Dependence of Fluorescence Quenching of CY3 Oligonucleotide Conjugates on the Oxidation Potential of the Stacking Base Pair
Abstract
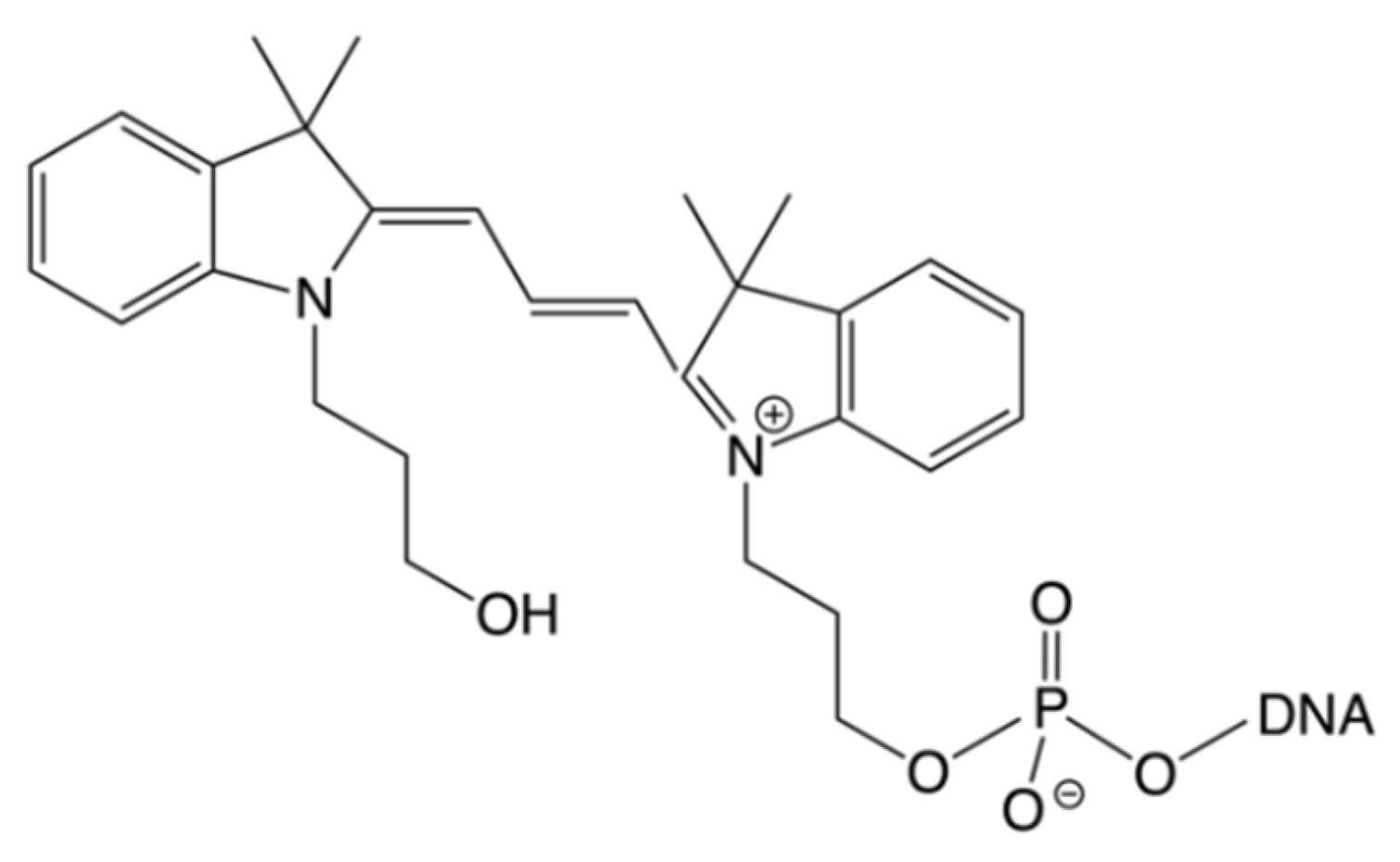
1. Introduction
2. Results
2.1. Optical Spectra
2.2. Surface Plasmon Resonance (SPR) Measurements
2.3. Single-Molecule Measurements
3. Discussion
4. Materials and Methods
4.1. Optical Measurements
4.2. Surface Plasmon Resonance Measurements
4.3. Single-Molecule Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- König, W. Über die Konstitution der Pinacyanole, ein Beitrag zur Chemie der Chinocyanine. Ber. Dtsch. Chem. Ges. 1922, 55, 3294–3313. [Google Scholar] [CrossRef][Green Version]
- Randolph, J.B.; Waggoner, A.S. Stability, specificity and fluorescence brightness of multiply-labeled fluorescent DNA probes. Nucleic Acids Res. 1997, 25, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Sanborn, M.E.; Connolly, B.K.; Gurunathan, K.; Levitus, M. Fluorescence properties and photophysics of the sulfoindocyanine Cy3 linked covalently to DNA. J. Phys. Chem. B 2007, 111, 11064–11074. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.J.; Perez, C.; Levitus, M. DNA sequence-dependent enhancement of Cy3 fluorescence. Photochem. Photobiol. Sci. 2009, 8, 1105. [Google Scholar] [CrossRef] [PubMed]
- Levitus, M.; Ranjit, S. Cyanine dyes in biophysical research: The photophysics of polymethine fluorescent dyes in biomolecular environments. Q. Rev. Biophys. 2011, 44, 123–151. [Google Scholar] [CrossRef]
- Stennett, E.M.S.; Ma, N.; van der Vaart, A.; Levitus, M. Photophysical and dynamical properties of doubly linked Cy3-DNA constructs. J. Phys. Chem. B 2014, 118, 152–163. [Google Scholar] [CrossRef]
- Jia, K.; Wan, Y.; Xia, A.; Li, S.; Gong, F.; Yang, G. Characterization of Photoinduced Isomerization and Intersystem Crossing of the Cyanine Dye Cy3. J. Phys. Chem. A 2007, 111, 1593–1597. [Google Scholar] [CrossRef]
- Ouellet, J.; Schorr, S.; Iqbal, A.; Wilson, T.J.; Lilley, D.M.J. Orientation of cyanine fluorophores terminally attached to DNA via long, flexible tethers. Biophys. J. 2011, 101, 1148–1154. [Google Scholar] [CrossRef]
- Linck, L.; Kapusta, P.; Resch-Genger, U. Spectroscopic and photophysical properties of dUTP and internally DNA bound fluorophores for optimized signal detection in biological formats. Photochem. Photobiol. 2012, 88, 867–875. [Google Scholar] [CrossRef]
- Linck, L.; Reiß, E.; Bier, F.; Resch-Genger, U. Direct labeling rolling circle amplification as a straightforward signal amplification technique for biodetection formats. Anal. Methods 2012, 4, 1215. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Science 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Kashida, H.; Morimoto, K.; Asanuma, H. A stem-less probe using spontaneous pairing between Cy3 and quencher for RNA detection. Sci. Technol. Adv. Mater. 2016, 17, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.; Heilemann, M. Single-molecule localization microscopy in Eukaryotes. Chem. Rev. 2017, 117, 7478–7509. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa-Ankerhold, H.C.H.; Ankerhold, R.R.; Drummen, G.P.C.G. Advanced fluorescence microscopy techniques—FRAP, FLIP, FLAP, FRET and FLIM. Molecules 2011, 17, 4047–4132. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.; Tinnefeld, P. Photophysics of fluorescent probes for single-molecule biophysics and super-resolution imaging. Annu. Rev. Phys. Chem. 2012, 63, 595–617. [Google Scholar] [CrossRef] [PubMed]
