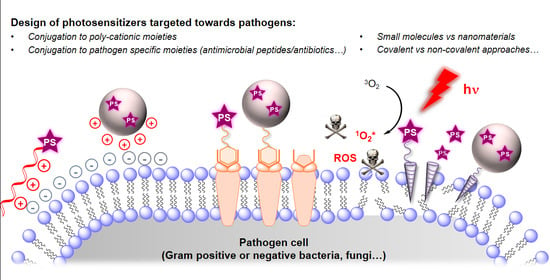
Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy
Abstract
1. Introduction
2. Mechanisms and Challenges in aPDT
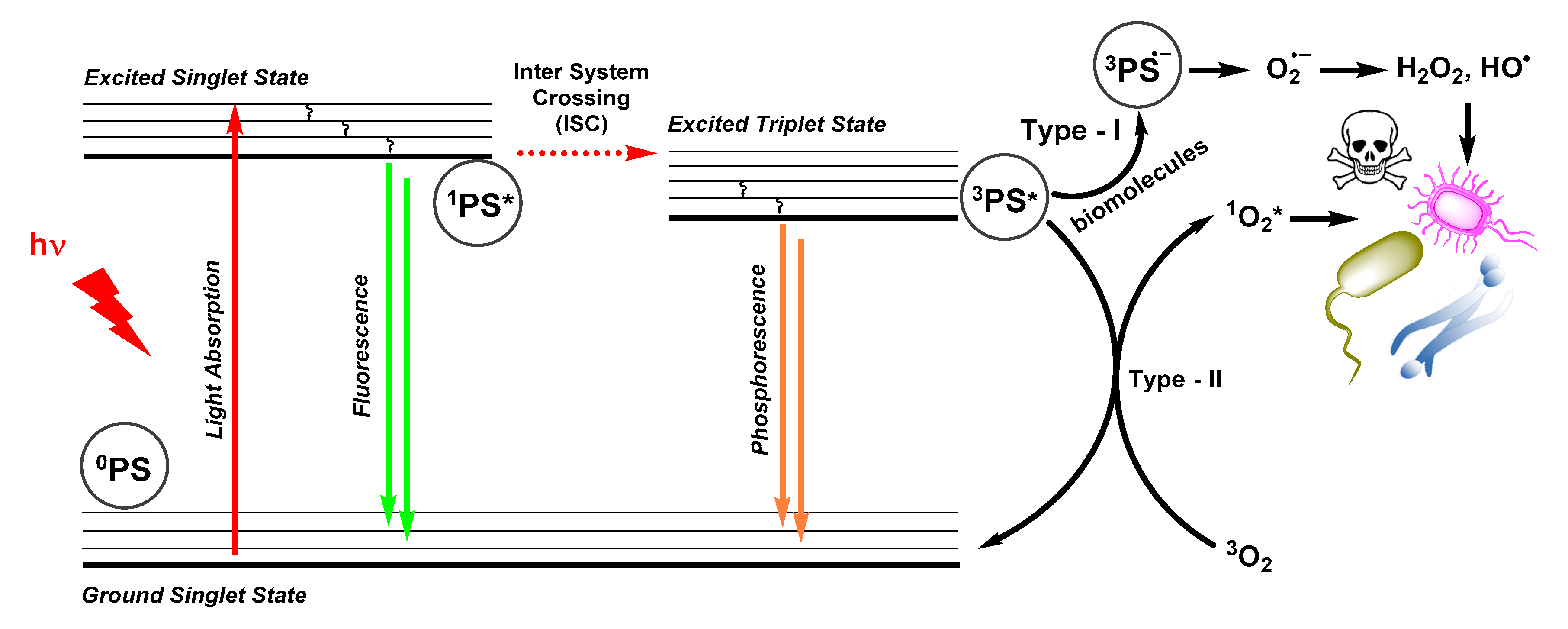
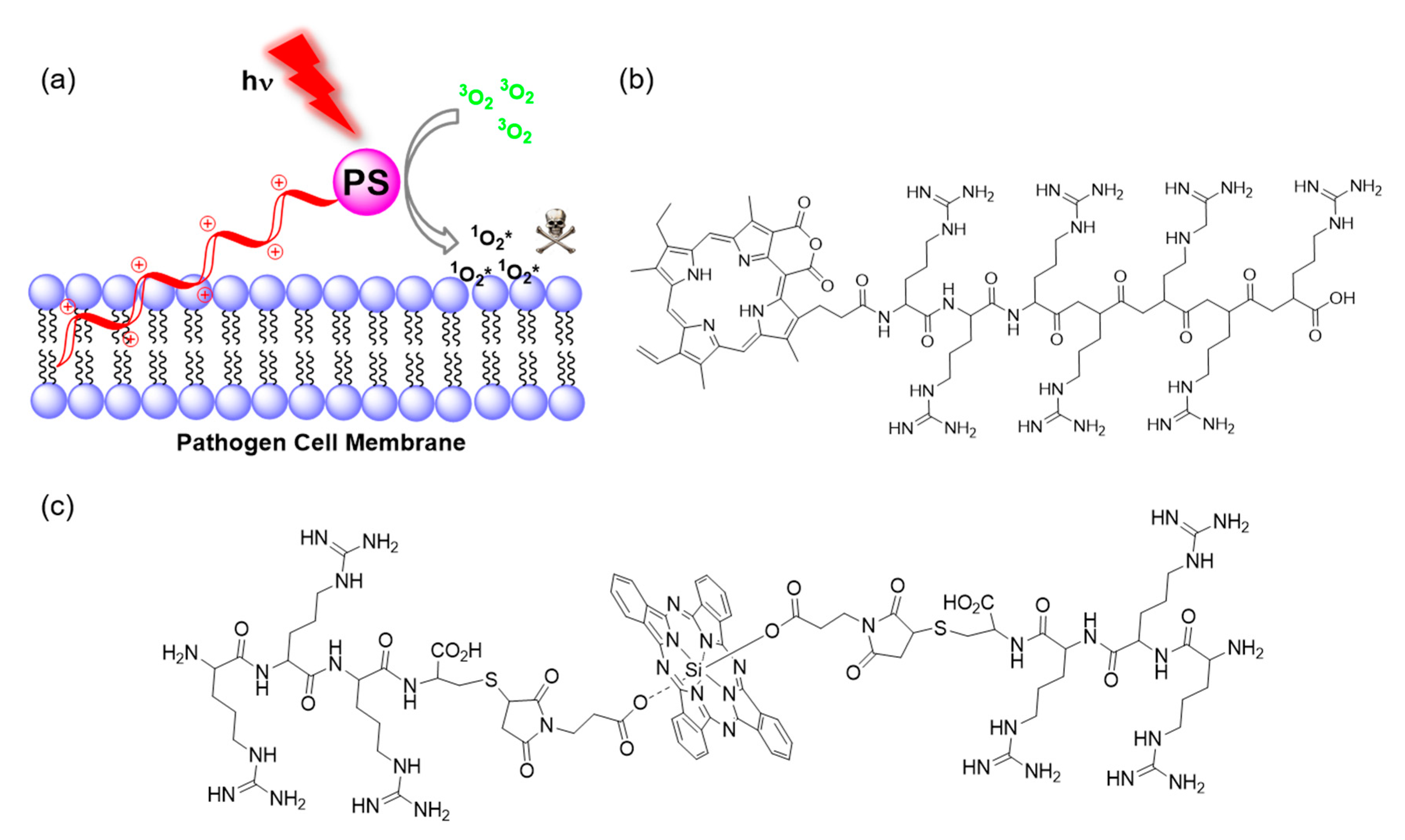
2.1. Photophysical Principles of PDT
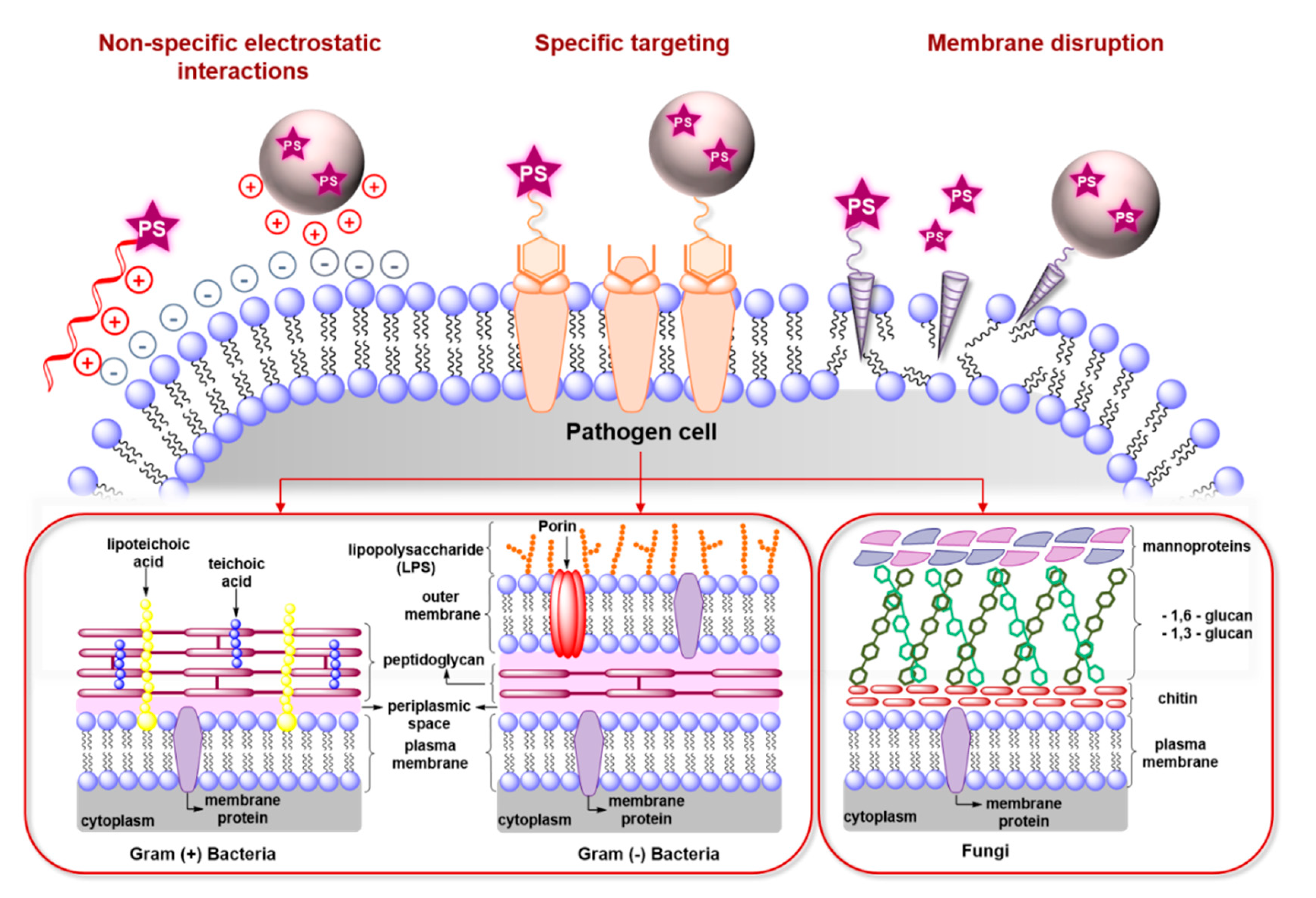
2.2. Antimicrobial Mechanisms and Challenges in aPDT
3. Recent Studies on Targeted aPDT
3.1. Small Molecules & Peptide Conjugates
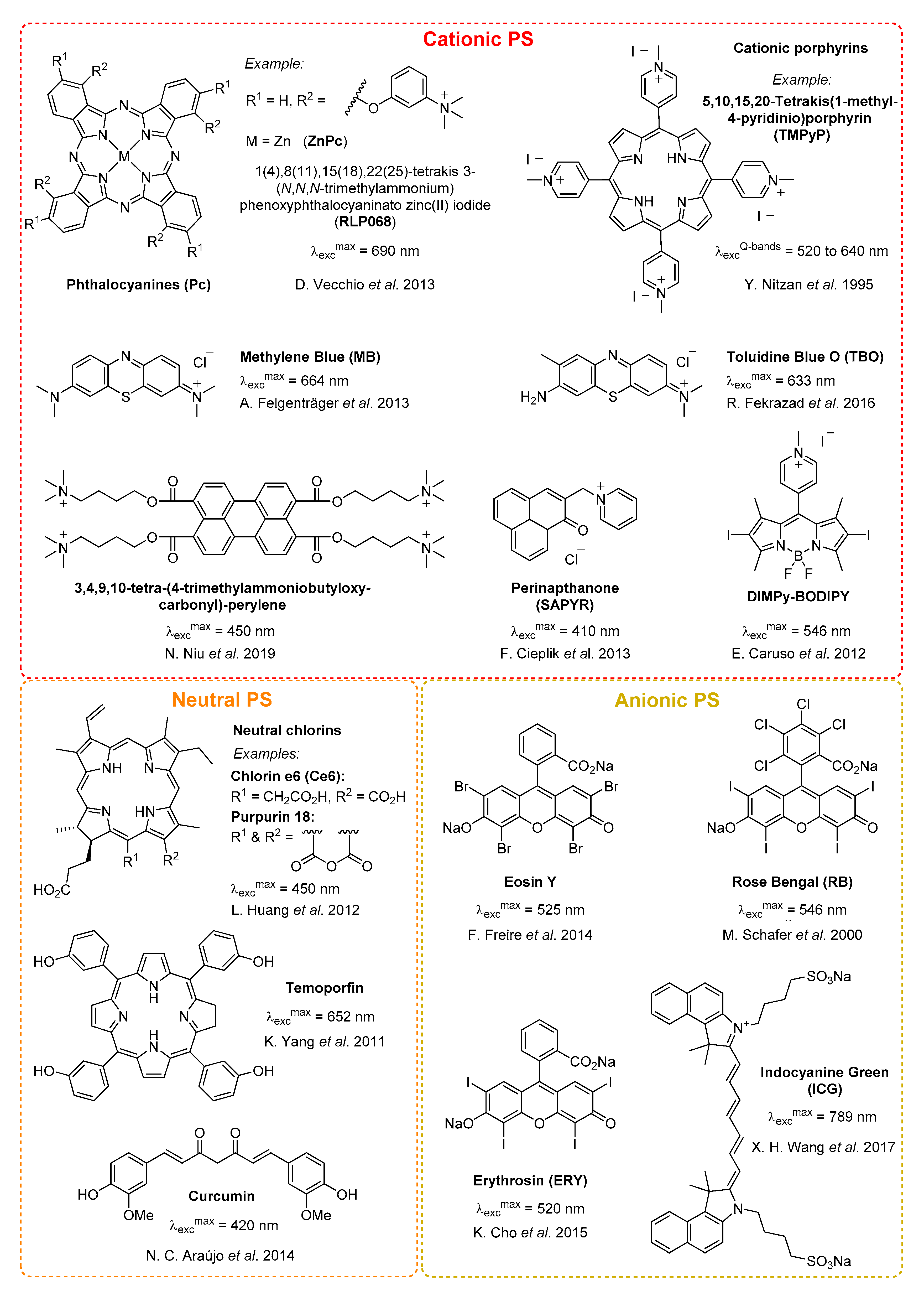
3.1.1. Conjugation of Small Cationic Groups for Electrostatic Interactions
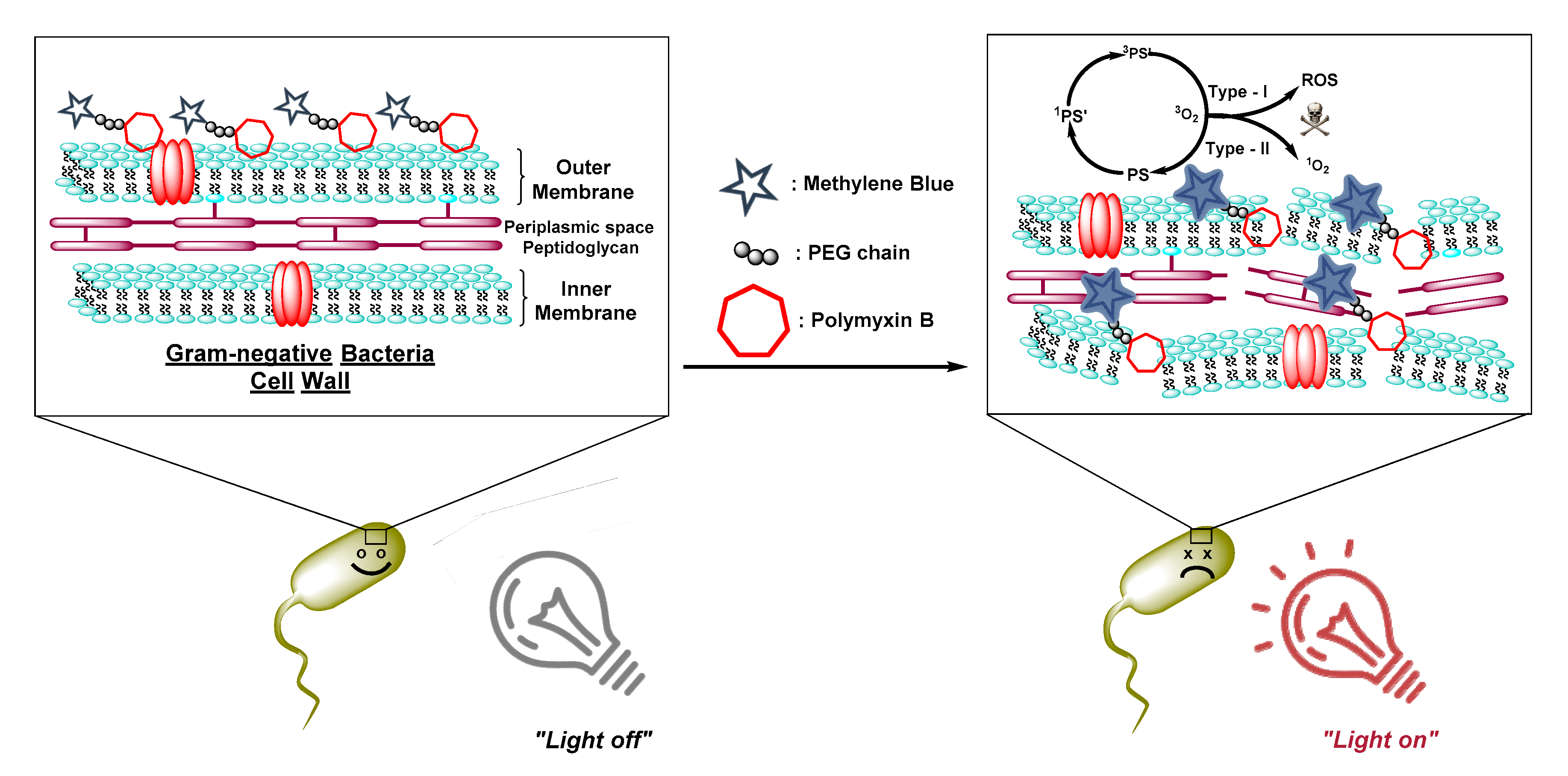
3.1.2. Antibiotics as Membrane-Disrupting Building-Blocks
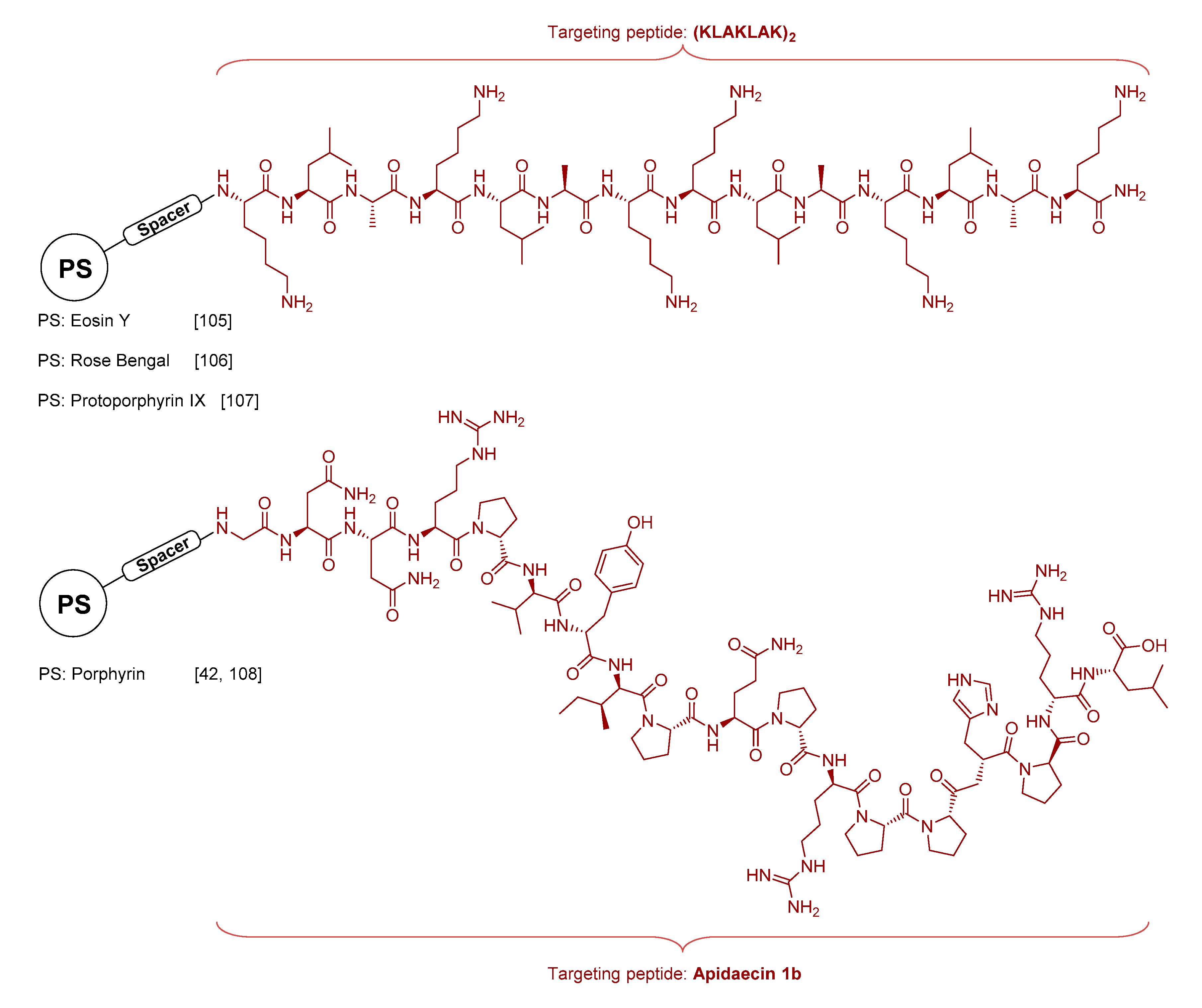
3.1.3. Antimicrobial Peptide Conjugates
3.2. Macro- and Nano-Photosensitizers
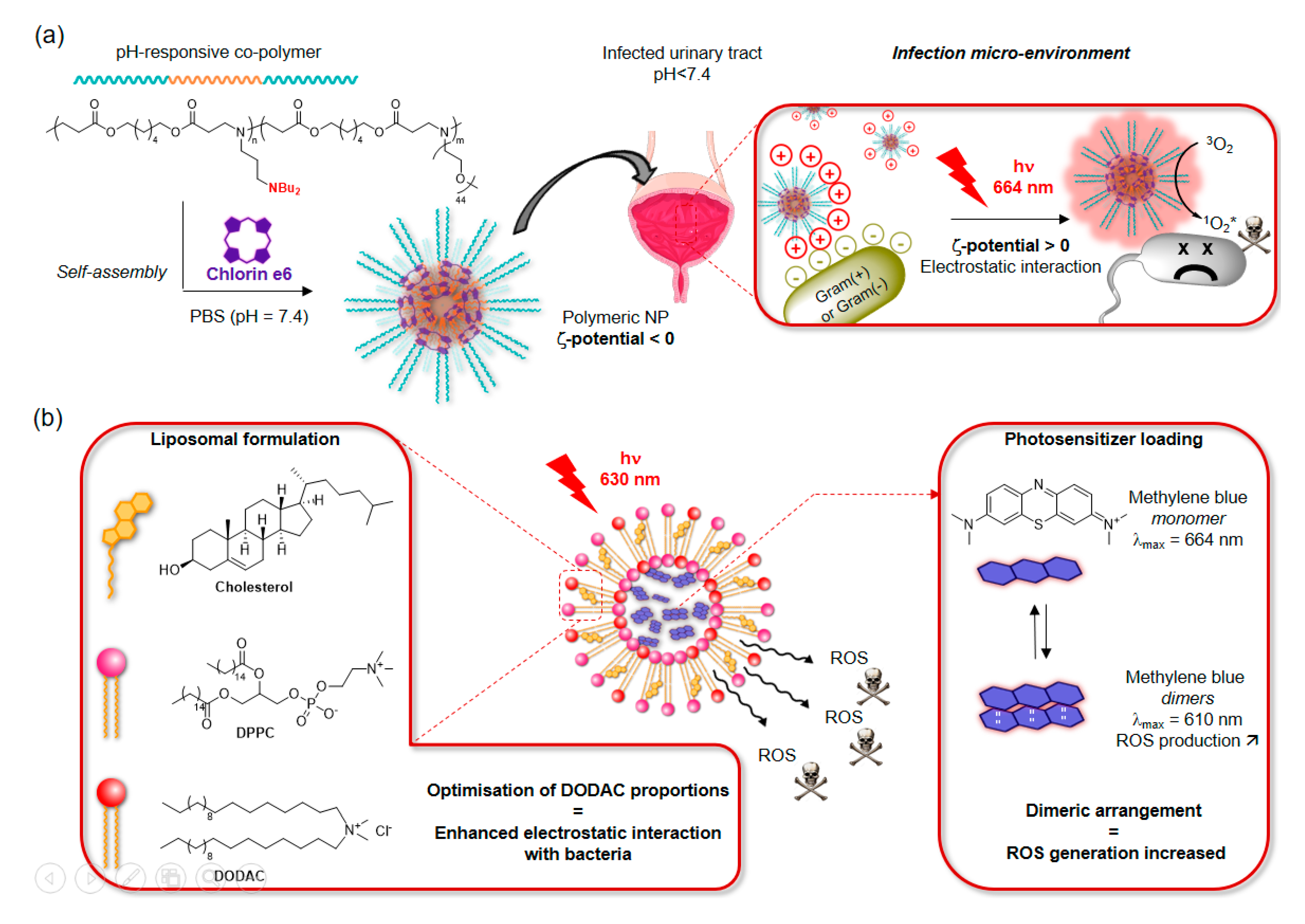
3.2.1. Micelles and Liposomes
3.2.2. Bio-Sourced Oligosaccharide Conjugates
3.2.3. Synthetic and other Bio-Inspired Polymer Conjugates
3.2.4. Hybrid and Inorganic Nanoparticles
3.2.5. Immunoconjugates and Protein Conjugates
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Spellberg, B.; Guidos, R.; Gilbert, D.; Bradley, J.; Boucher, H.W.; Scheld, W.M.; Bartlett, J.G.; Edwards, J., Jr.; The Infectious Diseases Society of America. The Epidemic of Antibiotic-Resistant Infections: A Call to Action for the Medical Community from the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 155–164. [Google Scholar] [CrossRef]
- Sarmah, P.; Dan, M.M.; Adapa, D.; Sarangi, T.K. A review on common pathogenic microorganisms and their impact on human health. Electron. J. Biol. 2018, 14, 50–58. [Google Scholar]
- Yoshikawa, T.T. Antimicrobial resistance and aging: Beginning of the end of the antibiotic era? J. Am. Geriatr. Soc. 2002, 50, 226–229. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; The Review on Antimicrobial Resistance; Government of the United Kingdom: London, UK, 2016.
- Aminov, R.I. A Brief History of the Antibiotic Era: Lessons Learned and Challenges for the Future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis. Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Daniell, M.D.; Hill, J.S. A History of Photodynamic Therapy. ANZ J. Surg. 1991, 61, 340–348. [Google Scholar] [CrossRef]
- DeRosa, M.C. Photosensitized singlet oxygen and its applications. Co-ord. Chem. Rev. 2002, 233, 351–371. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Nonell, S.; Flors, C. Singlet Oxygen: Applications in Biosciences and Nanosciences; Royal Society of Chemistry (Great Britain): London, UK, 2016; ISBN 978-1-78262-038-9. [Google Scholar]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, H.; Meyers, A.D.; Musani, A.I.; Wang, L.; Tagg, R.; Barqawi, A.B.; Chen, Y.K. Photodynamic Therapy for Treatment of Solid Tumors—Potential and Technical Challenges. Technol. Cancer Res. Treat. 2008, 7, 309–320. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Tampa, M.; Sarbu, M.-I.; Matei, C.; Mitran, C.-I.; Mitran, M.-I.; Caruntu, C.; Constantin, C.; Neagu, M.; Georgescu, S.-R. Photodynamic therapy: A hot topic in dermato-oncology (Review). Oncol. Lett. 2019, 17, 4085–4093. [Google Scholar] [CrossRef] [PubMed]
- Tandon, Y.K.; Yang, M.F.; Baron, E.D. Role of photodynamic therapy in psoriasis: A brief review. Photodermatol. Photoimmunol. Photomed. 2008, 24, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Plotino, G.; Grande, N.M.; Mercade, M. Photodynamic therapy in endodontics. Int. Endod. J. 2018, 52, 760–774. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Photodynamic therapy for localized infections—State of the art. Photodiagnosis Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef]
- Maisch, T.; Hackbarth, S.; Regensburger, J.; Felgenträger, A.; Bäumler, W.; Landthaler, M.; Röder, B. Photodynamic inactivation of multi-resistant bacteria (PIB)—A new approach to treat superficial infections in the 21st century. J. Dtsch. Dermatol. Ges. 2010, 9, 360–366. [Google Scholar] [CrossRef]
- Demidova, T.N.; Hamblin, M.R. Photodynamic Therapy Targeted to Pathogens. Int. J. Immunopathol. Pharmacol. 2004, 17, 245–254. [Google Scholar] [CrossRef]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Tavares, A.; Carvalho, C.M.B.; Faustino, M.A.; Neves, M.G.P.M.S.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Gomes, N.C.M.; Alves, E.; et al. Antimicrobial Photodynamic Therapy: Study of Bacterial Recovery Viability and Potential Development of Resistance after Treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.; Rossi, M.T.; Sala, R.; Venturini, M. Photodynamic Antifungal Chemotherapy†. Photochem. Photobiol. 2012, 88, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Samaranayake, L.P.; Romanos, G.E. Treatment of oral fungal infections using antimicrobial photodynamic therapy: A systematic review of currently available evidence. Photochem. Photobiol. Sci. 2014, 13, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, L.M.; Eray, A.; Santos, D.A.; Cisalpino, P.S.; Friedman, A.J.; Nosanchuk, J.D. Antimicrobial photodynamic therapy: An effective alternative approach to control fungal infections. Front. Microbiol. 2015, 6, 202. [Google Scholar] [CrossRef] [PubMed]
- Santus, R.; Grellier, P.; Schrevel, J.; Mazière, J.C.; Stoltz, J.F. Photodecontamination of blood components: Advantages and drawbacks. Clin. Hemorheol. Microcirc. 1998, 18, 299–308. [Google Scholar]
- Carpenter, B.L.; Situ, X.; Scholle, F.; Bartelmess, J.; Weare, W.W.; Ghiladi, R.A. Antiviral, Antifungal and Antibacterial Activities of a BODIPY-Based Photosensitizer. Molecules 2015, 20, 10604–10621. [Google Scholar] [CrossRef]
