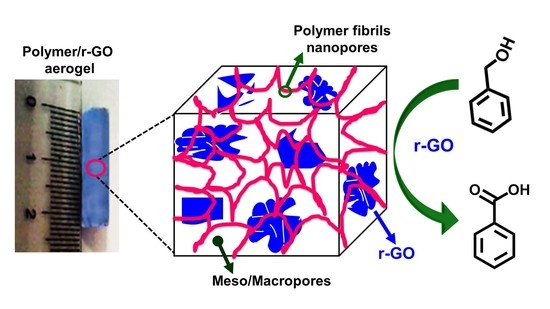
Nanoporous Crystalline Composite Aerogels with Reduced Graphene Oxide
Abstract
1. Introduction
2. Results
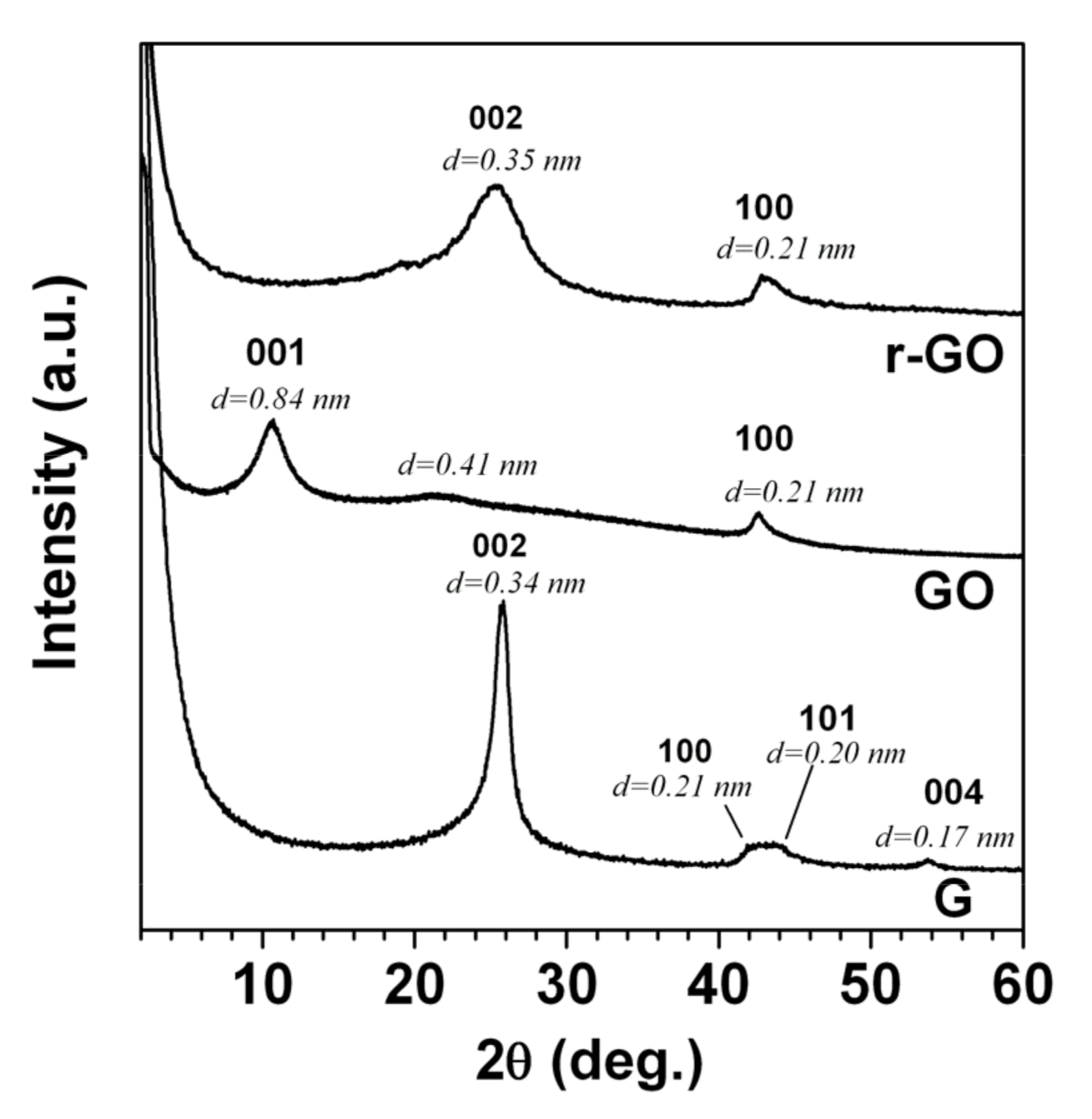
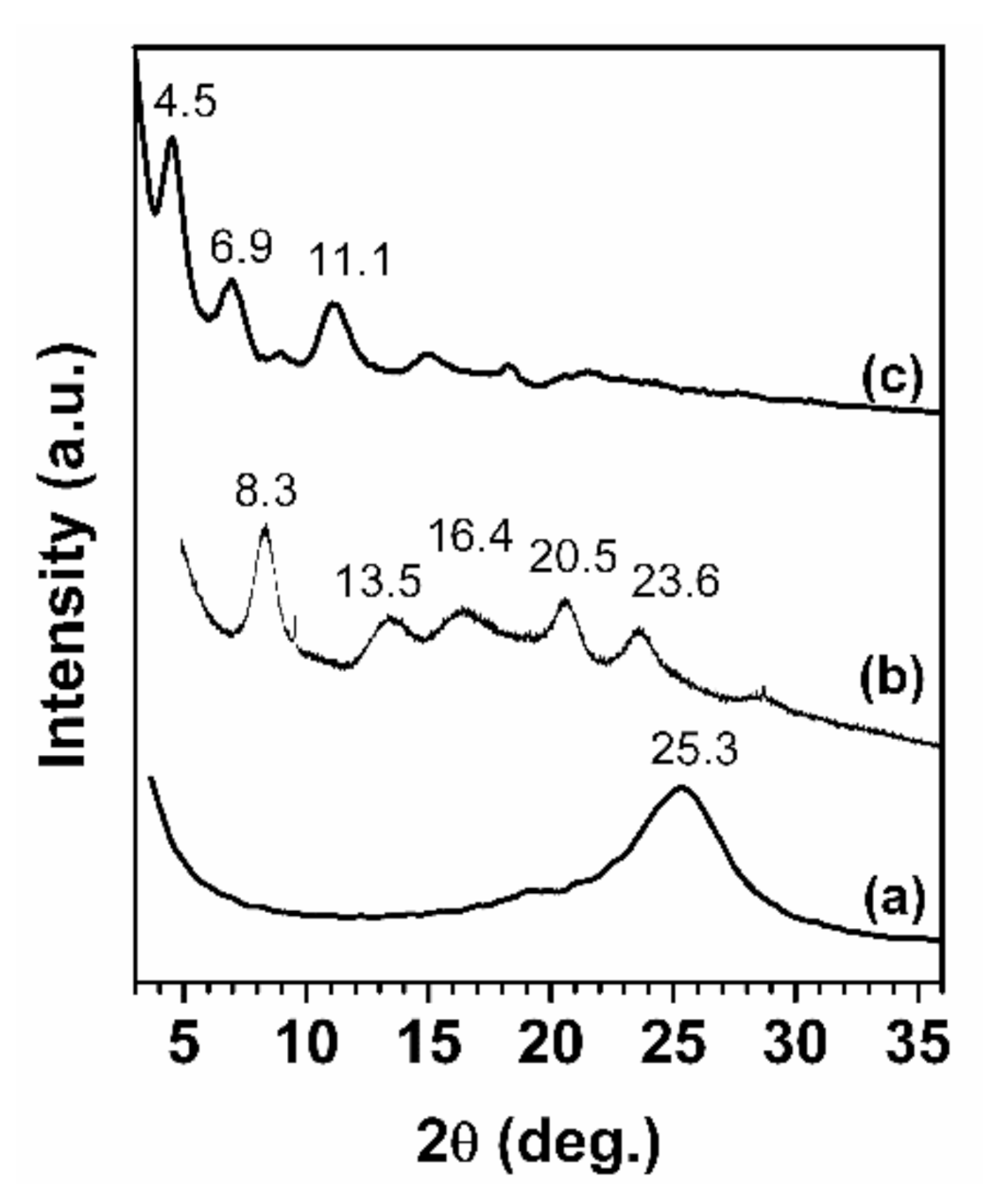
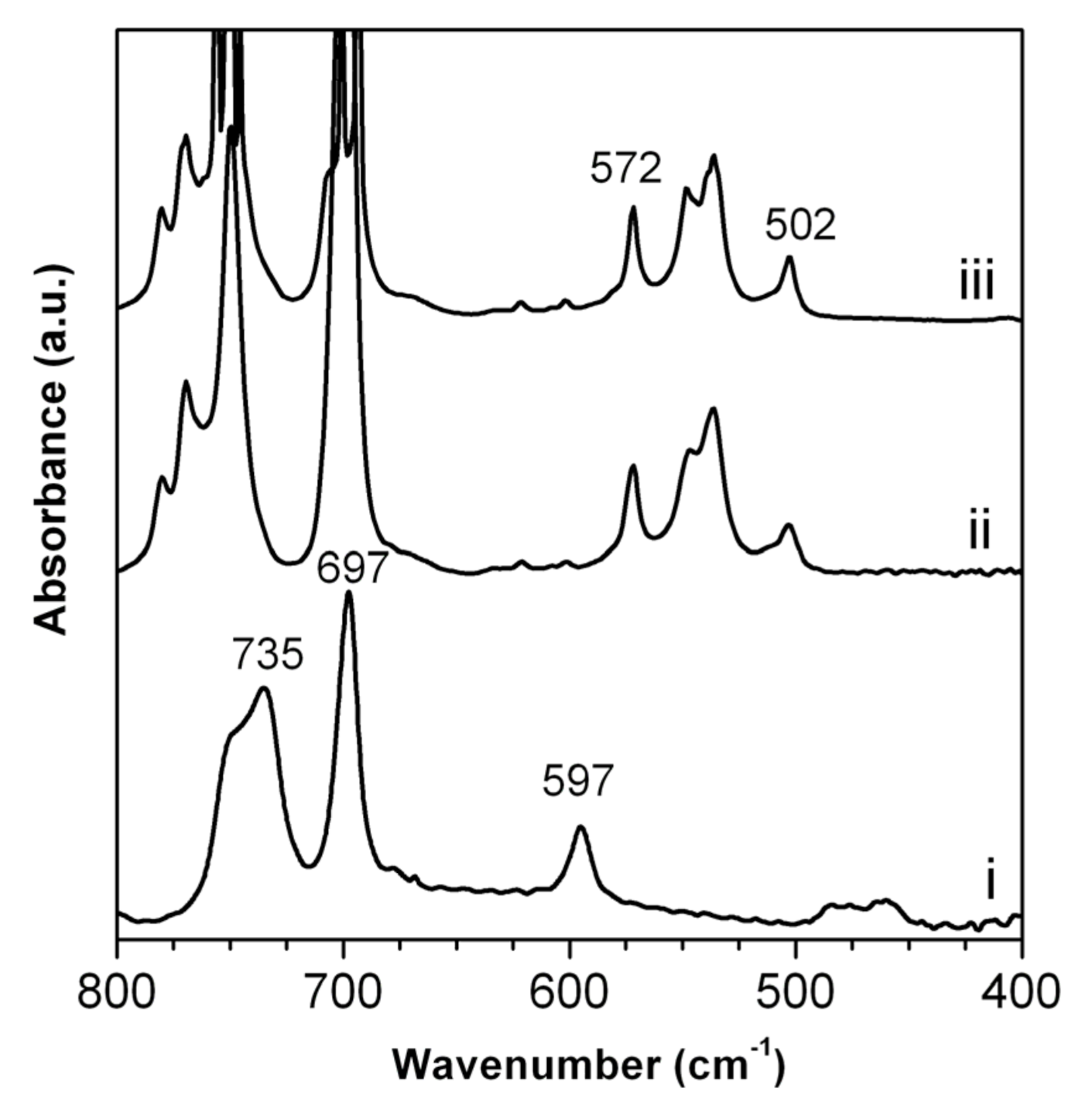
2.1. Reduced Graphite Oxide (r-GO) Preparation and Characterization
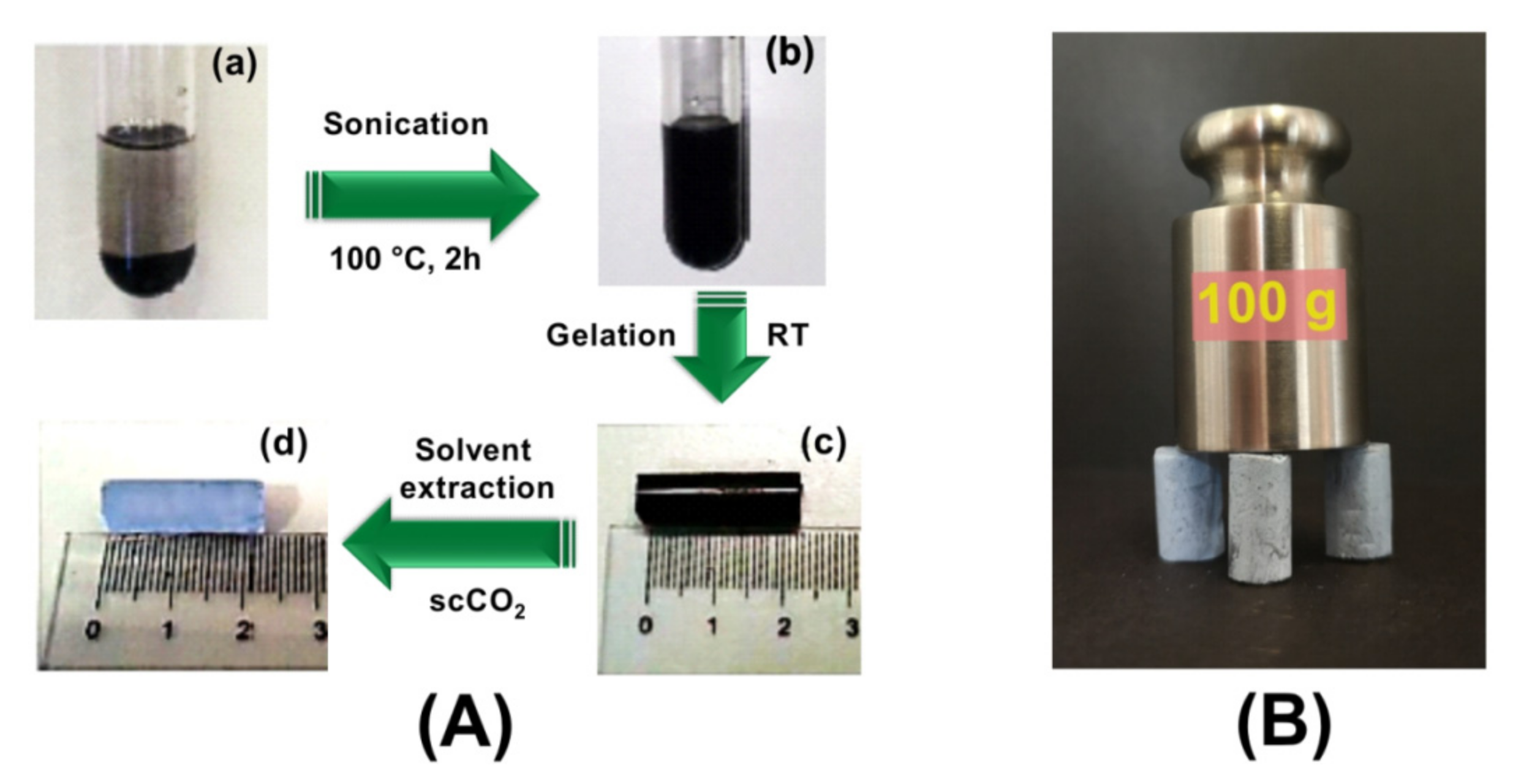
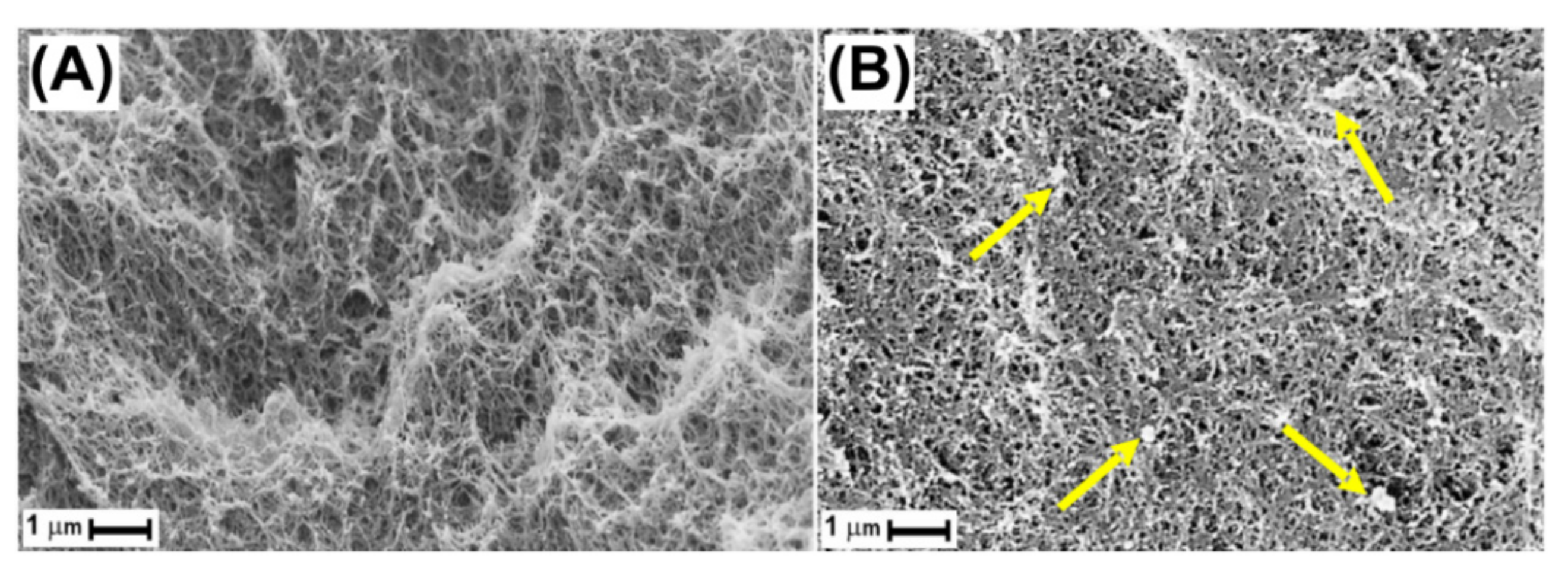
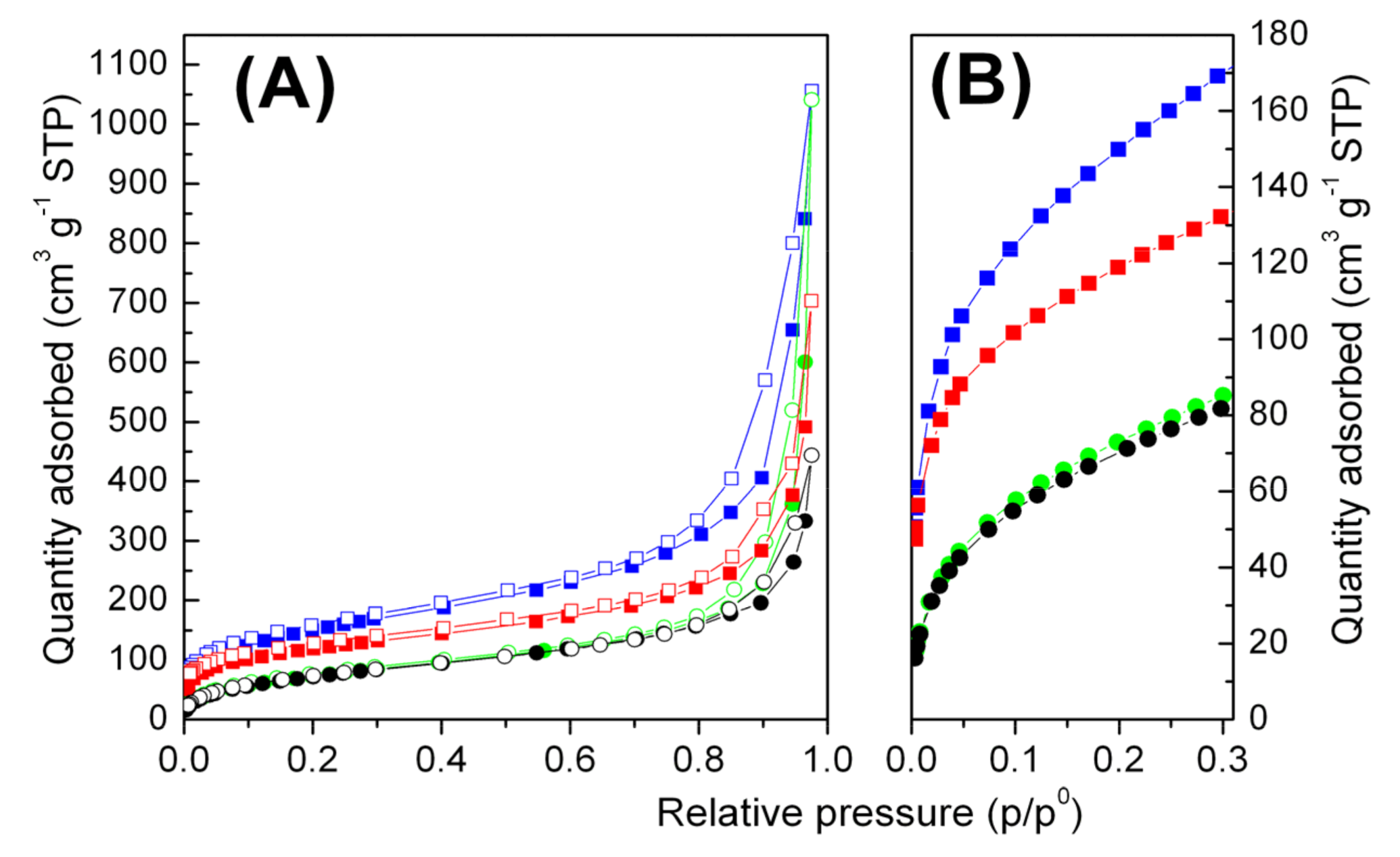
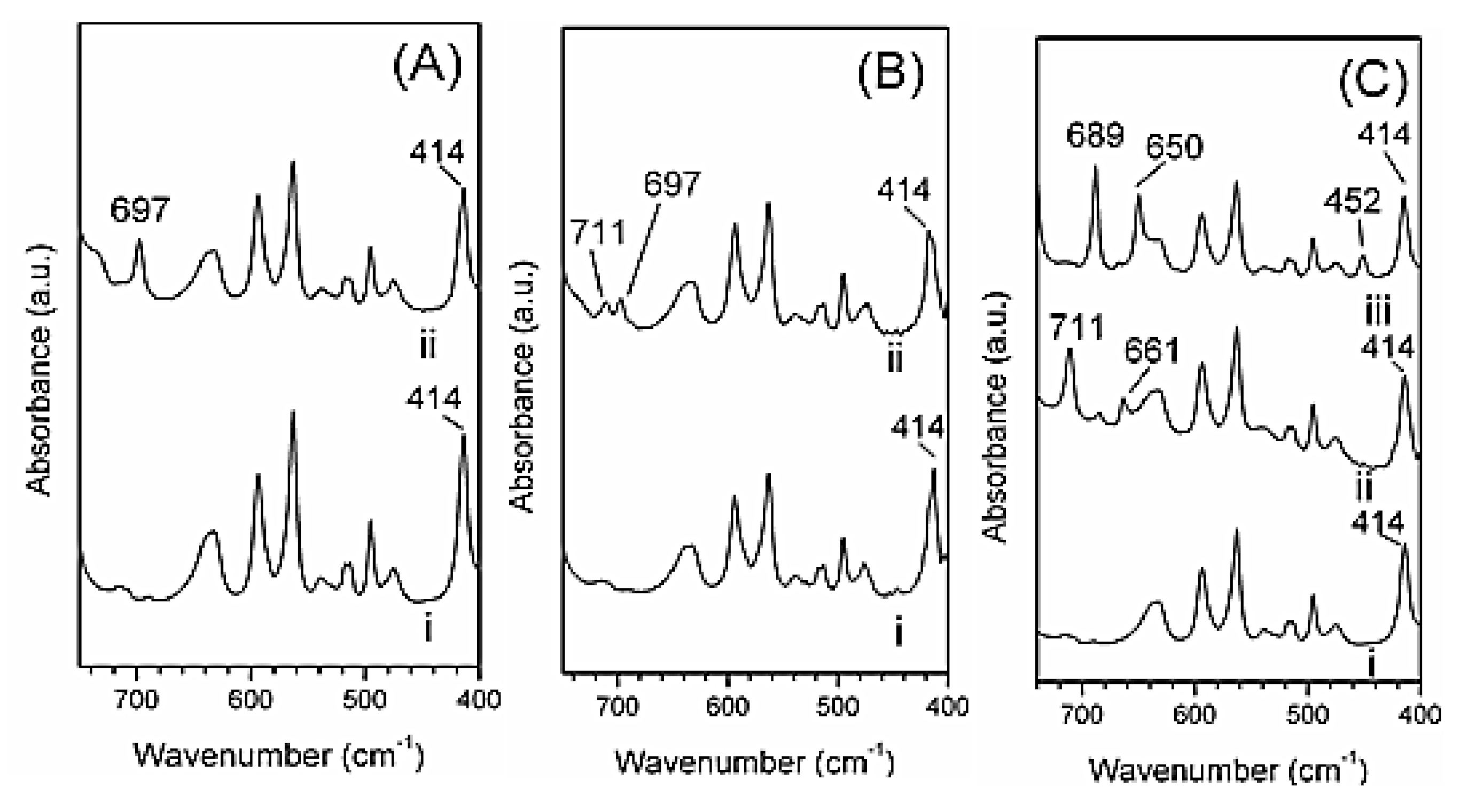
2.2. Polymer/r-GO Monolithic Aerogel Preparation and Characterization
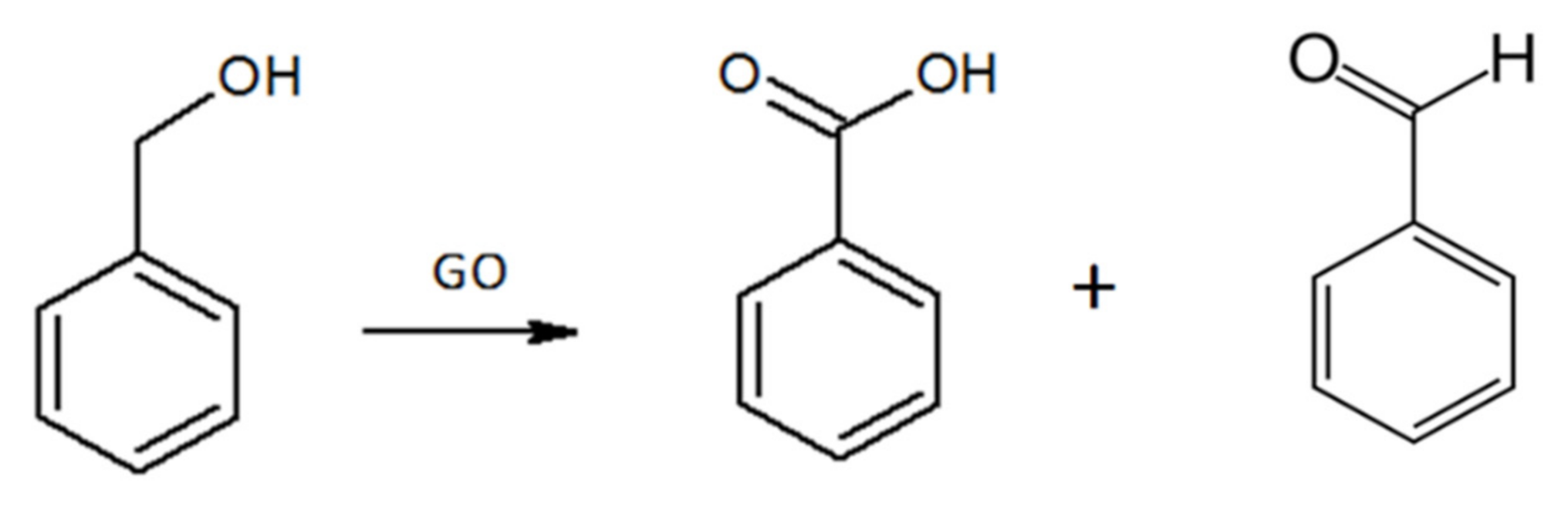
2.3. Catalytic Activity of the Polymer/r-GO Monolithic Aerogels
3. Materials and Methods
3.1. Materials
3.2. Graphene Oxide and Reduced Graphene Oxide Synthesis
3.3. Monolithic Polymer Aerogels Fabrication
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pekala, R.W. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Pekala, R.W.; Alviso, C.T.; Kong, F.M.; Hulsey, S.S. Aerogels derived from multifunctional organic monomers. J. Non-Cryst. Solids 1992, 145, 90–98. [Google Scholar] [CrossRef]
- Pekala, R.W.; Alviso, C.T.; Lu, X.; Gross, J.; Fricke, J. New organic aerogels based upon a phenolic-furfural reaction. J. Non-Cryst. Solids 1995, 188, 34–40. [Google Scholar] [CrossRef]
- Biesmans, G.; Mertens, A.; Duffours, L.; Woignier, T.; Phalippou, J. Polyurethane based organic aerogels and their transformation into carbon aerogels. J. Non-Cryst. Solids 1998, 225, 64–68. [Google Scholar] [CrossRef]
- Rigacci, A.; Marechal, J.C.; Repoux, M.; Moreno, M.; Achard, P.J. Preparation of polyurethane-based aerogels and xerogels for thermal superinsulation. J. Non-Cryst. Solids 2004, 350, 372–378. [Google Scholar] [CrossRef]
- Quignard, F.; Valentin, R.; Di Renzo, F. Aerogel materials from marine polysaccharides. New J. Chem. 2008, 32, 1300–1310. [Google Scholar] [CrossRef]
