Coptis chinensis Franch Directly Inhibits Proteolytic Activation of Kallikrein 5 and Cathelicidin Associated with Rosacea in Epidermal Keratinocytes
Abstract
1. Introduction
2. Results
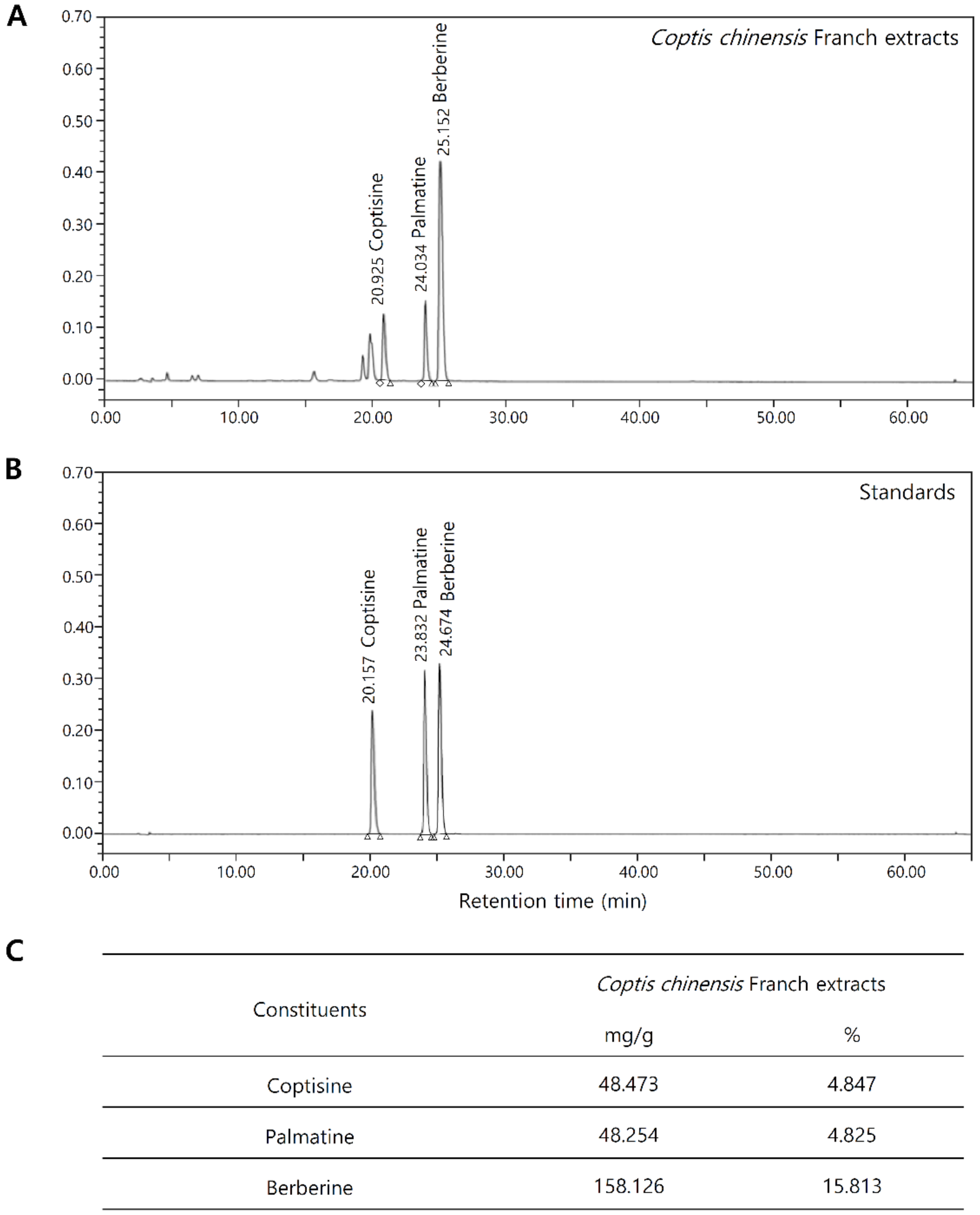
2.1. Chemical Composition Analysis of Coptis Chinensis Franch
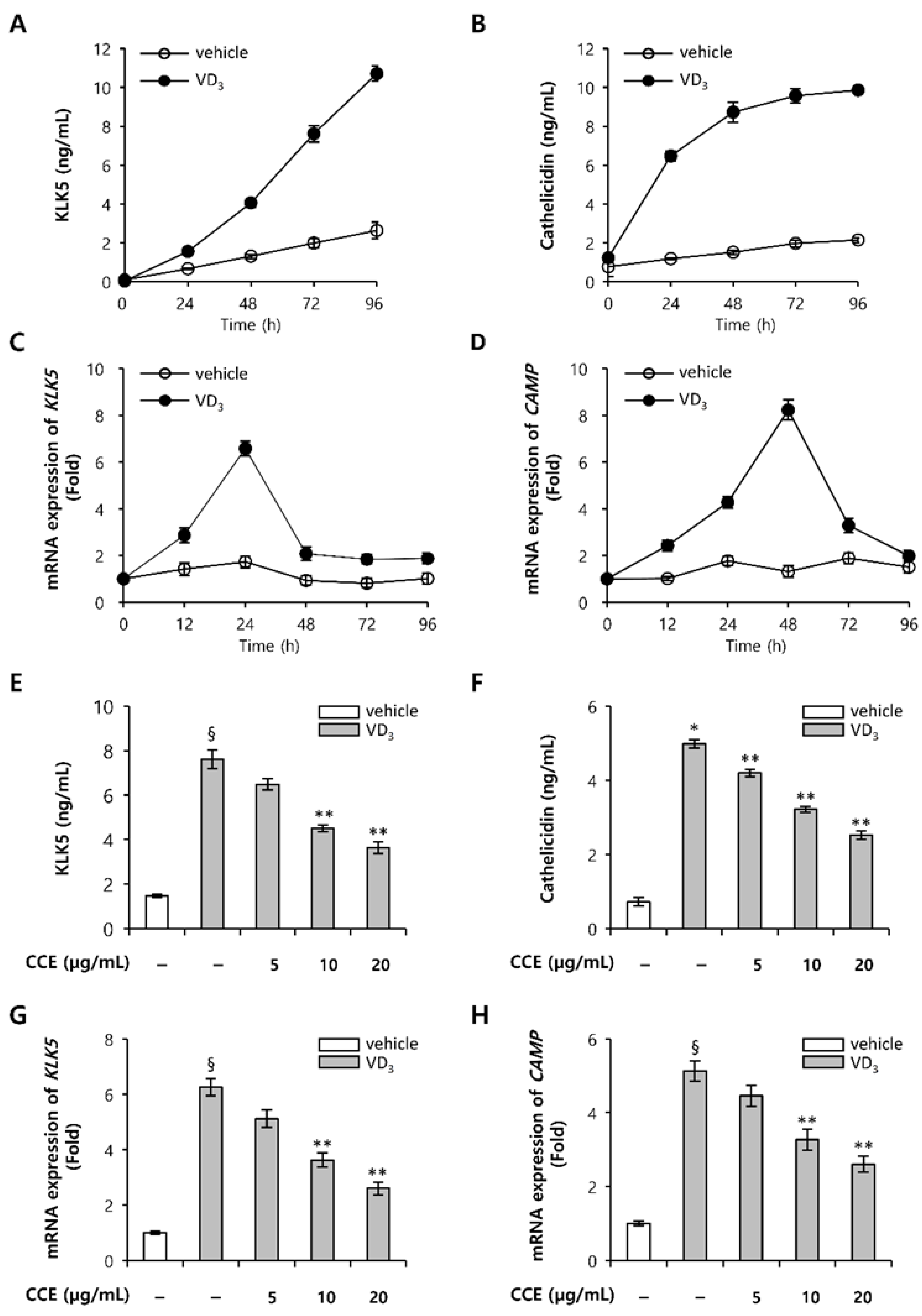
2.2. CCE Decrease KLK5 and Cathelicidin Expression in Epidermal Keratinocytes In Vitro
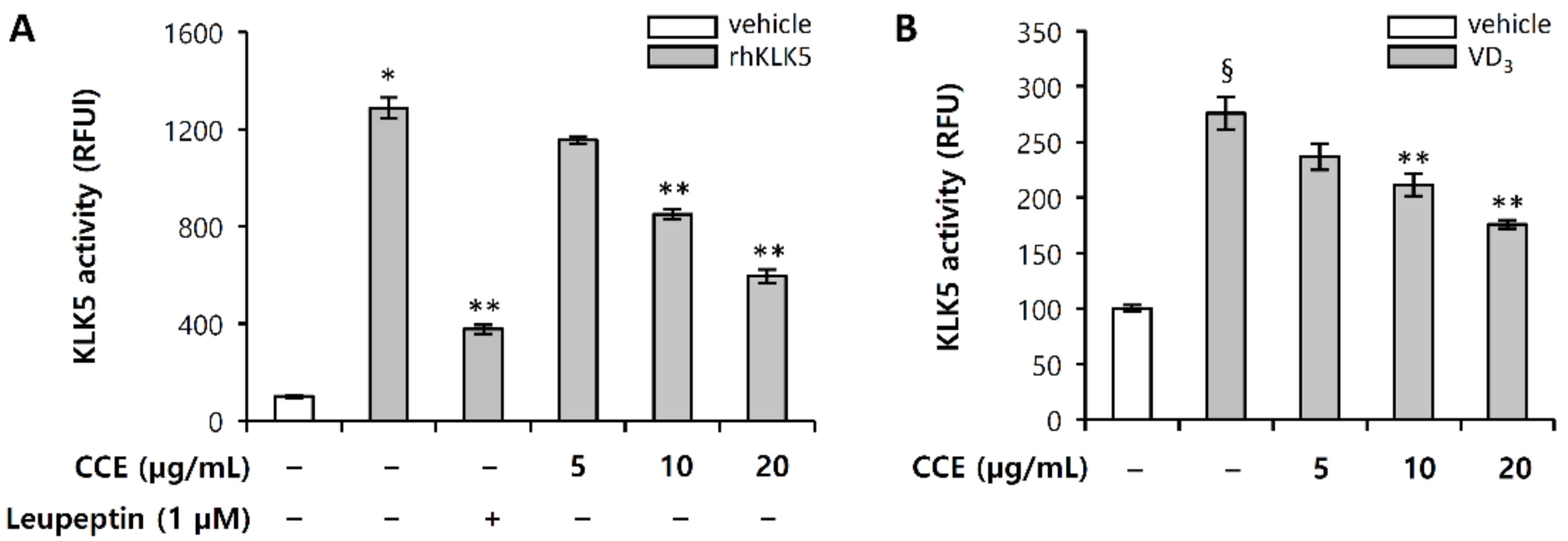
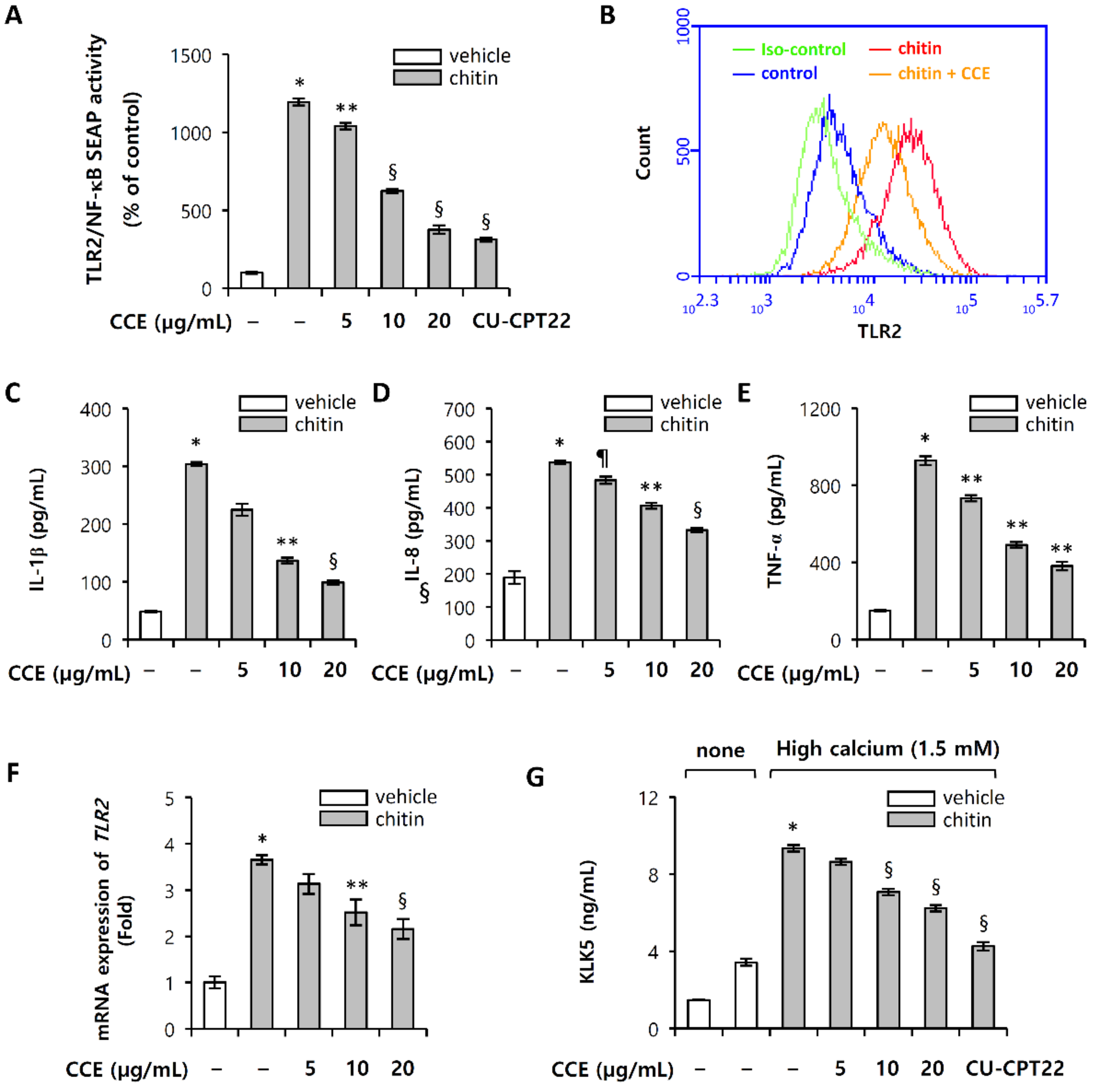
2.3. CCE Decrease Cutaneous Trypsin-Like Serine Protease (KLK5) Activity
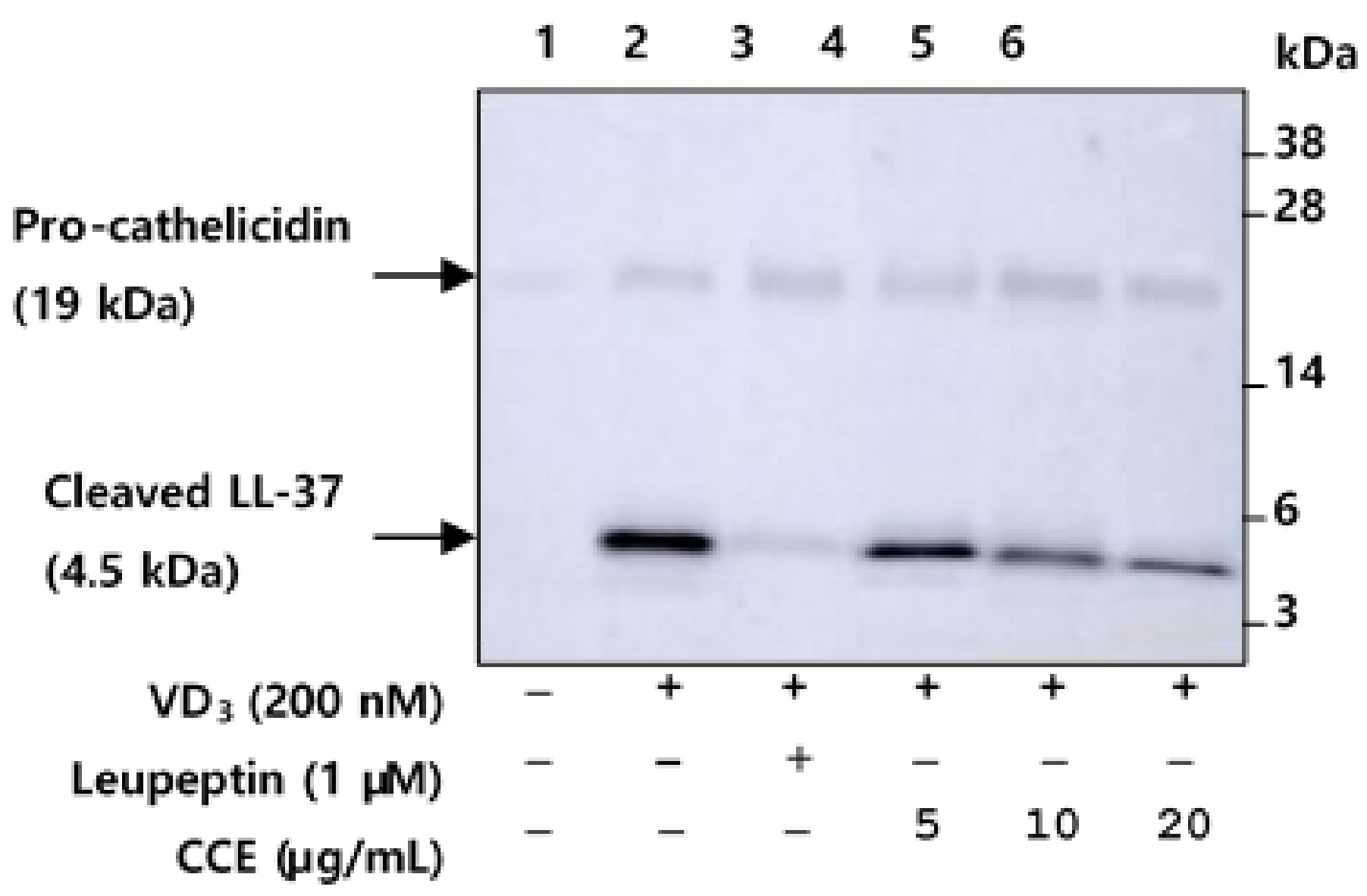
2.4. CCE Inhibit the Proteolytic Cleavage of Cathelicidin by Inhibiting KLK5 Expression and Protease Activity
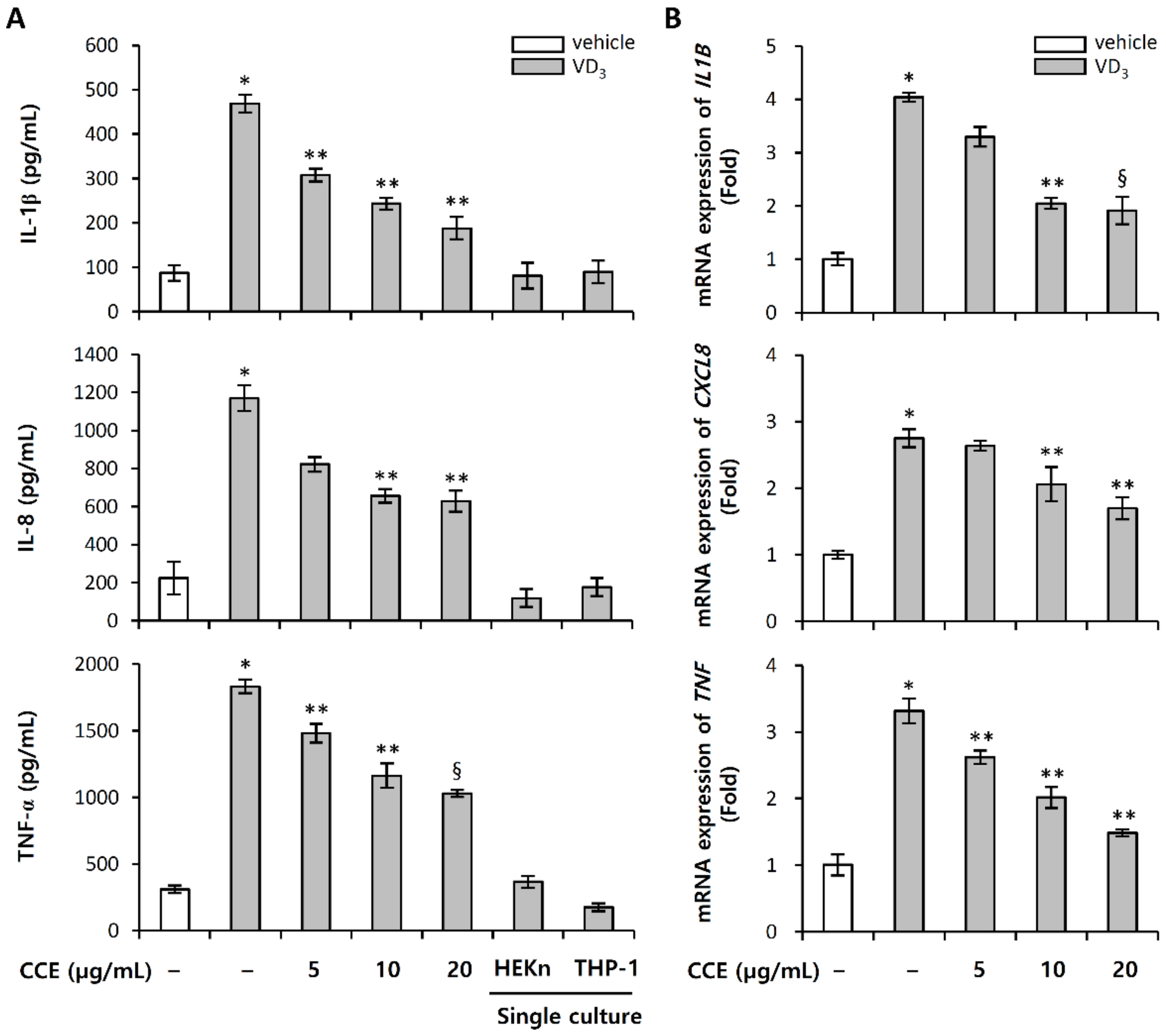
2.5. CCE Decrease the Expression of Pro-Inflammatory Cytokines Induced by VD3
2.6. Chitin-Induced Inflammatory Responses Are Inhibited by CCE
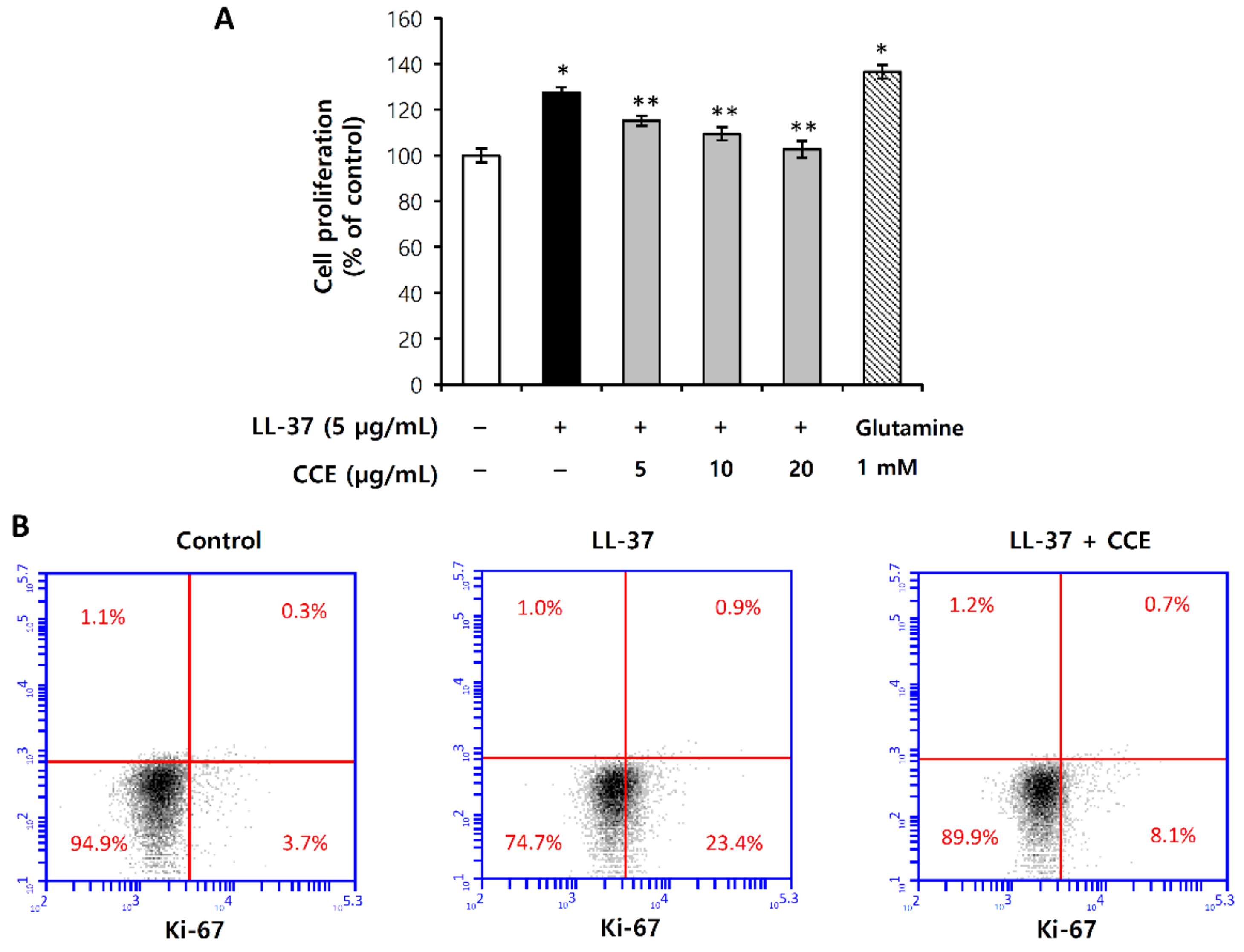
2.7. LL-37-Induced Vascular Endothelial Cell Proliferation Was Inhibited by CCE
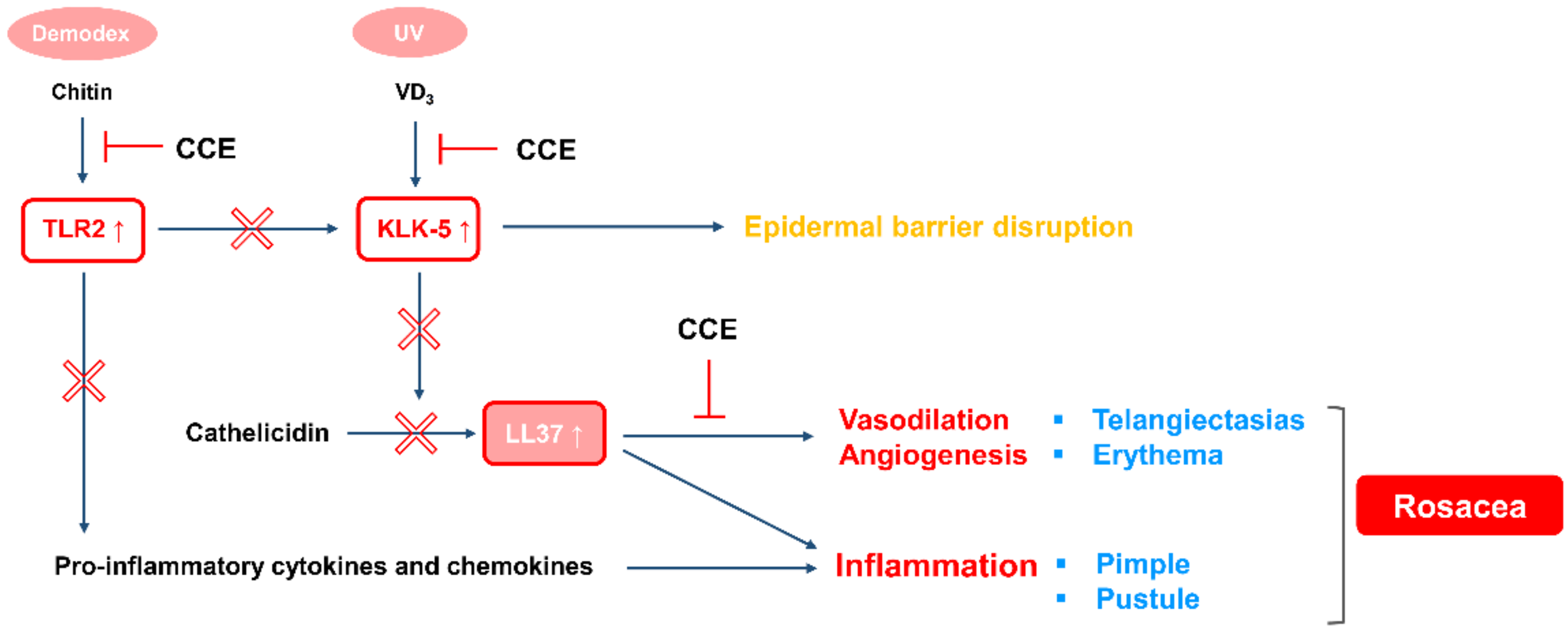
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of Coptis Chinensis Franch Extract (CCE)
4.3. Cell Culture
4.4. High-Performance Liquid Chromatography (HPLC)
4.4.1. Chemical Standards and Solvents
4.4.2. Preparation of Standard and CCE Solution
4.4.3. Validation of the Method
4.5. TLR2/NF-κB/SEAP Activity
4.6. TLR2 Expression Analysis
4.7. HMEC-1 Proliferation Assay
4.7.1. WST Assay
4.7.2. Ki-67 Assay
4.8. Preparation of Chitin Particles
4.9. KLK5 Protease Activity
4.10. Cathelicidin Cleavage Assay
4.11. Co-Culture Conditions
4.12. Total RNA Extraction, cDNA Synthesis, and Quantitative PCR
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| KLK5 | Kallikrein-related peptidase 5 |
| VD3 | 1,25 (OH)2 vitamin D3 |
| TLR2 | Toll-like receptor 2 |
