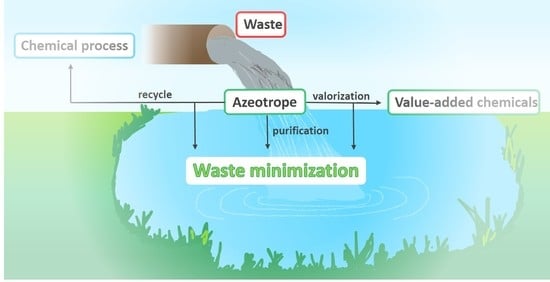
Azeotropes as Powerful Tool for Waste Minimization in Industry and Chemical Processes
Abstract
1. Introduction
- Reduction at the source: generally requiring an important process modification to reduce or eliminate the generation of waste and the use of hazardous materials.
- Recycling: consisting of contaminant removal from a useful fraction of waste to allow it to be reused.
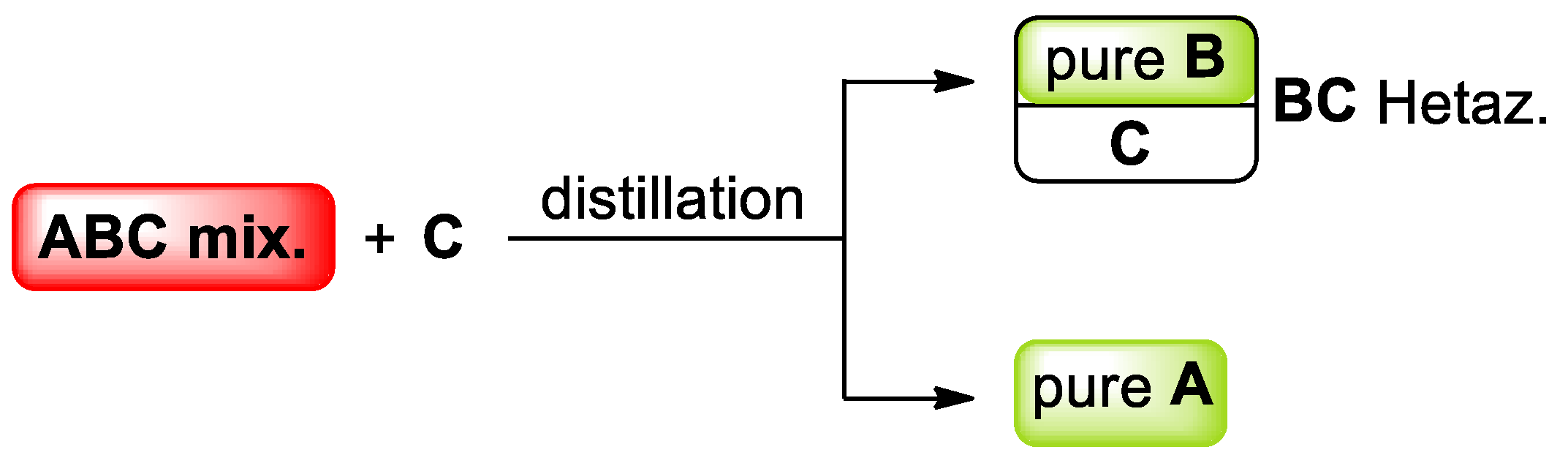
2. Azeotropes for Waste Recovery and Reuse
2.1. Homogeneous Azeotropic Distillation
2.2. Heterogeneous Azeotropic Distillation
2.3. Hybrid Extractive-Heterogeneous Azeotropic Distillation
- Group 1: acetone does not form any azeotropes with other components; from the economic point of view it is preferable to separate it with conventional distillation before proceeding through extractive heterogenous distillation to separate the remaining solvents by using additional water as auto-entrainer.
- Group 2: when a couple of solvents which do not form azeotropes, but their azeotropes are present with other components, extractive-heterogeneous azeotropic distillation with two subsequent conventional distillations is the preferred way to recover single components.
- Group 3: when a binary azeotrope is present between the components of the mixtures, the best way for solvent separation is two extractive-heterogeneous azeotropic distillations with subsequent conventional distillation.
2.4. Azeotropes and Membrane Technologies in Water-Containing Waste Treatment
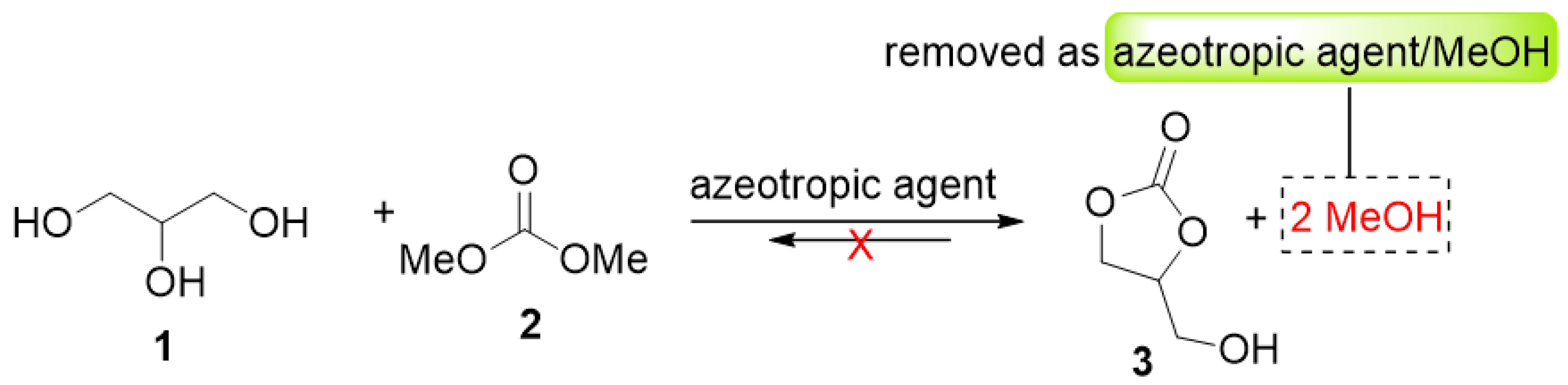
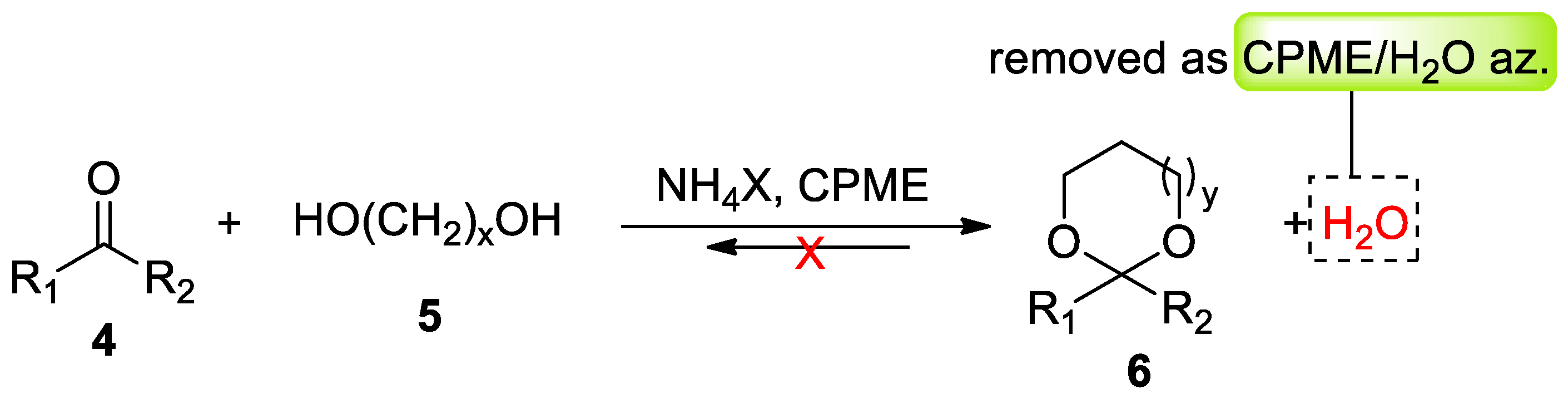
3. Azeotropes in Organic Synthesis for Waste Minimization
3.1. Azeotropes Steer Organic Synthesis
3.2. Azeotropes in Purification Process
3.3. Azeotropes Enable Waste Prevention
4. Azeotropes in Material Synthesis for Waste Valorization
5. Conclusions and Future Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brundtland, C.G. Our Common Features, The World Commission on Environmental Development; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Burnett, M.L. The Pollution Prevention Act of 1990: A Policy Whose Time Has Come or Symbolic Legislation? Environ. Manag. 1998, 22, 213–224. [Google Scholar] [CrossRef]
- Farr, J.R. A Hat, a Rapier, a Knife, and a Dagger. In A Tale of Two Murders; Duke University Press: Durham, NC, USA, 2005; pp. 40–53. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Report to Congress: Minimization of Hazardous Waste; EPA/530-SW-86-033; OSW and ER: Washington, DC, USA, 1986.
- Anastas, P.; Warner, J. Green Chemistry Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Constable, D.J.C.; Curzons, A.D.; Cunningham, V.L. Metrics to ‘green’ chemistry—Which are the best? Green Chem. 2002, 4, 521–527. [Google Scholar] [CrossRef]
