Characterization of Inherently Chiral Electrosynthesized Oligomeric Films by Voltammetry and Scanning Electrochemical Microscopy (SECM)
Abstract
1. Introduction
2. Results and Discussion
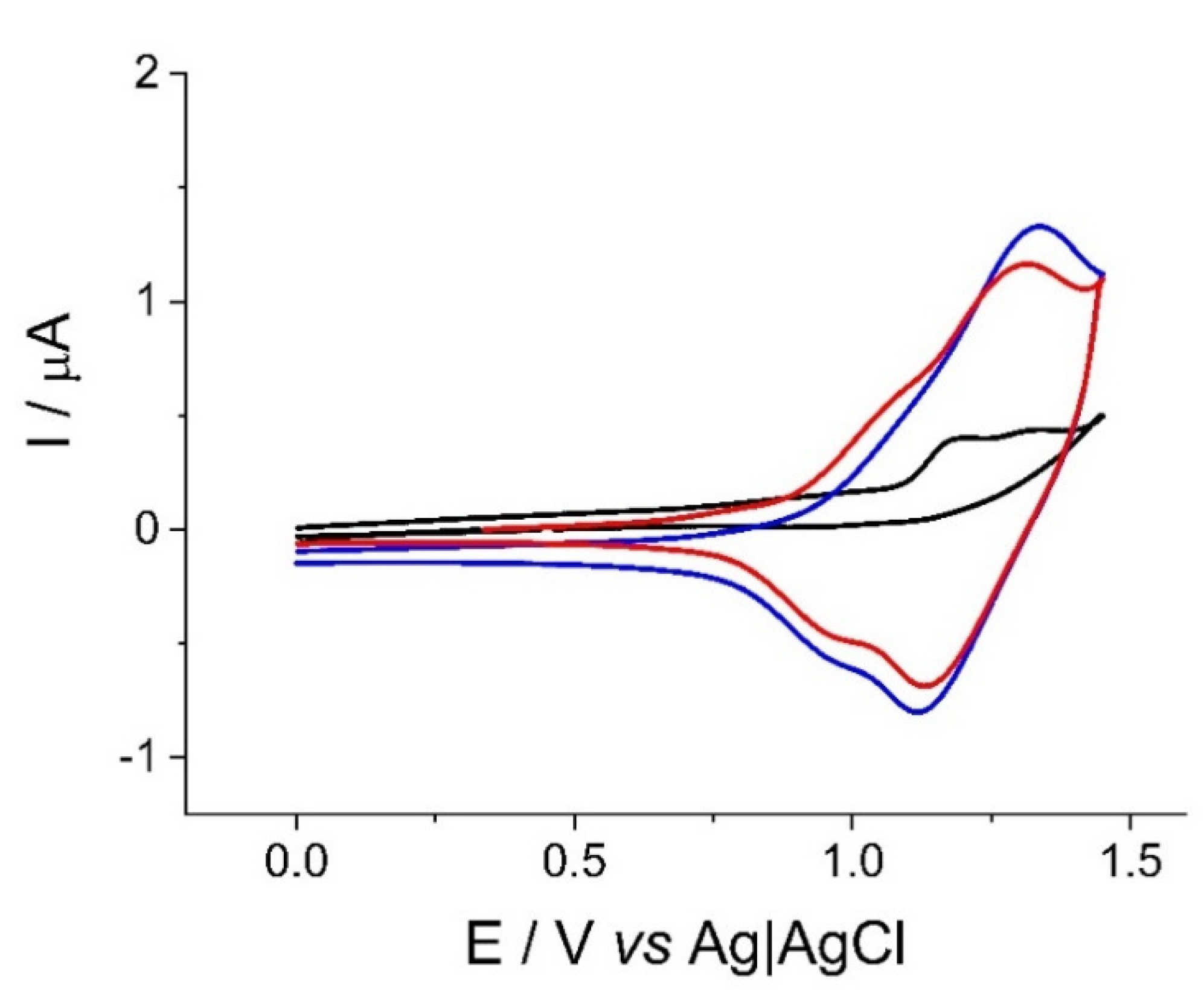
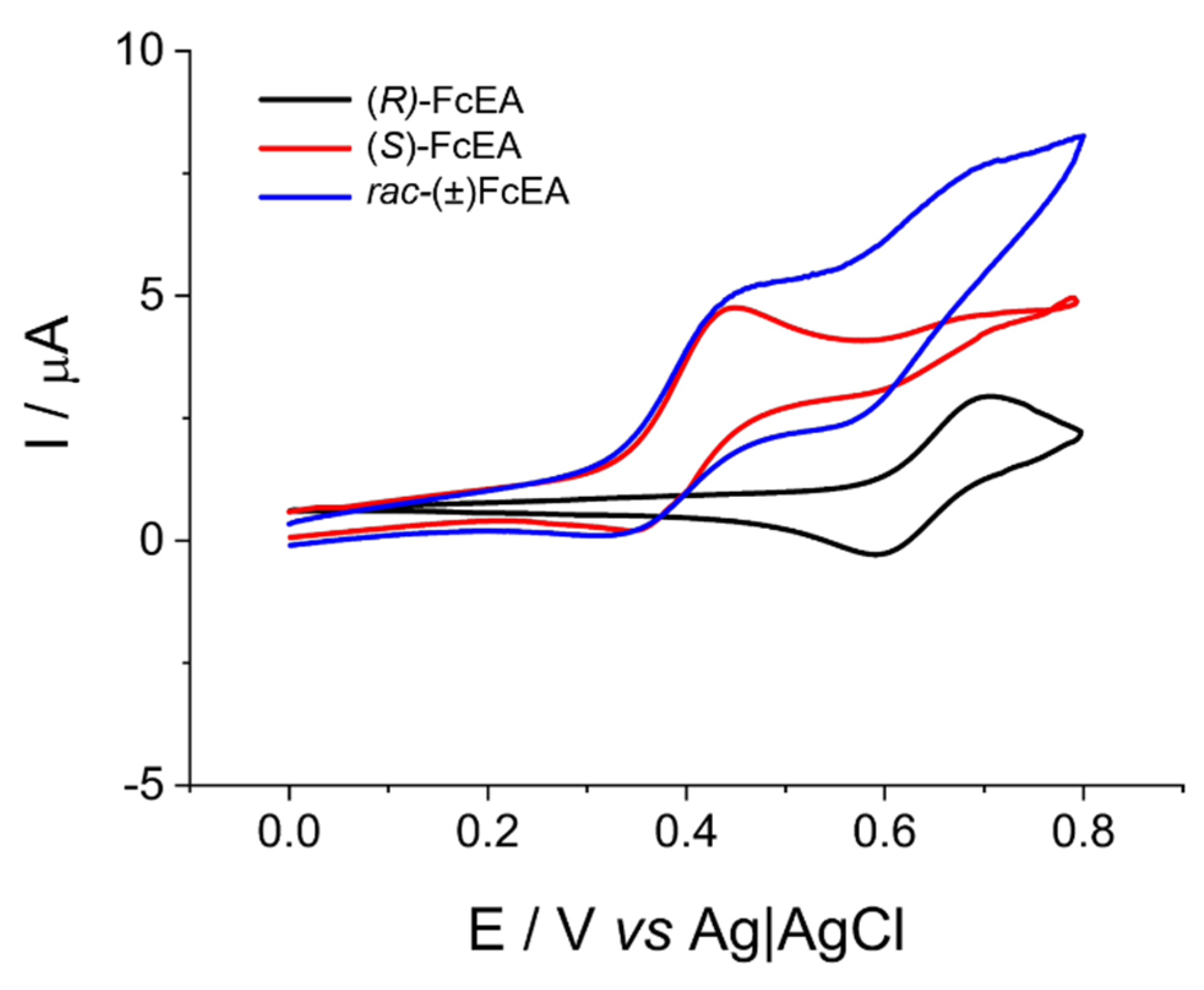
2.1. Electrosynthesis and Voltammetric Characterization of the Oligomeric BT2T4 Film
2.2. SECM Characterization of the Bare Au Surface and Oligo-(S)-BT2T4-Au Substrate
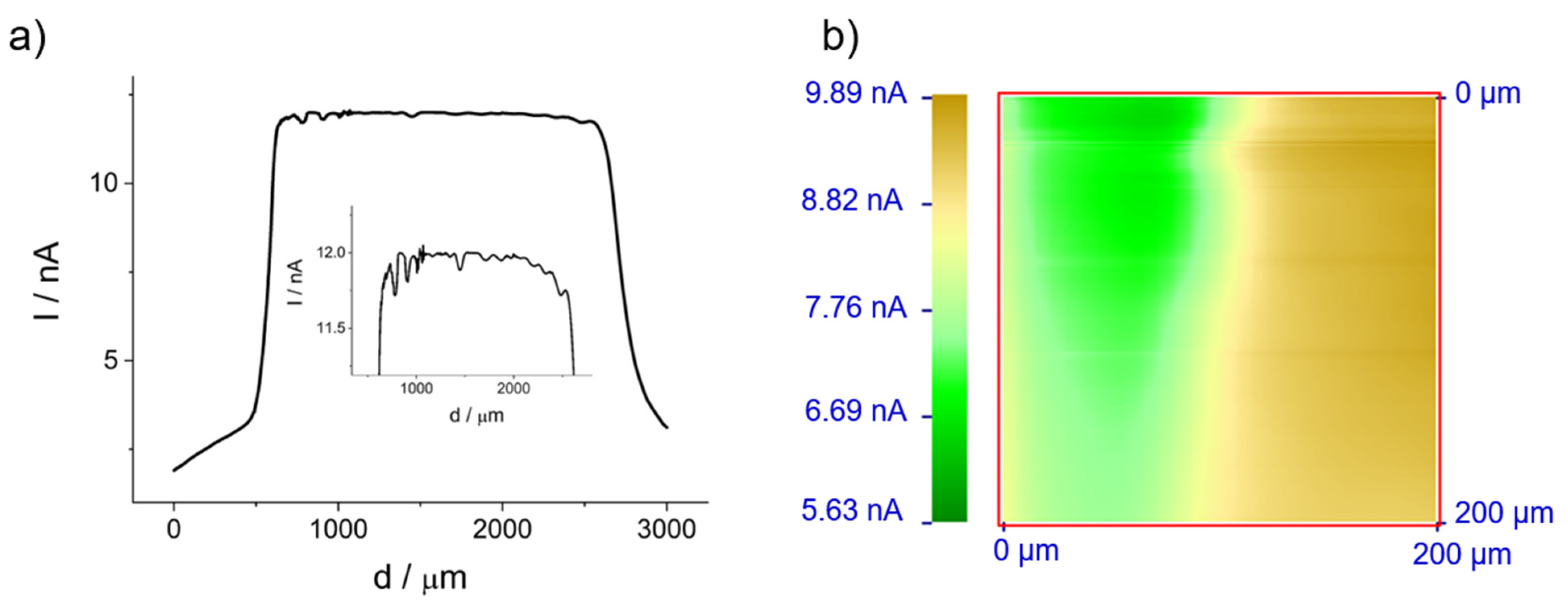
2.2.1. General Aspects
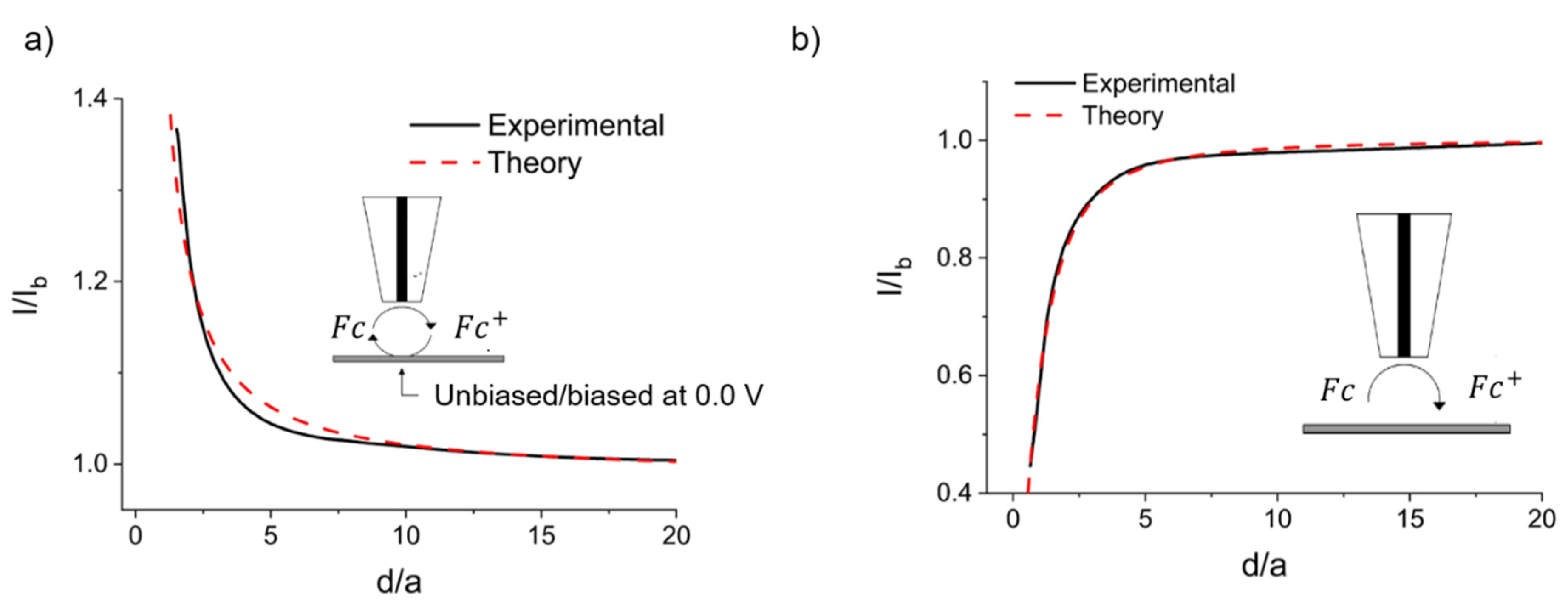
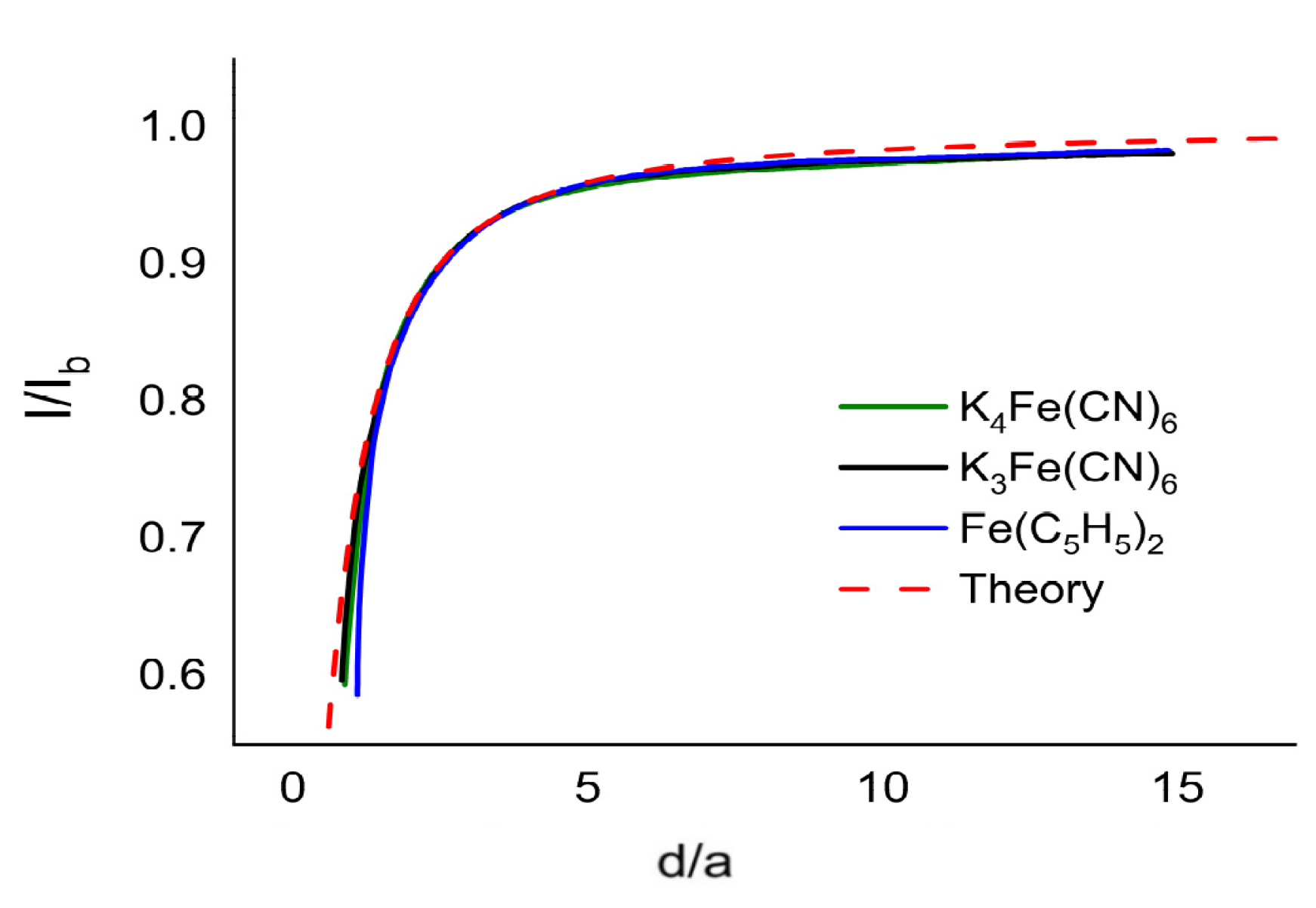
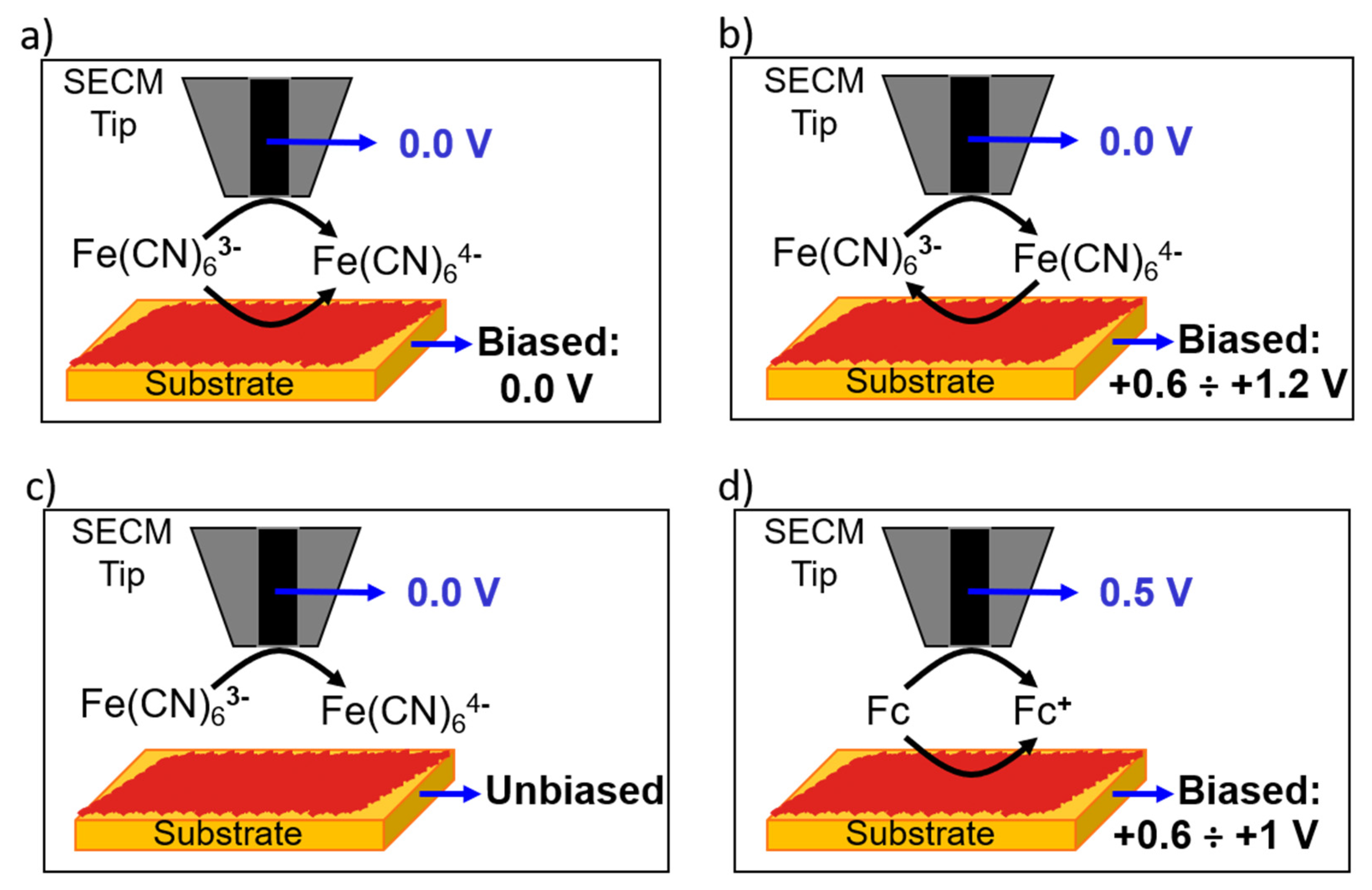
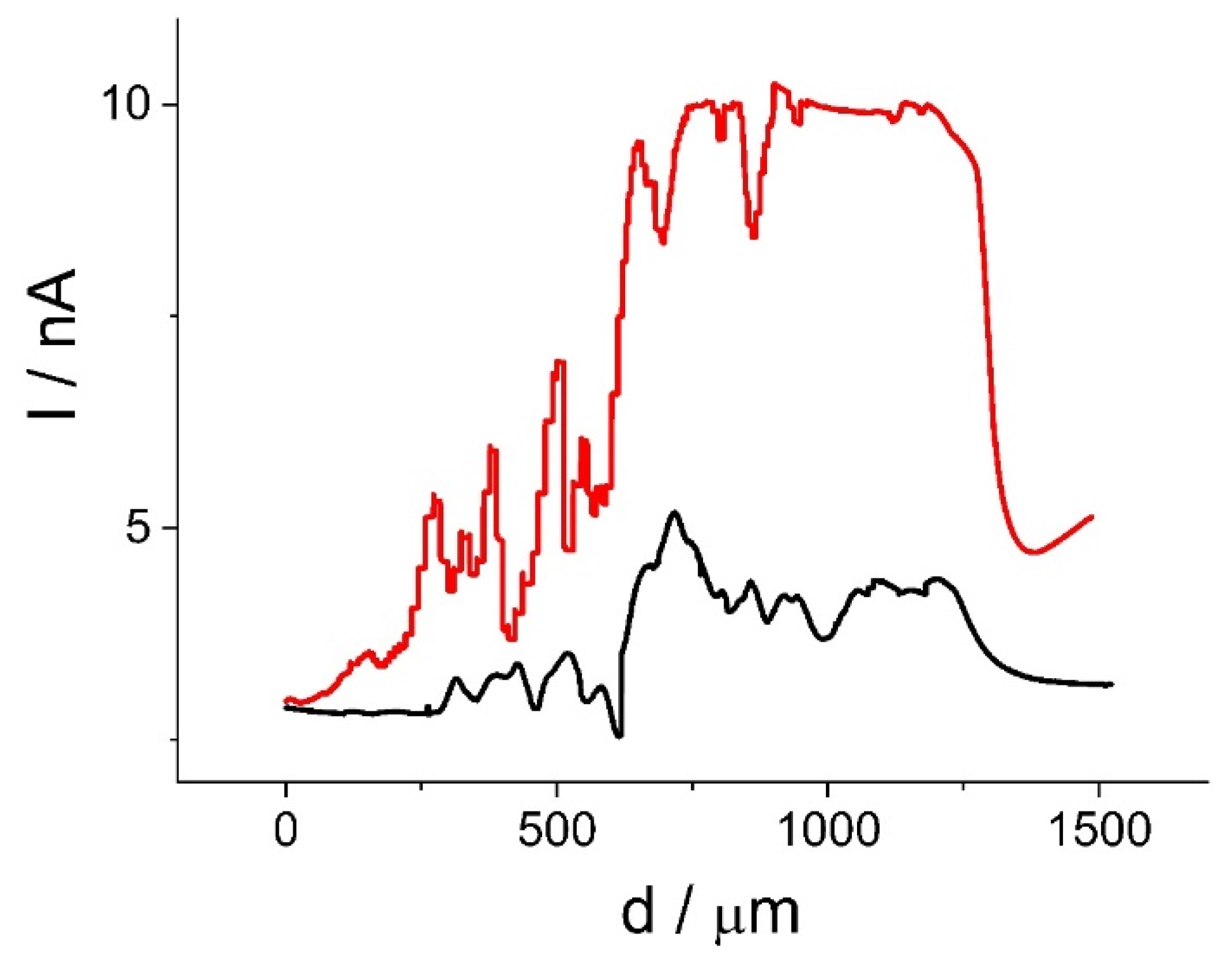
2.2.2. Achiral Redox Mediators
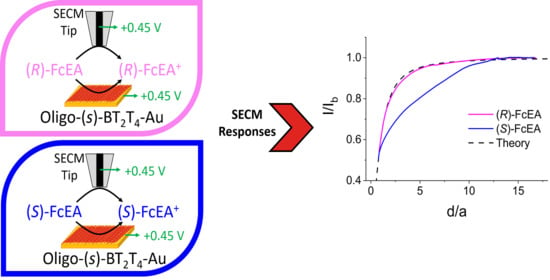
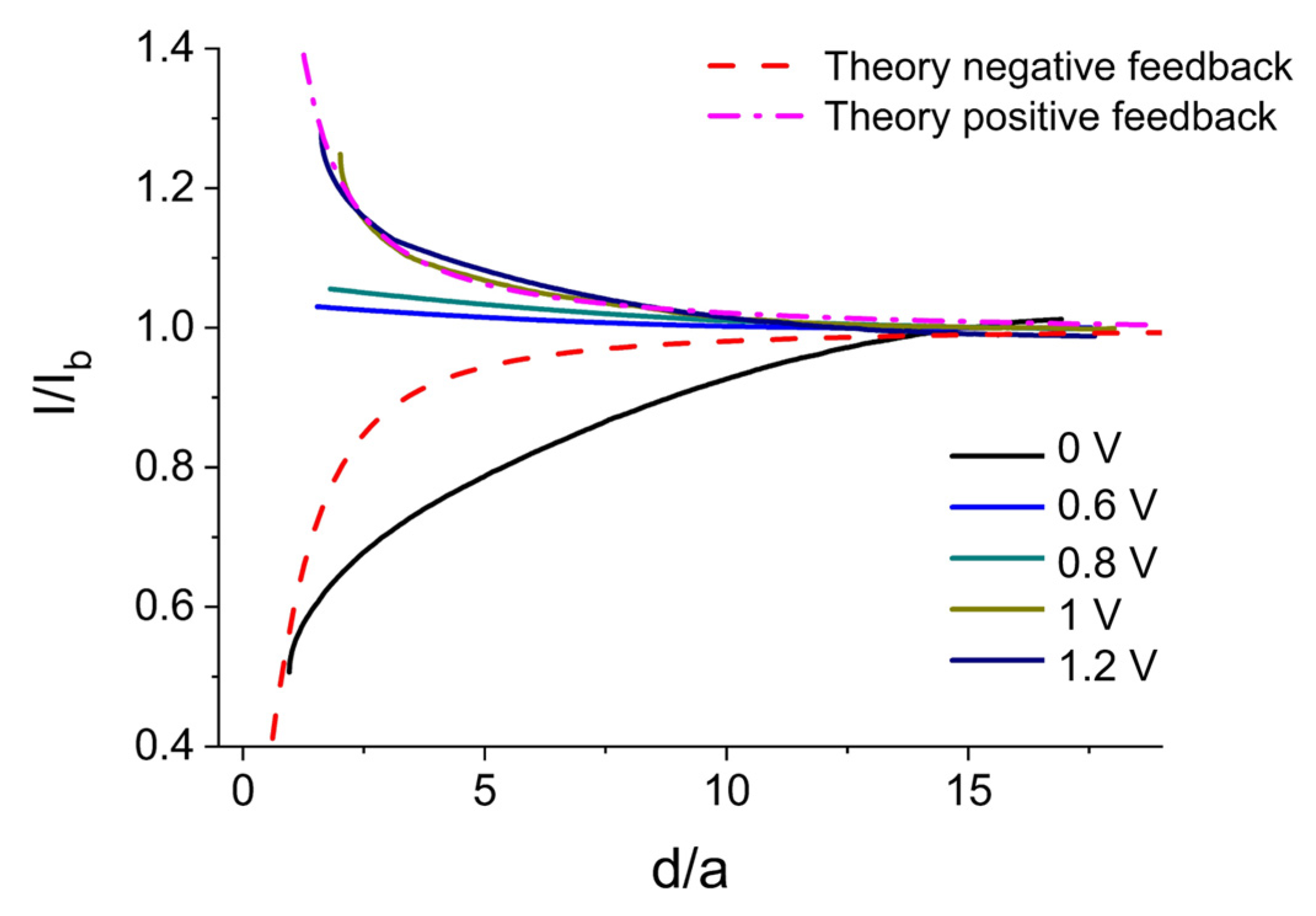
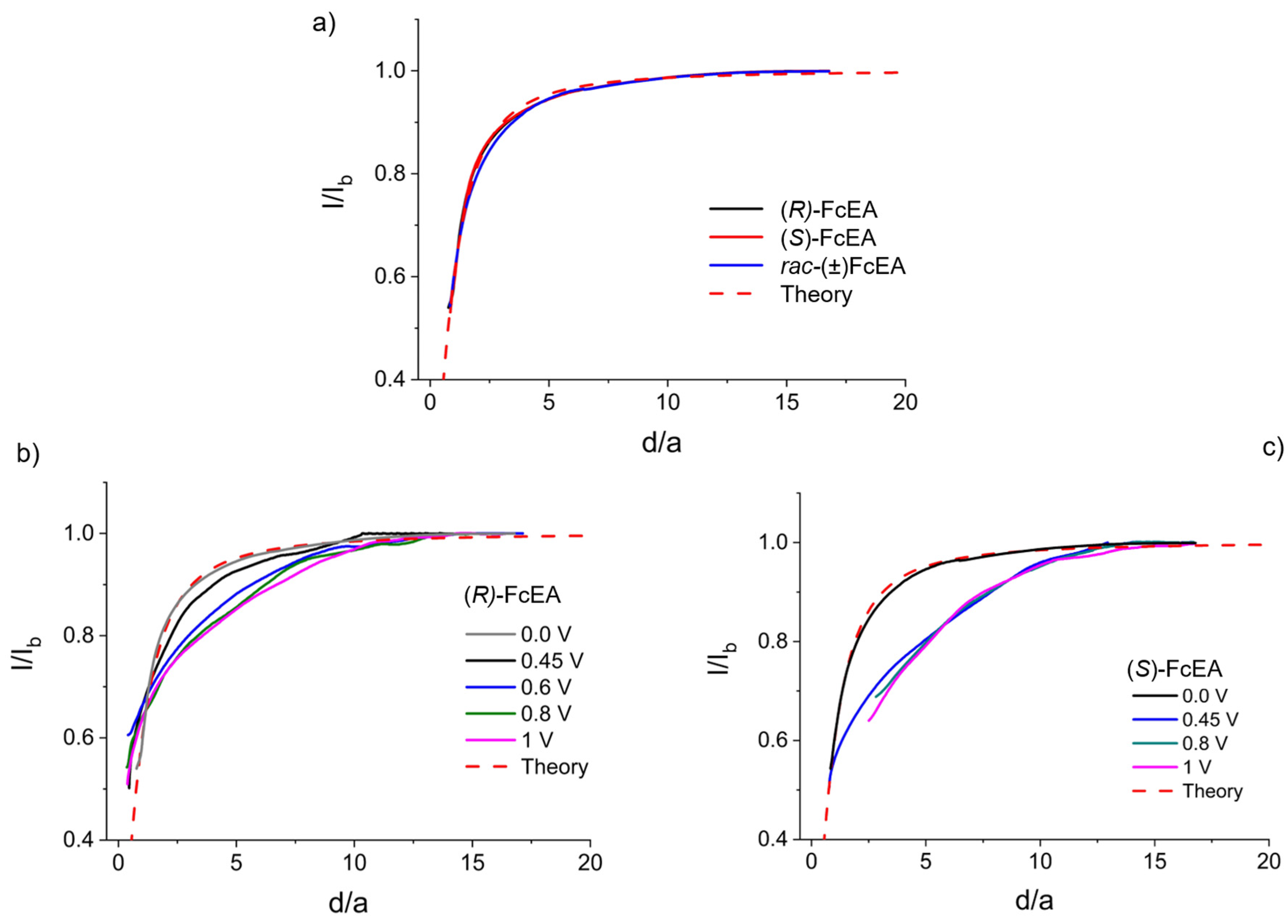
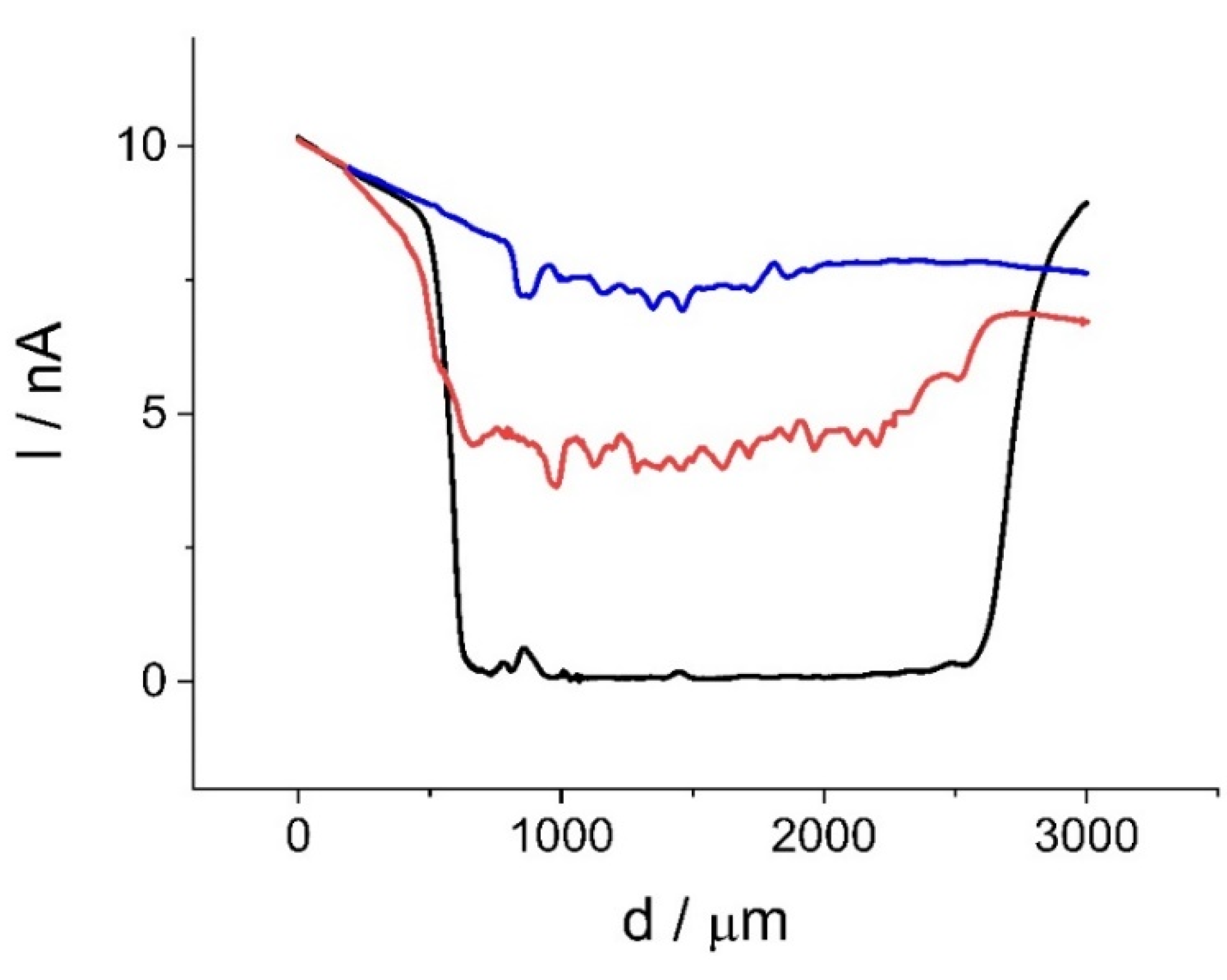
2.2.3. Chiral Redox Mediators
3. Materials and Methods
3.1. Chemicals
3.2. Apparatus and Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of conducting polymers-persistent models and new concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef]
- Tsakova, V.; Seeber, R. Conducting polymers in electrochemical sensing: Factors influencing the electroanalytical signal. Anal. Bioanal. Chem. 2016, 408, 7231–7241. [Google Scholar] [CrossRef]
