Terpenoids from Marine Soft Coral of the Genus Xenia in 1977 to 2019
Abstract
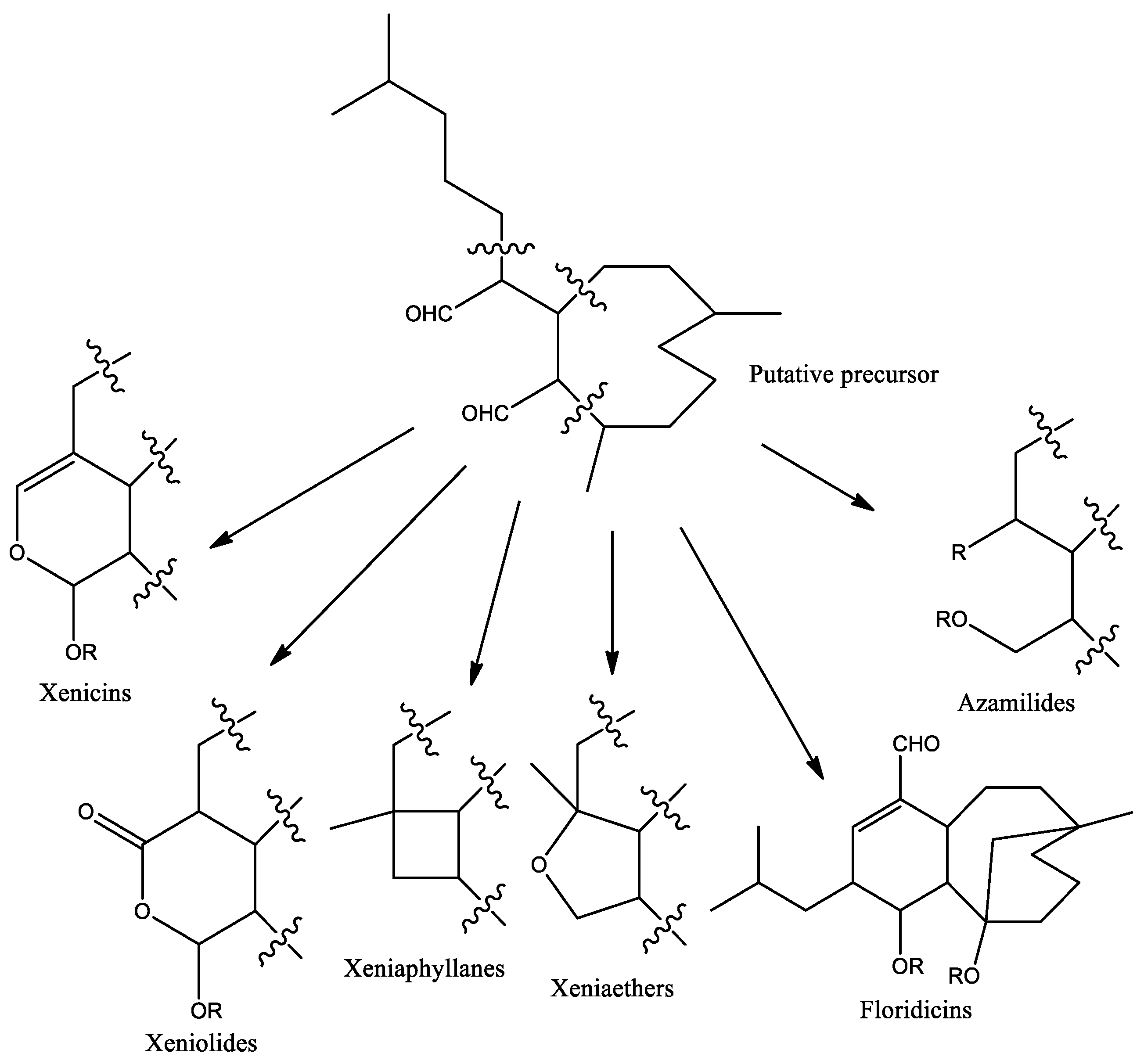
1. Introduction
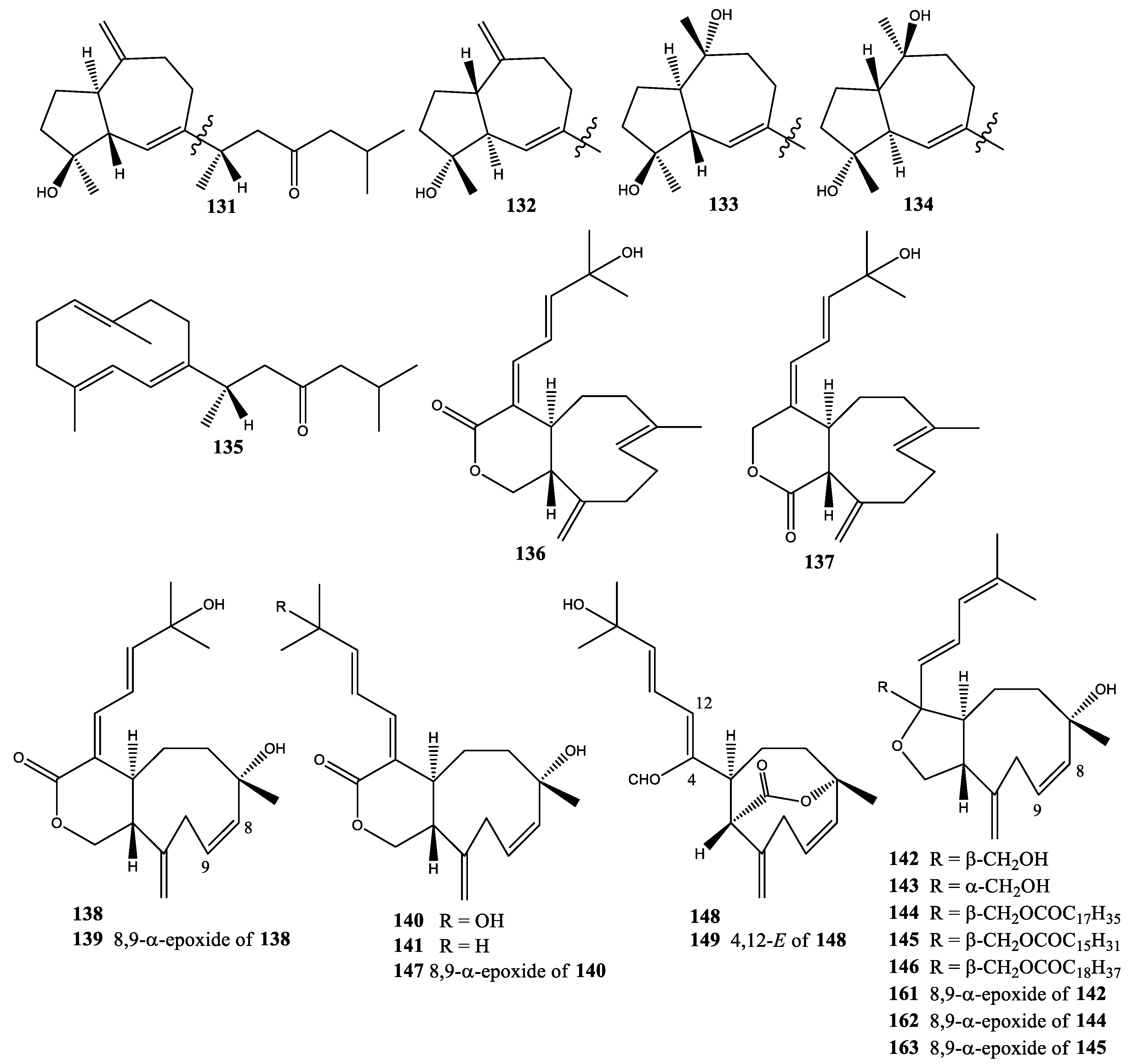
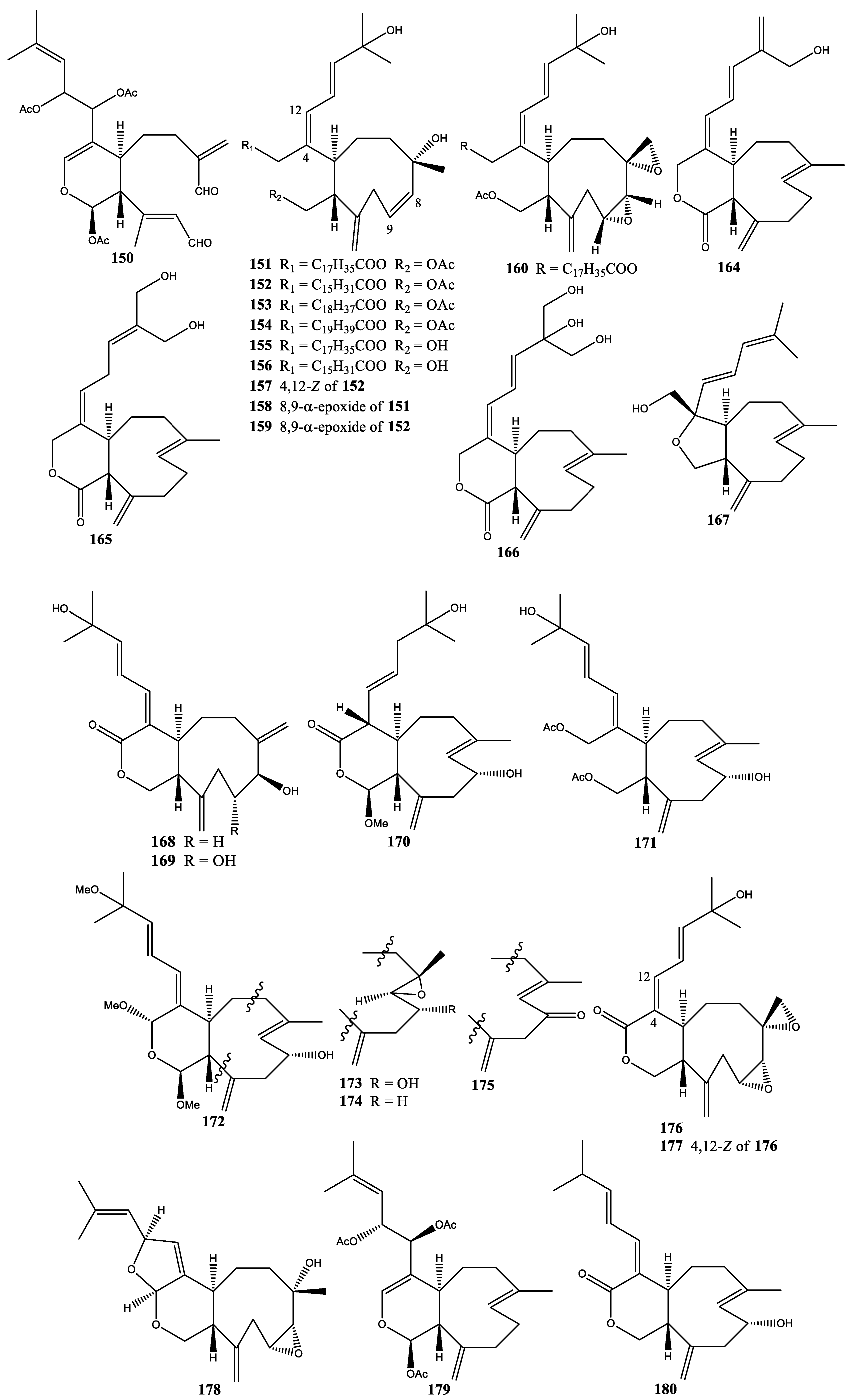
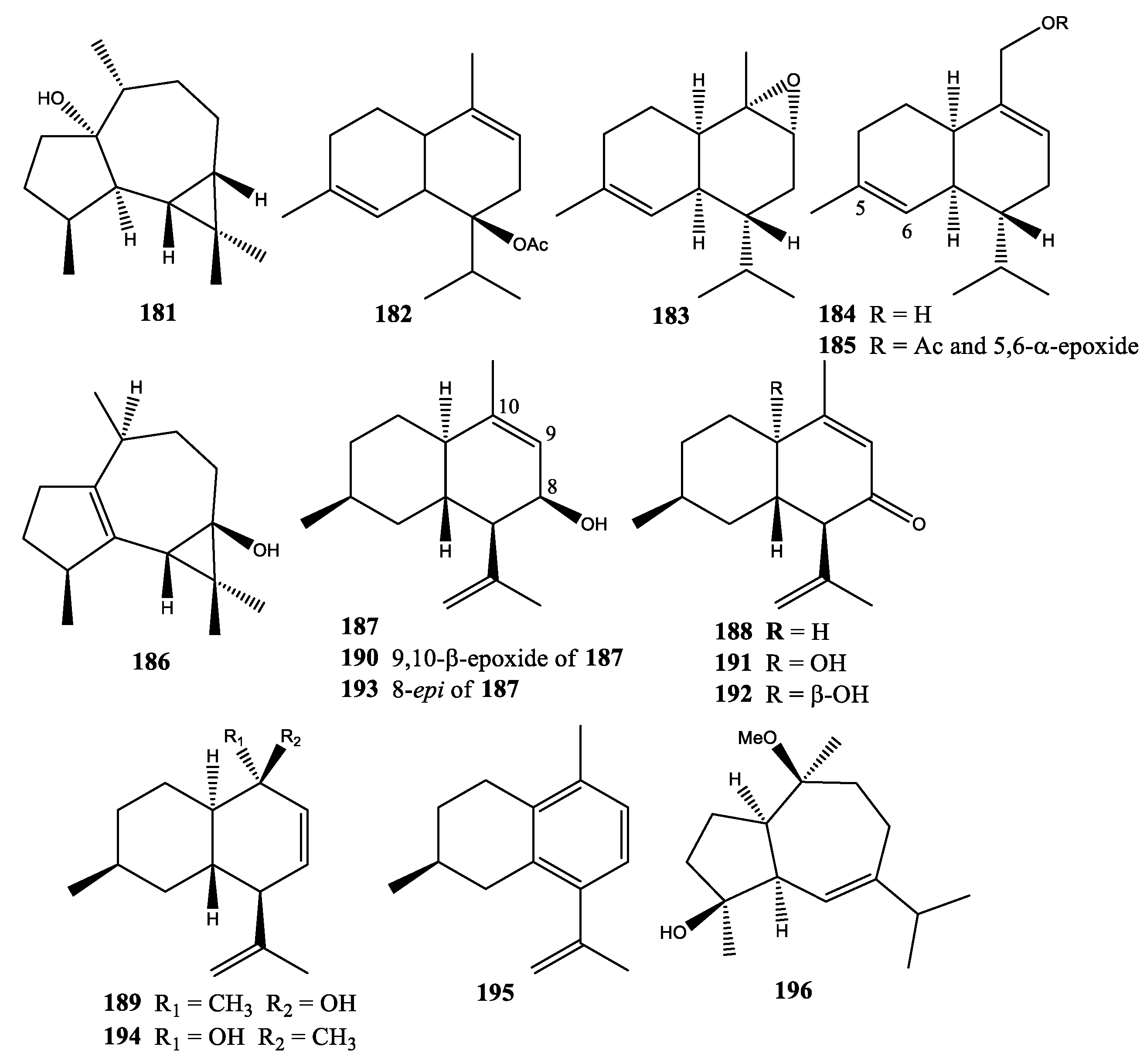
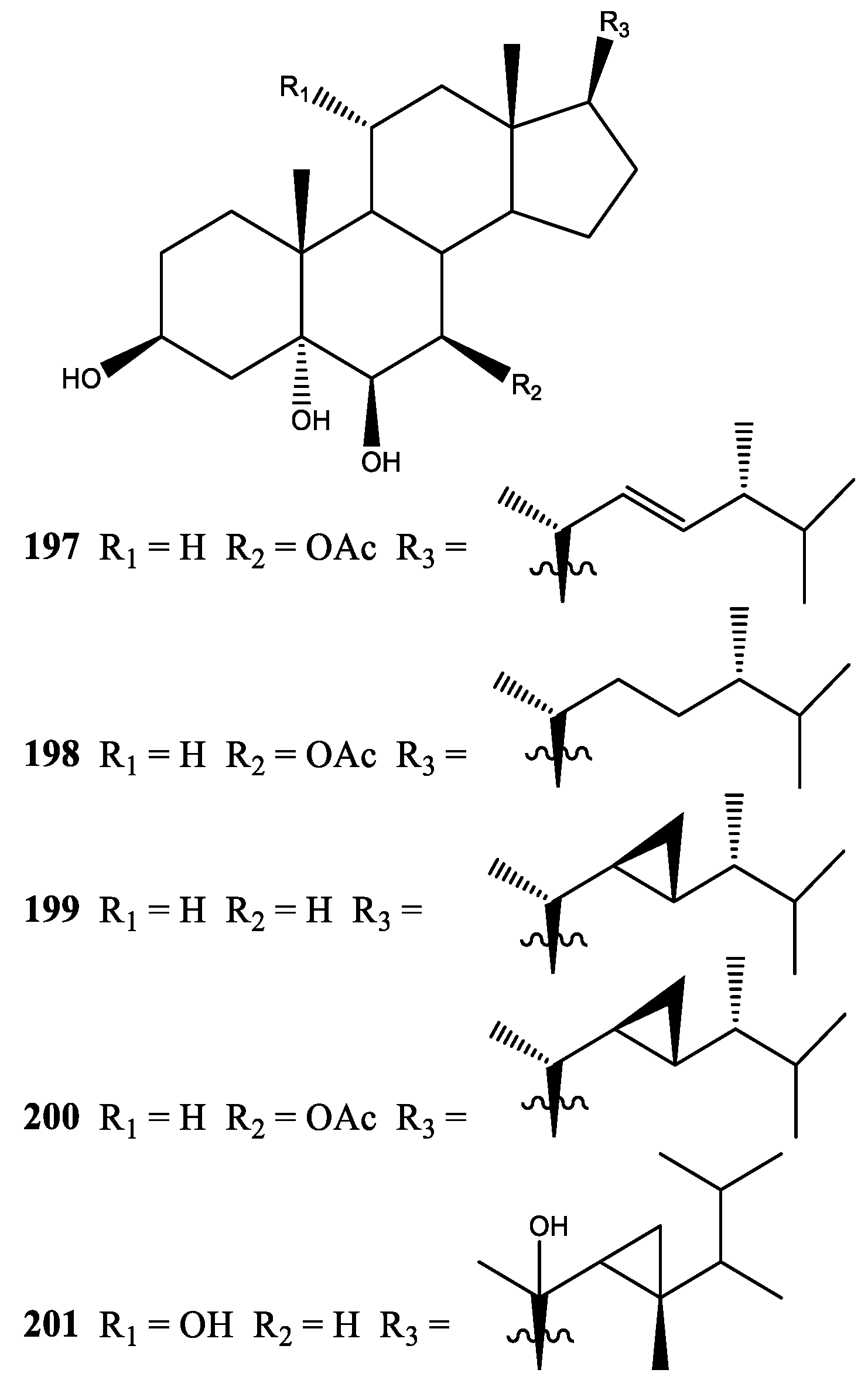
2. Diterpenes
2.1. Xenia Blumi
2.2. Xenia Crassa
2.3. Xenia Elongata
2.4. Xenia Faraunensis
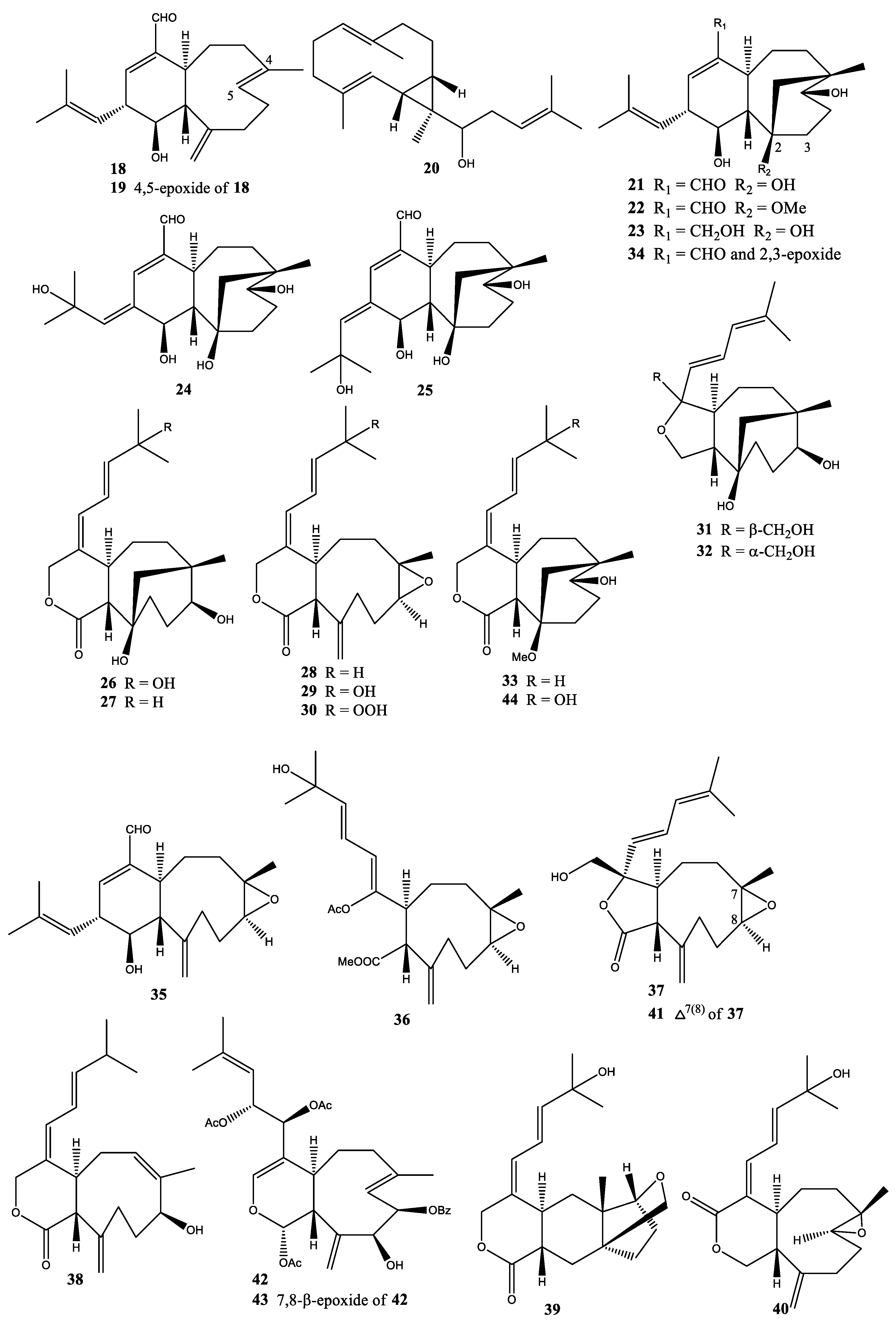
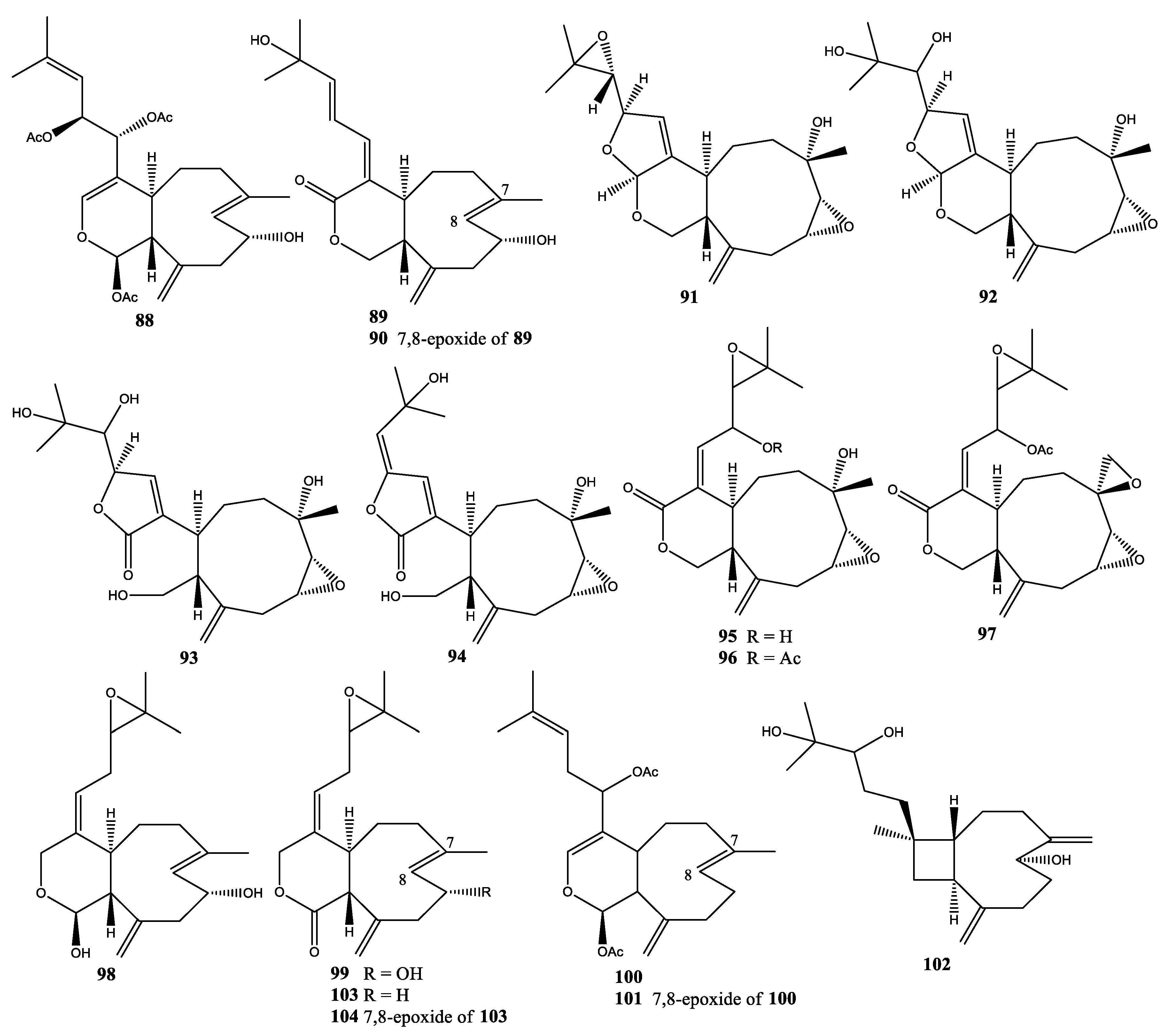
2.5. Xenia Florida
2.6. Xenia Lilielae
2.7. Xenia Macrospiculata
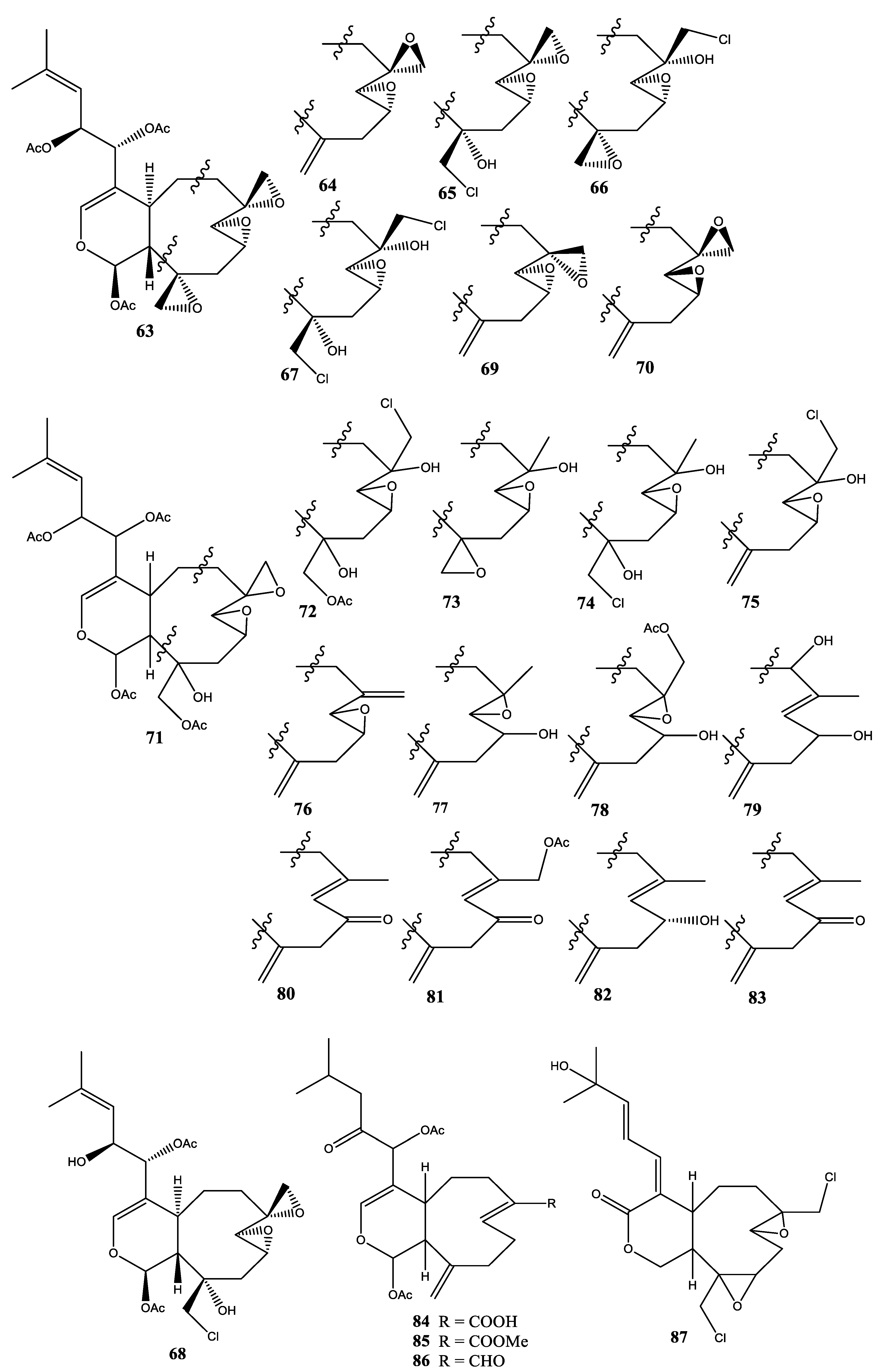
2.8. Xenia Membranacea
2.9. Xenia Novae-Britanniae
2.10. Xenia Obscuronata
2.11. Xenia Umbellata
2.12. Xenia Viridis
2.13. Xenia sp.
3. Sesquiterpenes
4. Steroids
5. Synthesis of Xenia Diterpenoids
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, A.F.; Chen, Y.W.; Huang, C.Y.; Tseng, Y.J.; Lin, C.C.; Dai, C.F.; Wu, Y.C.; Shen, J.H. Isolation and structure elucidation of cembranoids from a Dongsha Atoll soft coral Sacrophyton stellatum. Mar. Drugs 2018, 16, 210. [Google Scholar] [CrossRef]
- Huang, C.Y.; Tseng, W.R.; Ahmed, A.F.; Chiang, P.L.; Tai, C.L.; Hwang, T.L.; Dai, C.F.; Sheu, J.H. Anti-inflammatory polyoxygenated steroids from the soft coral Lobophytum michaelae. Mar. Drugs 2018, 16, 93. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Chen, W.T.; Yao, L.G.; Guo, Y.W. Two new cytotoxic steroids from the Chinese soft coral Sinularia sp. Steroids 2018, 136, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.F.; Kurtán, T.; Mándi, A.; Yao, L.G.; Li, J.; Lan, L.F.; Guo, Y.W. Structural, stereochemical, and bioactive studies of cembranoids from Chinese soft coral Sarcophyton trocheliophorum. Tetrahedron 2018, 74, 1933–1941. [Google Scholar] [CrossRef]
- Phan, C.S.; Kamada, T.; Kobayashi, K.; Hamada, T.; Vairappan, C.S. 15-Deoxy-isoxeniolide-A, new diterpenoid from a Bornean soft coral, Xenia sp. Nat. Prod. Res. 2018, 32, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.S.; Kamada, T.; Ishii, T.; Hamada, T.; Vairappan, C.S. A new guaiane-type sesquiterpenoid from a Bornean soft coral, Xenia stellifera. Nat. Prod. Commun. 2018, 13, 15–16. [Google Scholar] [CrossRef]
