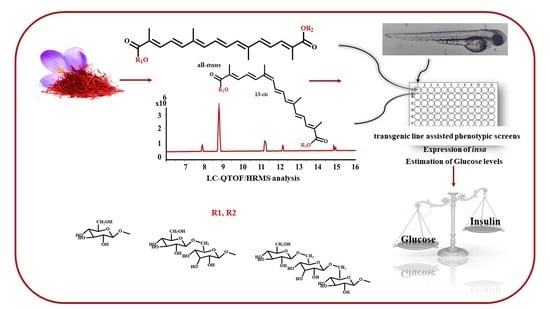
Crocins from Crocus sativus L. in the Management of Hyperglycemia. In Vivo Evidence from Zebrafish
Abstract
1. Introduction
2. Results
2.1. LC-QTOF HRMS Analysis
2.2. Determination of LC50
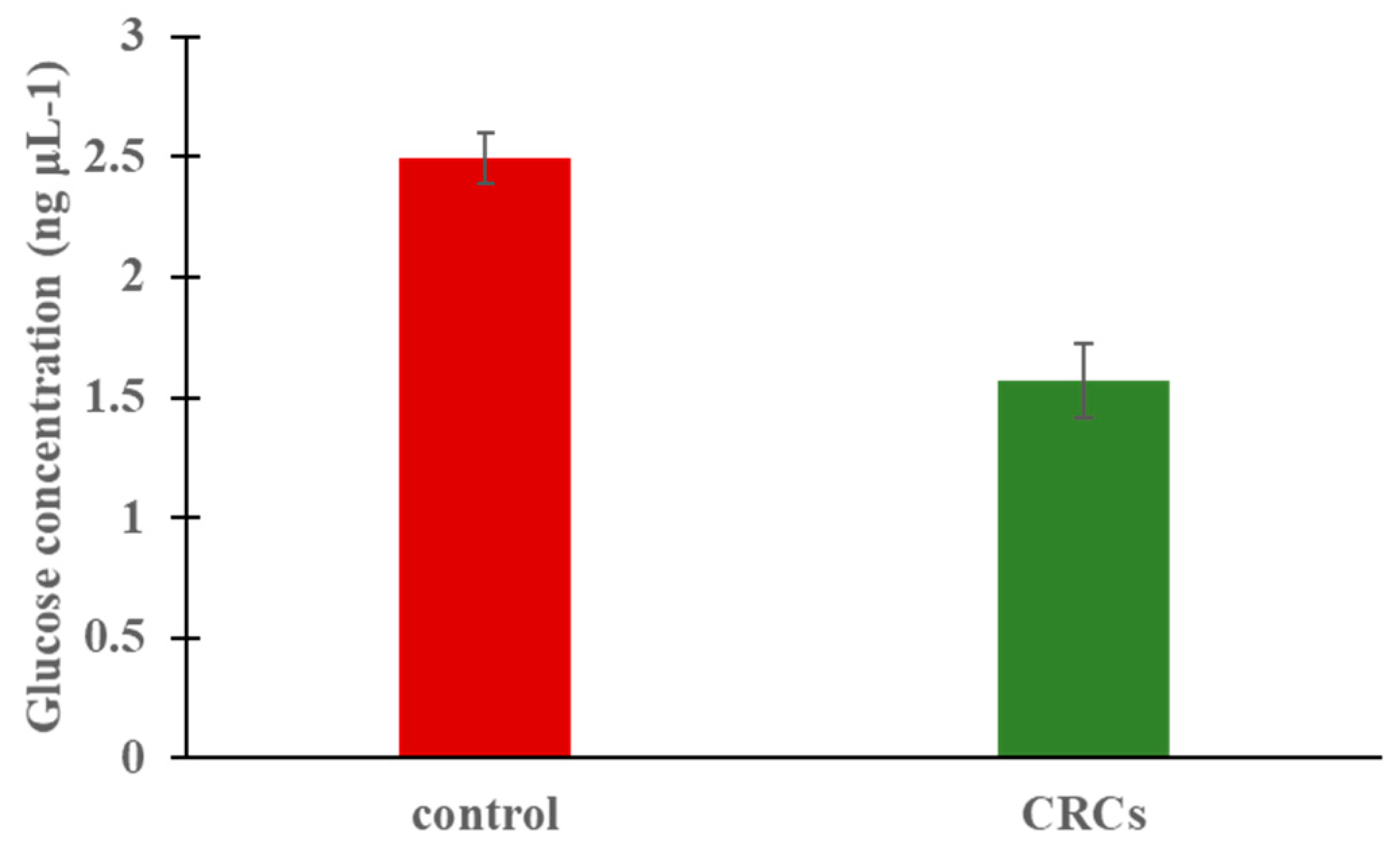
2.3. Zebrafish Glucose Levels Are Lowered by CRCs
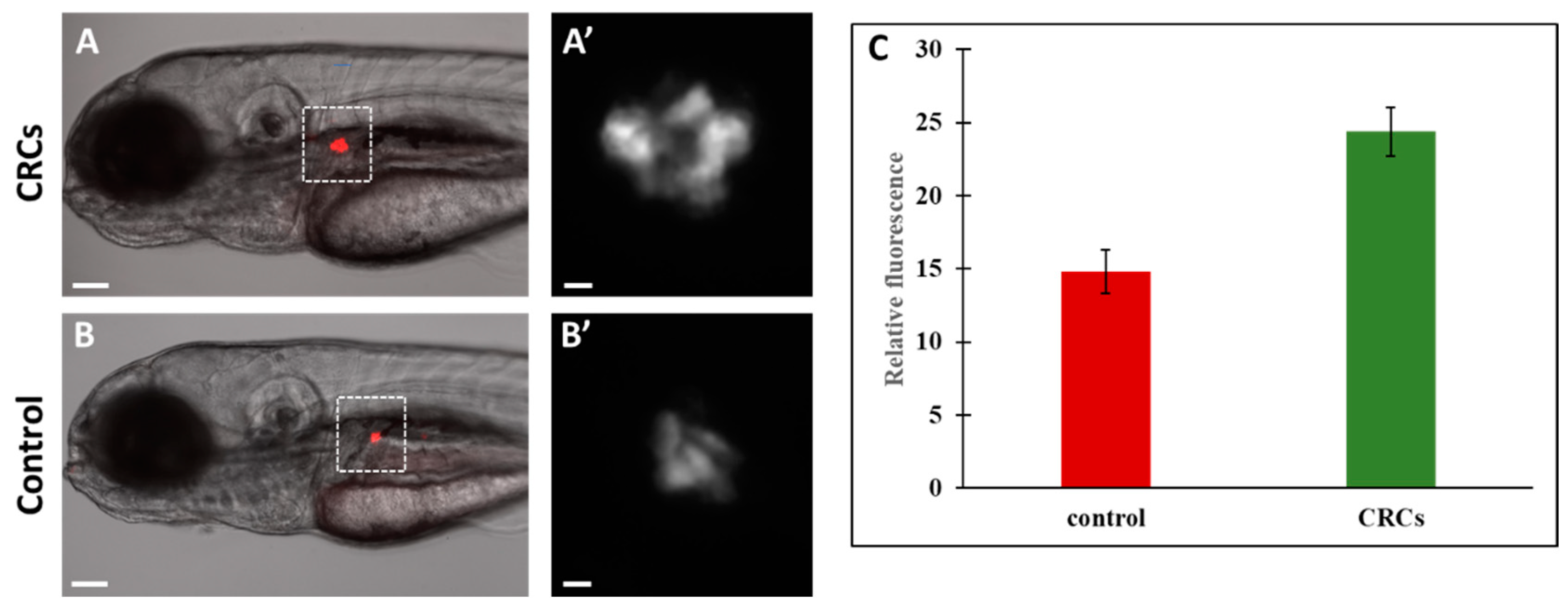
2.4. Enhanced Fluorescence of β-Cells Indicate Insa Upregulation
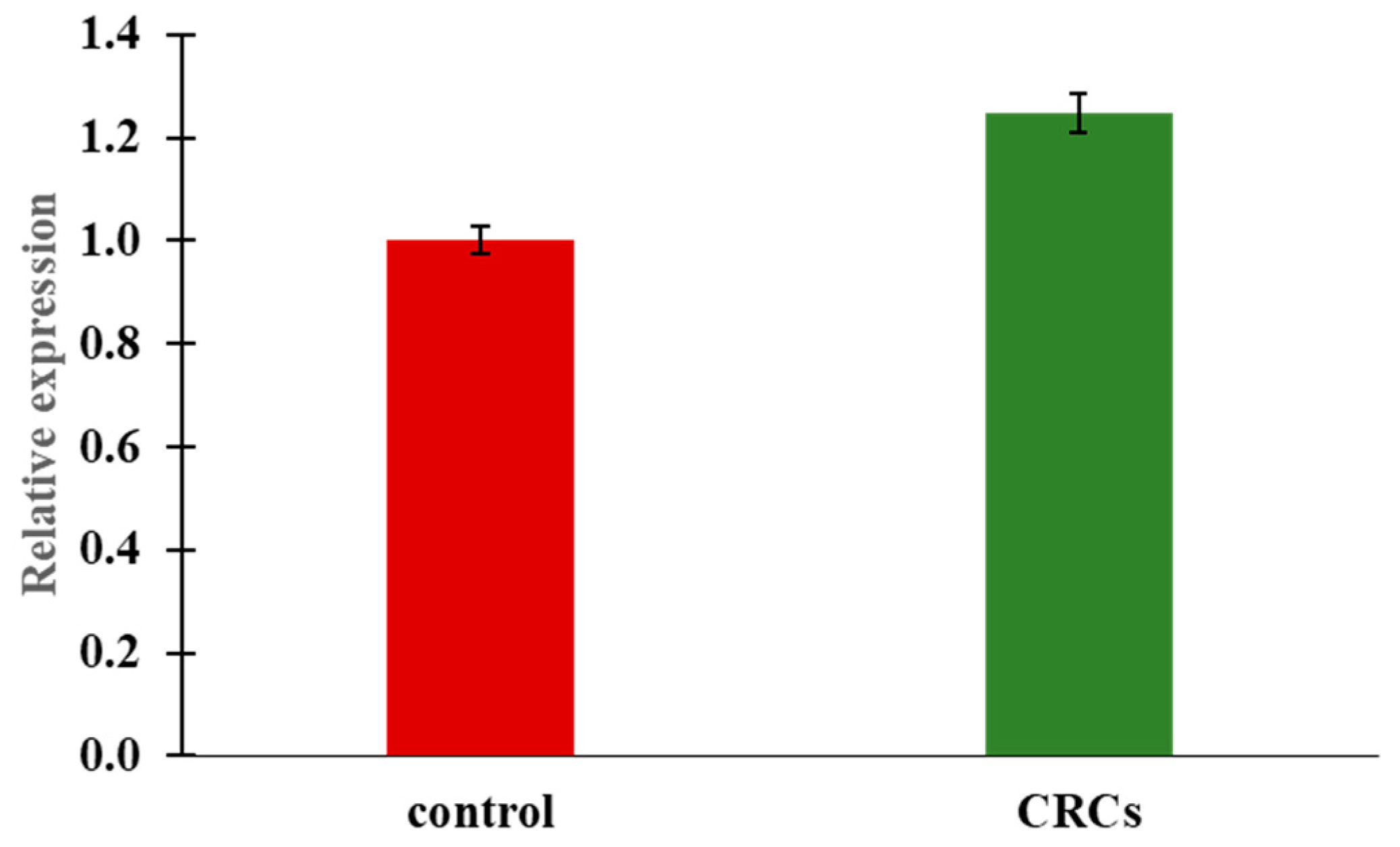
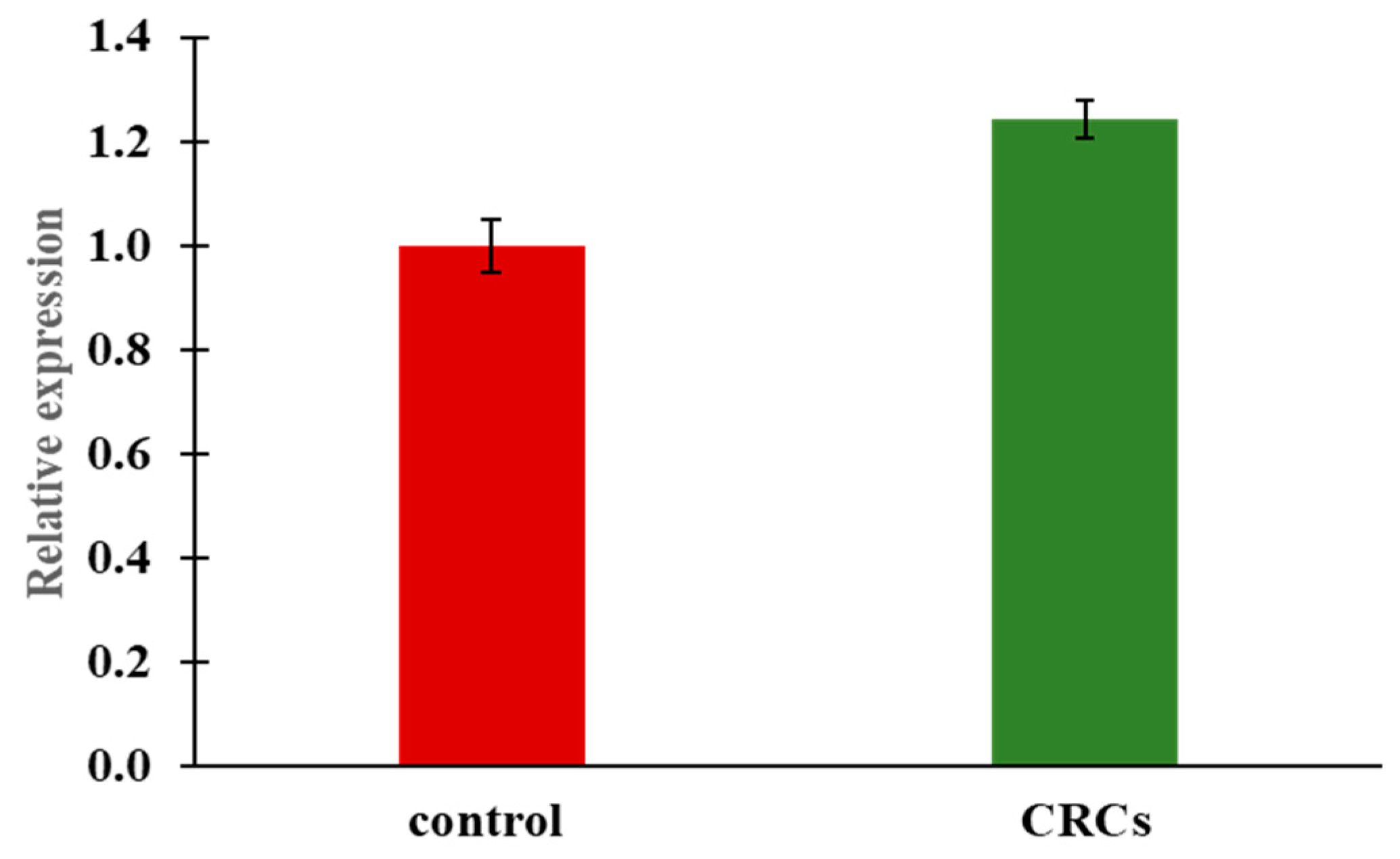
2.5. Insulin Expression by Quantitative Real-Time PCR
2.6. Pck1 Expression Induced as a Response to Lower Glucose Levels
3. Discussion
4. Materials and Methods
4.1. Extraction and Chemical Analysis of Crocins
4.2. Determination of LC50
4.3. Zebrafish Maintenance and Breeding
4.4. Measurement of Glucose Levels
4.5. RNA Isolation and cDNA Synthesis
4.6. Quantitative Real-Time PCR
4.7. Monitoring Pancreas Development
Author Contributions
Funding
Conflicts of Interest
References
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J. Tradit. Complement. Med. 2017, 8, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Kakouri, E.; Sarikurkcu, T.R.; Tarantilis, P.A. Study on the chemical composition, enzyme inhibition and antioxidant activity of Ziziphora taurica subsp. cleonioides. Appl. Sci. 2019, 9, 5515. [Google Scholar] [CrossRef]
- Prince, P.S.M.; Kannan, N.K. Protective effect of rutin on lipids, lipoproteins, lipid metabolizing enzymes and glycoproteins in streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2006, 58, 1373–1383. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Haddad, P.S. The Antidiabetic Potential of Quercetin: Underlying Mechanisms. Curr. Med. Chem. 2017, 24, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Ortega, H.; Pereda-Miranda, R.; Riveron-Negrete, L.; Hernandez, J.M.; Medécigo-Ríos, M.; Castillo-Villanueva, A.; Abdullaev, F.I. Chemical composition of saffron (Crocus sativus L.) from four countries. Acta Hortic. 2004, 650, 321–326. [Google Scholar] [CrossRef]
