Effects of Grape Polyphenols on the Life Span and Neuroinflammatory Alterations Related to Neurodegenerative Parkinson Disease-Like Disturbances in Mice
Abstract
1. Introduction
2. Results
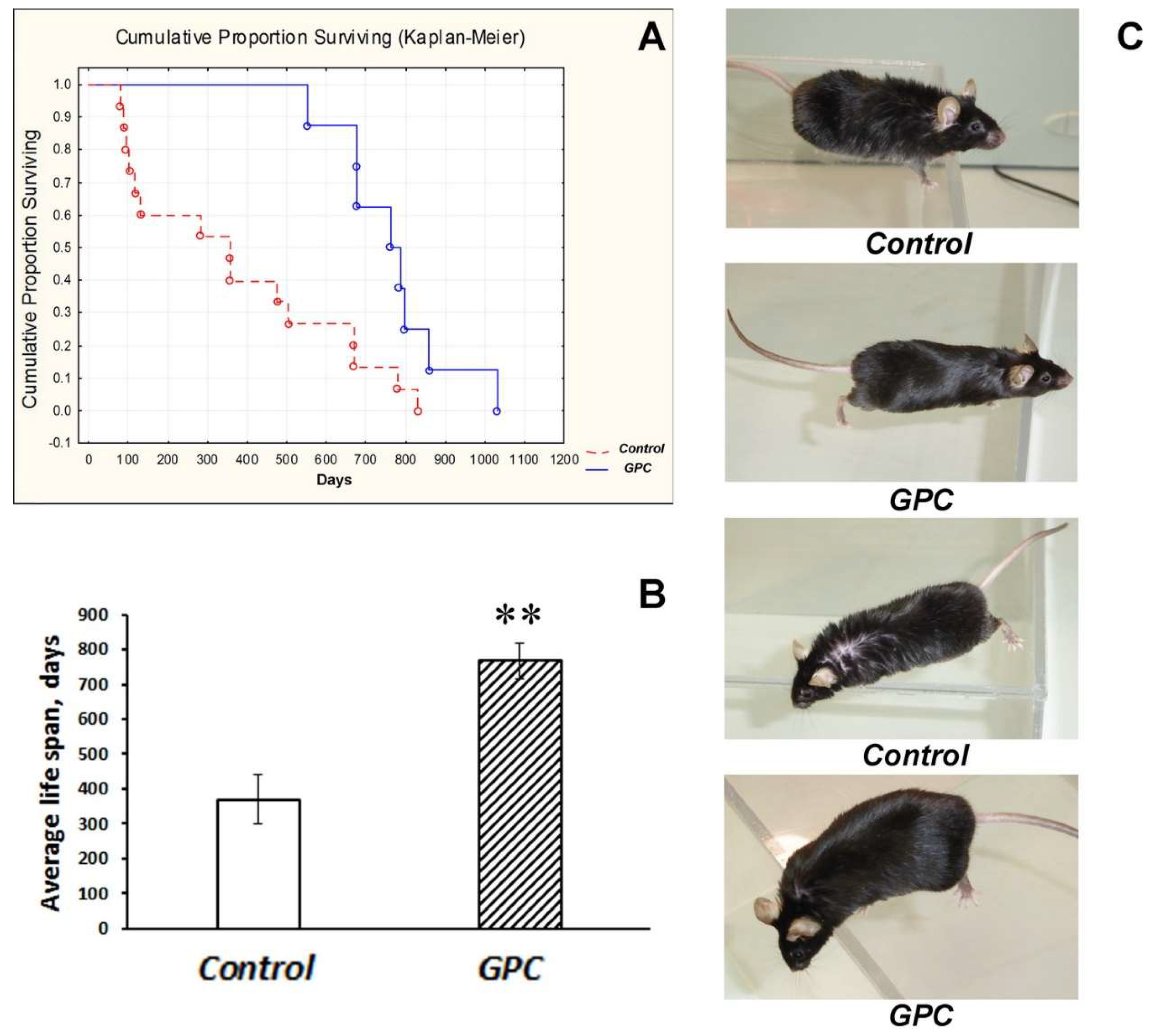
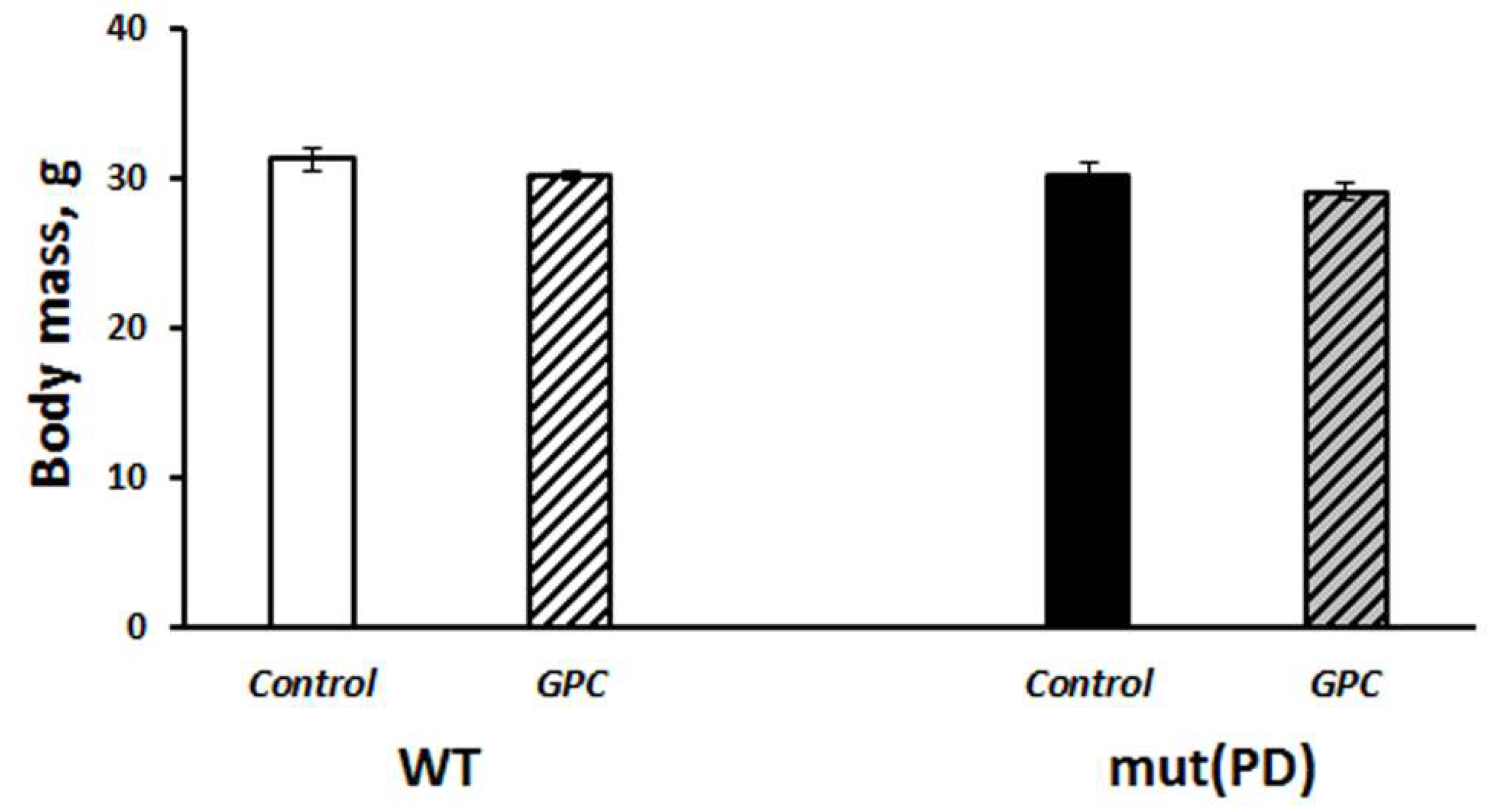
2.1. Diet Tolerance and the Effects on Life Expectancy and Body Weight Gain
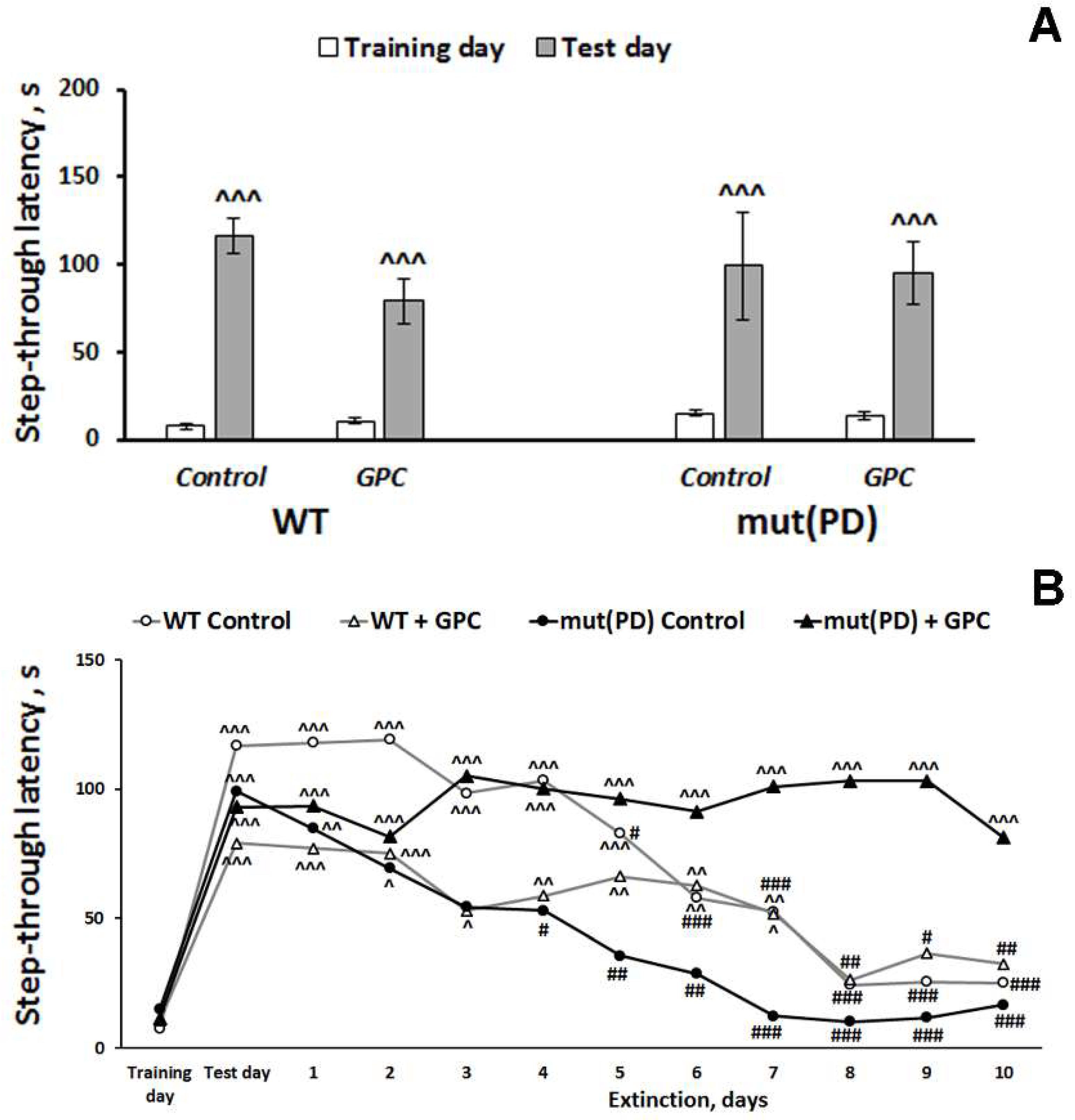
2.2. Behavioral Effects
2.2.1. The Open Field Test
2.2.2. The Passive Avoidance Test
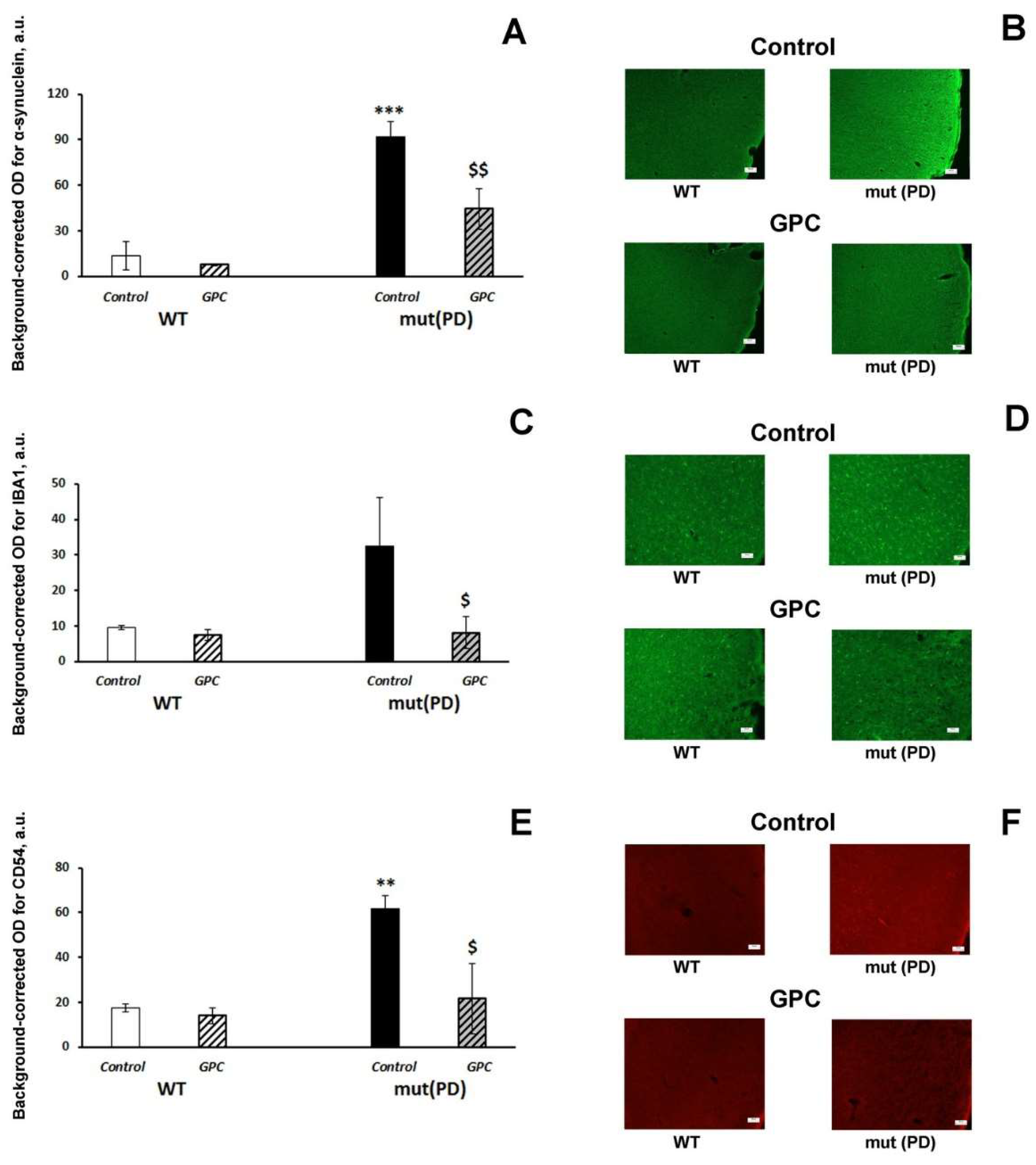
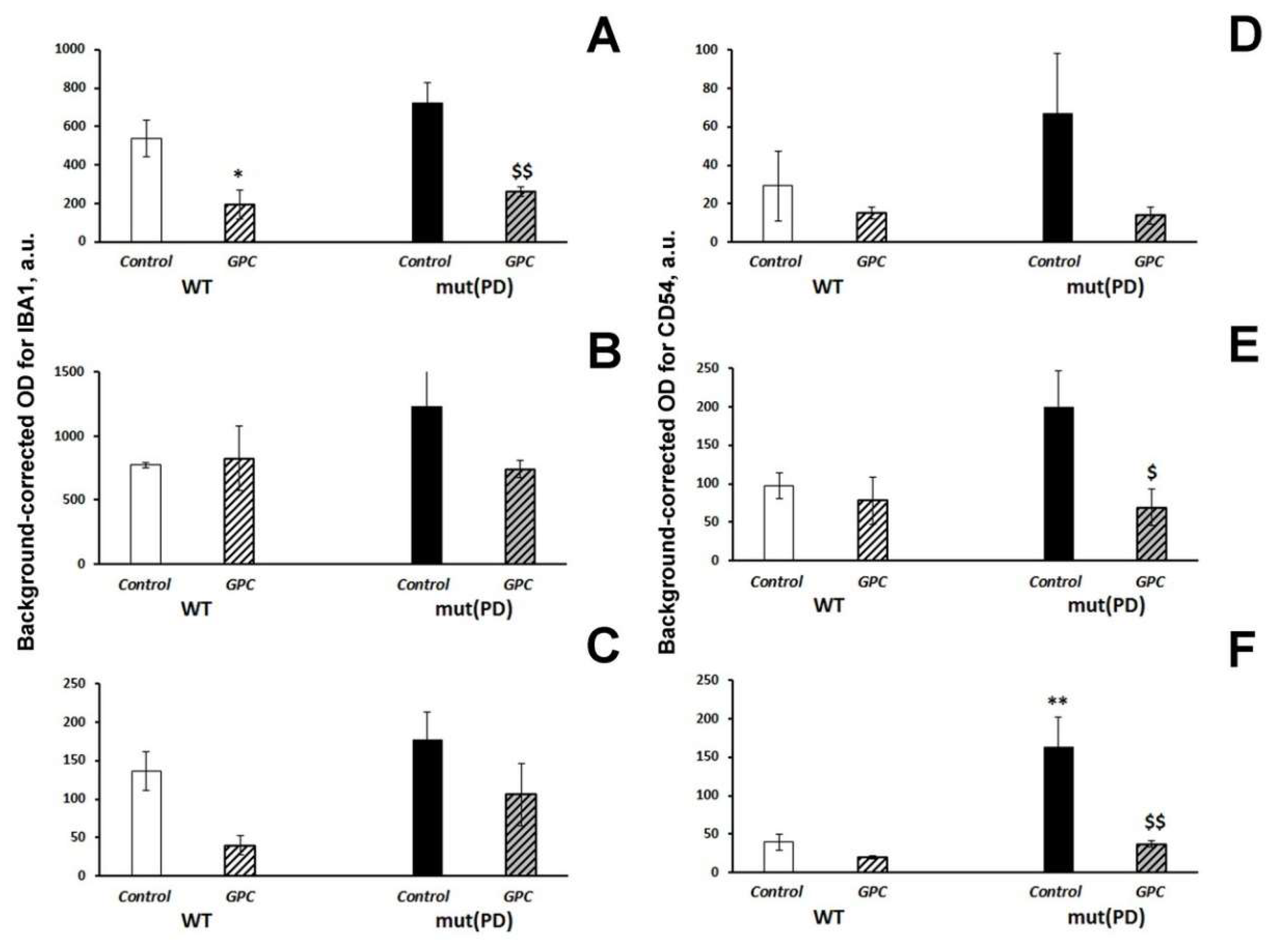
2.3. Immunohistochemical Analysis
3. Discussion
3.1. Safety of Long-Term Supplementation with Grape Polyphenols and Their Effect on Life Span in Mice
3.2. Polyphenols Attenuate Neuropathological Changes Associated with Aging and PD-Like Disturbances
4. Materials and Methods
4.1. Experimental Animal and Design
4.2. Behavioral Tests
4.2.1. The Open Field Test
4.2.2. The Passive Avoidance Test
4.3. Immunohistochemical Analysis
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, W.G.; Zhang, X.Y.; Wu, Y.J.; Tian, X. Anti-inflammatory effect and mechanism of proanthocyanidins from grape seeds. Acta Pharmacol. Sin. 2001, 22, 1117–1120. [Google Scholar]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef]
- Seo, K.-H.; Kim, H.; Chon, J.-W.; Kim, D.-H.; Nah, S.-Y.; Arvik, T.; Yokoyama, W. Flavonoid-rich Chardonnay grape seed flour supplementation ameliorates diet-induced visceral adiposity, insulin resistance, and glucose intolerance via altered adipose tissue gene expression. J. Funct. Foods 2015, 17, 881–891. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20 (Suppl. S2), 1–42. [Google Scholar] [CrossRef]