| CC | Coptis chinensis Franch |
| CCE | Coptis chinensis Franch water Extract |
| HEKn | Human Epidermal Keratinocytes |
| CAMP | Cathelicidin Antimicrobial Peptides |
| Boc-V-P-R-AMC | Boc-Val-Pro-Arg-7-amido-4-methylcoumarin hydrochloride |
| SCTE | Stratum Corneum Tryptic Enzyme |
| RFU | Relative Fluorescence Units |
| rhKLK5 | recombinant human KLK5 |
| FITC | Fluorescein Isothiocyanate |
| HMEC-1 | Human Microvascular Endothelial Cells |
| HPLC | High-Performance Liquid Chromatography |
| TFA | Trifluoroacetic Acid |
| ICH | International Conference on Harmonisation |
| WST | Water-soluble Tetrazolium salt |
References
- Two, A.M.; Del Rosso, J.Q. Kallikrein 5-mediated inflammation in rosacea: Clinically relevant correlations with acute and chronic manifestations in rosacea and how individual treatments may provide therapeutic benefit. J. Clin. Aesthet. Dermatol. 2014, 7, 20–25. [Google Scholar]
- Yamasaki, K.; Schauber, J.; Coda, A.; Lin, H.; Dorschner, R.A.; Schechter, N.M.; Bonnart, C.; Descargues, P.; Hovnanian, A.; Gallo, R.L. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006, 20, 2068–2080. [Google Scholar] [CrossRef]
- Meyer-Hoffert, U.; Schroder, J.M. Epidermal proteases in the pathogenesis of rosacea. J. Investig. Dermatol. Symp. Proc. 2011, 15, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Gallo, R.L. Rosacea as a disease of cathelicidins and skin innate immunity. J. Investig. Dermatol. Symp. Proc. 2011, 15, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Kanada, K.; Macleod, D.T.; Borkowski, A.W.; Morizane, S.; Nakatsuji, T.; Cogen, A.L.; Gallo, R.L. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J. Invest. Dermatol. 2011, 131, 688–697. [Google Scholar] [CrossRef]
