Synthesis of Polyheterocyclic Dimers Containing Restricted and Constrained Peptidomimetics via IMCR-Based Domino/Double CuAAC Click Strategy
Abstract
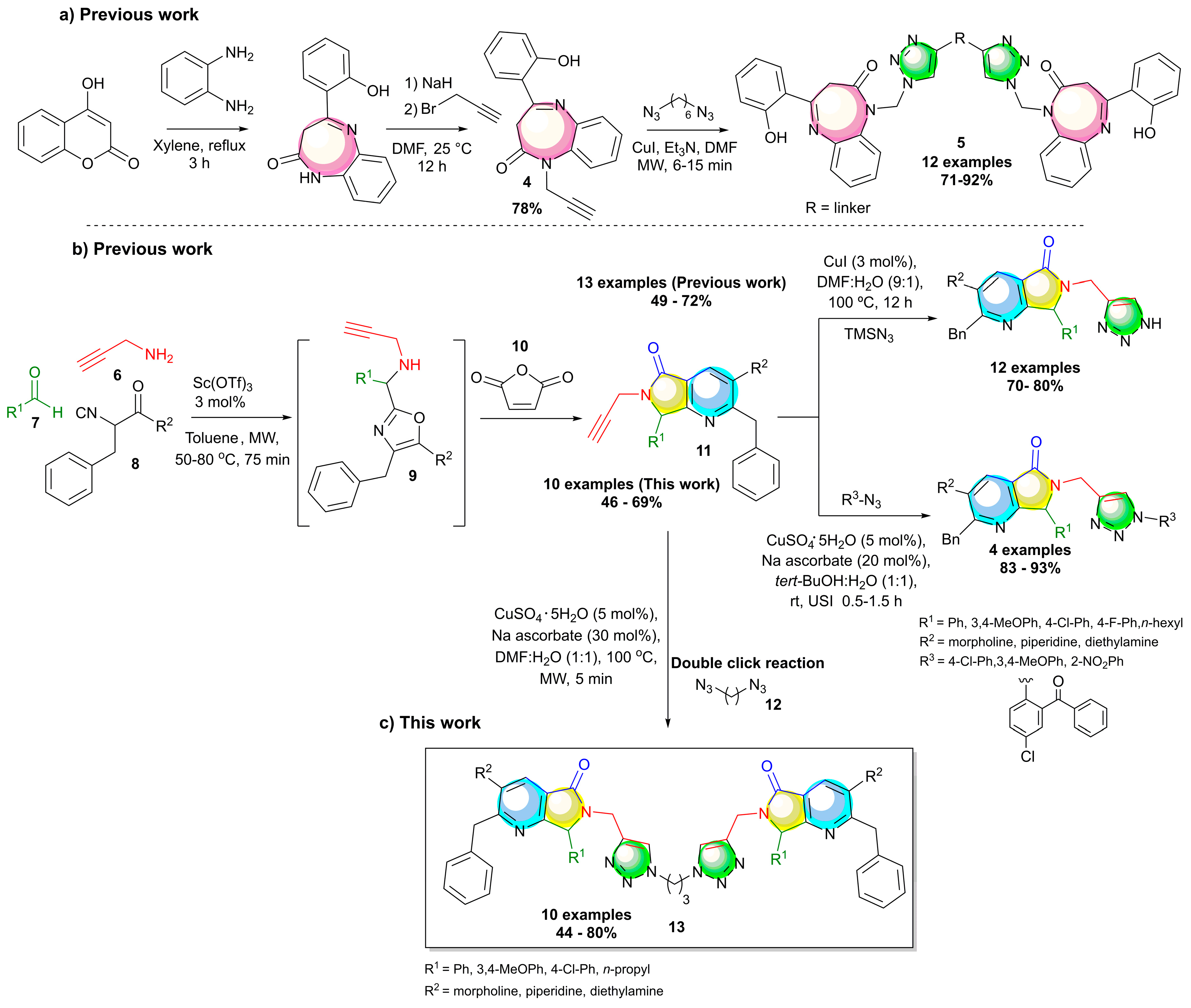
1. Introduction
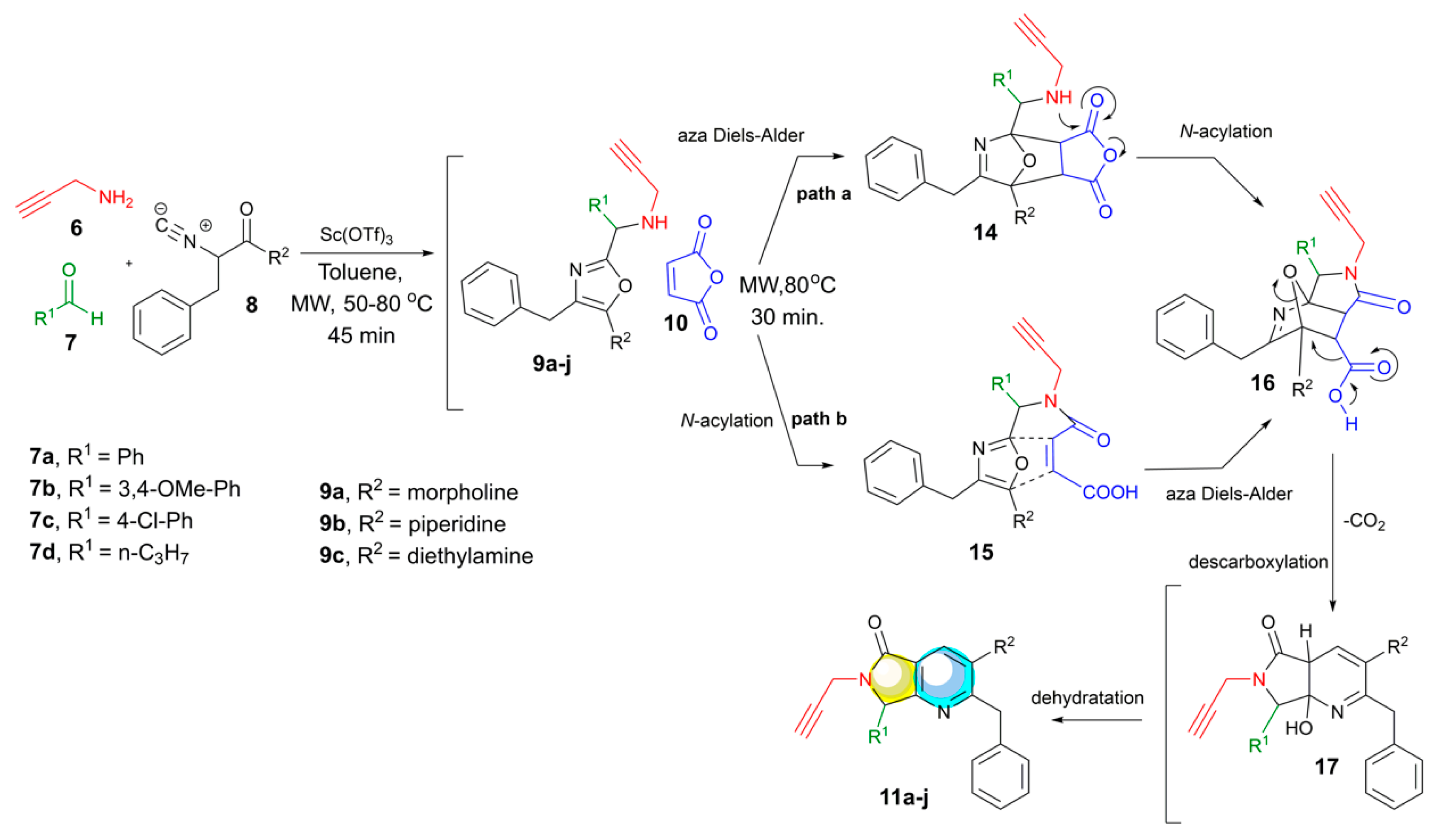
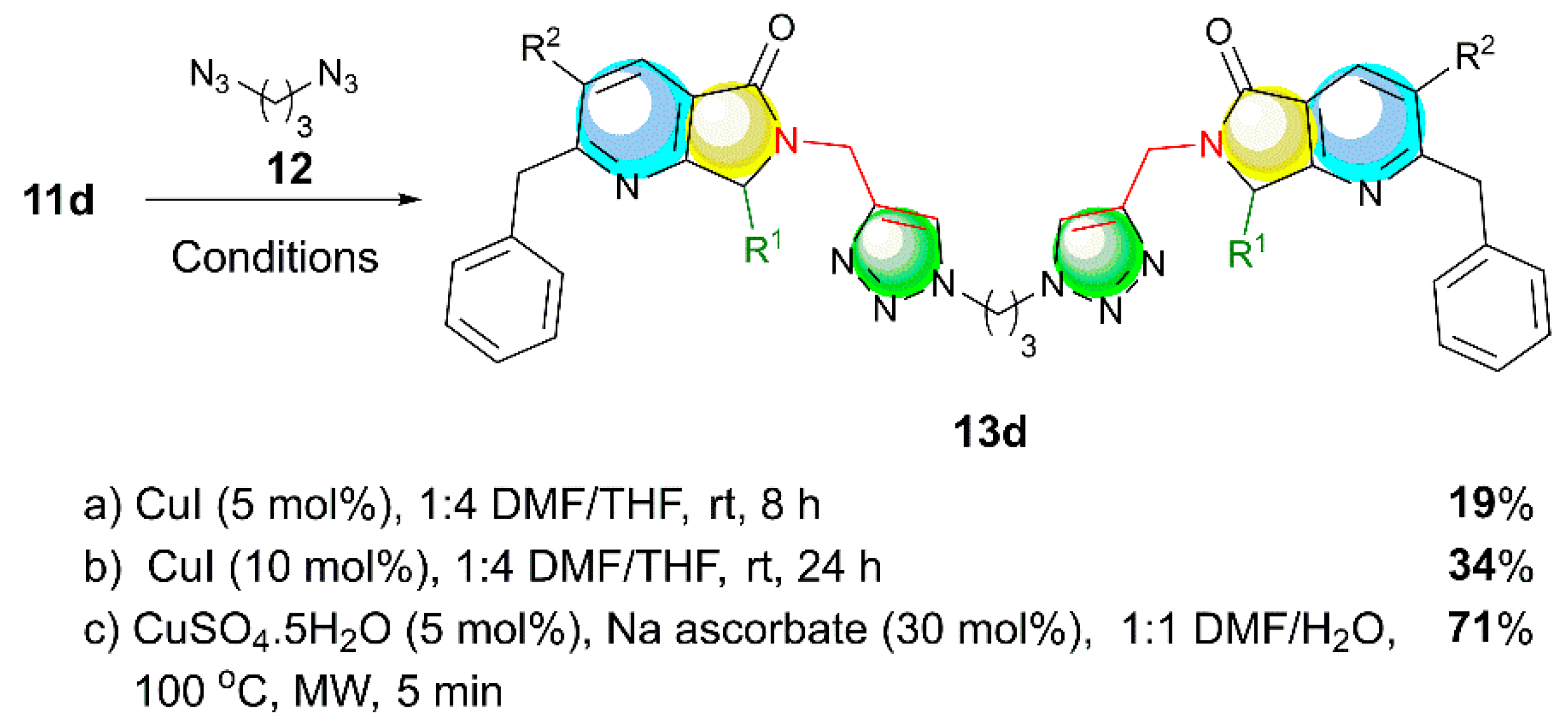
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthetic Procedures
3.2.1. General procedure for the synthesis and characterization of the 6-Propargyl-pyrrolo[3,4-b]pyridin-5-ones 11a–j (GP-1)
3.2.2. General procedure for the synthesis and characterization of the Propane-linked bis-Triazolyl-pyrrolo[3,4-b]pyridin-5-ones 13a–j (GP-2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koopmanschap, G.; Ruijter, E.; Orru, R.V. Isocyanide-based multicomponent reactions towards cyclic constrained peptidomimetics. Beilstein J. Org. Chem. 2014, 10, 544–598. [Google Scholar] [CrossRef] [PubMed]
- Vagner, J.; Qu, H.; Hruby, V.J. Peptidomimetics, a synthetic tool of drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A.A. Click chemistry reactions in medicinal chemistry: Applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 2008, 28, 278–308. [Google Scholar] [CrossRef]
- Terracciano, S.; Chini, M.G.; Piaz, F.D.; Vassallo, A.; Riccio, R.; Bruno, I.; Bifulco, G. Dimeric and trimeric triazole based molecules as a new class of Hsp90 molecular chaperone inhibitors. Eur. J. Med. Chem. 2013, 65, 464–476. [Google Scholar] [CrossRef]
