C. The situation and prospect of research on Bergenia purpurascens. J. -Yunnan Agric. Univ. 2006, 21, 845. [Google Scholar]
- Pokhrel, P.; Parajuli, R.R.; Tiwari, A.K.; Banerjee, J. A short glimpse on promising pharmacological effects of Begenia ciliata. J. Appl. Pharm. Res. 2014, 2, 1–6. [Google Scholar]
- Xie, G.; Zhou, J.; Yan, X. Encyclopedia of Traditional Chinese Medicines: Molecular Structures, Pharmacological Activities, Natural Sources and Applications; Springer: Berlin/Heidelberg, Germany, 2011; Volume 2. [Google Scholar]
- Chen, Y.; Jia, X.; Zhang, Y. Studies on chemical compositions of Bergenia scopulosa T. P. Wang. J. Chin. Med. Mater. 2008, 31, 1006–1007. [Google Scholar]
- Hasan, A.; Husain, A.; Khan, M.A. Flavonol glycosides from leaves of Bergenia himalaica. Asian J. Chem. 2005, 17, 822. [Google Scholar]
- Saijyo, J.; Suzuki, Y.; Okuno, Y.; Yamaki, H.; Suzuki, T.; Miyazawa, M. A-glucosidase inhibitor from Bergenia ligulata. J. Oleo Sci. 2008, 57, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Xin-Min, C.; Yoshida, T.; Hatano, T.; Fukushima, M.; Okuda, T. Galloylarbutin and other polyphenols from Bergenia purpurascens. Phytochemistry 1987, 26, 515–517. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Z.; Wang, Z.; Li, R. Analysis of nutritive components and mineral element of Bergenae pacumbis intibet. J. Chang. Veg. 2009, 22, 57–58. [Google Scholar]
- Carmen, P.; Vlase, L.; Tamas, M. Natural resources containing arbutin. Determination of arbutin in the leaves of Bergenia crassifolia (L.) fritsch. Acclimated in romania. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 129–132. [Google Scholar]
- Chen, J.; Li, Y.; Cai, L. Determination of total flavonoids in Bergenia emeiensis leaf and rhizome by spectrophotometry. J. China West Norm. Univ. (Nat. Sci.) 2008, 29, 141–143. [Google Scholar]
- Lu, X. Studies on chemical compositions of Bergenia scopulosa TP Wang. Zhong Yao Cai 2003, 26, 791–792. [Google Scholar]
- Wang, J.; Lu, X. Studies on chemical compositions of Bergenia scopulosa T. P. Wang. J. Chin. Med. Mater. 2005, 28, 23–24. [Google Scholar] [CrossRef]
- Lim, Y.-J.; Lee, E.H.; Kang, T.H.; Ha, S.K.; Oh, M.S.; Kim, S.M.; Yoon, T.-J.; Kang, C.; Park, J.-H.; Kim, S.Y. Inhibitory effects of arbutin on melanin biosynthesis of α-melanocyte stimulating hormone-induced hyperpigmentation in cultured brownish guinea pig skin tissues. Arch. Pharm. Res 2009, 32, 367–373. [Google Scholar] [CrossRef]
- Samant, S.; Pant, S. Diversity, distribution pattern and conservation status of the plants used in liver diseases/ailments in Indian himalayan region. J. Mt. Sci. 2006, 3, 28–47. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, F.; Zhang, L.; Chen, Y.; Yang, S. Comparison of bergenin contents of Bergenia purpurascens among different regions in yunnan province. J. Yunnan Agric. Univ. 2010, 25, 895–898. [Google Scholar]
- Siddiqui, B.S.; Hasan, M.; Mairaj, F.; Mehmood, I.; Hafizur, R.M.; Hameed, A.; Shinwari, Z.K. Two new compounds from the aerial parts of Bergenia himalaica boriss and their anti-hyperglycemic effect in streptozotocin-nicotinamide induced diabetic rats. J. Ethnopharmacol. 2014, 152, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.A.; Nomura, K.; Malfanov, I.L.; Sklyar, I.V.; Ptitsyn, L.R. Isolation of a novel catechin from bergenia rhizomes that has pronounced lipase-inhibiting and antioxidative properties. Fitoterapia 2011, 82, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Dharmender, R.; Madhavi, T.; Reena, A.; Sheetal, A. Simultaneous quantification of bergenin,(+)-catechin, gallicin and gallic acid; and quantification of β-sitosterol using hptlc from Bergenia ciliata (haw.) sternb. Forma ligulata yeo (pasanbheda). Pharm. Anal. Acta 2010, 1, 104. [Google Scholar] [CrossRef]
- Sinha, S.; Murugesan, T.; Maiti, K.; Gayen, J.R.; Pal, M.; Saha, B. Evaluation of anti-inflammatory potential of Bergenia ciliata sternb. Rhizome extract in rats. J. Pharm. Pharmacol. 2001, 53, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Kakub, G.; Gulfraz, M. Cytoprotective effects of Bergenia ciliata sternb, extract on gastric ulcer in rats. Phytother. Res. 2007, 21, 1217–1220. [Google Scholar] [CrossRef]