- Cardea, S.; Gugliuzza, A.; Sessa, M.; Aceto, M.C.; Drioli, E.; Reverchon, E. Supercritical Gel Drying: A Powerful Tool for Tailoring Symmetric Porous PVDF−HFP Membranes. ACS Appl. Mater. Interfaces 2009, 1, 171–180. [Google Scholar] [CrossRef]
- Zhang, X.; Chang, D.; Liu, J.; Luo, Y. Conducting polymer aerogels from supercritical CO2 drying PEDOT-PSS hydrogels. J. Mater. Chem. 2010, 20, 5080–5085. [Google Scholar] [CrossRef]
- Korhonen, J.T.; Kettunen, M.; Ras, R.H.A.; Ikkala, O. Hydrophobic Nanocellulose Aerogels as Floating, Sustainable, Reusable, and Recyclable Oil Absorbents. ACS Appl. Mater. Interfaces 2011, 3, 1813–1816. [Google Scholar] [CrossRef]
- Salerno, A.; Domingo, C. Making microporous nanometre-scale fibrous PLA aerogels with clean and reliable supercritical CO2 based approaches. Microporous Mesoporous Mater. 2014, 184, 162–168. [Google Scholar] [CrossRef]
- Del Gaudio, P.; Auriemma, G.; Mencherini, T.; Porta, G.D.; Reverchon, E.; Aquino, R.P. Design of Alginate-Based Aerogel for Nonsteroidal Anti-Inflammatory Drugs Controlled Delivery Systems Using Prilling and Supercritical-Assisted Drying. J. Pharm. Sci. 2013, 102, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Meador, M.A.B.; McMillon, E.; Sandberg, A.; Barrios, E.; Wilmoth, N.G.; Mueller, C.H.; Miranda, F.A. Dielectric and Other Properties of Polyimide Aerogels Containing Fluorinated Blocks. ACS Appl. Mater. Interfaces 2014, 6, 6062–6068. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring Mechanical Properties of Aerogels for Aerospace Applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jana, S.C. Synergistic Hybrid Organic-Inorganic Aerogels. ACS Appl. Mater. Interfaces 2013, 5, 6423–6429. [Google Scholar] [CrossRef]
- Chen, H.-B.; Hollinger, E.; Wang, Y.-Z.; Schiraldi, D.A. Facile fabrication of poly(vinyl alcohol) gels and derivative aerogels. Polymer 2014, 55, 380–384. [Google Scholar] [CrossRef]
