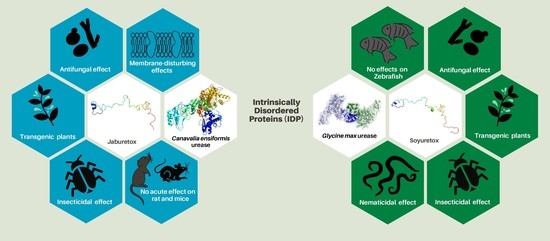
Structure-Function Insights of Jaburetox and Soyuretox: Novel Intrinsically Disordered Polypeptides Derived from Plant Ureases
Abstract
1. Introduction
2. Jaburetox and Soyuretox: Historical Aspects and Potential as Biopesticides
2.1. Transgenic Plants Expressing Biocide Polypeptides and Plant Defense
2.2. Plant Proteins and Peptides with Insecticidal and Fungitoxic Properties
2.3. Ureases and Derived Peptides as Sources of Insecticidal and Fungitoxic (Poly)Peptides
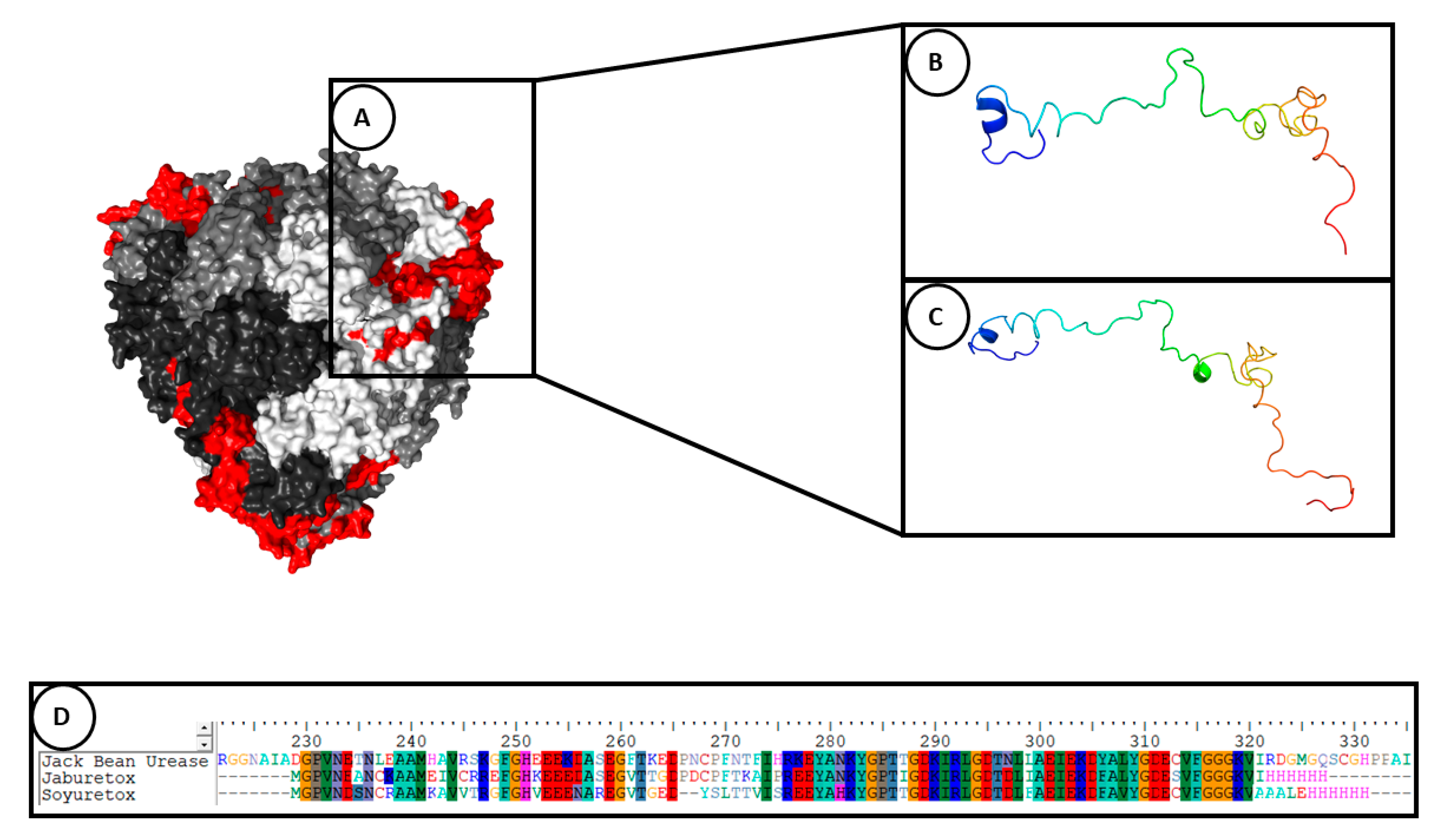
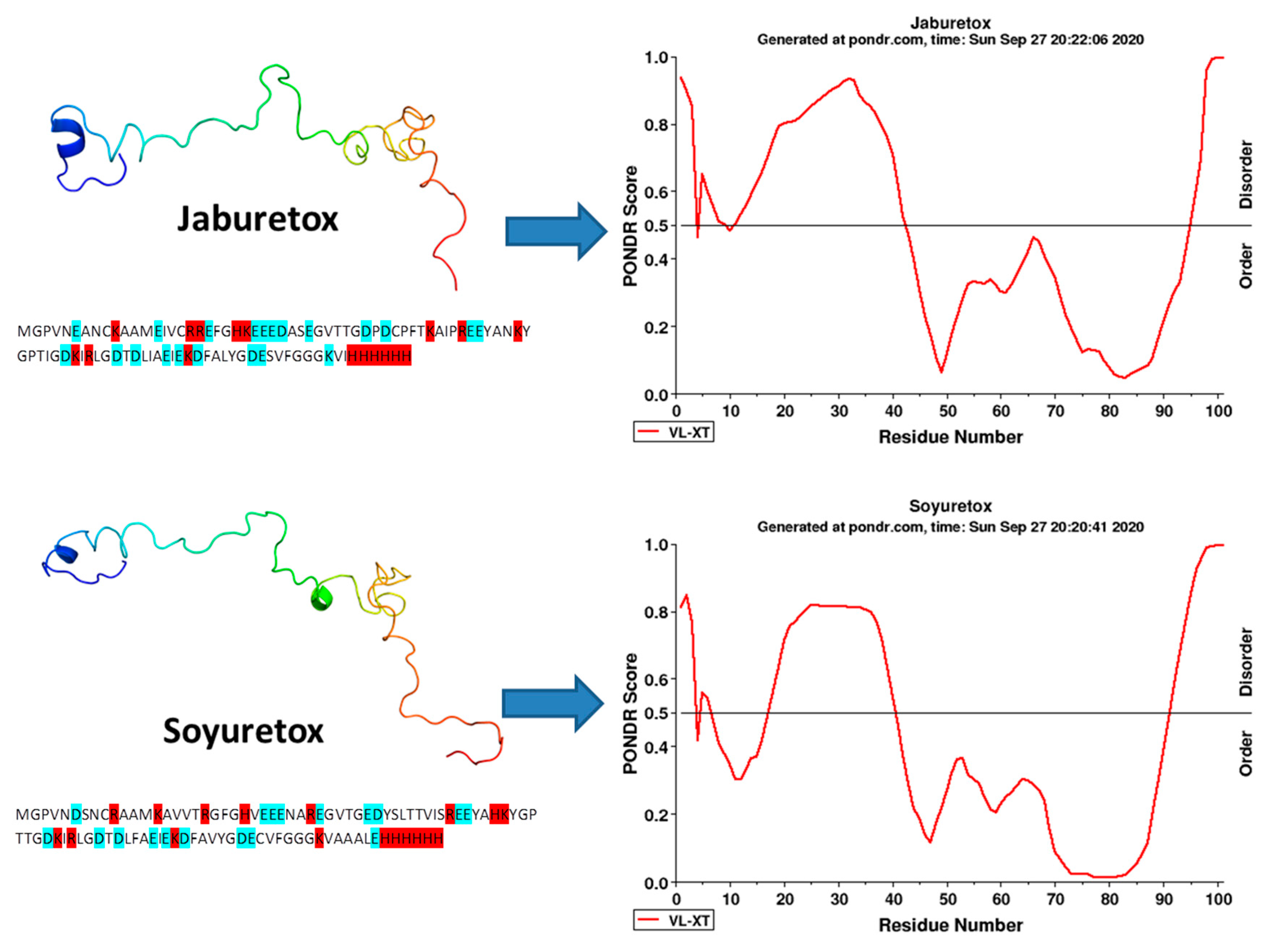
3. Structural Aspects of Jaburetox and Soyuretox and Its Interaction with Membranes
4. Biological Studies
4.1. Entomotoxic Effects
4.1.1. Lethality
4.1.2. Effects on the Central Nervous and Neuromuscular Systems
4.1.3. Effects on Behavior
4.1.4. Effects on Enzymatic Pathways
4.1.5. Effects on Diuresis
4.1.6. Effects on the Immune System
4.2. Antifungal and Antibacterial Activity
5. Structural Aspects of Other Intrinsically Disordered Bioactive Polypeptides
6. Biotechnological Applications and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oldfield, C.J.; Dunker, A.K. Intrinsically disordered proteins and intrinsically disordered protein regions. Annu. Rev. Biochem. 2014, 83, 553–584. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Intrinsically disordered proteins and their environment: Effects of strong denaturants, temperature, pH, Counter ions, membranes, binding partners, osmolytes, and macromolecular crowding. Protein J. 2009, 28, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Intrinsically disordered proteins and their “Mysterious” (meta)physics. Front. Phys. 2019, 7, 8–23. [Google Scholar] [CrossRef]
- Uversky, V.N. Intrinsically Disordered Proteins: Targets for the Future? Struct. Biol. Drug Discov. Methods Tech. Pract. 2020, 587–612. [Google Scholar] [CrossRef]
- Uversky, V.N. Unusual biophysics of intrinsically disordered proteins. Biochim. Biophys. Acta 2013, 1834, 932–951. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. A decade and a half of protein intrinsic disorder: Biology still waits for physics. Protein Sci. 2013, 22, 693–724. [Google Scholar] [CrossRef]
- Uversky, V.N. What does it mean to be natively unfolded? Eur. J. Biochem. 2002, 269, 2–12. [Google Scholar] [CrossRef]
- Tompa, P.; Fuxreiter, M. Fuzzy complexes: Polymorphism and structural disorder in protein--protein interactions. Trends Biochem. Sci. 2008, 33, 2–8. [Google Scholar] [CrossRef]
- Uversky, V.N. Multitude of binding modes attainable by intrinsically disordered proteins: A portrait gallery of disorder-based complexes. Chem. Soc. Rev. 2011, 40, 1623–1634. [Google Scholar] [CrossRef]
- Dunker, A.K.; Babu, M.M.; Barbar, E.; Blackledge, M.; Bondos, S.E.; Dosztányi, Z.; Dyson, H.J.; Forman-Kay, J.; Fuxreiter, M.; Gsponer, J.; et al. What’s in a name? Why these proteins are intrinsically disordered. Intrinsically Disord. Proteins 2013, 1, e24157. [Google Scholar] [CrossRef]
- Eliezer, D. Biophysical characterization of intrinsically disordered proteins. Curr. Opin. Struct. Biol. 2009, 19, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Chen, J. Targeting Intrinsically Disordered Proteins through Dynamic Interactions. Biomolecules 2020, 10, 743. [Google Scholar] [CrossRef]
- Mitchell, C.; Brennan, R.M.; Graham, J.; Karley, A.J. Plant defense against herbivorous pests: Exploiting resistance and tolerance traits for sustainable crop protection. Front. Plant Sci. 2016, 7, 1132. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Gregory, P.J.; Johnson, S.N.; Newton, A.C.; Ingram, J.S.I. Integrating pests and pathogens into the climate change/food security debate. J. Exp. Bot. 2009, 60, 2827–2838. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Ashraf, M.; Younis, M.; Hu, X.; Kumar, A.; Akram, N.A.; Al-Qurainy, F. Role of transgenic plants in agriculture and biopharming. Biotechnol. Adv. 2012, 30, 524–540. [Google Scholar] [CrossRef]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P.; Caballero, P. Bacillus thuringiensis toxins: An overview of their biocidal activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef]
- Chougule, N.P.; Bonning, B.C. Toxins for transgenic resistance to hemipteran pests. Toxins 2012, 4, 405–429. [Google Scholar] [CrossRef]
