Synthesis, Kinetic and Conformational Studies of 2-Substituted-5-(β-d-glucopyranosyl)-pyrimidin-4-ones as Potential Inhibitors of Glycogen Phosphorylase
Abstract
1. Introduction
2. Results
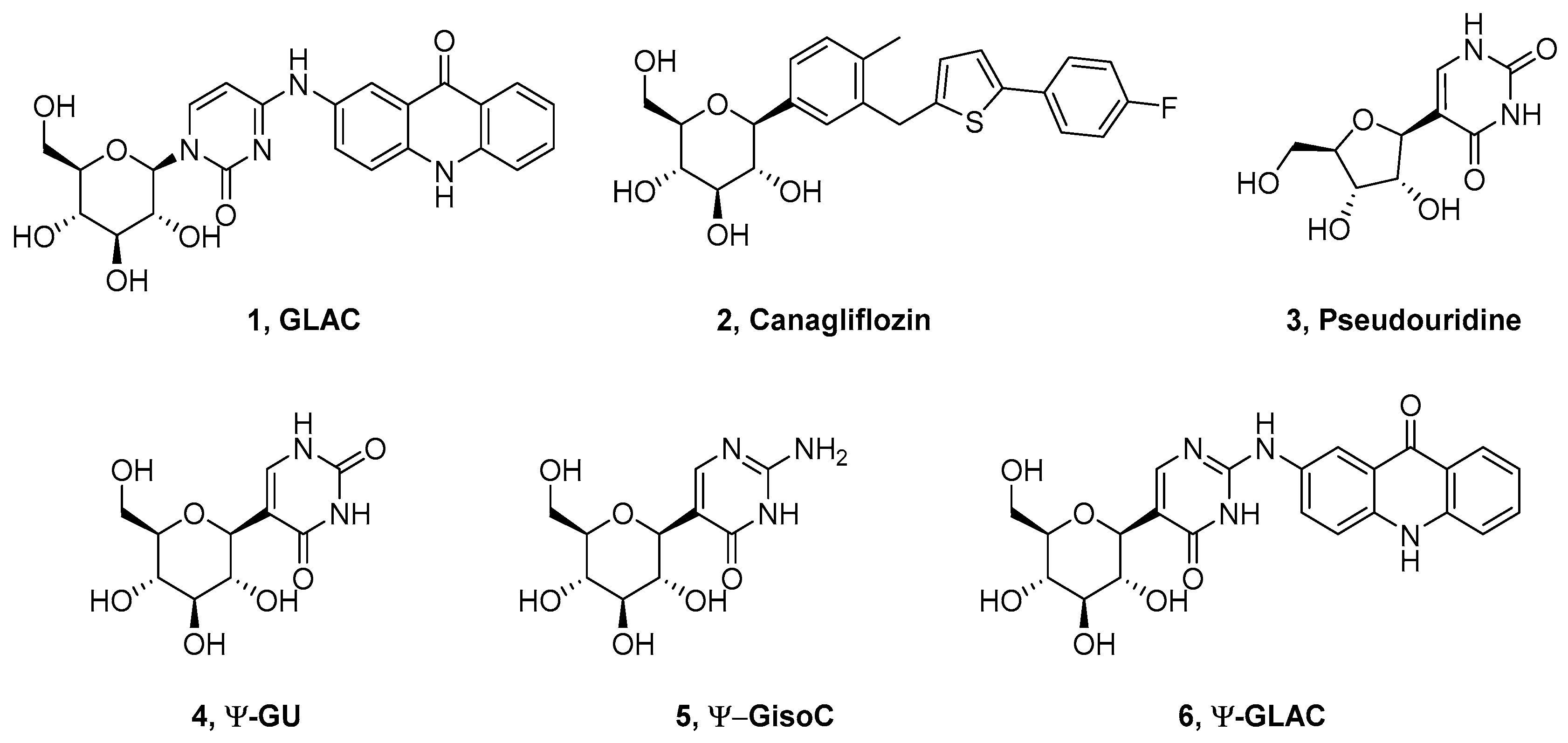
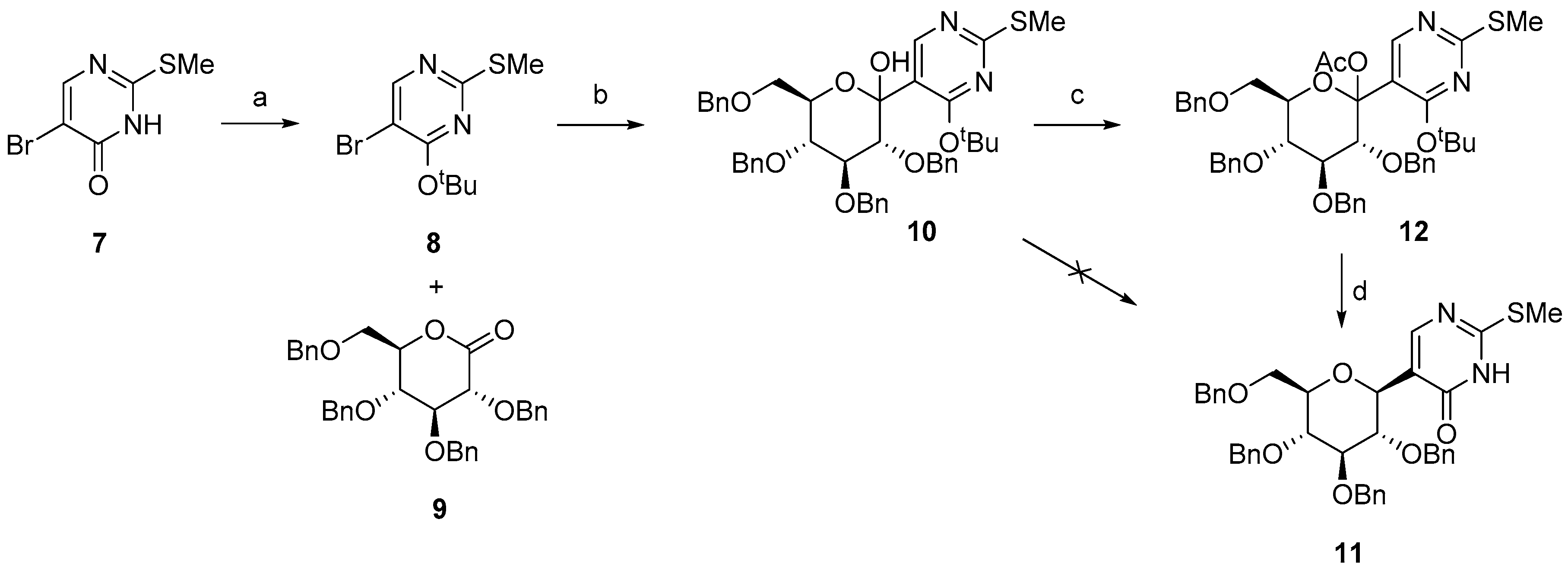
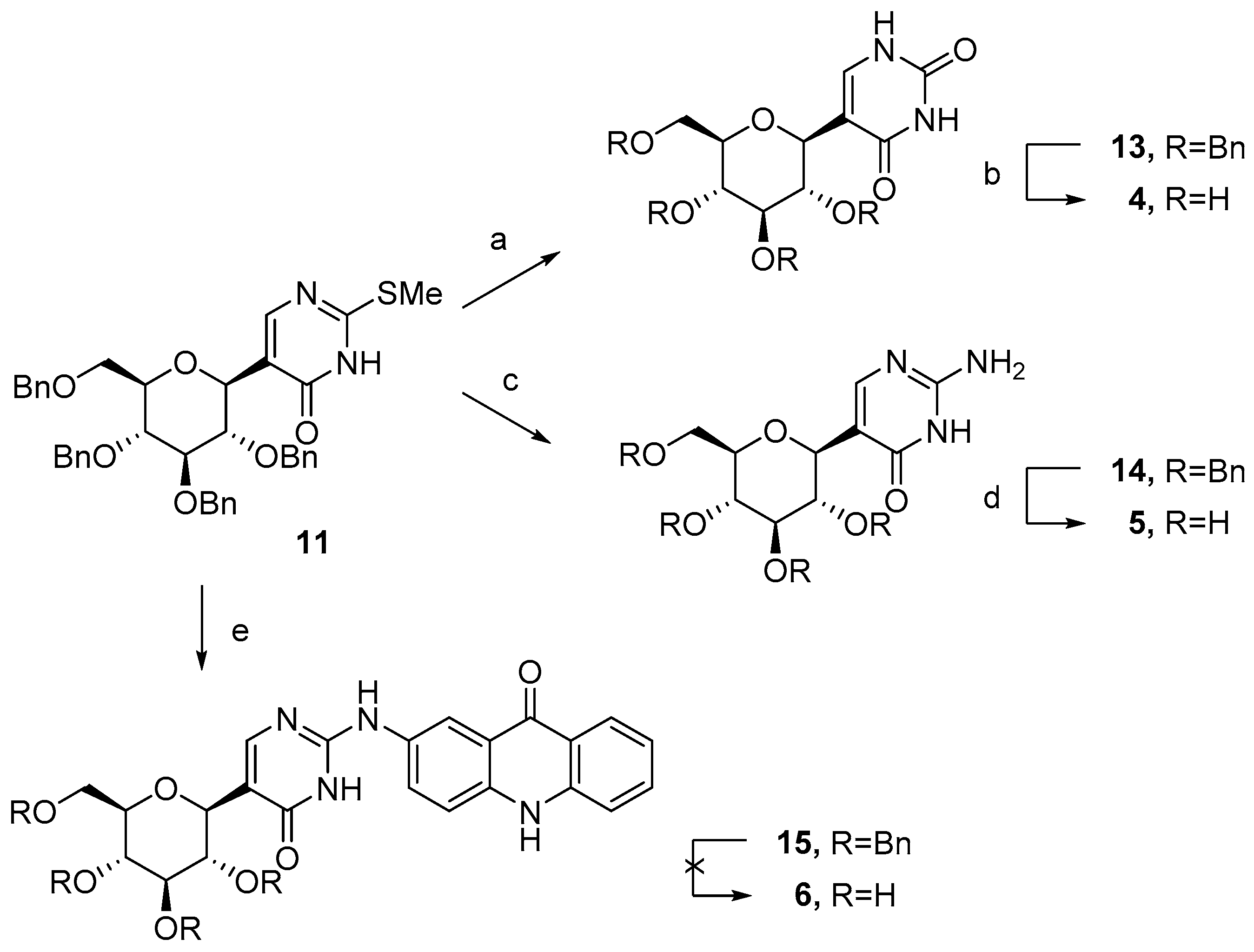
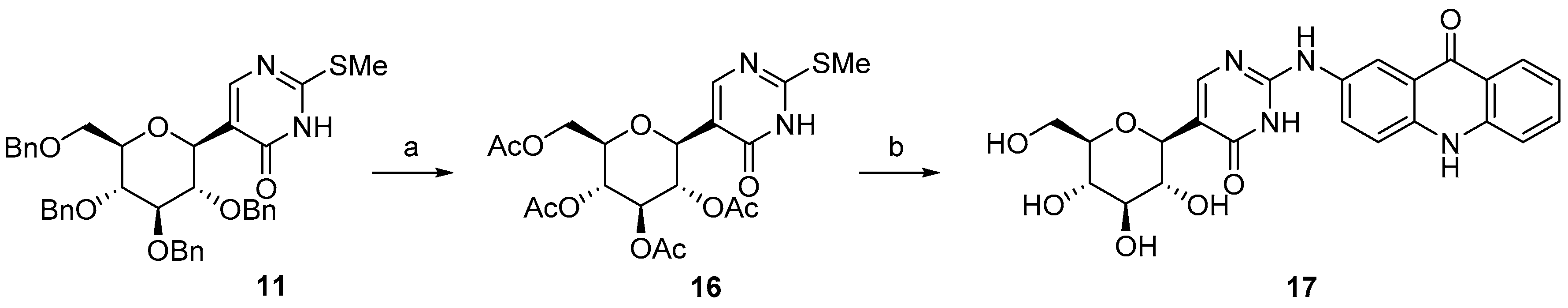
2.1. Synthesis
2.2. Enzyme Kinetics
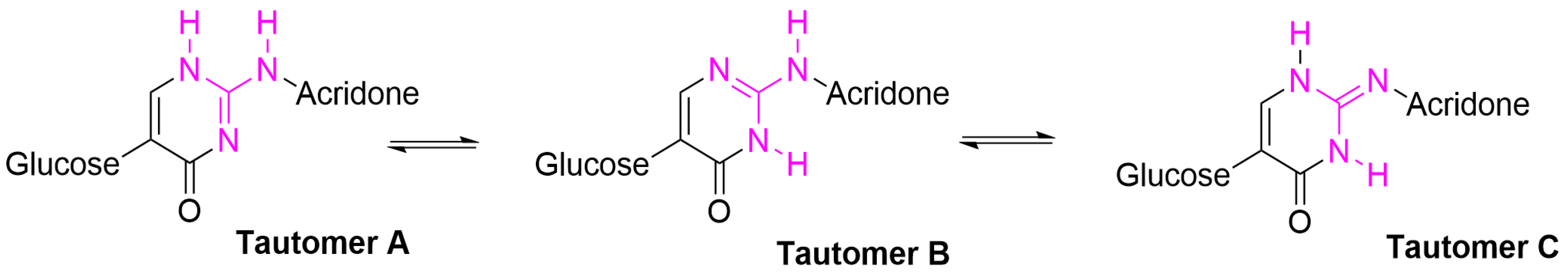
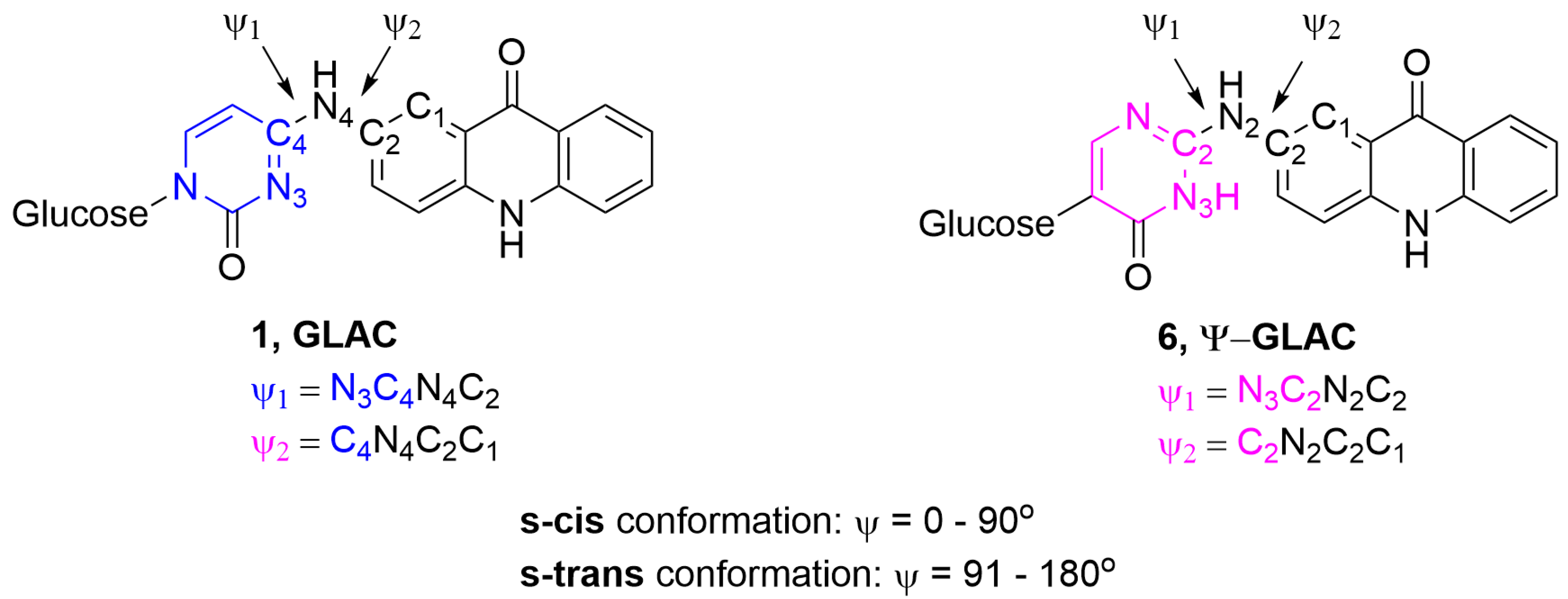
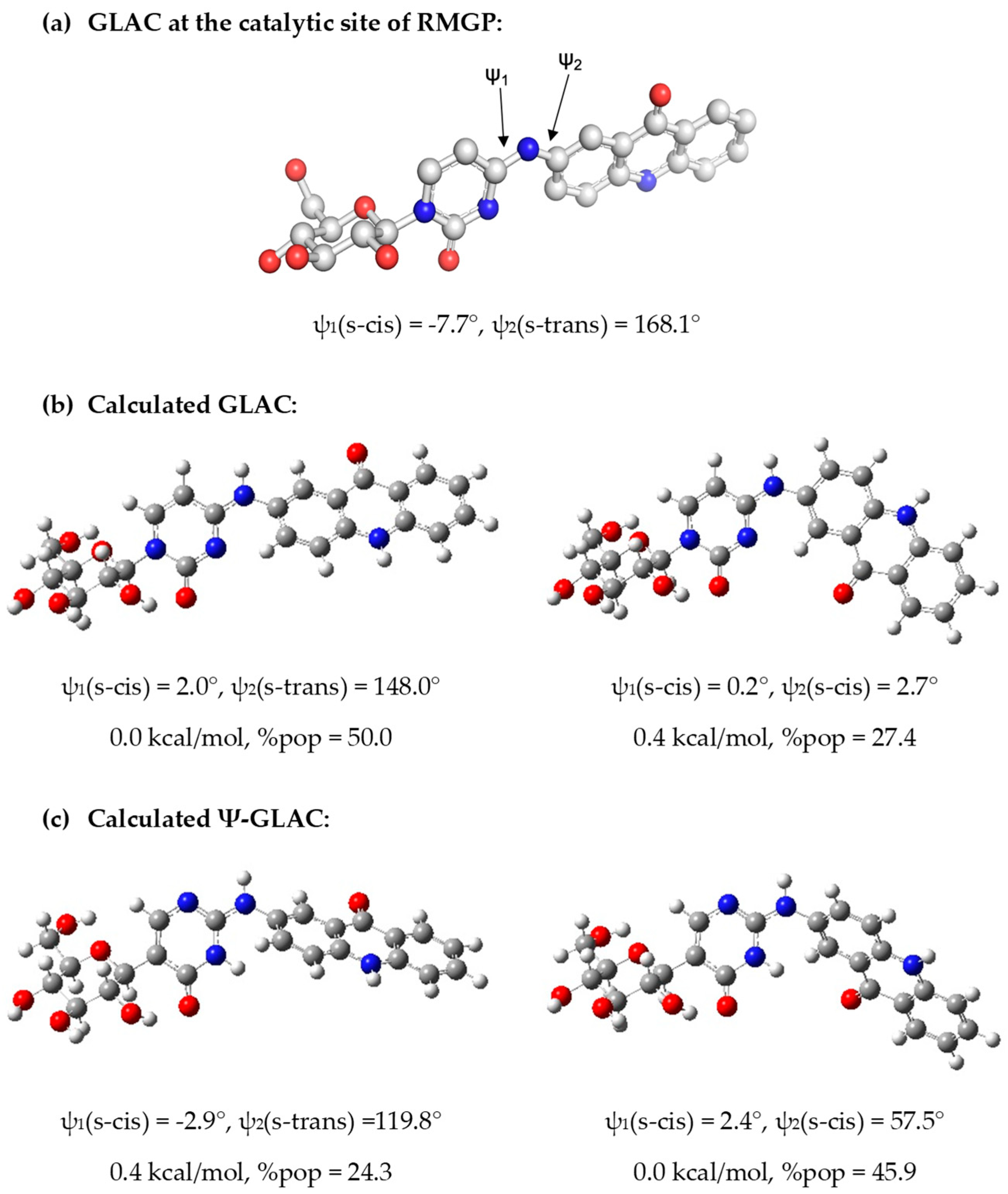
3. Discussion
Computational Studies
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Belete, T.M. A recent achievement in the discovery and development of novel targets for the treatment of type-2 diabetes mellitus. J. Exp. Pharmacol. 2020, 12, 1–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Praly, J.-P.; Vidal, S. Inhibition of glycogen phosphorylase in the context of type 2 diabetes, with focus on recent inhibitors bound at the active site. Mini Rev. Med. Chem. 2010, 10, 1102–1126. [Google Scholar] [CrossRef] [PubMed]
- Treadway, J.L.; Mendys, P.; Hoover, D.J. Expert opinion on investigational drugs glycogen phosphorylase inhibitors for treatment of type 2 diabetes mellitus. Expert Opin. Investig. Drugs 2001, 10, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Rousset, M.; Chevalier, G.; Rousset, J.P.; Dussaulx, E.; Zweibaum, A. Presence and cell growth-related variations of glycogen in human colorectal adenocarcinoma cell lines in culture. Cancer Res. 1979, 39, 531–534. [Google Scholar]
- Rousset, M.; Zweibaum, A.; Fogh, J. Presence of glycogen and growth-related variations in 58 cultured human tumor cell lines of various tissue origins. Cancer Res. 1981, 41, 1165–1170. [Google Scholar]
- Schnier, J.B.; Nishi, K.; Monks, A.; Gorin, F.A.; Bradbury, E.M. Inhibition of glycogen phosphorylase (GP) by CP-91,149 induces growth inhibition correlating with brain GP expression. Biochem. Biophys. Res. Commun. 2003, 309, 126–134. [Google Scholar] [CrossRef]