- Tan-Phat, H.; Sharma, P.S.; Sosnowska, M.; D’Souza, F.; Kutner, W. Functionalized polythiophenes: Recognition materials for chemosensors and biosensors of superior sensitivity, selectivity, and detectability. Prog. Polym. Sci. 2015, 47, 1–25. [Google Scholar]
- Bottari, D.; Pigani, L.; Zanardi, C.; Terzi, F.; Paturcă, S.V.; Grigorescu, S.D.; Matei, C.; Lete, C.; Lupu, S. Electrochemical Sensing of Caffeic Acid Using Gold Nanoparticles Embedded in Poly(3,4-ethylenedioxythiophene) Layer by Sinusoidal Voltage Procedure. Chemosensors 2019, 7, 65. [Google Scholar] [CrossRef]
- Kane-Maguire, L.A.P.; Wallace, G.G. Chiral conducting polymers. Chem. Soc. Rev. 2010, 39, 2545–2576. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Grecchi, S.; Magni, M.; Mussini, P. Electroactive chiral oligo- and polymer layers for electrochemical enantiorecognition. Curr. Opin. Electrochem. 2018, 7, 188–199. [Google Scholar] [CrossRef]
- Sannicolò, F.; Arnaboldi, S.; Benincori, T.; Bonometti, V.; Cirilli, R.; Dunsch, L.; Kutner, W.; Longhi, G.; Mussini, P.R.; Panigati, M.; et al. Potential-driven chirality manifestations and impressive enantioselectivity by inherently chiral electroactive organic films. Angew. Chem. Int. Ed. 2014, 53, 2623–2627. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Vigo, D.; Longhi, M.; Orsini, F.; Riva, S.; Grecchi, S.; Giacovelli, E.; Guglielmi, V.; Cirilli, R.; Longhi, G.; et al. Self-Standing Membranes Consisting of Inherently Chiral Electroactive Oligomers: Electrosynthesis, Characterization and Preliminary Tests in Potentiometric Setups. ChemElectroChem 2019, 6, 4202–4214. [Google Scholar] [CrossRef]
- Sannicolò, F.; Mussini, P.R.; Benincori, T.; Cirilli, R.; Abbate, S.; Arnaboldi, S.; Casolo, S.; Castiglioni, E.; Longhi, G.; Martinazzo, R.; et al. Inherently chiral macrocyclic oligothiophenes: Easily accessible electrosensitive cavities with outstanding enantioselection performances. Chem. Eur. J. 2014, 20, 15298–15302. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Benincori, T.; Cirilli, R.; Kutner, W.; Magni, M.; Mussini, P.R.; Noworyta, K.; Sannicolò, F. Inherently chiral electrodes: The tool for chiral voltammetry. Chem. Sci. 2015, 6, 1706–1711. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Benincori, T.; Cirilli, R.; Grecchi, S.; Santagostini, L.; Sannicolò, F.; Mussini, P.R. “Inherently chiral” thiophene-based electrodes at work: A screening of enantioselection ability toward a series of pharmaceutically relevant phenolic or catecholic amino acids, amino esters, and amine. Anal. Bioanal. Chem. 2016, 408, 7243–7254. [Google Scholar] [CrossRef] [PubMed]