- Curzons, A.D.; Mortimer, D.N.; Constable, D.J.C.; Cunningham, V.L. So you think your process is green, how do you know? Using principles of sustainability to determine what is green–a corporate perspective. Green Chem. 2001, 3, 1–6. [Google Scholar] [CrossRef]
- Sheldon, R.A. Organic Synthesis; Past, Present and Future. Chem. Ind. 1992, 903–906. [Google Scholar]
- Constable, D.J.C.; Curzons, A.D.; Dos Santos, L.M.F.; Geen, G.R.; Kitteringham, J.; Smith, P.; Hannah, R.; McGuire, M.A.; Webb, R.L.; Yu, M.; et al. Green Chemistry Measures for Process Research and Development. Green Chem. 2001, 3, 7–9. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, C.; Ponder, C.S.; Broxterman, Q.B.; Manley, J.B. Using the Right Green Yardstick: Why Process Mass Intensity Is Used in the Pharmaceutical Industry To Drive More Sustainable Processes. Org. Process. Res. Dev. 2011, 15, 912–917. [Google Scholar] [CrossRef]
- Curzons, A.; Constable, D.; Cunningham, V. Solvent selection guide: A guide to the integration of environmental, health and safety criteria into the selection of solvents. Clean Technol. Environ. Policy 1999, 1, 82–90. [Google Scholar] [CrossRef]
- Fien, G.-J.A.F.; Liu, Y.A. Heuristic Synthesis and Shortcut Design of Separation Processes Using Residue Curve Maps: A Review. Ind. Eng. Chem. Res. 1994, 33, 2505–2522. [Google Scholar] [CrossRef]
- Fink, J. Processes; Elsevier: Amsterdam, The Netherlands, 2016; pp. 185–223. [Google Scholar]
- Szanyi, A.; Mizsey, P.; Fonyo, Z. Novel hybrid separation processes for solvent recovery based on positioning the extractive heterogeneous-azeotropic distillation. Chem. Eng. Process. Process. Intensif. 2004, 43, 327–338. [Google Scholar] [CrossRef]
- Young, S. LXXIII.—The preparation of absolute alcohol from strong spirit. J. Chem. Soc., Trans. 1902, 81, 707. [Google Scholar] [CrossRef]
- Liu, H.-L. Waste Minimization At A Nitrocellulose Manufacturing Facility. Int. J. Environ. Stud. 2003, 60, 353–361. [Google Scholar] [CrossRef]
- Zhou, H.; Cai, Y.; You, F. Systems Design, Modeling, and Thermoeconomic Analysis of Azeotropic Distillation Processes for Organic Waste Treatment and Recovery in Nylon Plants. Ind. Eng. Chem. Res. 2018, 57, 9994–10010. [Google Scholar] [CrossRef]
- Wasylkiewicz, S.K.; Kobylka, L.C.; Castillo, F.J. Optimal design of complex azeotropic distillation columns. Chem. Eng. J. 2000, 79, 219–227. [Google Scholar] [CrossRef]
- Pham, H.N.; Doherty, M.F. Design and synthesis of heterogeneous azeotropic distillations—III. Column sequences. Chem. Eng. Sci. 1990, 45, 1845–1854. [Google Scholar] [CrossRef]