- Rousset, M.; Paris, H.; Chevalier, G.; Terrain, B.; Murat, J.C.; Zweibaum, A. Growth-related enzymatic control of glycogen metabolism in cultured human tumor cells. Cancer Res. 1984, 44, 154–160. [Google Scholar] [CrossRef]
- Uhlén, M.; Björling, E.; Agaton, C.; Szigyarto, C.A.K.; Amini, B.; Andersen, E.; Andersson, A.C.; Angelidou, P.; Asplund, A.; Asplund, C.; et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol. Cell. Proteomics 2005, 4, 1920–1932. [Google Scholar] [CrossRef]
- Favaro, E.; Bensaad, K.; Chong, M.G.; Tennant, D.A.; Ferguson, D.J.P.; Snell, C.; Steers, G.; Turley, H.; Li, J.-L.L.; Günther, U.L.; et al. Glucose utilization via glycogen phosphorylase sustains proliferation and prevents premature senescence in cancer cells. Cell Metab. 2012, 16, 751–764. [Google Scholar] [CrossRef]
- Shen, G.-M.; Zhang, F.-L.; Liu, X.-L.; Zhang, J.-W. Hypoxia-inducible factor 1-mediated regulation of PPP1R3C promotes glycogen accumulation in human MCF-7 cells under hypoxia. FEBS Lett. 2010, 584, 4366–4372. [Google Scholar] [CrossRef]
- Ros, S.; Schulze, A. Linking glycogen and senescence in cancer cells. Cell Metab. 2012, 16, 687–688. [Google Scholar] [CrossRef] [PubMed]
- Zois, C.E.; Favaro, E.; Harris, A.L. Glycogen metabolism in cancer. Biochem. Pharmacol. 2014, 92, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zois, C.E.; Harris, A.L. Glycogen metabolism has a key role in the cancer microenvironment and provides new targets for cancer therapy. J. Mol. Med. 2016, 94, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Bak, L.K.; Walls, A.B. Astrocytic glycogen metabolism in the healthy and diseased brain. J. Biol. Chem. 2018, 293, 7108–7116. [Google Scholar] [CrossRef]
- Swanson, R.A. Brain glycogen–Vestigial no more. Metab. Brain Dis. 2015, 30, 251–253. [Google Scholar] [CrossRef][Green Version]
