Dewatering Green Sapwood Using Carbon Dioxide Undergoing Cyclical Phase Change between Supercritical Fluid and Gas
Abstract
1. Introduction
1.1. Conventional Processes for Drying Green Industrial Wood
1.2. New Approaches to Industrial Wood Manufacture Using Dewatering Processes
1.3. Theory for a Supercritical Carbon Dioxide-to-Gas Phase-Change Process for Green Wood Dewatering
2. Review of Science Studies on Dewatering Green Wood Using the Supercritical Carbon Dioxide-to-Gas Phase-Change Process
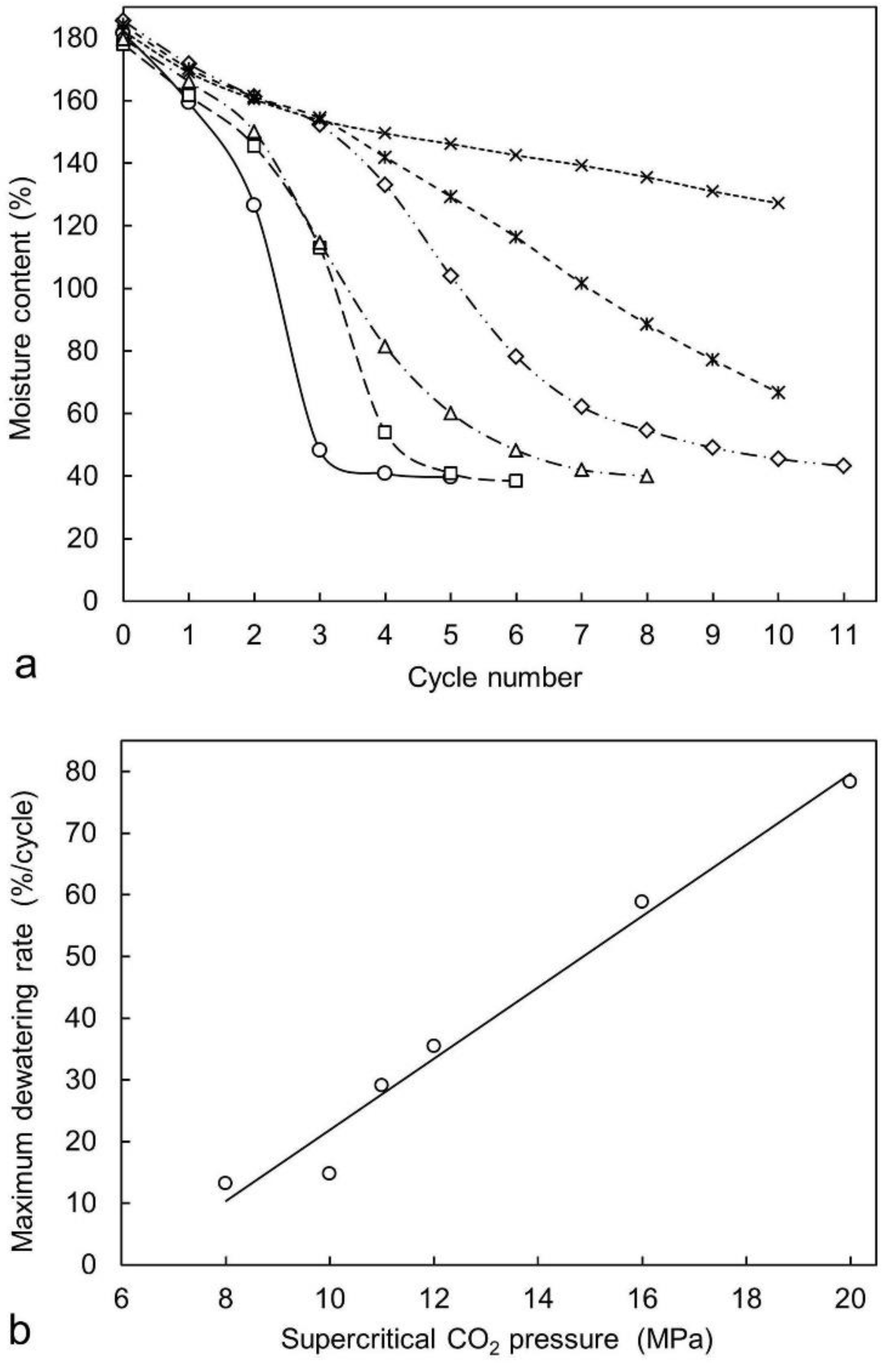
2.1. Laboratory Experiments to Study Dewatering of Radiata Pine Sapwood Specimens
2.2. Laboratory Experiments Dewatering Wood Specimens Sampled from Hardwood Timber Species and Softwood Species Other Than Radiata Pine
2.3. Dewatering Radiata Pine Sapwood Using Industrial-Scale Specimens
2.4. Sap (Cell Water) Chemistry
2.5. Magnetic Resonance Imaging (MRI) and Nuclear Magnetic Resonance (NMR) Spectroscopy Studies of the Supercritical Carbon Dioxide to Gas Phase-Change Dewatering Process
2.6. Predictive Models Developed for Supercritical Carbon Dioxide Dewatering of Green Wood
3. Commercialisation of Green Wood Dewatering Using Supercritical Carbon Dioxide
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kininmonth, J.A. Wood-Water Relationships. In Properties and Uses of New Zealand Radiata Pine, Volume 1—Wood Properties; Kininmonth, J.A., Whitehouse, L.J., Eds.; Forest Research Institute: Rotorua, New Zealand, 1991; pp. 7.1–7.23. [Google Scholar]
- Shusheng, P.; Keey, R.B. Drying kinetics of radiata pine boards at elevated temperatures. Dry Technol. 1995, 13, 1395–1409. [Google Scholar] [CrossRef]
- McDonald, A.G.; Dare, P.H.; Gifford, J.S.; Steward, D.; Riley, S. Assessment of air emissions from industrial drying of radiata pine wood. Holz Roh Werkst. 2002, 60, 181–190. [Google Scholar] [CrossRef]
- Li, X.-J.; Zhang, B.-G.; Li, W.-J. Microwave-Vacuum Drying of Wood: Model Formulation and Verification. Dry. Technol. 2008, 26, 1382–1387. [Google Scholar] [CrossRef]
- Avramidis, S.; Liu, F. Drying Characteristics of Thick Lumber in a Laboratory Radio-Frequency/Vacuum Dryer. Dry. Technol. 1994, 12, 1963–1981. [Google Scholar] [CrossRef]
- Meder, R.; Codd, S.L.; Franich, R.A.; Callaghan, P.T.; Pope, J. Observation of anisotropic water movement in Pinus radiata D. Don sapwood above fiber saturation using magnetic resonance micro-imaging. Holz Roh Werkst. 2003, 61, 251–256. [Google Scholar] [CrossRef]
- Choat, B.; Cobb, A.R.; Jansen, S. Structure and function of bordered pits: New discoveries and impacts on whole-plant hydraulic function. New Phytol. 2008, 177, 608–626. [Google Scholar] [CrossRef]
- Pang, S.; Wiberg, P. Model predicted and CT scanned moisture distribution in a Pinus radiata board during drying. Holz Roh Werkst. 1998, 56, 9–14. [Google Scholar] [CrossRef]
