Application of Functionalized DVB-co-GMA Polymeric Microspheres in the Enhanced Sorption Process of Hazardous Dyes from Dyeing Baths
Abstract
1. Introduction
2. Results
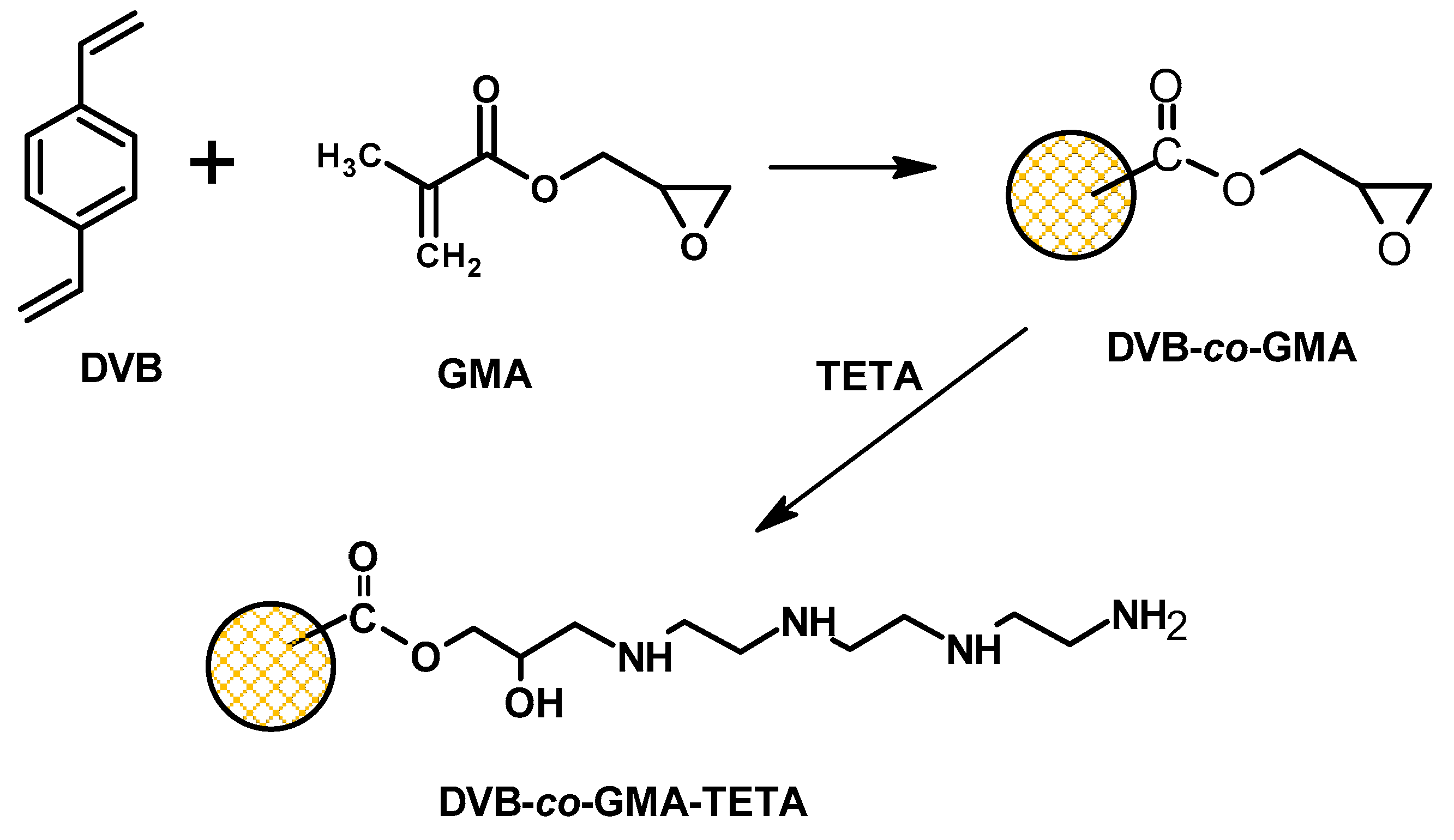
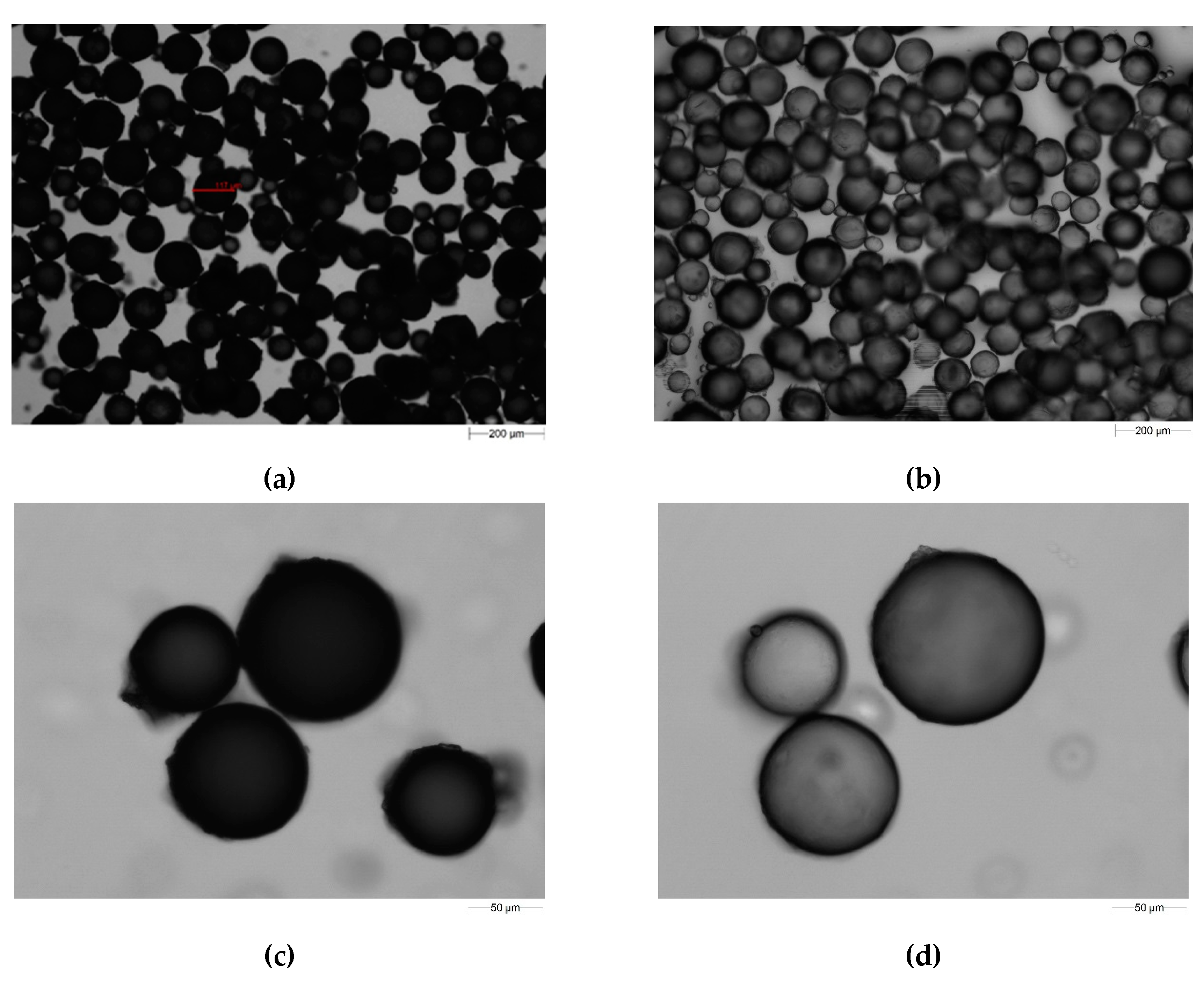
2.1. DVB-co-GMA-TETA Synthesis and Preliminary Characteristics
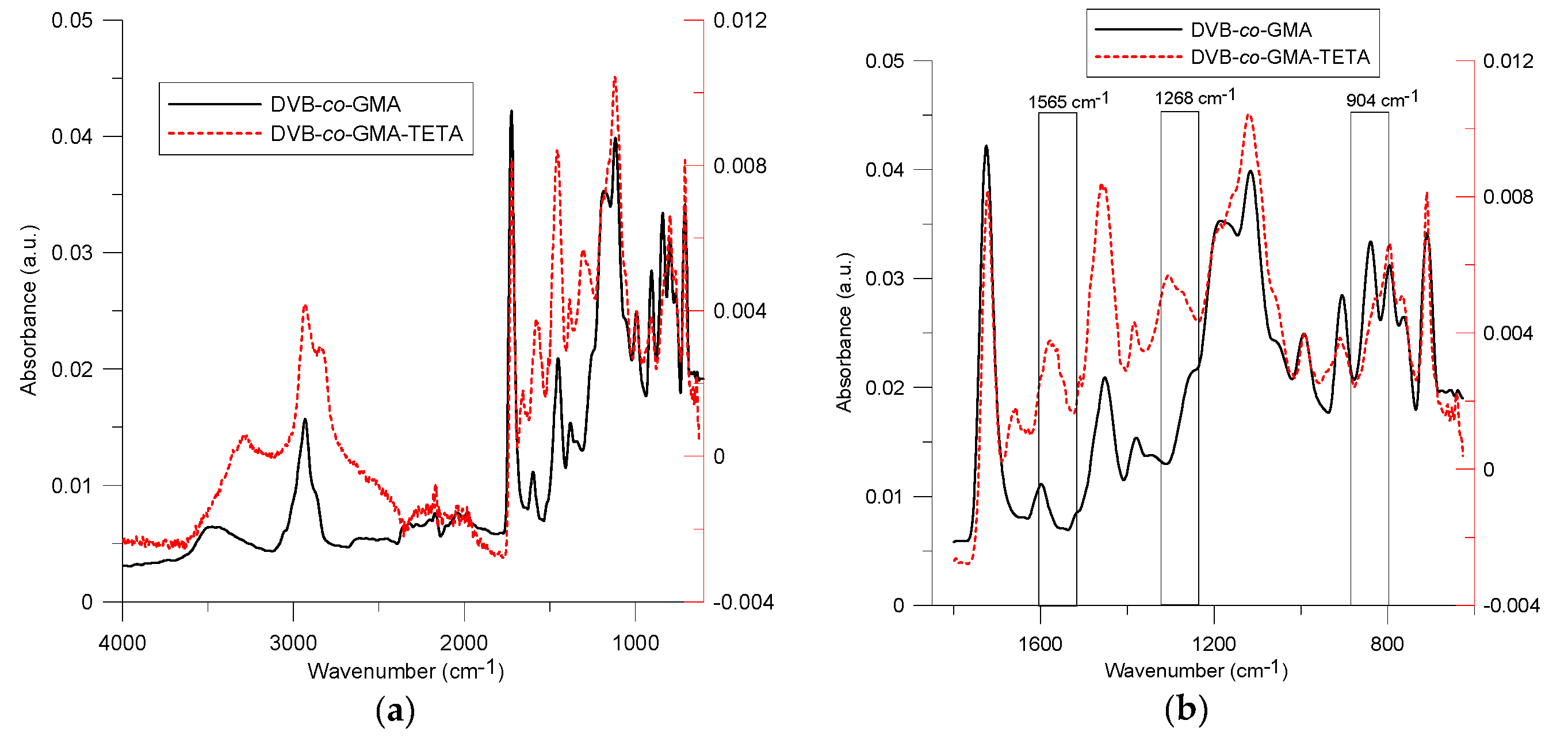
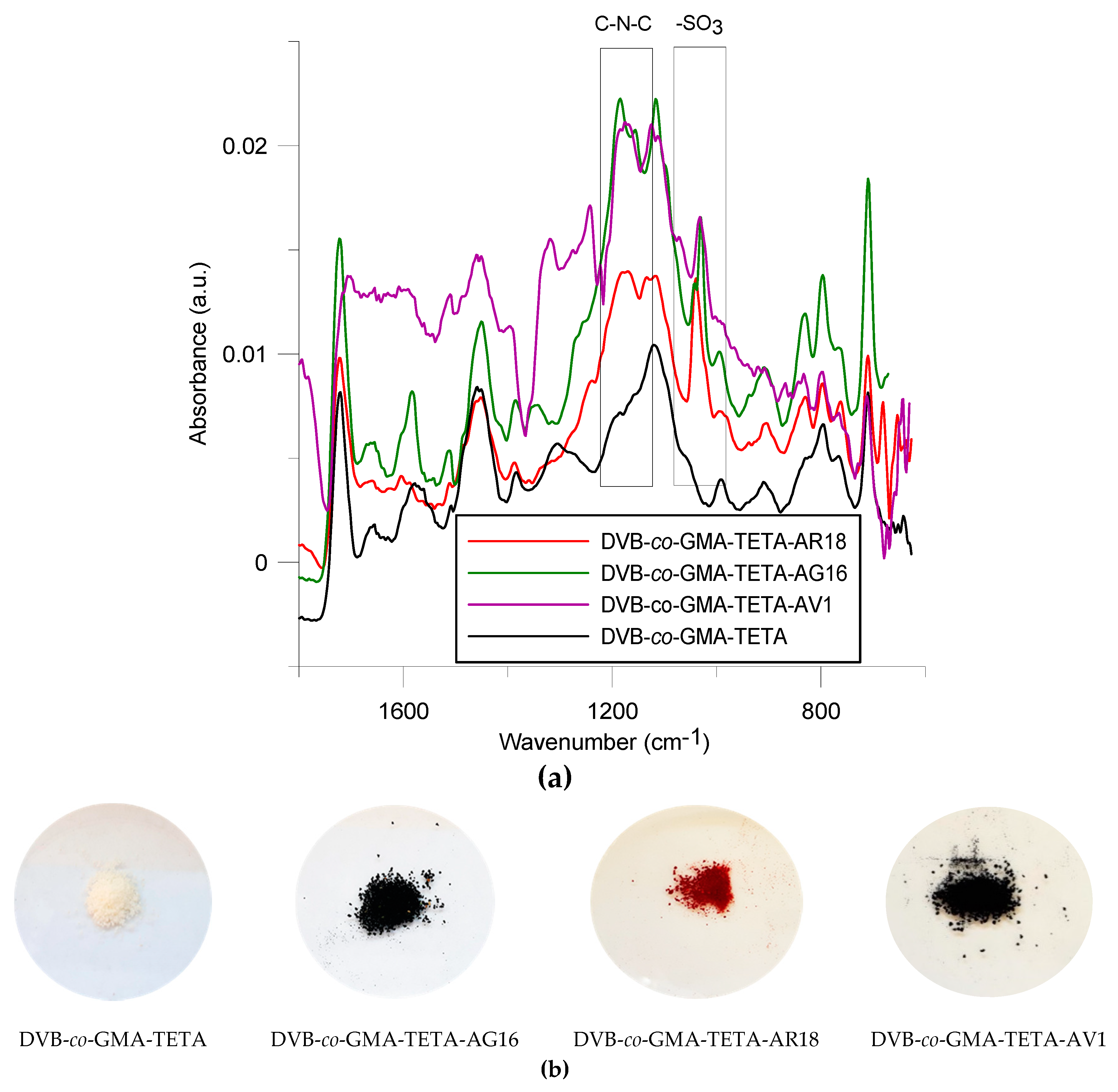
ATR-FT-IR Spectroscopy
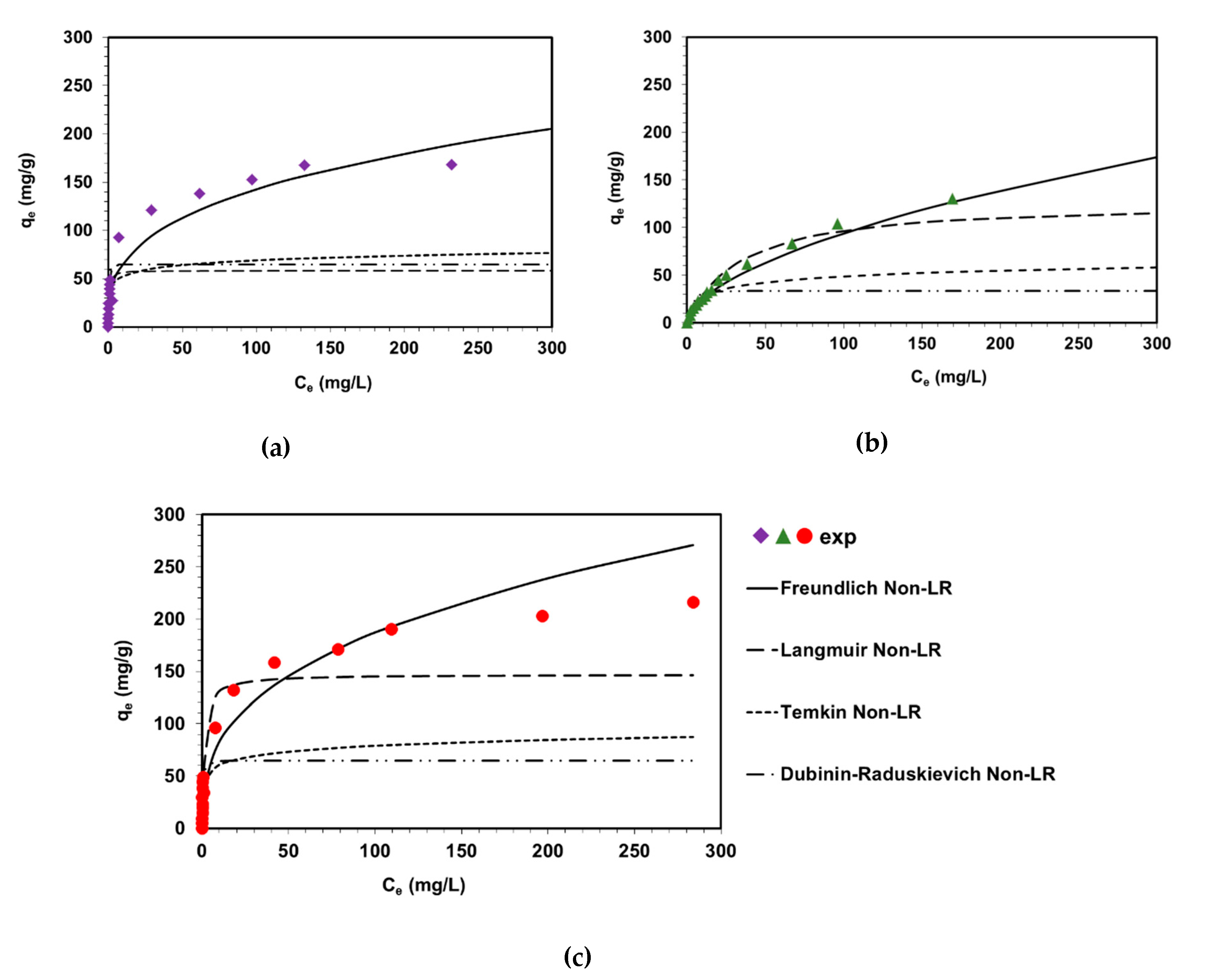
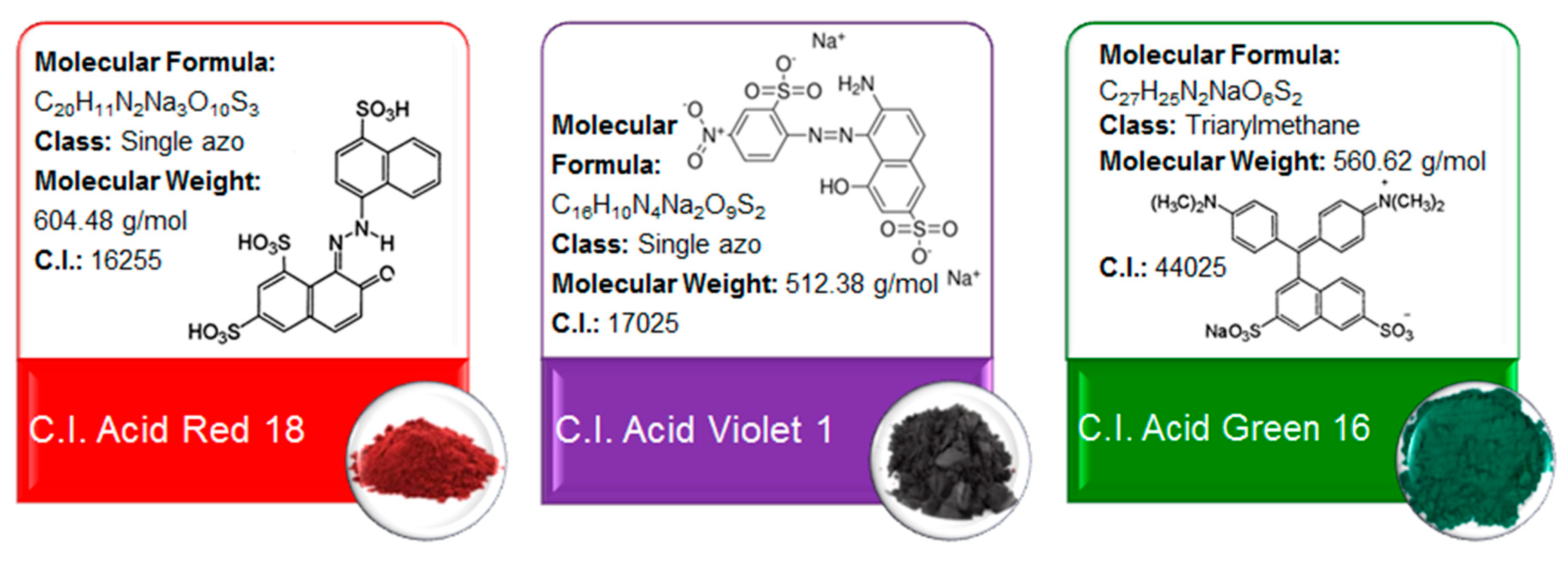
2.2. Determination of DVB-co-GMA-TETA Sorption Capacity
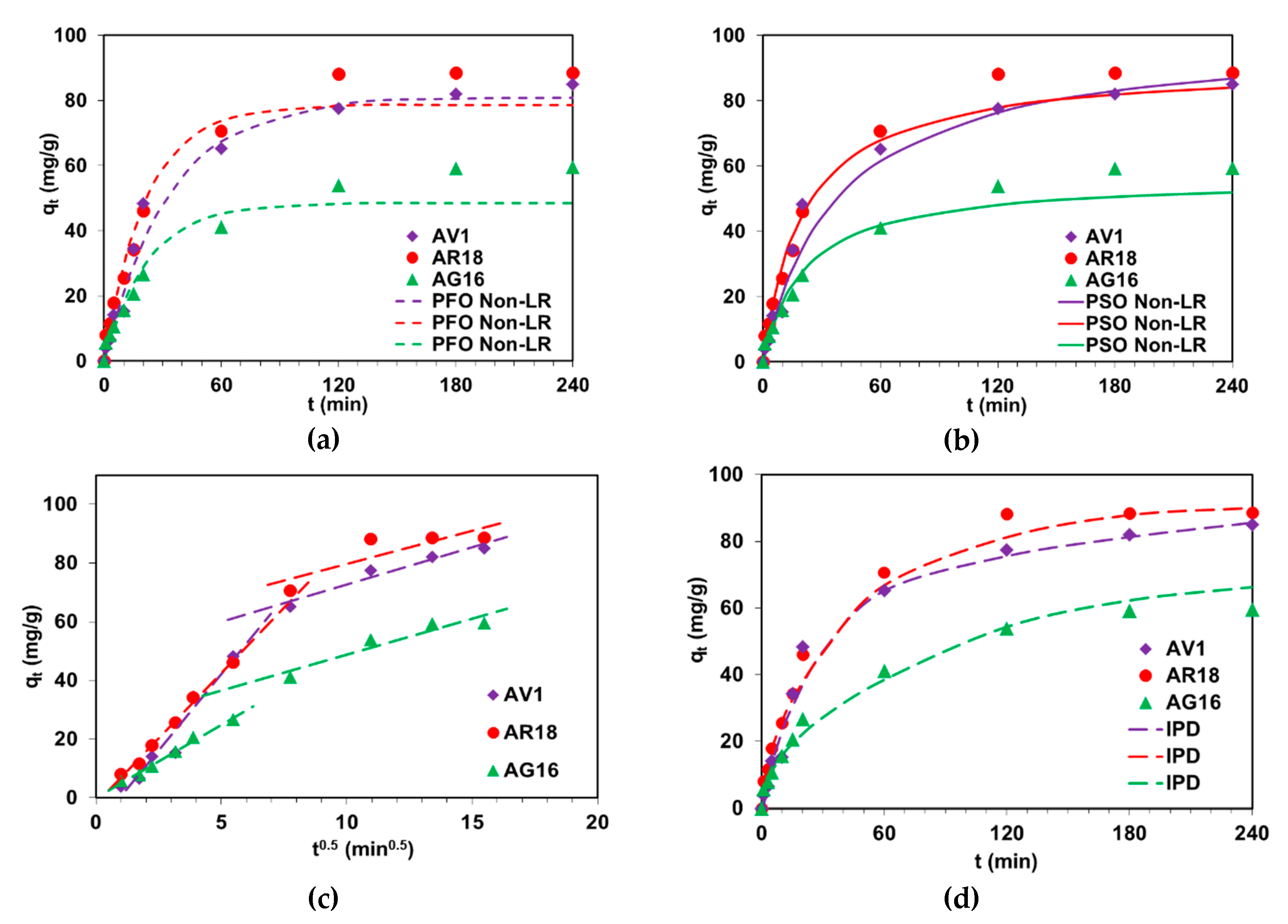
2.3. Determination of Kinetic Parameters
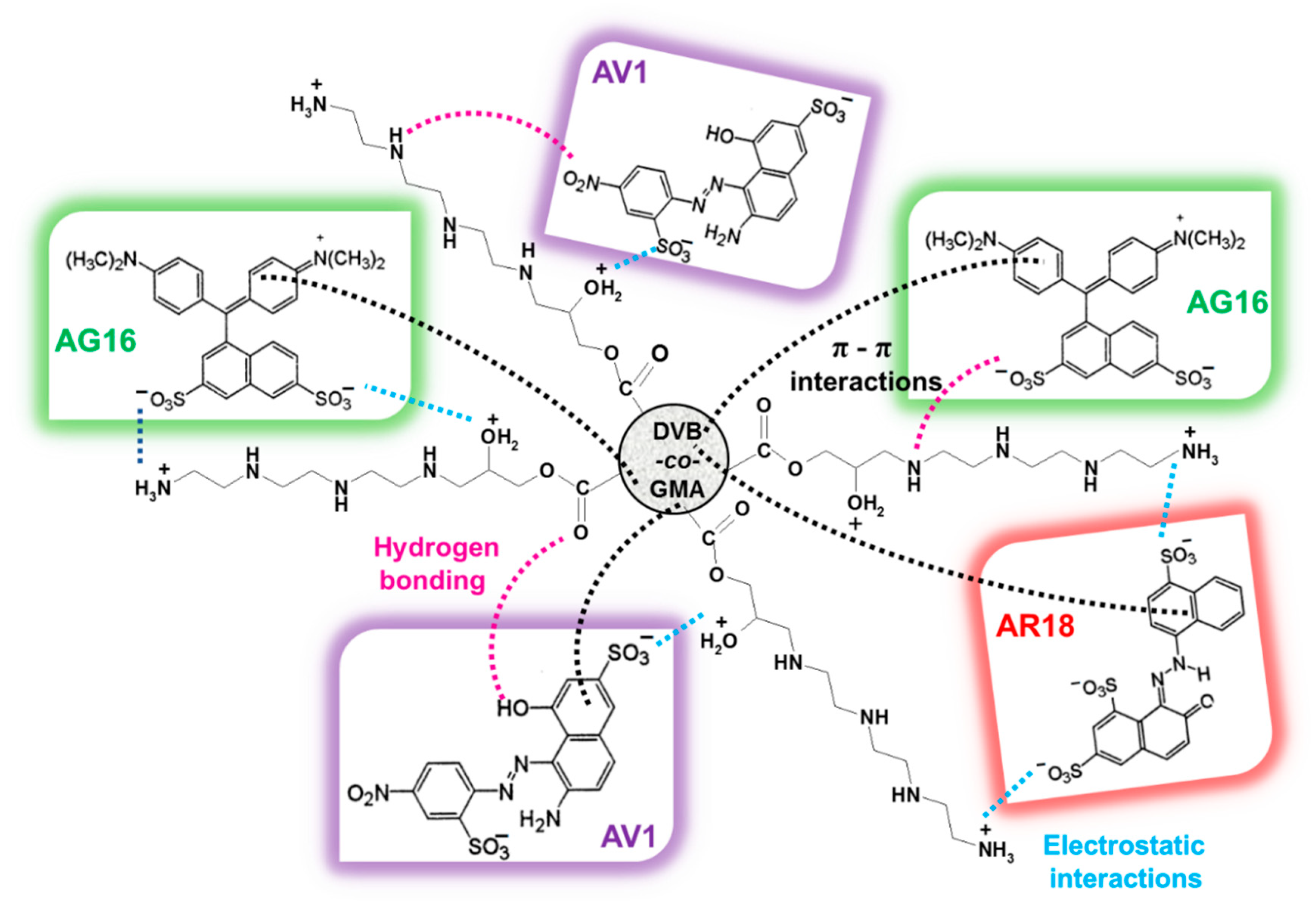