- Kliukiené, R.; Maroziené, A.; Cénas, N.; Becker, K.; Blanchard, J.S. Photoinactivation of Trypanothione Reductase and Glutathione Reductase by A1-Phthalocyanine Tetrasulfonate and Hematoporphyrin. Biochem. Biophys. Res. Commun. 1996, 218, 629–632. [Google Scholar] [CrossRef]
- Grellier, P.; Santus, R.; Mouray, E.; Agmon, V.; Mazière, J.-C.; Rigomier, D.; Dagan, A.; Gatt, S.; Schrével, J. Photosensitized Inactivation of Plasmodium falciparum- and Babesia divergens-Infected Erythrocytes in Whole Blood by Lipophilic Pheophorbide Derivatives. Vox Sang. 1997, 72, 211–220. [Google Scholar] [CrossRef]
- Tim, M. Strategies to optimize photosensitizers for photodynamic inactivation of bacteria. J. Photochem. Photobiol. B Biol. 2015, 150, 2–10. [Google Scholar] [CrossRef]
- Tekdaş, D.A.; Viswanathan, G.; Topal, S.Z.; Looi, C.Y.; Wong, W.F.; Tan, G.M.Y.; Zorlu, Y.; Gürek, A.G.; Lee, H.B.; Dumoulin, F. Antimicrobial activity of a quaternized BODIPY against Staphylococcus strains. Org. Biomol. Chem. 2016, 14, 2665–2670. [Google Scholar] [CrossRef]
- Mamone, L.; Ferreyra, D.; Gandara, L.; Di Venosa, G.; Vallecorsa, P.; Sáenz, D.; Calvo, G.; Batlle, A.; Buzzola, F.; Durantini, E.N.; et al. Photodynamic inactivation of planktonic and biofilm growing bacteria mediated by a meso-substituted porphyrin bearing four basic amino groups. J. Photochem. Photobiol. B Biol. 2016, 161, 222–229. [Google Scholar] [CrossRef]
- Li, M.; Mai, B.; Wang, A.; Gao, Y.; Zheng, H.; Liu, X.; Song, S.; Liu, Q.; Weiab, S.; Wang, P. Photodynamic antimicrobial chemotherapy with cationic phthalocyanines against Escherichia coli planktonic and biofilm cultures. RSC Adv. 2017, 7, 40734–40744. [Google Scholar] [CrossRef]
- Bresolí-Obach, R.; Gispert, I.; Peña, D.G.; Boga, S.; Gulias, Ó.; Agut, M.; Vázquez, M.E.; Nonell, S. Triphenylphosphonium cation: A valuable functional group for antimicrobial photodynamic therapy. J. Biophotonics 2018, 11, e201800054. [Google Scholar] [CrossRef]
- Niu, N.; Zhou, H.; Liu, N.; Jiang, H.; Hu, Z.; Yu, C. A perylene-based membrane intercalating conjugated oligoelectrolyte with efficient photodynamic antimicrobial activity. Chem. Commun. 2019, 55, 4395–4398. [Google Scholar] [CrossRef] [PubMed]
- Morley, S.; Griffiths, J.; Philips, G.; Moseley, H.; O’Grady, C.; Mellish, K.; Lankester, C.; Faris, B.; Young, R.; Brown, S.B.; et al. Phase IIa randomized, placebo-controlled study of antimicrobial photodynamic therapy in bacterially colonized, chronic leg ulcers and diabetic foot ulcers: A new approach to antimicrobial therapy. Br. J. Dermatol. 2013, 168, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Tardivo, J.P.; Adami, F.; Correa, J.A.; Pinhal, M.A.S.; Baptista, M.S. A clinical trial testing the efficacy of PDT in preventing amputation in diabetic patients. Photodiagnosis Photodyn. Ther. 2014, 11, 342–350. [Google Scholar] [CrossRef]
- Lei, X.; Liu, B.; Huang, Z.; Wu, J. A clinical study of photodynamic therapy for chronic skin ulcers in lower limbs infected with Pseudomonas aeruginosa. Arch. Dermatol. Res. 2014, 307, 49–55. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Wojtunik-Kulesza, K.A.; Oniszczuk, T.; Kasprzak, K. The potential of photodynamic therapy (PDT)—Experimental investigations and clinical use. Biomed. Pharmacother. 2016, 83, 912–929. [Google Scholar] [CrossRef]
- Naranjo, A.; Arboleda, A.; Martinez, J.D.; Durkee, H.; Aguilar, M.C.; Relhan, N.; Nikpoor, N.; Galor, A.; Dubovy, S.R.; Leblanc, R.; et al. Rose Bengal Photodynamic Antimicrobial Therapy for Patients With Progressive Infectious Keratitis: A Pilot Clinical Study. Am. J. Ophthalmol. 2019, 208, 387–396. [Google Scholar] [CrossRef]
- Embleton, M.L.; Nair, S.P.; Cookson, B.D.; Wilson, M. Selective lethal photosensitization of methicillin-resistant Staphylococcus aureus using an IgG-tin (IV) chlorin e6 conjugate. J. Antimicrob. Chemother. 2002, 50, 857–864. [Google Scholar] [CrossRef]
- Dosselli, R.; Gobbo, M.; Bolognini, E.; Campestrini, S.; Reddi, E. Porphyrin−Apidaecin Conjugate as a New Broad Spectrum Antibacterial Agent. ACS Med. Chem. Lett. 2010, 1, 35–38. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Huang, Y.-Y.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Kill Gram-negative Bacteria. Recent Patents Anti-Infective Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.R.; Gan, H.; Smith, B.D. Bacterial imaging and photodynamic inactivation using zinc(ii)-dipicolylamine BODIPY conjugates. Photochem. Photobiol. Sci. 2015, 14, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.C.F.R.; Silva, D.F.; Castro, R.C.F.R. Effect of photodynamic therapy on surface decontamination in clinical orthodontic instruments. Photodiagnosis Photodyn. Ther. 2018, 24, 123–128. [Google Scholar] [CrossRef]
- Maldonado-Carmona, N.; Ouk, T.S.; Calvete, M.J.; Pereira, M.M.; Villandier, N.; Leroy-Lhez, S. Conjugating biomaterials with photosensitizers: Advances and perspectives for photodynamic antimicrobial chemotherapy. Photochem. Photobiol. Sci. 2020, 19, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 2005, 38, 2543–2555. [Google Scholar] [CrossRef]
- Felgenträger, A.; Maisch, T.; Dobler, D.; Späth, A. Hydrogen Bond Acceptors and Additional Cationic Charges in Methylene Blue Derivatives: Photophysics and Antimicrobial Efficiency. BioMed Res. Int. 2012, 2013, 1–12. [Google Scholar] [CrossRef]