- Yamasaki, K.; Di Nardo, A.; Bardan, A.; Murakami, M.; Ohtake, T.; Coda, A.; Dorschner, R.A.; Bonnart, C.; Descargues, P.; Hovnanian, A.; et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. 2007, 13, 975–980. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Q.; Schmidt, A.P.; Anderson, G.M.; Wang, J.M.; Wooters, J.; Oppenheim, J.J.; Chertov, O. LL-37, the Neutrophil granule–and epithelial cell–derived cathelicidin, utilizes formyl peptide receptor–like 1 (Fprl1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 2000, 192, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Wang, Z.; Vanderberghe, M.; Two, A.; Gallo, R.L.; Nardo, A.D. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J. Invest. Dermatol. 2014, 134, 2728–2736. [Google Scholar] [CrossRef]
- Reinholz, M.; Ruzicka, T.; Schauber, J. Cathelicidin LL-37: An antimicrobial peptide with a role in inflammatory skin disease. Ann. Dermatol. 2012, 24, 126–135. [Google Scholar] [CrossRef]
- Steinhoff, M.; Schauber, J.; Leyden, J. New insights into rosacea pathophysiology: A review of recent findings. J. Am. Acad. Dermatol. 2013, 69, s15–s26. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, Y.J.; Lim, B.J.; Sohn, H.J.; Shin, D.; Oh, S.H. Increased expression of cathelicidin by direct activation of protease-activated receptor 2: Possible implications on the pathogenesis of rosacea. Yonsei. Med. J. 2014, 55, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Fioranelli, M.; Roccia, M.G.; di Nardo, V.; Aracena, C.J.; Lotti, T. Vitamin D supplementation for childhood atopic dermatitis. Dermatol. Ther. 2016, 29, 303. [Google Scholar] [CrossRef] [PubMed]
- Bonnar, E.; Eustace, P.; Powell, F.C. The Demodex mite population in rosacea. J. Am. Acad. Dermatol. 1993, 28, 443–448. [Google Scholar] [CrossRef]
- Forton, F.; Seys, B. Density of Demodex folliculorum in rosacea: A case-control study using standardized skin-surface biopsy. Br. J. Dermatol. 1993, 128, 650–659. [Google Scholar] [CrossRef]
