Cyanobacteria and Microalgae as Sources of Functional Foods to Improve Human General and Oral Health
Abstract
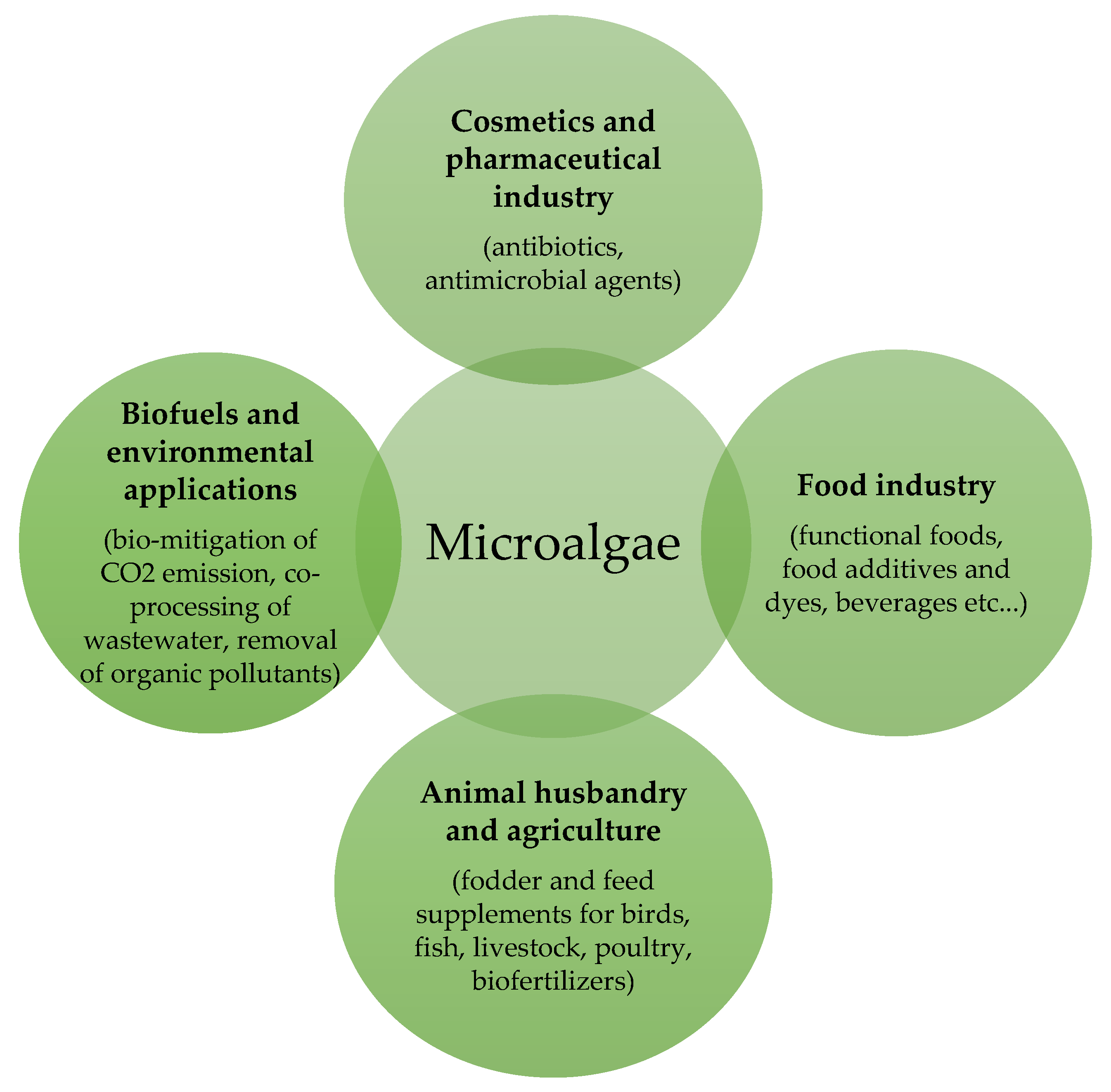
1. Introduction
2. Health Beneficial Effects of Cyanobacteria and Microalgae Crude Extracts and Fractions
2.1. A Taxonomic Controversy: Arthrospira vs. Spirulina
2.2. Chlorella sp.: A Multifunctional Dietary Supplement with Diverse Medical Properties
3. Microalgal Effects on Oral Health
3.1. Antiviral Activity
3.2. Oral Cancer Chemoprevention
3.3. Oral Antimicrobial Activity
3.4. Potential Effects in the Treatment of Periodontitis
3.5. Control of Oral Submucous Fibrosis
3.6. Salivary Secretion Improving
3.7. More Oral Benefits from Chlorella sp. and Other Algal Extracts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mundt, S.; Kreitlow, S.; Nowotny, A.; Effmert, U. Biochemical and pharmacological investigations of selected cyanobacteria. Int. J. Hyg. Environ. Health 2001, 203, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Safonova, E.; Kvitko, K.; Kuschk, P.; Möder, M.; Reisser, W. Biodegradation of Phenanthrene by the Green Alga Scenedesmus obliquus ES-55. Eng. Life Sci. 2005, 5, 234–239. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Mohagheghzadeh, A.; Mohammad, H.M.; Moradian, A.; Shadman, S. Antifungal and Antibacterial Activity of the Microalgae Collected from Paddy Fields of Iran: Characterization of Antimicrobial Activity of Chroococcus disperses. J. Biol. Sci. 2007, 7, 904–910. [Google Scholar]
- Prakash, O.; Hussain, K.; Aneja, K.R.; Sharma, C. Synthesis and Antimicrobial Activity of Some New 2-(3-(4-Aryl)-1-phenyl-1H-pyrazol-4-yl) Chroman-4-ones. Indian J. Pharm. Sci. 2011, 73, 586–590. [Google Scholar] [CrossRef] [PubMed]
- De Natale, A.; Pollio, A. Plants species in the folk medicine of Montecorvino Rovella (inland Campania, Italy). J. Ethnopharmacol. 2007, 109, 295–303. [Google Scholar] [CrossRef]
- Perrin, S.; Fougnies, C.; Grill, J.P.; Jacobs, H.; Schneider, F. Fermentation of chicory fructo-oligosaccharides in mixtures of different degrees of polymerization by three strains of bifidobacteria. Can. J. Microbiol. 2002, 48, 759–763. [Google Scholar] [CrossRef]
- Lee, M.H.; Kwon, H.A.; Kwon, D.Y.; Park, H.; Sohn, D.H.; Kim, Y.C.; Eo, S.K.; Kang, H.Y.; Kim, S.W.; Lee, J.H. Antibacterial activity of medicinal herb extracts against Salmonella. Int. J. Food Microbiol. 2006, 111, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, G. Pharmacological considerations on herbal medicine use. Ann. Ist. Super. Sanita 2005, 41, 23–26. [Google Scholar]