- Schiraldi, D.A. Polymer Aerogels. In Encyclopedia of Polymer Science and Technology; John and Wiley and Sons: Hoboken, NJ, USA, 2015; pp. 1–18. [Google Scholar]
- Leonhardt, E.E.; Meador, M.A.B.; Wooley, K.L. β-Cyclodextrin-Derived Monolithic, Hierarchically Porous Polyimides Designed for Versatile Molecular Separation Applications. Chem. Mater. 2018, 30, 6226–6230. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. Nanostructured chitosan–gelatin hybrid aerogels produced by supercritical gel drying. Polym. Eng. Sci. 2018, 58, 1494–1499. [Google Scholar] [CrossRef]
- Li, M.; Qin, Z.; Cui, Y.; Yang, C.; Deng, C.; Wang, Y.; Kang, J.S.; Xia, H.; Hu, Y. Ultralight and Flexible Monolithic Polymer Aerogel with Extraordinary Thermal Insulation by A Facile Ambient Process. Adv. Mater. Interfaces 2019, 6, 1900314. [Google Scholar] [CrossRef]
- Baldino, L.; Zuppolini, S.; Cardea, S.; Diodato, L.; Borriello, A.; Reverchon, E.; Nicolais, L. Production of biodegradable superabsorbent aerogels using a supercritical CO2 assisted drying. J. Supercrit. Fluids 2020, 156, 104681. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, J.; Ge, S.; Jiang, C.; Guo, T.; Peng, T.; Huang, T.; Xie, L. Biocompatible, hydrophobic and resilience graphene/chitosan composite aerogel for efficient oil−water separation. Surf. Coat. Technol. 2020, 385, 125361. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef] [PubMed]
- Phadtare, V.D.; Parale, V.G.; Lee, K.-Y.; Kim, T.; Puri, V.R.; Park, H.-H. Flexible and lightweight Fe3O4/polymer foam composites for microwave-absorption applications. J. Alloy. Compaunds 2019, 805, 120–129. [Google Scholar] [CrossRef]
- Phadtare, V.D.; Parale, V.G.; Kim, T.; Lee, K.-Y.; Pathak, S.; Park, H.-H. Facile synthesis of a lightweight three-dimensional polymer scaffold dip-coated with multiple layers of TiO2 aerogel for X-band microwave absorption applications. J. Alloys Compd. 2020, 823, 153847. [Google Scholar] [CrossRef]