- Grecchi, S.; Arnaboldi, S.; Korb, M.; Cirilli, R.; Araneo, S.; Guglielmi, V.; Tomboni, G.; Magni, M.; Benincori, T.; Lang, H.; et al. Widening the scope of “inherently chiral” electrodes: Enantiodiscrimination of chiral electroactive probes with planar stereogenicity. ChemElectroChem 2020, 7, 3429–3438. [Google Scholar] [CrossRef]
- Benincori, T.; Gàmez-Valenzuela, S.; Goll, M.; Bruchlos, K.; Malacrida, C.; Arnaboldi, S.; Mussini, P.R.; Panigati, M.; López Navarrete, J.T.; Ruiz Delgado, M.C.; et al. Electrochemical studies of a new, low-band gap inherently chiral ethylenedioxythiophene-based oligothiophene. Electrochim. Acta 2018, 284, 513–525. [Google Scholar] [CrossRef]
- Bard, A.J.; Mirkin, M.V. (Eds.) Scanning Electrochemical Microscopy, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. (Eds.) Electrochemical Methods, Fundamentals and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Caniglia, G.; Kranz, C. Scanning electrochemical microscopy and its potential for studying biofilms and antimicrobial coatings. Anal. Bioanal. Chem. 2020, 412, 6133–6148. [Google Scholar] [CrossRef]
- Souto, R.M.; González-García, Y.; Battistel, D.; Daniele, S. In Situ Scanning Electrochemical Microscopy (SECM) Detection of Metal Dissolution during Zinc Corrosion by Means of Mercury Sphere-Cap Microelectrode Tips. Chem. Eur. J. 2012, 18, 230–236. [Google Scholar] [CrossRef]