- Xu, W.; Diwekar, U.M. Environmentally Friendly Heterogeneous Azeotropic Distillation System Design: Integration of EBS Selection and IPS Recycling. Ind. Eng. Chem. Res. 2005, 44, 4061–4067. [Google Scholar] [CrossRef]
- Chien, I.-L.; Zeng, K.-L.; Chao, H.-Y.; Liu, J.H. Design and control of acetic acid dehydration system via heterogeneous azeotropic distillation. Chem. Eng. Sci. 2004, 59, 4547–4567. [Google Scholar] [CrossRef]
- Chien, I.-L.; Kuo, C.-L. Investigating the need of a pre-concentrator column for acetic acid dehydration system via heterogeneous azeotropic distillation. Chem. Eng. Sci. 2006, 61, 569–585. [Google Scholar] [CrossRef]
- Ooms, T.; Vreysen, S.; Van Baelen, G.; Gerbaud, V.; Rodríguez-Donis, I. Separation of ethyl acetate–isooctane mixture by heteroazeotropic batch distillation. Chem. Eng. Res. Des. 2014, 92, 995–1004. [Google Scholar] [CrossRef]
- Asprion, N.; Kaibel, G. Dividing wall columns: Fundamentals and recent advances. Chem. Eng. Process. Process. Intensif. 2010, 49, 139–146. [Google Scholar] [CrossRef]
- Chaniago, Y.D.; Harvianto, G.R.; Bahadori, A.; Lee, M. Enhanced recovery of PGME and PGMEA from waste photoresistor thinners by heterogeneous azeotropic dividing-wall column. Process. Saf. Environ. Prot. 2016, 103, 413–423. [Google Scholar] [CrossRef]
- Sun, L.; Chang, X.-W.; Qi, C.-X.; Li, Q.-S. Implementation of Ethanol Dehydration Using Dividing-Wall Heterogeneous Azeotropic Distillation Column. Sep. Sci. Technol. 2011, 46, 1365–1375. [Google Scholar] [CrossRef]
- Kiss, A.A.; Suszwalak, D.J.P.C. Enhanced bioethanol dehydration by extractive and azeotropic distillation in dividing-wall columns. Sep. Purif. Technol. 2012, 86, 70–78. [Google Scholar] [CrossRef]
- Le, Q.-K.; Halvorsen, I.J.; Pajalic, O.; Skogestad, S. Dividing wall columns for heterogeneous azeotropic distillation. Chem. Eng. Res. Des. 2015, 99, 111–119. [Google Scholar] [CrossRef]
- Wu, Y.C.; Lee, H.-Y.; Huang, H.-P.; Chien, I.-L. Energy-Saving Dividing-Wall Column Design and Control for Heterogeneous Azeotropic Distillation Systems. Ind. Eng. Chem. Res. 2014, 53, 1537–1552. [Google Scholar] [CrossRef]
- Benko, T.; Szanyi, A.; Mizsey, P.; Fonyo, Z. Environmental and economic comparison of waste solvent treatment options. Open Chem. 2006, 4, 92–110. [Google Scholar] [CrossRef]
- Goedkoop, M.; Spriensma, R. The Eco-indicator 99, a Damage Oriented Method for Life Cycle Assessment; Methodolgy Report; Pre Consultants: Amersfoort, The Netherlands, 2000. [Google Scholar]
- Toth, A.J.; Szilagyi, B.; Haaz, E.; Solti, S.; Nagy, T.; Szanyi, A.; Nagy, J.; Mizsey, P. Enhanced separation of maximum boiling azeotropic mixtures with extractive heterogeneous-azeotropic distillation. Chem. Eng. Res. Des. 2019, 147, 55–62. [Google Scholar] [CrossRef]
