Effects of Sucrose Palmitate on the Physico-Chemical and Mucoadhesive Properties of Buccal Films
Abstract
1. Introduction
2. Results and Discussion
2.1. Tensile Strength and Deformation Process
2.2. Surface Free Energy
2.3. Spreading Coefficient
2.4. Mucoadhesive Force
2.5. X-ray Powder Diffraction (XRPD)
2.6. Positron Annihilation Lifetime Spectroscopy (PALS)
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Samples
3.2.2. Tensile Strength
3.2.3. Surface Free Energy (SFE)
3.2.4. Spreading Coefficient
3.2.5. Mucoadhesive Force and Mucoadhesive Work
3.2.6. X-ray Powder Diffractometry (XRPD)
3.2.7. Positron Annihilation Lifetime Spectroscopy (PALS)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mojsiewicz-Pienkowska, K.; Jamrógiewicz, M.; Zebrowska, M.; Mikolaszek, B.; Sznitowska, M. Double layer adhesive silicone dressing as a potential dermal drug delivery film in scar treatment. Int. J. Pharm. 2015, 481, 18–26. [Google Scholar] [CrossRef]
- Mikolaszek, B.; Jamrógiewicz, M.; Mojsiewicz-Pienkowska, K.; Zebrowska, M.; Sznitowska, M.; Strankowska, J. Physical and Mechanical Evaluation of Silicone-Based Double-Layer Adhesive Patch Intended for Keloids and Scar Treatment Therapy. Polymers 2016, 8, 398. [Google Scholar] [CrossRef]
- Kerec, M.; Bogataj, M.; Mugerle, B.; Gašperlin, M.; Mrhar, A. Mucoadhesion on pig vesical mucosa: Influence of polycarbophil/calcium interactions. Int. J. Pharm. 2002, 241, 135–143. [Google Scholar] [CrossRef]
- Sandri, G.; Motta, S.; Bonferoni, M.C.; Brocca, P.; Rossi, S.; Ferrari, F.; Rondelli, V.; Cantù, L.; Caramella, C.; Del Favero, E. Chitosan-coupled solid lipid nanoparticles: Tuning nanostructure and mucoadhesion. Eur. J. Pharm. Biopharm. 2017, 110, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Sandri, G.; Ferrari, F.; Rossi, S.; Larghi, V.; Zambito, Y.; Caramella, C. Comparison of different in vitro and ex vivo methods to evaluate mucoadhesion of glycol-palmitoyl chitosan micelles. J. Drug Deliv. Sci. Technol. 2010, 20, 419–424. [Google Scholar] [CrossRef]