- Tartaglione, L.; Gambuti, A.; De Cicco, P.; Ercolano, G.; Ianaro, A.; Taglialatela-Scafati, O.; Moio, L.; Forino, M. NMR-based phytochemical analysis of Vitis vinifera cv Falanghina leaves. Characterization of a previously undescribed biflavonoid with antiproliferative activity. Fitoterapia 2018, 125, 13–17. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Di Francesco, S.; Savio, M.; Bloise, N.; Borroni, G.; Stivala, L.A.; Borroni, R.G. Red grape (Vitis vinifera L.) flavonoids down-regulate collagen type III expression after UV-A in primary human dermal blood endothelial cells. Exp. Dermatol. 2018, 27, 973–980. [Google Scholar] [CrossRef]
- Khlestkina, E.K. The adaptive role of flavonoids: Emphasis on cereals. Cereal Res. Commun. 2013, 41, 185–198. [Google Scholar] [CrossRef]
- Amato, A.; Cavallini, E.; Walker, A.R.; Pezzotti, M.; Bliek, M.; Quattrocchio, F.; Koes, R.; Ruperti, B.; Bertini, E.; Zenoni, S.; et al. The MYB5-driven MBW complex recruits a WRKY factor to enhance the expression of targets involved in vacuolar hyper-acidification and trafficking in grapevine. Plant J. 2019, 99, 1220–1241. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Vélez, M.L.; Martínez-Martínez, F.; Del Valle-Ribes, C. The Study of Phenolic Compounds as Natural Antioxidants in Wine. Crit. Rev. Food Sci. Nutr. 2003, 43, 233–244. [Google Scholar] [CrossRef]
- Jesus, M.S.; Genisheva, Z.; Romaní, A.; Pereira, R.N.C.; Teixeira, J.A.; Domingues, L. Bioactive compounds recovery optimization from vine pruning residues using conventional heating and microwave-assisted extraction methods. Ind. Crop. Prod. 2019, 132, 99–110. [Google Scholar] [CrossRef]
- Jesus, M.S.; Ballesteros, L.F.; Pereira, R.N.C.; Genisheva, Z.; Carvalho, A.C.; Pereira-Wilson, C.; Teixeira, J.A.; Domingues, L. Ohmic heating polyphenolic extracts from vine pruning residue with enhanced biological activity. Food Chem. 2020, 316, 126298. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Carey, A.; Simon, L.; Mark, D.A.; Joseph, J.A. Effects of Concord grape juice on cognitive and motor deficits in aging. Nutrition 2006, 22, 295–302. [Google Scholar] [CrossRef]