- Janganati, V.; Ponder, J.; Balasubramaniam, M.; Bhat-Nakshatri, P.; Bar, E.E.; Nakshatri, H.; Jordan, C.T.; Crooks, P.A. MMB triazole analogs are potent NF-κB inhibitors and anti-cancer agents against both hematological and solid tumor cells. Eur. J. Med. Chem. 2018, 157, 562–581. [Google Scholar] [CrossRef]
- Zhu, X.; Wong, I.L.K.; Chan, K.-F.; Cui, J.; Law, M.C.; Chong, T.C.; Hu, X.; Chow, L.M.C.; Chan, T.H. Triazole Bridged Flavonoid Dimers as Potent, Nontoxic, and Highly Selective Breast Cancer Resistance Protein (BCRP/ABCG2) Inhibitors. J. Med. Chem. 2019, 62, 8578–8608. [Google Scholar] [CrossRef]
- Granchi, C.; Roy, S.; Del Fiandra, C.; Tuccinardi, T.; Lanza, M.; Betti, L.; Giannaccini, G.; Lucacchini, A.; Martinelli, A.; Macchia, M.; et al. Triazole-substituted N-hydroxyindol-2-carboxylates as inhibitors of isoform 5 of human lactate dehydrogenase (hLDH5). Med. Chem. Commun. 2011, 2, 638–643. [Google Scholar] [CrossRef]
- Celik, F.; Unver, Y.; Barut, B.; Ozel, A.; Sancak, K. Synthesis, Characterization and Biological Activities of New Symmetric Bis-1,2,3-Triazoles with Click Chemistry. Med. Chem. 2018, 14, 230–241. [Google Scholar] [CrossRef]