- Fekrazad, R.; Zare, H.; Vand, S.M.S. Photodynamic therapy effect on cell growth inhibition induced by Radachlorin and toluidine blue O on Staphylococcus aureus and Escherichia coli: An in vitro study. Photodiagnosis Photodyn. Ther. 2016, 15, 213–217. [Google Scholar] [CrossRef]
- Wainwright, M.; Phoenix, D.; Marland, J.; Wareing, D.; Bolton, F. A study of photobactericidal activity in the phenothiazinium series. FEMS Immunol. Med. Microbiol. 1997, 19, 75–80. [Google Scholar] [CrossRef]
- Nitzan, Y.; Dror, R.; Ladan, H.; Malik, Z.; Kimel, S.; Gottfried, V. Structure-Activity Relationship Of Porphines For Photoinactivation Of Bacteria. Photochem. Photobiol. 1995, 62, 342–347. [Google Scholar] [CrossRef]
- Merchat, M.; Spikes, J.; Bertoloni, G.; Jori, G. Studies on the mechanism of bacteria photosensitization by meso-substituted cationic porphyrins. J. Photochem. Photobiol. B Biol. 1996, 35, 149–157. [Google Scholar] [CrossRef]
- Vecchio, D.; Dai, T.; Huang, L.; Fantetti, L.; Roncucci, G.; Hamblin, M.R. Antimicrobial photodynamic therapy with RLP068 kills methicillin-resistantStaphylococcus aureusand improves wound healing in a mouse model of infected skin abrasion PDT with RLP068/Cl in infected mouse skin abrasion. J. Biophotonics 2013, 6, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Yow, C.M.N.; Tang, H.M.; Chu, E.S.M.; Huang, Z. Hypericin-mediated Photodynamic Antimicrobial Effect on Clinically Isolated Pathogens†. Photochem. Photobiol. 2012, 88, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Araújo, N.C.; Fontana, C.R.; Bagnato, V.S.; Gerbi, M.E.M. Photodynamic antimicrobial therapy of curcumin in biofilms and carious dentine. Lasers Med. Sci. 2013, 29, 629–635. [Google Scholar] [CrossRef]
- Schäfer, M.; Schmitz, C.; Facius, R.; Horneck, G.; Milow, B.; Funken, K.-H.; Ortner, J. Systematic Study of Parameters Influencing the Action of Rose Bengal with Visible Light on Bacterial Cells: Comparison Between the Biological Effect and Singlet-Oxygen Production. Photochem. Photobiol. 2000, 71, 514–523. [Google Scholar] [CrossRef]
- Freire, F.; Costa, A.C.B.P.; Pereira, C.A.; Junior, M.B.; Junqueira, J.C.; Jorge, A.O.C. Comparison of the effect of rose bengal- and eosin Y-mediated photodynamic inactivation on planktonic cells and biofilms of Candida albicans. Lasers Med. Sci. 2014, 29, 949–955. [Google Scholar] [CrossRef]
- Cho, K.; Lee, S.Y.; Chang, B.-S.; Um, H.-S.; Lee, J. The effect of photodynamic therapy on Aggregatibacter actinomycetemcomitans attached to surface-modified titanium. J. Periodontal Implant. Sci. 2015, 45, 38–45. [Google Scholar] [CrossRef]
- Boehm, T.K.; Ciancio, S.G. Diode laser activated indocyanine green selectively kills bacteria. J. Int. Acad. Periodontol. 2011, 13, 58–63. [Google Scholar]
- Caruso, E.; Banfi, S.; Barbieri, P.; Leva, B.; Orlandi, V. Synthesis and antibacterial activity of novel cationic BODIPY photosensitizers. J. Photochem. Photobiol. B Biol. 2012, 114, 44–51. [Google Scholar] [CrossRef]
- Cieplik, F.; Späth, A.; Regensburger, J.; Gollmer, A.; Tabenski, L.; Hiller, K.-A.; Bäumler, W.; Maisch, T.; Schmalz, G. Photodynamic biofilm inactivation by SAPYR—An exclusive singlet oxygen photosensitizer. Free. Radic. Biol. Med. 2013, 65, 477–487. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; De Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.E.; Ceri, H.; Morck, D.W.; Buret, A.G.; Read, R.R. Biofilm bacteria: Formation and comparative susceptibility to antibiotics. Can. J. Veter. Res. Rev. Can. Rech. Veter. 2002, 66, 86–92. [Google Scholar]
- Preston, A.; Mandrell, R.E.; Gibson, B.W.; Apicella, M.A. The Lipooligosaccharides of Pathogenic Gram-Negative Bacteria. Crit. Rev. Microbiol. 1996, 22, 139–180. [Google Scholar] [CrossRef] [PubMed]
- Minnock, A.; Vernon, D.I.; Schofield, J.; Griffiths, J.; Parish, J.H.; Brown, S.B. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both Gram-negative and Gram-positive bacteria. J. Photochem. Photobiol. B Biol. 1996, 32, 159–164. [Google Scholar] [CrossRef]
- Tegos, G.P.; Anbe, M.; Yang, C.; Demidova, T.N.; Satti, M.; Mroz, P.; Janjua, S.; Gad, F.; Hamblin, M.R. Protease-Stable Polycationic Photosensitizer Conjugates between Polyethyleneimine and Chlorin(e6) for Broad-Spectrum Antimicrobial Photoinactivation. Antimicrob. Agents Chemother. 2006, 50, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Xu, Z.; Hong, G.; Zhao, L.; Zhao, Z.; Guo, J.; Ji, H.; Liu, T. Synthesis, characterization and in vitro photodynamic antimicrobial activity of basic amino acid–porphyrin conjugates. Eur. J. Med. Chem. 2015, 92, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Gao, Y.; Meng, S.; Yang, B.; Pang, L.; Wang, C.; Liu, T. Mechanism and In Vivo Evaluation: Photodynamic Antibacterial Chemotherapy of Lysine-Porphyrin Conjugate. Front. Microbiol. 2016, 7, 242. [Google Scholar] [CrossRef]
- Zhou, J.; Qi, G.-B.; Wang, H. A purpurin-peptide derivative for selective killing of Gram-positive bacteria via insertion into cell membrane. J. Mater. Chem. B 2016, 4, 4855–4861. [Google Scholar] [CrossRef]
- Zhao, Y.; Ying, J.-W.; Sun, Q.; Ke, M.-R.; Zheng, B.-Y.; Huang, J.-D. A novel silicon(IV) phthalocyanine-oligopeptide conjugate as a highly efficient photosensitizer for photodynamic antimicrobial therapy. Dye. Pigment. 2020, 172, 107834. [Google Scholar] [CrossRef]
- Sahu, K.; Sharma, M.; Bansal, H.; Dube, A.; Gupta, P.K. Topical photodynamic treatment with poly-l-lysine–chlorin p6 conjugate improves wound healing by reducing hyperinflammatory response in Pseudomonas aeruginosa-infected wounds of mice. Lasers Med. Sci. 2012, 28, 465–471. [Google Scholar] [CrossRef]