- Pang, S.; Herritsch, A. Physical properties of earlywood and latewood of Pinus radiata D. Don: Anisotropic shrinkage, equilibrium moisture content and fibre saturation point. Holzforschung 2005, 59, 654–661. [Google Scholar] [CrossRef]
- Kreber, B.; Fernández, M.; McDonald, A.G. Migration of Kiln Brown Stain Precursors During the Drying of Radiata Pine Sapwood. Holzforschung 1998, 52, 441–446. [Google Scholar] [CrossRef]
- Wingate-Hill, R.; Cunningham, R.B. Compression drying of sapwood. Wood Fiber Sci. 1986, 18, 315–326. [Google Scholar]
- Kozhin, V.P. Centrifugal dewatering and drying of high-moisture wood. J. Eng. Phys. Thermophys. 2012, 85, 1278–1283. [Google Scholar] [CrossRef]
- Stahl, M.; Bentz, M. High-pressure treatment of wood–Combination of mechanical and thermal drying in the “I/D process”. Chem. Eng. Technol. 2004, 27, 1216–1221. [Google Scholar] [CrossRef]
- Kreber, B.; Stahl, M.R.; Haslett, A.N. Application of a novel de-watering process to control kiln brown stain in radiata pine. Holz Roh Werkst. 2001, 59, 29–34. [Google Scholar] [CrossRef]
- McHugh, M.A.; Krukonis, V.J. Supercritical Fluid Extraction: Principles and Practice; Butterworths: Stoneham, MA, USA, 1986. [Google Scholar]
- Diamond, L.W.; Akinfiev, N.N. Solubility of CO2 in water from −1.5 to 100 °C and from 0.1 to 100 MPa: Evaluation of literature data and thermodynamic modeling. Fluid Phase Equilibria 2003, 208, 265–290. [Google Scholar] [CrossRef]
- Morrison, G. Effect of water on the critical points of carbon dioxide and ethane. J. Phys. Chem. 1981, 85, 759–761. [Google Scholar] [CrossRef]
- Tamimi, A.; Rinker, E.B.; Sandall, O.C. Diffusion Coefficients for Hydrogen Sulfide, Carbon Dioxide, and Nitrous Oxide in Water over the Temperature Range 293‒368 K.J. Chem. Eng. Data 1994, 39, 330–332. [Google Scholar] [CrossRef]
- Franich, R.; Gallagher, S.; Kroese, H.W. Dewatering green sapwood using carbon dioxide cycled between supercritical fluid and gas phase. J. Supercrit. Fluids 2014, 89, 113–118. [Google Scholar] [CrossRef]
- Dawson, B.S.; Pearson, H.; Kroese, H.W.; Sargent, R. Effect of specimen dimension and pre-heating temperature on supercritical CO2 dewatering of radiata pine sapwood. Holzforschung 2015, 69, 421–430. [Google Scholar] [CrossRef]
- Dawson, B.; Pearson, H. Effect of supercritical CO2 dewatering followed by oven-drying of softwood and hardwood timbers. Wood Sci. Technol. 2017, 51, 771–784. [Google Scholar] [CrossRef]
- Yang, L.; Liu, H. A Review of Eucalyptus Wood Collapse and its Control during Drying. Bioresource 2018, 13, 2171–2181. [Google Scholar] [CrossRef]
- Dawson, B.; Pearson, H.; Kimberley, M.O.; Davy, B.; Dickson, A. Effect of supercritical CO2 treatment and kiln drying on collapse in Eucalyptus nitens wood. Holz Roh Werkst. 2020, 78, 209–217. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Liu, H.-H.; Yang, H.; Yang, L. Drying characteristics of Eucalyptus urophylla × E. grandis with supercritical CO2. Materials 2020, 13, 3989. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, H. Effect of Supercritical CO2 Drying on Moisture Transfer and Wood Property of Eucalyptus urophydis. Forests 2020, 11, 1115. [Google Scholar] [CrossRef]