- Jaafar, Z.; Chniti, S.; Sassi, A.B.; Dziri, H.; Marque, S.; Lecouvey, M.; Gharbi, R.; Msaddek, M. Design and microwave-assisted synthesis of dimers of 1,5-benzodiazepine-1,2,3-triazole hybrids bearing alkyl/aryl spacers and their biological assessment. J. Mol. Struct. 2019, 1195, 689–701. [Google Scholar] [CrossRef]
- Kaushik, C.P.; Kumar, K.; Singh, D.; Singh, S.K.; Jindal, D.K.; Luxmi, R. Synthesis, Characterization, and Antimicrobial Potential of Some 1,4-Disubstituted 1,2,3-Bistriazoles. Synth. Commun. 2015, 45, 1977–1985. [Google Scholar] [CrossRef]
- Popov, A.B.; Stolić, I.; Krstulović, L.; Taylor, M.C.; Kelly, J.M.; Tomić, S.; Tumir, L.; Bajić, M.; Raić-Malića, S. Novel symmetric bis-benzimidazoles: Synthesis, DNA/RNA binding and antitrypanosomal activity. Eur. J. Med. Chem. 2019, 173, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Kühhorn, J.; Hübner, H.; Gmeiner, P. Bivalent Dopamine D2 Receptor Ligands: Synthesis and Binding Properties. J. Med. Chem. 2011, 54, 13, 4896–4903. [Google Scholar] [CrossRef]
- Crowley, J.D.; McMorran, D.A. “Click-Triazole” Coordination Chemistry: Exploiting 1,4-Disubstituted-1,2,3-Triazoles as Ligands. In Click Triazoles. Topics in Heterocyclic Chemistry; Košmrlj, J., Ed.; Springer: Berlin, Germany, 2012; Volume 28, pp. 31–83. [Google Scholar] [CrossRef]
- Schulzeab, B.; Schubert, U.S. Beyond click chemistry—supramolecular interactions of 1,2,3-triazoles. Chem. Soc. Rev. 2014, 43, 2522–2571. [Google Scholar] [CrossRef] [PubMed]