- Carmona, M.; Zalacain, A.; Sanchez, A.M.; Novella, J.L.; Alonso, G.L. Crocetin esters, picrocrocin and its related compounds present in Crocus sativus stigmas and Gardenia jasminoides fruits. Tentative identification of seven new compounds by LC-ESI-MS. J. Agric. Food Chem. 2006, 54, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Recio, M.C.; Giner, R.M.; Máñez, S. An Update Review of Saffron and its Active Constituents. Phytother. Res. 1996, 10, 189–193. [Google Scholar] [CrossRef]
- Tarantilis, P.A.; Tsoupras, G.; Polissiou, M. Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV-visible photodiode-array detection-mass spectrometry. J. Chromatogr. A 1995, 699, 107–118. [Google Scholar] [CrossRef]
- Mohajeri, S.A.; Hosseinzadeh, H.; Keyhanfar, F.; Aghamohammadian, J. Extraction of crocin from saffron (Crocus sativus) using molecularly imprinted polymer solid-phase extraction. J. Sep. Sci. 2010, 33, 2302–2309. [Google Scholar] [CrossRef] [PubMed]
- Karkoula, E.; Angelis Koulakiotis, N.S.; Gikas, E.; Halabalaki, M.; Tsarbopoulos, A.; Skaltsounis, A.L. Rapid isolation and characterization of crocins, picrocrocin, and crocetin from saffron using centrifugal partition chromatography and LC-MS. J. Sep. Sci. 2018, 41, 4105–4114. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, C.D.; Daferera, D.J.; Tarantilis, P.A.; Polissiou, M.G. Qualitative determination of volatile compounds and quantitative evaluation of safranal and 4-hydroxy-2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde (HTCC) in Greek saffron. J. Agric. Food Chem. 2004, 52, 4515–4521. [Google Scholar] [CrossRef] [PubMed]