- Selim, A.; Toth, A.J.; Fozer, D.; Haaz, E.; Mizsey, P. Pervaporative desalination of concentrated brine solution employing crosslinked PVA/silicate nanoclay membranes. Chem. Eng. Res. Des. 2020, 155, 229–238. [Google Scholar] [CrossRef]
- Toth, A.J. Modelling and Optimisation of Multi-Stage Flash Distillation and Reverse Osmosis for Desalination of Saline Process Wastewater Sources. Membranes 2020, 10, 265. [Google Scholar] [CrossRef]
- Valentínyi, N.; Cséfalvay, E.; Mizsey, P. Modelling of pervaporation: Parameter estimation and model development. Chem. Eng. Res. Des. 2013, 91, 174–183. [Google Scholar] [CrossRef]
- Haáz, E.; Valentinyi, N.; Tarjani, A.J.; Fozer, D.; Andre, A.; Mohamed, S.A.K.; Rahimli, F.; Nagy, T.; Mizsey, P.; Deák, C.; et al. Platform Molecule Removal from Aqueous Mixture with Organophilic Pervaporation: Experiments and Modelling. Period. Polytech. Chem. Eng. 2018, 63, 138–146. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Galiano, F.; Figoli, A. Recent advances in pervaporation hollow fiber membranes for dehydration of organics. Chem. Eng. Res. Des. 2020, 164, 68–85. [Google Scholar] [CrossRef]
- Valentínyi, N.; Mizsey, P. Comparison of pervaporation models with simulation of hybrid separation processes. Period. Polytech. Chem. Eng. 2014, 58, 7–14. [Google Scholar] [CrossRef]
- Toth, A.J.; Gergely, F.; Mizsey, P. Physicochemical treatment of pharmaceutical process wastewater: Distillation and membrane processes. Period. Polytech. Chem. Eng. 2011, 55, 59. [Google Scholar] [CrossRef]
- Valentinyi, N.; Andre, A.; Haaz, E.; Fozer, D.; Toth, A.J.; Nagy, T.; Mizsey, P. Experimental investigation and modeling of the separation of ternary mixtures by hydrophilic pervaporation. Sep. Sci. Technol. 2019, 55, 601–617. [Google Scholar] [CrossRef]
- Szilágyi, B.; Trang, D.T.H.; Fózer, D.; Selim, A.; Haáz, E.; Toth, A.J. Modelling of Hybrid Method for VOC Removal from Process Wastewater: Distillation and Hydrophilic Pervaporation. Period. Polytech. Chem. Eng. 2020, 64, 364–370. [Google Scholar] [CrossRef]
- Toth, A.J. Comprehensive evaluation and comparison of advanced separation methods on the separation of ethyl acetate-ethanol-water highly non-ideal mixture. Sep. Purif. Technol. 2019, 224, 490–508. [Google Scholar] [CrossRef]
- Haaz, E.; Szilagyi, B.; Fozer, D.; Toth, A.J. Combining extractive heterogeneous-azeotropic distillation and hydrophilic pervaporation for enhanced separation of non-ideal ternary mixtures. Front. Chem. Sci. Eng. 2020, 14, 913–927. [Google Scholar] [CrossRef]