- Bhandari, M.R.; Jong-Anurakkun, N.; Hong, G.; Kawabata, J. α-glucosidase and α-amylase inhibitory activities of nepalese medicinal herb pakhanbhed (Bergenia ciliata, haw.). Food Chem. 2008, 106, 247–252. [Google Scholar] [CrossRef]
- Mazhar-Ul-Islam, I.A.; Mazhar, F.; Usmanghani, K.; Gill, M.A. Evaluation of antibacterial activity of Bergenia ciliata. Pak. J. Pharm. Sci. 2002, 15, 21–27. [Google Scholar]
- Bagul, M.S.; Ravishankara, M.; Padh, H.; Rajani, M. Phytochemical evaluation and free radical scavenging properties of rhizome of Bergenia ciliata (haw.) sternb. Forma ligulata yeo. J. Nat. Remedies 2003, 3, 83–89. [Google Scholar]
- Sticher, O.; Soldati, F.; Lehmann, D. High-performance liquid chromatographic separation and quantitative determination of arbutin, methylarbutin, hydroquinone and hydroquinone-monomethylether in arctostaphylos, bergenia, calluna and vaccinium species [blueberry]. Planta Med. 1979, 35, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Miyaichi, Y.; Tomimori, T. Studies on nepalese crude drugs. XXII: On the phenolic constituents of the rhizome of Bergenia ciliata (haw.) sternb. Nat. Med. 1996, 50, 404–407. [Google Scholar]
- Sinha, S.; Murugesan, T.; Maiti, K.; Gayen, J.; Pal, B.; Pal, M.; Saha, B. Antibacterial activity of Bergenia ciliata rhizome. Fitoterapia 2001, 72, 550–552. [Google Scholar] [CrossRef]
- Mazhar-Ul-Islam, I.A.; Usmanghani, K.; Shahab-ud-Din, A. Antifungal activity evaluation of Bergenia ciliata. Pak. J. Pharmacol. 2002, 19, 1–6. [Google Scholar]
- Chowdhary, S.; Verma, K. Some peculiar structures in bergenia species growing in western himalaya. Nat. Sci. 2010, 8, 1–4. [Google Scholar]
- Rajkumar, V.; Guha, G.; Kumar, R.A.; Mathew, L. Evaluation of antioxidant activities of Bergenia ciliata rhizome. Rec. Nat. Prod. 2010, 4, 38–48. [Google Scholar]
- Zhang, Y.; Liao, C.; Li, J.; Liu, X. A review on resource status, bioactive ingredients, clinical applications and biological progress in bergenia. J. Med. Plant Res. 2011, 5, 4396–4399. [Google Scholar]
- Chauhan, R.; Ruby, K.; Dwivedi, J. Bergenia ciliata mine of medicinal properties: A review. Int. J. Pharm. Sci. Rev. Res 2012, 15, 20–23. [Google Scholar]
- Chauhan, R.; Ruby, K.; Dwivedi, J. Golden herbs used in piles treatment: A concise report. Int. J. Drug Dev. Res. 2012, 4, 50–68. [Google Scholar]
- Ruby, K.; Chauhan, R.; Sharma, S.; Dwivedi, J. Polypharmacological activities of bergenia species. Int. J. Pharm. Sci. Rev. Res. 2012, 13, 100–110. [Google Scholar]
- Patel, A.M.; Savadi, R.V. Pharmacognostic and phytochemical evaluation of Bergenia ciliata rhizome. Int. J. Pharm. Rev. Res. 2014, 4, 52–55. [Google Scholar]
- Ruby, K.; Sharma, S.; Chauhan, R.; Dwivedi, J. In-vitro antioxidant and hemorrhoidal potential of hydroethanolic leaf extracts of Bergenia ciliata, Bergenia ligulata and Bergenia stracheyi. Asian J. Plant Sci. Res. 2015, 5, 34–46. [Google Scholar]
- Srivastava, N.; Srivastava, A.; Srivastava, S.; Rawat, A.K.S.; Khan, A.R. Simultaneous quantification of syringic acid and kaempferol in extracts of bergenia species using validated high-performance thin-layer chromatographic-densitometric method. J. Chromatogr. Sci. 2015, 54, 460–465. [Google Scholar] [PubMed]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarova, M.N.; Makarov, V.G.; Wagner, H. Bergenia crassifolia (l.) Fritsch-pharmacology and phytochemistry. Phytomedicine 2014, 21, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Vaishali, A.S.; Vikas, M.D.; Krishnapriya, M.; Sanjeevani, G. Identification of potential antioxidants by in-vitro activity guided fractionation of Bergenia ligulata. Pharmacogn. Mag. 2008, 4, 79–84. [Google Scholar]
- Pozharitskaya, O.N.; Ivanova, S.A.; Shikov, A.N.; Makarov, V.G. Separation and evaluation of free radical-scavenging activity of phenol components of Emblica officinalis extract by using an HPTLC–DPPH method. J. Sep. Sci. 2007, 30, 1250–1254. [Google Scholar] [CrossRef]
- Vasi, I.; Kalintha, V. Chemical analysis of Bergenia lingulata roots. Comp. Physiol. Ecol. 1981, 6, 127–128. [Google Scholar]