- Xu, L.; Sun, H. Pharmacological manipulation of brain glycogenolysis as a therapeutic approach to cerebral ischemia. Mini Rev. Med. Chem. 2010, 10, 1188–1193. [Google Scholar] [CrossRef]
- Donnier-Maréchal, M.; Vidal, S. Glycogen phosphorylase inhibitors: A patent review (2013–2015). Expert Opin. Ther. Pat. 2016, 26, 199–212. [Google Scholar] [CrossRef]
- D Chrysina, E.; Chajistamatiou, A.; Chegkazi, M. From structure–based to knowledge-based drug design through X-ray protein crystallography: Sketching glycogen phosphorylase binding sites. Curr. Med. Chem. 2011, 18, 2620–2629. [Google Scholar] [CrossRef]
- Gimisis, T. Synthesis of N-glucopyranosidic derivatives as potential inhibitors that bind at the catalytic site of glycogen phosphorylase. Mini Rev. Med. Chem. 2010, 10, 1127–1138. [Google Scholar] [CrossRef]
- Somsak, L.; Nagy, V.; Hadady, Z.; Docsa, T.; Gergely, P. Glucose analog inhibitors of glycogen phosphorylases as potential antidiabetic agents: Recent developments. Curr. Pharm. Des. 2003, 9, 1177–1189. [Google Scholar] [CrossRef]
- Somsak, L.; Czifrak, K.; Toth, M.; Bokor, E.; Chrysina, E.; Alexacou, K.-M.; Hayes, J.; Tiraidis, C.; Lazoura, E.; Leonidas, D.; et al. New inhibitors of glycogen phosphorylase as potential antidiabetic agents. Curr. Med. Chem. 2008, 15, 2933–2983. [Google Scholar] [CrossRef] [PubMed]
- Oikonomakos, N.G.; Somsák, L. Advances in glycogen phosphorylase inhibitor design. Curr. Opin. Investig. Drugs 2008, 9, 379–395. [Google Scholar] [PubMed]
- Somsák, L. Glucose derived inhibitors of glycogen phosphorylase. Comptes Rendus Chim. 2011, 14, 211–223. [Google Scholar] [CrossRef]
- Bokor, É. N- and C-Glycopyranosyl heterocycles as glycogen phosphorylase inhibitors. In Recent Trends in Carbohydrate Chemistry; Rauter, A.P., Christensen, B.E., Somsák, L., Kosma, P., Adamo, R., Eds.; Elsevier: New York, NY, USA, 2020; pp. 253–300. [Google Scholar] [CrossRef]
