The Strong Anti-Kinetoplastid Properties of Bee Propolis: Composition and Identification of the Active Agents and Their Biochemical Targets
Abstract
1. Introduction
2. The Chemistry of Propolis
2.1. General Composition
2.1.1. Flavonoids
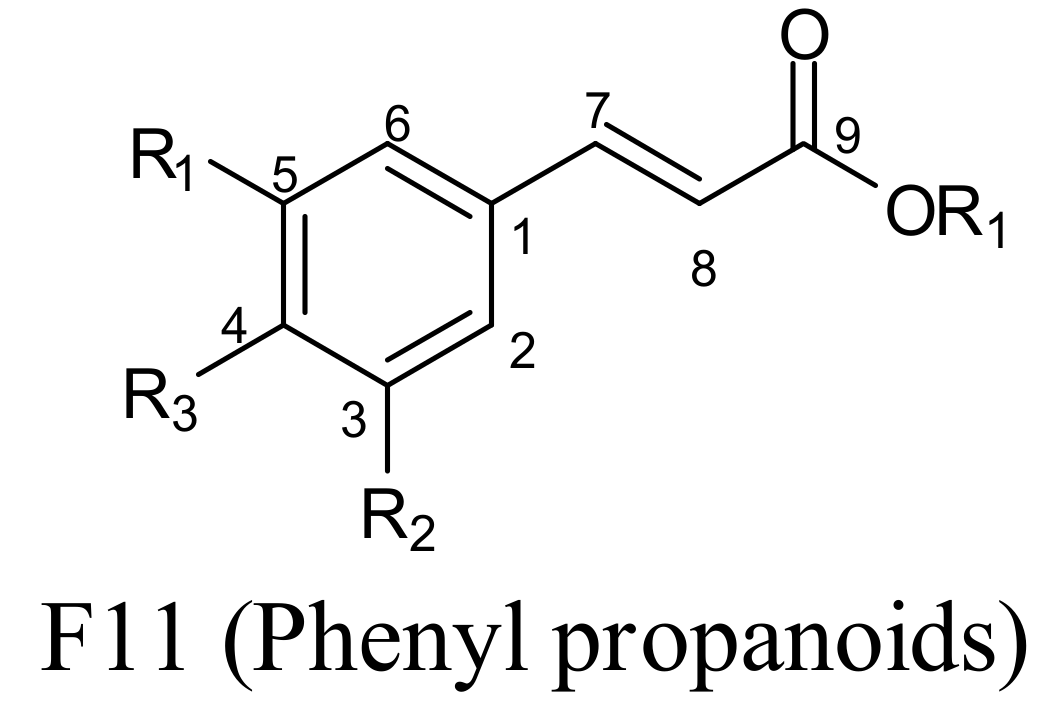
2.1.2. Phenyl Propanoids
2.1.3. Other Constituents
2.2. Composition Based on Geographical Origin
3. Evidence for Propolis Protection against Bee Infections
4. Propolis as an Anti-Kinetoplastid Agent
4.1. Antitrypanosomal Activity of Propolis