- Branco, T.M.; Valério, N.C.; Jesus, V.I.R.; Dias, C.J.; Neves, M.G.; Faustino, M.A.F.; Almeida, A. Single and combined effects of photodynamic therapy and antibiotics to inactivate Staphylococcus aureus on skin. Photodiagnosis Photodyn. Ther. 2018, 21, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Iluz, N.; Maor, Y.; Keller, N.; Malik, Z. The synergistic effect of PDT and oxacillin on clinical isolates of Staphylococcus aureus. Lasers Surg. Med. 2018, 50, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Ilizirov, Y.; Formanovsky, A.; Mikhura, I.; Paitan, Y.; Nakonechny, F.; Nisnevitch, M. Effect of Photodynamic Antibacterial Chemotherapy Combined with Antibiotics on Gram-Positive and Gram-Negative Bacteria. Molecules 2018, 23, 3152. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, A.; Rapacka-Zdonczyk, A.; Mutters, N.T.; Grinholc, M.S. Antimicrobials Are a Photodynamic Inactivation Adjuvant for the Eradication of Extensively Drug-Resistant Acinetobacter baumannii. Front. Microbiol. 2019, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Magacho, C.C.; Pinto, J.G.; Souza, B.M.N.; Pereira, A.H.C.; Strixino, J.F. Comparison of photodynamic therapy with methylene blue associated with ceftriaxone in gram-negative bacteria; an in vitro study. Photodiagnosis Photodyn. Ther. 2020, 30, 101691. [Google Scholar] [CrossRef]
- Weinstein, M.J.; Luedemann, G.M.; Oden, E.M.; Wagman, G.H.; Rosselet, J.P.; Marquez, J.A.; Coniglio, C.T.; Charney, W.; Herzog, H.L.; Black, J. Gentamicin,1a New Antibiotic Complex from Micromonospora. J. Med. Chem. 1963, 6, 463–464. [Google Scholar] [CrossRef]
- Daniels, P.J.L.; Rane, D.F.; McCombie, S.W.; Testa, R.T.; Wright, J.J.; Nagabhushan, T.L. Chemical and Biological Modification of Antibiotics of the Gentamicin Group. In Proceedings of the ACS Symposium Series; American Chemical Society (ACS): Washington, DC, USA, 1980; Volume 125, pp. 371–392. [Google Scholar]
- Rajasekaran, P.; Crich, D. Synthesis of Gentamicin Minor Components: Gentamicin B1 and Gentamicin X2. Org. Lett. 2020, 22, 3850–3854. [Google Scholar] [CrossRef]
- Pérez-Laguna, V.; García-Luque, I.; Ballesta, S.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; Samper, S.; Soria-Lozano, P.; Rezusta, A.; Gilaberte, Y. Antimicrobial photodynamic activity of Rose Bengal, alone or in combination with Gentamicin, against planktonic and biofilm Staphylococcus aureus. Photodiagnosis Photodyn. Ther. 2018, 21, 211–216. [Google Scholar] [CrossRef]
- Nieves, I.; Hally, C.; Viappiani, C.; Agut, M.; Nonell, S. A porphycene-gentamicin conjugate for enhanced photodynamic inactivation of bacteria. Bioorganic Chem. 2020, 97, 103661. [Google Scholar] [CrossRef]
- Butler, M.S.; Hansford, K.; Blaskovich, M.A.T.; Halai, R.A.; Cooper, M. Glycopeptide antibiotics: Back to the future. J. Antibiot. 2014, 67, 631–644. [Google Scholar] [CrossRef]
- Borman, S. Vancomycin triple threat antibiotic. C&EN Glob. Enterp. 2017, 95, 7. [Google Scholar] [CrossRef]
- Van Oosten, M.; Schäfer, T.; Gazendam, J.A.C.; Ohlsen, K.; Tsompanidou, E.; De Goffau, M.C.; Harmsen, H.J.M.; Crane, L.M.A.; Lim, E.; Francis, K.P.; et al. Real-time in vivo imaging of invasive- and biomaterial-associated bacterial infections using fluorescently labelled vancomycin. Nat. Commun. 2013, 4, 2584. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Jiang, T.; Bi, W.; Yang, Y.; Li, L.; Ma, M.; Chang, C.K.; Xu, B.; Yeow, E.K.L. Multifunctional divalent vancomycin: The fluorescent imaging and photodynamic antimicrobial properties for drug resistant bacteria. Chem. Commun. 2011, 47, 1601–1603. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-Z.; Yang, K.-W.; Wu, X.-L.; Liu, J.-Y.; Feng, L.; Xiao, J.-M.; Zhou, L.-S.; Jia, C.; Shi, Z. Novel Conjugation of Norvancomycin–Fluorescein for Photodynamic Inactivation ofBacillus subtilis. Bioconjugate Chem. 2011, 22, 2217–2221. [Google Scholar] [CrossRef]
- Choi, K.-H.; Lee, H.-J.; Park, B.J.; Wang, K.-K.; Shin, E.P.; Park, J.-C.; Kim, Y.K.; Oh, M.-K.; Kim, Y.-R. Photosensitizer and vancomycin-conjugated novel multifunctional magnetic particles as photoinactivation agents for selective killing of pathogenic bacteria. Chem. Commun. 2012, 48, 4591–4593. [Google Scholar] [CrossRef]
- Liu, B.; Yuan, Y.; Fang, H.; Zhang, R.; Xing, B.; Zhang, G.; Zhang, D.; Liu, B. A light-up probe with aggregation-induced emission characteristics (AIE) for selective imaging, naked-eye detection and photodynamic killing of Gram-positive bacteria. Chem. Commun. 2015, 51, 12490–12493. [Google Scholar] [CrossRef]
- Zhai, L.; Yang, K.-W. Porphyrin-vancomycin: A highly promising conjugate for the identification and photodynamic inactivation of antibiotic resistant Gram-positive pathogens. Dye. Pigment. 2015, 120, 228–238. [Google Scholar] [CrossRef]
- Huang, L.; Wang, M.; Huang, Y.-Y.; El-Hussein, A.; Wolf, L.M.; Chiang, L.Y.; Hamblin, M.R. Progressive cationic functionalization of chlorin derivatives for antimicrobial photodynamic inactivation and related vancomycin conjugates. Photochem. Photobiol. Sci. 2018, 17, 638–651. [Google Scholar] [CrossRef]
- Baltzer, S.A.; Brown, M.H. Antimicrobial Peptides—Promising Alternatives to Conventional Antibiotics. J. Mol. Microbiol. Biotechnol. 2011, 20, 228–235. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2015, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, Y.; Gutterman, M.; Malik, Z.; Ehrenberg, B. Inactivation of Gram-Negative Bacteria by Photosensitized Porphyrins. Photochem. Photobiol. 1992, 55, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Qin, W.; Lin, J.; Fang, S.; Qiu, J. Antibacterial Mechanisms of Polymyxin and Bacterial Resistance. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rabanal, F.; Cajal, Y. Recent advances and perspectives in the design and development of polymyxins. Nat. Prod. Rep. 2017, 34, 886–908. [Google Scholar] [CrossRef] [PubMed]