- Wu, C.H.; Chai, C.H.; Huang, T.Z.; Huang, C.Y.; Hang, T.L.; Dai, C.F.; Sheu, J.H. Cembranoid-related metabolites and biological activities from the soft coral Sinularia flexibilis. Mar. Drugs 2018, 16, 278. [Google Scholar] [CrossRef]
- Ye, F.; Li, J.; Wu, Y.; Zhu, Z.D.; Mollo, E.; Gavagnin, M.; Gu, Y.C.; Zhu, W.L.; Li, X.W.; Guo, Y.W. Sarinfacetamides A and B, nitrogenous diterpenoids with tricycle[6.3.1.01,5]dodecane scaffold from the South China Sea soft coral Sarcophyton infundibuliforme. Org. Lett. 2018, 20, 2637–2640. [Google Scholar] [CrossRef]
- Green, D.; Carmely, S.; Benayahu, Y.; Kashman, Y. Antheliolide A & B: Two new C24-acetoacetylated diterpenoids of the soft coral Anthelia glauca. Tetrahedron 1988, 29, 1605–1608. [Google Scholar]
- Lin, Y.S.; Fazary, A.E.; Chen, C.H.; Kuo, Y.H.; Shen, Y.C. Bioactive xenicane diterpenoids from the Taiwanese soft coral Asterospicularia laurae. Chem. Biodivers. 2011, 8, 1310–1317. [Google Scholar] [CrossRef]
- Hooper, G.J.; Davies-Coleman, M.T. New metabolites from the south African soft coral Capnella thyrsoidea. Tetrahedron 1995, 51, 9973–9984. [Google Scholar] [CrossRef]
- Hooper, G.J.; Davies-Coleman, M.T.; Schleyer, M. New diterpenes from the South African soft coral Eleutherobia aurea. J. Nat. Prod. 1997, 60, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Ogawa, N.; Liang, J.; Higa, T. Helioxenicins A-C: Diterpenes from the blue coral Heliopora coerulea. Tetrahedron 1994, 50, 9989–9996. [Google Scholar] [CrossRef]
- Chen, S.P.; Su, J.H.; Ahmed, A.F.; Dai, C.F.; Wu, Y.C.; Sheu, J.H. Xeniaphyllane-derived terpenoids from the Formosan soft coral Sinularia gibberosa. Chem. Pharm. Bull. 2007, 55, 1471–1475. [Google Scholar] [CrossRef]
- Schwartz, R.E.; Scheuer, P.J.; Zabel, V.; Watson, W.H. The coraxeniolides, constituents of pink coral, corallium sp. Tetrahedron 1981, 37, 2725–2733. [Google Scholar] [CrossRef]
- Fusetani, N.; Asano, M.; Matsunaga, S.; Hashimoto, K. Acalycixeniolides, novel norditerpenes with allene functionality from two gorgornians of the genus Acalycigorgia. Tetrahedron 1989, 45, 1647–1652. [Google Scholar] [CrossRef]