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Cantile, T.; Roberto, L.; Ingenito, A.; Catania, M.R.; Roscetto, E.; Palumbo, G.; Zarrelli, A.; Pollio, A. Determination of the in vitro and in vivo antimicrobial activity on salivary Streptococci and Lactobacilli and chemical characterisation of the phenolic content of a Plantago lanceolata infusion. Biomed. Res. Int. 2015, 2015, 286817. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Roberto, L.; Catania, M.R.; Chiaviello, A.; De Natale, A.; Roscetto, E.; Pinto, G.; Pollio, A.; Ingenito, A.; Palumbo, G. Screening and Scoring of Antimicrobial and Biological Activities of Italian Vulnerary Plants against Major Oral Pathogenic Bacteria. Evid. Based Complement. Altern. Med. 2013, 316280. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules 2011, 16, 1486–1507. [Google Scholar] [CrossRef]
- Galasso, C.; Gentile, A.; Orefice, I.; Ianora, A.; Bruno, A.; Noonan, D.M.; Sansone, C.; Albini, A.; Brunet, C. Microalgal Derivatives as Potential Nutraceutical and Food Supplements for Human Health: A Focus on Cancer Prevention and Interception. Nutrients 2019, 11, 1226. [Google Scholar] [CrossRef]
- Apone, F.; Barbulova, A.; Colucci, M.G. Plant and Microalgae Derived Peptides Are Advantageously Employed as Bioactive Compounds in Cosmetics. Front. Plant Sci. 2019, 10, 756. [Google Scholar] [CrossRef] [PubMed]
- Camacho, F.; Macedo, A.; Malcata, F. Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From Plants to Food and Feed Industries. Methods Mol. Biol. 2018, 1852, 57–71. [Google Scholar]
- Papapanagiotou, G.; Gkelis, S. Taxonomic revision of commercially used Arthrospira (Cyanobacteria) strains: A polyphasic approach. Eur. J. Phycol. 2019, 54, 595–608. [Google Scholar] [CrossRef]
- Blinkova, L.P.; Gorobets, O.B.; Baturo, A.P. Biological activity of Spirulina. Zh Mikrobiol. Epidemiol. Immunobiol. 2001, 2, 114–118. [Google Scholar]
- Deng, R.; Chow, T.J. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc. Ther. 2010, 28, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, Immunomodulating, and Microbial-Modulating Activities of the Sustainable and Ecofriendly Spirulina. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Marangoni, A.; Foschi, C.; Micucci, M.; Nahui Palomino, R.A.; Gallina Toschi, T.; Vitali, B.; Camarda, L.; Mandrioli, M.; De Giorgio, M.; Aldini, R.; et al. In vitro activity of Spirulina platensis water extract against different Candida species isolated from vulvo-vaginal candidiasis cases. PLoS ONE 2017, 12, 11. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Gunes, S.; Tamburaci, S.; Dalay, M.C.; Deliloglu Gurhan, I. In vitro evaluation of Spirulina platensis extract incorporated skin cream with its wound healing and antioxidant activities. Pharm. Biol. 2017, 55, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Kiziltan, H.S.; Gunes Bayir, A.; Taspinar, O.; Yucesan, G.; Tastekin, D.; Sonmez, F.C.; Coban, G.; Kilic, G.; Eris, A.H.; Aydin, T.; et al. Radioprotectant and Cytotoxic Effects of Spirulina in Relapsed Verrucous Vulvar Cancer: A Case Report. Altern. Ther. Health Med. 2015, 21, 68–72. [Google Scholar]