- Heckel, D.G. How do toxins from Bacillus thuringiensis kill insects? An evolutionary perspective. Arch. Insect Biochem. Physiol. 2020, 104, e21673. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; An, S. The progress in insect cross-resistance among Bacillus thuringiensis toxins. Arch. Insect Biochem. Physiol. 2019, 102, e21547. [Google Scholar] [CrossRef]
- Grossi-de-Sá, M.F.; Pelegrini, P.B.; Vasconcelos, I.M.; Carlini, C.R.; Silva, M.S. Entomotoxic Plant Proteins: Potential Molecules to Develop Genetically Modified Plants Resistant to Insect-Pests. In Plant Toxins; Carlini, C.R., Ligabue-Braun, R., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2017; pp. 415–447. ISBN 978-94-007-6464-4. [Google Scholar] [CrossRef]
- Tsaneva, M.; Van Damme, E.J.M. 130 years of Plant Lectin Research. Glycoconj. J. 2020, 37, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Polito, L. Ribosome-inactivating proteins from plants: A historical overview. Molecules 2016, 21, 1627. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Zeng, R. Insect Response to Plant Defensive Protease Inhibitors. Annu. Rev. Entomol. 2015, 60, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Souza Cândido, E.; Pinto, M.F.S.; Pelegrini, P.B.; Lima, T.B.; Silva, O.N.; Pogue, R.; Grossi-de-Sá, M.F.; Franco, O.L. Plant storage proteins with antimicrobial activity: Novel insights into plant defense mechanisms. FASEB J. 2011, 25, 3290–3305. [Google Scholar] [CrossRef] [PubMed]
- Sales, M.P.; Gerhardt, I.R.; Grossi-de-Sa, M.F.; Xavier-Filho, J. Do legume storage proteins play a role in defending seeds against Bruchids? Plant Physiol. 2000, 124, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Vriens, K.; Cammue, B.P.A.; Thevissen, K. Antifungal plant defensins: Mechanisms of action and production. Molecules 2014, 19, 12280–12303. [Google Scholar] [CrossRef]
- Carvalho, A.d.O.; Gomes, V.M. Role of plant lipid transfer proteins in plant cell physiology—A concise review. Peptides 2007, 28, 1144–1153. [Google Scholar] [CrossRef]
- Grover, A. Plant Chitinases: Genetic Diversity and Physiological Roles. CRC. Crit. Rev. Plant Sci. 2012, 31, 57–73. [Google Scholar] [CrossRef]
- Breitenbach Barroso Coelho, L.C.; Marcelino dos Santos Silva, P.; Felix de Oliveira, W.; de Moura, M.C.; Viana Pontual, E.; Soares Gomes, F.; Guedes Paiva, P.M.; Napoleão, T.H.; dos Santos Correia, M.T. Lectins as antimicrobial agents. J. Appl. Microbiol. 2018, 125, 1238–1252. [Google Scholar] [CrossRef]
- Stec, B. Plant thionins—The structural perspective. Cell. Mol. Life Sci. 2006, 63, 1370–1385. [Google Scholar] [CrossRef]
- Khan, H.; Mubarak, M.S.; Amin, S. Antifungal Potential of Alkaloids as an Emerging Therapeutic Target. Curr. Drug Targets 2016, 18. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; Van Damme, E.J.M. Toxic proteins in plants. Phytochemistry 2015, 117, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Carlini, C.R.; Grossi-De-Sá, M.F. Plant toxic proteins with insecticidal properties. A review on their potentialities as bioinsecticides. Toxicon 2002, 40, 1515–1539. [Google Scholar] [CrossRef]
- Carlini, C.R.; Ligabue-Braun, R. Ureases as multifunctional toxic proteins: A review. Toxicon 2016, 110, 90–109. [Google Scholar] [CrossRef]
- Carlini, C.R.; Polacco, J.C. Toxic properties of urease. Crop Sci. 2008, 48, 1665–1672. [Google Scholar] [CrossRef]
- Stanisçuaski, F.; Carlini, C.R. Plant ureases and related peptides: Understanding their entomotoxic properties. Toxins 2012, 4, 55–67. [Google Scholar] [CrossRef]
- Kappaun, K.; Piovesan, A.R.; Carlini, C.R.; Ligabue-Braun, R. Ureases: Historical aspects, catalytic, and non-catalytic properties—A review. J. Adv. Res. 2018, 13, 3–17. [Google Scholar] [CrossRef]
- Ligabue-Braun, R.; Carlini, C.R. Moonlighting Toxins: Ureases and Beyond. In Plant Toxins; Springer Netherlands: Dordrecht, The Netherlands, 2015; pp. 1–21. [Google Scholar] [CrossRef]
- Mazzei, L.; Musiani, F.; Ciurli, S. Urease. In RSC Metallobiology series “The Biological Chemistry of Nickel”; The Royal Society of Chemistry: London, UK, 2017; ISBN 1555812139. [Google Scholar] [CrossRef]
- Mobley, H.L.T.; Island, M.D.; Hausinger, R.P. Molecular biology of microbial ureases. Microbiol. Rev. 1995, 59, 451–480. [Google Scholar] [CrossRef]
- Carlini, C.R.; Guimarães, J.A. Isolation and characterization of a toxic protein from Canavalia ensiformis (jack bean) seeds, distinct from concanavalin A. Toxicon 1981, 19, 667–675. [Google Scholar] [CrossRef]