- Mamais, M.; Degli Esposti, A.; Kouloumoundra, V.; Gustavsson, T.; Monti, F.; Venturini, A.; Chrysina, E.D.; Markovitsi, D.; Gimisis, T. A new potent inhibitor of glycogen phosphorylase reveals the basicity of the catalytic site. Chem. A Eur. J. 2017, 23, 8800–8805. [Google Scholar] [CrossRef]
- Kantsadi, A.L.; Bokor, É.; Kun, S.; Stravodimos, G.A.; Chatzileontiadou, D.S.M.; Leonidas, D.D.; Juhász-Tóth, É.; Szakács, A.; Batta, G.; Docsa, T.; et al. Synthetic, enzyme kinetic, and protein crystallographic studies of C-β-d-glucopyranosyl pyrroles and imidazoles reveal and explain low nanomolar inhibition of human liver glycogen phosphorylase. Eur. J. Med. Chem. 2016, 123, 737–745. [Google Scholar] [CrossRef]
- Bokor, É.; Kun, S.; Goyard, D.; Tóth, M.; Praly, J.-P.; Vidal, S.; Somsák, L. C-glycopyranosyl arenes and hetarenes: Synthetic methods and bioactivity focused on antidiabetic potential. Chem. Rev. 2017, 117, 1687–1764. [Google Scholar] [CrossRef]
- Deeks, E.D.; Scheen, A.J. Canagliflozin: A review in type 2 diabetes. Drugs 2017, 77, 1577–1592. [Google Scholar] [CrossRef]
- Somsák, L.; Bokor, É.; Juhász, L.; Kun, S.; Lázár, L.; Juhász-Tóth, É.; Tóth, M. New syntheses towards C-glycosyl type glycomimetics. Pure Appl. Chem. 2019, 91, 1159–1175. [Google Scholar] [CrossRef]
- Bokor, É.; Kun, S.; Docsa, T.; Gergely, P.; Somsák, L. 4(5)-Aryl-2-C-glucopyranosyl-imidazoles as new nanomolar glucose analogue inhibitors of glycogen phosphorylase. ACS Med. Chem. Lett. 2015, 6, 1215–1219. [Google Scholar] [CrossRef]
- Mamais, M.; Kouloumoundra, V.; Smyrli, E.; Grammatopoulos, P.; Chrysina, E.D.; Gimisis, T. Synthesis of N4-aryl-β-d-glucopyranosylcytosines: A methodology study. Tetrahedron Lett. 2015, 56, 5549–5552. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Yu, B. Recent advances in the chemical synthesis of C-glycosides. Chem. Rev. 2017, 117, 12281–12356. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.D.; Cha, J.K.; Kishi, Y. Highly stereoselective approaches to α- and β-C-glycopyranosides. J. Am. Chem. Soc. 1982, 104, 4976–4978. [Google Scholar] [CrossRef]
