Liquid-Liquid Extraction of Indium(III) from the HCl Medium by Ionic Liquid A327H+Cl− and Its Use in a Supported Liquid Membrane System
Abstract
1. Introduction
2. Results and Discussion
2.1. Generation of Ionic Liquids
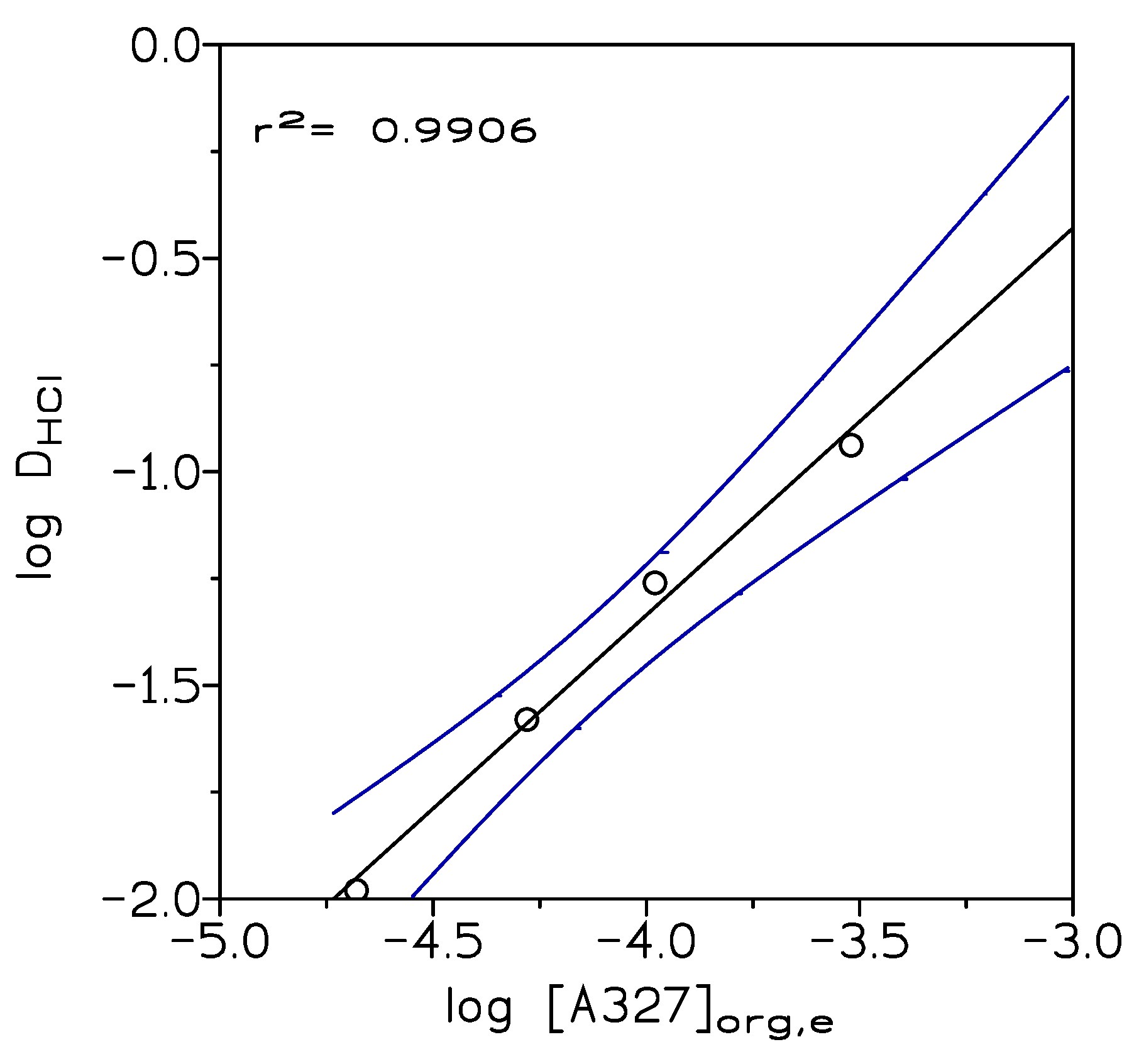
2.2. Indium(III) Liquid-Liquid Extraction Results
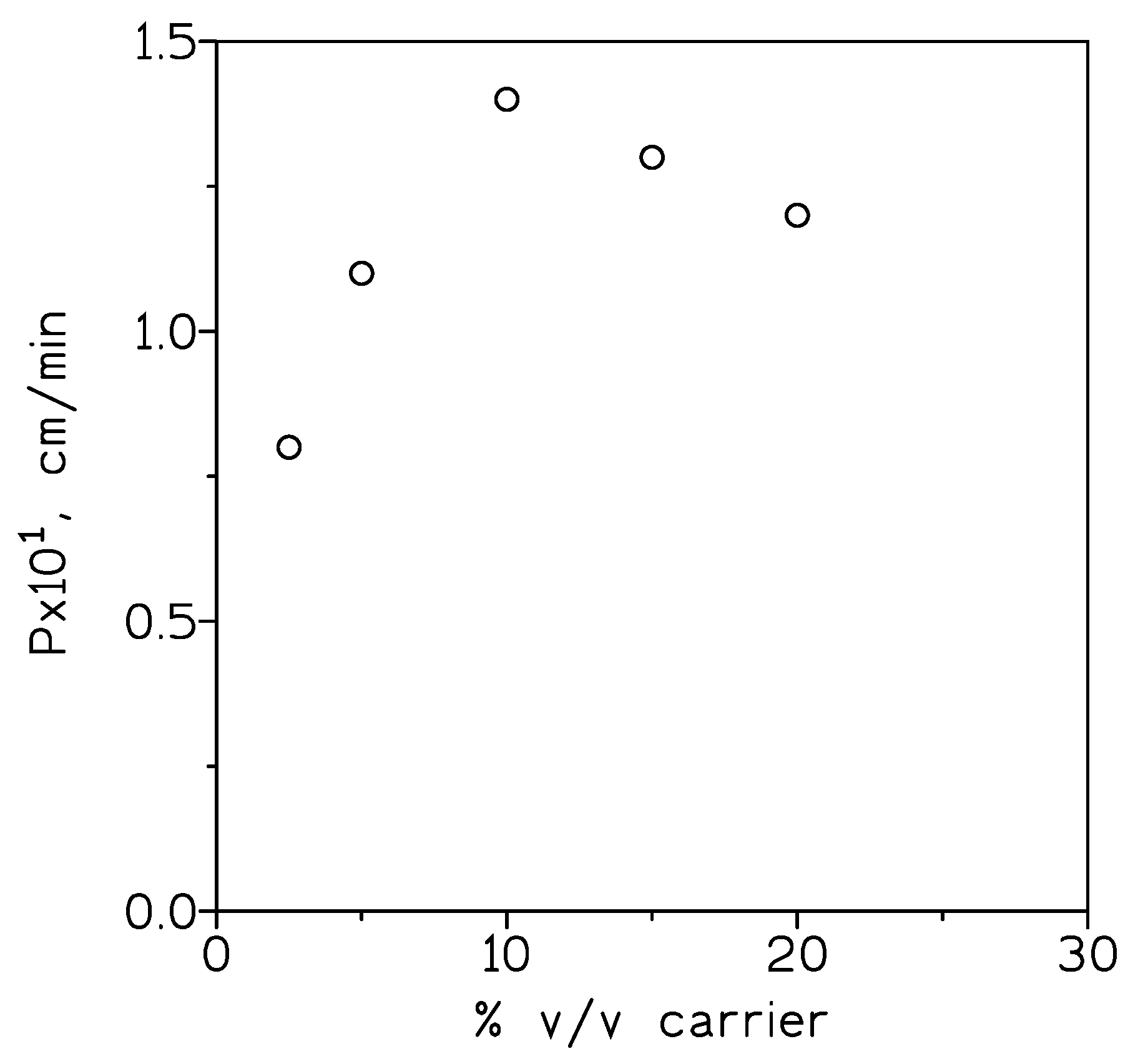
2.3. Indium(III) Transport Results
3. Materials and Methods
3.1. Liquid-Liquid Extraction Experiments
3.2. Supported Liquid Membrane Experiments
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Pradhan, D.; Panda, S.; Sukla, L.B. Recent advances in indium metallurgy: A review. Min. Proc. Extract. Metall. Rev. 2018, 39, 167–180. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Ali Kucuker, M.; Kuchta, K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes—A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212–275. [Google Scholar] [CrossRef]
- Houssaine Moutiy, E.; Tran, L.-H.; Mueller, K.K.; Coudert, L.; Blais, J.-F. Optimized indium solubilization from LCD panels using H2SO4 leaching. Waste Manag. 2020, 114, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Virolainen, S.; Huhtanen, T.; Laitinen, A.; Sainio, T. Two alternative process routes for recovering pure indium from waste liquid crystal display panels. J. Clean. Prod. 2020, 243, 118599. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, N.; Li, Y.; Luo, D.; Wu, P.; Dang, Z. Rapid and green process for valuable materials recovery from waste liquid crystal displays. Res. Cons. Rec. 2020, 153, 104544. [Google Scholar] [CrossRef]
- Zürner, P.; Frisch, G. Leaching and Selective Extraction of Indium and Tin from Zinc Flue Dust Using an Oxalic Acid-Based Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2019, 7, 5300–5308. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. A Review on Separation of Gallium and Indium from Leach Liquors by Solvent Extraction and Ion Exchange. Min. Proc. Extr. Metall. Rev. 2019, 40, 278–291. [Google Scholar] [CrossRef]