- Khan, S.; Boateng, J. Effects of Cyclodextrins (β and γ) and L-Arginine on Stability and Functional Properties of Mucoadhesive Buccal Films Loaded with Omeprazole for Pediatric Patients. Polymers 2018, 10, 157. [Google Scholar] [CrossRef]
- De Souza Cintra, G.A.; Pinto, L.A.; Calixto, G.M.F.; Soares, C.P.; de Souza Von Zuben, E.; Scarpa, M.V.; Gremião, M.P.D.; Chorilli, M. Bioadhesive Surfactant Systems for Methotrexate Skin Delivery. Molecules 2016, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Frade, M.L.; de Annunzio, S.R.; Calixto, G.M.F.; Victorelli, F.D.; Chorilli, M.; Fontana, C.R. Assessment of Chitosan-Based Hydrogel and Photodynamic Inactivation against Propionibacterium acnes. Molecules 2018, 23, 473. [Google Scholar]
- Preis, M.; Woertz, C.; Schneider, K.; Kukawka, J.; Broscheit, J.; Roewer, N.; Breitkreutz, J. Design and evaluation of bilayered buccal film preparations for local administration of lidocaine hydrochloride. Eur. J. Pharm. Biopharm. 2014, 86, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, C.; Fini, A.; Ospitali, F. Mucoadhesive multiparticulate patch for the intrabuccal controlled delivery of lidocaine. Eur. J. Pharm. Biopharm. 2013, 83, 405–414. [Google Scholar] [CrossRef]
- Morales, J.O.; McConville, J.T. Manufacture and characterization of mucoadhesive buccal films. Eur. J. Pharm. Biopharm. 2011, 77, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, C.; Brigidi, P.; Fini, A. Ex-vivo and in-vitro assessment of mucoadhesive patches containing the gel-forming polysaccharide psyllium for buccal delivery of chlorhexidine base. Int. J. Pharm. 2015, 496, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Shiledar, R.R.; Tagalpallewar, A.A.; Kokare, C.R. Formulation and in vitro evaluation of xanthan gum-based bilayered mucoadhesive buccal patches of zolmitriptan. Carbohydr. Polym. 2014, 101, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Mennini, N.; Kosalec, I.; Furlanetto, S.; Orlandini, S.; Jug, M. Amidated pectin-based wafers for econazole buccal delivery: Formulation optimization and antimicrobial efficacy estimation. Carbohydr. Polym. 2015, 121, 231–240. [Google Scholar] [CrossRef]