- Devasthale, P.; Wang, Y.; Wang, W.; Fevig, J.; Feng, J.; Wang, A.; Harrity, T.; Egan, D.; Morgan, N.; Cap, M.; et al. Optimization of activity, selectivity, and liability profiles in 5-oxopyrrolopyridine DPP4 inhibitors leading to clinical candidate (Sa)-2-(3-(aminomethyl)-4-(2,4-dichlorophenyl)-2-methyl-5-oxo-5H-pyrrolo[3,4-b]pyridin-6(7H)-yl)-N,N-dimethylacetamide (BMS-767778). J. Med. Chem. 2013, 56, 7343–7357. [Google Scholar] [CrossRef]
- Unverferth, K.; Arnold, T.; Lankau, H.; Rostock, A.; Tober, C.; Dost, R.; Rundfeldt, C.; Gasparic, A. 6,7-Dihydro-Pirrolo[3,4-b]Pyridin-5-One With an Anticonvulsive Action and Methods for the Same World Intellectual WO 02/18381 A1, Geneva, Switzerland. 2002. Available online: https://worldwide.espacenet.com/patent/search/family/007653968/publication/WO0218381A1?q=WO0218381 (accessed on 29 October 2020).
- Chang, H.-F.; Chapdelaine, M.; Dembofsky, B.T.; Herzog, K.J.; Horschler, C.; Schmiesing, R.J. Compounds and Uses Thereof−848. Patent Cooperation Treaty (PCT). WO2008/155572 A1, 2008. Geneva: World Intellectual Property Organization. Property Organization. A: Geneva, Switzerland. 2008. Available online: https://worldwide.espacenet.com/patent/search/family/040084145/publication/WO2008155572A2?q=WO2008155572A2 (accessed on 29 October 2020).