- Lv, Y.; Xing, P.; Ma, B.; Liu, B.; Wang, C.; Zhang, Y.; Zhang, W. Separation and Recovery of Valuable Elements from Spent CIGS Materials. ACS Sustain. Chem. Eng. 2019, 7, 19816–19823. [Google Scholar] [CrossRef]
- Alguacil, F.J.; López, F.A. Dispersion-free extraction of In(III) from HCl solutions using a supported liquid membrane containing the HA324H+Cl− ionic liquid as the carrier. Sci. Rep. 2020, 10, 13868. [Google Scholar] [CrossRef]
- Dang, N.T.T.; Wang, D.-M.; Huang, S.-Y.; Tran, K.T. Indium recovery from aqueous solution containing oxalic acid—Enhancement by using hydrophobic membranes. Sep. Purif. Technol. 2020, 235, 116300. [Google Scholar] [CrossRef]
- Kinoshita, T.; Ishigaki, Y.; Kitagawa, S.; Ichino, R.; Kamimoto, Y. Selective recovery of indium via continuous counter-current foam separation from sulfuric acid solutions II—Optimization of operational parameters on separation performance. Sep. Purif. Technol. 2020, 238, 116490. [Google Scholar] [CrossRef]
- Ciro, E.; Dell’Era, A.; Pasquali, M.; Lupi, C. Indium electrowinning study from sulfate aqueous solution using different metal cathodes. J. Environ. Chem. Eng. 2020, 8, 103688. [Google Scholar] [CrossRef]
- Schulze, M.M.; Löwe, R.; Pollex, R.; Mazik, M. Structure–extractability relationships for substituted 8-hydroxyquinolines: Solvent extraction of indium ions from acidic aqueous media. Monats. Chem. 2019, 150, 983–990. [Google Scholar] [CrossRef]
- Cao, Y.; Li, F.; Li, G.; Huang, J.; Zhu, H.; He, W. Leaching and purification of indium from waste liquid crystal display panel after hydrothermal pretreatment: Optimum conditions determination and kinetic analysis. Waste Manag. 2020, 102, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Jian, D.; Guo, X.; Li, X.; Deng, Z.; Wei, C.; Li, M.; Fan, G. Extraction of indium with N,N-di(1-methylheptyl)acetamide, di(l-methylheptyl)methyl phosphate, and tributylphosphate by solvent extraction in hydrochloric acid solution. Min. Eng. 2020, 156, 106510. [Google Scholar] [CrossRef]