- Koller, B.; Muller-Wiefel, A.S.; Rupec, R.; Korting, H.C.; Ruzicka, T. Chitin modulates innate immune responses of keratinocytes. PLoS ONE 2011, 6, e16594. [Google Scholar] [CrossRef]
- Casas, C.; Paul, C.; Lahfa, M.; Livideanu, B.; Lejeune, O.; Alvarez-Georges, S.; Saint-Martory, C.; Degouy, A.; Mengeaud, V.; Ginisty, H.; et al. Quantification of Demodex folliculorum by PCR in rosacea and its relationship to skin innate immune activation. Exp. Dermatol. 2012, 21, 906–910. [Google Scholar] [CrossRef]
- Culp, B.; Scheinfeld, N. Rosacea: A review. P. T. 2009, 34, 38–45. [Google Scholar]
- Matsubara, M.; Matsumoto, Y.; Koseki, J.; Kaneko, A.; Aiba, S.; Yamasaki, K. Inhibition of human Kallikrein 5 protease by triterpenoids from natural sources. Molecules 2017, 22, 1829. [Google Scholar] [CrossRef]
- Kathy, K.A.; Joshua, K.K. Coptis chinensis inhibits hepatocellular carcinoma cell growth through nonsteroidal anti-inflammatory drug-activated gene activation. Int. J. Mol. Med. 2009, 24, 571–577. [Google Scholar]
- Enk, R.; Ehehalt, R.; Graham, J.E.; Bierhaus, A.; Remppis, A.; Greten, H.J. Differential effect of Rhizoma coptidis and its main alkaloid compound berberine on TNF-alpha induced NFkappaB translocation in human keratinocytes. J. Ethnopharmacol. 2007, 109, 170–175. [Google Scholar] [CrossRef]
- Zhen, Z.; Chang, B.; Li, M.; Lian, F.M.; Chen, L.; Dong, L.; Wang, J.; Yu, B.; Liu, W.K.; Li, X.Y.; et al. Anti-diabetic effects of a Coptis chinensis containing new traditional Chinese medicine formula in type 2 diabetic rats. Am. J. Chin. Med. 2011, 39, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Friedemann, T.; Schumacher, U.; Tao, Y.; Kai-Man, L.A.; Schröder, S. Neuroprotective activity of coptisine from Coptis chinensis (Franch). Evid. Based Complement. Alternat. Med. 2015, 2015, 827308. [Google Scholar] [CrossRef]