- McDonald, A.G.; Fernandez, M.; Kreber, B.; Laytner, F. The Chemical Nature of Kiln Brown Stain in Radiata Pine. Holzforschung 2000, 54, 12–22. [Google Scholar] [CrossRef]
- Franich, R.A.; Kroese, H.; Gallagher, S.; Steward, D.; Isak, I. Solutes in sap obtained from supercritical CO2 dewatering of radiata pine sapwood, and a new role of sap cyclitols in brown stain formation during kiln drying of green wood. Holzforschung 2019, 73, 947–956. [Google Scholar] [CrossRef]
- McCurdy, M.C.; Pang, S. Optimization of Kiln Drying for Softwood through Simulation of Wood Stack Drying, Energy Use, and Wood Color Change. Dry. Technol. 2007, 25, 1733–1740. [Google Scholar] [CrossRef]
- Behr, V.C.; Schmid, M.W.; Franich, R.A.; Meder, R. An advanced, integrated large-volume high-pressure autoclave and 1h/13c double-tuned resonator for chemistry and materials nuclear magnetic resonance spectroscopy and microscopy investigations. Concepts Magn. Reson. Part B Magn. Reson. Eng. 2013, 43, 49–58. [Google Scholar] [CrossRef]
- Behr, V.C.; Hill, S.J.; Meder, R.; Sandquist, D.; Hindmarsh, J.P.; Franich, R.; Newman, R.H. Carbon-13 NMR chemical-shift imaging study of dewatering of green sapwood by cycling carbon dioxide between the supercritical fluid and gas phases. J. Supercrit. Fluids 2014, 95, 535–540. [Google Scholar] [CrossRef]
- Newman, R.H.; Franich, R.A.; Meder, R.; Hill, S.J.; Kroese, H.W.; Sandquist, D.; Hindmarsh, J.P.; Schmid, M.W.; Fuchs, J.; Behr, V.C. Proton magnetic resonance imaging used to investigate dewatering of green sapwood by cycling carbon dioxide between supercritical fluid and gas phase. J. Supercrit. Fluids 2016, 111, 36–42. [Google Scholar] [CrossRef]
- Franich, R.A.; Meder, R.; Falge, M.; Fuchs, J.; Behr, V.C. Uncovering supercritical CO2 wood dewatering via interleaved 1H-imaging and 13C-spectroscopy with real-time reconstruction. J. Supercrit. Fluids 2019, 144, 56–62. [Google Scholar] [CrossRef]
- Meder, R.; Franich, R.A.; Callaghan, P.T.; Behr, V.C. A comparative study of dewatering of Pinus radiata sapwood using supercritical CO2 and conventional forced air-drying via in situ magnetic resonance microimaging (MRI). Holzforschung 2015, 69, 1137–1142. [Google Scholar] [CrossRef]
- Pearson, H.; Dawson, B.; Kimberley, M.; Davy, B. Predictive modelling of supercritical CO2 dewatering of capillary tubes. J. Supercrit. Fluids 2019, 143, 198–204. [Google Scholar] [CrossRef]
- Pearson, H.; Dawson, B.; Kimberley, M.; Davy, B. Modeling and optimization of ceramic and wood dewatering using supercritical CO2. J. Supercrit. Fluids 2019, 146, 15–22. [Google Scholar] [CrossRef]
- Superwood A/S, Palsgaardvej 3, DK-7362, Hampen. Available online: https://www.superwood.dk/rejsen/gennemimpraegnering/ (accessed on 15 October 2020).