- Vanderah, D.J.; Steudler, P.A.; Ciereszko, L.S.; Schmitz, F.J.; Ekstrand, J.D.; van der Helm, D. Marine natural products. Xenicin: A diterpenoid possessing a nice-membered ring from the soft coral, Xenia elongata. J. Am. Chem. Soc. 1977, 99, 5780–5784. [Google Scholar] [CrossRef]
- Kashman, Y.; Groweiss, A. Xeniolide-A and xeniolide-B, two new diterpenoids from the soft-coral Xenia macrospiculata. Tetrahedron Lett. 1978, 19, 4833–4836. [Google Scholar] [CrossRef]
- Kashman, Y.; Groweiss, A. New diterpenoids from the soft corals Xenia macrospiculata and Xenia obscuronata. J. Org. Chem. 1980, 45, 3824–3827. [Google Scholar] [CrossRef]
- Iwagawa, T.; Kawasaki, J.; Hase, T.; Yu, C.M.; Walter, J.A.; Wright, J.L.C. A new tricarbocyclic diterpene structure from the soft coral Xenia florida. J. Chem. Soc. Chem. Commun. 1994, 18, 2073–2074. [Google Scholar] [CrossRef]
- Iwagawa, T.; Amano, Y.; Hase, T.; Shiro, M. New xenia diterpenoids from a soft coral, xenia species 1. Tetrahedron 1995, 51, 11111–11118. [Google Scholar] [CrossRef]
- Iwagawa, T.; Amano, Y.; Nakatani, M.; Hase, T. New xenia diterpenoids from a soft coral, Xenia species containing fatty acyl side chains. Bull. Chem. Soc. Jpn. 1996, 69, 1309–1312. [Google Scholar] [CrossRef]
- El-Gamal, A.A.H.; Chiang, C.Y.; Huang, S.H.; Wang, S.K.; Duh, C.Y. Xenia diterpenoids from the Formosan soft coral Xenia blumi. J. Nat. Prod. 2005, 68, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, A.A.H.; Wang, S.K.; Duh, C.Y. Umbellactal, a novel diterpenoid from the Formosan soft coral Xenia umbellata. Tetrahedron Lett. 2005, 46, 6095–6096. [Google Scholar] [CrossRef]
- El-Gamal, A.A.H.; Wang, S.K.; Duh, C.Y. Xenibellols A and B, new diterpenoids from the Formosan soft coral Xenia umbellata. Org. Lett. 2005, 7, 2023–2025. [Google Scholar] [CrossRef]
- El-Gamal, A.A.H.; Wang, S.K.; Duh, C.Y. Xenibellal, a novel norditerpenoid from the Formosan soft coral Xenia umbellata. Tetrahedron Lett. 2005, 46, 4499–4500. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; Ditzel, E.; Mitchell, S.J.; Robinson, W.T. Studies of Australian soft corals. XXVIII. The structure determination of two new diterpenes from the genus Xenia (Alcyonacea). Aust. J. Chem. 1982, 35, 997–1002. [Google Scholar] [CrossRef]