- Follmer, C.; Barcellos, G.B.S.; Zingali, R.B.; Machado, O.L.T.; Alves, E.W.; Barja-Fidalgo, C.; Guimarães, J.A.; Carlini, C.R. Canatoxin, a toxic protein from jack beans (Canavalia ensiformis), is a variant form of urease (EC 3.5.1.5): Biological effects of urease independent of its ureolytic activity. Biochem. J. 2001, 360, 217–224. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Durairajpandian, V.; Elumalai, S.; Mathivanan, N.; Munirajan, A.K.; Ponnuraj, K. Structural and functional studies on urease from pigeon pea (Cajanus cajan). Int. J. Biol. Macromol. 2013, 58, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Follmer, C.; Real-Guerra, R.; Wasserman, G.E.; Olivera-Severo, D.; Carlini, C.R. Jackbean, soybean and Bacillus pasteurii ureases: Biological effects unrelated to ureolytic activity. Eur. J. Biochem. 2004, 271, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Real-Guerra, R.; Staniscuaski, F.; Carlini, C.R. Soybean Urease: Over a Hundred Years of Knowledge. In A Comprehensive Survey of International Soybean Research—Genetics, Physiology, Agronomy and Nitrogen Relationships; Board, J.E., Ed.; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Becker-Ritt, A.B.; Martinelli, A.H.S.; Mitidieri, S.; Feder, V.; Wassermann, G.E.; Santi, L.; Vainstein, M.H.; Oliveira, J.T.A.; Fiuza, L.M.; Pasquali, G.; et al. Antifungal activity of plant and bacterial ureases. Toxicon 2007, 50, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, A.H.S.; Lopes, F.C.; Broll, V.; Defferrari, M.S.; Ligabue-Braun, R.; Kappaun, K.; Tichota, D.M.; Fruttero, L.L.; Moyetta, N.R.; Demartini, D.R.; et al. Soybean ubiquitous urease with purification facilitator: An addition to the moonlighting studies toolbox. Process Biochem. 2017, 53, 245–258. [Google Scholar] [CrossRef]
- Follmer, C. Insights into the role and structure of plant ureases. Phytochemistry 2008, 69, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Carlini, C.R.; Oliveira, A.E.; Azambuja, P.; Xavier-Filho, J.; Wells, M.A. Biological effects of canatoxin in different insect models: Evidence for a proteolytic activation of the toxin by insect cathepsinlike enzymes. J. Econ. Entomol. 1997, 90, 340–348. [Google Scholar] [CrossRef]
- Oliveira, A.E.A.; Gomes, V.M.; Sales, M.P.; Fernandes, K.V.S.; Carlini, C.R.; Xavier-Filho, J. The toxicity of jack bean [Canavalia ensiformis (L.) DC.] canatoxin to plant pathogenic fungi. Rev. Bras. Biol. 1999, 59, 59–62. [Google Scholar] [CrossRef]
- Ferreira-DaSilva, C.T.; Gombarovits, M.E.C.; Masuda, H.; Oliveira, C.M.; Carlini, C.R. Proteolytic activation of canatoxin, a plant toxic protein, by insect cathepsin-like enzymes. Arch. Insect Biochem. Physiol. 2000, 44, 162–171. [Google Scholar] [CrossRef]
- Polgár, L.; Csoma, C. Dissociation of ionizing groups in the binding cleft inversely controls the endo- and exopeptidase activities of cathepsin B. J. Biol. Chem. 1987, 262, 14448–14453. [Google Scholar]
- Turk, D.; Gunčar, G.; Podobnik, M.; Turk, B. Revised definition of substrate binding sites of papain-like cysteine proteases. Biol. Chem. 1998, 379, 137–147. [Google Scholar] [CrossRef]
- Sun, H.; Lou, X.; Shan, Q.; Zhang, J.; Zhu, X.; Zhang, J.; Wang, Y.; Xie, Y.; Xu, N.; Liu, S. Proteolytic Characteristics of Cathepsin D Related to the Recognition and Cleavage of Its Target Proteins. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Piovesan, A.R.; Stanisçuaski, F.; Marco-Salvadori, J.; Real-Guerra, R.; Defferrari, M.S.; Carlini, C.R. Stage-specific gut proteinases of the cotton stainer bug Dysdercus peruvianus: Role in the release of entomotoxic peptides from Canavalia ensiformis urease. Insect Biochem. Mol. Biol. 2008, 38, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Mulinari, F.; Stanisçuaski, F.; Bertholdo-Vargas, L.R.; Postal, M.; Oliveira-Neto, O.B.; Rigden, D.J.; Grossi-de-Sá, M.F.; Carlini, C.R. Jaburetox-2Ec: An insecticidal peptide derived from an isoform of urease from the plant Canavalia ensiformis. Peptides 2007, 28, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, A.H.S.; Kappaun, K.; Ligabue-Braun, R.; Defferrari, M.S.; Piovesan, A.R.; Stanisçuaski, F.; Demartini, D.R.; Dal Belo, C.A.; Almeida, C.G.M.; Follmer, C.; et al. Structure-function studies on jaburetox, a recombinant insecticidal peptide derived from jack bean (Canavalia ensiformis) urease. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 935–944. [Google Scholar] [CrossRef]