- Masek, J.; Lubasova, D.; Lukac, R.; Turanek-Knotigova, P.; Kulich, P.; Plockova, J.; Maskova, E.; Prochazka, L.; Koudelka, S.; Sasithorn, N.; et al. Multi-layered nanofibrous mucoadhesive films for buccal and sublingual administration of drug-delivery and vaccination nanoparticles—important step towards effective mucosal vaccines. J. Control. Release 2017, 249, 183–195. [Google Scholar] [CrossRef]
- Anarjan, N.; Tan, C.P. Effects of Selected Polysorbate and Sucrose Ester Emulsifiers on the Physicochemical Properties of Astaxanthin Nanodispersions. Molecules 2013, 18, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.H.Y.; Regdon, G., Jr.; Kristó, K.; Kelemen, A.; Adam, M.E.; Hamedelniel, E.I.; Sovány, T. Design and characterization of chitosan/citrate films as carrier for oral macromolecule delivery. Eur. J. Pharm. Sci. 2020, 146, 105270. [Google Scholar] [CrossRef]
- Kumria, R.; Nair, A.B.; Al-Dhubiab, B.E. Loratidine buccal films for allergic rhinitis: Development and evaluation. Drug Dev. Ind. Pharm. 2014, 40, 625–631. [Google Scholar] [CrossRef]
- Perioli, L.; Ambrogi, V.; Angelici, F.; Ricci, M.; Giovagnoli, S.; Capuccella, M.; Rossi, C. Development of mucoadhesive patches for buccal administration of ibuprofen. J. Control. Release 2004, 99, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Okeke, O.C.; Boateng, J.S. Composite HPMC and sodium alginate based buccal formulations for nicotine replacement therapy. Int. J. Biol. Macromol. 2016, 91, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Szűts, A.; Pallagi, E.; Regdon, G., Jr.; Aigner, Z.; Szabó-Révész, P. Study of thermal behaviour of sugar esters. Int. J. Pharm. 2007, 336, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Gottnek, M.; Süvegh, K.; Pintye-Hódi, K.; Regdon, G. Effects of excipients on the tensile strength, surface properties and free volume of Klucel® free films of pharmaceutical importance. Radiat. Phys. Chem. 2013, 89, 57–63. [Google Scholar] [CrossRef]