- Zhuang, X.; Huang, Y.; Zhang, D.; Tao, L.; Li, Y. Research status and prospect on hot water extract of Chlorella: The high value-added bioactive substance from Chlorella. Sheng Wu Gong Cheng Xue Bao 2015, 31, 24–42. [Google Scholar]
- Halperin, S.A.; Smith, B.; Nolan, C.; Shay, J.; Kralovec, J. Safety and immunoenhancing effect of a Chlorella-derived dietary supplement in healthy adults undergoing influenza vaccination: Randomized, double-blind, placebo-controlled trial. CMAJ 2003, 169, 111–117. [Google Scholar]
- Panahi, Y.; Darvishi, B.; Jowzi, N.; Beiraghdar, F.; Sahebkar, A. Chlorella vulgaris: A Multifunctional Dietary Supplement with Diverse Medicinal Properties. Curr. Pharm. Des. 2016, 22, 164–173. [Google Scholar] [CrossRef]
- Panahi, Y.; Mostafazadeh, B.; Abrishami, A.; Saadat, A.; Beiraghdar, F.; Tavana, S.; Pishgoo, B.; Parvin, S.; Sahebkar, A. Investigation of the effects of Chlorella vulgaris supplementation on the modulation of oxidative stress in apparently healthy smokers. Clin. Lab. 2013, 59, 579–587. [Google Scholar] [CrossRef]
- Panahi, Y.; Badeli, R.; Karami, G.R.; Badeli, Z.; Sahebkar, A. A randomized controlled trial of 6-week Chlorella vulgaris supplementation in patients with major depressive disorder. Complement. Ther. Med. 2015, 23, 598–602. [Google Scholar] [CrossRef]
- Ebrahimi-Mameghani, M.; Sadeghi, Z.; Abbasalizad Farhangi, M.; Vaghef-Mehrabany, E.; Aliashrafi, S. Glucose homeostasis, insulin resistance and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: Beneficial effects of supplementation with microalgae Chlorella vulgaris: A double-blind placebo-controlled randomized clinical trial. Clin. Nutr. 2017, 36, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Entesar, A. Antimicrobial activity of microalgal extracts isolated from Baharia Oasis, Egypt. Glob. Adv. Res. J. Microbiol. 2016, 5, 033–041. [Google Scholar]
- Cannell, R.J.; Kellam, S.J.; Owsianka, A.M.; Walker, J.M. Results of a large scale screen of microalgae for the production of protease inhibitors. Planta Med. 1988, 54, 10–14. [Google Scholar] [CrossRef]
- Aremu, A.O.; Neményi, M.; Stirk, W.A.; Ördög, V.; van Staden, J. Manipulation of nitrogen levels and mode of cultivation are viable methods to improve the lipid, fatty acids, phytochemical content, and bioactivities in Chlorella minutissima. J. Phycol. 2015, 51, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Tsai, C.T.; Chuang, W.L.; Chao, Y.H.; Pan, I.H.; Chen, Y.K.; Lin, C.C.; Wang, B.Y. Chlorella sorokiniana induces mitochondrial-mediated apoptosis in human non-small cell lung cancer cells and inhibits xenograft tumor growth in vivo. BMC Complement. Altern. Med. 2017, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Suzich, J.B.; Cliffe, A.R. Strength in diversity: Understanding the pathways to herpes simplex virus reactivation. Virology 2018, 522, 81–91. [Google Scholar] [CrossRef]