- Andre, A.; Nagy, T.; Toth, A.J.; Haaz, E.; Fozer, D.; Tarjani, J.A.; Mizsey, P. Distillation contra pervaporation: Comprehensive investigation of isobutanol-water separation. J. Clean. Prod. 2018, 187, 804–818. [Google Scholar] [CrossRef]
- Sarney, D.B.; Barnard, M.J.; Virto, M.; Vulfson, E.N. Enzymatic synthesis of sorbitan esters using a low-boiling-point azeotrope as a reaction solvent. Biotechnol. Bioeng. 1997, 54, 351–356. [Google Scholar] [CrossRef]
- Li, J.; Wang, T. Coupling reaction and azeotropic distillation for the synthesis of glycerol carbonate from glycerol and dimethyl carbonate. Chem. Eng. Process. Process. Intensif. 2010, 49, 530–535. [Google Scholar] [CrossRef]
- Jindapon, W.; Ruengyoo, S.; Kuchonthara, P.; Ngamcharussrivichai, C.; Vitidsant, T. Continuous production of fatty acid methyl esters and high-purity glycerol over a dolomite-derived extrudate catalyst in a countercurrent-flow trickle-bed reactor. Renew. Energy 2020, 157, 626–636. [Google Scholar] [CrossRef]
- Gérardy, R.; Debecker, D.P.; Estager, J.; Luis, P.; Monbaliu, J.-C.M. Continuous Flow Upgrading of Selected C2–C6 Platform Chemicals Derived from Biomass. Chem. Rev. 2020, 120, 7219–7347. [Google Scholar] [CrossRef] [PubMed]
- Azzena, U.; Carraro, M.; Mamuye, A.D.; Murgia, I.; Pisano, L.; Zedde, G. Cyclopentyl methyl ether–NH4X: A novel solvent/catalyst system for low impact acetalization reactions. Green Chem. 2015, 17, 3281–3284. [Google Scholar] [CrossRef]
- Mujahed, S.; Valentini, F.; Cohen, S.; Vaccaro, L.; Gelman, D. Polymer-Anchored Bifunctional Pincer Catalysts for Chemoselective Transfer Hydrogenation and Related Reactions. ChemSusChem 2019, 12, 4693–4699. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; Jolley, K.E.; Clarkson, G.J.; Wills, M. Direct Formation of Tethered Ru(II) Catalysts Using Arene Exchange. Org. Lett. 2013, 15, 5110–5113. [Google Scholar] [CrossRef] [PubMed]
- Zimbron, J.M.; Dauphinais, M.; Charette, A.B. Noyori–Ikariya catalyst supported on tetra-arylphosphonium salt for asymmetric transfer hydrogenation in water. Green Chem. 2015, 17, 3255–3259. [Google Scholar] [CrossRef]
- He, B.; Zheng, L.; Phansavath, P.; Ratovelomanana-Vidal, V. Rh III -Catalyzed Asymmetric Transfer Hydrogenation of α-Methoxy β-Ketoesters through DKR in Water: Toward a Greener Procedure. ChemSusChem 2019, 12, 3032–3036. [Google Scholar] [CrossRef]
- Soni, R.; Cheung, F.K.; Clarkson, G.C.; Martins, J.E.D.; Graham, M.A.; Wills, M. The importance of the N–H bond in Ru/TsDPEN complexes for asymmetric transfer hydrogenation of ketones and imines. Org. Biomol. Chem. 2011, 9, 3290–3294. [Google Scholar] [CrossRef]
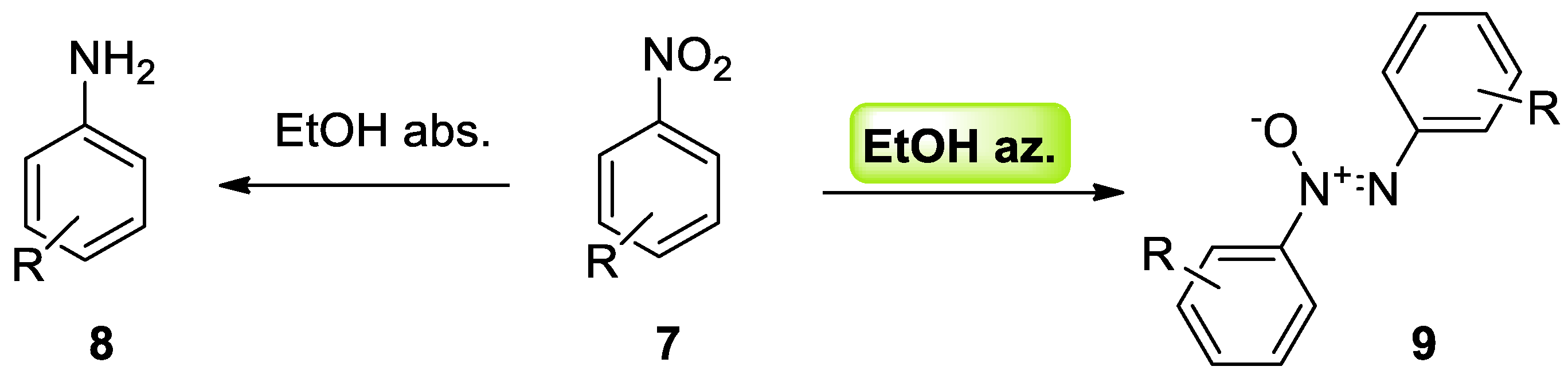