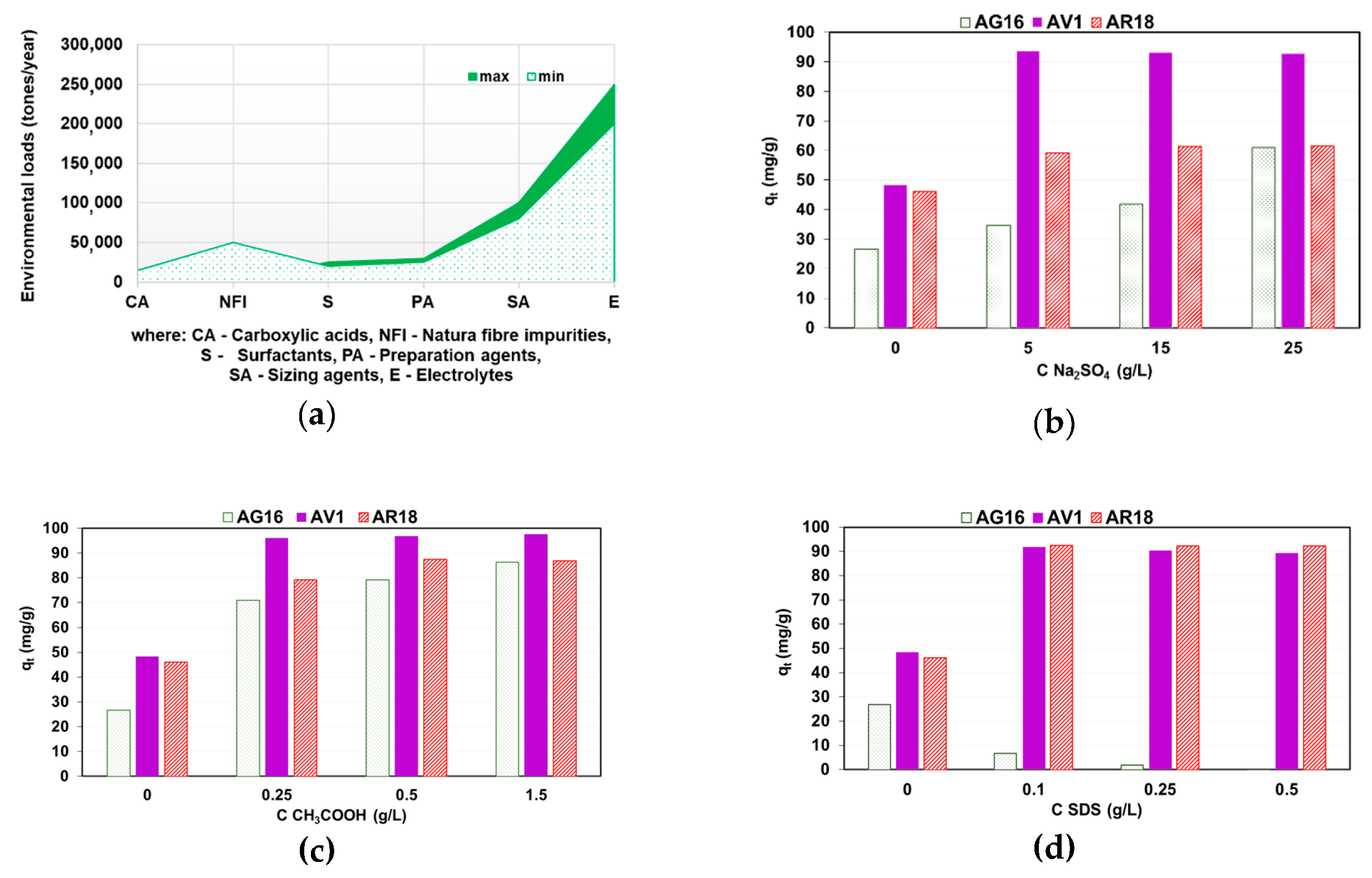
2.4. Auxiliaries Presence on Dye Adsorption Effectiveness
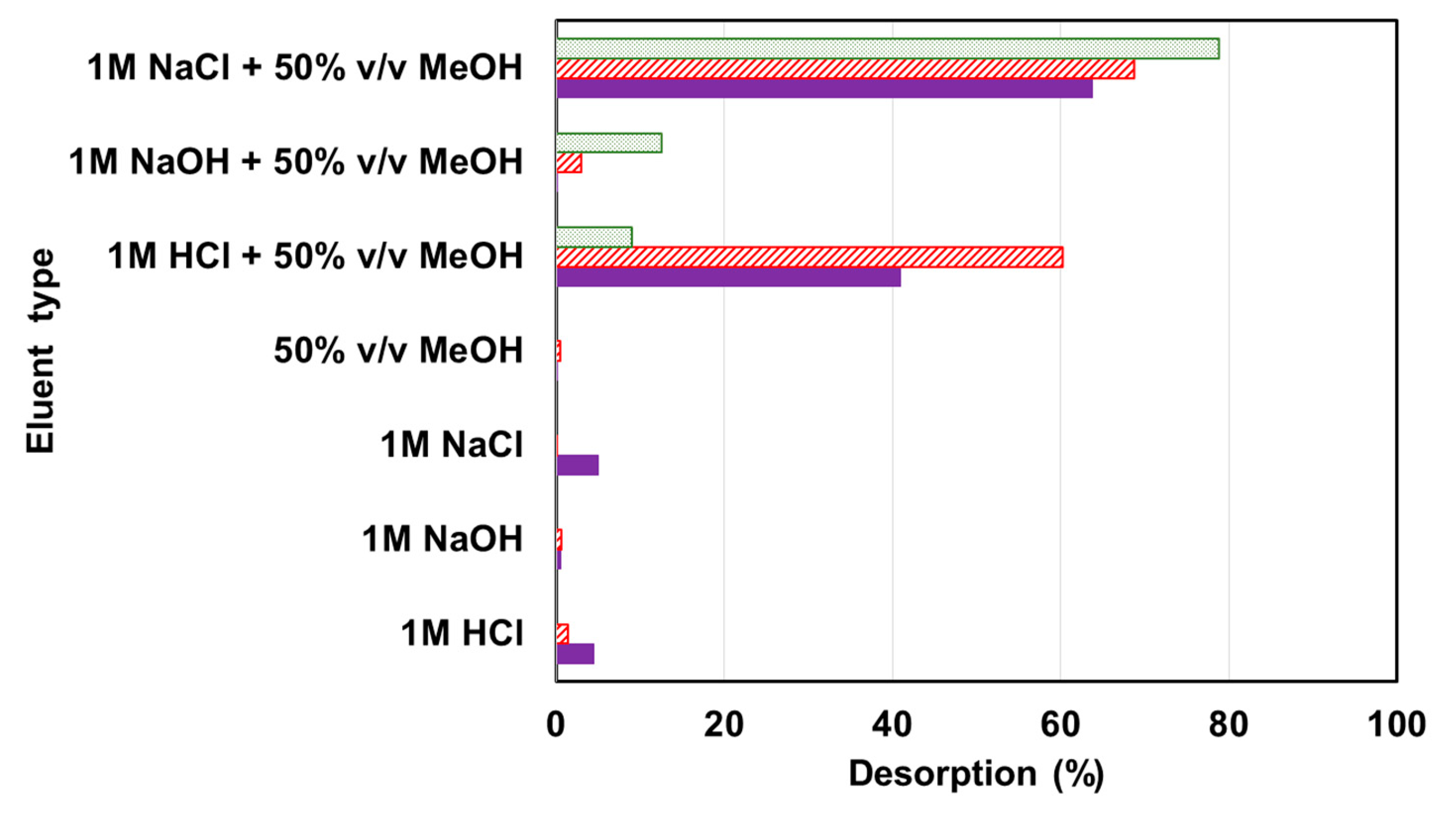
2.5. Regeneration Studies
3. Materials and Methods
3.1. Chemicals and Eluents
3.2. Preparation of Microspheres
3.3. Functionalization
3.4. Characterization
3.5. Adsorption Experiments
3.5.1. Determination of DVB-co-GMA-TETA Sorption Capacity
3.5.2. Kinetic Studies and Impact of Auxiliaries on Dye Uptake
3.5.3. Resin Regeneration
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Afroze, S.; Sen, T.K. A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Badeenezhad, A.; Azhdarpoor, A.; Bahrami, S.; Yousefinejad, S. Removal