- Tsionsky, M.; Bard, A.J.; Dini, D.; Decker, F. Polymer films on electrodes. 28. Scanning electrochemical microscopy study of electron transfer at poly(alkylterthiophene) films. Chem. Mater. 1998, 10, 2120–2126. [Google Scholar] [CrossRef]
- Arca, M.; Mirkin, M.V.; Bard, A.J. Polymer Films on Electrodes. 26. Study of Ion Transport and Electron Transfer at Polypyrrole Films by Scanning Electrochemical Microscopy. J. Phys. Chem. 1995, 99, 5040–5050. [Google Scholar] [CrossRef]
- Mandler, D.; Unwin, P.R. Measurement of Lateral Charge Propagation in Polyaniline Layers with the Scanning Electrochemical Microscope. J. Phys. Chem. B 2003, 107, 407–410. [Google Scholar] [CrossRef]
- Quartapelle Procopio, E.; Benincori, T.; Appoloni, G.; Mussini, P.R.; Arnaboldi, S.; Carbonera, C.; Cirilli, R.; Cominetti, A.; Longo, L.; Martinazzo, R.; et al. A family of solution-processable macrocyclic and open-chain oligothiophenes with atropoisomeric scaffolds: Structural and electronic features for potential energy applications. New J. Chem. 2017, 41, 10009–10019. [Google Scholar] [CrossRef]
- Moss, G.P. Basic terminology of stereochemistry. Pure Appl. Chem. 1996, 88, 2193–2222. [Google Scholar] [CrossRef]
- Bard, A.J.; Mirkin, M.V.; Unwin, P.R.; Wipf, D.O. Scanning electrochemical microscopy. 12. Theory and experiment of the feedback mode with finite heterogeneous electron-transfer kinetics and arbitrary substrate size. J. Phys. Chem. 1992, 96, 1861–1868. [Google Scholar] [CrossRef]