- Mullen, W.; Marks, S.C.; Crozier, A. Evaluation of Phenolic Compounds in Commercial Fruit Juices and Fruit Drinks. J. Agric. Food Chem. 2007, 55, 3148–3157. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35. [Google Scholar] [CrossRef]
- Chou, L.-M.; Lin, C.-I.; Chen, Y.-H.; Liao, H.; Lin, S.-H. A diet containing grape powder ameliorates the cognitive decline in aged rats with a long-term high-fructose-high-fat dietary pattern. J. Nutr. Biochem. 2016, 34, 52–60. [Google Scholar] [CrossRef]
- Herman, F.; Westfall, S.; Brathwaite, J.; Pasinetti, G.M. Suppression of Presymptomatic Oxidative Stress and Inflammation in Neurodegeneration by Grape-Derived Polyphenols. Front. Pharmacol. 2018, 9, 867. [Google Scholar] [CrossRef]
- Ben Youssef, S.; Brisson, G.; Doucet-Beaupré, H.; Castonguay, A.-M.; Gora, C.; Amri, M.; Lévesque, M. Neuroprotective benefits of grape seed and skin extract in a mouse model of Parkinson’s disease. Nutr. Neurosci. 2019, 1–15. [Google Scholar] [CrossRef]
- Rocha, E.M.; De Miranda, B.; Sanders, L.H. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef]
- Farrell, K.F.; Krishnamachari, S.; Villanueva, E.; Lou, H.; Alerte, T.N.M.; Peet, E.; Drolet, R.E.; Perez, R.G. Non-motor parkinsonian pathology in aging A53T α-Synuclein mice is associated with progressive synucleinopathy and altered enzymatic function. J. Neurochem. 2014, 128, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Paumier, K.L.; Rizzo, S.J.S.; Berger, Z.; Chen, Y.; Gonzales, C.; Kaftan, E.; Li, L.; Lotarski, S.; Monaghan, M.; Shen, W.; et al. Behavioral Characterization of A53T Mice Reveals Early and Late Stage Deficits Related to Parkinson’s Disease. PLoS ONE 2013, 8, e70274. [Google Scholar] [CrossRef] [PubMed]