- Pajouhesh, H.; Hollan, R.; Zhou, Y.; Zhu, Y.; Grimwood, M.E.; Chahal, N. Bisaylsufone and Dialkylarylsulfone Compounds as Calcium Channel Blockers. Patent Cooperation Treaty (PCT) WO2012/061926 A1, 18 May 2012. Available online: https://worldwide.espacenet.com/patent/search/family/046050268/publication/WO2012061926A1?q=WO2012061926A1 (accessed on 29 October 2020).
- Lindsley, C.W.; Conn, P.J.; Wood, M.R.; Hopkins, C.R.; Melancon, B.J.; Poslusney, M.S.; Engers, D.W. Substituted 2-(4-heterocyclylbenzyl)Isoindolin-1-One Analogs as Positive Allosteric Modulators of Muscarinic Acethylcholine ReceptorM1. Patent Cooperation Treaty (PCT) WO2013/063549 A1, 2 May 2013. Available online: https://worldwide.espacenet.com/patent/search/family/048168630/publication/WO2013063549A1?q=pn%3DWO2013063549A1 (accessed on 29 October 2020).
- Wager, T.T.; Galatsis, P.; Chandrasekaran, R.Y.; Butler, T.W.; Li, J.; Zhang, L.; Mente, S.; Subramanyam, C.; Liu, S.; Doran, A.C.; et al. Identification and Profiling of a Selective and Brain Penetrant Radioligand for in Vivo Target Occupancy Measurement of Casein Kinase 1 (CK1) Inhibitors. ACS Chem. Neurosci. 2017, 8, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Tantcheva, L.; Petkov, V.V.; Karamukova, G.; Abarova, S.; Chekalarova, Y.; Tsekova, D.; Escuder, B.; Miravet, J.; Lyubomirova, K. Neuropharmacological activity of newly synthesized derivatives of valin. Bulg. Chem. Comm. 2006, 38, 54–57. [Google Scholar]
- Tsekova, D.S.; Makakova, E.; Alov, P.; Gorneva, G.; Pajeva, I.; Tancheva, L.; Petkov, V.; Surleva, A.; Escuder, B.; Miravet, J.; et al. Structure-activity relationships of new L-Valine derivatives with neuropharmacological effects. Bulg. Chem. Comm. 2009, 41, 133–137. [Google Scholar]
- Tancheva, L.P.; Encheva, E.N.; Tsekova, D.S.; Alova, L.G.; Stancheva, S.L.; Petkov, V.V.; Novoselski, M.T.; Klisurov, R. New L- valine peptide mimetics as potential neuropharmacological agents. Bulg. Chem. Comm. 2012, 44, 262–266. [Google Scholar]
- Tsekova, D.S.; Klisurov, R.; Tancheva, L.; Encheva, E.; Genadieva, M. The influence of solvents on the molecular structure and biological activity Of N,N/-Bis(N-nicotinoyl-l-valyl)-diaminohexane. J. Chem. Technol. Metall. 2013, 48, 637–641. [Google Scholar]
- Liu, Q.; Zhang, S.W.; Wei, Y.G.; Shao, M.C. Anticancer and Antifertility Agents. II. N,N’-Hexamethylenebis(3-pyridinecarboxamide). Acta Crystallogr. Sect. C: Cryst. Struct. Commun. 1996, 52, 2260–2261. [Google Scholar] [CrossRef]
- Lubell, W.D. Topics in Heterocyclic Chemistry: Peptidomimetics I; Springer: Cham, Switzerland, 2017; Volume 48, pp. 1–305. [Google Scholar] [CrossRef]
- Wessjohann, L.A.; Rhoden, C.R.B.; Rivera, D.G.; Vercillo, O.E. Cyclic Peptidomimetics and Pseudopeptides from Multicomponent Reactions. In Synthesis of Heterocycles via Multicomponent Reactions I. Topics in Heterocyclic Chemistry; Orru, R., Ruijter, E., Eds.; Springer: Berlin, Germany, 2010; Volume 23, pp. 199–226. [Google Scholar] [CrossRef]
- Ruijter, E.; Scheffelaar, R.; Orru, R.V.A. Multicomponent Reaction Design in the Quest for Molecular Complexity and Diversity. Angew. Chem. Int. Ed. 2011, 50, 6234–6246. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, J. Multicomponent Domino Process: Rational Design and Serendipity. In Domino Reactions: Concepts for Efficient Organic Synthesis; Tietze, L.F., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA, Boschstr: Weinheim, Germany, 2013; pp. 579–610. [Google Scholar] [CrossRef]