- Hocevar, S.B.; Daniele, S.; Bragato, C.; Ogorevc, B. Reactivity at the film/solution interface of ex situ prepared bismuth film electrodes: A scanning electrochemical microscopy (SECM) and atomic force microscopy (AFM) investigation. Electrochim. Acta 2007, 53, 555–560. [Google Scholar] [CrossRef]
- Liljeroth, P.; Vanmaekelbergh, D.; Ruiz, V.; Konturri, K.; Jiang, H.; Kauppinen, E.; Quinn, B.M. Electron Transport in Two-Dimensional Arrays of Gold Nanocrystals Investigated by Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2004, 126, 7126–7132. [Google Scholar] [CrossRef]
- Whitworth, A.L.; Mandler, D.; Unwin, P.R. Theory of scanning electrochemical microscopy (SECM) as a probe of surface conductivity. Phys. Chem. Chem. Phys. 2005, 7, 356–365. [Google Scholar] [CrossRef]
- Bard, A.J.; Fan, F.-R.F.; Kwak, J.; Lev, O. Scanning Electrochemical Microscopy. Introduction and Principles. Anal. Chem. 1989, 61, 132–138. [Google Scholar] [CrossRef]
- Wipf, D.O.; Bard, A.J. Scanning Electrochemical Microscopy: X. High Resolution Imaging of Active Sites on an Electrode Surface. J. Electrochem. Soc. 1991, 138, L4–L6. [Google Scholar] [CrossRef]