of methylene blue dye from aqueous solutions by natural clinoptilolite and clinoptilolite modified by iron oxide nanoparticles. Mol. Simul. 2019, 45, 564–571. [Google Scholar] [CrossRef]
- Ali, S.M.; Eskandrani, A.A. The Sorption Performance of Cetyl Trimethyl Ammonium Bromide-Capped La0.9Sr0.1FeO3 Perovskite for Organic Pollutants from Industrial Processes. Molecules 2020, 25, 1640. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2018, 17, 195–213. [Google Scholar] [CrossRef]
- Gupta, V.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Hassan, S.S.; El-Shafie, A.S.; Zaher, N.; Elazazy, M.S. Application of Pineapple Leaves as Adsorbents for Removal of Rose Bengal from Wastewater: Process Optimization Operating Face-Centered Central Composite Design (FCCCD). Molecules 2020, 25, 3752. [Google Scholar] [CrossRef] [PubMed]
- Wawrzkiewicz, M. Removal of C.I. Basic Blue 3 dye by sorption onto cation exchange resin, functionalized and non-functionalized polymeric sorbents from aqueous solutions and wastewaters. Chem. Eng. J. 2013, 217, 414–425. [Google Scholar] [CrossRef]
- Karcher, S.; Kornmüller, A.; Jekel, M. Screening of commercial sorbents for the removal of reactive dyes. Dye. Pigment. 2001, 51, 111–125. [Google Scholar] [CrossRef]