- Sasaki, Y. Various metal extraction from hydrochloric acid into n-dodecane by methylimino-dioctylacetamide (MIDOA). Sep. Sci. Technol. 2020, 55, 708–715. [Google Scholar] [CrossRef]
- Deferm, C.; Onghena, B.; Nguyen, V.T.; Banerjee, D.; Fransaer, J.; Binnemans, K. Non-aqueous solvent extraction of indium from an ethylene glycol feed solution by the ionic liquid Cyphos IL 101: Speciation study and continuous counter-current process in mixer-settlers. RSC Adv. 2020, 10, 24595–24612. [Google Scholar] [CrossRef]
- Dhiman, S.; Gupta, B. Cyphos IL 104 assisted extraction of indium and recycling of indium, tin and zinc from discarded LCD screen. Sep. Purif. Technol. 2020, 237, 116407. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Escudero, E. Solvent extraction of indium(III) from HCl solutions by the ionic liquid (A324H+)(Cl−) dissolved in Solvesso 100. Hydrometallurgy 2019, 189, 105104. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Garcia-Diaz, I.; Escudero, E. Extraction of indium(III) from sulphuric acid medium by the ionic liquid (PJMTH+HSO4−). Sep. Purif. Technol. 2019, 211, 764–767. [Google Scholar] [CrossRef]
- Cen, P.; Spahiu, K.; Tyumentsev, M.S.; Foreman, M.R.S.J. Metal extraction from a deep eutectic solvent, an insight into activities. Phys. Chem. Chem. Phys. 2020, 22, 11012–11024. [Google Scholar] [CrossRef] [PubMed]
- Billard, I. Green solvents in urban mining. Curr. Op. Green Sustain. Chem. 2019, 18, 37–41. [Google Scholar] [CrossRef]
- Rzelewska-Piekut, M.; Regel-Rosocka, M. Separation of Pt(IV), Pd(II), Ru(III) and Rh(IV) from model chloride solutions by liquid-liquid extraction with phosphonium ionic liquids. Sep. Purif. Technol. 2019, 212, 791–801. [Google Scholar] [CrossRef]