- Kraus, G.A.; Molina, M.T.; Teresa Molina, M. A direct synthesis of C-glycosyl compounds. J. Org. Chem. 1988, 53, 752–753. [Google Scholar] [CrossRef]
- Horton, D.; Priebe, W. Synthetic routes to higher-carbon sugars. Reaction of lactones with 2-lithio-,3-dithiane. Carbohydr. Res. 1981, 94, 27–41. [Google Scholar] [CrossRef]
- RajanBabu, T.V.; Reddy, G.S. 1-methylene sugars as C-glycoside precursors. J. Org. Chem. 1986, 51, 5458–5461. [Google Scholar] [CrossRef]
- Tibiletti, F.; Simonetti, M.; Nicholas, K.M.; Palmisano, G.; Parravicini, M.; Imbesi, F.; Tollari, S.; Penoni, A. One-pot synthesis of meridianins and meridianin analogues via indolization of nitrosoarenes. Tetrahedron 2010, 66, 1280–1288. [Google Scholar] [CrossRef]
- Barrett, H.W.; Goodman, I.; Dittmer, K. The synthesis of 5-halogeno-2-thiouracil and 6-methyl-5-halogeno-2-thiouracil derivatives 1. J. Am. Chem. Soc. 1948, 70, 1753–1756. [Google Scholar] [CrossRef]
- Hanessian, S.; Machaalani, R. A highly stereocontrolled and efficient synthesis of α- and β-pseudouridines. Tetrahedron Lett. 2003, 44, 8321–8323. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.L.V. Syntheses with pyrimidine-lithium compounds. Tetrahedron 1959, 6, 225–231. [Google Scholar] [CrossRef]
- Boudet, N.; Knochel, P. Chemo- and regioselective functionalization of uracil derivatives. Applications to the synthesis of oxypurinol and emivirine. Org. Lett. 2006, 8, 3737–3740. [Google Scholar] [CrossRef]