- Gombarovits, M. Peptídeos entomotóxicos gerados a partir da CNTX: Obtenção, isolamento, propriedades biológicas e caracterização físico-química. Ph.D. Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 1999. [Google Scholar]
- Becker-Ritt, A.B.; Portugal, C.S.; Carlini, C.R. Jaburetox: Update on a urease-derived peptide. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Postal, M.; Martinelli, A.H.S.; Becker-Ritt, A.B.; Ligabue-Braun, R.; Demartini, D.R.; Ribeiro, S.F.F.; Pasquali, G.; Gomes, V.M.; Carlini, C.R. Antifungal properties of Canavalia ensiformis urease and derived peptides. Peptides 2012, 38, 22–32. [Google Scholar] [CrossRef]
- Broll, V.; Martinelli, A.H.S.; Lopes, F.C.; Fruttero, L.L.; Zambelli, B.; Salladini, E.; Dobrovolska, O.; Ciurli, S.; Carlini, C.R. Structural analysis of the interaction between Jaburetox, an intrinsically disordered protein, and membrane models. Colloids Surfaces B Biointerfaces 2017, 159, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Wiebke-Strohm, B.; Pasquali, G.; Margis-Pinheiro, M.; Bencke, M.; Bücker-Neto, L.; Becker-Ritt, A.B.; Martinelli, A.H.S.; Rechenmacher, C.; Polacco, J.C.; Stolf, R.; et al. Ubiquitous urease affects soybean susceptibility to fungi. Plant Mol. Biol. 2012, 79, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Kappaun, K.; Martinelli, A.H.S.; Broll, V.; Zambelli, B.; Lopes, F.C.; Ligabue-Braun, R.; Fruttero, L.L.; Moyetta, N.R.; Bonan, C.D.; Carlini, C.R.; et al. Soyuretox, an intrinsically disordered polypeptide derived from soybean (Glycine max) ubiquitous urease with potential use as a biopesticide. Int. J. Mol. Sci. 2019, 20, 5401. [Google Scholar] [CrossRef]
- Sá, C.A.; Vieira, L.R.; Pereira Almeida Filho, L.C.; Real-Guerra, R.; Lopes, F.C.; Souza, T.M.; Vasconcelos, I.M.; Staniscuaski, F.; Carlini, C.R.; Urano Carvalho, A.F.; et al. Risk assessment of the antifungal and insecticidal peptide Jaburetox and its parental protein the Jack bean (Canavalia ensiformis) urease. Food Chem. Toxicol. 2020, 136, 110977. [Google Scholar] [CrossRef]
- Barros, P.R.; Stassen, H.; Freitas, M.S.; Carlini, C.R.; Nascimento, M.A.C.; Follmer, C. Membrane-disruptive properties of the bioinsecticide Jaburetox-2Ec: Implications to the mechanism of the action of insecticidal peptides derived from ureases. Biochim. Biophys. Acta Proteins Proteomics 2009, 1794, 1848–1854. [Google Scholar] [CrossRef]
- Micheletto, Y.M.S.; Moro, C.F.; Lopes, F.C.; Ligabue-Braun, R.; Martinelli, A.H.S.; Marques, C.M.; Schroder, A.P.; Carlini, C.R.; da Silveira, N.P. Interaction of jack bean (Canavalia ensiformis) urease and a derived peptide with lipid vesicles. Colloids Surfaces B Biointerfaces 2016, 145, 576–585. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Ponnuraj, K. Crystal Structure of the First Plant Urease from Jack Bean: 83 Years of Journey from Its First Crystal to Molecular Structure. J. Mol. Biol. 2010, 400, 274–283. [Google Scholar] [CrossRef]
- Lopes, F.C.; Dobrovolska, O.; Real-Guerra, R.; Broll, V.; Zambelli, B.; Musiani, F.; Uversky, V.N.; Carlini, C.R.; Ciurli, S. Pliable natural biocide: Jaburetox is an intrinsically disordered insecticidal and fungicidal polypeptide derived from jack bean urease. FEBS J. 2015, 282, 1043–1064. [Google Scholar] [CrossRef]
- Portugal, C.S. Avaliação dos efeitos do peptídeo recombinante Jaburetox em linhagens celulares e Drosophila melanogaster. Ph.D. Thesis, Universidade Luterana do Brasil, Canoas, Brazil, 2017. [Google Scholar]
- Sirko, A.; Brodzik, R. Plant ureases: Roles and regulation. Acta Biochim. Pol. 2000, 47, 1189–1195. [Google Scholar] [CrossRef]
- Kumar, V.; Wagenet, R.J. Urease activity and kinetics of urea transformation in soils. Soil Sci. 1984, 137, 263–269. [Google Scholar] [CrossRef]
- Sparks, T.C.; Crossthwaite, A.J.; Nauen, R.; Banba, S.; Cordova, D.; Earley, F.; Ebbinghaus-Kintscher, U.; Fujioka, S.; Hirao, A.; Karmon, D.; et al. Insecticides, biologics and nematicides: Updates to IRAC’s mode of action classification—A tool for resistance management. Pestic. Biochem. Physiol. 2020, 167, 104587. [Google Scholar] [CrossRef]
- Mall, D.; Larsen, A.E.; Martin, E.A. Investigating the (Mis)match between natural pest control knowledge and the intensity of pesticide use. Insects 2018, 9, 2. [Google Scholar] [CrossRef]
- Hardy, M.C. Resistance is not futile: It shapes insecticide discovery. Insects 2014, 5, 227–242. [Google Scholar] [CrossRef]