- Xu, X.-H.; Li, M.-L.; Yuan, Y. Treatment of Direct Blending Dye Wastewater and Recycling of Dye Sludge. Molecules 2012, 17, 2784–2795. [Google Scholar] [CrossRef] [PubMed]
- Hessel, C.; Allegre, C.; Maisseu, M.; Charbit, F.; Moulin, P. Guidelines and legislation for dye house effluents. J. Environ. Manag. 2007, 83, 171–180. [Google Scholar] [CrossRef]
- Podkościelna, B.; Gawdzik, B.; Bartnicki, A. Use of a new methacrylic monomer, 4,4′-di(2-hydroxy-3-methacryloyloxypropoxy)benzophenone, in the synthesis of porous microspheres. J. Polym. Sci. Part A 2006, 44, 7014–7026. [Google Scholar] [CrossRef]
- Podkościelna, B. Synthesis, modification, and porous properties of new glycidyl methacrylate copolymers. J. Appl. Polym. Sci. 2011, 120, 3020–3026. [Google Scholar] [CrossRef]
- Podkościelna, B. The Use of Bis[4(2-hydroxy-3-methacryloyloxypropoxy)phenyl]sulfide in Preparation of Microspheres with Pendant Amine Groups as a Heavy Metal Sorbent. Sep. Sci. Technol. 2013, 48, 1699–1708. [Google Scholar] [CrossRef]
- Trytek, M.; Fiedurek, J.; Podkościelna, B.; Gawdzik, B.; Skowronek, M. An efficient method for the immobilization of inulinase using new types of polymers containing epoxy groups. J. Ind. Microbiol. Biotechnol. 2015, 42, 985–996. [Google Scholar] [CrossRef]
- Amberlite XAD4 Industrial Grade Polymeric Resin, Product Datasheet; Rohm and Haas Company: Philadelphia, PA, USA, 2003.