- De Caro, V.; Murgia, D.; Seidita, F.; Bologna, E.; Alotta, G.; Zingales, M.; Campisi, G. Enhanced In Situ Availability of Aphanizomenon Flos-Aquae Constituents Entrapped in Buccal Films for the Treatment of Oxidative Stress-Related Oral Diseases: Biomechanical Characterization and In Vitro/Ex Vivo Evaluation. Pharmaceutics 2019, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, A.; Gottnek, M.; Regdon, G., Jr.; Pintye-Hódi, K. New equipment for measurement of the force of adhesion of mucoadhesive films. J. Adhes. Sci. Technol. 2015, 29, 1360–1367. [Google Scholar] [CrossRef]
- Buckton, G. Characterisation of small changes in the physical properties of powders of significance for dry powder inhaler formulations. Adv. Drug Deliv. Rev. 1997, 26, 17–27. [Google Scholar] [CrossRef]
- Bajdik, J.; Fehér, M.; Pintye-Hódi, K. Effect of plasticizer on surface of free films prepared from aqueous solutions of salts of cationic polymers with different plasticizers. Appl. Surf. Sci. 2007, 253, 7303–7308. [Google Scholar] [CrossRef]
- Kristó, K.; Bajdik, J.; Kleinebudde, P.; Pintye-Hódi, K. Effect of lubricant on spreading of coating liquid on surface of tablets containing pancreatin. Pharm. Dev. Technol. 2010, 15, 354–359. [Google Scholar] [CrossRef]
- Wu, S. Calculation of interfacial tension in polymer systems. J. Polym. Sci. 1971, 34, 19–30. [Google Scholar] [CrossRef]
- Ström, G. Krüss Users’ Manual. Krüss. K121. In Contact Angle and Adsorption Measuring System; Krüss GmbH: Hamburg, Germany, 1996; pp. 50–77. [Google Scholar]
- Rowe, R.C. Binder-substrate interactions in granulation: A theoretical approach based on surface free energy and polarity. Int. J. Pharm. 1989, 52, 149–154. [Google Scholar] [CrossRef]
- Tadmor, R.; Das, R.; Gulec, S.; Liu, J.; N’guessan, H.E.; Shah, M.; Wasnik, P.S.; Yadav, S.B. Solid–Liquid Work of Adhesion. Langmuir 2017, 33, 3594–3600. [Google Scholar] [CrossRef]
- Schrader, M.E. Young-Dupre Revisited. Langmuir 1995, 11, 3585–3589. [Google Scholar] [CrossRef]