- Mader, J.; Gallo, A.; Schommartz, T.; Handke, W.; Nagel, C.H.; Günther, P.; Brune, W.; Reich, K. Calcium spirulan derived from Spirulina platensis inhibits herpes simplex virus 1 attachment to human keratinocytes and protects against herpes labialis. J. Allergy Clin. Immunol. 2016, 137, 197–203. [Google Scholar] [CrossRef]
- Wong, T.; Wiesenfeld, D. Oral Cancer. Aust. Dent. J. 2018, 1, 91–99. [Google Scholar] [CrossRef]
- Shibahara, T. Oral cancer -diagnosis and therapy-. Clin. Calcium 2017, 27, 1427–1433. [Google Scholar]
- Mathew, B.; Sankaranarayanan, R.; Nair, P.P.; Varghese, C.; Somanathan, T.; Amma, B.P.; Amma, N.S.; Nair, M.K. Evaluation of chemoprevention of oral cancer with Spirulina fusiformis. Nutr. Cancer 1995, 24, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, M.; Matsuno, T.; Asano, K.; Mataga, I. Anti-inflammatory effects of astaxanthin in the human gingival keratinocyte line NDUSD-1. J. Clin. Biochem. Nutr. 2015, 56, 171–178. [Google Scholar] [CrossRef]
- Mori, H.; Tanaka, T.; Sugie, S.; Yoshimi, N.; Kawamori, T.; Hirose, Y.; Ohnishi, M. Chemoprevention by naturally occurring and synthetic agents in oral, liver, and large bowel carcinogenesis. J. Cell. Biochem. Suppl. 1997, 27, 35–41. [Google Scholar] [CrossRef]
- Tanaka, T.; Makita, H.; Ohnishi, M.; Mori, H.; Satoh, K.; Hara, A. Chemoprevention of rat oral carcinogenesis by naturally occurring xanthophylls, astaxanthin and canthaxanthin. Cancer Res. 1995, 55, 4059–4064. [Google Scholar]
- Grawish, M.E. Effects of Spirulina platensis extract on Syrian hamster cheek pouch mucosa painted with 7,12-dimethylbenz[a]anthracene. Oral Oncol. 2008, 44, 956–962. [Google Scholar] [CrossRef]
- Grawish, M.E.; Zaher, A.R.; Gaafar, A.I.; Nasif, W.A. Long-term effect of Spirulina platensis extract on DMBA-induced hamster buccal pouch carcinogenesis (immunohistochemical study). Med. Oncol. 2010, 27, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Shklar, G.; Reid, S.; Trickler, D. Prevention of experimental oral cancer by extracts of Spirulina-Dunaliella algae. Nutr. Cancer 1988, 11, 127–134. [Google Scholar] [CrossRef]