- Unger, E.L.; Eve, D.J.; Perez, X.A.; Reichenbach, D.K.; Xu, Y.; Lee, M.K.; Andrews, A.M. Locomotor hyperactivity and alterations in dopamine neurotransmission are associated with overexpression of A53T mutant human α-synuclein in mice. Neurobiol. Dis. 2006, 21, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Garelick, M.G.; Storm, D.R. The relationship between memory retrieval and memory extinction. Proc. Natl. Acad. Sci. USA 2005, 102, 9091–9092. [Google Scholar] [CrossRef] [PubMed]
- Pallauf, K.; Duckstein, N.; Rimbach, G. A literature review of flavonoids and lifespan in model organisms. Proc. Nutr. Soc. 2017, 76, 145–162. [Google Scholar] [CrossRef]
- Aires, D.J.; Rockwell, G.; Wang, T.; Frontera, J.; Wick, J.; Wang, W.; Tonkovic-Capin, M.; Lu, J.; Lezi, E.; Zhu, H.; et al. Potentiation of dietary restriction-induced lifespan extension by polyphenols. Biochim. Biophys. Acta. 2012, 1822, 522–526. [Google Scholar] [CrossRef]
- Kitani, K.; Osawa, T.; Yokozawa, T. The effects of tetrahydrocurcumin and green tea polyphenol on the survival of male C57BL/6 mice. Biogerontology 2007, 8, 567–573. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Cai, W.-J.; Huang, J.-H.; Wu, B.; Xia, S.-J.; Chen, X.-L.; Zhang, X.-M.; Shen, Z. Icariin, a natural flavonol glycoside, extends healthspan in mice. Exp. Gerontol. 2015, 69, 226–235. [Google Scholar] [CrossRef]
- Strong, R.; Miller, R.A.; Astle, C.M.; Baur, J.A.; De Cabo, R.; Fernandez, E.; Guo, W.; Javors, M.; Kirkland, J.L.; Nelson, J.F.; et al. Evaluation of Resveratrol, Green Tea Extract, Curcumin, Oxaloacetic Acid, and Medium-Chain Triglyceride Oil on Life Span of Genetically Heterogeneous Mice. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2012, 68, 6–16. [Google Scholar] [CrossRef]