- Kofink, C.C.; Knochel, P. Synthesis of functionalized diarylmethanes via a copper-catalyzed cross-coupling of arylmagnesium reagents with benzylic phosphates. Org. Lett. 2006, 8, 4121–4124. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, B.A.; Doyle, A.G.; Patel, M.; Caceres-Cortes, J.; Meng, W.; Deshpande, P.P.; Pullockaran, A.; Washburn, W.N. C-Arylglucoside synthesis: Triisopropylsilane as a selective reagent for the reduction of an anomeric C-phenyl ketal. Tetrahedron Asymm. 2003, 14, 3243–3247. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Singh, J.; Pullockaran, A.; Kissick, T.; Ellsworth, B.A.; Gougoutas, J.Z.; Dimarco, J.; Fakes, M.; Reyes, M.; Lai, C.; et al. A practical stereoselective synthesis and novel cocrystallizations of an amphiphatic SGLT-2 inhibitor. Org. Process Res. Dev. 2012, 16, 577–585. [Google Scholar] [CrossRef]
- Urabe, D.; Nagatomo, M.; Hagiwara, K.; Masuda, K.; Inoue, M. Symmetry-driven synthesis of 9-demethyl-10,15-dideoxyryanodol. Chem. Sci. 2013, 4, 1615. [Google Scholar] [CrossRef]
- Dondoni, A.; Scherrmann, M. Thiazole-based synthesis of formyl C-Glycosides. J. Org. Chem. 1994, 59, 6404–6412. [Google Scholar] [CrossRef]
- Xie, J.; Molina, A.; Czernecki, S. Alkylidenation of sugar lactones and further transformation to C-glycosides. J. Carbohydr. Chem. 1999, 18, 481–498. [Google Scholar] [CrossRef]
- Khalifa, N.M.; Al-Omar, M.A.; Amr, A.E. Synthesis and characterization of novel 5-allyl-6-{(benzo[d]thiazol-2-yl)methyl}-2-(alkylsulfanyl)oxopyrimidine derivatives. Russ. J. Gen. Chem. 2016, 86, 2752–2758. [Google Scholar] [CrossRef]
- Grosjean, S.; Triki, S.; Meslin, J.C.; Julienne, K.; Deniaud, D. Synthesis of nitrogen bicyclic scaffolds: Pyrimido[1,2-a]pyrimidine-2,6-diones. Tetrahedron 2010, 66, 9912–9924. [Google Scholar] [CrossRef]
- Cho, A.; Zhang, L.; Xu, J.; Babusis, D.; Butler, T.; Lee, R.; Saunders, O.L.; Wang, T.; Parrish, J.; Perry, J.; et al. Synthesis and characterization of 2′-C-Me branched C-nucleosides as HCV polymerase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 4127–4132. [Google Scholar] [CrossRef]