- Kowshik, J.; Baba, A.B.; Giri, H.; Deepak Reddy, G.; Dixit, M.; Nagini, S. Astaxanthin inhibits JAK/STAT-3 signaling to abrogate cell proliferation, invasion and angiogenesis in a hamster model of oral cancer. PLoS ONE 2014, 9, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Sannasimuthu, A.; Kumaresan, V.; Anilkumar, S.; Pasupuleti, M.; Ganesh, M.R.; Mala, K.; Paray, B.A.; Al-Sadoon, M.K.; Albeshr, M.F.; Arockiaraj, J. Design and characterization of a novel Arthrospira platensis glutathione oxido-reductase-derived antioxidant peptide GM15 and its potent anti-cancer activity via caspase-9 mediated apoptosis in oral cancer cells. Free Radic. Biol. Med. 2019, 135, 198–209. [Google Scholar] [CrossRef]
- Kavitha, K.; Kowshik, J.; Kishore, T.K.K.; Baba, A.B.; Nagini, S. Astaxanthin inhibits NF-κB and Wnt/β-catenin signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to induce intrinsic apoptosis in a hamster model of oral cancer. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 4433–4444. [Google Scholar] [CrossRef]
- Kowshik, J.; Nivetha, R.; Ranjani, S.; Venkatesan, P.; Selvamuthukumar, S.; Veeravarmal, V.; Nagini, S. Astaxanthin inhibits hallmarks of cancer by targeting the PI3K/NF-κΒ/STAT3 signalling axis in oral squamous cell carcinoma models. IUBMB Life 2019, 71, 1595–1610. [Google Scholar] [CrossRef]
- Azizi, A.; Aghayan, S.; Zaker, S.; Shakeri, M.; Entezari, N.; Lawaf, S. In Vitro Effect of Zingiber officinale Extract on Growth of Streptococcus mutans and Streptococcus sanguinis. Int. J. Dent. 2015, 2015, 489842. [Google Scholar] [CrossRef]
- Zhou, Y.; Millhouse, E.; Shaw, T.; Lappin, D.F.; Rajendran, R.; Bagg, J.; Lin, H.; Ramage, G. Evaluating Streptococcus mutans Strain Dependent Characteristics in a Polymicrobial Biofilm Community. Front. Microbiol. 2018, 9, 1498. [Google Scholar] [CrossRef]
- Rakic, M.; Galindo-Moreno, P.; Monje, A.; Radovanovic, S.; Wang, H.L.; Cochran, D.; Sculean, A.; Canullo, L. How frequent does peri-implantitis occur? A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 1805–1816. [Google Scholar] [CrossRef]
- Komiyama, E.Y.; Lepesqueur, L.S.; Yassuda, C.G.; Samaranayake, L.P.; Parahitiyawa, N.B.; Balducci, I.; Koga-Ito, C.Y. Enterococcus Species in the Oral Cavity: Prevalence, Virulence Factors and Antimicrobial Susceptibility. PLoS ONE 2016, 11, e0163001. [Google Scholar] [CrossRef]
- Davoodbasha, M.; Edachery, B.; Nooruddin, T.; Lee, S.Y.; Kim, J.W. An evidence of C16 fatty acid methyl esters extracted from microalga for effective antimicrobial and antioxidant property. Microb. Pathog. 2018, 115, 233–238. [Google Scholar] [CrossRef]
- Uma, R.; Sivasubramanian, V.; Niranjali Devaraj, S. Preliminary phycochemical analysis and in vitro antibacterial screening of green microalgae, Desmococcus Olivaceous, Chlorococcum humicola and Chlorella vulgaris. J. Algal Biomass Utln. 2011, 2, 74–81. [Google Scholar]