- Letrado, P.; de Miguel, I.; Lamberto, I.; Díez-Martínez, R.; Oyarzabal, J. Zebrafish: Speeding Up the Cancer Drug Discovery Process. Cancer Res. 2018, 78, 6048–6058. [Google Scholar] [CrossRef]
- Giardoglou, P.; Beis, D. On Zebrafish Disease Models and Matters of the Heart. Biomedicines 2019, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Bandmann, O.; Burton, A.E. Genetic zebrafish models of neurodegenerative diseases. Neurobiol. Dis. 2010, 40, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a Model for Obesity and Diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar] [CrossRef]
- Gut, P.; Baeza-Raja, B.; Andersson, O.; Hasenkamp, L.; Hsiao, J.; Hesselson, D.; Akassoglou, K.; Verdin, E.; Hirschey, M.D.; Stainier, Y.R.D. Whole-organism screening for gluconeogenesis identifies activators of fasting metabolism. Nat. Chem. Biol. 2012, 9, 97–104. [Google Scholar] [CrossRef]
- Wiley, D.S.; Redfield, S.E.; Zon, L.I. Chemical screening in zebrafish for novel biological and therapeutic discovery. Methods Cell Biol. 2017, 138, 651–679. [Google Scholar] [CrossRef]
- Papakyriakou, A.; Kefalos, P.; Sarantis, P.; Tsiamantas, C.; Xanthopoulos, K.P.; Vourloumis, D.; Beis, D. A zebrafish in vivo phenotypic assay to identify 3-aminothiophene-2-carboxylic acid-based angiogenesis inhibitors. Assay Drug Dev. Technol. 2014, 12, 527–535. [Google Scholar] [CrossRef]
- Agalou, A.; Thrapsianiotis, M.; Angelis, A.; Papakyriakou, A.; Skaltsounis, A.L.; Aligiannis, N.; Beis, D. Identification of Novel Melanin Synthesis Inhibitors from Crataegus pycnoloba Using an in Vivo Zebrafish Phenotypic Assay. Front. Pharmacol. 2018, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Jurczyk, A.; Roy, N.; Bajwa, R.; Gut, P.; Lipson, K.; Yang, C.; Covassin, L.; Racki, W.J.; Rossini, A.A.; Phillips, N.; et al. Dynamic glucoregulation and mammalian-like responses to metabolic and developmental disruption in zebrafish. Gen. Comp. Endocrinol. 