- Amberlite™ XAD7HP (XAD7)—Sigma Prod. Nos. XAD-4, XAD-7, XAD-16, 1-0377,1-0393, and 1-0379, Product Information Sheet. Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Product_Information_Sheet/1/xad7pis.pdf (accessed on 30 October 2020).
- Anaia, G.C.; Freitas, P.A.M.; Suareziha, M.E.V.; Rocha, F.R.P. Adsorption of 1-(2-Thiazolylazo)-2-Naphthol on Amberlite XAD-7 and Silica Gel: Isotherms and Kinetic Studies. J. Braz. Chem. Soc. 2014, 25, 648–657. [Google Scholar] [CrossRef]
- Emran, K.M.; Ali, S.M.; Al-Oufi, A.L.L. Synthesis and Characterization of Nano-Conducting Copolymer Composites: Efficient Sorbents for Organic Pollutants. Molecules 2017, 22, 772. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.; Dotto, G.; Salau, N. Experimental and mathematical modeling of hindered diffusion effect of cationic dye in the adsorption onto bentonite. J. Environ. Chem. Eng. 2019, 7, 102891. [Google Scholar] [CrossRef]
- Foo, K.; Hameed, B. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Kyzioł-Komosińska, J.; Rosik-Dulewska, C.; Pająk, M.; Czupioł, J.; Dzieniszewska, A.; Krzyżewska, I. Sorption of Acid Green 16 from aqueous solution onto low-moor peat and smectite clay co-occurring in lignite of Belchatow mine field. Annu. Set Environ. Prot. 2015, 17, 165–187. [Google Scholar]
- Chaleshtori, A.A.N.; Meghadddam, F.M.; Sadeghi, M.M.; Rahimi, R.R.; Hemati, S.; Ahmadi, A.A. Removal of acid red 18 (Azo-dye) from aqueous solution by adsorption onto activated charcoal prepared from almond shell. J. Environ. Sci. Manag. 2017, 20, 9–16. [Google Scholar]
- Lu, F.; Dong, A.; Ding, G.; Xu, K.; Li, J.; You, L. Magnetic porous polymer composite for high performance adsorption of acid red 18 based on melamine resin and chitosan. J. Mol. Liq. 2019, 294, 111515. [Google Scholar] [CrossRef]
- Krysztafkiewicz, A.; Binkowski, S.; Jesionowski, T. Adsorption of dyes on a silica surface. Appl. Surf. Sci. 2002, 199, 31–39. [Google Scholar] [CrossRef]
- Rossatto, D.; Netto, M.; Jahn, S.; Mallmann, E.; Dotto, G.; Foletto, E. Highly efficient adsorption performance of a novel magnetic geopolymer/Fe3O4 composite towards removal of aqueous acid green 16 dye. J. Environ. Chem. Eng. 2020, 8, 103804. [Google Scholar] [CrossRef]
- Foguel, M.V.; Pedro, N.T.B.; Wong, A.; Khan, S.; Zanoni, M.V.B.; Sotomayor, M.D.P.T. Synthesis and evaluation of a molecularly imprinted polymer for selective adsorption and quantification of Acid Green 16 textile dye in water samples. Talanta 2017, 170, 244–251. [Google Scholar] [CrossRef]
- Sankar, M.; Sekaran, G.; Sadulla, S.; Ramasami, T. Removal of diazo and triphenylmethane dyes from aqueous solutions through an adsorption process. J. Chem. Technol. Biotechnol. 1999, 74, 337–344. [Google Scholar] [CrossRef]
- Xing, T.; Kai, H.; Chen, G. Study of adsorption and desorption performance of acid dyes on anion exchange membrane. Color. Technol. 2012, 128, 295–299. [Google Scholar] [CrossRef]
- Wen, G.; Cookson, P.; Liu, X.; Wang, X. The effect of pH and temperature on the dye sorption of wool powders. J. Appl. Polym. Sci. 2010, 116, 2216–2226. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Wołowicz, A.; Wawrzkiewicz, M. Characteristics and Adsorptive Treatment of Wastewaters Containing Dyes. In Pollutants in Buildings, Water and Living Organisms; Springer: Cham, Switzerland, 2020; pp. 273–311. [Google Scholar]
- Wawrzkiewicz, M.; Hubicki, Z. Anion Exchange Resins as Effective Sorbents for Removal of Acid, Reactive, and Direct Dyes from Textile Wastewaters. In Ion Exchange—Studies and Applications; IntechOpen: London, UK, 2015. [Google Scholar]
- Dulman, V.; Cucu-Man, S.-M.; Bunia, I.; Dumitras, M. Batch and fixed bed column studies on removal of Orange G acid dye by a weak base functionalized polymer. Desalin. Water Treat. 2015, 57, 14708–14727. [Google Scholar] [CrossRef]
- Petcu, A.R.; Rogozea, E.A.; Lazar, C.A.; Olteanu, N.L.; Meghea, A.; Mihaly, M. Specific interactions within micelle microenvironment in different charged dye/surfactant systems. Arab. J. Chem. 2016, 9, 9–17. [Google Scholar] [CrossRef]
- Bielska, M.; Sobczyńska, A.; Prochaska, K. Dye–surfactant interaction in aqueous solutions. Dye. Pigment. 2009, 80, 201–205. [Google Scholar] [CrossRef]
- Klimiuk, E.; Kabardo, K.; Gusiatin, Z.; Filipkowska, U. The adsorption of reactive dyes from mixtures containing surfactants onto chitin. Pol. J. Environ. Stud. 2005, 14, 771–780. [Google Scholar]
- Chakraborty, J.N. Fundamentals and Practices in Colouration of Textiles; Elsevier BV: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Mielicki, J. Zarys chemicznej obróbki wyrobów włókienniczych; Wydawnictwa Naukowo-Techniczne: Warszawa, Poland, 1991. (In Polish) [Google Scholar]
- Marković, D.D.; Lekić, B.; Rajaković-Ognjanović, V.N.; Onjia, A.; Rajaković, L.V. A New Approach in Regression Analysis for Modeling Adsorption Isotherms. Sci. World J. 2014, 2014, 930879. [Google Scholar] [CrossRef]