- Madhumathi, V.; Deepa, P.; Jeyachandran, S.; Manoharan, C.; Vijayakumar, S. Antimicrobial Activity of Cyanobacteria Isolated from Freshwater Lake. Int. J. Microbiol. Res. 2011, 2, 213–216. [Google Scholar]
- Zarska, M.; Novotny, F.; Havel, F.; Sramek, M.; Babelova, A.; Benada, O.; Novotny, M.; Saran, H.; Kuca, K.; Musilek, K.; et al. Two-Step Mechanism of Cellular Uptake of Cationic Gold Nanoparticles Modified by (16-Mercaptohexadecyl) trimethylammonium Bromide. Bioconjug. Chem. 2016, 27, 2558–2574. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef]
- Salaheldin, T.A.; Loutfy, S.A.; Ramadan, M.A.; Youssef, T.; Mousa, S.A. IR-enhanced photothermal therapeutic effect of graphene magnetite nanocomposite on human liver cancer HepG2 cell model. Int. J. Nanomed. 2019, 14, 4397–4412. [Google Scholar] [CrossRef] [PubMed]
- Rashad, S.; El-Chaghaby, G.A.; Elchaghaby, M.A. Antibacterial activity of silver nanoparticles biosynthesized using Spirulina platensis microalgae extract against oral pathogens. Egypt. J. Aquat. Biol. Fish. 2019, 23, 261–266. [Google Scholar] [CrossRef]
- Bosshardt, D.D. The periodontal pocket: Pathogenesis, histopathology and consequences. Periodontology 2000 2018, 76, 43–50. [Google Scholar] [CrossRef]
- Deas, D.E.; Moritz, A.J.; Sagun, R.S., Jr.; Gruwell, S.F.; Powell, C.A. Scaling and root planing vs. conservative surgery in the treatment of chronic periodontitis. Periodontology 2000 2016, 71, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, J.; Mahendra, L.; Muthu, J.; John, L.; Romanos, G.E. Clinical effects of subgingivally delivered spirulina gel in chronic periodontitis cases: A placebo controlled clinical trial. J. Clin. Diagn. Res. 2013, 7, 2330–2333. [Google Scholar] [CrossRef] [PubMed]
- Balci Yuce, H.; Lektemur Alpan, A.; Gevrek, F.; Toker, H. Investigation of the effect of astaxanthin on alveolar bone loss in experimental periodontitis. J. Periodontal Res. 2018, 53, 131–138. [Google Scholar] [CrossRef]
- Hwang, Y.; Kwang-Jin, K.; Su-Jin, K.; Seul-Ki, M.; Seong-Gyeol, H.; Young-Jin, S.; Sung-Tae, Y. Suppression Effect of Astaxanthin on Osteoclast Formation In Vitro and Bone Loss In Vivo. Int. J. Mol. Sci. 2018, 19, 912. [Google Scholar] [CrossRef]
- Kose, O.; Arabaci, T.; Yemenoglu, H.; Kara, A.; Ozkanlar, S.; Kayis, S.; Duymus, Z.Y. Influences of Fucoxanthin on Alveolar Bone Resorption in Induced Periodontitis in Rat Molars. Mar. Drugs 2016, 14, 70. [Google Scholar] [CrossRef]
- Molteni, M.; Bosi, A.; Rossetti, C. The Effect of Cyanobacterial LPS Antagonist (CyP) on Cytokines and Micro-RNA Expression Induced by Porphyromonas gingivalis LPS. Toxins 2018, 10, 290. [Google Scholar] [CrossRef]
- Orpha.Net. Available online: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=IT&Expert=357154 (accessed on 10 February 2019).