- Francesca, S.L. Anti-microbial properties of Scutellaria baicalensis and Coptis chinensis, two traditional Chinese medicines. Biosci. Horiz. 2011, 4, 119–127. [Google Scholar]
- Ni, L.; Li, J.; Zhang, W.; Zhou, Z.; Liu, H.; Tian, J.; Liu, H.; Ren, H. Coptis chinensis inhibits growth and metastasis and induces cell apoptosis in non-small cell lung cancer cells. Int. J. Clin. Exp. Med. 2017, 10, 16037–16048. [Google Scholar]
- Aoyagi, T.; Takeuchi, T.; Matsuzaki, A.; Kawamura, K.; Kondo, S. Leupeptins, new protease inhibitors from Actinomycetes. J. Antibiot. 1969, 22, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, K.; Cardona, G.Y.; Wolz, O.O.; Herster, F.; Sharma, L.; Dillen, C.A.; Täumer, C.; Dickhöfer, S.; Bittner, Z.; Dang, T.M.; et al. The fungal ligand chitin directly binds TLR2 and triggers inflammation dependent on oligomer size. EMBO Rep. 2018, 19, e46065. [Google Scholar] [CrossRef] [PubMed]
- Koczulla, R.; von Degenfeld, G.; Kupatt, C.; Krötz, F.; Zahler, S.; Gloe, T.; Issbrücker, K.; Unterberger, P.; Zaiou, M.; Lebherz, C.; et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J. Clin. Invest. 2003, 111, 1665–1672. [Google Scholar] [CrossRef]
- Berkestedt, I.; Nelson, A.; Bodelsson, M. Endogenous antimicrobial peptide LL-37 induces human vasodilatation. Br. J. Anaesth. 2008, 100, 803–809. [Google Scholar] [CrossRef]
- Kim, B.; Li, J.; Jang, C.; Arany, Z. Glutamine fuels proliferation but not migration of endothelial cells. EMBO J. 2017, 36, 2321–2333. [Google Scholar] [CrossRef]
- Brattsand, M.; Egelrud, T. Purification, molecular cloning, and expression of a human stratum corneum trypsin-like serine protease with possible function in desquamation. J. Biol. Chem. 1999, 274, 30033–30040. [Google Scholar] [CrossRef]