- Sivarajasekar, N.; Baskar, R. Adsorption of Basic Magenta II onto H2SO4 activated immature Gossypium hirsutum seeds: Kinetics, isotherms, mass transfer, thermodynamics and process design. Arab. J. Chem. 2019, 12, 1322–1337. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Resin Name | Specific Surface Area SBET (m2/g) | Total Pore Volume VTOT (cm3/g) | Average Pore Diameter (nm) | Ref. |
|---|---|---|---|---|
| DVB-co-GMA DVB-co-GMA-TETA | 152 139 | 0.524 0.596 | 13.82 17.00 | This study |
| Amberlite XAD-4 (PS-DVB) | 750 | 0.5 | 5.0 | [15,16] |
| Amberlite XAD-7 (PA-DVB) | 450 | 1.14 | 9.0 | [16,17] |
| Polyaniline/SiO2 nano-composite Polypyrrole/SiO2 nano-composite | 72.4 122.81 | - | - | [18] |
| Bentonite (Mendoza, Argentina) | 8.77 | 0.315 | 7.3 | [19] |
| Equation No. | Isotherm | Non-Linear Isotherm Form | Calculated Parameters |
|---|---|---|---|
| (2) | Freundlich | (mg1−1/n L1/n/g) and n are the Freundlich constants related to adsorption capability and adsorption intensity, respectively | |
| (3) | Langmuir | kL (L/mg) is the constant parameter of adsorption equilibrium, Q0 (mg/g) is the monolayer adsorption capacity | |
| (4) | Temkin | bT (J g/mol mg) is the Temkin constant related to the heat of adsorption, A (L/mg) is the Temkin isotherm equilibrium binding constant | |
| (5) | Dubinin-Radushkevich | qm (mg/g) is the maximum adsorption capacity, kDR (mol2 J2) is the constant related to the adsorption energy, ε (J/mol) is the adsorption potential, E (J/mol) is the mean free energy for removing dye molecule from its adsorption site to the infinity |
| Isotherm | Parameters | Dyes | ||
|---|---|---|---|---|
| AV1 | AG16 | AR18 | ||
| Freundlich | (mg1−1/n L1/n/g) 1/n MPSD R2 | 30.5 0.33 1.487 0.935 0.925 | 6.7 0.59 0.359 0.934 0.925 | 36.3 0.36 1.472 0.951 0.944 |
| Langmuir | kL (L/mg) Q0 (mg/g) MPSD R2 | 2.8 58.3 4.165 0.661 0.612 | 0.030 129.2 0.536 0.665 0.617 | 0.77 143.7 3.482 0.895 0.880 |
| Temkin | bT (J g/mol mg) A (L/mg) MPSD R2 | 351.2 175.4 3.310 0.915 0.903 | 273 2.1 2.108 0.884 0.867 | 300.5 159.6 3.499 0.934 0.925 |
| Dubinin-Radushkevich | qm (mg/g) kDR (mol2 J2) E (kJ/mol) MPSD R2 | 64.7 3,8∙10−8 3.6 7.627 0.477 0.402 | 33.67 1.6∙10−6 0.57 3.863 0.340 0.246 | 63.7 3.6∙10−8 4.4 6.350 0.626 0.573 |
| Sorbent | Kinetic Studies | Equilibrium Studies | Ref. |
|---|---|---|---|
| AV1 | |||
| 3-aminopropyl-triethoxysilane N-2-(aminoethyl)-3-aminopropyltrimethoxysilane | Disposal extent: 52–99.6% Disposal extent: 79–99.8% | - | [24] |
| AG16 | |||
| Magnetic geopolimer | PSO, k2 = 0.001–0.005 g/mg min, pH = 2.3, a.d = 0.75 g/L | qe = 108 mg/g, T = 25 °C, pH = 2.3, a.d. = 0.75 g/L | [25] |
| Low-moor peat and smectite clay | PSO, k2 = 0.0086–0.010 g/mg min, a.d. = 1 g/20 mL | qe = 12.07–13.0 mg/g, a.d. = 1 g/20 mL T = 25 °C, pH = 6.23–6.59 | [13] |
| Molecularly imprinted polymers (MIP) | 86% of AG16 was bound on the MIP in 60 min | qe = 6.9 mg/g | [26] |
| RBAC (rice bran-based activated carbon) | AG16 removal: 85.52–99.22% pH = 3.5, T = 20–55 °C, a.d. = 1 g/50 mL | qe = 1.05–1.36 mg/g, T = 20–55 °C, pH = 2.0–3.5 | [27] |
| AR18 | |||
| Anion exchange membrane with quaternary ammonium groups (SB 6407) | PSO, h = 4.3 mg/g min1/2 | qe = 217.6 mg/g, T = 25 °C, pH = 7 | [28] |
| Wool powder (p.s. = 6.2 μm, s.a. = 5.91 m2/g) | AR18 removal: 90%, pH = 2.5 | qe = 100 mg/g, T = 25 °C, pH = 7 | [29] |
| Parameter | Dye | ||
|---|---|---|---|
| AV1 | AR18 | AG16 | |
| qexp (mg/g) | 85.0 | 88.7 | 59.9 |
| PFO | |||
| qe (mg/g) | 80.9 | 78.6 | 48.5 |
| k1 (1/min) | 0.0299 | 0.0464 | 0.0450 |
| MPSD | 0.361 | 0.499 | 0.689 |
| R2 | 0.991 | 0.951 | 0.916 |
| 0.987 | 0.936 | 0.892 | |
| PSO | |||
| qe (mg/g) | 100.6 | 91.3 | 56.5 |
| k2 (g/mg min) | 0.0003 | 0.0005 | 0.0008 |
| MPSD | 0.339 | 0.326 | 0.462 |
| R2 | 0.989 | 0.977 | 0.959 |
| 0.986 | 0.971 | 0.947 | |
| IPD | |||
| qe (mg/g) | 85.6 | 66.3 | 90.01 |
| ki (mg/g min0.5) | 10.39 | 8.88 | 4.95 |
| R2 | 0.939 | 0.991 | 0.989 |
| 0.922 | 0.990 | 0.986 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzkiewicz, M.; Podkościelna, B.; Podkościelny, P. Application of Functionalized DVB-co-GMA Polymeric Microspheres in the Enhanced Sorption Process of Hazardous Dyes from Dyeing Baths. Molecules 2020, 25, 5247. https://doi.org/10.3390/molecules25225247
Wawrzkiewicz M, Podkościelna B, Podkościelny P. Application of Functionalized DVB-co-GMA Polymeric Microspheres in the Enhanced Sorption Process of Hazardous Dyes from Dyeing Baths. Molecules. 2020; 25(22):5247. https://doi.org/10.3390/molecules25225247
Chicago/Turabian StyleWawrzkiewicz, Monika, Beata Podkościelna, and Przemysław Podkościelny. 2020. "Application of Functionalized DVB-co-GMA Polymeric Microspheres in the Enhanced Sorption Process of Hazardous Dyes from Dyeing Baths" Molecules 25, no. 22: 5247. https://doi.org/10.3390/molecules25225247
APA StyleWawrzkiewicz, M., Podkościelna, B., & Podkościelny, P. (2020). Application of Functionalized DVB-co-GMA Polymeric Microspheres in the Enhanced Sorption Process of Hazardous Dyes from Dyeing Baths. Molecules, 25(22), 5247. https://doi.org/10.3390/molecules25225247