2011, 170, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Curado, S.; Anderson, R.M.; Jungblut, B.; Mumm, J.; Schroeter, E.; Stainier, D.Y. Conditional targeted cell ablation in zebrafish: A new tool for regeneration studies. Dev. Dyn. 2007, 236, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Bosch, J.A.; Goll, M.G.; Hesselson, D.; Dong, P.D.; Shin, D.; Chi, N.C.; Shin, C.H.; Schlegel, A.; Halpern, M.; et al. Loss of Dnmt1 catalytic activity reveals multiple roles for DNA methylation during pancreas development and regeneration. Dev. Biol. 2009, 334, 213–223. [Google Scholar] [CrossRef]
- Elo, B.; Villano, C.M.; Govorko, D.; White, L.A. Larval zebrafish as a model for glucose metabolism: Expression of phosphoenolpyruvate carboxykinase as a marker for exposure to anti-diabetic compounds. J. Mol. Endocrinol. 2007, 38, 433–440. [Google Scholar] [CrossRef]
- Seth, A.; Stemple, D.L.; Barroso, I. The emerging use of zebrafish to model metabolic disease. Dis. Model Mech. 2013, 6, 1080–1088. [Google Scholar] [CrossRef]
- OECD (2013). Test No. 236: Fish Embryo Acute Toxicity (FET) Test, OECD Guidelines for the Testing of Chemicals, Section 2. Home Page. Available online: https://www.oecd-ilibrary.org/environment/test-no-236-fish-embryo-acute-toxicity-fet-test_9789264203709-en (accessed on 14 June 2020).
- Papasani, M.R.; Robison, B.D.; Hardy, R.W.; Hill, R.A. Early developmental expression of two insulins in zebrafish (Danio rerio). Physiol. Genom. 2006, 27, 79–85. [Google Scholar] [CrossRef]
- Irwin, D.M. Duplication and diversification of insulin genes in ray-finned fish. Zool. Res. 2019, 40, 185–197. [Google Scholar] [CrossRef]
- Koren, D.; Palladino, A. Hypoglycemia, in Genetic Diagnosis of Endocrine Disorders, 2nd ed.; Roy, E., Samuel Refetoff, W., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 31–75. [Google Scholar]