- Ferlin, F.; Cappelletti, M.; Vivani, R.; Pica, M.; Piermatti, O.; Vaccaro, L. Au@zirconium-phosphonate nanoparticles as an effective catalytic system for the chemoselective and switchable reduction of nitroarenes. Green Chem. 2019, 21, 614–626. [Google Scholar] [CrossRef]
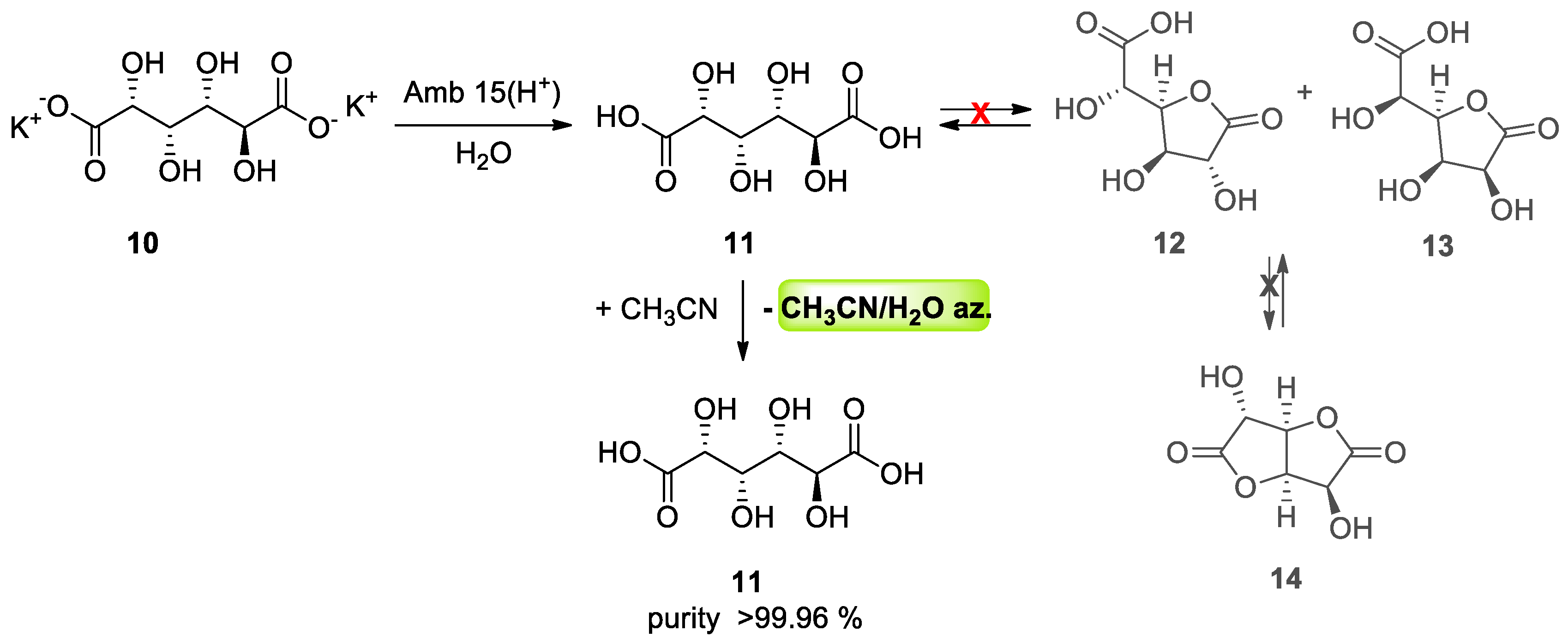
- Armstrong, R.D.; Kariuki, B.M.; Knight, D.W.; Hutchings, G.J. How to Synthesise High Purity, Crystalline d-Glucaric Acid Selectively. Eur. J. Org. Chem. 2017, 2017, 6811–6814. [Google Scholar] [CrossRef]
- Thaore, V.B.; Armstrong, R.D.; Hutchings, G.J.; Knight, D.W.; Chadwick, D.; Shah, N. Sustainable production of glucaric acid from corn stover via glucose oxidation: An assessment of homogeneous and heterogeneous catalytic oxidation production routes. Chem. Eng. Res. Des. 2020, 153, 337–349. [Google Scholar] [CrossRef]
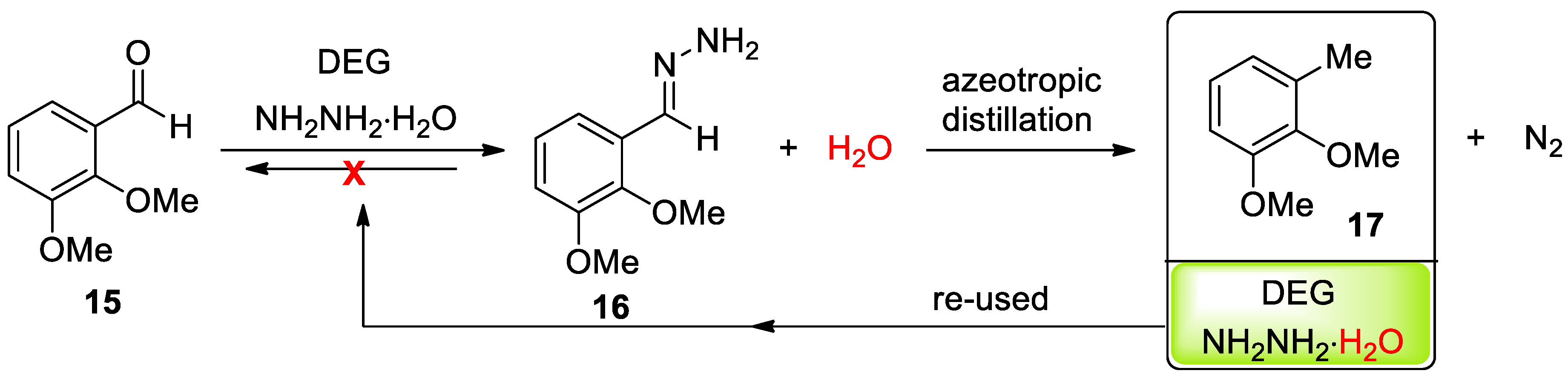
- Eisenbraun, E.J.; Payne, K.W.; Bymaster, J.S. Multiple-Batch, Wolff−Kishner Reduction Based on Azeotropic Distillation Using Diethylene Glycol. Ind. Eng. Chem. Res. 2000, 39, 1119–1123. [Google Scholar] [CrossRef]
- Herr, C.H.; Whitmore, F.C.; Schiessler, R.W. The Wolff-Kishner Reaction at Atmospheric Pressure. J. Am. Chem. Soc. 1945, 67, 2061–2063. [Google Scholar] [CrossRef]
- Soffer, M.D.; Soffer, M.B.; Sherk, K.W. A Low Pressure Method for Wolff—Kishner Reduction. J. Am. Chem. Soc. 1945, 67, 1435–1436. [Google Scholar] [CrossRef]
- Huang, M. A Simple Modification of the Wolff-Kishner Reduction. J. Am. Chem. Soc. 1946, 68, 2487–2488. [Google Scholar] [CrossRef]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Spörl, J.M.; Buchmeiser, M.R. Carbon Fibers: Precursor Systems, Processing, Structure, and Properties. Angew. Chem. Int. Ed. 2014, 53, 5262–5298. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, R.K. Fundamental Principles of Selective Heterogeneous Oxidation Catalysis. Top. Catal. 2002, 21, 79–88. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Tenhover, M.A. Ammoxidation. In Handbook of Heterogeneous Catalysis; Wiley: Hoboken, NJ, USA, 2008; pp. 3489–3517. [Google Scholar]
- Centi, G.; Perathoner, S.; Trifirò, F. V-Sb-oxide catalysts for the ammoxidation of propane. Appl. Catal. A Gen. 1997, 157, 143–172. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Trifirò, F. Acrylonitrile from Biomass: Still Far from Being a Sustainable Process. Top. Catal. 2016, 59, 1651–1658. [Google Scholar] [CrossRef]
- Karp, E.M.; Eaton, T.R.; Nogué, V.S.I.; Vorotnikov, V.; Biddy, M.J.; Tan, E.C.D.; Brandner, D.G.; Cywar, R.M.; Liu, R.; Manker, L.P.; et al. Renewable acrylonitrile production. Science 2017, 358, 1307–1310. [Google Scholar] [CrossRef]