- Ostrowska, B.; Gorecki, P.; Wolska, D. Investigation on possibility of utilization of bergenia leaves to therapeutics in place of arbutin and tannin raw materials deficiency. Pt. 2. Isolation of bergenin and a method of its quantitative determination [Bergenia crassifolia, Bergenia cordifolia]. Herba Polonica (Poland) 1989, 35, 117–122. [Google Scholar]
- Furmanowa, M.; Rapczewska, L. Bergenia crassifolia (l.) fritsch (bergenia): Micropropagation and arbutin contents. In Medicinal and Aromatic Plants IV; Springer: Berlin/Heidelberg, Germany, 1993; pp. 18–33. [Google Scholar]
- Golovchenko, V.; Bushneva, O.; Ovodova, R.; Shashkov, A.; Chizhov, A.; Ovodov, Y.S. Structural study of bergenan, a pectin from Bergenia crassifolia. Russ. J. Bioorg. Chem. 2007, 33, 47–56. [Google Scholar] [CrossRef]
- Roselli, M.; Lentini, G.; Habtemariam, S. Phytochemical, antioxidant and anti-α-glucosidase activity evaluations of Bergenia cordifolia. Phytother. Res. 2012, 26, 908–914. [Google Scholar] [CrossRef]
- Sun, X.; Huang, W.; Ma, M.; Guo, B.; Wang, G. Comparative studies on content of arbutin, bergenin and catechin in different part of Bergenia purpurascens and B. crassifolia. China J. Chin. Mater. Med. 2010, 35, 2079–2082. [Google Scholar]
- Chernetsova, E.S.; Crawford, E.A.; Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Morlock, G.E. ID-CUBE direct analysis in real time high-resolution mass spectrometry and its capabilities in the identification of phenolic components from the green leaves of Bergenia crassifolia L. Rapid Commun. Mass Spectrom. 2012, 26, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. The hidden treasure in europe’s garden plants: Case examples; Berberis darwinni and Bergenia cordifolia. Med. Aromat. Plants 2013, 2, 1–5. [Google Scholar]
- Chernetsova, E.S.; Shikov, A.N.; Crawford, E.A.; Grashorn, S.; Laakso, I.; Pozharitskaya, O.N.; Makarov, V.G.; Hiltunen, R.; Galambosi, B.; Morlock, G.E. Characterization of volatile and semi-volatile compounds in green and fermented leaves of Bergenia crassifolia L. By gas chromatography-mass spectrometry and id-cube direct analysis in real time-high resolution mass spectrometry. Eur. J. Mass Spectrom 2014, 20, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.-P.; Shikov, A.N.; Karonen, M.; Pozharitskaya, O.N.; Kim, J.; Makarov, V.G.; Hiltunen, R.; Galambosi, B. Rapid profiling of phenolic compounds of green and fermented Bergenia crassifolia l. Leaves by UPLC-DAD-QqQ-MS AND HPLC-DAD-ESI-QTOF-MS. Nat. Prod. Res. 2014, 28, 1530–1533. [Google Scholar] [CrossRef] [PubMed]
- Ogisu, M.; Rix, M. 572. Bergenia emeiensis: Saxifragaceae. Curtis’s Bot. Mag. 2007, 24, 2–6. [Google Scholar] [CrossRef]
- Bashir, S.; Gilani, A.H. Antiurolithic effect of Bergenia ligulata rhizome: An explanation of the underlying mechanisms. J. Ethnopharmacol. 2009, 122, 106–116. [Google Scholar] [CrossRef]
- Singh, N.; Juyal, V.; Gupta, A.K.; Gahlot, M. Evaluation of ethanolic extract of root of Bergenia ligulata for hepatoprotective, diuretic and antipyretic activities. J. Pharm. Res. 2009, 2, 958–960. [Google Scholar]
- Singh, N.; Juyal, V.; Gupta, A.; Gahlot, M.; Prashant, U. Antidiabetic activity of ethanolic extract of root of Bergenia ligulata in alloxan diabetic rats. Indian Drugs 2009, 46, 247–249. [Google Scholar]
- Kashima, Y.; Yamaki, H.; Suzuki, T.; Miyazawa, M. Insecticidal effect and chemical composition of the volatile oil from Bergenia ligulata. J. Agric. Food Chem. 2011, 59, 7114–7119. [Google Scholar] [CrossRef]
- Sajad, T.; Zargar, A.; Ahmad, T.; Bader, G.; Naime, M.; Ali, S. Antibacterial and anti-inflammatory potential Bergenia ligulata. Am. J. Biomed. Sci. 2010, 2, 313–321. [Google Scholar] [CrossRef]
- Nardev, S.; Gupta, A.; Vijay, J.; Renu, C. Study on antipyretic activity of extracts of Bergenia ligulata wall. Int. J. Pharma Bio Sci. 2010, 1, 1–5. [Google Scholar]
- Chauhan, R.; Saini, R.; Dwivedi, J. Secondary metabolites found in bergenia species: A compendious review. Int. J. Pharm. Pharm. Sci. 2013, 5, 9–16. [Google Scholar]
- Rajbhandari, M.; Wegner, U.; Schoepke, T.; Lindequist, U.; Mentel, R. Inhibitory effect of Bergenia ligulata on influenza virus A. Pharmazie 2003, 58, 268–271. [Google Scholar]
- Jain, M.; Gupta, K. Isolation of bergenin from Saxifraga ligulata wall. J. Ind. J. Chem. Soc. 1962, 39, 559–560. [Google Scholar]