- Spindler, S.R.; Mote, P.L.; Flegal, J.; Teter, B. Influence on Longevity of Blueberry, Cinnamon, Green and Black Tea, Pomegranate, Sesame, Curcumin, Morin, Pycnogenol, Quercetin, and Taxifolin Fed Iso-Calorically to Long-Lived, F1 Hybrid Mice. Rejuvenation Res. 2013, 16, 143–151. [Google Scholar] [CrossRef]
- Frozza, R.L.; Lourenco, M.V.; De Felice, F.G. Challenges for Alzheimer’s Disease Therapy: Insights from Novel Mechanisms Beyond Memory Defects. Front. Neurosci. 2018, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, B.; Shang, Y.; Yin, Q.; Wang, D.; Xu, S.; Hong, Y.; Hou, X.; Liu, X. Flavonoid-Rich Ethanol Extract from the Leaves of Diospyros kaki Attenuates D-Galactose-Induced Oxidative Stress and Neuroinflammation-Mediated Brain Aging in Mice. Oxidative Med. Cell. Longev. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.C.; Antunes, M.S.; Filho, C.B.; Del Fabbro, L.; De Gomes, M.G.; Goes, A.T.R.; Donato, F.; Prigol, M.; Boeira, S.P.; Jesse, C. Flavonoid Chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol. Biochem. Behav. 2015, 134, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Thomas, P.; Zhong, J.-H.; Bi, F.-F.; Kosaraju, S.; Pollard, A.; Fenech, M.; Zhou, X.-F. Consumption of Grape Seed Extract Prevents Amyloid-β Deposition and Attenuates Inflammation in Brain of an Alzheimer’s Disease Mouse. Neurotox. Res. 2009, 15, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Bensalem, J.; Dudonné, S.; Gaudout, D.; Servant, L.; Calon, F.; Desjardins, Y.; Layé, S.; Lafenetre, P.; Pallet, V. Polyphenol-rich extract from grape and blueberry attenuates cognitive decline and improves neuronal function in aged mice. J. Nutr. Sci. 2018, 7, e19. [Google Scholar] [CrossRef] [PubMed]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; A Kroon, P.