- Sun, X.; Janvier, P.; Zhao, G.; Bienayme, H.; Zhu, J. A Novel Multicomponent Synthesis of Polysubstituted 5-Aminooxazole and Its New Scaffold-Generating Reaction to Pyrrolo[3,4-b]pyridine. Org. Lett. 2001, 3, 877–880. [Google Scholar] [CrossRef]
- Rentería-Gómez, M.A.; Islas-Jácome, A.; Pharande, S.G.; Vosburg, D.A.; Gámez-Montaño, R. Synthesis of Tris-Heterocycles via a Cascade IMCR/Aza Diels-Alder + CuAAC Strategy. Front. Chem. 2019, 7, 546. [Google Scholar] [CrossRef]
- Islas-Jácome, A.; González-Zamora, E.; Gámez-Montaño, R. A short microwave-assisted synthesis of tetrahydroisoquinolin-pyrrolopyridinones by a triple process: Ugi-3CR–aza Diels–Alder/S-oxidation/Pummerer. Tetrahedron Lett. 2011, 52, 5245–5248. [Google Scholar] [CrossRef]
- Islas-Jácome, A.; Cárdenas-Galindo, L.E.; Jerezano, A.V.; Tamariz, J.; González-Zamora, E.; Gámez-Montaño, R. Synthesis of Nuevamine Aza-Analogues by a Sequence: I-MCR–Aza-Diels–Alder–Pictet–Spengler. Synlett 2012, 2951–2956. [Google Scholar] [CrossRef]
- Gordillo-Cruz, R.E.; Rentería-Gómez, A.; Islas-Jácome, A.; Cortes-García, C.J.; Díaz-Cervantes, E.; Robles, J.; Gámez-Montaño, R. Synthesis of 3-tetrazolylmethyl-azepino[4,5-b]indol-4-ones in two reaction steps: (Ugi-azide/N-acylation/SN2)/free radical cyclization and docking studies to a 5-Ht6 model. Org. Biomol. Chem. 2013, 11, 6470–6476. [Google Scholar] [CrossRef]
- Cárdenas-Galindo, L.E.; Islas-Jácome, A.; Alvárez-Rodríguez, N.V.; El Kaim, L.; Gámez-Montaño, R. aper Synthesis of 2-Tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines by a One-Pot Ugi-Azide/Pictet–Spengler Process. Synthesis 2014, 46, 49–56. [Google Scholar] [CrossRef][Green Version]
- Rentería-Gómez, A.; Islas-Jácome, A.; Díaz-Cervantes, E.; Villaseñor-Granados, T.; Robles, J.; Gámez-Montaño, R. Synthesis of azepino[4,5-b]indol-4-ones via MCR/free radical cyclization and in vitro–in silico studies as 5-Ht6R ligands. Bioorg. Med. Chem. 2016, 26, 2333–2338. [Google Scholar] [CrossRef]
- Rentería-Gómez, A.; Islas-Jácome, A.; Cruz-Jiménez, A.E.; Manzano-Velázquez, J.C.; Rojas-Lima, S.; Jiménez-Halla, J.O.C. Gámez-Montaño, R. Synthesis of 2-Tetrazolylmethyl-isoindolin-1-ones via a One-Pot Ugi-Azide/(N-Acylation/exo-Diels–Alder)/Dehydration Process. ACS Omega 2016, 1, 943–951. [Google Scholar] [CrossRef]
- Alvárez-Rodríguez, N.V.; Islas-Jácome, A.; Rentería-Gómez, A.; Cárdenas-Galindo, L.E.; Unnamatla, M.V.B.; Gámez-Montaño, R. Synthesis of 1′-tetrazolylmethyl-spiro[pyrrolidine-3,3′-oxindoles] via two coupled one-pot processes Ugi-azide/Pictet–Spengler and oxidative spiro-rearrangement. New J. Chem. 2018, 42, 1600–1603. [Google Scholar] [CrossRef]
- Claudio-Catalán, M.Á.; Pharande, S.G.; Quezada-Soto, A.; Kishore, K.G.; Rentería-Gómez, A.; Padilla-Vaca, F.; Gámez-Montaño, R. Solvent- and Catalyst-Free One-Pot Green Bound-Type Fused Bis-Heterocycles Synthesis via Groebke–Blackburn–Bienaymé Reaction/SNAr/Ring-Chain Azido-Tautomerization Strategy. ACS Omega 2018, 3, 5177–5186. [Google Scholar] [CrossRef]