- Daniel, C.; Longo, S.; Ricciardi, R.; Reverchon, E.; Guerra, G. Monolithic Nanoporous Crystalline Aerogels. Macromol. Rapid Commun. 2013, 34, 1194–1207. [Google Scholar] [CrossRef] [PubMed]
- Venditto, V.; Pellegrino, M.; Califano, R.; Guerra, G.; Daniel, C.; Ambrosio, L.; Borriello, A. Monolithic polymeric aerogels with VOCs sorbent nanoporous crystalline and water sorbent amorphous phases. ACS Appl. Mater. Interfaces 2015, 7, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.; Pellegrino, M.; Venditto, V.; Aurucci, S.; Guerra, G. Nanoporous-crystalline poly(2,6-dimethyl-1,4-phenylene)oxide (PPO) aerogels. Polymer 2016, 105, 96–103. [Google Scholar] [CrossRef]
- Manfredi, C.; Del Nobile, M.A.; Mensitieri, G.; Guerra, G.; Rapacciuolo, M. Vapor sorption in emptied clathrate samples of syndiotactic polystyrene. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 133–140. [Google Scholar] [CrossRef]
- Mahesh, K.P.O.; Sivakumar, M.; Yamamoto, Y.; Tsujita, Y.; Yoshimizu, H.; Okamoto, S. Structure and properties of the mesophase of syndiotactic polystyrene: IX. Preferential sorption behavior of sPS-p-chlorotoluene mesophase membrane in a mixture of solvents. J. Membr. Sci. 2005, 262, 11–19. [Google Scholar] [CrossRef]
- Daniel, C.; Longo, S.; Fasano, G.; Vitillo, J.G.; Guerra, G. Nanoporous Crystalline Phases of Poly(2,6-Dimethyl-1,4-phenylene)oxide. Chem. Mater. 2011, 23, 3195–3200. [Google Scholar] [CrossRef]
- Daniel, C.; Zhovner, D.; Guerra, G. Thermal Stability of Nanoporous Crystalline and Amorphous Phases of Poly(2,6-dimethyl-1,4-phenylene) Oxide. Macromolecules 2013, 46, 449–454. [Google Scholar] [CrossRef]
- Daniel, C.; Antico, P.; Yamaguchi, H.; Kogure, M.; Guerra, G. Microporous-crystalline Microfibers by Eco-friendly Guests: An Efficient Tool for Sorption of Volatile Organic Pollutants. Microporous Mesoporous Mater. 2016, 232, 205–210. [Google Scholar] [CrossRef]