- Miyamoto, T.; Takenaka, Y.; Yamada, K.; Higuchi, R. Bioactive diterpenoids from Octocorallia, deoxyxeniolide B, a novel ichthyotoxic diterpenoid from the soft coral Xenia elongata. J. Nat. Prod. 1995, 58, 924–928. [Google Scholar] [CrossRef]
- Andrianasolo, E.H.; Haramaty, L.; Degenhardt, K.; Mathew, R.; White, E.; Lutz, R.; Falkowski, P. Induction of apoptosis by diterpenes from the soft coral Xenia elongata. J. Nat. Prod. 2007, 70, 1551–1557. [Google Scholar] [CrossRef]
- Andrianasolo, E.H.; Haramaty, L.; White, E.; Lutz, R.; Falkowski, P. Mode of action of diterpene and characterization of related metabolites from the soft coral, Xenia elongata. Mar. Drugs 2014, 12, 1102–1115. [Google Scholar] [CrossRef]
- Kashman, Y.; Saltoun, M.; Rudi, A.; Benayahu, Y. Xeniafaraunol A and B, and faraunatin; three new cytotoxic diterpenes from the soft coral Xenia faraunensis. Tetrahedron Lett. 1994, 35, 8855–8858. [Google Scholar] [CrossRef]
- Iwagawa, T.; Kawasaki, J.; Hase, T.; Wright, J.L.C. New di- and tricarbocyclic diterpenes possessing a bicyclic [4.3.1] ring system isolated from the soft coral, Xenia florida. Tetrahedron 1997, 53, 6809–6816. [Google Scholar] [CrossRef]
- Iwagawa, T.; Kawasaki, J.; Hase, T. New xenia diterpenoids isolated from the soft coral, Xenia florida. J. Nat. Prod. 1998, 61, 1513–1515. [Google Scholar] [CrossRef] [PubMed]
- Iwagawa, T.; Nakamura, K.; Hirose, T.; Okamura, H.; Nakatani, M. New xenicane diterpenes isolated from the acetone extract of the soft coral Xenia florida. J. Nat. Prod. 2000, 63, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Lin, Y.C.; Ahmed, A.F.; Kuo, Y.H. New xenicane diterpenoids from Xenia florida. Tetrahedron Lett. 2005, 46, 4793–4796. [Google Scholar] [CrossRef]
- Cheng, Y.B.; Jang, J.Y.; Khalil, A.T.; Kuo, Y.H.; Shen, Y.C. Xenicane-type diterpenes with cytotoxicity from Xenia florida. J. Nat. Prod. 2006, 69, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Groweiss, A.; Kashman, Y. Eight new xenia diterpenoids from three soft corals of the Red Sea. Tetrahedron 1983, 39, 3385–3396. [Google Scholar] [CrossRef]