- Richter, P.; Krüger, M.; Prasad, B.; Gastiger, S.; Bodenschatz, M.; Wieder, F.; Burkovski, A.; Geißdörfer, W.; Lebert, M.; Strauch, S.M. Using Colistin as a Trojan Horse: Inactivation of Gram-Negative Bacteria with Chlorophyllin. Antibiotic 2019, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Le Guern, F.; Ouk, T.-S.; Grenier, K.; Joly, N.; Lequart, V.; Sol, V. Enhancement of photobactericidal activity of chlorin-e6-cellulose nanocrystals by covalent attachment of polymyxin B. J. Mater. Chem. B 2017, 5, 6953–6962. [Google Scholar] [CrossRef]
- Le Guern, F.; Sol, V.; Ouk, C.; Arnoux, P.; Frochot, C.; Ouk, T.-S. Enhanced Photobactericidal and Targeting Properties of a Cationic Porphyrin following the Attachment of Polymyxin, B. Bioconjugate Chem. 2017, 28, 2493–2506. [Google Scholar] [CrossRef]
- Le Guern, F.; Ouk, T.-S.; Ouk, C.; Vanderesse, R.; Champavier, Y.; Pinault, E.; Sol, V. Lysine Analogue of Polymyxin B as a Significant Opportunity for Photodynamic Antimicrobial Chemotherapy. ACS Med. Chem. Lett. 2017, 9, 11–16. [Google Scholar] [CrossRef]
- Bayat, F.; Karimi, A.R. Design of photodynamic chitosan hydrogels bearing phthalocyanine-colistin conjugate as an antibacterial agent. Int. J. Biol. Macromol. 2019, 129, 927–935. [Google Scholar] [CrossRef]
- Akram, A.R.; Chankeshwara, S.V.; Scholefield, E.; Aslam, T.; McDonald, N.; Megia-Fernandez, A.; Marshall, A.; Mills, B.; Avlonitis, N.; Craven, T.H.; et al. In situ identification of Gram-negative bacteria in human lungs using a topical fluorescent peptide targeting lipid A. Sci. Transl. Med. 2018, 10, eaal0033. [Google Scholar] [CrossRef]
- Ucuncu, M.; Mills, B.; Duncan, S.; Staderini, M.; Dhaliwal, K.; Bradley, M. Polymyxin-based photosensitizer for the potent and selective killing of Gram-negative bacteria. Chem. Commun. 2020, 56, 3757–3760. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.A.; Muthukrishnan, N.; Pellois, J.-P. Photoinactivation of Gram Positive and Gram Negative Bacteria with the Antimicrobial Peptide (KLAKLAK)2Conjugated to the Hydrophilic Photosensitizer Eosin, Y. Bioconjugate Chem. 2012, 24, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Costley, D.; Nesbitt, H.; Ternan, N.; Dooley, J.; Huang, Y.-Y.; Hamblin, M.R.; McHale, A.P.; Callan, J.F. Sonodynamic inactivation of Gram-positive and Gram-negative bacteria using a Rose Bengal–antimicrobial peptide conjugate. Int. J. Antimicrob. Agents 2017, 49, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.-N.; Wu, W.; Zhang, C.; Wang, Q.-Y.; Zhuang, Z.-N.; Cheng, H.; Zhang, X.-Z.; Zhang, A. A versatile bacterial membrane-binding chimeric peptide with enhanced photodynamic antimicrobial activity. J. Mater. Chem. B 2019, 7, 1087–1095. [Google Scholar] [CrossRef]
- Dosselli, R.; Tampieri, C.; Ruiz-González, R.; De Munari, S.; Ragàs, X.; Sánchez-García, D.; Agut, M.; Nonell, S.; Reddi, E.; Gobbo, M. Synthesis, Characterization, and Photoinduced Antibacterial Activity of Porphyrin-Type Photosensitizers Conjugated to the Antimicrobial Peptide Apidaecin 1b. J. Med. Chem. 2013, 56, 1052–1063. [Google Scholar] [CrossRef]
- De Freitas, L.M.; Lorenzón, E.N.; Santos-Filho, N.A.; Zago, L.H.D.P.; Uliana, M.P.; De Oliveira, K.T.; Cilli, E.M.; Fontana, C.R. Antimicrobial Photodynamic therapy enhanced by the peptide aurein 1.2. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Li, X.; Lee, S.; Yoon, J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. [Google Scholar] [CrossRef]
- Hackbarth, S.; Röder, B. Singlet oxygen luminescence kinetics in a heterogeneous environment – identification of the photosensitizer localization in small unilamellar vesicles. Photochem. Photobiol. Sci. 2015, 14, 329–334. [Google Scholar] [CrossRef]
- Tsai, T.; Yang, Y.-T.; Wang, T.-H.; Chien, H.-F.; Chen, C. Improved photodynamic inactivation of gram-positive bacteria using hematoporphyrin encapsulated in liposomes and micelles. Lasers Surg. Med. 2009, 41, 316–322. [Google Scholar] [CrossRef]
- Sharma, B.; Kaur, G.; Chaudhary, G.R.; Gawali, S.L.; Hassan, P. High antimicrobial photodynamic activity of photosensitizer encapsulated dual-functional metallocatanionic vesicles against drug-resistant bacteria S. aureus. Biomater. Sci. 2020, 8, 2905–2920. [Google Scholar] [CrossRef]
- Ferro, S.; Ricchelli, F.; Monti, D.; Mancini, G.; Jori, G. Efficient photoinactivation of methicillin-resistant Staphylococcus aureus by a novel porphyrin incorporated into a poly-cationic liposome. Int. J. Biochem. Cell Biol. 2007, 39, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Haldar, J.; Kondaiah, P.; Bhattacharya, S. Synthesis and Antibacterial Properties of Novel Hydrolyzable Cationic Amphiphiles. Incorporation of Multiple Head Groups Leads to Impressive Antibacterial Activity. J. Med. Chem. 2005, 48, 3823–3831. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-T.; Chien, H.-F.; Chang, P.-H.; Chen, Y.-C.; Jay, M.; Tsai, T.; Chen, C.-T. Photodynamic inactivation of chlorin e6-loaded CTAB-liposomes against Candida albicans. Lasers Surg. Med. 2013, 45, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qiao, S.; Li, L.; Qi, G.; Lin, Y.; Qiao, Z.; Wang, H.; Shao, C. Surface charge-conversion polymeric nanoparticles for photodynamic treatment of urinary tract bacterial infections. Nanotechnology 2015, 26, 495602. [Google Scholar] [CrossRef]