- Daniel, C.; Antico, P.; Guerra, G. Etched Fibers of Syndiotactic Polystyrene with Nanoporous-Crystalline Phases. Macromolecules 2018, 51, 6138–6148. [Google Scholar] [CrossRef]
- Joseph, A.M.; Nagendra, B.; Shaiju, P.; Surendran, K.P.; Gowd, E.B. Aerogels of Hierarchically Porous Syndiotactic Polystyrene with a Dielectric Constant Near to Air. J. Mater. Chem. C 2018, 6, 360–368. [Google Scholar] [CrossRef]
- Kim, S.J.; Chase, G.; Jana, S.C. Polymer aerogels for efficient removal of airborne nanoparticles. Sep. Purif. Technol. 2015, 156, 803–808. [Google Scholar] [CrossRef]
- Kim, S.J.; Chase, G.; Jana, S.C. The role of mesopores in achieving high efficiency airborne nanoparticle filtration using aerogel monoliths. Sep. Purif. Technol. 2016, 166, 48–54. [Google Scholar] [CrossRef]
- Alegre, C.; Sebastián, D.; Baquedano, E.; Gálvez, M.E.; Moliner, R.; Lázaro, M.J. Tailoring Synthesis Conditions of Carbon Xerogels towards Their Utilization as Pt-Catalyst Supports for Oxygen Reduction Reaction (ORR). Catalysts 2012, 2, 466–489. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Daniel, C.; Navarra, W.; Venditto, V. Removal of Phenol in Aqueous Media by N-doped TiO2 Based Photocatalytic Aerogels. Mater. Sci. Semicond. Process. 2018, 80, 104–110. [Google Scholar] [CrossRef]
- Wan, W.; Zhang, R.; Mab, M.; Zhou, Y. Monolithic Aerogel Photocatalysts: A Review. J. Mater. Chem. A 2018, 6, 754–775. [Google Scholar] [CrossRef]
- Maleki, H.; Hüsing, N. Current status, opportunities and challenges in catalytic and photocatalytic applications of aerogels: Environmental protection aspects. Appl. Catal. B Environ. 2018, 221, 530–555. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Daniel, C.; Navarra, W.; Venditto, V. Highly Robust and Selective System for Water Pollutants Removal: How to Transform a Traditional Photocatalyst into a Highly Robust and Selective System for Water Pollutants Removal. Nanomaterials 2019, 9, 1509. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Jia, H.-P.; Bielawski, C.W. Graphene Oxide: A Convenient Carbocatalyst for Facilitating Oxidation and Hydration Reactions. Angew. Chem. Int. Ed. 2010, 49, 6813–6816. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Carbocatalysis by Graphene-Based Materials. Chem. Rev. 2014, 114, 6179–6212. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zheng, Y.; Li, J.; Jin, Y.; Gao, C. Monolithic Neat Graphene Oxide Aerogel for Efficient Catalysis of S→O Acetyl Migration. ACS Catal. 2015, 5, 3387–3392. [Google Scholar] [CrossRef]
- Wen, G.; Wu, S.; Li, B.; Dai, C.; Su, D.S. Active Sites and Mechanisms for Direct Oxidation of Benzene to Phenol over Carbon Catalysts. Angew. Chem. Int. Ed. 2015, 54, 4105–4509. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Bielawski, C.W. Carbocatalysis: Heterogeneous carbons finding utility in synthetic chemistry. Chem. Sci. 2011, 2, 1233–1240. [Google Scholar] [CrossRef]
- Acocella, M.R.; Mauro, M.; Guerra, G. Regio- and Enantioselective Friedel–Crafts Reactions of Indoles to Epoxides Catalyzed by Graphene Oxide: A Green Approach. ChemSusChem 2014, 7, 3279–3283. [Google Scholar] [CrossRef]
- Acocella, M.R.; De Pascale, M.; Maggio, M.; Guerra, G. Graphite oxide as catalyst for diastereoselective Mukaiyama aldol reaction of 2-(trimethylsilyloxy)furan in solvent free conditions. J. Mol. Catal. A Chem. 2015, 408, 237–241. [Google Scholar] [CrossRef]
- Acocella, M.R.; Maggio, M.; Ambrosio, C.; Aprea, N.; Guerra, G. Oxidized Carbon Black as an Activator of Transesterification Reactions under Solvent-Free Conditions. ACS Omega 2017, 2, 7862–7867. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Soin, N.; Roy, S.S. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: A review. RSC Adv. 2014, 4, 3823–3851. [Google Scholar] [CrossRef]