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.-L. A theoretical physiologically based pharmacokinetic approach for modeling the fate of anthocyanins in vivo. Crit. Rev. Food Sci. Nutr. 2017, 57, 3197–3207. [Google Scholar] [CrossRef]
- Sandoval-Ramírez, B.A.; Catalán, Ú.; Fernández-Castillejo, S.; Rubió, L.; Macià, A.; Solà, R. Anthocyanin Tissue Bioavailability in Animals: Possible Implications for Human Health. A Systematic Review. J. Agric. Food Chem. 2018, 66, 11531–11543. [Google Scholar] [CrossRef]
- Janle, E.M.; Lila, M.A.; Grannan, M.; Wood, L.; Higgins, A.; Yousef, G.G.; Rogers, R.B.; Kim, H.; Jackson, G.S.; Ho, L.; et al. Pharmacokinetics and Tissue Distribution of14C-Labeled Grape Polyphenols in the Periphery and the Central Nervous System Following Oral Administration. J. Med. Food 2010, 13, 926–933. [Google Scholar] [CrossRef]
- Kisková, T.; Kubatka, P.; Büsselberg, D.; Kassayova, M. The Plant-Derived Compound Resveratrol in Brain Cancer: A Review. Biomolecules 2020, 10, 161. [Google Scholar] [CrossRef]
- Baell, J.B.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nat. Cell Biol. 2014, 513, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Ingólfsson, H.I.; Thakur, P.; Herold, K.F.; Hobart, E.A.; Ramsey, N.B.; Periole, X.; De Jong, D.H.; Zwama, M.; Yilmaz, D.; Hall, K.; et al. Phytochemicals Perturb Membranes and Promiscuously Alter Protein Function. ACS Chem. Biol. 2014, 9, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Teles, R.B.; Diniz, T.C.; Pinto, T.C.C.; de Oliveira Júnior, R.G.; E Silva, M.G.; de Lavor, É.M.; Fernandes, A.W.C.; de Oliveira, A.P.; de Almeida Ribeiro, F.P.R.; da Silva, A.A.M.; et al. Flavonoids as Therapeutic Agents in Alzheimer’s and Parkinson’s Diseases: A Systematic Review of Preclinical Evidences. Oxidative Med. Cell. Longev. 