- Hatziagapiou, K.; Kakouri, E.; Lambrou, G.I.; Bethanis, K.; Tarantilis, P.A. Antioxidant Properties of Crocus sativus L. and Its Constituents and Relevance to Neurodegenerative Diseases; Focus on Alzheimer’s and Parkinson’s Disease. Curr. Neuropharmacol. 2019, 17, 377–402. [Google Scholar] [CrossRef]
- Azimi, P.; Ghiasvand, R.; Feizi, A.; Hosseinzadeh, J.; Bahreynian, M.; Hariri, M.; Khosravi-Boroujeni, H. Effect of cinnamon, cardamom, saffron and ginger consumption on blood pressure and a marker of endothelial function in patients with type 2 diabetes mellitus: A randomized controlled clinical trial. Blood Press 2016, 25, 133–140. [Google Scholar] [CrossRef]
- Pitsikas, N.; Tarantilis, P.A. Effects of the active constituents of Crocus sativus L. crocins and their combination with memantine on recognition memory in rats. Behav. Pharmacol. 2018, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Ayatollahi, H.; Javan, A.O.; Khajedaluee, M.; Shahroodian, M.; Hosseinzadeh, H. Effect of Crocus sativus L. (saffron) on coagulation and anticoagulation systems in healthy volunteers. Phytother. Res. 2014, 28, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, D.; Mousavi, G.; Mesgari, M.; Doustar, Y.; Nouri, M.H.K. Subacute Toxicity of Crocus sativus L. (Saffron) Stigma Ethanolic Extract in Rats. Am. J. Pharmacol. Toxicol. 2007, 2, 189–193. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Sadeghi Shakib, S.; Khadem Sameni, A.; Taghiabadi, E. Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iran. J. Pharm. Res. IJPR 2013, 12, 93–99. [Google Scholar] [CrossRef]
- Mehri, S.; Razavi, B.M.; Hosseinzadeh, H. Safety and Toxicity of Saffron, in Saffron; Woodhead Publishing: Cambridge, UK, 2020; pp. 517–530. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency, Chemical Hazard Classification and Labeling: Comparison of OPP Requirements and the GHS: Draft. 2004. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/ghscriteria-summary.pdf (accessed on 30 October 2020).