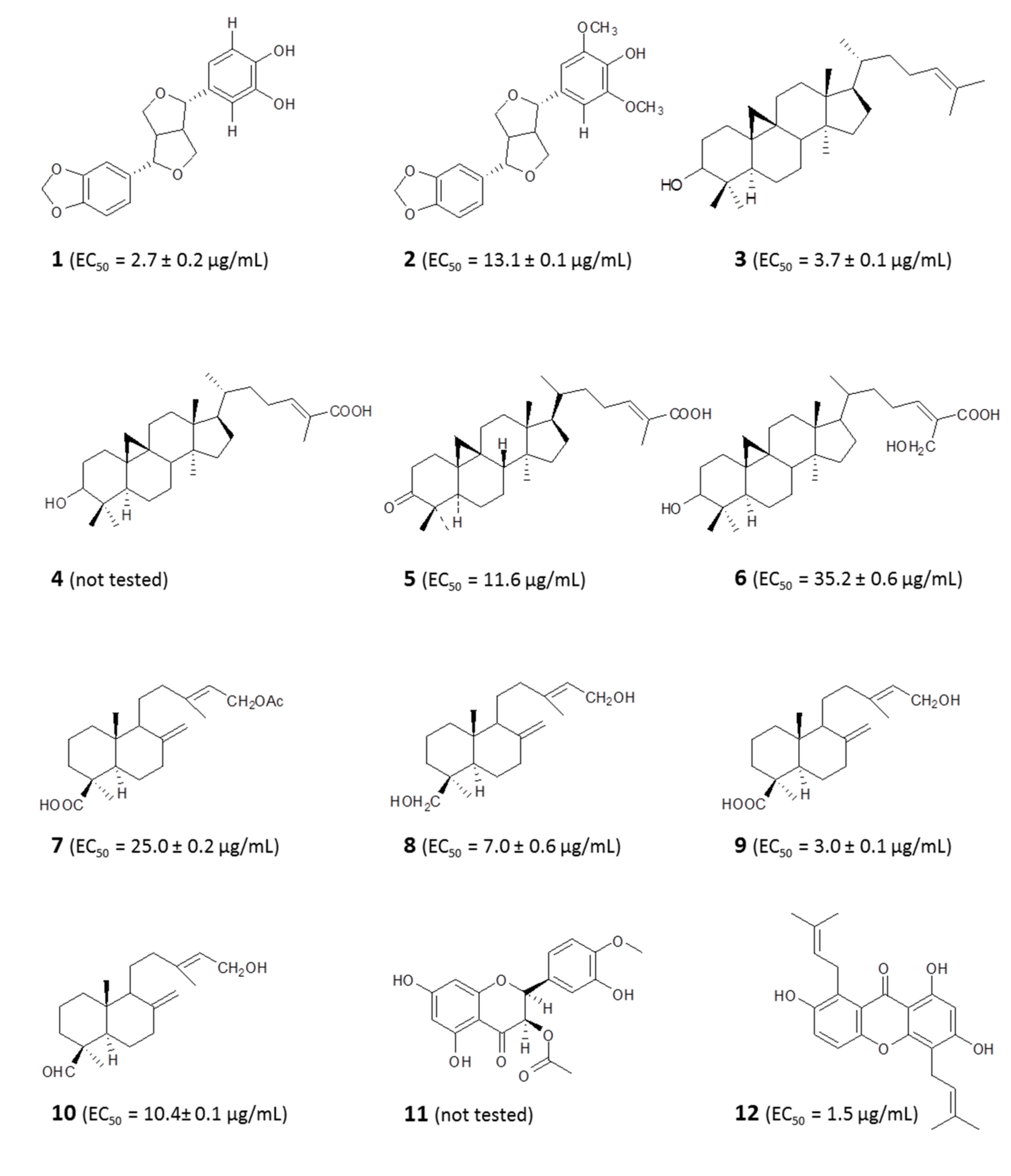
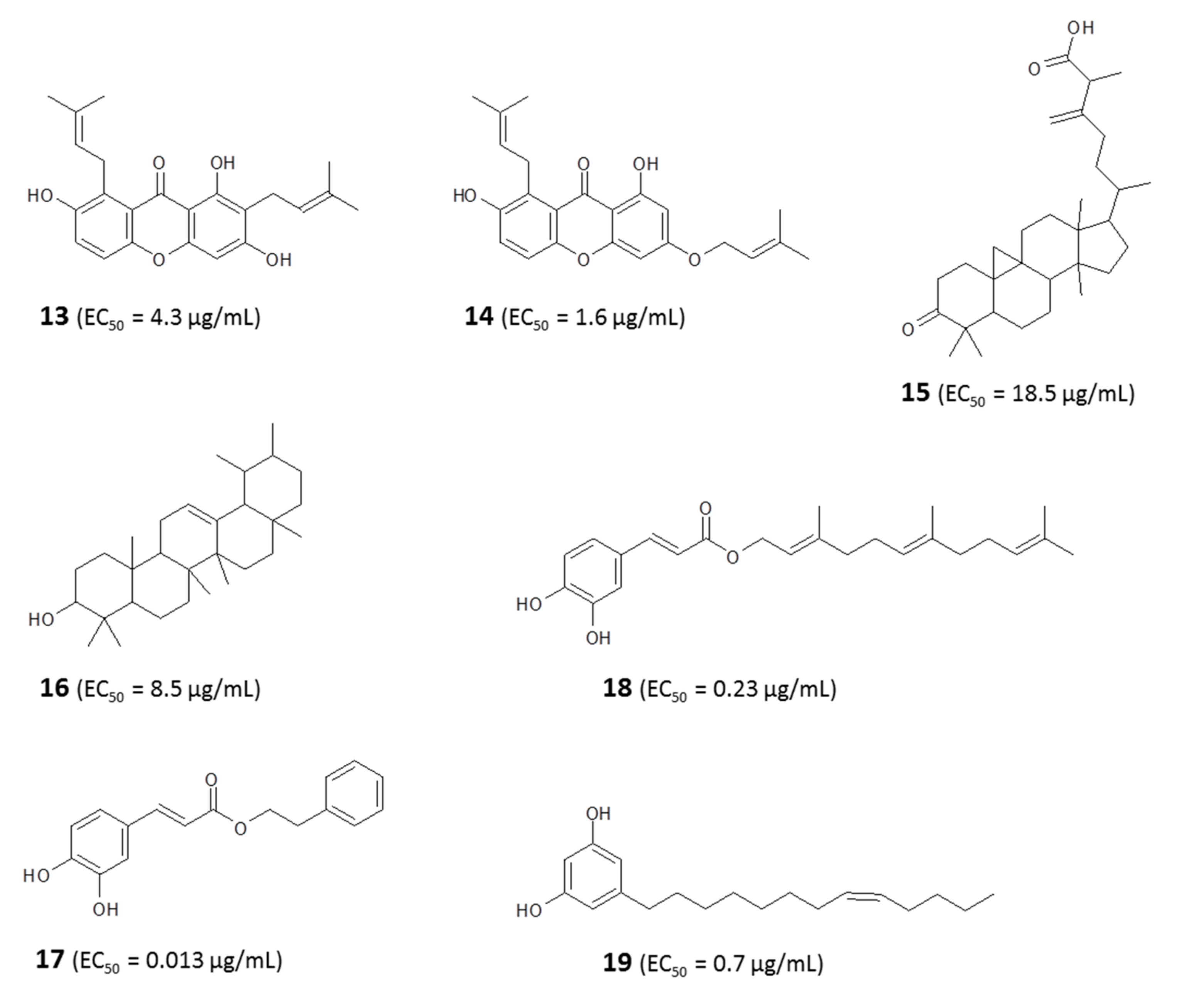
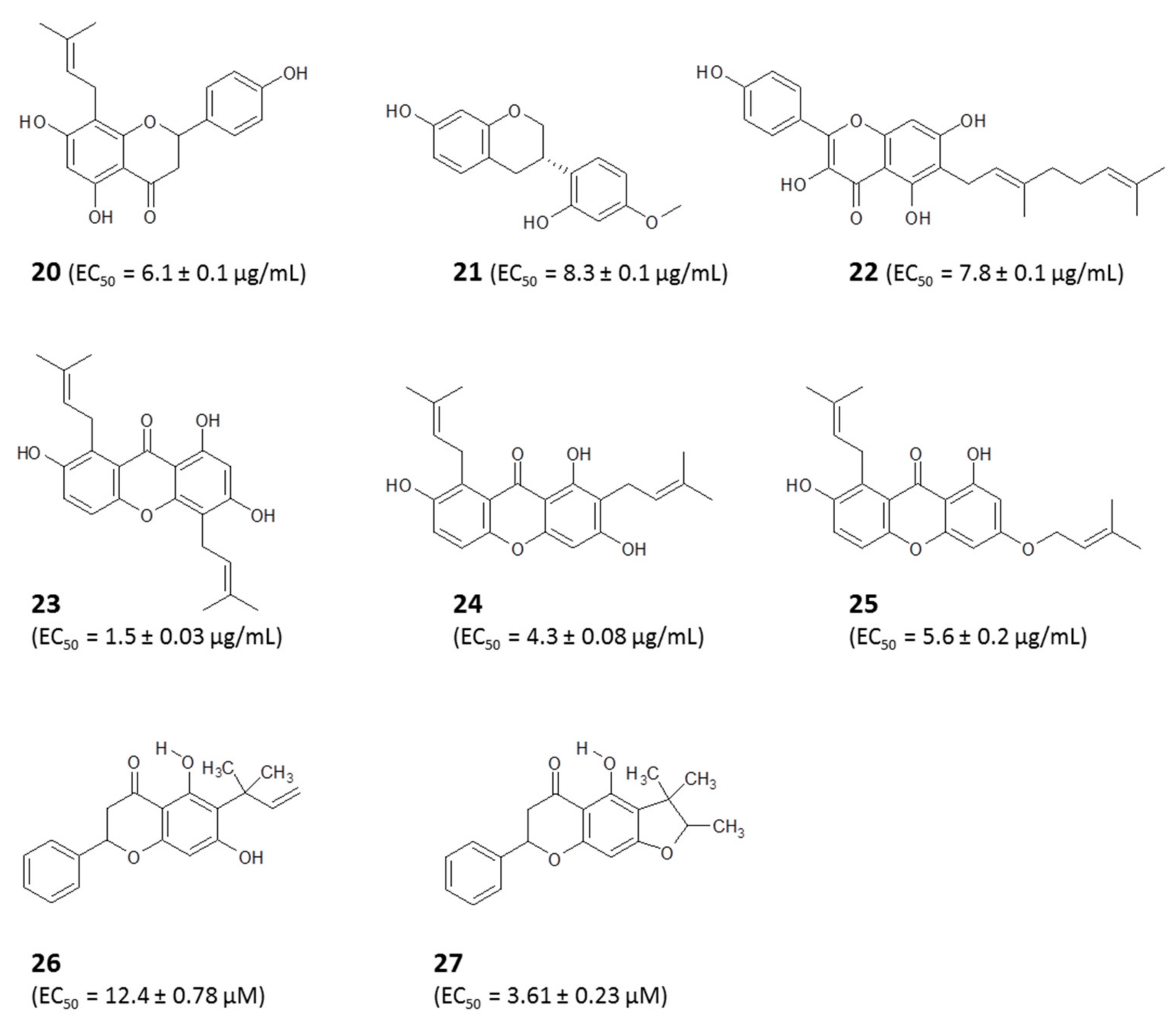
4.1.1. Identification of Bioactive Antitrypanosomal Compounds in Propolis Extracts
4.1.2. Metabolomic Profiling Revealed a Possible Mode of Action of Propolis In Vitro
4.1.3. Propolis Is Active against Drug-Sensitive and -Resistant Strains of T. brucei
4.1.4. Propolis Contains Antitrypanosomal Activities Regardless of Geographical Location
4.1.5. Propolis Is Active In Vitro and In Vivo Against Trypanosomes
4.1.6. Direct Antiparasitic Efficacy and Sites of Action of Propolis in T. cruzi
4.1.7. Indirect Antiparasite Efficacy of Propolis via Immune Modulation in T. cruzi Infection
4.2. Anti-Leishmania Effects of Propolis
4.2.1. Identification of Active Antileishmanial Compounds in Propolis Extracts
4.2.2. Effects of Propolis on Infected Macrophages
4.2.3. Propolis in Animal Models of Leishmaniasis
4.2.4. Synergy of Propolis and Mainstream Antileishmanials
4.2.5. Direct Antiparasite and Indirect Effects of Propolis on Intramacrophage Amastigotes via Immunomodulation
4.2.6. Nanotechnology in Delivering Propolis Therapy
4.3. Effects on Crithidia fasciculata
5. Conclusions
- (i)
- In some cases, the biological activity of flavonoids is not improved or sometimes increased by conjugation.
- (ii)
- At higher doses and in samples containing a mixture of flavonoids, there may be incomplete conjugation of particular flavonoids.
- (iii)
- It is possible that flavonoid metabolites can become deconjugated.