- Tucci, A.P.; Delle, F.M.; Marini-Bettolo, G.B. The occurrence of (+) afzelechin in Saxifraga ligulata wall. Ann. Ist Super Sanita 1969, 5, 555–556. [Google Scholar]
- Bahl, C.; Murari, R.; Parthasarathy, M.; Seshadri, T. Components of Bergenia strecheyi & Bergenia ligulata. Indian J. Chem. 1974, 12, 1038–1039. [Google Scholar]
- Gehlot, N.; Sharma, V.; Vyas, D. Some pharmacological studies on ethanolic extract of Bergenia ligulata. Indian J. Pharmacol. 1976, 8, 92–94. [Google Scholar]
- Dix, B.; Srivastava, S. Tannin constituents of Bergenia ligulata roots. Ind. J. Nat. Prod. 1989, 5, 24–25. [Google Scholar]
- Reddy, U.D.C.; Chawla, A.S.; Deepak, M.; Singh, D.; Handa, S.S. High pressure liquid chromatographic determination of bergenin and (+)-afzelechin from different parts of paashaanbhed (Bergenia ligulata yeo). Phytochem. Anal. 1999, 10, 44–47. [Google Scholar] [CrossRef]
- Chauhan, S.K.; Singh, B.; Agrawal, S. Simultaneous determination of bergenin and gallic acid in Bergenia ligulata wall by high-performance thin-layer chromatography. J. AOAC Int. 2000, 83, 1480–1483. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.S.; Parekh, B.B.; Joshi, M.J.; Vaidya, A.D. Inhibition of the growth of urinary calcium hydrogen phosphate dihydrate crystals with aqueous extracts of tribulus terrestris and Bergenia ligulata. Urol. Res. 2005, 33, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Herbaceous flora of Jaunsar-Bawar (Uttarkhand), India: Enumerations. Phytotaxonomy 2012, 12, 33–56. [Google Scholar]
- Goswami, P.K.; Samant, M.; Srivastava, R.S. Multi faceted Saxifraga ligulata. Int. J. Res. Ayurveda Pharm. 2013, 4, 608–611. [Google Scholar] [CrossRef]
- Jani, S.; Shukla, V.J.; Harisha, C. Comparative pharmacognostical and phytochemical study on Bergenia ligulata wall. and Ammania buccifera linn. Ayu 2013, 34, 406–410. [Google Scholar] [CrossRef]
- Agnihotri, V.; Sati, P.; Jantwal, A.; Pandey, A. Antimicrobial and antioxidant phytochemicals in leaf extracts of Bergenia ligulata: A himalayan herb of medicinal value. Nat. Prod. Res. 2015, 29, 1074–1077. [Google Scholar] [CrossRef]
- Messaoudi, D.; Bouriche, H.; Demirtas, I.; Senator, A. Phytochemical analysis and hepatoprotective activity of Algerian Santolina chamaecyparissus. Extracts. Annu. Res. Rev. Biol. 2018, 25, 1–12. [Google Scholar] [CrossRef]
- Pushpalatha, H.B.; Pramod, K.; Devanathan, R.; Sundaram, R. Use of bergenin as an analytical marker for standardization of the polyherbal formulation containing Saxifraga ligulata. Pharmacogn. Mag. 2015, 11, S60. [Google Scholar] [CrossRef]
- Zuo, G.-Y.; Li, Z.-Q.; Chen, L.-R.; Xu, X.-J. In vitro anti-hcv activities of Saxifraga melanocentra and its related polyphenolic compounds. Antivir. Chem. Chemother. 2005, 16, 393–398. [Google Scholar] [CrossRef]
- Bajracharya, G.B.; Maharjan, R.; Maharjan, B.L. Potential antibacterial activity of Bergenia purpurascens. Nepal J. Sci. Technol. 2011, 12, 157–162. [Google Scholar] [CrossRef]
- Chen, W.; Nie, M. HPLC determination of bergenin in Astilbe chinensis (maxim.) franch. Et sav. And Bergenia purpurascens (hook. F. Et thoms.) engl. Acta Pharm. Sin. 1988, 23, 606–609. [Google Scholar]
- Li, B.-H.; Wu, J.-D.; Li, X.-L. LC–MS/MS determination and pharmacokinetic study of bergenin, the main bioactive component of Bergenia purpurascens after oral administration in rats. J. Pharm. Anal. 2013, 3, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Cao, L.; Chang, L.; Zhi, X.; Yuan, L.; Sheng, N.; Zhang, L.-T. Simultaneous determination of nine compounds in Bergenia purpurascens by HPLC-MS. Chin. Pharm. J. 2013, 6, 477–481. [Google Scholar]
- Shi, X.; Li, X.; He, J.; Han, Y.; Li, S.; Zou, M. Study on the antibacterialactivity of Bergenia purpurascens extract. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 464–468. [Google Scholar] [CrossRef]
- Ma, L. The antibacterial activity and antibacterial mechanism of Bergenia scopulosa TP Wang extract. Adv. J. Food Sci. Technol. 2014, 6, 994–997. [Google Scholar] [CrossRef]
- Cui, Y. Chemical constituents from rhizomes of Bergenia scopulosa (ⅱ). Chin. Tradi. Herb. Drugs 2012, 43, 1704–1707. [Google Scholar]
- Yao-yuan, W. Chemical constituents from Bergenia scopulosa (I). Chin. J. Exp. Tradit. Med Formulae 2012, 9, 154–156. [Google Scholar]