- Kianbakht, S.; Hajiaghaee, R. Anti-hyperglycemic Effects of Saffron and its Active Constituents, Crocin and Safranal, in Alloxan-Induced Diabetic Rats. J. Med. Plants. 2011, 3, 82–89. [Google Scholar]
- Kang, C.; Lee, H.; Jung, E.S.; Seyedian, R.; Jo, M.; Kim, J.; Kim, J.S.; Kim, E. Saffron (Crocus sativus L.) increases glucose uptake and insulin sensitivity in muscle cells via multipathway mechanisms. Food Chem. 2012, 135, 2350–2358. [Google Scholar] [CrossRef]
- Dehghan, F.; Hajiaghaalipour, F.; Yusof, A.; Muniandy, S.; Hosseini, S.A.; Heydari, S.; Salim, L.Z.; Azarbayjani, M.A. Saffron with resistance exercise improves diabetic parameters through the GLUT4/AMPK pathway in-vitro and in-vivo. Sci. Rep. 2016, 6, 25139. [Google Scholar] [CrossRef]
- Milajerdi, A.; Jazayeri, S.; Hashemzadeh, N.; Shirzadi, E.; Derakhshan, Z.; Djazayeri, A.; Akhondzadeh, S. The effect of saffron (Crocus sativus L.) hydroalcoholic extract on metabolic control in type 2 diabetes mellitus: A triple-blinded randomized clinical trial. J. Res. Med. Sci. 2018, 20, 23. [Google Scholar] [CrossRef]
- Karimi-Nazari, E.; Nadjarzadeh, A.; Masoumi, R.; Marzban, A.; Mohajeri, S.A.; Ramezani-Jolfaie, N.; Salehi-Abargouei, A. Effect of saffron (Crocus sativus L.) on lipid profile, glycemic indices and antioxidant status among overweight/obese prediabetic individuals: A double-blinded, randomized controlled trial. Clin. Nutr. ESPEN 2019, 34, 130–136. [Google Scholar] [CrossRef]
- Moravej Aleali, A.; Amani, R.; Shahbazian, H.; Namjooyan, F.; Latifi, S.M.; Cheraghian, B. The effect of hydroalcoholic Saffron (Crocus sativus L.) extract on fasting plasma glucose, HbA1c, lipid profile, liver, and renal function tests in patients with type 2 diabetes mellitus: A randomized double-blind clinical trial. Phytother. Res. 2019, 33, 1648–1657. [Google Scholar] [CrossRef]