- Schaeffer, N.; Passos, H.; Billard, I.; Papaiconomou, N.; Coutinho, J.A.P. Recovery of metals from waste electrical and electronic equipment (WEEE) using unconventional solvents based on ionic liquids. Crit. Rev. Environ. Sci. Technol. 2018, 48, 859–922. [Google Scholar] [CrossRef]
- Svecova, L.; Papaiconomou, N.; Billard, I. Rh(III) aqueous speciation with chloride as a driver for its extraction by phosphonium based ionic liquids. Molecules 2019, 24, 1391. [Google Scholar] [CrossRef] [PubMed]
- De Agreda, D.; Garcia-Diaz, I.; López, F.A.; Alguacil, F.J. Supported liquid membranes technologies in metals removal from liquid effluents. Rev. Metal. Madr. 2011, 47, 146–168. [Google Scholar] [CrossRef]
- Amini, M.; Rahbar-Kelishami, A.; Alipour, M.; Vahidi, O. Supported liquid membrane in metal ion separation: An overview. J. Membr. Sci. Res. 2018, 4, 121–135. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Caravaca, C. Study of gold(III)-HCl-amine Alamine 304 extraction equilibrium system. Hydrometallurgy 1993, 34, 91–98. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Cobo, A.; Coedo, A.G.; Dorado, M.T.; Sastre, A. Extraction of platinum(IV) from hydrochloric acid solutions by amine alamine 304 in xylene. Estimation of the interaction coefficient between PtCl62− and H+. Hydrometallurgy 1997, 44, 203–212. [Google Scholar] [CrossRef]
- Deferm, C.; Onghena, B.; Vander Hoogerstraete, T.; Banerjee, D.; Luyten, J.; Oosterhorf, H.; Fransaer, J.; Binnemans, K. Speciation of indium(III) chloro complexes in the solvent extraction process from chloride aqueous solutions to ionic liquids. Dalton Trans. 2017, 46, 4412–4421. [Google Scholar] [CrossRef]
- Puigdomenech, I. MEDUSA Program. Available online: www/kth.se (accessed on 1 August 2020).