- Oleinick, A.I.; Battistel, D.; Daniele, S.; Svir, I.; Christian Amatore, C. Simple and Clear Evidence for Positive Feedback Limitation by Bipolar Behavior during Scanning Electrochemical Microscopy of Unbiased Conductors. Anal. Chem. 2011, 83, 4887–4893. [Google Scholar] [CrossRef]
- Amatore, C.; Saveant, J.M.; Tessier, D. Charge transfer at partially blocked surfaces. A model for microscopic active and inactive sites. J. Electroanal. Chem. 1983, 147, 39–51. [Google Scholar] [CrossRef]
- Saito, Y. Theoretical study on the diffusion current at the stationary electrodes of circular and narrow band types. Rev. Polarogr. 1968, 15, 177–187. [Google Scholar] [CrossRef]
- Battistel, D.; Pecchielan, G.; Daniele, S. Micropipette contact technique as a tool to reveal, characterize and modify nanopore electrodes. ChemElectroChem 2014, 1, 140–146. [Google Scholar] [CrossRef]
- Amphlett, J.L.; Denuault, G. Scanning Electrochemical Microscopy (SECM): An Investigation of the Effects of Tip Geometry on Amperometric Tip Response. J. Phys. Chem. B 1998, 102, 9946–9951. [Google Scholar] [CrossRef]
- Lindsey, G.; Abercrombie, S.; Denuault, G.; Daniele, S.; Eddy De Faveri, E. Scanning Electrochemical Microscopy: Approach Curves for Sphere-Cap Scanning Electrochemical Microscopy Tips. Anal. Chem. 2007, 79, 2952–2956. [Google Scholar] [CrossRef][Green Version]