- Piovesan, A.R.; Martinelli, A.H.S.; Ligabue-Braun, R.; Schwartz, J.L.; Carlini, C.R. Canavalia ensiformis urease, Jaburetox and derived peptides form ion channels in planar lipid bilayers. Arch. Biochem. Biophys. 2014, 547, 6–17. [Google Scholar] [CrossRef]
- Moyetta, N.R.; Broll, V.; Perin, A.P.A.; Uberti, A.F.; Coste Grahl, M.V.; Staniscuaski, F.; Carlini, C.R.; Fruttero, L.L. Jaburetox-induced toxic effects on the hemocytes of Rhodnius prolixus (Hemiptera: Reduviidae). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 200, 17–26. [Google Scholar] [CrossRef]
- Fruttero, L.L.; Moyetta, N.R.; Krug, M.S.; Broll, V.; Grahl, M.V.C.; Real-Guerra, R.; Stanisçuaski, F.; Carlini, C.R. Jaburetox affects gene expression and enzyme activities in Rhodnius prolixus, a Chagas’ disease vector. Acta Trop. 2017, 168, 54–63. [Google Scholar] [CrossRef]
- Wallmann, A.; Kesten, C. Common functions of disordered proteins across evolutionary distant organisms. Int. J. Mol. Sci. 2020, 21, 2105. [Google Scholar] [CrossRef]
- Tomazetto, G.; Mulinari, F.; Stanisçuaski, F.; Settembrini, B.; Carlini, C.R.; Záchia Ayub, M.A. Expression kinetics and plasmid stability of recombinant E. coli encoding urease-derived peptide with bioinsecticide activity. Enzyme Microb. Technol. 2007, 41, 821–827. [Google Scholar] [CrossRef]
- Stanisçuaski, F.; Te Brugge, V.; Carlini, C.R.; Orchard, I. In vitro effect of Canavalia ensiformis urease and the derived peptide Jaburetox-2Ec on Rhodnius prolixus Malpighian tubules. J. Insect Physiol. 2009, 55, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Fruttero, L.L.; Moyetta, N.R.; Uberti, A.F.; Grahl, M.V.C.; Lopes, F.C.; Broll, V.; Feder, D.; Carlini, C.R. Humoral and cellular immune responses induced by the urease-derived peptide Jaburetox in the model organism Rhodnius prolixus. Parasit. Vectors 2016, 9, 1–14. [Google Scholar] [CrossRef]
- Coste Grahl, M.V.; Perin, A.P.A.; Lopes, F.C.; Porto, B.N.; Uberti, A.F.; Canavoso, L.E.; Stanisçuaski, F.; Fruttero, L.L. The role of extracellular nucleic acids in the immune system modulation of Rhodnius prolixus (Hemiptera: Reduviidae). Pestic. Biochem. Physiol. 2020, 167, 104591. [Google Scholar] [CrossRef]
- Galvani, G.L.; Fruttero, L.L.; Coronel, M.F.; Nowicki, S.; Demartini, D.R.; Defferrari, M.S.; Postal, M.; Canavoso, L.E.; Carlini, C.R.; Settembrini, B.P. Effect of the urease-derived peptide Jaburetox on the central nervous system of Triatoma infestans (Insecta: Heteroptera). Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 255–262. [Google Scholar] [CrossRef]
- Dos Santos, D.S.; Zanatta, A.P.; Martinelli, A.H.S.; Rosa, M.E.; de Oliveira, R.S.; Pinto, P.M.; Peigneur, S.; Tytgat, J.; Orchard, I.; Lange, A.B.; et al. Jaburetox, a natural insecticide derived from Jack Bean Urease, activates voltage-gated sodium channels to modulate insect behavior. Pestic. Biochem. Physiol. 2019, 153, 67–76. [Google Scholar] [CrossRef]
- Perin, A.P.A.; Noronha, M.S.; Moyetta, N.R.; Coste Grahl, M.V.; Fruttero, L.L.; Staniscuaski, F. Jaburetox, a urease-derived peptide: Effects on enzymatic pathways of the cockroach Nauphoeta cinerea. Arch. Insect Biochem. Physiol. 2020, 105, e21731. [Google Scholar] [CrossRef]
- Didoné, D.A. Development of Jaburetox-expressing maize plants for resistance to pest lepidopterans. Ph.D. Thesis, Universidade de Passo Fundo, Passo Fundo, Brazil, 2018. [Google Scholar]
- Ceccon, C.C. Transgenic tobacco plants expressing hairpin and jaburetox RNA as strategies for Helicoverpa armigera control. Ph.D. Thesis, Universidade de Passo Fundo, Passo Fundo, Brazil, 2019. [Google Scholar]
- Chattopadhyay, P.; Banerjee, G. Recent advancement on chemical arsenal of Bt toxin and its application in pest management system in agricultural field. 3 Biotech. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mio, T.; Yabe, T.; Arisawa, M.; Yamada-Okabe, H. The Eukaryotic UDP-N-Acetylglucosamine Pyrophosphorylases: Gene Cloning, Protein Expression, and Catalytic Mechanism. J. Biol. Chem. 1998, 273, 14392–14397. [Google Scholar] [CrossRef] [PubMed]
- Barreto, Y.C.; Rosa, M.E.; Zanatta, A.P.; Borges, B.T.; Hyslop, S.; Vinadé, L.H.; Dal Belo, C.A. Entomotoxicity of jaburetox: Revisiting the neurotoxic mechanisms in insects. J. Venom Res. 2020, 10, 1–15. [Google Scholar]
- Radi, R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. USA 2004, 101, 4003–4008. [Google Scholar] [CrossRef]