- Morten, M.J.; Lopez, S.G.; Steinmark, I.E.; Rafferty, A.; Magennis, S.W. Stacking-induced fluorescence increase reveals allosteric interactions through DNA. Nucleic Acids Res. 2018, 46, 11618–11626. [Google Scholar] [CrossRef] [PubMed]
- Glembockyte, V.; Lincoln, R.; Cosa, G. Cy3 Photoprotection mediated by Ni2+ for extended single-molecule imaging: Old tricks for new techniques. J. Am. Chem. Soc. 2015, 137, 1116–1122. [Google Scholar] [CrossRef]
- Kawai, K.; Maruyama, A. Triple helix conformation-specific blinking of Cy3 in DNA. Chem. Commun. 2015, 51, 4861–4864. [Google Scholar] [CrossRef]
- Sobek, J.; Rehrauer, H.; Schauer, S.; Fischer, D.; Patrignani, A.; Landgraf, S.; Korlach, J.; Schlapbach, R. Single-molecule DNA hybridisation studied by using a modified DNA sequencer: A comparison with surface plasmon resonance data. Methods Appl. Fluoresc. 2016, 4, 015002. [Google Scholar] [CrossRef]
- Sobek, J.; Schmidt, M.; Grossmann, J.; Rehrauer, H.; Schmidt, L.; Schlapbach, R. Single-molecule chemistry. Part I: Monitoring oxidation of G in oligonucleotides using CY3 fluorescence. Methods Appl. Fluoresc. 2020, 8, 035010. [Google Scholar] [CrossRef]
- Harvey, B.; Levitus, M. Nucleobase-specific enhancement of Cy3 fluorescence. J. Fluoresc. 2009, 19, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Chen, X.; Aumiler, D.; Xia, A. Single molecule fluorescence fluctuations of the cyanine dyes linked covalently to DNA. Sci. China Ser. B Chem. 2009, 52, 1148–1153. [Google Scholar] [CrossRef]
- Mujumdar, R.B.; Ernst, L.A.; Mujumdar, S.R.; Lewis, C.J.; Waggoner, A.S. Cyanine dye labeling reagents: Sulfoindocyanine succinimidyl esters. Bioconjug. Chem. 1993, 4, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.M.; Banal, J.L.; Bathe, M.; Schlau-Cohen, G.S. Identification of nonradiative decay pathways in Cy3. J. Phys. Chem. Lett. 2020, 11, 5000–5007. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.; Ebner, A.; Briggs, M.; Burrows, M.; Gardner, N.; Richardson, R.; West, R. Cy3B: Improving the performance of cyanine dyes. J. Fluoresc. 2004, 14, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.M.; Gerowska, M.; Brown, T. A highly fluorescent DNA toolkit: Synthesis and properties of oligonucleotides containing new Cy3, Cy5 and Cy3B monomers. Nucleic Acids Res. 2012, 40, e108. [Google Scholar] [CrossRef]
- Stennett, E.M.S.; Ciuba, M.A.; Lin, S.; Levitus, M. Demystifying PIFE: The photophysics behind the protein-induced fluorescence enhancement phenomenon in Cy3. J. Phys. Chem. Lett. 2015, 6, 1819–1823. [Google Scholar] [CrossRef]
- Nazarenko, I.; Pires, R.; Lowe, B.; Obaidy, M.; Rashtchian, A. Effect of primary and secondary structure of oligodeoxyribonucleotides on the fluorescent properties of conjugated dyes. Nucleic Acids Res. 2002, 30, 2089–2195. [Google Scholar] [CrossRef]
- Seidel, C.; Schulz, A.; Sauer, M. Nucleobase-specific quenching of fluorescent dyes. 1. Nucleobase one-electron redox potentials and their correlation with static and dynamic quenching efficiencies. J. Phys. Chem. 1996, 100, 5541–5553. [Google Scholar] [CrossRef]