- Wei, L.; Si, M.; Long, M.; Zhu, L.; Li, C.; Shen, X.; Wang, Y.; Zhao, L.; Zhang, L. Rhizobacter bergeniae sp. Nov., isolated from the root of Bergenia scopulosa. Int. J. Syst. Evol. Microbiol. 2015, 65, 479–484. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Zhang, Y.; Xu, J. Experimental study on antitussive effect or arbutin. Yaoxue Tongbao 1982, 17, 720–722. [Google Scholar]
- Kumar, V.; Tyagi, D. Antifungal activity evaluation of different extracts of Bergenia stracheyi. Int. J. Curr. Microbiol. App. Sci. 2013, 2, 69–78. [Google Scholar]
- Kumar, V.; Tyagi, D. Phytochemical screening and free-radical scavenging activity of Bergenia stracheyi. J. Pharmacogn. Phytochem. 2013, 2, 175–180. [Google Scholar]
- Ali, I.; Bibi, S.; Hussain, H.; Bano, F.; Ali, S.; Khan, S.W.; Ahmad, V.U.; Al-Harrasi, A. Biological activities of Suaeda heterophylla and Bergenia stracheyi. Asian Pac. J. Trop. Dis. 2014, 4, S885–S889. [Google Scholar] [CrossRef]
- Yuldashev, M.; Batirov, È.K.; Malikov, V. Anthraquinones of Bergenia hissarica. Chem. Nat. Compd. 1993, 29, 543–544. [Google Scholar] [CrossRef]
- Izhaki, I. Emodin-a secondary metabolite with multiple ecological functions in higher plants. New Phytol. 2002, 155, 205–217. [Google Scholar] [CrossRef]
- Garimella, T.; Jolly, C.; Narayanan, S. In vitro studies on antilithiatic activity of seeds of Dolichos biflorus linn. and rhizomes of Bergenia ligulata wall. Phytother. Res. 2001, 15, 351–355. [Google Scholar] [CrossRef]
- Nagal, A.; Singla, R.K. Herbal resources with antiurolithiatic effects: A review. Indo Glob. J. Pharm. Sci. 2013, 3, 6–14. [Google Scholar]
- Satish, H.; Umashankar, D. Comparative study of methanolic extract of Bergenia ligulata yeo., with isolated constituent bergenin in urolithiatic rats. Biomed 2006, 1, 80–86. [Google Scholar]
- Voloboy, N.; Smirnov, I.; Bondarev, A. Features of diuretic activity of arbutin and hydroquinone. Sib. Med. J. 2012, 27, 131–134. [Google Scholar]
- Naik, S.; Kalyanpur, S.; Sheth, U. Effects of anti-inflammatory drugs on glutathione levels and liver succinic dehydrogenase activity in carrageenin edema and cotton pellet granuloma in rats. Biochem. Pharmacol. 1972, 21, 511–516. [Google Scholar] [CrossRef]
- Churin, A.; Masnaia, N.; Sherstoboev, E.Y.; Suslov, N. Effect of Bergenia crassifolia extract on specific immune response parameters under extremal conditions. Eksp. Klin. Farmakol. 2005, 68, 51–54. [Google Scholar]
- Popov, S.; Popova, G.Y.; Nikolaeva, S.Y.; Golovchenko, V.; Ovodova, R. Immunostimulating activity of pectic polysaccharide from Bergenia crassifolia (L.) fritsch. Phytother. Res. 2005, 19, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Nazir, N.; Koul, S.; Qurishi, M.A.; Taneja, S.C.; Ahmad, S.F.; Bani, S.; Qazi, G.N. Immunomodulatory effect of bergenin and norbergenin against adjuvant-induced arthritis-a flow cytometric study. J. Ethnopharmacol. 2007, 112, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Makarova, M.; Makarov, V. Molecular Biology of Flavonoids (Chemistry, Biochemistry, Pharmacology): Manual for Doctors; Lema Publishing: St-Petersburg, Russia, 2010; pp. 272–290. [Google Scholar]
- Ivanov, S.; Garbuz, S.; Malfanov, I.; Ptitsyn, L. Screening of Russian medicinal and edible plant extracts for angiotensin I-converting enzyme (ACE I) inhibitory activity. Russ. J. Bioorganic Chem. 2013, 39, 743–749. [Google Scholar] [CrossRef]
- Mansoor, M.; Bhagyarao, D.; Srinivasa Rao, D. Photochemical analysis and hepatoprotective activity of Saxifraga ligulata leaves extract. J. Sci. Res. Pharm. 2015, 4, 93–97. [Google Scholar]
- Shutov, D.V. Hepatoprotective effect of Bergenia crassifolia extract and silymarin at experimental inhibition of (3-oxidation of fatty acids caused by 4-pentenioc acid. Bull. Sib. Med. 2007, 7, 64–70. [Google Scholar]
- Rajkumar, V.; Guha, G.; Kumar, R.A. Anti-neoplastic activities of Bergenia ciliata rhizome. J. Pharm. Res. 2011, 4, 443–445. [Google Scholar]
- Zafar, R.; Ullah, H.; Zahoor, M.; Sadiq, A. Isolation of bioactive compounds from Bergenia ciliata (haw.) sternb rhizome and their antioxidant and anticholinesterase activities. BMC Complement. Altern. Med. 2019, 19, 296. [Google Scholar] [CrossRef]