- Kim, T.; Parale, V.G.; Jung, H.-N.-R.; Kim, Y.; Driss, Z.; Driss, D.; Bouabidi, A.; Euchy, S.; Park, H.-H. Facile Synthesis of SnO2 Aerogel/Reduced Graphene Oxide Nanocomposites via in Situ Annealing for the Photocatalytic Degradation of Methyl Orange. Nanomaterials 2019, 9, 358. [Google Scholar] [CrossRef]
- Xiang, C.; Cox, P.J.; Kukovecz, A.; Genorio, B.; Hashim, D.P.; Yan, Z.; Peng, Z.; Hwang, C.-C.; Ruan, G.; Samuel, E.L.G.; et al. Functionalized Low Defect Graphene Nanoribbons and Polyurethane Composite Film for Improved Gas Barrier and Mechanical Performances. ACS Nano 2013, 7, 10380–10386. [Google Scholar] [CrossRef] [PubMed]
- Nassira, H.; Sánchez-Ferrer, A.; Adamcik, J.; Handschin, S.; Mahdavi, H.; Taheri Qazvini, N.; Mezzenga, R. Gelatin–Graphene Nanocomposites with Ultralow Electrical Percolation Threshold. Adv. Mater. 2016, 28, 6914–6920. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.M.; Nagendra, B.; Surendran, K.P.; Gowd, E.B. Sustainable in Situ Approach to Covalently Functionalize Graphene Oxide with POSS Molecules Possessing Extremely Low Dielectric Behavior. Langmuir 2019, 35, 4672–4681. [Google Scholar] [CrossRef] [PubMed]
- Longo, S.; Mauro, M.; Daniel, C.; Musto, P.; Guerra, G. Rayleigh scattering by graphene-oxide in syndiotactic polystyrene aerogels. Carbon 2014, 77, 896–905. [Google Scholar] [CrossRef]
- De Rosa, C.; Guerra, G.; Petraccone, V.; Pirozzi, B. Crystal structure of the emptied clathrate form (δe form) of syndiotactic polystyrene. Macromolecules 1997, 30, 4147–4152. [Google Scholar] [CrossRef]
- Nagendra, B.; Cozzolino, A.; Daniel, C.; Rizzo, P.; Guerra, G.; Auriemma, F.; De Rosa, C.; D’Alterio, M.; Tarallo, O.; Nuzzo, A. Two nanoporous crystalline forms of poly (2,6-dimethyl-1,4-phenylene) oxide and related Co-crystalline forms. Macromolecules 2019, 52, 9646–9656. [Google Scholar] [CrossRef]