2018, 2018, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Strathearn, K.E.; Yousef, G.G.; Grace, M.H.; Roy, S.L.; Tambe, M.A.; Ferruzzi, M.G.; Wu, Q.-L.; Simon, J.E.; Lila, M.A.; Rochet, J.-C. Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinson’s disease. Brain Res. 2014, 1555, 60–77. [Google Scholar] [CrossRef]
- Macedo, D.; Tavares, L.; McDougall, G.J.; Miranda, H.V.; Stewart, D.; Ferreira, R.B.; Tenreiro, S.; Outeiro, T.F.; Santos, C.N. (Poly)phenols protect from α-synuclein toxicity by reducing oxidative stress and promoting autophagy. Hum. Mol. Genet. 2014, 24, 1717–1732. [Google Scholar] [CrossRef]
- Macedo, D.; Jardim, C.; Figueira, I.; Almeida, A.F.; McDougall, G.; Stewart, D.; Yuste, J.E.; Tomás-Barberán, F.A.; Tenreiro, S.; Outeiro, T.F.; et al. (Poly)phenol-digested metabolites modulate alpha-synuclein toxicity by regulating proteostasis. Sci. Rep. 2018, 8, 6965. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Álvarez-Fernández, M.A.; Cerezo, A.B.; Richard, T.; Troncoso, A.; García-Parrilla, M.C. Protocatechuic Acid: Inhibition of Fibril Formation, Destabilization of Preformed Fibrils of Amyloid-β and α-Synuclein, and Neuroprotection. J. Agric. Food Chem. 2016, 64, 7722–7732. [Google Scholar] [CrossRef]
- Pogačnik, L.; Pirc, K.; Palmela, I.; Skrt, M.; Kim, K.S.; Brites, D.; Brito, M.A.; Ulrih, N.P.; Silva, R.F. Potential for brain accessibility and analysis of stability of selected flavonoids in relation to neuroprotection in vitro. Brain Res. 2016, 1651, 17–26. [Google Scholar] [CrossRef]
- The Jackson Laboratory. Available online: https://www.jax.org/ (accessed on 13 November 2020).