Author Contributions
Funding
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Alcântara, L.M.; Ferreira, T.C.; Gadelha, F.R.; Miguel, D.C. Challenges in drug discovery targeting TriTryp diseases with an emphasis on leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.P.; Barrett, M.P.; Dranoff, G.; Faraday, C.J.; Gimpelewicz, C.R.; Hailu, A.; Jones, C.L.; Kelly, J.M.; Lazdins-Helds, J.K.; Mäser, P. Drug discovery for kinetoplastid diseases: Future directions. ACS Infect. Dis. 2018, 5, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.L.; Ford, L.; Taylor, M.J. Overcoming the challenges of drug discovery for neglected tropical diseases: The A·WOL experience. J. Biomol. Screen. 2014, 19, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Seifert, K.; Yardley, V. Current scenario of drug development for leishmaniasis. Indian J. Med. Res. 2006, 123, 399–410. [Google Scholar] [PubMed]
- Ebiloma, G.U.; Igoli, J.O.; Katsoulis, E.; Donachie, A.-M.; Eze, A.; Gray, A.I.; de Koning, H.P. Bioassay-guided isolation of active principles from Nigerian medicinal plants identifies new trypanocides with low toxicity and no cross-resistance to diamidines and arsenicals. J. Ethnopharmacol. 2017, 202, 256–264. [Google Scholar] [CrossRef]
- Ebiloma, G.U.; Katsoulis, E.; Igoli, J.O.; Gray, A.I.; De Koning, H.P. Multi-target mode of action of a Clerodane-type diterpenoid from Polyalthia longifolia targeting African trypanosomes. Sci. Rep. 2018, 8, 4613. [Google Scholar] [CrossRef]
- Stuart, K.; Brun, R.; Croft, S.; Fairlamb, A.; Gürtler, R.E.; McKerrow, J.; Reed, S.; Tarleton, R. Kinetoplastids: Related protozoan pathogens, different diseases. J. Clin. Investig. 2008, 118, 1301–1310. [Google Scholar] [CrossRef]
- World Health Organization. WHO Roadmap Inspires Unprecedented Support to Defeat Neglected Tropical Diseases. World Health Organization: Geneva, Switzerland. Available online: http://www.who.int/neglected_diseases/London_meeting_follow_up/en/ (accessed on 6 June 2020).
- Ichoron, N.; Tyoer, S.; James, E.J.; Igoli, J.O. A survey of medicinal plants used as traditional medicine in Ukum and Ogbadibo Local Government Areas of Benue state, Nigeria. Plants Environ. 2019, 1, 5–11. [Google Scholar]
- Gray, A.I.; Igoli, J.O.; Edrada-Ebel, R. Natural products isolation in modern drug discovery programs. In Natural Products Isolation; Springer: Berlin, Germany, 2012; pp. 515–534. [Google Scholar]
- Brahmachari, G. Therapeutics from natural products against neglected tropical diseases: An overview. In Discovery and Development of Therapeutics from Natural Products against Neglected Tropical Diseases; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–6. [Google Scholar]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Omar, R.; Igoli, J.O.; Zhang, T.; Gray, A.I.; Ebiloma, G.U.; Clements, C.J.; Fearnley, J.; Ebel, R.E.; Paget, T.; De Koning, H.P. The chemical characterization of Nigerian propolis samples and their activity against Trypanosoma brucei. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Omar, R.M.; Igoli, J.; Gray, A.I.; Ebiloma, G.U.; Clements, C.; Fearnley, J.; Edrada Ebel, R.A.; Zhang, T.; De Koning, H.P.; Watson, D.G. Chemical characterisation of Nigerian red propolis and its biological activity against Trypanosoma brucei. Phytochem. Anal. 2016, 27, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Siheri, W.; Zhang, T.; Ebiloma, G.U.; Biddau, M.; Woods, N.; Hussain, M.Y.; Clements, C.J.; Fearnley, J.; Ebel, R.E.; Paget, T. Chemical and antimicrobial profiling of propolis from different regions within Libya. PLoS ONE 2016, 11, e0155355. [Google Scholar] [CrossRef] [PubMed]