- Unnamatla, M.V.B.; Islas-Jácome, A.; Quezada-Soto, A.; Ramírez-López, S.C.; Flores-Álamo, M.; Gámez-Montaño, R. Multicomponent One-Pot Synthesis of 3-Tetrazolyl and 3-Imidazo[1,2-a]pyridin Tetrazolo[1,5-a]quinolines. J. Org. Chem. 2016, 81, 10576–10583. [Google Scholar] [CrossRef]
- Kaveti, B.; Ramírez-López, S.C.; Gámez Montaño, R. Ultrasound-assisted green one-pot synthesis of linked bis-heterocycle peptidomimetics via IMCR/post-transformation/tandem strategy. Tetrahedron Lett. 2018, 59, 4355–4358. [Google Scholar] [CrossRef]
- Shaabani, S.; Shaabani, A.; Ng, S.W. One-Pot Synthesis of Coumarin-3-carboxamides Containing a Triazole Ring via an Isocyanide-Based Six-Component Reaction. ACS Comb. Sci. 2014, 16, 176–183. [Google Scholar] [CrossRef]
- Qian, W.; Wang, D.; Wang, H.; Yu, P.; Liu, S.; Chen, S. One-pot synthesis of 3-triazolyl-quinolin-2-(1H)-ones via sequential Ugi-“click”-Knoevenagel condensations. Tetrahedron Lett. 2018, 59, 2167–2169. [Google Scholar] [CrossRef]
- Shaabani, A.; Hajishaabanha, F.; Mahyari, M.; Mofakham, H.; Ng, S.W. A novel one-pot pseudo-four-component isocyanide-based reaction: An unexpected approach for the synthesis of tetrahydrodiisoindoloquinoxalines and tetrahydrobenzodiisoindoloquinoxalines. Tetrahedron 2011, 67, 8360–8366. [Google Scholar] [CrossRef]
- Ghorbani-Vaghei, R.; Amiri, M.; Karimi-Nami, R.; Salimi, Z. Efficient one-pot synthesis of mono and bis-N-cyclohexyl-3-alkyl(aryl)-quinoxaline-2-amines using N-halo catalysts. RSC Advances 2013, 3, 25924–25929. [Google Scholar] [CrossRef]
- Cárdenas-Galindo, L.; Islas-Jácome, A.; Colmenero-Martínez, K.; Martínez-Richa, A.; Gámez-Montaño, R. Synthesis of Novel bis-1,5-Disubstituted-1H-Tetrazoles by an Efficient Catalyst-Free Ugi-Azide Repetitive Process. Molecules 2015, 20, 1519–1526. [Google Scholar] [CrossRef]
- Ghandi, M.; Sherafat, F. Expedient Access to Novel Bis-tetrazolopiperazines via Ugi-azide Reactions. J. Heterocycl. Chem. 2016, 54, 1396–1403. [Google Scholar] [CrossRef]
- Feng, Q.-Y.; Zhu, J.; Wang, M.-X.; Tong, S. Organocatalytic Double Ugi Reaction with Statistical Amplification of Product Enantiopurity: A Linker Cleavage Approach To Access Highly Enantiopure Ugi Products. Org. Lett. 2020, 22, 483–487. [Google Scholar] [CrossRef]
- Lu, K.; Luo, T.; Xiang, Z.; You, Z.; Fathi, R.; Chen, J.; Yang, Z. A Concise and Diversity-Oriented Strategy for the Synthesis of Benzofurans and Indoles via Ugi and Diels−Alder Reactions. J. Comb. Chem. 2005, 7, 958–967. [Google Scholar] [CrossRef]
- Pertejo, P.; García-Valverde, M.; Peña, P.; Cordero, N.A.; Torroba, T.; González-Ortega, A. Experimental and theoretical studies on the effect of the oxo group in 1,4-benzodiazepines. Org. Biomol. Chem. 2014, 12, 4905–4916. [Google Scholar] [CrossRef] [PubMed]
- Zamudio-Medina, A.; García-González, M.C.; Gutierrez-Carrillo, A.; González-Zamora, E. Synthesis of cyclic analogues of hexamethylenebis(3-pyridine)amide (HMBPA) in a one-pot process. Tetrahedron Lett. 2015, 56, 627–629. [Google Scholar] [CrossRef]
- Islas-Jácome, A.; Rentería-Gómez, A.; Rentería-Gómez, M.A.; González-Zamora, E. Jiménez-Halla, J.O.C. Gámez-Montaño, R. Selective reaction route in the construction of the pyrrolo[3,4-b]pyridin-5-one core from a variety of 5-aminooxazoles and maleic anhydride. A DFT study. Tetrahedron Lett. 2016, 57, 3496–3500. [Google Scholar] [CrossRef]