- Shilova, I.; Pisareva, S.; Krasnov, E.; Bruzhes, M.; Pyak, A. Antioxidant properties of Bergenia crassifolia extract. Pharm. Chem. J. 2006, 40, 620–623. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarova, M.N.; Kovaleva, M.A.; Laakso, I.; Dorman, H.D.; Hiltunen, R.; Makarov, V.G.; Galambosi, B. Effect of Bergenia crassifolia L. Extracts on weight gain and feeding behavior of rats with high-caloric diet-induced obesity. Phytomedicine 2012, 19, 1250–1255. [Google Scholar] [CrossRef]
- Janar, J.; Fang, L.; Wong, C.P.; Kaneda, T.; Hirasawa, Y.; Morita, H.; Shahmanovna, B.; Abduahitovich, A. A new galloylbergenin from Bergenia crassifolia with anti-lipid droplet accumulation activity. Heterocycles 2012, 86, 1591–1595. [Google Scholar]
- Panossian, A.; Wikman, G.; Wagner, H. Plant adaptogens III. Earlier and more recent aspects and concepts on their mode of action. Phytomedicine 1999, 6, 287–300. [Google Scholar] [CrossRef]
- Tsyrenzhapova, O.D.; Lubsandorzhieva, P.B.; Bryzgalov, G.Y. Conservation of Biological Diversity in the Baikal Region: Problems, Approaches, Practice; Korsunov, V.M., Ed.; Baikal Scientific Center SB RAS: Ulan-Ude, Russia, 1996; pp. 157–158. (In Russian) [Google Scholar]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarova, M.N.; Dorman, H.D.; Makarov, V.G.; Hiltunen, R.; Galambosi, B. Adaptogenic effect of black and fermented leaves of Bergenia crassifolia L. in mice. J. Funct. Foods 2010, 2, 71–76. [Google Scholar] [CrossRef]
- Bolshunova, E.; Lamazhapova, G.; Zhamsaranova, S. Research of liposomal form of Bergenia crassifolia (L.) fritsch influence on formation of adaptation potencial of the body. ESSUTM Bull. 2010, 4, 83–88. [Google Scholar]
- Mironova, G.; Shigaeva, M.; Belosludtseva, N.; Gritsenko, E.; Belosludtsev, K.; Germanova, E.; Lukyanova, L. Effect of several flavonoid-containing plant preparations on activity of mitochondrial ATP-dependent potassium channel. Bull. Exp. Biol. Med. 2008, 146, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Yaginuma, A.; Murata, K.; Matsuda, H. Beta-gulcan and Bergenia ligulata as cosmetics ingredient. Fragrance J. 2003, 31, 114–119. [Google Scholar]
- Guo, H.; Song, K.; Chen, Q. The synthesis of two arbutin derivatives and inhibitory effect of them on tyrosinase. J. Xiamen Univ. (Nat. Sci.) 2004, 43, 1–4. [Google Scholar]
- Lee, K.-T.; Lee, S.-y.; Lee, K.-S.; Jeong, J.-H. Cosmetic Composition for Remedying Skin Wrinkles Comprising Bergenia emeiensis Extract as Active Ingredient. Google Patents US20040115286A1, 2004. [Google Scholar]
- Shrestha, U.K.; Pant, B. Production of bergenin, an active chemical constituent in the callus of Bergenia ciliate (Haw.) Sternb. Botanica Orientalis. J. Plant Sci. 2011, 8, 40–44. [Google Scholar]
- Verma, R.; Parkash, V.; Kumar, D. Ethnomedicinal uses of some plants of Kanag Hill in Shimla, Himachal Pradesh, India. Int. J. Res. Ayurveda Pharm. 2012, 3, 319–322. [Google Scholar]
- Rafi, S.; Kamili, A.N.; Ganai, B.A.; Mir, M.Y.; Parray, J.A. In vitro Culture and Biochemical Attributes of Bergenia ciliate (Haw.) Sternb. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 609–619. [Google Scholar] [CrossRef]
- Liu, M.; Hao, X.Y.; Xu, Q.; Bo, L.T.; Kang, X.L.; Wang, X.J. Tissue culture of wild flower Bergenia crassifolia and establishment of its regeneration system. J. Anhui. Agric. Sci. 2009, 37, 3455–3456. [Google Scholar]
- Parveen, S.; Kamili, A.N. In vitro shoot multiplication response from shoot tips of Bergenia ligulata Engl. on different nutrient media-A comparative study. Int. J. Innov. Res. Dev. 2013, 2, 65–67. [Google Scholar]
- Lu, X.M.; Wang, J.X. Research advancement on Bergenia genus plants. Chin. Med. Mat. 2003, 26, 58–60. [Google Scholar]

| Bergenia Species | Distribution | Medicinal Property | Part Used | Chemical Constituents (Structure Number) | Reference(s) |
|---|---|---|---|---|---|