- Santos da Silva, S.; da Silva Thomé, G.; Cataneo, A.H.D.; Miranda, M.M.; Felipe, I.; Andrade, C.G.T.D.J.; Watanabe, M.A.E.; Piana, G.M.; Sforcin, J.M.; Pavanelli, W.R. Brazilian propolis antileishmanial and immunomodulatory effects. Evid. Based Complement. Alternat. Med. 2013, 2013, 673058. [Google Scholar]
- Otoguro, K.; Iwatsuki, M.; Ishiyama, A.; Namatame, M.; Nishihara-Tsukashima, A.; Kiyohara, H.; Hashimoto, T.; Asakawa, Y.; Ōmura, S.; Yamada, H. In vitro antitrypanosomal activity of some phenolic compounds from propolis and lactones from Fijian Kawa (Piper methysticum). J. Nat. Med. 2012, 66, 558–561. [Google Scholar] [CrossRef]
- Amarante, M.K.; Watanabe, M.A.E.; Conchon-Costa, I.; Fiori, L.L.; Oda, J.M.M.; Búfalo, M.C.; Sforcin, J.M. The effect of propolis on CCL5 and IFN-γ expression by peripheral blood mononuclear cells from leishmaniasis patients. J. Pharm. Pharmacol. 2012, 64, 154–160. [Google Scholar] [CrossRef]
- Fidalgo, L.M.; Ramos, I.S.; Parra, M.G.; Cuesta-Rubio, O.; Hernández, I.M.; Fernández, M.C.; Piccinelli, A.L.; Rastrelli, L. Activity of Cuban propolis extracts on Leishmania amazonensis and Trichomonas vaginalis. Nat. Prod. Commun. 2011, 6, 973–976. [Google Scholar] [CrossRef]
- Ozbilge, H.; Kaya, E.G.; Albayrak, S.; Silici, S. Anti-leishmanial activities of ethanolic extract of Kayseri propolis. Afr. J. Microbiol. Res. 2010, 4, 556–560. [Google Scholar]
- Duran, G.; Duran, N.; Culha, G.; Ozcan, B.; Oztas, H.; Ozer, B. In vitro antileishmanial activity of Adana propolis samples on Leishmania tropica: A preliminary study. Parasitol. Res. 2008, 102, 1217–1225. [Google Scholar] [CrossRef]
- Pontin, K.; Da Silva Filho, A.A.; Santos, F.F.; e Silva, M.L.A.; Cunha, W.R.; Nanayakkara, N.D.; Bastos, J.K.; de Albuquerque, S. In vitro and in vivo antileishmanial activities of a Brazilian green propolis extract. Parasitol. Res. 2008, 103, 487–492. [Google Scholar] [CrossRef]
- de Carvalho Machado, G.M.; Leon, L.L.; de Castro, S.L. Activity of Brazilian and Bulgarian propolis against different species of Leishmania. Mem. Inst. Oswaldo Cruz 2007, 102, 73–77. [Google Scholar] [CrossRef]
- Ahangari, Z.; Naseri, M.; Vatandoost, F. Propolis: Chemical composition and its applications in endodontics. Iran. Endodont. J. 2018, 13, 285. [Google Scholar]
- Falcão, S.I.; Vale, N.; Cos, P.; Gomes, P.; Freire, C.; Maes, L.; Vilas-Boas, M. In vitro evaluation of Portuguese propolis and floral sources for antiprotozoal, antibacterial and antifungal activity. Phytother. Res. 2014, 28, 437–443. [Google Scholar] [CrossRef]
- Šturm, L.; Ulrih, N.P. Advances in the propolis chemical composition between 2013 and 2018: A review. eFood 2019, 1, 24–37. [Google Scholar] [CrossRef]
- Pujirahayu, N.; Suzuki, T.; Katayama, T. Cycloartane-type triterpenes and botanical origin of propolis of stingless Indonesian bee Tetragonula sapiens. Plants 2019, 8, 57. [Google Scholar] [CrossRef]
- Santos, L.M.; Fonseca, M.S.; Sokolonski, A.R.; Deegan, K.R.; Araújo, R.P.; Umsza-Guez, M.A.; Barbosa, J.D.; Portela, R.D.; Machado, B.A. Propolis: Types, composition, biological activities, and veterinary product patent prospecting. J. Sci. Food Agric. 2020, 100, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Blicharska, N.; Seidel, V. Chemical diversity and biological activity of African propolis. In Progress in the Chemistry of Organic Natural Products 109; Springer: Berlin, Germany, 2019; pp. 415–450. [Google Scholar]