- Li, Y.; Manickam, G.; Ghoshal, A.; Subramaniam, P. More efficient palladium catalyst for hydrogenolysis of benzyl groups. Synth. Commun. 2006, 36, 925–928. [Google Scholar] [CrossRef]
- Maddess, M.L.; Carter, R. SNAr Reactions of 2-Methylthio-4-pyrimidinones in Pivalic Acid: Access to Functionalized Pyrimidinones and Pyrimidines. Synthesis. 2012, 44, 1109–1118. [Google Scholar] [CrossRef]
- Alzeer, J.; Vasella, A. Oligosaccharide analogues of polysaccharides. Part 2. Regioselective deprotection of monosaccharide-derived monomers and dimers. Helv. Chim. Acta 1995, 78, 177–193. [Google Scholar] [CrossRef]
- Oikonomakos, N.G.; Kontou, M.; Zographos, S.E.; Watson, K.A.; Johnson, L.N.; Bichard, C.J.F.; Fleet, G.W.J.; Acharya, K.R. N-acetyl-β-d-glucopyranosylamine: A potent T-state inhibitor of glycogen phosphorylase. A comparison with α-d-glucose. Protein Sci. 1995, 4, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, V.; Mavreas, K.; Monti, F.; Mamais, M.; Gustavsson, T.; Chrysina, E.D.; Markovitsi, D.; Gimisis, T.; Venturini, A. Multiscale time-resolved fluorescence study of a glycogen phosphorylase inhibitor combined with quantum chemistry calculations. Phys. Chem. Chem. Phys. 2019, 21, 7685–7696. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. G16_c01; Gaussian, Inc.: Oxfordshire, UK, 2016. [Google Scholar]
- Szennyes, E.; Gyémánt, G.; Somsák, L.; Bokor, É. Synthesis of new series of 2-C-(β-d-glucopyranosyl)-pyrimidines and their evaluation as inhibitors of some glycoenzymes. Molecules 2020, 25, 701. [Google Scholar] [CrossRef]
- Szennyes, E.; Bokor, É.; Langer, P.; Gyémánt, G.; Docsa, T.; Sipos, Á.; Somsák, L. The first general synthesis of 2-C-(β-d-glycopyranosyl)pyrimidines and their evaluation as inhibitors of some glycoenzymes. New J. Chem. 2018, 42, 17439–17446. [Google Scholar] [CrossRef]