Bergenia ciliata (Haw.) Sternb. | Central Asia, Afghanistan to China, Himalayan region. Altitude range (1800–3000 m) | Analgesic, Antiarrhythmic, Antiwrinkle, Antiasthma, Antibacterial, Anticancer, Antidiabetic, Antidiarrheal, Antidotary, Antiepileptic, Antiflatulent, Antifungal, Anti-haemorrhoidal, Antiviral, Anti-inflammatory, Antilithiatic, Antimalaria; Antimenorrhagic, Antiobesity, Antiophthalmia, Antioxidant, Antipyretic, Antispasmodic, Antiulcer, Burn wound healing, Deobstruent, Cerebroprotective, Diuretic, Ecbolic, Emmenagogue, Expectorant, Hepatoprotective, Immunomodulatory, Pulmonary affection | Whole plant | Bergenin (1) a Catechin (2) a Gallic acid (3) a β-Sitosterol (4) d Catechin-7-O-glucoside (5) a Afzelechin (6) a Quercetin-3-O-β-d-xylopyranoside (7) a Quercetin-3-O-α-l-arbinofuranoxide (8) a Eryodictiol-7-O-β-d-glucopyranoside (9) a Arbutin (10) c 6-O-p-Hydroxybenzoyl arbutin (11) a 4-O-Galloylbergenin (12) a 11-O-Galloylbergenin (13) a p-Hydroxybenzoic acid (14) f Protocatechuic acid (15) a 6-O-Protocatechuoyl arbutin (16) a 11-O-p-Hydroxybenzoyl bergenin (17) a 11-O-Protocatechuoyl bergenin (18) a 6-O-p-Hydroxybenzoyl parasorboside (19) a | [11,16,31,43,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91] |
Bergenia crassifolia (L.) Fritsch [Synonym: Bergenia cordifolia (Haw.) Sternb.] | North Eastern Asia. Altitude range (200–2000 m) | Antihypertensive, Anti-inflammatory, Antilithiatic, Antiobesity, Antioxidant, Antipyretic, Cerebroprotective, Diuretic, Hepatoprotective, Immunomodulatory | Whole plant | Ellagitannins (20) a Gallic acid (3) a Arbutin (10) c Bergenin (1) a Caffeoylquinic acid (21) c Monogalloylquinic acid (22) c 2,4,6-Tri-O-galloyl-β-d-glucose (23) a Pedunculagin (24) a Tellimagrandin I (25) a Catechin-7-O-β-d-glucoside (5) a Paashanolactone (26) b Catechin (2) a β-Sitosterol (4) b 2,4-Heptadienal (27) f Benzaldehyde (28) f Benzeneacetaldehyde (29) f Decadienal (30) f Decanal (31) f Dimethylcyclohexene acetaldehyde (32) f (E)-2-Decenal (33) f (E)-2-Nonenal (34) f Nonanal (35) f p-Menthenal (36) f (E)-β-Damascenone (37) e (E)-β-Damascone (38) e 3-Thujen-2-one (39) e Caryophyllene (40) e Cedranol (41) e (E)-2-Decenol (42) e Farnesol (43) e Farnesyl acetone (44) e Geraniol (45) e Geranyl acetone (46) e Hexahydrofarnesyl acetone (47) e Ionone (48) e Linalool (49) e m-Mymene (50) e Nerolidol (51) e Phytol (52) e p-Menth-1-en-4-ol (53) e Prenol (54) e Thymol (55) e α-Bisabolol (56) e α-Bisabololoxide B (57) e α-Cadinol (58) e α-Terpineol (59) e β-Elemene (60) e β-Eudesmol (61) e δ-Cadinene (62) e 11-O-(p-Hydroxybezoyl)bergenin (63) a 3,11-Di-O-galloylbergenin (64) a 4,11-Di-O-galloylbergenin (65) a Bergapten (66) a Kaempferol-3-O-xylosylgalactoside (67) a Kaempferol-3-O-xylosylglucoside (68) a Kaempferol-3-O-arabinoside (69) a Kaempferol-3-O-rutinoside (70) a Norathyriol (71) a Norbergenin (72) a Quercetin-3-O-xylosylgalactoside (73) a Quercetin-3-O-xylosylglucoside (74) a Quercetin-3-O-arabinoside (75) a Quercetin-3-O-galactoside (76) a Quercetin-3-O-glucoside (77) a Quercetin-3-O-rhamnoside (78) a Quercetin-3-O-rutinoside (79) a Quercetin-3-O-xyloside (80) a Trihydroxycoumarin (81) a (+)-Catechin-3,5-di-O-gallate (82) a (+)-Catechin-3-O-gallate (83) a 1,2,4,6-Tetra-O-galloy-β-d-glucopyranose (84) a 1-O-Galloylglucose (85) a 2-O-Caffeoylarbutin (86) a 6-O-Galloylarbutin (87) a Ellagic acid (88) a Hydroquinone (89) a p-Galloyloxyphenyl-β-d-glucoside (90) a Pyrogallol (91) a Acetylsalicylic acid (92) f Fumaric acid (93) f Furancarboxylic acid (94) f Protocatechuic acid (15) f Malic acid (95) f Quinic acid (96) f 4-Methoxystyrene (97) f 9,12-Octadecadienoic acid (98) f 9-Octadecenoic acid (99) f Decanoic acid (100) f Dodecanoic acid (101) f Hexadecanoic acid (102) f n-Cetyl alcohol (103) f n-Eicosanol (104) f n-Hentriacontane (105) f n-Heptacosane (106) f n-Nonacosane (107) f Nonanoic acid (108) f n-pentacosane (109) f Pentadecanoic acid (110) f Rhododendrin (111) f Stearic acid (112) f Tetradecanoic acid (113) f Tetramethyl hexadecenol (114) f Trimethyl dihydronaphthalene (115) f Trimethyl-3-methylene hexadecatetraene (116) f | [14,28,31,64,79,85,92,93,94,95,96,97,98,99,100,101,102,103,104] |
Bergenia emeiensis C.Y. Wu ex J.T. Pan. | China. Altitude range (1600–4200 m) | Antiwrinkle, Anti-inflammatory, Antiobesity, Antioxidant | Whole plant | Bergenin (1) a Tannic acid (117) a Arbutin (10) c | [31,65,85,105] |