- Betances-Salcedo, E.; Revilla, I.; Vivar-Quintana, A.M.; González-Martín, M.I. Flavonoid and antioxidant capacity of propolis prediction using near infrared spectroscopy. Sensors 2017, 17, 1647. [Google Scholar] [CrossRef] [PubMed]
- da Silva Cunha, I.B.; Salomão, K.; Shimizu, M.; Bankova, V.S.; Custódio, A.R.; de Castro, S.L.; Marcucci, M.C. Antitrypanosomal activity of Brazilian propolis from Apis mellifera. Chem. Pharm. Bull. 2004, 52, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Righi, A.A.; Negri, G.; Salatino, A. Comparative chemistry of propolis from eight Brazilian localities. Evid. Based Complement. Alternat. Med. 2013, 2013, 267878. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.Q.; Hu, F.-L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem. Cent. J. 2014, 8, 28. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- Sami, B.; Okoro, H.; Igoli, N.P.; Igoli, J.O. Isolation of isosativan from Nigerian red propolis. Trop. J. Nat. Prod. Res. 2020, 4, 77–79. [Google Scholar] [CrossRef]
- Falcão, S.I.; Lopes, M.; Vilas-Boas, M. A first approach to the chemical composition and antioxidant potential of Guinea-Bissau propolis. Nat. Prod. Commun. 2019, 14, 1–5. [Google Scholar] [CrossRef]
- Alaribe, C.S.; Esposito, T.; Sansone, F.; Sunday, A.; Pagano, I.; Piccinelli, A.L.; Celano, R.; Cuesta Rubio, O.; Coker, H.A.; Nabavi, S.M. Nigerian propolis: Chemical composition, antioxidant activity and α-amylase and α-glucosidase inhibition. Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef]
- Do Nascimento Araujo, C.; Sivero Mayworm, M.A.; Yatsuda, R.; Negri, G.; Faria Salatino, M.L.; Salatino, A.; Timenetsky, J.; Campos, G.B. Chemical composition and antimycoplasma activity of a brown propolis from southern Brazil. J. Food Sci. Technol. 2020, 57, 4228–4235. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Bayaqoob, N.I.; Rushdi, A.I.; Alattal, Y.; Simoneit, B.R.; El-Mubarak, A.H.; Al-Mutlaq, K.F. Chemical compositions and characteristics of organic compounds in propolis from Yemen. Saudi J. Biol. Sci. 2017, 24, 1094–1103. [Google Scholar] [CrossRef]
- Bonamigo, T.; Campos, J.F.; Alfredo, T.M.; Balestieri, J.B.P.; Cardoso, C.A.L.; Paredes-Gamero, E.J.; de Picoli Souza, K.; dos Santos, E.L. Antioxidant, cytotoxic, and toxic activities of propolis from two native bees in Brazil: Scaptotrigona depilis and Melipona quadrifasciata anthidioides. Oxid. Med. Cell. Longev. 2017, 2017, 1038153. [Google Scholar] [CrossRef]
- Hodel, K.V.; Machado, B.A.; Santos, N.R.; Costa, R.G.; Menezes-Filho, J.A.; Umsza-Guez, M.A. Metal content of nutritional and toxic value in different types of Brazilian propolis. Sci. World J. 2020, 2020, 395496. [Google Scholar] [CrossRef]
- Souza, E.; Zaluski, R.; Veiga, N.; Orsi, R. Effects of seasonal variations and collection methods on the mineral composition of propolis from Apis mellifera Linnaeus beehives. Braz. J. Biol. 2016, 76, 396–401. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, M.I.; Escuredo, O.; Revilla, I.; Vivar-Quintana, A.M.; Coello, M.C.; Riocerezo, C.P.; Moncada, G.W. Determination of the mineral composition and toxic element contents of propolis by near infrared spectroscopy. Sensors 2015, 15, 27854–27868. [Google Scholar] [CrossRef]
- da Silva, M.V.; de Moura, N.G., Jr.; Motoyama, A.B.; Ferreira, V.M. A review of the potential therapeutic and cosmetic use of propolis in topical formulations. J. Appl. Pharmacol. Sci. 2019, 10, 131–141. [Google Scholar]
- Popova, M.; Giannopoulou, E.; