Benefits of Anthocyanin-Rich Black Rice Fraction and Wood Sterols to Control Plasma and Tissue Lipid Concentrations in Wistar Kyoto Rats Fed an Atherogenic Diet
Abstract
1. Introduction
2. Results
2.1. Body Weight Gain and Heart and Liver Weights
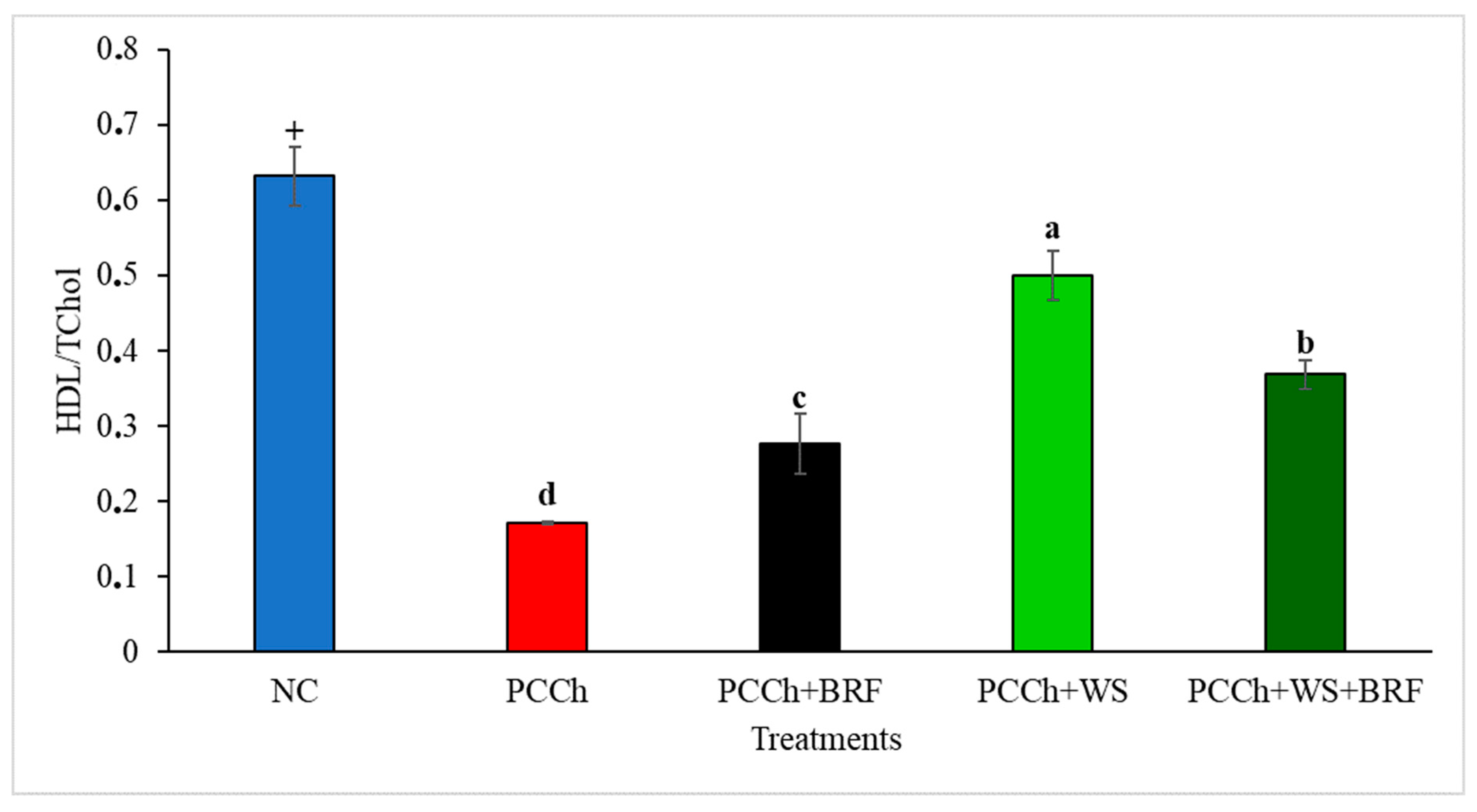
2.2. Blood Constituent Analysis
2.3. Tissue Lipid Concentrations
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Experimental Design and Plasma Analysis
4.3. Liver, Heart, and Aorta Tissue Analyses
4.4. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Adams, R.J.; Berry, J.D.; Brown, T.M.; Carnethon, M.R.; Dai, S.; de Simone, G.; Ford, E.S.; et al. Heart Disease and Stroke Statistics—2011 Update. A Report from the American Heart Association. Circulation 2011, 123, e18–e209. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. (WHO). Noncommunicable Diseases Country Profiles 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Prabhakaran, D.; Jeemon, P.; Roy, A. Cardiovascular diseases in India: Current epidemiology and future directions. Circulation 2016, 133, 1605–1620. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, D.D.; Ley, S.H.; Green-Howard, A.; He, Y.; Lu, Y.; Danaei, G.; Hu, F.B. Potential impact of time trend of life-style factors on cardiovascular disease burden in China. J. Am. Col. Cardiol. 2016, 68, 818–833. [Google Scholar] [CrossRef]
- World Health Organization. (WHO). Global Health Observatory (GHO) Data: Raised Cholesterol. Situation and Trends. 2017. Available online: https://www.who.int/gho/ncd/risk_factors/cholesterol_text/en/ (accessed on 21 June 2020).
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegård, L.; Jessup, W.; Jones, P.J.; Lütjohann, D.; Maerz, W.; Masana, L.; et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014, 232, 346–360. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Ras, R.T.; Gagliardi, A.C.; Mangili, L.C.; Trautwein, E.A.; Santos, R.D. Effects of phytosterols on markers of inflammation: A systematic review and meta-analysis. Atherosclerosis 2016, 248, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.J.H.; Shamloo, M.; MacKay, D.S.; Rideout, T.C.; Myrie, S.B.; Plat, J.; Roullet, J.-B.; Baer, D.J.; Calkins, K.L.; Davis, H.R.; et al. Progress and perspectives in plant sterol and plant stanol research. Nutr. Rev. 2018, 76, 725–746. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Ling, W.H.; Kitts, D.D.; Zawistowski, J. Supplementation of diets with black rice pigment fraction attenuates atherosclerotic plaque formation in apolipoprotein E deficient mice. J. Nutr. 2003, 133, 744–751. [Google Scholar] [CrossRef]
- Hu, C.; Zawistowski, J.; Ling, W.; Kitts, D.D. Black rice (Oryza sativia L. indica) pigmented fraction suppresses both reactive oxygen species and nitric oxide in chemical and biological model system. J. Agric. Food Chem. 2003, 51, 5271–5277. [Google Scholar] [CrossRef]
- Yousuf, B.; Gul, K.; Abas Wani, A.; Singh, P. Health benefits of anthocyanins and their encapsulation for potential use in food systems: A review. Crit. Rev. Food Sci. Nutr. 2016, 13, 2223–2230. [Google Scholar] [CrossRef]
- Wallace, T.C.; Slavin, M.; Frankenfeld, C.L. Systematic review of anthocyanins and markers of cardiovascular disease. Nutrients 2016, 32, 32. [Google Scholar] [CrossRef]
- Cabral, C.E.; Klein, M.R.S.T. Phytosterols in the treatment of hypercholesterolemia and prevention of cardiovascular diseases. Arq. Bras. Cardiol. 2017, 109, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Lawless, A.L.; Reeves, M.S.; Kelley, K.M.; Dicklin, M.R.; Jenks, B.H.; Shneyvas, E.; Brooks, J.R. Lipid effects of a dietary supplement softgel capsule containing plant sterols/stanols in primary hypercholesterolemia. Nutrition 2013, 29, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Jaceldo-Siegel, K.; Lütjohann, D.; Sirirat, R.; Mashchak, A.; Fraser, G.E.; Haddad, E. Variations in dietary intake and plasma concentrations of plant sterols across plant-based diets among North American adults. Mol. Nutr. Food Res. 2017, 61, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vanstone, C.A.; Raeini-Sarjaz, M.; Parson, W.E.; Jones, P.J.H. Unesterified plant sterols and stanols lower LDL-cholesterol concentrations equivalently in hypercholesterolemic persons. Am. J. Clin. Nutr. 2002, 76, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Rouyanne, T.; Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomized controlled studies. Br. J. Nutr. 2014, 28, 214–219. [Google Scholar]
- Kalliny, S.; Zawistowski, J. Phytosterols and phytosterols. In Encyclopedia of Food Chemistry; Aluko, R., Birch, E.J., Larsen, D., Melton, L., Rogers, M., Shahidi, F., Stadler, R., Sun-Waterhouse, D., Varelis, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 3, pp. 289–299. [Google Scholar]
- Wilson, T.A.; Idreis, H.M.; Taylor, C.M.; Nicolosi, R.J. Whole fat rice bran reduces the development of aortic atherosclerosis in hypercholesterolemic hamsters compared with wheat bran. Nutr. Res. 2002, 22, 1319–1332. [Google Scholar] [CrossRef]
- Ausman, L.M.; Rong, N.; Nicolosi, R.J. Hypocholesterolemic effect of physically refined rice bran oil: Studies of cholesterol metabolism and early atherosclersis in hyprcholesterolemic hamsters. J. Nutr. Biochem. 2005, 16, 521–529. [Google Scholar] [CrossRef]
- Zawistowski, J.; Kopeć, A.; Kitts, D.D. Effect of a black rice extract (Oryza sativa L. indica) on cholesterol levels and plasma lipid parameters in Wistar Kyoto rats. J. Funct. Foods 2009, 1, 50–56. [Google Scholar] [CrossRef]
- Ling, W.H.; Cheng, Q.X.; Ma, J.; Wang, T. Red and black rice decrease atherosclerotic plaque formation and increase antioxidant status in rabbits. J. Nutr. 2001, 131, 1421–1426. [Google Scholar] [CrossRef]
- Xia, X.; Ling, W.H.; Ma, J.; Xia, M.; Hou, M.; Wang, Q.; Zhu, H. An anthocyanin-rich extract from black rice enhances atherosclerosis plaque stabilization in apolipoprotein E-deficient mice. J. Nutr. 2006, 136, 2220–2225. [Google Scholar] [CrossRef]
- Novotny, J.A.; Baer, D.J.; Khoo, K.; Gebauer, S.K.; Charron, C.S. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating c-reactive protein, triglyceride, and glucose concentrations in adults. J. Nutr. 2015, 45, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Qin, P.; Zhang, Y.; Cui, S.; Ren, G. Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics. Food Res. Int. 2013, 50, 691–697. [Google Scholar] [CrossRef]
- Graf, D.; Seifert, S.; Jaudszus, A.; Bub, A.; Watzl, B. Anthocyanin-rich juice lowers serum cholesterol, leptin and resistin and improves plasma fatty acid composition in Fischer rats. PLoS ONE 2013, 8, e66690. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.H.; Wang, L.L.; Ma, J. Supplementation of the black rice outer layer fraction to rabbits decreases atherosclerotic plaque formation and increases antioxidant status. J. Nutr. 2002, 132, 20–26. [Google Scholar] [CrossRef]
- Yang, Y.; Andrews, M.C.; Hu, Y.; Wang, D.; Qin, Y.; Zhu, Y.; Ni, H.; Ling, W. Anthocyanin extract from black rice significantly ameliorates platelet hyperactivity and hypertriglyceridemia in dyslipidemic rats induced by high fat diets. J. Agric. Food. Chem. 2011, 59, 6759–6764. [Google Scholar] [CrossRef]
- Guo, H.; Ling, W.; Wang, Q.; Liu, C.; Hu, Y.; Xia, M.; Feng, X.; Xia, X. Effect of anthocyanin-rich extract from black rice (Oryza sativa L. indica) on hyperlipidemia and insulin resistance in fructose-fed rats. Plant Foods Human Nutr. 2007, 62, 1–6. [Google Scholar] [CrossRef]
- Jang, H.H.; Park, M.Y.; Kim, H.W.; Leen, Y.M.; Hwang, K.A.; Park, J.H.; Park, D.S.; Kwon, O. Black rice (Oryza sativa L.) extract attenuates hepatic steatosis in C57BL/6 J mice fed a high-fat diet via fatty acid oxidation. Nutr. Metab. 2012, 9, 27–38. [Google Scholar] [CrossRef]
- Pritchard, P.H.; Li, M.; Zamfir, K.; Lukic, T.; Novak, E.; Moghadasin, M. Comparison of cholesterol-lowering efficacy and anti-atherogenic properties of hydrogenated versus non-hydrogenated (PhytrolTM) tall oil-derived phytrosterols in apo E-deficient mice. Cardiovasc. Drugs Ther. 2003, 17, 443–449. [Google Scholar] [CrossRef]
- Fang, N.; Yu, S.; Bader, T. Characterization of triterpene alkohol and serol ferulates in rice bran using LC-MS/MS. J. Agric. Food Chem. 2003, 51, 3260–3267. [Google Scholar] [CrossRef]
- He, W.S.; Zhu, H.; Chen, Z.Y. Plant Sterols: Chemical and enzymatic structural modifications and effects on their cholesterol-lowering activity. J. Agric. Food Chem. 2018, 66, 3047–3062. [Google Scholar] [CrossRef] [PubMed]
- Redan, B.W.; Albaugh, G.P.; Charron, C.S.; Novotny, J.A.; Ferruzzi, M.G. Adaptation in Caco-2 human intestinal cell differentiation and phenolic transport with chronic exposure to blackberry (Rubus sp) extract. J. Agric. Food Chem. 2017, 65, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pittman, H.E.; Prior, R.L. Fate anhocyanins and antioxidant capacity in contents of the gastrointestinal tract of weanling pigs following black raspberry consumption. J. Agric. Food Chem. 2006, 54, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Elisia, I.; Kitts, D.D. Anthocyanins inhibit peroxyl radical-induced apoptosis in Caco-2 cells. Mol. Cell Biochem. 2008, 312, 139–145. [Google Scholar] [CrossRef]
- Vaskonsen, T.; Mervaala, E.; Sumuvuori, V.; Seppän-Laakso, T.; Karppanen, H. Effect of calcium and plant sterols on serum lipids in obese Zucker rats on a low-fat diet. Br. J. Nutr. 2002, 87, 239–245. [Google Scholar] [CrossRef]
- Devaraj, R.; Autret, B.C.; Jialal, I. Reduced-calorie orange juice beverage with plant sterols lowers C-reactive protein concentrations and improves the lipid profile in human volunteers. Am. J. Clin. Nutr. 2006, 84, 756–761. [Google Scholar] [CrossRef]
- AbuMweis, S.S.; Vanstone, C.A.; Ebine, N.; Kassis, A.; Ausman, L.M.; Jones, P.J.H.; Lichtenstein, A.H. Intake of single morning dose of standard and novel plant sterol preparations for 4 weeks odes not dramatically affected plasma lipid concentratins in humans. J. Nutr. 2006, 136, 1012–1016. [Google Scholar] [CrossRef]
- Fumeron, F.; Bard, J.M.; Lecerf, J.M. Interindividual variability in the cholesterol-lowering effect of supplementation with plant sterols or stanols. Nutr. Rev. 2017, 75, 134–145. [Google Scholar] [CrossRef]
- Calpe-Berdiel, L.; Escola-Gil, J.C.; Blanco-Vaca, F. Phytosterol-mediated inhibition of intestinal cholesterol absorption is independent of ATP-binding cassette transporter A1. Br. J. Nutr. 2006, 95, 618–622. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–168. [Google Scholar] [CrossRef]
- Reis, J.F.; Monteiro, V.V.S.M.; de Souza Gomes, R.; do Carmo, M.M.; da Costa, G.V.; Ribera, P.C.; Chagas Monteiro, M. Action mechanism and cardiovascular effect of anthocyanins: A systematic review of animal and human studies. J. Transl. Med. 2016, 14, 315–331. [Google Scholar] [CrossRef]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Comp. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Pong, C.H.; Liu, L.K.; Chuang, C.M.; Chyau, C.C.; Huang, C.N.; Wang, C.J. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J. Agric. Food Chem. 2011, 59, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Mekki, N.; Dubois, C.; Charbonier, M.; Cara, L.; Senft, M.; Pauli, A.M.; Portugal, H.; Gassin, A.M.; Lafon, H.; Lairon, D. Effects of lowering fat and increasing dietary fiber on fasting and postprandial plasma lipids in hypercholsterolemic subjects consuming a mixed Mediterranean-Western diet. Am. J. Clin. Nutr. 1997, 66, 1443–1451. [Google Scholar] [CrossRef]
- Behall, K.M.; Scholfield, D.J.; Hallfrisch, J. Diets containing barley significantly reduce lipids in mildly hypercholesterolemic men and women. Am. J. Clin. Nutr. 2004, 80, 1185–1193. [Google Scholar] [CrossRef]
- Kontush, A. HDL-mediated mechanisms of protection in cardiovascular disease. Cardiovasc. Res. 2014, 103, 341–349. [Google Scholar] [CrossRef]
- Morgan, A.E.; Mooney, K.M.; Wilkinson, S.J.; Pickles, N.A.; Mc Auley, M.T. Cholesterol metabolism: A review of how ageing disrupts the biological mechanisms responsible for its regulation. Aging Res. Rev. 2016, 27, 108–124. [Google Scholar] [CrossRef]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.T.; Liu, J.; Mou, H.Y.; Cao, L.; Ling, W.H. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef]
- Roohinejad, S.; Omidizadeh, A.; Mirhosseini, H.; Saari, N.; Mustafa, S.; Yusof, R.M.; Hussin, A.S.M.; Hamid, A.; Yazid, M.; Manap, A. Effect of pre-germination time of brown rice on serum cholesterol levels of hypercholesterolaemic rats. J. Sci. Food Agric. 2010, 90, 245–251. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, M.P.; Hansson, G.K. Progress and challenges in transating the biology of artherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Ma, Y.; Griffith, J.A.; Chasan-Taber, L.; Olendzki, B.C.; Jackson, E.; Stanek, E.J.; Li, W.; Pagoto, S.L.; Hafner, A.R.; Ockene, I.S. Association between dietary fiber and serum C-reactive protein. Am. J. Clin. Nutr. 2006, 83, 754–759. [Google Scholar] [CrossRef]
- Oi, L.; van Dam, R.M.; Liu, S.; Franz, M.; Mantzoros, C.; Hu, F.B. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care 2006, 29, 207–211. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Canadian Council of Animal Care. Guide to the Care and Use of Experimental Animals; CCAC: Ottawa, ON, Canada, 1983; Volume 2, p. 180. [Google Scholar]
- Kitts, D.D.; Hu, C. Biological and chemical assessment of antioxidant activity of sugar-lysine model Maillard Reaction Products. Ann. N. Y. Acad. Sci. 2005, 1043, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Carlson, S.E.; Goldfarb, S. A sensitive enzymatic method for the determination of free and esterified tissue cholesterol. Clin. Chim. Acta 1979, 79, 575–584. [Google Scholar] [CrossRef]
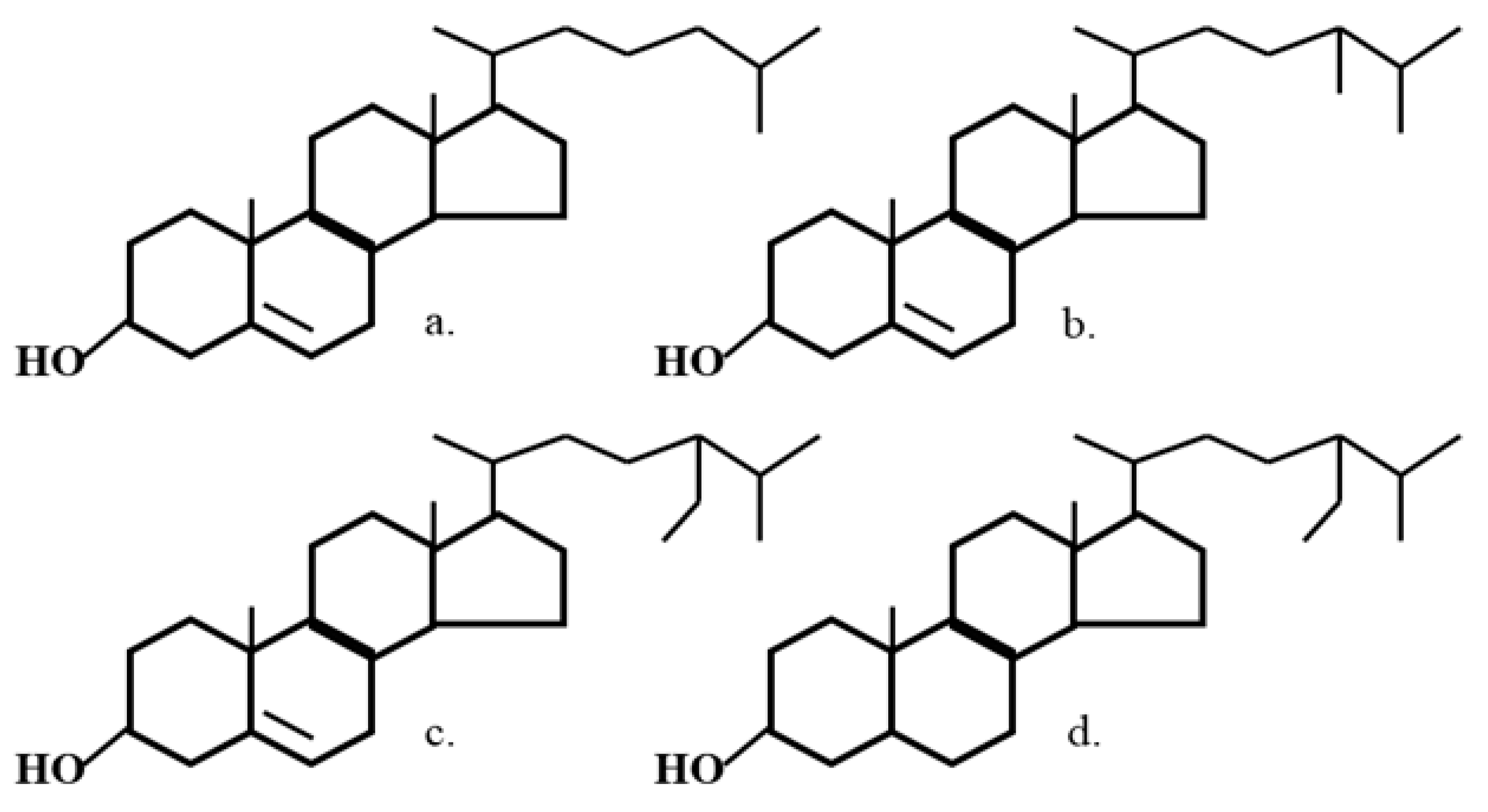
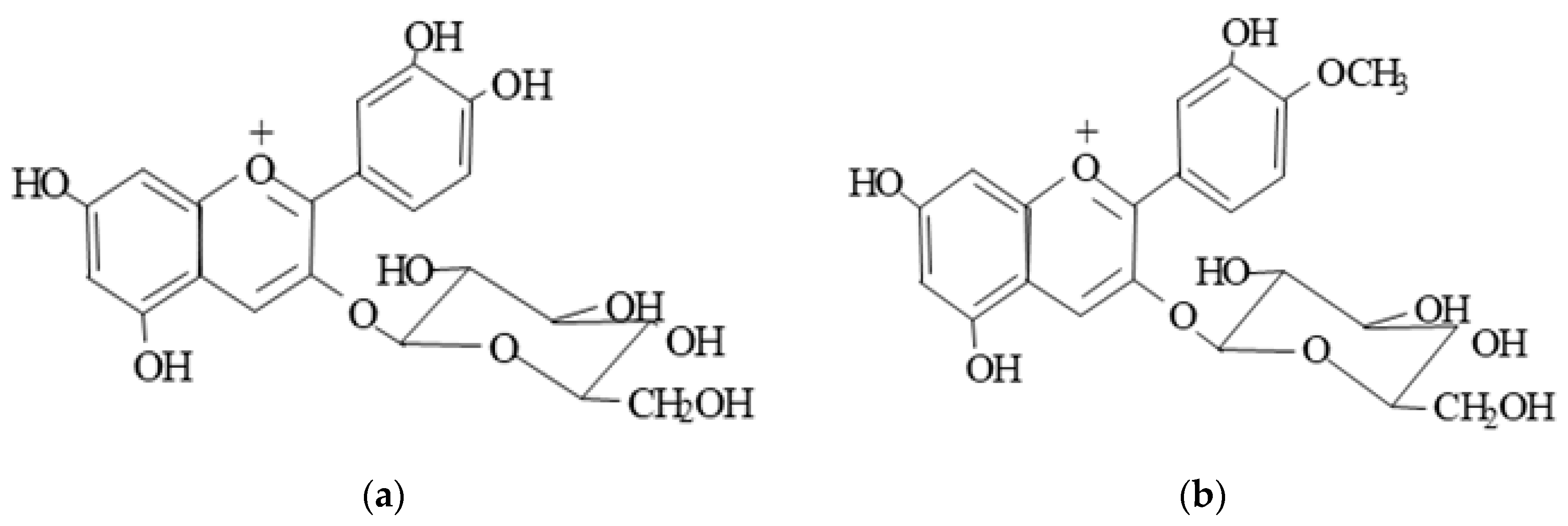

| Diet Treatment | TC | HDL | Non-HDL | TAG | PL |
|---|---|---|---|---|---|
| NC | 1.75 ± 0.10 + | 1.12 ± 0.03 + | 0.67 ± 0.09 + | 0.52 ± 0.04 | 111.8 ± 9.0 |
| PCCh | 4.21 ± 0.11 a | 0.74 ± 0.03 a | 3.47 ± 0.12 a | 0.56 ± 0.03 a | 96.97 ± 4.67 a |
| PCCh + BRF | 2.67 ± 0.12 b | 0.74 ± 0.06 a | 1.74 ± 0.13 b | 0.45 ± 0.04 b | 89.78 ± 2.1 a |
| PCCh + WS | 2.07 ± 0.06 c | 1.08 ± 0.05 b | 1.05 ± 0.12 c | 0.74 ± 0.03 c | 115 ± 4.6 b |
| PCCh + WS + BRF | 2.08 ± 0.07 c | 0.73 ± 0.08 a | 1.33 ± 0.14 c | 0.47 ± 0.02 b | 112.5 ± 5.7 b |
| Diet Treatment | Liver CL | Liver TC | Liver TAG | Heart TC | Aorta TC |
|---|---|---|---|---|---|
| NC | 58 ± 2.83 + | 6.19 ± 0.59 + | 0.53 ± 0.33 + | 0.157 ± 0.01 + | 1.14 ± 0.03 + |
| PCCh | 390 ± 20 a | 15.92 ± 0.56 a | 9.53 ± 0.64 a | 0.256 ± 0.03 a | 5.86 ± 0.83 a |
| PCCh + BRF | 280 ± 12 b | 11.86 ± 1.36 b | 6.29 ± 0.95 b | 0.186 ± 0.03 b | 2.14 ± 0.20 b |
| PCCh + WS | 96 ± 15 c | 8.30 ± 0.58 c | 10.17 ± 0.50 a | 0.245 ± 0.01 a | 0.55 ± 0.10 d |
| PCCh + WS + BRF | 120 ± 10 c | 8.17 ± 0.71 c | 7.73 ± 0.56 b | 0.234 ± 0.02 a | 1.51 ± 0.15 c |
| Ingredient | g/100 g d.m. |
|---|---|
| Protein | 17 |
| Fat | 15 |
| Ash | 8.3 |
| Digestible carbohydrates | 50.02 |
| Soluble dietary fiber | 3.08 |
| Insoluble dietary fiber | 6.60 |
| Anthocyanins (%) | |
| cyanidin-3-O-glucoside | 97.86 |
| peonidin-3-O-glucoside | 2.14 |
| Total anthocyanins (mg/g) | 31.3 |
| Oryzanol (% of total fat) | 3.83 |
| Total Sterol Content | 99.6 |
|---|---|
| Sitostanol | 16 |
| Sitosterol | 73 |
| Campasterol | 6 |
| Stigmasterol | 1 |
| Other sterols | 2 |
| Ingredient | Negative Control (NC) | PCCh | PCCh + BRF | PCCh + WS | PCCh + WS + BRF |
|---|---|---|---|---|---|
| Casein | 25.5 | 25.5 | 25.5 | 25·5 | 25.5 |
| Corn starch | 47 | 45.95 | 45.5 | 43.5 | 43.5 |
| Sucrose | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Cellulose | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Monophosphate | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Vitamin mix | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Choline chloride | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| D-L Methionine | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Mineral mix | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Canola Oil | 10 | 7.0 | 7.0 | 7.0 | 7.0 |
| Butter | - | 3.0 | 3.0 | 3.0 | 3.0 |
| Wood sterols | 0 | - | - | 2.0 | 2.0 |
| Cholesterol | 0 | 0.50 | 0.50 | 0.50 | 0.50 |
| Cholic acid | 0 | 0.050 | 0.05 | 0.05 | 0.05 |
| BRF | - | - | 1.0 | - | 1.0 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopeć, A.; Zawistowski, J.; Kitts, D.D. Benefits of Anthocyanin-Rich Black Rice Fraction and Wood Sterols to Control Plasma and Tissue Lipid Concentrations in Wistar Kyoto Rats Fed an Atherogenic Diet. Molecules 2020, 25, 5363. https://doi.org/10.3390/molecules25225363
Kopeć A, Zawistowski J, Kitts DD. Benefits of Anthocyanin-Rich Black Rice Fraction and Wood Sterols to Control Plasma and Tissue Lipid Concentrations in Wistar Kyoto Rats Fed an Atherogenic Diet. Molecules. 2020; 25(22):5363. https://doi.org/10.3390/molecules25225363
Chicago/Turabian StyleKopeć, Aneta, Jerzy Zawistowski, and David D. Kitts. 2020. "Benefits of Anthocyanin-Rich Black Rice Fraction and Wood Sterols to Control Plasma and Tissue Lipid Concentrations in Wistar Kyoto Rats Fed an Atherogenic Diet" Molecules 25, no. 22: 5363. https://doi.org/10.3390/molecules25225363
APA StyleKopeć, A., Zawistowski, J., & Kitts, D. D. (2020). Benefits of Anthocyanin-Rich Black Rice Fraction and Wood Sterols to Control Plasma and Tissue Lipid Concentrations in Wistar Kyoto Rats Fed an Atherogenic Diet. Molecules, 25(22), 5363. https://doi.org/10.3390/molecules25225363