- Azambuja, P.; Garcia, E.S.; Waniek, P.J.; Vieira, C.S.; Figueiredo, M.B.; Gonzalez, M.S.; Mello, C.B.; Castro, D.P.; Ratcliffe, N.A. Rhodnius prolixus: From physiology by Wigglesworth to recent studies of immune system modulation by Trypanosoma cruzi and Trypanosoma rangeli. J. Insect Physiol. 2017, 97, 45–65. [Google Scholar] [CrossRef]
- Hillyer, J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016, 58, 102–118. [Google Scholar] [CrossRef]
- Defferrari, M.S.; Da Silva, R.; Orchard, I.; Carlini, C.R. Jack bean (Canavalia ensiformis) urease induces eicosanoid-modulated hemocyte aggregation in the Chagas’ disease vector Rhodnius prolixus. Toxicon 2014, 82, 18–25. [Google Scholar] [CrossRef][Green Version]
- Nascimento, M.T.C.C.; Silva, K.P.; Garcia, M.C.F.F.; Medeiros, M.N.; Machado, E.A.; Nascimento, S.B.; Saraiva, E.M. DNA extracellular traps are part of the immune repertoire of Periplaneta americana. Dev. Comp. Immunol. 2018, 84, 62–70. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Uversky, V.N.; Gillespie, J.R.; Fink, A.L. Why are “natively unfolded” proteins unstructured under physiologic conditions? Proteins Struct. Funct. Bioinform. 2000, 41, 415–427. [Google Scholar] [CrossRef]
- Dunker, A.K.; Lawson, J.D.; Brown, C.J.; Williams, R.M.; Romero, P.; Oh, J.S.; Oldfield, C.J.; Campen, A.M.; Ratliff, C.M.; Hipps, K.W.; et al. Intrinsically disordered protein. J. Mol. Graph. Model. 2001, 19, 26–59. [Google Scholar] [CrossRef]
- Tompa, P. Intrinsically unstructured proteins. Trends Biochem. Sci. 2002, 27, 527–533. [Google Scholar] [CrossRef]
- Wright, P.E.; Dyson, H.J. Intrinsically unstructured proteins: Re-assessing the protein structure-function paradigm. J. Mol. Biol. 1999, 293, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.C.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q.Y. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2015, 40, 133–159. [Google Scholar] [CrossRef]
- White, D.C.; Frerman, F.E. Extraction, characterization, and cellular localization of the lipids of Staphylococcus aureus. J. Bacteriol. 1967, 94, 1854–1867. [Google Scholar] [CrossRef]
- Löffler, J.; Einsele, H.; Hebart, H.; Schumacher, U.; Hrastnik, C.; Daum, G. Phospholipid and sterol analysis of plasma membranes of azole-resistant Candida albicans strains. FEMS Microbiol. Lett. 2000, 185, 59–63. [Google Scholar] [CrossRef]
- Narayani, M.; Babu, R.; Chadha, A.; Srivastava, S. Production of bioactive cyclotides: A comprehensive overview. Phytochem. Rev. 2020, 19, 787–825. [Google Scholar] [CrossRef]
- Huang, Y.H.; Colgrave, M.L.; Daly, N.L.; Keleshian, A.; Martinac, B.; Craik, D.J. The biological activity of the prototypic cyclotide Kalata B1 is modulated by the formation of multimeric pores. J. Biol. Chem. 2009, 284, 20699–20707. [Google Scholar] [CrossRef]
- Saether, O.; Craik, D.J.; Campbell, I.D.; Sletten, K.; Juul, J.; Norman, D.G. Elucidation of the Primary and Three-Dimensional Structure of the Uterotonic Polypeptide Kalata B1. Biochemistry 1995, 34, 4147–4158. [Google Scholar] [CrossRef] [PubMed]
- Plan, M.R.R.; Göransson, U.; Clark, R.J.; Daly, N.L.; Colgrave, M.L.; Craik, D.J. The cyclotide fingerprint in Oldenlandia affinis: Elucidation of chemically modified, linear and novel macrocyclic peptides. ChemBioChem 2007, 8, 1001–1011. [Google Scholar] [CrossRef]
- Daly, N.L.; Gunasekera, S.; Clark, R.J.; Lin, F.; Wade, J.D.; Anderson, M.A.; Craik, D.J. The N-terminal pro-domain of the kalata B1 cyclotide precursor is intrinsically unstructured. Biopolymers 2016, 106, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Marín, M.; Ott, T. Intrinsic disorder in plant proteins and phytopathogenic bacterial effectors. Chem. Rev. 2014, 114, 6912–6932. [Google Scholar] [CrossRef]
- Oppenheim, F.G.; Xu, T.; McMillian, F.M.; Levitz, S.M.; Diamond, R.D.; Offner, G.D.; Troxler, R.F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 1988, 263, 7472–7477. [Google Scholar]
- Jephthah, S.; Staby, L.; Kragelund, B.B.; Skepö, M. Temperature Dependence of Intrinsically Disordered Proteins in Simulations: What are We Missing? J. Chem. Theory Comput. 2019, 15, 2672–2683. [Google Scholar] [CrossRef]
- Tsai, H.; Raj, P.A.; Bobek, L.A. Candidacidal activity of recombinant human salivary histatin-5 and variants. Infect. Immun. 1996, 64, 5000–5007. [Google Scholar] [CrossRef]
- Puri, S.; Edgerton, M. How does it kill?: Understanding the candidacidal mechanism of salivary histatin 5. Eukaryot. Cell 2014, 13, 958–964. [Google Scholar] [CrossRef]