Sample Availability: Not available. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donnici, M.; Toniolo, R.; Arnaboldi, S.; Mussini, P.R.; Benincori, T.; Cirilli, R.; Daniele, S. Characterization of Inherently Chiral Electrosynthesized Oligomeric Films by Voltammetry and Scanning Electrochemical Microscopy (SECM). Molecules 2020, 25, 5368. https://doi.org/10.3390/molecules25225368
Donnici M, Toniolo R, Arnaboldi S, Mussini PR, Benincori T, Cirilli R, Daniele S. Characterization of Inherently Chiral Electrosynthesized Oligomeric Films by Voltammetry and Scanning Electrochemical Microscopy (SECM). Molecules. 2020; 25(22):5368. https://doi.org/10.3390/molecules25225368
Chicago/Turabian StyleDonnici, Margherita, Rosanna Toniolo, Serena Arnaboldi, Patrizia R. Mussini, Tiziana Benincori, Roberto Cirilli, and Salvatore Daniele. 2020. "Characterization of Inherently Chiral Electrosynthesized Oligomeric Films by Voltammetry and Scanning Electrochemical Microscopy (SECM)" Molecules 25, no. 22: 5368. https://doi.org/10.3390/molecules25225368
APA StyleDonnici, M., Toniolo, R., Arnaboldi, S., Mussini, P. R., Benincori, T., Cirilli, R., & Daniele, S. (2020). Characterization of Inherently Chiral Electrosynthesized Oligomeric Films by Voltammetry and Scanning Electrochemical Microscopy (SECM). Molecules, 25(22), 5368. https://doi.org/10.3390/molecules25225368