- Caubet, C. Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J. Invest. Dermatol. 2004, 122, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. Stratum corneum defensive functions: An integrated view. J. Invest. Dermatol. 2005, 125, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Hachem, J.P.; Houben, E.; Crumrine, D.; Man, M.Q.; Schurer, N.; Roelandt, T.; Choi, E.H.; Uchida, Y.; Brown, B.E.; Feingold, K.R.; et al. Serine protease signaling of epidermal permeability barrier homeostasis. J. Invest. Dermatol. 2006, 126, 2074–2086. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, N.; Takata, M.; Otsuki, N.; Ohka, R.; Amano, O.; Takehara, K.; Saijoh, K. Elevated stratum corneum hydrolytic activity in netherton syndrome suggests an inhibitory regulation of desquamation by SPINK5-derived peptides. J. Invest. Dermatol. 2002, 118, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mu, W.; Shang, H.; Lin, J.; Lei, X. The antihyperglycemic effects of Rhizoma Coptidis and mechanism of actions: A review of systematic reviews and pharmacological research. BioMed. Res. Int. 2014, 2014, 798093. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeng, M.; Zhang, Y.; Bhat, I.; Wazer, D.E.; Band, H.; Band, V. The human kallikrein 10 promoter contains a functional retinoid response element. Biol. Chem. 2006, 387, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Matigian, N.; Harvey, T.J.; Samaratunga, H.; Hooper, J.D.; Clements, J.A. Tissue-specific promoter utilisation of the kallikrein-related peptidase genes, KLK5 and KLK7, and cellular localisation of the encoded proteins suggest roles in exocrine pancreatic function. Biol. Chem. 2008, 389, 99–109. [Google Scholar] [CrossRef]