- Melino, S.; Santone, C.; Di Nardo, P.; Sarkar, B. Histatins: Salivary peptides with copper (II)-and zinc (II)-binding motifs: Perspectives for biomedical applications. FEBS J. 2014, 281, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Meyer-Hoffert, U.; Reithmayer, K.; Paus, R.; Hansmann, B.; He, Y.; Bartels, J.; Gläser, R.; Harder, J.; Schröder, J.M. Highly complex peptide aggregates of the S100 fused-type protein hornerin are present in human skin. J. Investig. Dermatol. 2009, 129, 1446–1458. [Google Scholar] [CrossRef]
- Latendorf, T.; Gerstel, U.; Wu, Z.; Bartels, J.; Becker, A.; Tholey, A.; Schröder, J.M. Cationic Intrinsically Disordered Antimicrobial Peptides (CIDAMPs) Represent a New Paradigm of Innate Defense with a Potential for Novel Anti-Infectives. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gerstel, U.; Latendorf, T.; Bartels, J.; Becker, A.; Tholey, A.; Schröder, J.M. Hornerin contains a Linked Series of Ribosome-Targeting Peptide Antibiotics. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tyczewska, A.; Woźniak, E.; Gracz, J.; Kuczyński, J.; Twardowski, T. Towards Food Security: Current State and Future Prospects of Agrobiotechnology. Trends Biotechnol. 2018, 36, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Brévault, T.; Carrière, Y. Insect resistance to Bt crops: Lessons from the first billion acres. Nat. Biotechnol. 2013, 31, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Rechenmacher, C.; Wiebke-Strohm, B.; de Oliveira-Busatto, L.A.; Weber, R.L.M.; Corso, M.C.M.; Lopes-Caitar, V.S.; Silva, S.M.H.; Dias, W.P.; Marcelino-Guimarães, F.C.; Carlini, C.R.; et al. Endogenous soybean peptide overexpression: An alternative to protect plants against root-knot nematodes. Biotechnol. Res. Innov. 2019, 3, 10–18. [Google Scholar] [CrossRef]
- Biłas, R.; Szafran, K.; Hnatuszko-Konka, K.; Kononowicz, A.K. Cis-regulatory elements used to control gene expression in plants. Plant Cell. Tissue Organ Cult. 2016, 127, 269–287. [Google Scholar] [CrossRef]
- Muthusamy, S.K.; Sivalingam, P.N.; Sridhar, J.; Singh, D.; Haldhar, S.M. Biotic stress inducible promoters in crop plants-a review. J. Agric. Ecol. 2017, 4, 14–24. [Google Scholar]

| Approach | Peptide(s) | Data Obtained | Reference |
|---|---|---|---|
| Ab initio modeling | Jaburetox | A β-hairpin motif was observed in the C-terminal region of the molecule. It was hypothesized that the β-hairpin motif could be involved in the entomotoxic activity. | [57,66] |
| Molecular dynamics simulation (in aqueous system for 500 ns) | Jaburetox, its N- and C-termini peptides and Soyuretox | Jaburetox became more unstructured in its N-terminal portion, containing a few secondary structural elements and the major part of molecule in random coil. The β-hairpin structure was conserved in the C-terminal domain. The N-terminal peptide became totally unfolded and C-terminal showed a stabilization with β-sheet structures. Soyuretox became more globular in solution and showed changes in its secondary structure, with loss of helices and beta strands. | [58,64] |
| Dynamic Light Scattering and Small Angle X-ray Scattering Dynamic Light Scattering | Jaburetox Jaburetox | Demonstrated the ability of Jaburetox to interact with lipids using platelet-like multilamellar liposomes (PML). Jaburetox in a neutral solution is found in a single oligomeric form, exhibiting a large hydrodynamic radius, suggestive of a disordered polypeptide. | [67] [69] |
| Circular dichroism (CD) spectroscopy | Jaburetox and Soyuretox | Jaburetox showed a typical random coil conformation and small amount of secondary structure under native state. Jaburetox increased its secondary structure content when in contact with SDS-micelles and large unilamellar vesicles (LUVs) composed by phospholipids of different net charges. Jaburetox and Soyuretox showed disordered behavior at pH 6.5. Soyuretox acquired some secondary structure at pH 8. | [69] [62] [64] |
| Nuclear Magnetic Resonance (NMR) spectroscopy | Jaburetox Soyuretox | The heteronuclear single quantum coherence (HSQC) spectrum unveiled low signal dispersion in the proton dimension; the SSP analysis of chemical shifts predicted that Jaburetox is widely disordered with a small tendency to form α-structures. The 3D structure obtained from nuclear Overhauser enhancement (NOE) do not demonstrated the presence of a stable tertiary structure. The HSQC NMR spectrum obtained for Soyuretox showed a low signal dispersion in the proton dimension. A more ordered structure of Soyuretox in the presence of SDS micelles (10 mM) was also confirmed in the peptide’s HSQC NMR spectrum, demonstrating a widening of signal dispersion. | [69] [64] |