- Rajaei, Z.; Hadjzadeh, M.A.; Nemati, H.; Hosseini, M.; Ahmadi, M.; Shafiee, S. Antihyperglycemic and antioxidant activity of crocin in streptozotocin-induced diabetic rats. J. Med. Food 2013, 16, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Mohammadi, M.T.; Sahebkar, A. Crocin potentiates antioxidant defense system and improves oxidative damage in liver tissue in diabetic rats. Biomed. Pharmacother. 2018, 98, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Shirali, S.; Zahra Bathaie, S.; Nakhjavani, M. Effect of crocin on the insulin resistance and lipid profile of streptozotocin-induced diabetic rats. Phytother. Res. 2013, 27, 1042–1047. [Google Scholar] [CrossRef]
- Razavi, B.M.; Hosseinzadeh, H. Saffron: A promising natural medicine in the treatment of metabolic syndrome. J. Sci. Food Agric. 2017, 97, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Samarghandian, S. The effect of saffron (Crocus sativus L.) and its ingredients on the management of diabetes mellitus and dislipidemia. Afr. J. Pharm. Pharmacol. 2014, 8, 541–549. [Google Scholar] [CrossRef][Green Version]
- Nemati, Z.; Harpke, D.; Gemicioglu, A.; Kerndorff, H.; Blattner, F.R. Saffron (Crocus sativus) is an autotriploid that evolved in Attica (Greece) from wild Crocus cartwrightianus. Mol. Phylogenet. Evol. 2019, 136, 14–20. [Google Scholar] [CrossRef]
- Aleström, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and husbandry recommendations. Lab. Anim. 2020, 54, 213–224. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| tR | Compound Name * | Chemical Formula | m/z Theoretical | m/z Observed [M − H]− | % of CRCs | Δm |
|---|---|---|---|---|---|---|