- Meek, K.M.; Eaton, T.R.; Rorrer, N.A.; Brandner, D.G.; Manker, L.P.; Karp, E.M.; Biddy, M.J.; Bratis, A.D.; Beckham, G.T.; Naskar, A.K. Emulsion polymerization of acrylonitrile in aqueous methanol. Green Chem. 2018, 20, 5299–5310. [Google Scholar] [CrossRef]
- Pavia, C.; Ballerini, E.; Bivona, L.A.; Giacalone, F.; Aprile, C.; Vaccaro, L.; Gruttadauria, M. Palladium Supported on Cross-Linked Imidazolium Network on Silica as Highly Sustainable Catalysts for the Suzuki Reaction under Flow Conditions. Adv. Synth. Catal. 2013, 355, 2007–2018. [Google Scholar] [CrossRef]
- Kozell, V.; Giannoni, T.; Nocchetti, M.; Vivani, R.; Piermatti, O.; Vaccaro, L. Immobilized Palladium Nanoparticles on Zirconium Carboxy-Aminophosphonates Nanosheets as an Efficient Recoverable Heterogeneous Catalyst for Suzuki–Miyaura and Heck Coupling. Catalyst 2017, 7, 186. [Google Scholar] [CrossRef]
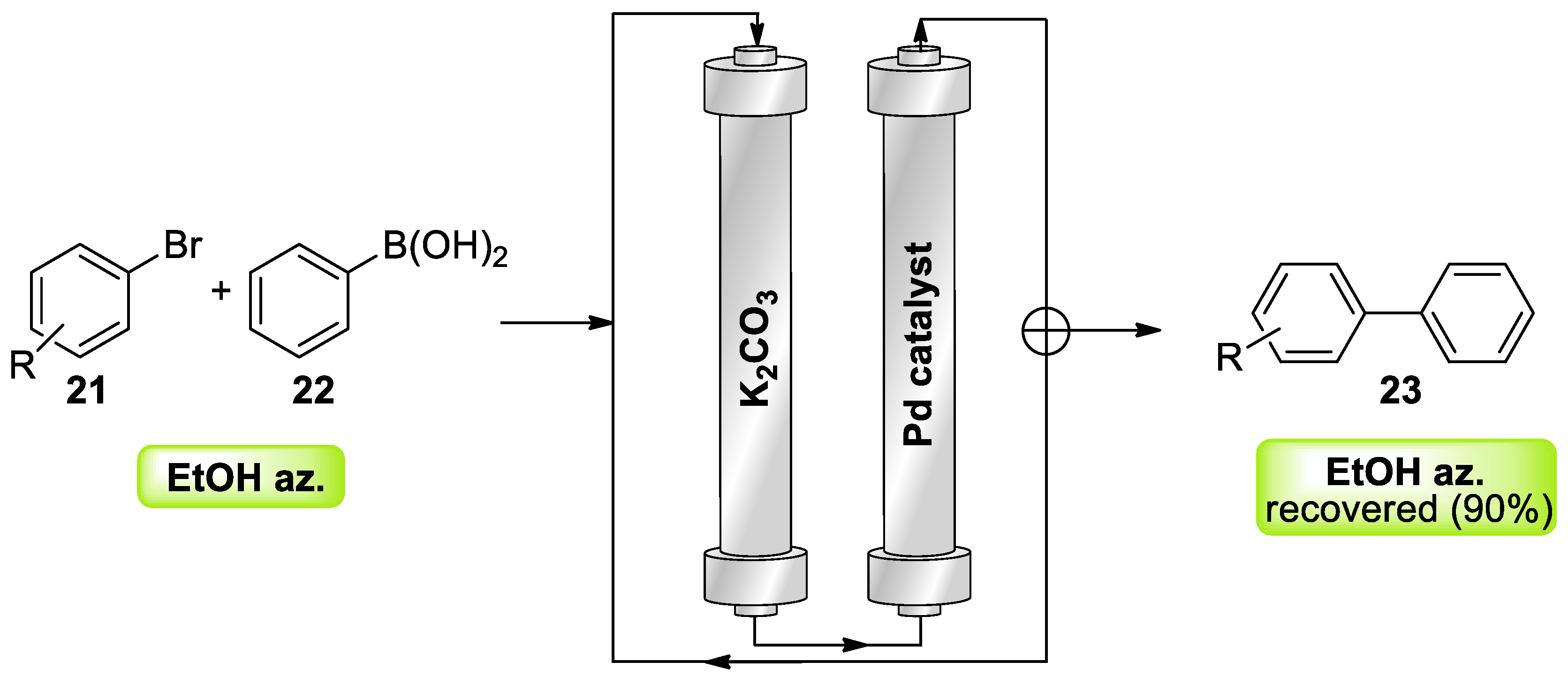
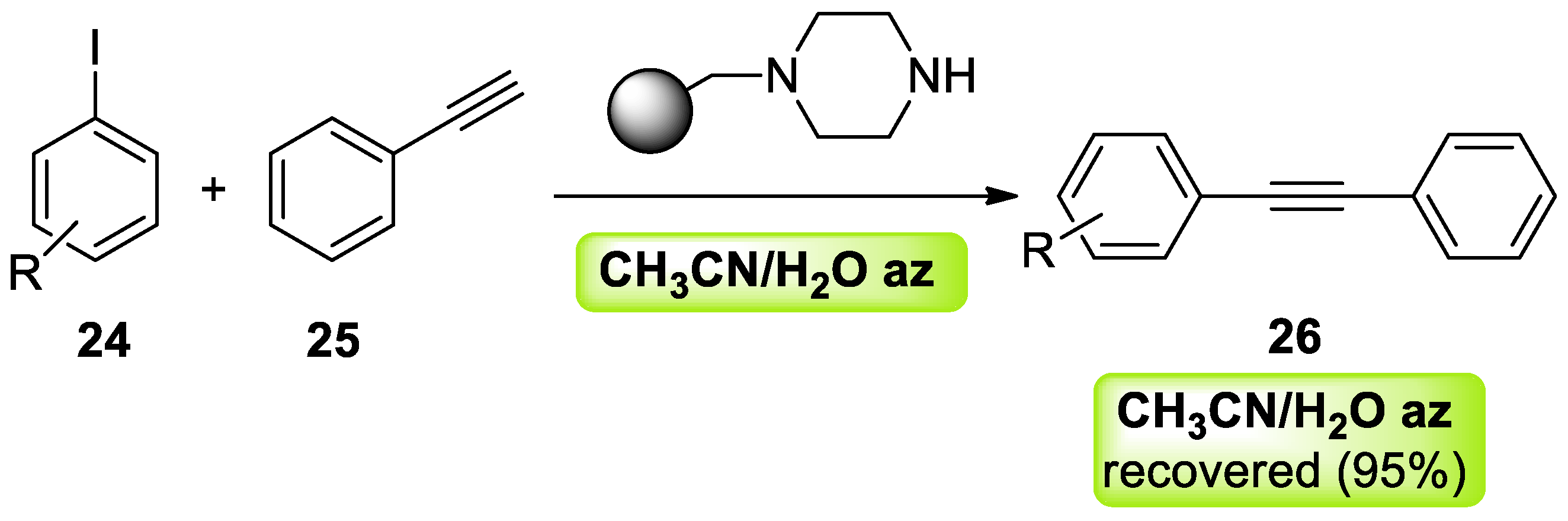
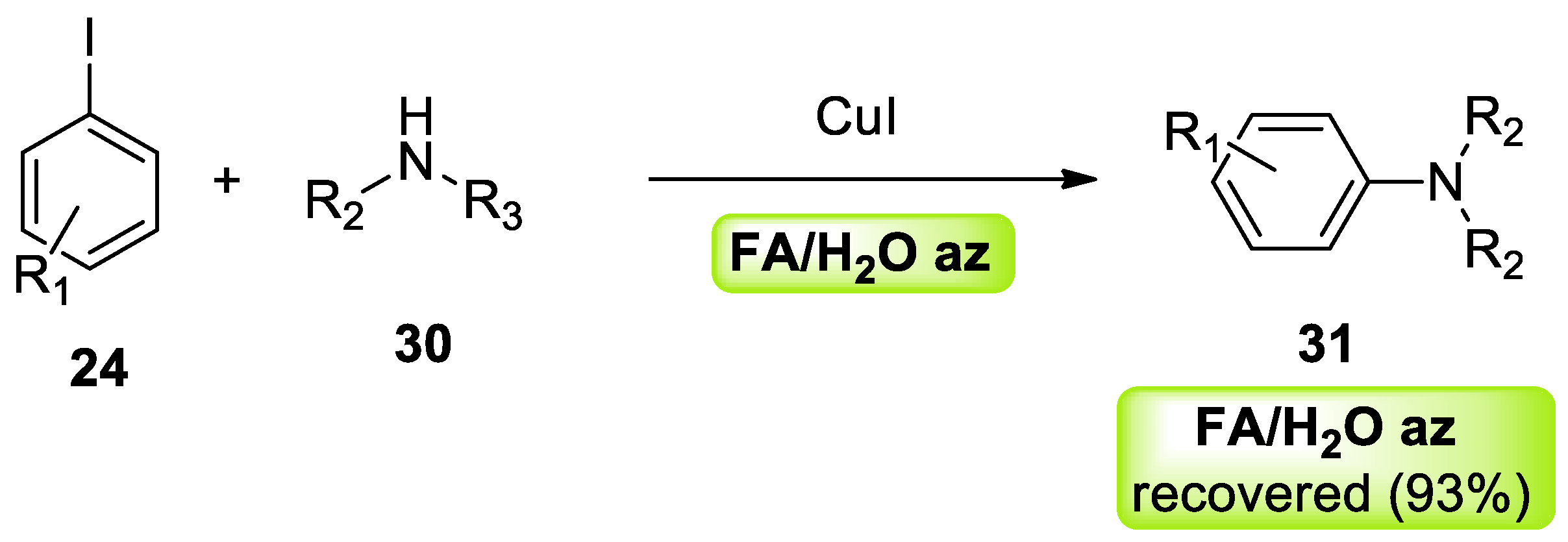
- Kozell, V.; McLaughlin, M.; Strappaveccia, G.; Santoro, S.; Bivona, L.A.; Aprile, C.; Gruttadauria, M.; Vaccaro, L. Sustainable Approach to Waste-Minimized Sonogashira Cross-Coupling Reaction Based on Recoverable/Reusable Heterogeneous Catalytic/Base System and Acetonitrile Azeotrope. ACS Sustain. Chem. Eng. 2016, 4, 7209–7216. [Google Scholar] [CrossRef]
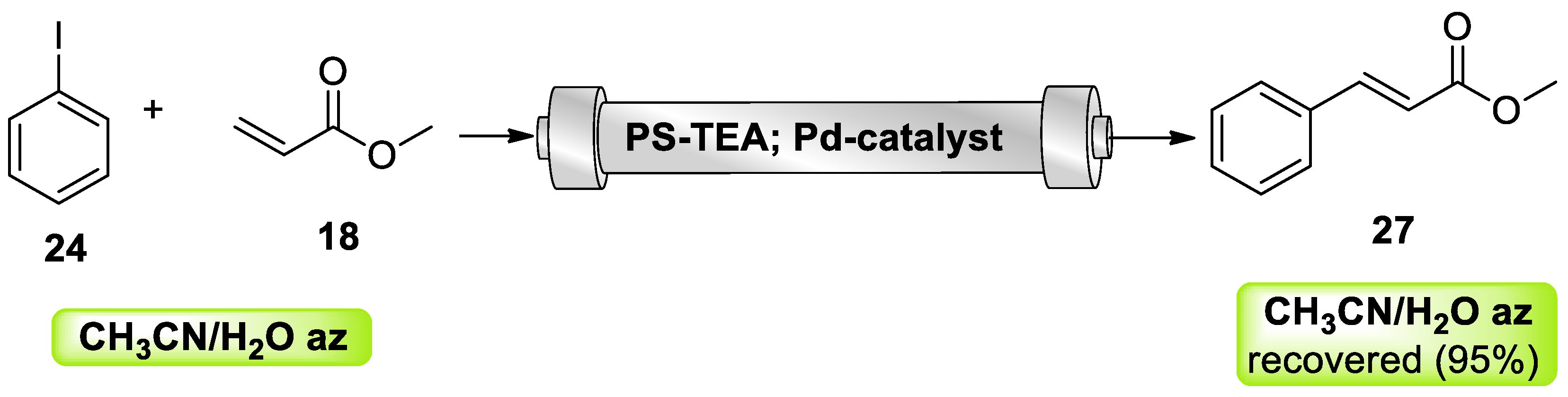
- Petrucci, C.; Strappaveccia, G.; Giacalone, F.; Gruttadauria, M.; Pizzo, F.; Vaccaro, L. An E-Factor Minimized Protocol for a Sustainable and Efficient Heck Reaction in Flow. ACS Sustain. Chem. Eng. 2014, 2, 2813–2819. [Google Scholar] [CrossRef]
- Petrucci, C.; Cappelletti, M.; Piermatti, O.; Nocchetti, M.; Pica, M.; Pizzo, F.; Vaccaro, L. Immobilized palladium nanoparticles on potassium zirconium phosphate as an efficient recoverable heterogeneous catalyst for a clean Heck reaction in flow. J. Mol. Catal. A Chem. 2015, 401, 27–34. [Google Scholar] [CrossRef]
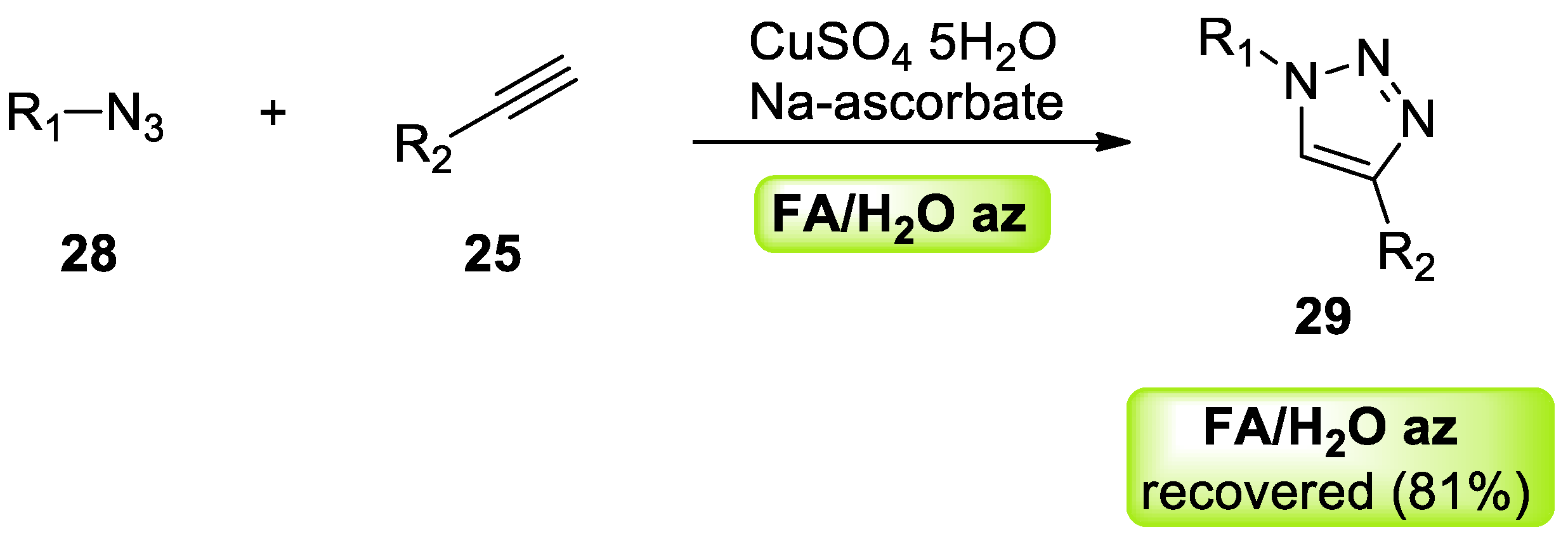
- Rasina, D.; Lombi, A.; Santoro, S.; Ferlin, F.; Vaccaro, L. Searching for novel reusable biomass-derived solvents: Furfuryl alcohol/water azeotrope as a medium for waste-minimised copper-catalysed azide–alkyne cycloaddition. Green Chem. 2016, 18, 6380–6386. [Google Scholar] [CrossRef]
- Ferlin, F.; Trombettoni, V.; Luciani, L.; Fusi, S.; Piermatti, O.; Santoro, S.; Vaccaro, L. A waste-minimized protocol for copper-catalyzed Ullmann-type reaction in a biomass derived furfuryl alcohol/water azeotrope. Green Chem. 2018, 20, 1634–1639. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Giacalone, F.; Noto, R. “Release and catch” catalytic systems. Green Chem. 2013, 15, 2608–2618. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Valentini, F.; Ferlin, F.; Bivona, L.A.; Anastasiou, I.; Fusaro, L.; Aprile, C.; Marrocchi, A.; Vaccaro, L. A tailored polymeric cationic tag–anionic Pd(ii) complex as a catalyst for the low-leaching Heck–Mizoroki coupling in flow and in biomass-derived GVL. Green Chem. 2019, 21, 355–360. [Google Scholar] [CrossRef]