- Kanjani, V.; Annigeri, R.G.; Revanappa, M.M.; Rani, A. Efficacy of Spirulina along with Different Physiotherapeutic Modalities in the Management of Oral Submucous Fibrosis. Ann. Maxillofac. Surg. 2019, 9, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.; Shenai, P.; Chatra, L.; Rao, P.K. Efficacy of spirulina as an antioxidant adjuvant to corticosteroid injection in management of oral submucous fibrosis. Indian J. Dent. Res. 2013, 24, 347–350. [Google Scholar] [CrossRef]
- Mulk, B.S.; Deshpande, P.; Velpula, N.; Chappidi, V.; Chintamaneni, R.L.; Goyal, S. Spirulina and pentoxyfilline-a novel approach for treatment of oral submucous fibrosis. J. Clin. Diagn. Res. 2013, 7, 3048–3050. [Google Scholar]
- Kuraji, M.; Matsuno, T.; Satoh, T. Astaxanthin affects oxidative stress and hyposalivation in aging mice. J. Clin. Biochem. Nutr. 2016, 59, 79–85. [Google Scholar] [CrossRef]
- Yamada, T.; Ryo, K.; Tai, Y.; Tamaki, Y.; Inoue, H.; Mishima, K.; Tsubota, K.; Saito, I. Evaluation of Therapeutic Effects of Astaxanthin on Impairments in Salivary Secretion. J. Clin. Biochem. Nutr. 2010, 47, 130–137. [Google Scholar] [CrossRef][Green Version]
- Otsuki, T.; Shimizu, K.; Zempo-Miyaki, A.; Maeda, S. Changes in salivary flow rate following Chlorella-derived multicomponent supplementation. J. Clin. Biochem. Nutr. 2016, 59, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.J.; Parmigiani-Izquierdo, J.M.; Toledano Gasca, A.; Cabaña-Muñoz, M.E. The Long-Term Algae Extract (Chlorella and Fucus sp.) and Aminosulphurate Supplementation Modulate SOD-1 Activity and Decrease Heavy Metals (Hg++, Sn) Levels in Patients with Long-Term Dental Titanium Implants and Amalgam Fillings Restorations. Antioxidants 2019, 8, 101. [Google Scholar] [CrossRef]
- Cicco, S.R.; Vona, D.; Leone, G.; De Giglio, E.; Bonifacio, M.A.; Cometa, S.; Fiore, S.; Palumbo, F.; Ragni, R.; Farinola, G.M. In vivo functionalization of diatom biosilica with sodium alendronate as osteoactive material. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109897. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 36. [Google Scholar] [CrossRef]
| S. platensis’s Medicinal Properties | Ref. |
|---|---|
| Highly nutrient | [19] |
| Immunostimulating | [20] |
| Antiviral | [18] |
| Antimicotic | [18] |
| Anti-cancer | [18,24] |
| Antioxidant | [22] |
| Skin-beneficial | [23] |
| Chlorella sp. Medicinal Properties | Ref. |
|---|---|
| Anti-cancer | [25,27,34] |
| Antioxidant | [27,28,29] |
| Anti-inflammatory | [30] |
| Antibacterial, antimicotic | [31,32,33] |
| Species/Source | Type of Study | Results | Potential Application | Ref. |
|---|---|---|---|---|
| S. platensis extract containing Calcium spirulan (Ca-SP) | In vitro | Inhibition of HSV-1, Kaposi sarcoma-associated herpes virus, and human herpes virus 8. | Prophylactic treatment of herpes viruses infections. | [36] |
| S. fusiformis | In vivo | Complete regression of precancerous lesions in 45% of subjects. | Chemopreventive therapy for tobacco-induced oral leucoplakia. | [39] |
| Astaxanthin | In vitro | Decrease of IL-6 and TNF-α and increase of cell proliferation in oral lichen planus. | Anti-inflammatory treatment for oral lichen planus. | [40] |
| Astaxanthin | In vivo | Decrease of the incidence of oral pre-neoplastic lesions and cell proliferation activity in rats after 8 weeks of treatment. | Prevention of 4-NQO-induced tongue carcinogenesis. | [41,42] |
| S. platensis | In vivo | Decrease of dysplastic changes on hamsters’ buccal pouch after 14 weeks of treatment. | Oral cancer preventive therapy. | [43,44] |
| Spirulina-Dunaliella extract containing astaxanthin | In vivo | Inhibition of JAK-2/STAT-3 downstream events in hamster buccal pouch tumor progression. | Oral cancer therapy. | [45,46] |
| GM15 peptide from Spirulina Arthrospira platensis | In vitro | Scavenge of superoxide and hydroxyl radicals and reduction of intracellular oxidative stress. | Antioxidant treatment for oral cancer. | [47] |