- Skłodowska, A.; Woźniak, M.; Matlakowska, R. The method of contact angle measurements and estimation of work of adhesion in bioleaching of metals. Biol. Proced. Online 1999, 1, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Ojewole, E.; Pillay, V.; Kumar, P.; Rambharose, S.; Govender, T. Monolayered multipolymeric buccal films with drug and polymers of opposing solubilities for ARV therapy: Physico-mechanical evaluation and molecular mechanics modelling. Int. J. Pharm. 2013, 455, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Costa Idos, S.; Abranches, R.P.; Garcia, M.T.; Pierre, M.B. Chitosan-based mucoadhesive films containing 5-aminolevulinic acid for buccal cancer’s treatment. J. Photochem. Photobiol. B 2014, 140, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Avachat, A.M.; Gujar, K.N.; Wagh, K.V. Development and evaluation of tamarind seed xyloglucan-based mucoadhesive buccal films of rizatriptan benzoate. Carbohydr. Polym. 2013, 91, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Sebe, I.; Szabó, B.; Zelkó, R. A poziron annihilációs élettartam spektroszkópia és gyógyszerészeti alkalmazása. Acta Pharm. Hung. 2012, 82, 23–32. [Google Scholar]
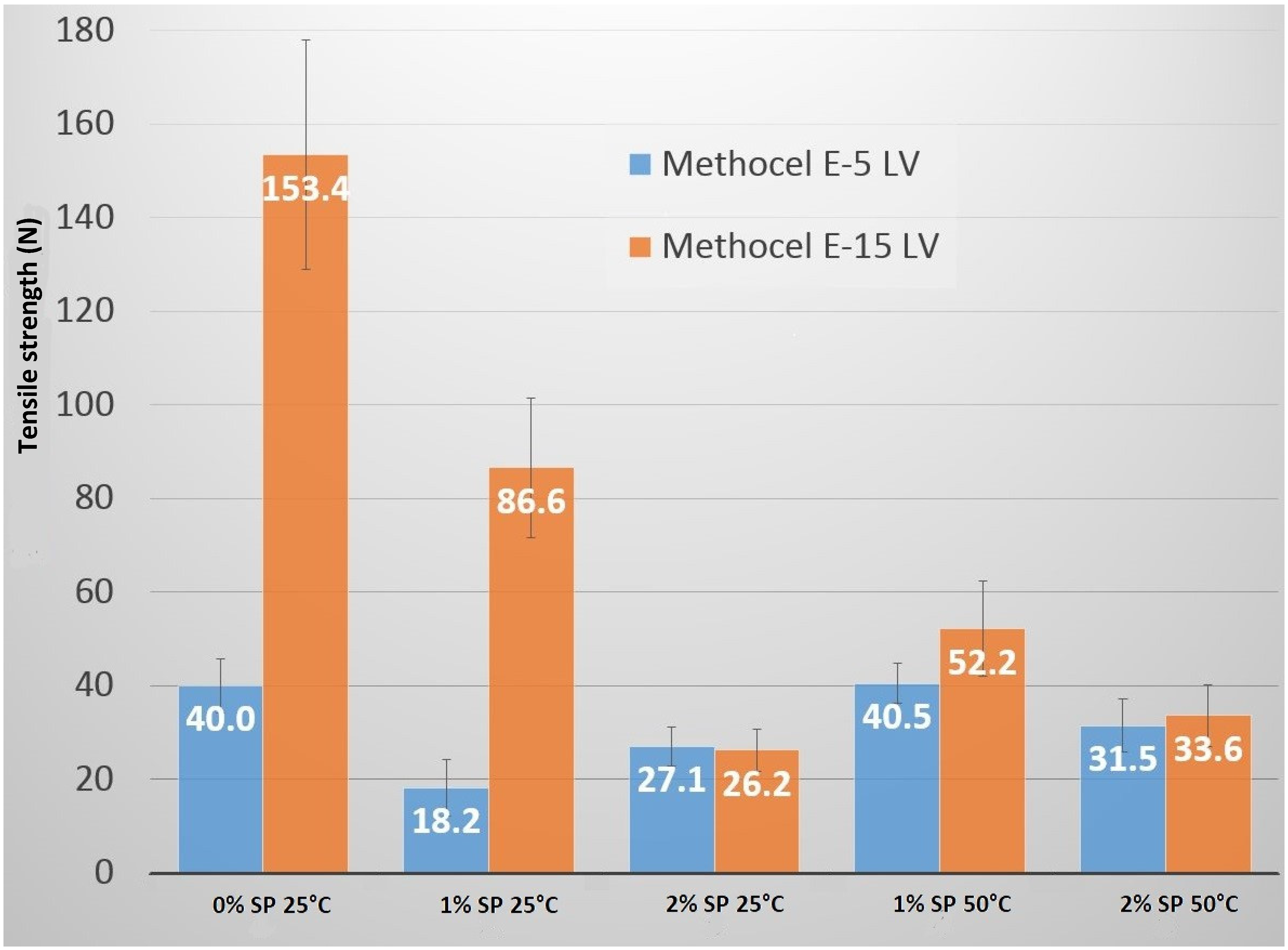
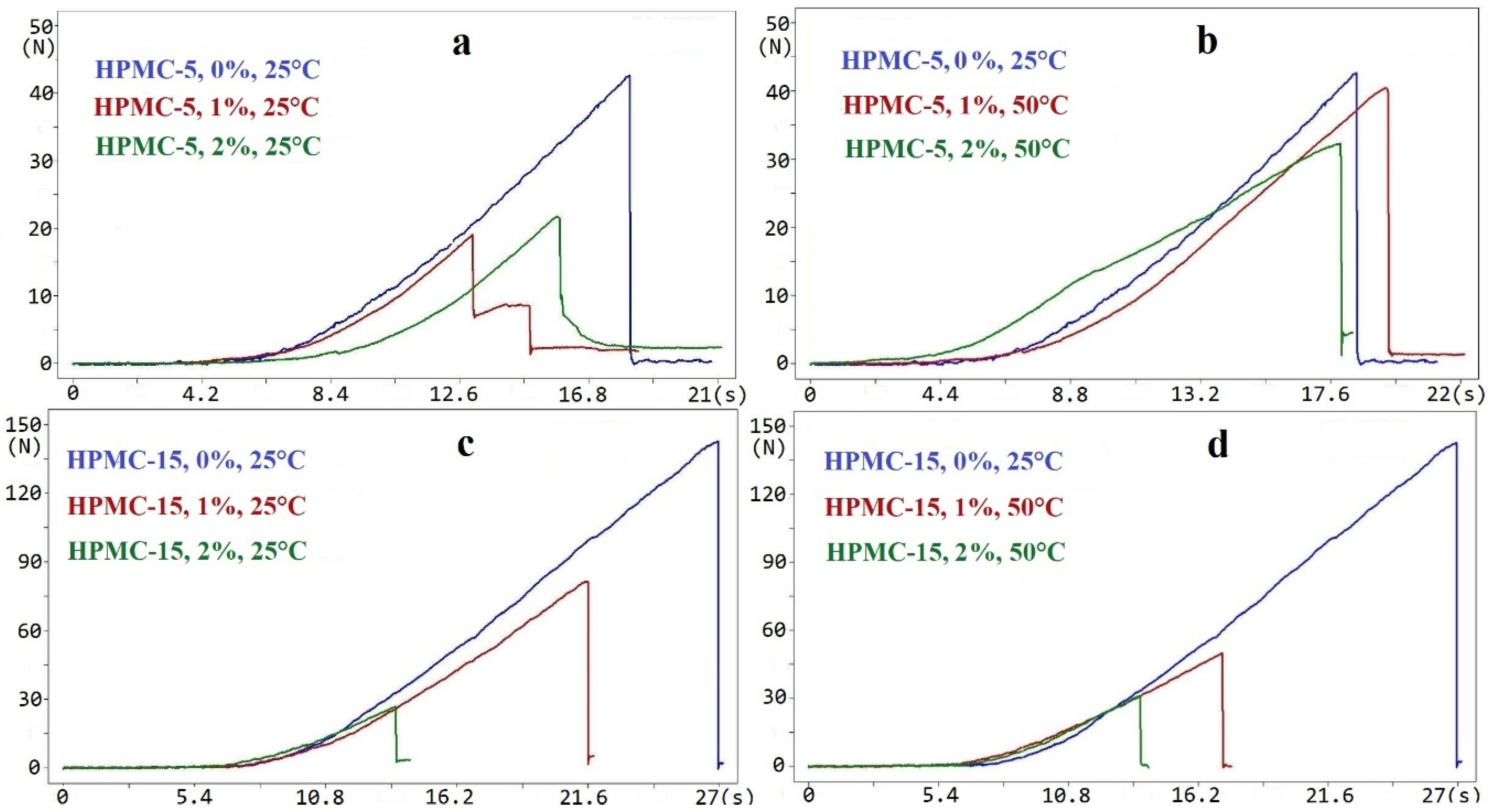



| Sample | γtot (mN/m) | γd (mN/m) | γp (mN/m) |
|---|---|---|---|
| HPMC-5-0%-25 °C | 64.40 | 38.01 | 26.39 |
| HPMC-5-1%-25 °C | 67.18 | 30.30 | 36.87 |
| HPMC-5-2%-25 °C | 67.68 | 37.08 | 30.60 |
| HPMC-5-1%-50 °C | 68.50 | 30.13 | 38.36 |
| HPMC-5-2%-50 °C | 70.54 | 32.08 | 38.46 |
| HPMC-15-0%-25 °C | 55.74 | 37.22 | 18.52 |
| HPMC-15-1%-25 °C | 65.59 | 35.35 | 30.24 |
| HPMC-15-2%-25 °C | 67.07 | 34.28 | 32.79 |
| HPMC-15-1%-50 °C | 64.79 | 35.84 | 28.95 |
| HPMC-15-2%-50 °C | 70.16 | 30.98 | 39.18 |
| Material | γtot (mN/m) | γd (mN/m) | γp (mN/m) |
|---|---|---|---|
| HPMC | 55.19 ± 2.50 | 39.40 ± 2.02 | 15.79 ± 1.47 |
| SP | 71.11 ± 2.33 | 27.01 ± 1.50 | 44.10 ± 1.79 |
| Material 1 | Material 2 | S12 (mN/m) | S21 (mN/m) |
|---|---|---|---|
| SP | HPMC | −7.91 | 0.09 |
| Sample | Mucoadhesive Force (N) | Standard Deviation (N) | Significant Difference (p < 0.05) |
|---|---|---|---|
| HPMC-5-0% | 12.58 | 0.94 | * |
| HPMC-5-1%-25 °C | 9.96 | 2.00 | No |
| HPMC-5-2%-25 °C | 11.2 | 0.48 | No |
| HPMC-5-1%-50 °C | 10.35 | 1.39 | No |
| HPMC-5-2%-50 °C | 11.34 | 1.81 | No |
| HPMC-15-0% | 9.81 | 1.19 | * |