| Species | Stage(s) | Assay | Toxic Peptide(s) | Effect(s) | Reference |
|---|---|---|---|---|---|
| Dysdercus peruvianus | Nymphs | Feeding | Jaburetox | Lethality | [57,80] |
| Nymphs | Feeding and injection | Soyuretox | Lethality | [64] | |
| Oncopeltus fasciatus | Nymphs | Injection | Jaburetox | Lethality | [58] |
| Nymphs | Injection, feeding | Jaburetox | Lethality | [58,80] | |
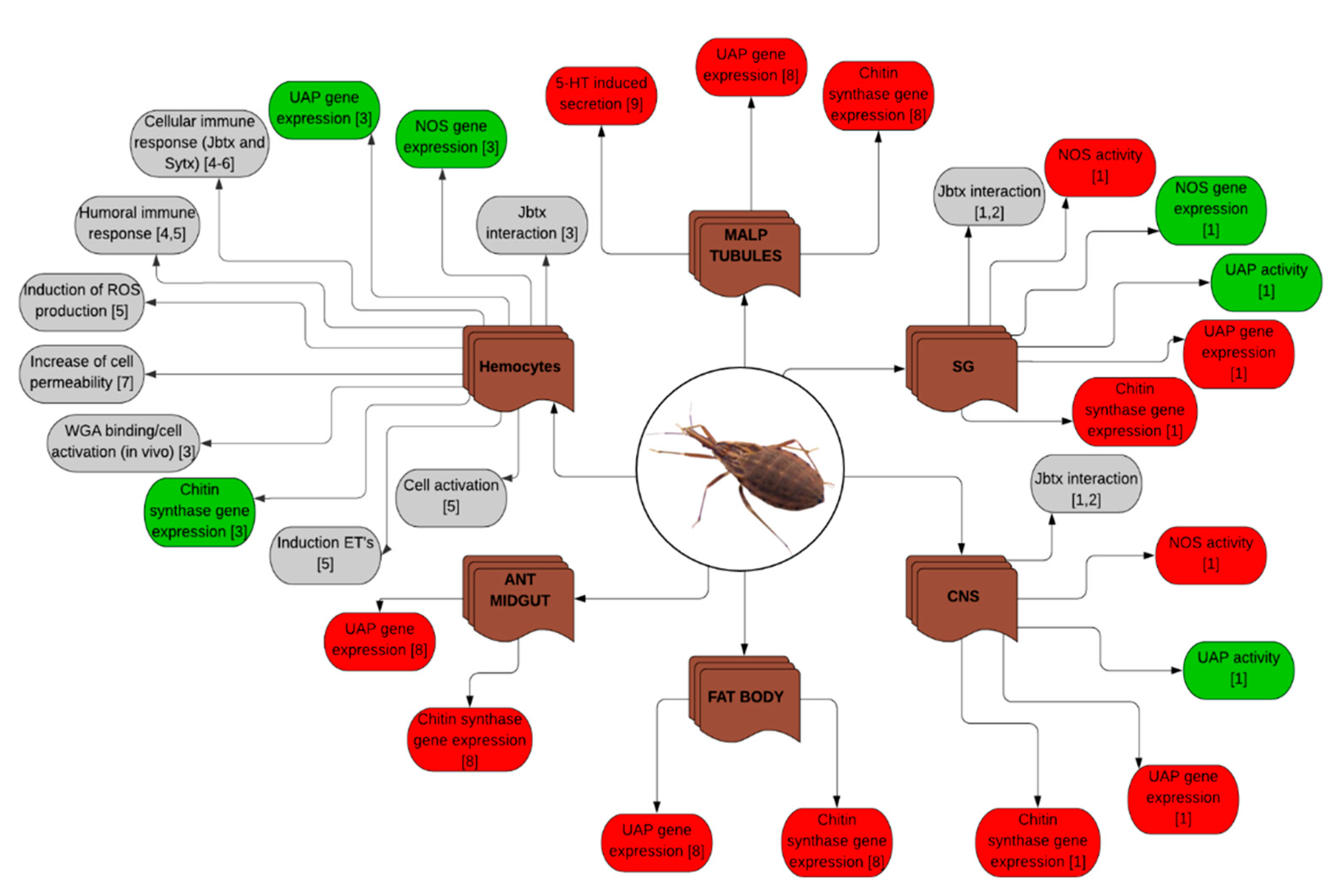
| Rhodnius prolixus | Nymphs and adults | Injection, feeding, in vitro | Jaburetox and Soyuretox | Effects on diuresis, enzymatic activities, expression of genes, cell activation and immune response, interaction of Jaburetox with the central nervous system and the salivary glands, among others (see Figure 3) | [58,64,77,78,81,82,83], unpublished results |
| Nymphs and adults | Injection | Jaburetox | Lethality | [80] | |
| Triatoma infestans | Adults | Injection | Jaburetox | Lethality, behavioral alterations, neurotoxicity, localization of the peptide in the central nervous system, interaction of the peptide with UDP-N-acetylglucosamine pyrophosphorylase (UAP), inhibition of nitric oxide synthase (NOS) activity | [84] |
| Phoetalia pallida | Adults | Injection | Jaburetox | Blockade of evoked contractions of coxal muscle | [58] |
| In vitro | Jaburetox | Interaction of the peptide with the central nervous system | [62] | ||
| Nauphoeta cinerea | Adults | Injection | Jaburetox | Alteration of locomotor behavior, leg and antennae grooming, neuromuscular blockade, cardiotoxicity and alterations in nerve and muscle electrophysiological profiles | [85] |
| Adults | Feeding, injection, in vitro | Jaburetox | Absence of lethality, modulation of NOS, UAP and acetylcholinesterase activity in the central nervous system | [86] | |
| Blatella germanica | Nymphs | Feeding | Jaburetox | Lethality | [57] |
| Larvae | Feeding | Jaburetox | Lethality and weight reduction | [57] | |
| Spodoptera frugiperda | Larvae | Feeding on transgenic corn plants | Jaburetox | Weight reduction, reduced feed consumption, sterility of females and lethality | [87] |
| Larvae | Feeding | Jaburetox | Lethality and delay in larval development | [87] | |
| Helicoverpa armigera | Larvae | Feeding on transgenic tobacco plants | Jaburetox | Lethality and reduced feed consumption | [88] |
| Aedes aegypti | Larvae | Feeding | Jaburetox | Lethality | [60] |
| IDP | Source | Biological Activity | Disorder Region | Reference |
|---|---|---|---|---|
| Kalata B1 | African plant Oldelandia affinis | Signaling, ability to bind and to form pores in membranes | N-terminal pro-domain | [111] |
| Histatin 5 | Mammalian saliva | Antifungal activity | No defined structure in solution | [116,117] |
| Hornerin | Human skin | Antibacterial activity | Almost all the protein is unstructured, except the N-terminus. Cationic peptides generated from hornerin present antimicrobial activity | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grahl, M.V.C.; Lopes, F.C.; Martinelli, A.H.S.; Carlini, C.R.; Fruttero, L.L. Structure-Function Insights of Jaburetox and Soyuretox: Novel Intrinsically Disordered Polypeptides Derived from Plant Ureases. Molecules 2020, 25, 5338. https://doi.org/10.3390/molecules25225338
Grahl MVC, Lopes FC, Martinelli AHS, Carlini CR, Fruttero LL. Structure-Function Insights of Jaburetox and Soyuretox: Novel Intrinsically Disordered Polypeptides Derived from Plant Ureases. Molecules. 2020; 25(22):5338. https://doi.org/10.3390/molecules25225338
Chicago/Turabian StyleGrahl, Matheus V. Coste, Fernanda Cortez Lopes, Anne H. Souza Martinelli, Celia R. Carlini, and Leonardo L. Fruttero. 2020. "Structure-Function Insights of Jaburetox and Soyuretox: Novel Intrinsically Disordered Polypeptides Derived from Plant Ureases" Molecules 25, no. 22: 5338. https://doi.org/10.3390/molecules25225338
APA StyleGrahl, M. V. C., Lopes, F. C., Martinelli, A. H. S., Carlini, C. R., & Fruttero, L. L. (2020). Structure-Function Insights of Jaburetox and Soyuretox: Novel Intrinsically Disordered Polypeptides Derived from Plant Ureases. Molecules, 25(22), 5338. https://doi.org/10.3390/molecules25225338