- Groweiss, A.; Kashman, Y. Xeniculin, xeniaphyllenol and xeniaphyllenol oxide, new diterpenoids from the soft-coral Xenia macrospiculata. Tetrahedron Lett. 1978, 19, 2205–2208. [Google Scholar] [CrossRef]
- Lelong, H.; Ahond, A.; Chiaroni, A.; Poupat, C.; Riche, C.; Potier, P.; Pusset, J.; Pusset, M.; Laboute, P.; Menou, J.L. Invertébrés marins du lagon Néo-Calédonien, VIII. Métabolites terpéniques de Xenia membranacea. J. Nat. Prod. 1987, 50, 203–210. [Google Scholar] [CrossRef]
- Almourabit, A.; Ahond, A.; Chiaroni, A.; Poupat, C.; Riche, C.; Potier, P.; Laboute, P.; Menou, J.L. Invertébrés marins du lagon Néo-Calédonien, IX. Havannachlorhydrines, nouveaux métabolites de Xenia membranacea: Étude structural et configuration absolue. J. Nat. Prod. 1988, 51, 282–292. [Google Scholar] [CrossRef]
- Almourabit, A.; Gillet, B.; Ahond, A.; Beloeil, J.C.; Poupat, C.; Potier, P. Invertébrés marins du lagon Néo-Calédonien, XI. Les desoxyhavannahines, nouveaux métabolites de Xenia membranacea. J. Nat. Prod. 1989, 52, 1080–1087. [Google Scholar] [CrossRef]
- Almourabit, A.; Ahond, A.; Poupat, C.; Potier, P. Invertébrés marins du lagon Néo-Calédonien, XII. Isolement et étude structural de nouveaux diterpénes extraits de l’alcyonaire Xenia membranacea. J. Nat. Prod. 1990, 53, 894–908. [Google Scholar] [CrossRef]
- Braekman, J.C.; Daloze, D.; Tursch, B.; Declercq, J.P.; Germain, G.; van Meerssche, M. Chemical studies of marine invertebrates. XXXIX. Three novel diterpenoids from the soft coral Xenia novae-britanniae. Bull. Soc. Chem. Belg. 1979, 88, 71–77. [Google Scholar] [CrossRef]
- Bishara, A.; Rudi, A.; Goldberg, I.; Benayahu, Y.; Kashman, Y. Novaxenicins A-D and xeniolides I-K, seven new diterpenes from the soft coral Xenia novaebrittanniae. Tetrahedron 2006, 62, 12092–12097. [Google Scholar] [CrossRef]
- Duh, C.Y.; El-Gamal, A.A.H.; Chiang, C.Y.; Chu, C.J.; Wang, S.K.; Dai, C.F. New cytotoxic xenia diterpenoids from the Formosan soft coral Xenia umbellata. J. Nat. Prod. 2002, 65, 1882–1885. [Google Scholar] [CrossRef]