- Janvier, P.; Sun, X.; Bienayme, H.; Zhu, J. Ammonium Chloride-Promoted Four-Component Synthesis of Pyrrolo[3,4-b]pyridin-5-one. J. Am. Chem. Soc. 2002, 124, 2560–2567. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Sugiura, M.; Kitagawa, H.; Lam, W.W.-L. Kobayashi S. Scandium triflate in organic synthesis. Chem. Rev. 2002, 102, 2227–2302. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Bock, V.D.; Hiemstra, H.; van Maarseveen, J.H. CuI -Catalyzed Alkyne–Azide “Click” Cycloadditions from a Mechanistic and Synthetic Perspective. Eur. J. Org. Chem. 2006, 51–68. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide–alkynecycloaddition (CuAAC) and beyond: New reactivity of copper(i) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- Lauria, A.; Delisi, R.; Mingoia, F.; Terenzi, A.; Martorana, A.; Barone, G.; Almerico, A.M. 1,2,3-Triazole in Heterocyclic Compounds, Endowed with Biological Activity, through 1,3-Dipolar Cycloadditions. Eur. J. Org. Chem. 2014, 3289–3306. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xue, P.; Sun, H.H.Y.; Williams, I.D.; Sharpless, K.B.; Fokin, V.V.; Jia, G. Ruthenium-Catalyzed Cycloaddition of Alkynes and Organic Azides. J. Am. Chem. Soc. 2005, 127, 15998–15999. [Google Scholar] [CrossRef]
- Johansson, J.R.; Beke-Somfai, T.; Stålsmeden, S.A.; Kann, N. Ruthenium-Catalyzed Azide Alkyne Cycloaddition Reaction: Scope, Mechanism, and Applications. Chem. Rev. 2016, 116, 14726–14768. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 13a–j are available from the authors. |


| Entry | R1 | R2 | 11 (%) a | 13 (%) a |
|---|---|---|---|---|
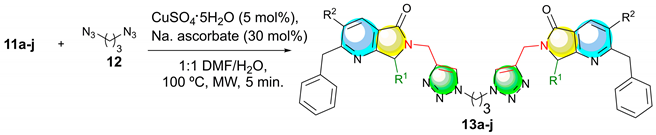 | ||||
| 1 | Ph | morpholine | 11a, 64 | 13a, 50 |
| 2 | 3,4-MeOPh | morpholine | 11b, 62 | 13b, 63 |
| 3 | 4-Cl-Ph | morpholine | 11c, 53 | 13c, 79 |
| 4 | n-propyl | morpholine | 11d, 47 | 13d, 71 |
| 5 | 3,4-MeOPh | piperidine | 11e, 66 | 13e, 80 |
| 6 | 4-Cl-Ph | piperidine | 11f, 56 | 13f, 69 |
| 7 | Ph | diethylamine | 11g, 59 | 13g, 49 |
| 8 | 3,4-MeOPh | diethylamine | 11h, 63 | 13h, 53 |
| 9 | 4-Cl-Ph | diethylamine | 11i, 46 | 13i, 44 |
| 10 | n-propyl | diethylamine | 11j, 50 | 13j, 57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pharande, S.G.; Rentería-Gómez, M.A.; Gámez-Montaño, R. Synthesis of Polyheterocyclic Dimers Containing Restricted and Constrained Peptidomimetics via IMCR-Based Domino/Double CuAAC Click Strategy. Molecules 2020, 25, 5246. https://doi.org/10.3390/molecules25225246
Pharande SG, Rentería-Gómez MA, Gámez-Montaño R. Synthesis of Polyheterocyclic Dimers Containing Restricted and Constrained Peptidomimetics via IMCR-Based Domino/Double CuAAC Click Strategy. Molecules. 2020; 25(22):5246. https://doi.org/10.3390/molecules25225246
Chicago/Turabian StylePharande, Shrikant G., Manuel A. Rentería-Gómez, and Rocío Gámez-Montaño. 2020. "Synthesis of Polyheterocyclic Dimers Containing Restricted and Constrained Peptidomimetics via IMCR-Based Domino/Double CuAAC Click Strategy" Molecules 25, no. 22: 5246. https://doi.org/10.3390/molecules25225246
APA StylePharande, S. G., Rentería-Gómez, M. A., & Gámez-Montaño, R. (2020). Synthesis of Polyheterocyclic Dimers Containing Restricted and Constrained Peptidomimetics via IMCR-Based Domino/Double CuAAC Click Strategy. Molecules, 25(22), 5246. https://doi.org/10.3390/molecules25225246