- Brattsand, M.; Stefansson, K.; Lundh, C.; Haasum, Y.; Egelrud, T.A. Proteolytic cascade of kallikreins in the stratum corneum. J. Invest. Dermatol. 2005, 124, 198–203. [Google Scholar] [CrossRef]
- Rather, P.A.; Hassan, I. Human Demodex Mite: The Versatile Mite of Dermatological Importance. Indian. J. Dermatol. 2014, 59, 60–66. [Google Scholar] [CrossRef]
- Steinhoff, M.; Déret, S.; Rosignoli, C.; Metze, D.; Luger, T.A.; Voegel, J.J. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J. Investig. Dermatol. Symp. Proc. 2011, 15, 2–11. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. Management of facial erythema of rosacea: What is the role of topical α-adrenergic receptor agonist therapy? J. Am. Acad. Dermatol. 2013, 69, S44–S56. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.X.; Moore, A.Y. Brimonidine tartrate for the treatment of facial flushing and erythema in rosacea. Expert. Rev. Clin. Pharmacol. 2014, 7, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.U.; Shukla, S.; Zaki, J.; Feldman, S.R. Oxymetazoline hydrochloride cream for facial erythema associated with rosacea. Expert. Rev. Clin. Pharmacol. 2017, 10, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, F.; Liu, J.; Lee, F.S.; Wang, X.; Yang, H. Analysis of alkaloids in Coptis chinensis Franch by accelerated solvent extraction combined with ultra performance liquid chromatographic analysis with photodiode array and tandem mass spectrometry detections. Anal. Chim. Acta. 2008, 613, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Shigwan, H.; Saklani, A.; Hamrapurkar, P.D.; Mane, T.; Bhatt, P. HPLC method development and validation for quantification of berberine from Berberis aristata and Berberis tinctoria. Int. J. Appl. Sci. Eng. 2013, 2, 203–211. [Google Scholar]