| 10.658 | Crocin 5 (trans 5GGG) | C50H74O29 | 1137.4230 | 1137.4218 | 0.73 | −1.05 |
| 13.677 | Crocin 5 (cis 5GGG) | C50H74O29 | 1137.4230 | 1137.4220 | 1.34 | −0.88 |
| 14.971 | Crocin 4 (trans 4GG) | C44H64O24 | 975.37148 | 975.3707 | 46.84 | −0.80 |
| 15.047 | Crocin 2 (trans 2G) | C32H44O14 | 651.26583 | 651.2639 | 29.30 | −2.96 |
| 15.402 | Crocin 4 (cis 4GG) | C44H64O24 | 975.37148 | 975.3699 | 14.96 | −1.62 |
| 15.783 | Crocin 3 (trans 3Gg) | C38H54O19 | 813.31865 | 813.317 | 18.29 | −2.02 |
| 16.062 | Crocin 3 (cis 3Gg) | C38H54O19 | 813.31865 | 813.3172 | 78.09 | −1.78 |
| 16.645 | Crocin 1 (trans 1g) | C26H34O9 | 489.21301 | 489.2117 | 4.84 | −2.68 |
| 18.142 | Crocin 2 (cis 2G) | C32H44O14 | 651.26583 | 651.2639 | 5.61 | −2.96 |
Sample Availability: Samples of the CRCs is available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakouri, E.; Agalou, A.; Kanakis, C.; Beis, D.; Tarantilis, P.A. Crocins from Crocus sativus L. in the Management of Hyperglycemia. In Vivo Evidence from Zebrafish. Molecules 2020, 25, 5223. https://doi.org/10.3390/molecules25225223
Kakouri E, Agalou A, Kanakis C, Beis D, Tarantilis PA. Crocins from Crocus sativus L. in the Management of Hyperglycemia. In Vivo Evidence from Zebrafish. Molecules. 2020; 25(22):5223. https://doi.org/10.3390/molecules25225223
Chicago/Turabian StyleKakouri, Eleni, Adamantia Agalou, Charalabos Kanakis, Dimitris Beis, and Petros A. Tarantilis. 2020. "Crocins from Crocus sativus L. in the Management of Hyperglycemia. In Vivo Evidence from Zebrafish" Molecules 25, no. 22: 5223. https://doi.org/10.3390/molecules25225223
APA StyleKakouri, E., Agalou, A., Kanakis, C., Beis, D., & Tarantilis, P. A. (2020). Crocins from Crocus sativus L. in the Management of Hyperglycemia. In Vivo Evidence from Zebrafish. Molecules, 25(22), 5223. https://doi.org/10.3390/molecules25225223