- Boccalini, G.; Conti, L.; Montis, C.; Bani, D.; Bencini, A.; Berti, D.; Giorgi, C.; Mengoni, A.; Valtancoli, B. Methylene blue-containing liposomes as new photodynamic anti-bacterial agents. J. Mater. Chem. B 2017, 5, 2788–2797. [Google Scholar] [CrossRef]
- Yang, K.; Gitter, B.; Rüger, R.; Wieland, G.D.; Chen, M.; Liu, X.; Albrecht, V.; Fahr, A. Antimicrobial peptide-modified liposomes for bacteria targeted delivery of temoporfin in photodynamic antimicrobial chemotherapy. Photochem. Photobiol. Sci. 2011, 10, 1593–1601. [Google Scholar] [CrossRef]
- Morgado, L.F.; Trávolo, A.R.F.; Muehlmann, L.A.; Narcizo, P.S.; Nunes, R.B.; Pereira, P.A.G.; Py-Daniel, K.R.; Jiang, C.-S.; Figueiró, L.J.P.; Azevedo, R.B.; et al. Photodynamic Therapy treatment of onychomycosis with Aluminium-Phthalocyanine Chloride nanoemulsions: A proof of concept clinical trial. J. Photochem. Photobiol. B Biol. 2017, 173, 266–270. [Google Scholar] [CrossRef]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Helander, I.; Nurmiaho-Lassila, E.-L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Chen, C.-P.; Chen, C.-T.; Tsai, T. Chitosan Nanoparticles for Antimicrobial Photodynamic Inactivation: Characterization and In Vitro Investigation†. Photochem. Photobiol. 2012, 88, 570–576. [Google Scholar] [CrossRef] [PubMed]
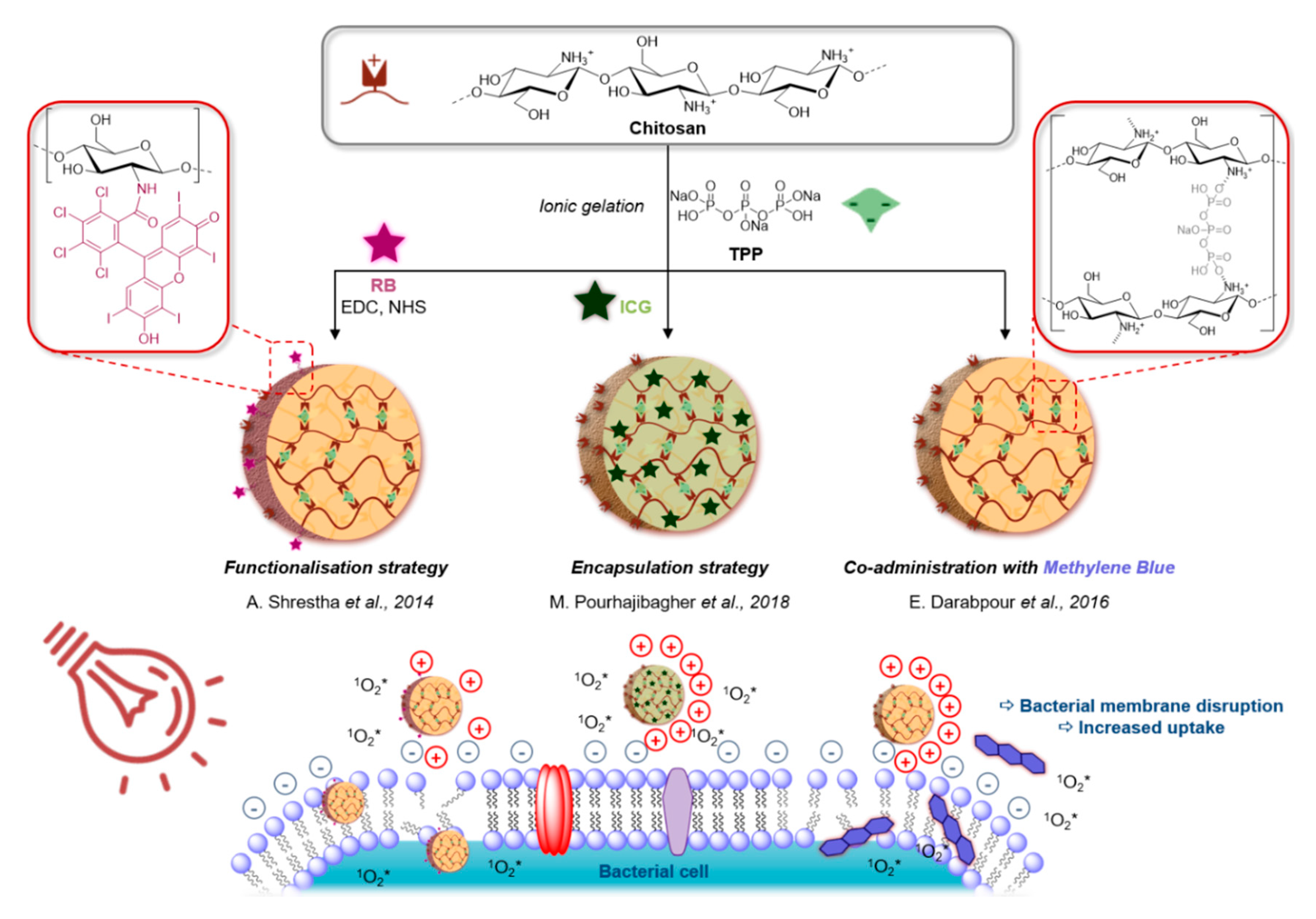
- Shrestha, A.; Kishen, A. Polycationic Chitosan-Conjugated Photosensitizer for Antibacterial Photodynamic Therapy†. Photochem. Photobiol. 2011, 88, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Darabpour, E.; Kashef, N.; Mashayekhan, S. Chitosan nanoparticles enhance the efficiency of methylene blue-mediated antimicrobial photodynamic inactivation of bacterial biofilms: An in vitro study. Photodiagnosis Photodyn. Ther. 2016, 14, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.-X.; Li, H.-C.; Ren, X.-D.; Sun, Y.; Xie, W.; Wang, C.-Y.; Zheng, B.-Y.; Ke, M.-R.; Huang, J.-D. Preparation and antifungal properties of monosubstituted zinc(П) phthalocyanine-chitosan oligosaccharide conjugates and their quaternized derivatives. Dye. Pigment. 2018, 159, 439–448. [Google Scholar] [CrossRef]
- Cavalcante, L.L.R.; Tedesco, A.C.; Takahashi, L.A.U.; Curylofo-Zotti, F.A.; Souza-Gabriel, A.E.; Corona, S.A.M. Conjugate of chitosan nanoparticles with chloroaluminium phthalocyanine: Synthesis, characterization and photoinactivation of Streptococcus mutans biofilm. Photodiagnosis Photodyn. Ther. 2020, 30, 101709. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Rokn, A.R.; Rostami-Rad, M.; Barikani, H.R.; Bahador, A. Monitoring of Virulence Factors and Metabolic Activity in Aggregatibacter Actinomycetemcomitans Cells Surviving Antimicrobial Photodynamic Therapy via Nano-Chitosan Encapsulated Indocyanine Green. Front. Phys. 2018, 6, 6. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Rokn, A.R.; Barikani, H.R.; Bahador, A. Photo-sonodynamic antimicrobial chemotherapy via chitosan nanoparticles-indocyanine green against polymicrobial periopathogenic biofilms: Ex vivo study on dental implants. Photodiagnosis Photodyn. Ther. 2020, 31, 101834. [Google Scholar] [CrossRef]
- Ribeiro, C.P.; Gamelas, S.R.; Faustino, M.A.; Gomes, A.T.; Tomé, J.P.; Almeida, A.; Lourenço, L.M. Unsymmetrical cationic porphyrin-cyclodextrin bioconjugates for photoinactivation of Escherichia coli. Photodiagnosis Photodyn. Ther. 2020, 31, 101788. [Google Scholar] [CrossRef]
- Hanakova, A.; Bogdanova, K.; Tomankova, K.; Pizova, K.; Malohlava, J.; Binder, S.; Bajgar, R.; Langova, K.; Kolar, M.; Mosinger, J.; et al. The application of antimicrobial photodynamic therapy on S. aureus and E. coli using porphyrin photosensitizers bound to cyclodextrin. Microbiol. Res. 2014, 169, 163–170. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Zagami, R.; Casaletto, M.P.; Martel, B.; Trapani, M.; Romeo, A.; Villari, V.; Sciortino, M.T.; Grasso, L.; Guglielmino, S.; et al. Poly(carboxylic acid)-Cyclodextrin/Anionic Porphyrin Finished Fabrics as Photosensitizer Releasers for Antimicrobial Photodynamic Therapy. Biomacromolecules 2017, 18, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Zagami, R.; Franco, D.; Pipkin, J.D.; Antle, V.; De Plano, L.; Patanè, S.; Guglielmino, S.; Scolaro, L.M.; Mazzaglia, A. Sulfobutylether-β-cyclodextrin/5,10,15,20-tetrakis(1-methylpyridinium-4-yl)porphine nanoassemblies with sustained antimicrobial phototherapeutic action. Int. J. Pharm. 2020, 585, 119487. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.; Kakatkar, A.S.; Chatterjee, S.; Barooah, N.; Kunwar, A.; Bhasikuttan, A.C.; Mohanty, J. Supramolecular Nanorods of (N-Methylpyridyl) Porphyrin With Captisol: Effective Photosensitizer for Anti-bacterial and Anti-tumor Activities. Front. Chem. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, J.; Hu, D.; Deng, Y.; Chen, T.; Jin, Q.; Ji, J. Bacteria-Targeted Supramolecular Photosensitizer Delivery Vehicles for Photodynamic Ablation Against Biofilms. Macromol. Rapid Commun. 2018, 40, 1800763. [Google Scholar] [CrossRef]
- Mora, S.J.; Cormick, M.P.; Milanesio, M.E.; Durantini, E.N. The photodynamic activity of a novel porphyrin derivative bearing a fluconazole structure in different media and against Candida albicans. Dye. Pigment. 2010, 87, 234–240. [Google Scholar] [CrossRef]
- Jia, R.; Tian, W.; Bai, H.; Zhang, J.; Wang, S.; Zhang, J. Sunlight-Driven Wearable and Robust Antibacterial Coatings with Water-Soluble Cellulose-Based Photosensitizers. Adv. Heal. Mater. 2019, 8, e1801591. [Google Scholar] [CrossRef]