- Iversen, S.B.; Larsen, T.; Henriksen, O.; Feisvang, K. Supercritical Fluids. In Proceedings of the International Symposium on Visual Information Communication and Interaction, Tianjin, China, 3–5 August 2013. [Google Scholar]
- Kjellow, A.W.; Henriksen, O. Supercritical wood impregnation. J. Supercrit. Fluids 2009, 50, 297–304. [Google Scholar] [CrossRef][Green Version]
- Fernandes, J.; Kjellow, A.W.; Henriksen, O. Modeling and optimization of the supercritical wood impregnation process—Focus on pressure and temperature. J. Supercrit. Fluids 2012, 66, 307–314. [Google Scholar] [CrossRef]
- Gabitov, R.F.; Khairutdinov, V.; Gumerov, F.M.; Gabitov, F.R.; Zaripov, Z.I.; Gaifullina, R.; Farakhov, M.I. Drying and Impregnation of Wood with Propiconazole Using Supercritical Carbon Dioxide. Russ. J. Phys. Chem. B 2017, 11, 1223–1230. [Google Scholar] [CrossRef]
- Meder, R.; Franich, R.; Callaghan, P.T. 11B magnetic resonance imaging and MAS spectroscopy of trimethylborate-treated radiata pine wood. Solid State Nucl. Magn. Reson. 1999, 15, 69–72. [Google Scholar] [CrossRef]
- Franich, R.; Nicholson, B.K.; Kroese, H.W.; Gallagher, S.S.; Meder, R.; Lane, J.R.; Kelly, B.D. Synthesis, characterization and crystal structures of the boratranes: 2,10,11-trioxa-6-aza-1-boratricyclo [4.4.4.01,6] tetradecane (tri-n-propanolamine borate), and 3-(4-methoxy)phenoxymethyl-7,10-dimethyl-2,8,9-trioxa-5-aza-1-boratricyclo[3.3.3.01,5]-undecane. Polyhedron 2011, 30, 2884–2889. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franich, R.A.; Meder, R.; Behr, V.C. Dewatering Green Sapwood Using Carbon Dioxide Undergoing Cyclical Phase Change between Supercritical Fluid and Gas. Molecules 2020, 25, 5367. https://doi.org/10.3390/molecules25225367
Franich RA, Meder R, Behr VC. Dewatering Green Sapwood Using Carbon Dioxide Undergoing Cyclical Phase Change between Supercritical Fluid and Gas. Molecules. 2020; 25(22):5367. https://doi.org/10.3390/molecules25225367
Chicago/Turabian StyleFranich, Robert A., Roger Meder, and Volker C. Behr. 2020. "Dewatering Green Sapwood Using Carbon Dioxide Undergoing Cyclical Phase Change between Supercritical Fluid and Gas" Molecules 25, no. 22: 5367. https://doi.org/10.3390/molecules25225367
APA StyleFranich, R. A., Meder, R., & Behr, V. C. (2020). Dewatering Green Sapwood Using Carbon Dioxide Undergoing Cyclical Phase Change between Supercritical Fluid and Gas. Molecules, 25(22), 5367. https://doi.org/10.3390/molecules25225367