- Torres, F.J.; Civalleri, B.; Meyer, A.; Musto, P.; Albunia, A.R.; Rizzo, P.; Guerra, G. Normal Vibrational Analysis of the Syndiotactic Polystyrene s(2/1)2 Helix. J. Phys. Chem. B 2009, 113, 5059–5071. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Ko, E.I. Aerogels in Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 1998; pp. 1–22. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| sPS | sPS0.8/r-GO0.2 | PPO | PPO0.8/r-GO0.2 | |
|---|---|---|---|---|
| Apparent density (g cm−3) | 0.15 | 0.16 | 0.18 | 0.20 |
| Total porosity a (cm3 g−1/%) | 6.66/86 | 6.25/80 | 5.55/82 | 5/76 |
| SBET b (m2/g) | 290 ± 20 | 250 ± 20 | 550 ± 10 | 440 ± 10 |
| VtotN2 c (cm3 g−1) | 0.56 | 0.41 | 1.013 | 0.628 |
| Vmeso d (cm3 g−1) | 0.49 | 0.33 | 0.86 | 0.48 |
| Average mesopore radius d (nm) | 9.0 | 6.3 | 8.9 | 8.7 |
| Vmicro e (cm3 g−1) | 0.049 | 0.046 | 0.13 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniel, C.; Nagendra, B.; Acocella, M.R.; Cascone, E.; Guerra, G. Nanoporous Crystalline Composite Aerogels with Reduced Graphene Oxide. Molecules 2020, 25, 5241. https://doi.org/10.3390/molecules25225241
Daniel C, Nagendra B, Acocella MR, Cascone E, Guerra G. Nanoporous Crystalline Composite Aerogels with Reduced Graphene Oxide. Molecules. 2020; 25(22):5241. https://doi.org/10.3390/molecules25225241
Chicago/Turabian StyleDaniel, Christophe, Baku Nagendra, Maria Rosaria Acocella, Esther Cascone, and Gaetano Guerra. 2020. "Nanoporous Crystalline Composite Aerogels with Reduced Graphene Oxide" Molecules 25, no. 22: 5241. https://doi.org/10.3390/molecules25225241
APA StyleDaniel, C., Nagendra, B., Acocella, M. R., Cascone, E., & Guerra, G. (2020). Nanoporous Crystalline Composite Aerogels with Reduced Graphene Oxide. Molecules, 25(22), 5241. https://doi.org/10.3390/molecules25225241