| FAME extracted from S. intermedius | In vitro | Inhibition of S. aureus, S. mutans, B. cereus, E. coli, P. aeruginosa, A. parasiticus, and C. albicans. | Antimicrobial therapy against Gram-positive bacteria, Gram-negative bacteria, and fungi. | [54] |
| C. calcitrans, S. costatum, C. turgidus, and N. oceanica | In vitro | Inhibition of S. aureus, S. pyogenes, B. subtilis, S. costatum, and C. turgidus showed also antimycotic action. | Antibiotics and fungicides. | [55] |
| Acetone extracts of O. latevirens | In vitro | Inhibition of S. aureus, S. mutans, and C. albicans. | Antibiotic and fungicide. | [56] |
| Ethanol extracts of Phormidium corium | In vitro | Inhibition of M. mutans and S. aureus. | Antibiotic. | [56] |
| Extract of L. martensiana | In vitro | Inhibition of B. subtilis, S. aureus, E. coli. | Antibiotic. | [56] |
| Extract of C. minor and M. aeruginosa | In vitro | Inhibition of C. albicans. | Fungicides. | [56] |
| Silver nanoparticles biosynthesized from S. platensis extract | In vitro | Inhibition of S. mutans, E. faecalis, and S. aureus. | Antibiotic. | [60] |
| Locally derived S. platensis gel | In vivo | Beneficials in the treatment of chronic periodontitis. | Co-adjuvant in the non-surgical treatment of periodontitis. | [63] |
| Systemic astaxanthin administration | In vivo | Reduction of alveolar bone loss in ligature-induced periodontitis in rats. | Treatment of periodontitis. | [64] |
| Cyp, an Oscillatoria planktothrix FP1-derived lipopolysaccharide | In vitro | Inhibition of TNF-α, IL-1β, and IL-8 expression. | Treatment of periodontitis. | [67] |
| Systemic S. platensis administration | In vivo | Significant improvement in oral submucous fibrosis symptoms after 3 months of therapy. | Adjuvant therapy in the management of oral submucous fibrosis. | [69] |
| Systemic S. platensis administration in addition to corticosteroid injections | In vivo | Highly significant clinical improvements in oral submucous fibrosis after 3 months of therapy. | Adjuvant therapy in the management of oral submucous fibrosis. | [70] |
| Astaxanthin | In vivo | Increase of saliva flow after 72 weeks of treatment. | Hyposalivation treatment. | [72] |
| Astaxanthin | In vitro and in vivo | Increase of saliva flow and decrease of oxidative stress markers. | Hyposalivation treatment. | [73] |
| Chlorella-derived multicomponent supplementation | In vivo | Increase of saliva flow in subjects with lower levels of saliva secretion. | Hyposalivation treatment. | [74] |
| C. vulgaris extract in conjunction with amminosulphurate supplementation | In vivo | Reduction of Hg++, Ag, Sn, and Pb in subjects with long-term titanium dental implants and/or amalgam fillings. | Heavy metal detoxyfing agents. | [75] |
| Sodium alendronate incorporated into biosilica shells of cultured Thalassiosira weissflogii diatoms | In vitro | Decrease of metabolic activity of J774 osteoclast-like cells. | Drug-carrier for bifosphonates. | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrazzano, G.F.; Papa, C.; Pollio, A.; Ingenito, A.; Sangianantoni, G.; Cantile, T. Cyanobacteria and Microalgae as Sources of Functional Foods to Improve Human General and Oral Health. Molecules 2020, 25, 5164. https://doi.org/10.3390/molecules25215164
Ferrazzano GF, Papa C, Pollio A, Ingenito A, Sangianantoni G, Cantile T. Cyanobacteria and Microalgae as Sources of Functional Foods to Improve Human General and Oral Health. Molecules. 2020; 25(21):5164. https://doi.org/10.3390/molecules25215164
Chicago/Turabian StyleFerrazzano, Gianmaria Fabrizio, Cristina Papa, Antonino Pollio, Aniello Ingenito, Giancarla Sangianantoni, and Tiziana Cantile. 2020. "Cyanobacteria and Microalgae as Sources of Functional Foods to Improve Human General and Oral Health" Molecules 25, no. 21: 5164. https://doi.org/10.3390/molecules25215164
APA StyleFerrazzano, G. F., Papa, C., Pollio, A., Ingenito, A., Sangianantoni, G., & Cantile, T. (2020). Cyanobacteria and Microalgae as Sources of Functional Foods to Improve Human General and Oral Health. Molecules, 25(21), 5164. https://doi.org/10.3390/molecules25215164