- Sauer, M.; Drexhage, K.; Lieberwirth, U.; Muller, R.; Nord, S.; Zander, C. Dynamics of the electron transfer reaction between an oxazine dye and DNA oligonucleotides monitored on the single-molecule level. Chem. Phys. Lett. 1998, 284, 153–163. [Google Scholar] [CrossRef]
- Widengren, J.; Dapprich, J.; Rigler, R. Fast interactions between Rh6G and dGTP in water studied by fluorescence correlation spectroscopy. Chem. Phys. 1997, 216, 417–426. [Google Scholar] [CrossRef]
- Unruh, J.R.; Gokulrangan, G.; Wilson, G.S.; Johnson, C.K. Fluorescence properties of fluorescein, tetramethylrhodamine and Texas Red linked to a DNA aptamer. Photochem. Photobiol. 2005, 81, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Kroutil, O.; Romancová, I.; Šíp, M.; Chval, Z. Cy3 and Cy5 dyes terminally attached to 5′C end of DNA: Structure, dynamics, and energetics. J. Phys. Chem. B 2014, 118, 13564–13572. [Google Scholar] [CrossRef] [PubMed]
- Spiriti, J.; Binder, J.K.; Levitus, M.; van der Vaart, A. Cy3-DNA stacking interactions strongly depend on the identity of the terminal basepair. Biophys. J. 2011, 100, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Norman, D.G.; Grainger, R.J.; Uhrín, D.; Lilley, D.M.J. Location of Cyanine-3 on double-stranded DNA: Importance for fluorescence resonance energy transfer studies. Biochemistry 2000, 39, 6317–6324. [Google Scholar] [CrossRef]
- Moreira, B.G.; You, Y.; Owczarzy, R. Cy3 and Cy5 dyes attached to oligonucleotide terminus stabilize DNA duplexes: Predictive thermodynamic model. Biophys. Chem. 2015, 198, 36–44. [Google Scholar] [CrossRef]
- Guckian, K.M.; Schweitzer, B.A.; Ren, R.X.F.; Sheils, C.J.; Tahmassebi, D.C.; Kool, E.T. Factors contributing to aromatic stacking in water: Evaluation in the context of DNA. J. Am. Chem. Soc. 2000, 122, 2213–2222. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 4th ed.; Springer Science & Business Media LLC: New York, NY, USA, 2006. [Google Scholar]
- Torimura, M.; Kurata, S.; Yamada, K.; Yokomaku, T.; Kamagata, Y.; Kanagawa, T.; Kurane, R. Fluorescence-quenching phenomenon by photoinduced electron transfer between a fluorescent dye and a nucleotide base. Anal. Sci. 2001, 17, 155–160. [Google Scholar] [CrossRef]
- Kawai, K.; Osakada, Y.; Fujitsuka, M.; Majima, T. Charge separation in acridine- and phenothiazine-modified DNA. J. Phys. Chem. B 2008, 112, 2144–2149. [Google Scholar] [CrossRef]
- Adhikary, A.; Khanduri, D.; Sevilla, M.D. Direct observation of the hole protonation state and hole localization site in DNA-oligomers. J. Am. Chem. Soc. 2009, 131, 8614–8619. [Google Scholar] [CrossRef]
- Steenken, S. Electron-transfer-induced acidity basicity and reactivity changes of purine and pyrimidine-bases—Consequences of redox processes for DNA-base pairs. Free Radic. Res. Commun. 1992, 16, 349–379. [Google Scholar] [CrossRef] [PubMed]
- Candeias, L.P.; Steenken, S. Structure and acid-base properties of one-electron-oxidized deoxyguanosine, guanosine, and 1-methylguanosine. J. Am. Chem. Soc. 1989, 111, 1094–1099. [Google Scholar] [CrossRef]
- Caruso, T.; Carotenuto, M.; Vasca, E.; Peluso, A. Direct experimental observation of the effect of the base pairing on the oxidation potential of guanine. J. Am. Chem. Soc. 2005, 127, 15040–15041. [Google Scholar] [CrossRef] [PubMed]
- Caruso, T.; Capobianco, A.; Peluso, A. The oxidation potential of adenosine and adenosine-thymidine base pair in chloroform solution. J. Am. Chem. Soc. 2007, 129, 15347–15353. [Google Scholar] [CrossRef]
- Crespo-Hernández, C.E.; Close, D.M.; Gorb, L.; Leszczynski, J. Determination of redox potentials for the watson−crick base pairs, DNA nucleosides, and relevant nucleoside analogues. J. Phys. Chem. B 1915, 111, 5386–5395. [Google Scholar] [CrossRef]
- Paukku, Y.; Hill, G. Theoretical determination of one-electron redox potentials for DNA bases, base pairs, and stacks. J. Phys. Chem. A 2011, 115, 4804–4810. [Google Scholar] [CrossRef]
- Colson, A.O.; Besler, B.; Sevilla, M.D. Ab initio molecular orbital calculations on DNA base pair radical ions: Effect of base pairing on proton-transfer energies, electron affinities, and ionization potentials. J. Phys. Chem. 1992, 96, 9787–9794. [Google Scholar] [CrossRef]
- Hutter, M.; Clark, T. On the enhanced stability of the guanine-cytosine base-pair radical cation. J. Am. Chem. Soc. 1996, 118, 7574–7577. [Google Scholar] [CrossRef]
- Kawai, K.; Wata, Y.; Hara, M.; Tojo, S.; Majima, T. Regulation of one-electron oxidation rate of guanine by base pairing with cytosine derivatives. J. Am. Chem. Soc. 2002, 124, 3586–3590. [Google Scholar] [CrossRef]
- Kan, Y.; Schuster, G.B. Long-range guanine damage in single-stranded DNA: Charge transport through a duplex bridge and in a single-stranded overhang. J. Am. Chem. Soc. 1999, 121, 10857–10864. [Google Scholar] [CrossRef]
- Burrows, C.J.; Muller, J.G. Oxidative nucleobase modifications leading to strand scission. Chem. Rev. 1998, 98, 1109–1152. [Google Scholar] [CrossRef] [PubMed]
- Kanvah, S.; Joseph, J.; Schuster, G.B.; Barnett, R.N.; Cleveland, C.L.; Landman, U. Oxidation of DNA: Damage to nucleobases. Acc. Chem. Res. 2010, 43, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Muren, N.B.; Olmon, E.D.; Barton, J.K. Solution, surface, and single molecule platforms for the study of DNA-mediated charge transport. Phys. Chem. Chem. Phys. 2012, 14, 13754. [Google Scholar] [CrossRef][Green Version]
- Genereux, J.C.; Barton, J.K. Mechanisms for DNA charge transport. Chem. Rev. 2010, 110, 1642–1662. [Google Scholar] [CrossRef] [PubMed]
- Giese, B. Hole injection and hole transfer through DNA: The hopping mechanism. In Longe-Range Charge Transfer in DNA I; Springer: Berlin/Heidelberg, Germany, 2004; pp. 151–164. [Google Scholar]
- Cadet, J.; Wagner, J.R. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, E.N.; Stull, F.; Al-Hashimi, H.M. Guanine to inosine substitution leads to large increases in the population of a transient G·C Hoogsteen base pair. Biochemistry 2014, 53, 7145–7147. [Google Scholar] [CrossRef]
- Modrich, P. DNA mismatch correction. Annu. Rev. Biochem. 1987, 56, 435–466. [Google Scholar] [CrossRef]
- Kingsland, A.; Maibaum, L. DNA base pair mismatches induce structural changes and alter the free-energy landscape of base flip. J. Phys. Chem. B 2018, 122, 12251–12259. [Google Scholar] [CrossRef]
- Wan, C.; Fiebig, T.; Schiemann, O.; Barton, J.K.; Zewail, A.H. Femtosecond direct observation of charge transfer between bases in DNA. Proc. Natl. Acad. Sci. USA 2000, 97, 14052–14055. [Google Scholar] [CrossRef]
- Sheu, C.; Foote, C.S. Photosensitized oxygenation of a 7,8-Dihydro-8-oxoguanosine derivative. Formation of dioxetane and hydroperoxide intermediates. J. Am. Chem. Soc. 1995, 117, 474–477. [Google Scholar] [CrossRef]
- Levene, M.J.; Korlach, J.; Turner, S.W.; Foquet, M.; Craighead, H.G.; Webb, W.W. Zero-mode waveguides for single-molecule analysis at high concentrations. Science 2003, 299, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Eid, J.; Fehr, A.; Gray, J.; Luong, K.; Lyle, J.; Otto, G.; Peluso, P.; Rank, D.; Baybayan, P.; Bettman, B.; et al. Real-time DNA sequencing from single polymerase molecules. Science 2009, 323, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dalal, R.V.; Petrov, A.N.; Tsai, A.; O’Leary, S.E.; Chapin, K.; Cheng, J.; Ewan, M.; Hsiung, P.-L.; Lundquist, P.; et al. High-throughput platform for real-time monitoring of biological processes by multicolor single-molecule fluorescence. Proc. Natl. Acad. Sci. USA 2014, 111, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.; Foote, C.S. Reactivity toward singlet oxygen of a 7,8-Dihydro-8-oxoguanosine (“8-Hydroxyguanosine”) formed by photooxidation of a guanosine derivative. J. Am. Chem. Soc. 1995, 117, 6439–6442. [Google Scholar] [CrossRef]
- Ravanat, J.-L.; Saint-Pierre, C.; Cadet, J. One-electron oxidation of the guanine moiety of 2‘-deoxyguanosine: Influence of 8-Oxo-7,8-dihydro-2‘-deoxyguanosine. J. Am. Chem. Soc. 2003, 125, 2030–2031. [Google Scholar] [CrossRef]
- Fleming, A.M.; Muller, J.G.; Ji, I.; Burrows, C.J. Characterization of 2′-deoxyguanosine oxidation products observed in the Fenton-like system Cu(ii)/H2O2/reductant in nucleoside and oligodeoxynucleotide contexts. Org. Biomol. Chem. 2011, 9, 3338. [Google Scholar] [CrossRef]
- Fleming, A.M.; Burrows, C.J. 8-Oxo-7,8-dihydro-2′-deoxyguanosine and abasic site tandem lesions are oxidation prone yielding hydantoin products that strongly destabilize duplex DNA. Org. Biomol. Chem. 2017, 15, 8341–8353. [Google Scholar] [CrossRef]
- Jean Cadet, T.D.A.J.-L.R. Oxidatively generated damage to the guanine moiety of DNA: Mechanistic aspects and formation in cells. Acc. Chem. Res. 2008, 41, 1075–1083. [Google Scholar] [CrossRef]
- Kanvah, S.; Schuster, G.B. One-electron oxidation of DNA: Thymine versus guanine reactivity. Org. Biomol. Chem. 2010, 8, 1340. [Google Scholar] [CrossRef]
- Ghosh, A.; Joy, A.; Schuster, G.B.; Douki, T.; Cadet, J. Selective one-electron oxidation of duplex DNA oligomers: Reaction at thymines. Org. Biomol. Chem. 2008, 6, 916. [Google Scholar] [CrossRef]
- Li, X.; Yin, Y.; Yang, X.; Zhi, Z.; Zhao, X.S. Temperature dependence of interaction between double stranded DNA and Cy3 or Cy5. Chem. Phys. Lett. 2011, 513, 271–275. [Google Scholar] [CrossRef]
- Iqbal, A.; Arslan, S.; Okumus, B.; Wilson, T.J.; Giraud, G.; Norman, D.G.; Ha, T.; Lilley, D.M.J. Orientation dependence in fluorescent energy transfer between Cy3 and Cy5 terminally attached to double-stranded nucleic acids. Proc. Natl. Acad. Sci. USA 2008, 105, 11176–11181. [Google Scholar] [CrossRef] [PubMed]
- Milas, P.; Gamari, B.D.; Parrot, L.; Krueger, B.P.; Rahmanseresht, S.; Moore, J.; Goldner, L.S. Indocyanine dyes approach free rotation at the 3′ terminus of A-RNA: A comparison with the 5′ terminus and consequences for fluorescence resonance energy transfer. J. Phys. Chem. B 2013, 117, 8649–8658. [Google Scholar] [CrossRef] [PubMed]
- Sjöback, R.; Nygren, J.; Kubista, M. Characterization of fluorescein-oligonucleotide conjugates and measurement of local electrostatic potential. Biopolymers 1998, 46, 445–453. [Google Scholar] [CrossRef]
- Neeley, W.L.; Essigmann, J.M. Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem. Res. Toxicol. 2006, 19, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.M. Reactivity of nucleic acid radicals. In Advances in Physical Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; Volume 50, pp. 119–202. [Google Scholar]
- Margolin, Y.; Shafirovich, V.; Geacintov, N.E.; DeMott, M.S.; Dedon, P.C. DNA sequence context as a determinant of the quantity and chemistry of guanine oxidation produced by hydroxyl radicals and one-electron oxidants. J. Biol. Chem. 2008, 283, 35569–35578. [Google Scholar] [CrossRef] [PubMed]
- Douki, T.; Martini, R.; Ravanat, J.L.; Turesky, R.J.; Cadet, J. Measurement of 2,6-diamino-4-hydroxy-5-formamidopyrimidine and 8-oxo-7,8-dihydroguanine in isolated DNA exposed to gamma radiation in aqueous solution. Carcinogenesis 1997, 18, 2385–2391. [Google Scholar] [CrossRef]
- Bauer, N.C.; Corbett, A.H.; Doetsch, P.W. The current state of eukaryotic DNA base damage and repair. Nucleic Acids Res. 2015, 43, 10083–10101. [Google Scholar] [CrossRef]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.; Di Mascio, P.; Wagner, J.R. Free radical biology and medicine. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef]
- Fleming, A.M.; Burrows, C.J. Formation and processing of DNA damage substrates for the hNEIL enzymes. Free Radic. Biol. Med. 2017, 107, 35–52. [Google Scholar] [CrossRef]
- Kasai, H.; Yamaizumi, Z.; Berger, M.; Cadet, J. Photosensitized formation of 7,8-dihydro-8-oxo-2“-deoxyguanosine (8-hydroxy-2-”deoxyguanosine) in DNA by riboflavin: A nonsinglet oxygen-mediated reaction. J. Am. Chem. Soc. 1992, 114, 9692–9694. [Google Scholar] [CrossRef]
- Rokhlenko, Y.; Geacintov, N.E.; Shafirovich, V. Lifetimes and reaction pathways of guanine radical cations and neutral guanine radicals in an oligonucleotide in aqueous solutions. J. Am. Chem. Soc. 2012, 134, 4955–4962. [Google Scholar] [CrossRef] [PubMed]
- Rokhlenko, Y.; Cadet, J.; Geacintov, N.E.; Shafirovich, V. Mechanistic aspects of hydration of guanine radical cations in DNA. J. Am. Chem. Soc. 2014, 136, 5956–5962. [Google Scholar] [CrossRef] [PubMed]
- Devasagayam, T.P.A.; Sundquist, A.R.; Di Mao, P.; Kaiser, S.; Sies, H. Activity of thiols as singlet molecular-oxygen quenchers. J. Photochem. Photobiol. B Biol. 1991, 9, 105–116. [Google Scholar] [CrossRef]
- Rehm, D.; Weller, A. Kinetics of fluorescence quenching by electron and H-atom transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Stein, I.H.; Capone, S.; Smit, J.H.; Baumann, F.; Cordes, T.; Tinnefeld, P. Linking single-molecule blinking to chromophore structure and redox potentials. ChemPhysChem 2012, 13, 931–937. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron-transfer reactions in chemistry—Theory and experiment (Nobel lecture). Angew. Chem. Int. Ed. Engl. 1993, 32, 1111–1121. [Google Scholar] [CrossRef]
- Batchelor-McAuley, C.; Li, Q.; Dapin, S.M.; Compton, R.G. Voltammetric characterization of DNA intercalators across the full pH range: Anthraquinone-2,6-disulfonate and anthraquinone-2-sulfonate. J. Phys. Chem. B 2010, 114, 4094–4100. [Google Scholar] [CrossRef]
- Grotz, K.K.; Nueesch, M.F.; Holmstrom, E.D.; Heinz, M.; Stelzl, L.S.; Schuler, B.; Hummer, G. Dispersion correction alleviates dye stacking of single-stranded DNA and RNA in simulations of single-molecule fluorescence experiments. J. Phys. Chem. B 2018, 122, 11626–11639. [Google Scholar] [CrossRef]
- Capobianco, A.; Caruso, T.; Celentano, M.; D’Ursi, A.M.; Scrima, M.; Peluso, A. Stacking interactions between adenines in oxidized oligonucleotides. J. Phys. Chem. B 2013, 117, 8947–8953. [Google Scholar] [CrossRef]
- Capobianco, A.; Caruso, T.; Peluso, A. Hole delocalization over adenine tracts in single stranded DNA oligonucleotides. Phys. Chem. Chem. Phys. 2015, 17, 4750–4756. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, A.; Caruso, T.; D’Ursi, A.M.; Fusco, S.; Masi, A.; Scrima, M.; Chatgilialoglu, C.; Peluso, A. Delocalized hole domains in guanine-rich DNA oligonucleotides. J. Phys. Chem. B 2015, 119, 5462–5466. [Google Scholar] [CrossRef] [PubMed]
- Doose, S.; Neuweiler, H.; Sauer, M. Fluorescence quenching by photoinduced electron transfer: A reporter for conformational dynamics of macromolecules. ChemPhysChem 2009, 10, 1389–1398. [Google Scholar] [CrossRef]
- Rashid, F.; Raducanu, V.-S.; Zaher, M.S.; Tehseen, M.; Habuchi, S.; Hamdan, S.M. Initial state of DNA-Dye complex sets the stage for protein induced fluorescence modulation. Nat. Commun. 2019, 10, 2104. [Google Scholar] [CrossRef] [PubMed]
- Crockett, A.O.; Wittwer, C.T. Fluorescein-labeled oligonucleotides for real-time PCR: Using the inherent quenching of deoxyguanosine nucleotides. Anal. Biochem. 2001, 290, 89–97. [Google Scholar] [CrossRef]
- Noble, J.E.; Wang, L.; Cole, K.D.; Gaigalas, A.K. The effect of overhanging nucleotides on fluorescence properties of hybridising oligonucleotides labelled with Alexa-488 and FAM fluorophores. Biophys. Chem. 2005, 113, 255–263. [Google Scholar] [CrossRef]
- Clark, T.A.; Spittle, K.E.; Turner, S.W.; Korlach, J. Direct detection and sequencing of damaged DNA bases. Genome Integr. 2011, 2, 10. [Google Scholar] [CrossRef]







| Sample | λmax (abs) | λmax (fl) |
|---|---|---|
| CY3-26 | 550 | 566 |
| TLG | 549 | 564 |
| TLoG | 549 | 565 |
| TLA | 550 | 565 |
| TLI | 549 | 566 |
| ON34 | 550 | 565 |
| Probe | Analyte | KD/nM | KD Ratio | ∆G/kJ/mol |
|---|---|---|---|---|
| TLG | 7m | 2230(90) | ||
| 3CY3-7m | 107(3) | 20.8 | 7.4 | |
| 5CY3-7m | 77.4(23) | 28.8 | 8.2 | |
| TLoG | 7m | 4401(229) | ||
| 3CY3-7m | 253(6) | 17.4 | 7.0 | |
| 5CY3-7m | 155(3) | 28.4 | 8.2 | |
| TLA | 7m | 9475(1832) | 27.3 | 8.0 |
| 3CY3-7m | 347(12) | |||
| TLI | 7m | 8832(866) | 28.8 | 8.0 |
| 3CY3-7m | 307(5) | |||
| TLG | 8mG | 320(16) | 12.2 | 6.1 |
| 3CY3-8mG | 26.2(20) | |||
| TLoG | 8mG | 230(13) | 10.6 | 5.8 |
| 3CY3-8mG | 21.6(24) |
| Probe | Analyte | Dye Class | Dye Charge | KD/nM | KD Ratio | ∆G/kJ/mol |
|---|---|---|---|---|---|---|
| Bio34G | 7n | - | - | 978(25) | - | - |
| 5CY3-7n | sym. cyanine | +1 | 32.8(41) | 29.8 | 8.3 | |
| 3CY3-7n | sym. cyanine | +1 | 46.4(15) | 21.1 | 7.5 | |
| CY3-7nPEG | sym. cyanine | +1 | 84.6(56) | 11.6 | 6.0 | |
| CY3B-7n | sym. cyanine | 0 | 92.9(42) | 10.5 | 5.8 | |
| DY547-7n | sym. cyanine | −1 | 206(6) | 4.5 | 3.7 | |
| DY530-7n | rhodamine | −1 | 217(9) | 4.5 | 3.7 | |
| TAMRA-7n | rhodamine | 0 | 278(5) | 3.5 | 3.1 | |
| TexasRed-7n | rhodamine | 0 | 94.0(51) | 10.4 | 5.7 | |
| ATTO532-7n | rhodamine | −1 | 200(8) | 4.9 | 3.9 | |
| ATTO550-7n | rhodamine | +1 | 39.1(44) | 25.0 | 7.9 | |
| CY5-7n | sym. cyanine | +1 | 28.8(17) | 34.0 | 8.6 | |
| DY630-7n | asym. cyanine | 0 | 84.4(27) | 11.6 | 6.0 | |
| ATTO647N-7n | carbopyronine | 0 | 32.2(41) | 30.3 | 8.4 | |
| MB-7n | phenothiazine | +1 | 29.6(18) | 33.0 | 8.6 | |
| FAM-7n | fluorescein | −2 | 881(69) | 1.1 | 0.3 | |
| Bio34r | 7r | - | - | 506(210) | - | - |
| DY548-7r | sym. cyanine | −2 | 423(49) | 1.2 | 0.4 | |
| DY549-7r | sym. cyanine | −3 | 534(68) | 0.95 | −0.1 |
| Probe | Analyte | KD/nM | KD Ratio | ∆G/kJ/mol |
|---|---|---|---|---|
| Bio34G | 7T | 3025(68) | 19.7 | 7.3 |
| CY3-7T | 153(3) | |||
| Bio34I | 7n | 7419(61) | 28.8 | 8.2 |
| CY3-7n | 257(10) | |||
| Bio34U | 7n | 14,830(494) | 7.4 | 4.9 |
| CY3-7n | 1987(42) | |||
| Bio34ap | 7n | 45,111(24,127) | ||
| CY3-7n | 15,015(6605) | 3.0 | 2.7 | |
| 3CY3-7n | 1744(124) | 25.9 | 8.0 |
| Oligonucleotide | Sequence |
|---|---|
| TLG |  |
| TLoG |  |
| TLA |  |
| TLI |  |
| 7m | 5′CACGGTC |
| 5CY3-7m | 5′CY3–CACGGTC |
| 3CY3-7m | 5′CACGGTC–CY3 |
| 8mG | 5′GGCACGGTG |
| 3CY3-8mG | 5′GCACGGTG–CY3 |
| Bio25LNA | 5′AGTACAGATCATGCGTCGGGTTTTT-Biotin |
| CY3-26 | 5′CY3–CGATCAAGTACAGATCATGCGTCGGG |
| CY3-27 | 5′CY3–TCGATCAAGTACAGATCATGCGTCGGG |
| ON34 | TTTTTGGAAACTGTATTGGCACTGAGTAGACTCC |
| Bio34G | 5′Biotin-TTTTTGGAAACTGTATTGGCACTGAGTAGACTCC |
| Bio34I | 5′Biotin-TTTTTGGAAACTGTATTGGCACTIAGTAGACTCC |
| Bio34U | 5′Biotin-TTTTTGGAAACTGTATTGGCACTUAGTAGACTCC |
| Bio34ap | 5′Biotin-TTTTTGGAAACTGTATTGGCACTapAGTAGACTCC |
| 7n | CAGTGCC |
| 5CY3-7n | 5′CY3-CAGTGCC |
| 3CY3-7n | 5′CAGTGCC-CY3 |
| DYE-7n | 5′DYE-CAGTGCC |
| 7T | 5′CY3-TCAGTGC |
| CY3-7T | 5′CY3-TCAGTGC |
| Bio34r | 5′Biotin-TTTTTGGAAACTGTATTGTCACGGAGTAGACTTT |
| 7r | 5′CCGTGAC |
| DY548-7r | 5′DY548-CCGTGAC |
| DY549-7r | 5′DY549-CCGTGAC |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobek, J.; Schlapbach, R. Dependence of Fluorescence Quenching of CY3 Oligonucleotide Conjugates on the Oxidation Potential of the Stacking Base Pair. Molecules 2020, 25, 5369. https://doi.org/10.3390/molecules25225369
Sobek J, Schlapbach R. Dependence of Fluorescence Quenching of CY3 Oligonucleotide Conjugates on the Oxidation Potential of the Stacking Base Pair. Molecules. 2020; 25(22):5369. https://doi.org/10.3390/molecules25225369
Chicago/Turabian StyleSobek, Jens, and Ralph Schlapbach. 2020. "Dependence of Fluorescence Quenching of CY3 Oligonucleotide Conjugates on the Oxidation Potential of the Stacking Base Pair" Molecules 25, no. 22: 5369. https://doi.org/10.3390/molecules25225369
APA StyleSobek, J., & Schlapbach, R. (2020). Dependence of Fluorescence Quenching of CY3 Oligonucleotide Conjugates on the Oxidation Potential of the Stacking Base Pair. Molecules, 25(22), 5369. https://doi.org/10.3390/molecules25225369