- RESSFOOD. Available online: http://enoant.info/ (accessed on 13 November 2020).
- Pupyshev, A.B.; Tikhonova, M.A.; Akopyan, A.A.; Tenditnik, M.V.; Dubrovina, N.I.; Korolenko, T.A. Therapeutic activation of autophagy by combined treatment with rapamycin and trehalose in a mouse MPTP-induced model of Parkinson’s disease. Pharmacol. Biochem. Behav. 2019, 177, 1–11. [Google Scholar] [CrossRef]
| Parameter | Group | F, p | |||
|---|---|---|---|---|---|
| WT | Mut (PD) | ||||
| Control | GPC | Control | GPC | ||
| Distance travelled, cm | 3773 ± 266 | 3704 ± 297 | 4964 ± 602 | 5236 ± 492 && | G: F(1, 28) = 10.8, p < 0.01 |
| D: F(1, 28) < 1 | |||||
| D × G: F(1, 28) < 1 | |||||
| Rearings, n | 74.7 ± 6.3 | 67.0 ± 8.9 | 76.4 ± 7.3 | 91.8 ± 12.3 | G: F(1, 28) = 1.8, p > 0.05 |
| D: F(1, 28) < 1 | |||||
| D × G: F(1, 28) = 1.4, p > 0.05 | |||||
| Time in the center, s | 31.5 ± 4.8 | 33.2 ± 4.5 | 36.0 ± 9.8 | 30.6 ± 6.6 | G: F(1, 28) < 1 |
| D: F(1, 28) < 1 | |||||
| D × G: F(1, 28) < 1 | |||||
| Fecal boli, n | 2.22 ± 0.74 | 2.89 ± 0.75 | 2.8 ± 1.16 | 1.0 ± 0.58 | G: F(1, 28) < 1 |
| D: F(1, 28) < 1 | |||||
| D × G: F(1, 28) = 2.4, p > 0.05 | |||||
Sample Availability: Samples of the compounds are not available from the author. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikhonova, M.A.; Tikhonova, N.G.; Tenditnik, M.V.; Ovsyukova, M.V.; Akopyan, A.A.; Dubrovina, N.I.; Amstislavskaya, T.G.; Khlestkina, E.K. Effects of Grape Polyphenols on the Life Span and Neuroinflammatory Alterations Related to Neurodegenerative Parkinson Disease-Like Disturbances in Mice. Molecules 2020, 25, 5339. https://doi.org/10.3390/molecules25225339
Tikhonova MA, Tikhonova NG, Tenditnik MV, Ovsyukova MV, Akopyan AA, Dubrovina NI, Amstislavskaya TG, Khlestkina EK. Effects of Grape Polyphenols on the Life Span and Neuroinflammatory Alterations Related to Neurodegenerative Parkinson Disease-Like Disturbances in Mice. Molecules. 2020; 25(22):5339. https://doi.org/10.3390/molecules25225339
Chicago/Turabian StyleTikhonova, Maria A., Nadezhda G. Tikhonova, Michael V. Tenditnik, Marina V. Ovsyukova, Anna A. Akopyan, Nina I. Dubrovina, Tamara G. Amstislavskaya, and Elena K. Khlestkina. 2020. "Effects of Grape Polyphenols on the Life Span and Neuroinflammatory Alterations Related to Neurodegenerative Parkinson Disease-Like Disturbances in Mice" Molecules 25, no. 22: 5339. https://doi.org/10.3390/molecules25225339
APA StyleTikhonova, M. A., Tikhonova, N. G., Tenditnik, M. V., Ovsyukova, M. V., Akopyan, A. A., Dubrovina, N. I., Amstislavskaya, T. G., & Khlestkina, E. K. (2020). Effects of Grape Polyphenols on the Life Span and Neuroinflammatory Alterations Related to Neurodegenerative Parkinson Disease-Like Disturbances in Mice. Molecules, 25(22), 5339. https://doi.org/10.3390/molecules25225339