- Ferlin, F.; Lanari, D.; Vaccaro, L. Sustainable flow approaches to active pharmaceutical ingredients. Green Chem. 2020, 22, 5937–5955. [Google Scholar] [CrossRef]
- Vaccaro, L. Green Shades in Organic Synthesis. Eur. J. Org. Chem. 2020, 2020, 4273–4283. [Google Scholar] [CrossRef]
- Gao, F.; Bai, R.; Ferlin, F.; Vaccaro, L.; Li, M.; Gu, Y. Replacement strategies for non-green dipolar aprotic solvents. Green Chem. 2020, 22, 6240–6257. [Google Scholar] [CrossRef]
- Sun, D.; Sato, S.; Ueda, W.; Primo, A.; García, H.; Corma, A. Production of C4 and C5 alcohols from biomass-derived materials. Green Chem. 2016, 18, 2579–2597. [Google Scholar] [CrossRef]
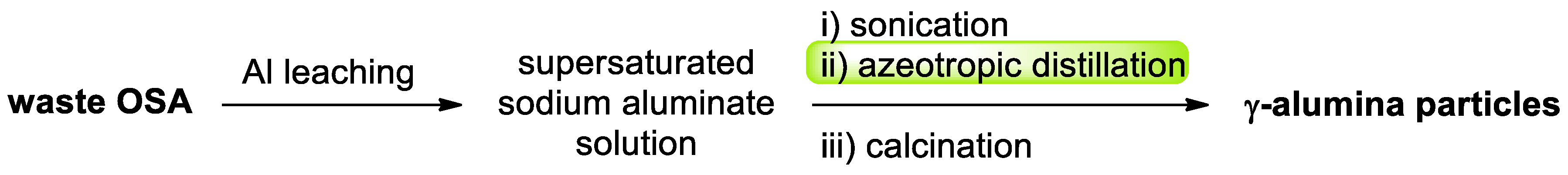
- An, B.; Ji, G.; Wang, W.; Gan, S.; Xu, J.; Gao, G.; Li, G. Azeotropic distillation-assisted preparation of nanoscale gamma-alumina powder from waste oil shale ash. Chem. Eng. J. 2010, 157, 67–72. [Google Scholar] [CrossRef]
- Mangalara, S.C.H.; Varughese, S. Green Recycling Approach To Obtain Nano- and Microparticles from Expanded Polystyrene Waste. ACS Sustain. Chem. Eng. 2016, 4, 6095–6100. [Google Scholar] [CrossRef]

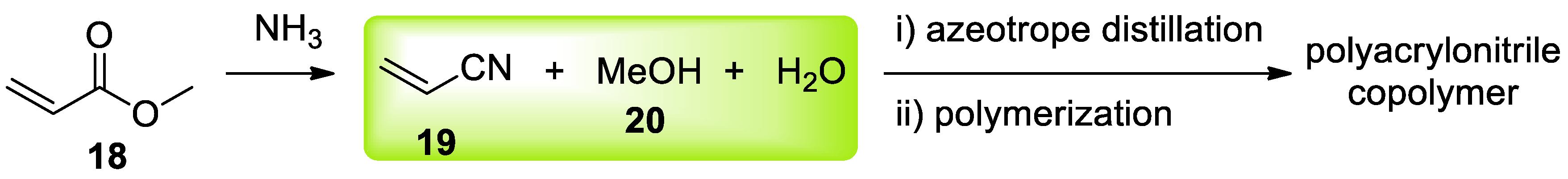

| Mixture | n. Binary Azeotropes | n. Ternary Azeotropes | |
|---|---|---|---|
| Group 1 | Water, EtOH, MEK, acetone | 3 | 1 |
| Water, EtOH, EtOAc, acetone | 3 | 1 | |
| Group 2 | Water, EtOH, EtOAc, IPOAc | 5 | 2 |
| Water, EtOH, MEK, IPOAc | 5 | 2 | |
| Group 3 | Water, EtOH, EtOAc, MEK | 6 | 3 |
| Water, IPOH, EtOAc, MEK | 6 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentini, F.; Vaccaro, L. Azeotropes as Powerful Tool for Waste Minimization in Industry and Chemical Processes. Molecules 2020, 25, 5264. https://doi.org/10.3390/molecules25225264
Valentini F, Vaccaro L. Azeotropes as Powerful Tool for Waste Minimization in Industry and Chemical Processes. Molecules. 2020; 25(22):5264. https://doi.org/10.3390/molecules25225264
Chicago/Turabian StyleValentini, Federica, and Luigi Vaccaro. 2020. "Azeotropes as Powerful Tool for Waste Minimization in Industry and Chemical Processes" Molecules 25, no. 22: 5264. https://doi.org/10.3390/molecules25225264
APA StyleValentini, F., & Vaccaro, L. (2020). Azeotropes as Powerful Tool for Waste Minimization in Industry and Chemical Processes. Molecules, 25(22), 5264. https://doi.org/10.3390/molecules25225264