- El-Gamal, A.A.H.; Wang, S.K.; Duh, C.Y. Cytotoxic xenia diterpenoids from the soft coral Xenia umbellata. J. Nat. Prod. 2006, 69, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Chen, Y.H.; Cheng, Y.B.; Kuo, Y.H.; Khalil, A.T. Two new xeniolides from a Taiwanese Xenia soft coral. Nat. Prod. Res. 2007, 21, 1171–1177. [Google Scholar] [CrossRef]
- Ayyad, S.E.N.; Alarif, W.M.; Al-Footy, K.O.; Selim, E.A.; Ghandourah, M.A.; Aly, M.M.; Alorfi, H.S. Isolation, antimicrobial and antitumor activities of a new polyhydroxysteroid and a new diterpenoid from the soft coral Xenia umbellata. Z. Nat. 2016, 72, 27–34. [Google Scholar] [CrossRef]
- Janairo, J.R.; Janairo, G.C.; Ragasa, C.Y.; Bowden, B.F. New xenicanes from Xenia viridis. Nat. Prod. Res. 2007, 21, 1067–1072. [Google Scholar] [CrossRef]
- Kobayashi, M.; Zheng, C.; Yang, C.; Kyogoku, Y.; Kitagawa, I. Xeniolone and isoxeniolone two new diterpenes having a perhydroazulene skeleton from an Okinawan soft coral of Xenia sp. Chem. Pharm. Bull. 1985, 34, 4641–4652. [Google Scholar] [CrossRef][Green Version]
- Kitagawa, I.; Zheng, C.; Yang, C.; Kobayashi, M.; Kyogoku, Y. Marine natural products. XVI. Structures of five new diterpenes from an Okinawan soft coral of Xenia sp. (Xeniidae). Chem. Pharm. Bull. 1986, 34, 4641–4652. [Google Scholar] [CrossRef]
- Vervoort, H.C.; Fenical, W. Antibacterial diterpenoids from an undescribed soft-coral of the genus Xenia. Nat. Prod. Lett. 1995, 6, 49–55. [Google Scholar] [CrossRef]
- Iwagawa, T.; Masuda, T.; Okamura, H.; Nakatani, M. New xenia diterpenoids from a Xenia species of a soft coral. Tetrahedron 1996, 52, 13121–13128. [Google Scholar] [CrossRef]
- Iwagawa, T.; Amano, Y.; Okamura, H.; Nakatani, M.; Hase, T. New xenia diterpenoids from a soft coral Xenia species. Heterocycles 1996, 43, 1271–1277. [Google Scholar] [CrossRef]
- Miyaoka, H.; Nakano, M.; Iguchi, K.; Yamada, Y. Three new xenicane diterpenoids from Okinawan soft coral of the genus Xenia. Tetrahedron 1999, 55, 12977–12982. [Google Scholar] [CrossRef]