| HPMC-15-1%-25 °C | 8.41 | 1.37 | No |
| HPMC-15-2%-25 °C | 8.38 | 0.99 | No |
| HPMC-15-1%-50 °C | 7.82 | 0.68 | No |
| HPMC-15-2%-50 °C | 9.66 | 0.39 | No |
| Sample Name | Net Area |
|---|---|
| SP Powder | 13.42 |
| HPMC-5-1%-25 °C | 0.821 |
| HPMC-5-2%-25 °C | 5.855 |
| HPMC-5-1%-50 °C | 0.763 |
| HPMC-5-2%-50 °C | 4.309 |
| HPMC-15-1%-25 °C | 4.72 |
| HPMC-15-2%-25 °C | 5.27 |
| HPMC-15-1%-50 °C | 2.13 |
| HPMC-15-2%-50 °C | 4.975 |
| Sample Name | HPMC (w/w%) | SP (w/w%) | Temperature (°C) |
|---|---|---|---|
| HPMC-5-0% | 8.5 | 0 | 25 |
| HPMC-5-1%-25 °C | 7.5 | 1 | 25 |
| HPMC-5-2%-25 °C | 6.5 | 2 | 25 |
| HPMC-5-1%-50 °C | 7.5 | 1 | 50 |
| HPMC-5-2%-50 °C | 6.5 | 2 | 50 |
| HPMC-15-0% | 8.5 | 0 | 25 |
| HPMC-15-1%-25 °C | 7.5 | 1 | 25 |
| HPMC-15-2%-25 °C | 6.5 | 2 | 25 |
| HPMC-15-1%-50 °C | 7.5 | 1 | 50 |
| HPMC-15-2%-50 °C | 6.5 | 2 | 50 |
Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelemen, A.; Katona, B.; Módra, S.; Aigner, Z.; Sebe, I.; Pintye-Hódi, K.; Zelkó, R.; Regdon, G., Jr.; Kristó, K. Effects of Sucrose Palmitate on the Physico-Chemical and Mucoadhesive Properties of Buccal Films. Molecules 2020, 25, 5248. https://doi.org/10.3390/molecules25225248
Kelemen A, Katona B, Módra S, Aigner Z, Sebe I, Pintye-Hódi K, Zelkó R, Regdon G Jr., Kristó K. Effects of Sucrose Palmitate on the Physico-Chemical and Mucoadhesive Properties of Buccal Films. Molecules. 2020; 25(22):5248. https://doi.org/10.3390/molecules25225248
Chicago/Turabian StyleKelemen, András, Bálint Katona, Szilvia Módra, Zoltán Aigner, István Sebe, Klára Pintye-Hódi, Romána Zelkó, Géza Regdon, Jr., and Katalin Kristó. 2020. "Effects of Sucrose Palmitate on the Physico-Chemical and Mucoadhesive Properties of Buccal Films" Molecules 25, no. 22: 5248. https://doi.org/10.3390/molecules25225248
APA StyleKelemen, A., Katona, B., Módra, S., Aigner, Z., Sebe, I., Pintye-Hódi, K., Zelkó, R., Regdon, G., Jr., & Kristó, K. (2020). Effects of Sucrose Palmitate on the Physico-Chemical and Mucoadhesive Properties of Buccal Films. Molecules, 25(22), 5248. https://doi.org/10.3390/molecules25225248