Bergenia ligulata Wall. Engl. [Accepted name: Bergenia pacumbis (Buch.-Ham. Ex D. Don.) C.Y. Wu & J.T. Pan] | Temperate Himalayas. Altitude range (2134–3048 m) | Analgesic, Antiarrhythmic, Anticancer, Antidiabetic, Antifungal, Anti-haemorrhoidal, Antiviral, Anti-inflammatory, Antilithiatic, Antiprotozoal, Antipyretic, Antiscorbutic, Antispasmodic, Antitumor, Antiulcer, Astringent, burn wound healing, Cerebroprotective, Diuretic, Expectorant, Hepatoprotective, Immunomodulatory | Root, Rhizome | Bergenin (1) a Gallic acid (3) a Tannic acid (117) a Arbutin (10) c Catechin (2) a β-Sitosterol (4) b Stigmasterol (118) d Afzelechin (6) a 1,8-Cineole (119) e Isovaleric acid (120) f (+)-(6S)-Parasorbic acid (121) b Terpinen-4-ol (122) e (Z)-asarone (123) f Leucocyanidin (124) a Methyl gallate (125) a Sitoinoside I (126) d β-Sitosterol-d-glucoside (127) d Avicularin (128) a Eriodictyol-7-O-β-d-glucopyranoside (9) a Reynoutrin (129) a 11-O-Galloylbergenin (13) a Pashaanolactone (26) b Catechin-7-O-glucoside (5) a Coumarin (130) b 11-O-p-Hydroxybenzoyl bergenin (17) a 11-O-Protocatechuoyl bergenin (18) a 4-O-Galloylbergenin (12) a 6-O-p-Hydroxybenzoyl arbutin (11) a Hexan-5-olide (131) b Quercetin (132) a β-Sitosterol-d-glucoside (127) d | [3,16,23,24,26,37,61,73,78,83,85,86,87,88,90,95,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127] |
Bergenia purpurascens (Hook.f. & Thomson) Engl. | Eastern Himalayas. Altitude range (2800–4800 m) | Antibacterial, Anti-inflammatory, Antilithiatic, Antipyretic | Rhizome | Catechin (2) a Gallic acid (3) a Bergenin (1) a Arbutin (10) c 1,2,3,4,6-Penta-O-galloyl-β-d-glucose (133) a 4,6-Di-O-galloyl-β-d-glucose (134) a 6-O-Galloylarbutin (87) a 11-O-Galloylbergenin (13) a 4-O-Galloylbergenin (12) a 2,3,4,6-Tetra-O-galloyl-β-d-glucose (84) a Procyanidin B1 (135) a 2,4,6-Tri-O-galloyl-β-d-glucose (23) a Procyanidin B3 (136) a | [31,44,56,62,85,95,100,128,129,130,131,132,133] |
Bergenia scopulosa (T.P. Wang) | China. Altitude range (2400–3600 m) | Anti-hypertensive, Anti-inflammatory, Antiobesity, Antioxidant, Antitussive, Cerebroprotective, Diuretic, Hepatoprotective, Immunomodulatory | Leaf, Root, Rhizome | Bergenin (1) a Arbutin (10) c Catechin (2) a β-Sitosterol (4) d 6-O-Galloylarbutin (87) a Catechin-7-O-β-d-glucopyranoside (5) a Phenylalanine (137) f Succinic acid (138) f Protocatechuic acid (15) a Gallic acid (3) a Methyl gallate (125) a Quercetin (133) a Hyperoside (139) a Quercetin-3-O-rutinoside (79) a Afzelin (140) a Chrysophanol-8-O-β-d-glucopyranoside (141) c 11-O-Galloylbergenin (13) a | [31,59,66,67,85,134,135,136,137,138] |
Bergenia stracheyi (Hook. f. & Thomas) Engl. | Afghanistan, Pakistan, Nepal. Altitude range (3000–4600 m) | Antifungal, Anti-haemorrhoidal, Anti-inflammatory, Antilithiatic, Antiobesity, Antioxidant, Poultice to treat the stiff joints | Rhizome | Bergenin (1) a (+)-Catechin-3-O-gallate (83) a Gallic acid (3) a Tannic acid (117) a Phytol (142) e Caryophyllene (40) e Damascenone (143) f β-Eudesmol (144) e 3-Methyl-2-buten-1-ol (145) e | [13,83,85,86,88,90,116,139,140,141] |
Bergenia hissarica (A. Boriss.) | Central Asia, Uzbekistan, Hissar. Altitude range (1200–1600 m) | Stimulantlaxative, Neuroprotective, Antioxidant | Root, Rhizome | Aloe emodin (146) c Aloeemodin-8-O-β-d-glucoside (147) c Chrysophanein (148) c Emodin-1-O-β-d-glucoside (149) c Physeion (150) d | [142,143] |
Bergenia tianquanesis (J.T. Pan) | China. Altitude range (2200–3400 m) | Not reported | [29,32] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koul, B.; Kumar, A.; Yadav, D.; Jin, J.-O. Bergenia Genus: Traditional Uses, Phytochemistry and Pharmacology. Molecules 2020, 25, 5555. https://doi.org/10.3390/molecules25235555
Koul B, Kumar A, Yadav D, Jin J-O. Bergenia Genus: Traditional Uses, Phytochemistry and Pharmacology. Molecules. 2020; 25(23):5555. https://doi.org/10.3390/molecules25235555
Chicago/Turabian StyleKoul, Bhupendra, Arvind Kumar, Dhananjay Yadav, and Jun-O. Jin. 2020. "Bergenia Genus: Traditional Uses, Phytochemistry and Pharmacology" Molecules 25, no. 23: 5555. https://doi.org/10.3390/molecules25235555
APA StyleKoul, B., Kumar, A., Yadav, D., & Jin, J.-O. (2020). Bergenia Genus: Traditional Uses, Phytochemistry and Pharmacology. Molecules, 25(23), 5555. https://doi.org/10.3390/molecules25235555