- Miyaoka, H.; Mitome, H.; Nakano, M.; Yamada, Y. Xeniaoxolane: A new xenicane-type diterpenoid from the Okinawan soft coral, Xenia sp.; absolute configurations of xeniaoxolane, xeniolide-A and xenialactol. Tetrahedron 2000, 25, 7737–7740. [Google Scholar] [CrossRef]
- Anta, C.; González, N.; Santafé, G.; Rodríguez, J.; Jiménez, C. New xenia diterpenoids from the Indonesian soft coral Xenia sp. J. Nat. Prod. 2002, 65, 766–768. [Google Scholar] [CrossRef]
- Miyaoka, H.; Nakano, M.; Iguchi, K.; Yamada, Y. Two new xenicane diterpenoids from Okinawan soft coral of the genus Xenia. Heterocycles 2003, 61, 189–196. [Google Scholar] [CrossRef]
- Fattorusso, E.; Romano, A.; Taglialatela-Scafati, O.; Achmad, M.J.; Bavestrello, G.; Cerrano, C. Xenimanadins A-D, a family of xenicane diterpenoids from the Indonesian soft coral Xenia sp. Tetrahedron 2008, 64, 3141–3146. [Google Scholar] [CrossRef]
- Maarisit, W.; Yano, K.; Miyazato, T.; Roy, P.K.; Taira, J.; Ueda, K. Structures and bioactivities of xenicanes from an Okinawan soft coral Xenia sp. Heterocycles 2015, 91, 505–514. [Google Scholar]
- Phan, C.S.; Kamada, T.; Ishii, T.; Hamada, T.; Vairappan, C.S. 12-Epi-9-deacetoxyxenicin, new cytotoxic diterpenoid from Bornean soft coral, Xenia sp. Nat. Prod. Res. 2017, 33, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Bowden, B.F.; Coll, J.C.; Willis, R.H. Studies of Australian soft corals. XXXIX. New sesquiterpene metabolites from several Xenia species (Xeniidae, Octocorallia, Anthozoa). Aust. J. Chem. 1986, 39, 1717–1722. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; Engelhardt, L.M.; Heaton, A.; White, A.H. Studies of Australian soft corals. XLI structure determination of a new sesquiterpene from Xenia novae-britanniae and an investigation of a Xenia species. Aust. J. Chem. 1987, 40, 1483–1489. [Google Scholar] [CrossRef]
- Duh, C.Y.; Chien, S.C.; Song, P.Y.; Wang, S.K.; El-Gamal, A.A.H.; Dai, C.F. New cadinene sesquiterpenoids from the Formosan soft coral Xenia puerto-galerae. J. Nat. Prod. 2002, 65, 1853–1856. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Tzeng, G.L.; Chen, M.H.; Khalil, A.T. Three new cadinene derivatives from Xenia puerto-galerae. J. Chin. Chem. Soc. 2007, 54, 1347–1352. [Google Scholar] [CrossRef]