- Waschke, D.; Thimm, J.; Thiem, J. Highly efficient synthesis of ketoheptoses. Org. Lett. 2011, 13, 3628–3631. [Google Scholar] [CrossRef]
- Damager, I.; Erik Olsen, C.; Lindberg Møller, B.; Saddik Motawia, M. Chemical synthesis of 6’’’-α-maltotriosyl-maltohexaose as substrate for enzymes in starch biosynthesis and degradation. Carbohydr. Res. 1999, 320, 19–30. [Google Scholar] [CrossRef]
- Saheki, S.; Takeda, A.; Shimazu, T. Assay of inorganic phosphate in the mild pH range, suitable for measurement of glycogen phosphorylase activity. Anal. Biochem. 1985, 148, 277–281. [Google Scholar] [CrossRef]


Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavreas, K.F.; Neofytos, D.D.; Chrysina, E.D.; Venturini, A.; Gimisis, T. Synthesis, Kinetic and Conformational Studies of 2-Substituted-5-(β-d-glucopyranosyl)-pyrimidin-4-ones as Potential Inhibitors of Glycogen Phosphorylase. Molecules 2020, 25, 5463. https://doi.org/10.3390/molecules25225463
Mavreas KF, Neofytos DD, Chrysina ED, Venturini A, Gimisis T. Synthesis, Kinetic and Conformational Studies of 2-Substituted-5-(β-d-glucopyranosyl)-pyrimidin-4-ones as Potential Inhibitors of Glycogen Phosphorylase. Molecules. 2020; 25(22):5463. https://doi.org/10.3390/molecules25225463
Chicago/Turabian StyleMavreas, Konstantinos F., Dionysios D. Neofytos, Evangelia D. Chrysina, Alessandro Venturini, and Thanasis Gimisis. 2020. "Synthesis, Kinetic and Conformational Studies of 2-Substituted-5-(β-d-glucopyranosyl)-pyrimidin-4-ones as Potential Inhibitors of Glycogen Phosphorylase" Molecules 25, no. 22: 5463. https://doi.org/10.3390/molecules25225463
APA StyleMavreas, K. F., Neofytos, D. D., Chrysina, E. D., Venturini, A., & Gimisis, T. (2020). Synthesis, Kinetic and Conformational Studies of 2-Substituted-5-(β-d-glucopyranosyl)-pyrimidin-4-ones as Potential Inhibitors of Glycogen Phosphorylase. Molecules, 25(22), 5463. https://doi.org/10.3390/molecules25225463