- Kazusaki, M.; Ueda, S.; Takeuchi, N.; Ohgami, Y. Validation of analytical procedures by high−performance liquid chromatography for pharmaceutical analysis. Chromatography 2012, 33, 65–73. [Google Scholar] [CrossRef]
- Shibata, Y.; Metzer, W.J.; Myrvik, Q.N. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: Mannose receptor-mediated phagocytosis initiates IL-12 production. J. Immunol. 1997, 159, 2462–2467. [Google Scholar]
- Da Silva, C.A.; Chalouni, C.; Williams, A.; Hartl, D.; Lee, C.G.; Elias, J.A. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J. Immunol. 2009, 182, 3573–3582. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roh, K.-B.; Ryu, D.-H.; Cho, E.; Weon, J.B.; Park, D.; Kweon, D.-H.; Jung, E. Coptis chinensis Franch Directly Inhibits Proteolytic Activation of Kallikrein 5 and Cathelicidin Associated with Rosacea in Epidermal Keratinocytes. Molecules 2020, 25, 5556. https://doi.org/10.3390/molecules25235556
Roh K-B, Ryu D-H, Cho E, Weon JB, Park D, Kweon D-H, Jung E. Coptis chinensis Franch Directly Inhibits Proteolytic Activation of Kallikrein 5 and Cathelicidin Associated with Rosacea in Epidermal Keratinocytes. Molecules. 2020; 25(23):5556. https://doi.org/10.3390/molecules25235556
Chicago/Turabian StyleRoh, Kyung-Baeg, De-Hun Ryu, Eunae Cho, Jin Bae Weon, Deokhoon Park, Dae-Hyuk Kweon, and Eunsun Jung. 2020. "Coptis chinensis Franch Directly Inhibits Proteolytic Activation of Kallikrein 5 and Cathelicidin Associated with Rosacea in Epidermal Keratinocytes" Molecules 25, no. 23: 5556. https://doi.org/10.3390/molecules25235556
APA StyleRoh, K.-B., Ryu, D.-H., Cho, E., Weon, J. B., Park, D., Kweon, D.-H., & Jung, E. (2020). Coptis chinensis Franch Directly Inhibits Proteolytic Activation of Kallikrein 5 and Cathelicidin Associated with Rosacea in Epidermal Keratinocytes. Molecules, 25(23), 5556. https://doi.org/10.3390/molecules25235556