- Kitagawa, I.; Kobayashi, M.; Cui, Z.; Kiyota, Y.; Ohnishi, M. Marine natural products. XV. Chemical constituents of an Okinawan soft coral of Xenia sp. (Xeniidae). Chem. Pharm. Bull. 1986, 34, 4590–4596. [Google Scholar] [CrossRef]
- Huber, T.; Weisheit, L.; Magauer, T. Synthesis of Xenia diterpenoids and related metabolites from marine organisms. Beilstein J. Org. Chem. 2015, 11, 2521–2539. [Google Scholar] [CrossRef]
- Koido, T.; Imahara, Y.; Fukami, H. High species diversity of the soft coral family Xeniidae (Octocorallia, Alcyonacea) in the temperate region of Japan revealed by morphological and molecular analyses. ZooKeys 2019, 862, 1–22. [Google Scholar] [CrossRef]
- Maloney, K.N.; Botts, R.T.; Davis, T.S.; Okada, B.K.; Maloney, E.M.; Leber, C.A.; Alvarado, O.; Brayton, C.; Caraballo-Rodríguez, M.A.; Chari, J.V.; et al. Cryptic species account for the seemingly idiosyncratic secondary metabolism of Sarcophyton glaucum specimens collected in Palau. J. Nat. Prod. 2020, 83, 693–705. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, S.-Y.; Phan, C.-S.; Ishii, T.; Kamada, T.; Hamada, T.; Vairappan, C.S. Terpenoids from Marine Soft Coral of the Genus Xenia in 1977 to 2019. Molecules 2020, 25, 5386. https://doi.org/10.3390/molecules25225386
Ng S-Y, Phan C-S, Ishii T, Kamada T, Hamada T, Vairappan CS. Terpenoids from Marine Soft Coral of the Genus Xenia in 1977 to 2019. Molecules. 2020; 25(22):5386. https://doi.org/10.3390/molecules25225386
Chicago/Turabian StyleNg, Shean-Yeaw, Chin-Soon Phan, Takahiro Ishii, Takashi Kamada, Toshiyuki Hamada, and Charles Santhanaraju Vairappan. 2020. "Terpenoids from Marine Soft Coral of the Genus Xenia in 1977 to 2019" Molecules 25, no. 22: 5386. https://doi.org/10.3390/molecules25225386
APA StyleNg, S.-Y., Phan, C.-S., Ishii, T., Kamada, T., Hamada, T., & Vairappan, C. S. (2020). Terpenoids from Marine Soft Coral of the Genus Xenia in 1977 to 2019. Molecules, 25(22), 5386. https://doi.org/10.3390/molecules25225386