- Dai, X.; Chen, X.; Zhao, Y.; Yu, Y.; Wei, X.; Zhang, X.; Li, C. A Water-Soluble Galactose-Decorated Cationic Photodynamic Therapy Agent Based on BODIPY to Selectively Eliminate Biofilm. Biomacromolecules 2017, 19, 141–149. [Google Scholar] [CrossRef]
- Hao, J.; Lu, Z.S.; Li, C.M.; Xu, L.Q. A maltoheptaose-decorated BODIPY photosensitizer for photodynamic inactivation of Gram-positive bacteria. New J. Chem. 2019, 43, 15057–15065. [Google Scholar] [CrossRef]
- Dabrzalska, M.; Zablocka, M.; Mignani, S.; Majoral, J.P.; Klajnert-Maculewicz, B. Phosphorus dendrimers and photodynamic therapy. Spectroscopic studies on two dendrimer-photosensitizer complexes: Cationic phosphorus dendrimer with rose bengal and anionic phosphorus dendrimer with methylene blue. Int. J. Pharm. 2015, 492, 266–274. [Google Scholar] [CrossRef]
- Sztandera, K.; Marcinkowska, M.; Gorzkiewicz, M.; Janaszewska, A.; Laurent, R.; Zabłocka, M.; Mignani, S.; Majoral, J.-P.; Klajnert-Maculewicz, B. In Search of a Phosphorus Dendrimer-Based Carrier of Rose Bengal: Tyramine Linker Limits Fluorescent and Phototoxic Properties of a Photosensitizer. Int. J. Mol. Sci. 2020, 21, 4456. [Google Scholar] [CrossRef]
- Staegemann, M.H.; Gitter, B.; Dernedde, J.; Kuehne, C.; Haag, R.; Wiehe, A. Mannose-Functionalized Hyperbranched Polyglycerol Loaded with Zinc Porphyrin: Investigation of the Multivalency Effect in Antibacterial Photodynamic Therapy. Chem. A Eur. J. 2017, 23, 3918–3930. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, R.; Geddes, C.D. Carbon Nanodots in Photodynamic Antimicrobial Therapy: A Review. Materials 2020, 13, 4004. [Google Scholar] [CrossRef] [PubMed]
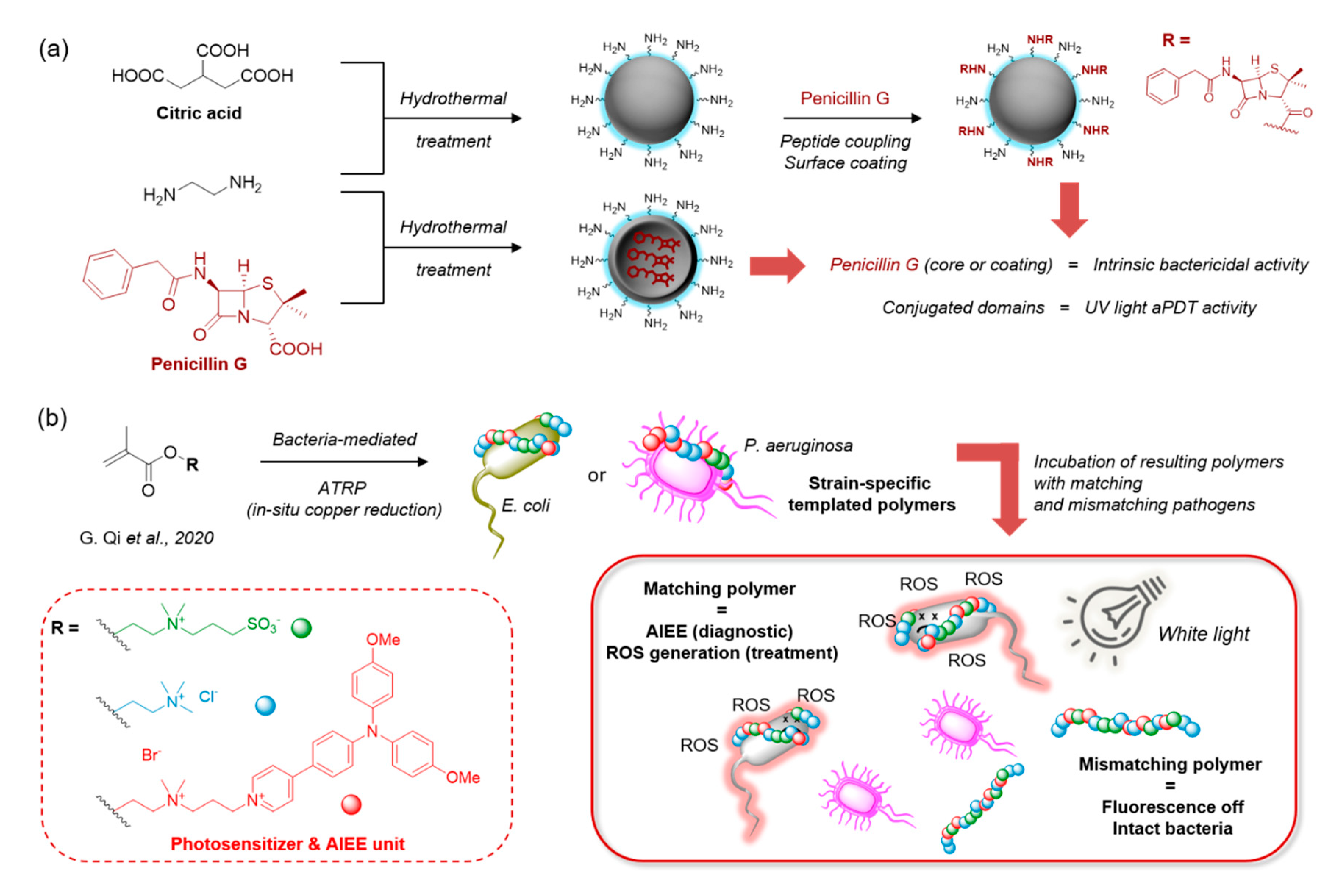
- Ning, L.G.; Liu, P.; Lu, Z.; Li, C.M.; Kang, E.-T.; Lu, Z.S.; Hu, X.; Xu, L.Q. Hydrothermal derived protoporphyrin IX nanoparticles for inactivation and imaging of bacteria strains. J. Colloid Interface Sci. 2019, 549, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, J.S.; Mayank; Pandiyan, T.; Kaur, N.; Arora, H.S. The Photochemical Degradation of Bacterial Cell Wall Using Penicillin-Based Carbon Dots: Weapons Against Multi-Drug Resistant (MDR) Strains. ChemistrySelect 2017, 2, 9277–9283. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, D.; Wan, Y.; Yang, Y. One-step synthesis of carbon dots for selective bacterial inactivation and bacterial differentiation. Anal. Bioanal. Chem. 2020, 412, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Prasad, S.R.; Mandal, D.; Das, P. Bovine Serum Albumin Amplified Reactive Oxygen Species Generation from Anthrarufin-Derived Carbon Dot and Concomitant Nanoassembly for Combination Antibiotic–Photodynamic Therapy Application. ACS Appl. Mater. Interfaces 2019, 11, 33273–33284. [Google Scholar] [CrossRef]
- Li, C.; Lin, F.; Sun, W.; Wu, F.-G.; Yang, H.; Lv, R.; Zhu, Y.-X.; Jia, H.-R.; Wang, C.; Gao, G.; et al. Self-Assembled Rose Bengal-Exopolysaccharide Nanoparticles for Improved Photodynamic Inactivation of Bacteria by Enhancing Singlet Oxygen Generation Directly in the Solution. ACS Appl. Mater. Interfaces 2018, 10, 16715–16722. [Google Scholar] [CrossRef]
- Qi, G.; Hu, F.; Kenry; Chong, K.C.; Wu, M.; Gan, Y.H.; Liu, B. Bacterium-Templated Polymer for Self-Selective Ablation of Multidrug-Resistant Bacteria. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Yan, R.; Wang, J.; Wu, H.; Wang, Y.; Chen, A.; Shao, S.; Li, Y.-Q. A bacteria-activated photodynamic nanosystem based on polyelectrolyte-coated silica nanoparticles. J. Mater. Chem. B 2017, 5, 3572–3579. [Google Scholar] [CrossRef]
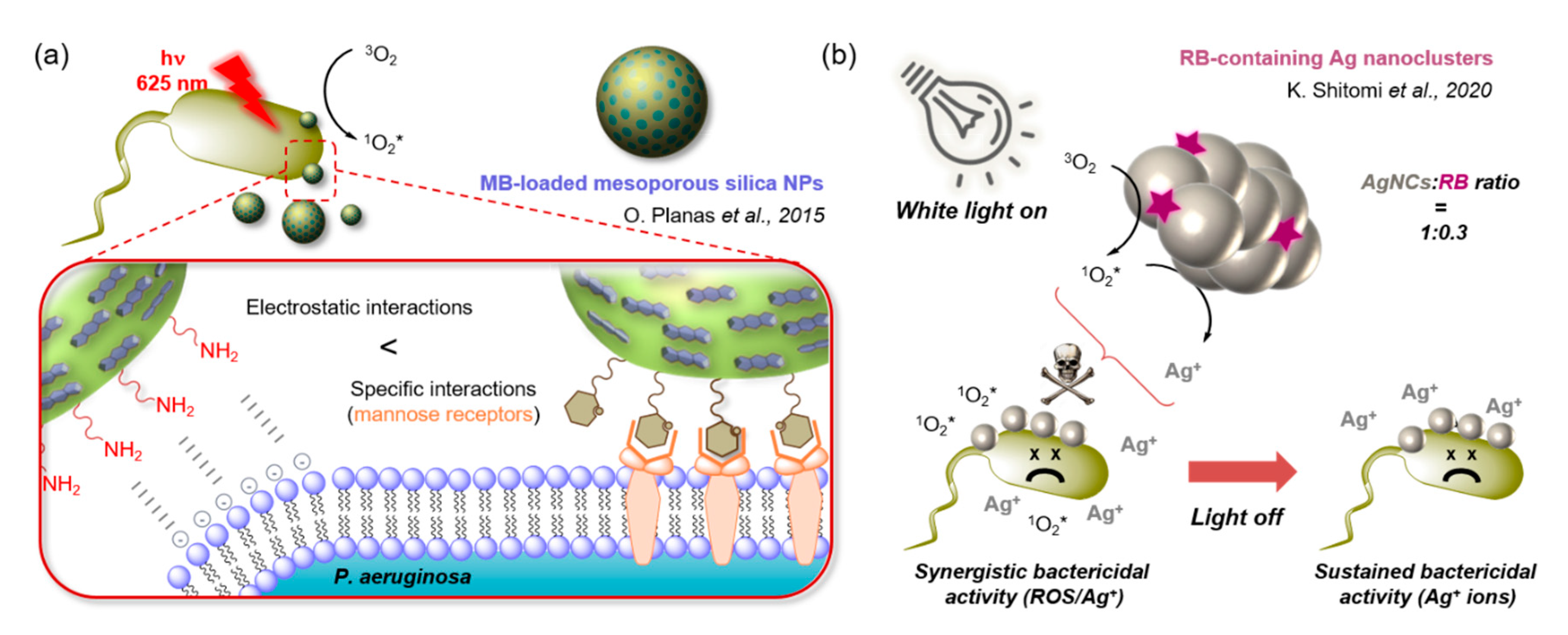
- Planas, O.; Bresolí-Obach, R.; Nos, J.; Gallavardin, T.; Ruiz-Gonzalez, R.; Agut, M.; Nonell, S. Synthesis, Photophysical Characterization, and Photoinduced Antibacterial Activity of Methylene Blue-loaded Amino- and Mannose-Targeted Mesoporous Silica Nanoparticles. Molecules 2015, 20, 6284–6298. [Google Scholar] [CrossRef]
- Grüner, M.C.; Arai, M.S.; Carreira, M.; Inada, N.; De Camargo, A.S.S.; De Camargo, A.S.S. Functionalizing the Mesoporous Silica Shell of Upconversion Nanoparticles To Enhance Bacterial Targeting and Killing via Photosensitizer-Induced Antimicrobial Photodynamic Therapy. ACS Appl. Bio Mater. 2018, 1, 1028–1036. [Google Scholar] [CrossRef]
- Aslan, K.; Lakowicz, J.R.; Geddes, C.D. Nanogold-plasmon-resonance-based glucose sensing. Anal. Biochem. 2004, 330, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Kaittanis, C.; Tinkham, A.; Perez, J.M. Dextran-Coated Gold Nanoparticles for the Assessment of Antimicrobial Susceptibility. Anal. Chem. 2008, 80, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Khan, S.N.; Meena, R.; Dar, A.M.; Pal, R.; Khan, A.U. Photoinactivation of multidrug resistant bacteria by monomeric methylene blue conjugated gold nanoparticles. J. Photochem. Photobiol. B Biol. 2017, 174, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Shitomi, K.; Miyaji, H.; Miyata, S.; Sugaya, T.; Ushijima, N.; Akasaka, T.; Kawasaki, H. Photodynamic inactivation of oral bacteria with silver nanoclusters/rose bengal nanocomposite. Photodiagnosis Photodyn. Ther. 2020, 30, 101647. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, P.; R. Y, T.; Shaji, C.; Sharan, A.; Bahkali, A.H.; Al-Harthi, H.F.; Syed, A.; Anju, V.; Dyavaiah, M.; Siddhardha, B. Biogenic Silver Nanoparticles Decorated with Methylene Blue Potentiated the Photodynamic Inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Pharmaceutics 2020, 12, 709. [Google Scholar] [CrossRef]