- Alguacil, F.J. Facilitated chromium(VI) transport across an ionic liquid membrane impregnated with Cyphos IL102. Molecules 2019, 24, 2437. [Google Scholar] [CrossRef] [PubMed]
- Alguacil, F.J.; Alonso, M.M.; Sastre, A.M. Modelling of mass transfer in facilitated supported liquid membrane transport of copper(II) using MOC-55TD in Iberfluid. J. Membr. Sci. 2001, 184, 117–122. [Google Scholar] [CrossRef]
- Huang, T.C.; Juang, R.S. Rate and mechanism of divalent metal transport through supported liquid membrane containing di(2-ethylhexyl) phosphoric acid as a mobile carrier. J. Chem. Technol. Biotechnol. 1988, 42, 3–17. [Google Scholar] [CrossRef]

| Temperature, °C | DIn |
|---|---|
| 20 | 15.7 |
| 30 | 13.3 |
| 40 | 11.5 |
| 50 | 9.0 |
| 60 | 7.3 |
| [In]0, g/L | DIn |
|---|---|
| 0.010 | 15.7 |
| 0.075 | 15.7 |
| 0.15 | 15.9 |
| Ionic Liquid Concentration, % v/v | DIn |
|---|---|
| 5 | 500 |
| 2.5 | 40.7 |
| 1.25 | 15.7 |
| 0.50 | 3.3 |
| O/A | %St |
|---|---|
| 0.5 | 99 |
| 1 | 79 |
| 2 | 64 |
| 3 | 45 |
| 4 | 36 |
| Stirring Speed, min−1 | PIn × 101, cm/min |
|---|---|
| 600 | 1.0 |
| 850 | 1.4 |
| 1000 | 1.4 |
| 1200 | 1.2 |
| Time, min | [In]f,t, mg/L | [In]r,t, mg/L |
|---|---|---|
| 0 | 10.0 | 10.0 |
| 15 | 8.9 | 11.0 |
| 30 | 7.8 | 12.0 |
| 45 | 6.7 | 13.2 |
| 60 | 6.1 | 13.8 |
| 120 | 3.7 | 15.9 |
| 180 | 2.3 | 16.7 |
| [In]0, g/L | PIn × 101, cm/min | J × 107, mol/cm2 min | %R a |
|---|---|---|---|
| 0.01 | 1.4 | 0.12 | 85 |
| 0.05 | 1.3 | 0.57 | 89 |
| 0.1 | 1.1 | 0.96 | 96 |
| 0.2 | 0.94 | 1.6 | 90 |
Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alguacil, F.J. Liquid-Liquid Extraction of Indium(III) from the HCl Medium by Ionic Liquid A327H+Cl− and Its Use in a Supported Liquid Membrane System. Molecules 2020, 25, 5238. https://doi.org/10.3390/molecules25225238
Alguacil FJ. Liquid-Liquid Extraction of Indium(III) from the HCl Medium by Ionic Liquid A327H+Cl− and Its Use in a Supported Liquid Membrane System. Molecules. 2020; 25(22):5238. https://doi.org/10.3390/molecules25225238
Chicago/Turabian StyleAlguacil, Francisco José. 2020. "Liquid-Liquid Extraction of Indium(III) from the HCl Medium by Ionic Liquid A327H+Cl− and Its Use in a Supported Liquid Membrane System" Molecules 25, no. 22: 5238. https://doi.org/10.3390/molecules25225238
APA StyleAlguacil, F. J. (2020). Liquid-Liquid Extraction of Indium(III) from the HCl Medium by Ionic Liquid A327H+Cl− and Its Use in a Supported Liquid Membrane System. Molecules, 25(22), 5238. https://doi.org/10.3390/molecules25225238