- Sah, U.; Sharma, K.; Chaudhri, N.; Sankar, M.; Gopinath, P. Antimicrobial photodynamic therapy: Single-walled carbon nanotube (SWCNT)-Porphyrin conjugate for visible light mediated inactivation of Staphylococcus aureus. Colloids Surfaces B Biointerfaces 2018, 162, 108–117. [Google Scholar] [CrossRef]
- Anju, V.T.; Paramanantham, P.; Sruthil Lal, S.B.; Sharan, A.; Syed, A.; Bahkali, N.A.; Alsaedi, M.H.; Kaviyarasu, K.; Busi, S. Antimicrobial photodynamic activity of toluidine blue-carbon nanotube conjugate against Pseudomonas aeruginosa and Staphylococcus aureus—Understanding the mechanism of action. Photodiagnosis Photodyn. Ther. 2019, 27, 305–316. [Google Scholar] [CrossRef]
- Parasuraman, P.; Anju, V.T.; Lal, S.B.S.; Sharan, A.; Busi, S.; Kaviyarasu, K.; Arshad, M.; Dawoud, T.M.S.; Syed, A. Synthesis and antimicrobial photodynamic effect of methylene blue conjugated carbon nanotubes on E. coli and S. aureus. Photochem. Photobiol. Sci. 2019, 18, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Akbari, T.; Pourhajibagher, M.; Hosseini, F.; Chiniforush, N.; Gholibegloo, E.; Khoobi, M.; Shahabi, S.; Bahador, A. The effect of indocyanine green loaded on a novel nano-graphene oxide for high performance of photodynamic therapy against Enterococcus faecalis. Photodiagnosis Photodyn. Ther. 2017, 20, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Peng, S.; Sui, M.; Chen, S.; Huang, L.; Xu, H.; Jiang, T. Multifunctional nanocomplex for surface-enhanced Raman scattering imaging and near-infrared photodynamic antimicrobial therapy of vancomycin-resistant bacteria. Colloids Surfaces B Biointerfaces 2018, 161, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Zoua, Z.; Suna, J.; Lia, Q.; Pub, Y.; Liua, J.; Suna, R.; Wanga, L.; Jiang, T.-T. Vancomycin modified copper sulfide nanoparticles for photokilling of vancomycin-resistant enterococci bacteria. Colloids Surfaces B Biointerfaces 2020, 189, 110875. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.M.; Fischman, A.J.; Stevens, E.; Lee, T.T.; Strong, L.; Tompkins, R.G.; Yarmush, M.L. Sn-chlorin e6 antibacterial immunoconjugates. J. Immunol. Methods 1992, 156, 85–99. [Google Scholar] [CrossRef]
- Berthiaume, F.; Reiken, S.R.; Toner, M.; Tompkins, R.G.; Yarmush, M.L. Antibody-targeted Photolysis of Bacteria In Vivo. Nat. Biotechnol. 1994, 12, 703–706. [Google Scholar] [CrossRef]
- Gross, S.; Brandis, A.; Chen, L.; Roehrs, S.; Scherz, A.; Salomon, Y.; Rosenbach-Belkin, V. Protein-A-mediated Targeting of Bacteriochlorophyll-IgG to Staphylococcus aureus: A Model for Enhanced Site-Specific Photocytotoxicity. Photochem. Photobiol. 1997, 66, 872–878. [Google Scholar] [CrossRef]
- Suci, P.A.; Varpness, Z.; Gillitzer, E.; Douglas, T.; Young, M. Targeting and Photodynamic Killing of a Microbial Pathogen Using Protein Cage Architectures Functionalized with a Photosensitizer. Langmuir 2007, 23, 12280–12286. [Google Scholar] [CrossRef]
- Kim, G.; Karbaschi, M.; Cooke, M.; Gaitas, A. Light-based methods for whole blood bacterial inactivation enabled by a recirculating flow system. Photochem. Photobiol. 2018, 94, 744–751. [Google Scholar] [CrossRef]
- Cantelli, A.; Piro, F.; Pecchini, P.; Di Giosia, M.; Danielli, A.; Calvaresi, M. Concanavalin A-Rose Bengal bioconjugate for targeted Gram-negative antimicrobial photodynamic therapy. J. Photochem. Photobiol. B Biol. 2020, 206, 111852. [Google Scholar] [CrossRef]
- Gao, S.; Yan, X.; Xie, G.; Zhu, M.; Ju, X.; Stang, P.J.; Tian, Y.; Niu, Z. Membrane intercalation-enhanced photodynamic inactivation of bacteria by a metallacycle and TAT-decorated virus coat protein. Proc. Natl. Acad. Sci. USA 2019, 116, 23437–23443. [Google Scholar] [CrossRef] [PubMed]
- Delcanale, P.; Montali, C.; Rodríguez-Amigo, B.; Abbruzzetti, S.; Bruno, S.; Bianchini, P.; Diaspro, A.; Agut, M.; Nonell, S.; Viappiani, C. Zinc-Substituted Myoglobin Is a Naturally Occurring Photo-antimicrobial Agent with Potential Applications in Food Decontamination. J. Agric. Food Chem. 2016, 64, 8633–8639. [Google Scholar] [CrossRef] [PubMed]
- Hally, C.; Delcanale, P.; Nonell, S.; Viappiani, C.; Abbruzzetti, S. Photosensitizing proteins for antibacterial photodynamic inactivation. Transl. Biophotonics 2020, 2. [Google Scholar] [CrossRef]
- Mordon, S.; Cochrane, C.; Tylcz, J.B.; Betrouni, N.; Mortier, L.; Koncar, V. Light emitting fabric technologies for photodynamic therapy. Photodiagnosis Photodyn. Ther. 2015, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hempstead, J.; Jones, D.P.; Ziouche, A.; Cramer, G.M.; Rizvi, I.; Arnason, S.; Hasan, T.; Celli, J.P. Low-cost photodynamic therapy devices for global health settings: Characterization of battery-powered LED performance and smartphone imaging in 3D tumor models. Sci. Rep. 2015, 5, 10093. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klausen, M.; Ucuncu, M.; Bradley, M. Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy. Molecules 2020, 25, 5239. https://doi.org/10.3390/molecules25225239
Klausen M, Ucuncu M, Bradley M. Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy. Molecules. 2020; 25(22):5239. https://doi.org/10.3390/molecules25225239
Chicago/Turabian StyleKlausen, Maxime, Muhammed Ucuncu, and Mark Bradley. 2020. "Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy" Molecules 25, no. 22: 5239. https://doi.org/10.3390/molecules25225239
APA StyleKlausen, M., Ucuncu, M., & Bradley, M. (2020). Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy. Molecules, 25(22), 5239. https://doi.org/10.3390/molecules25225239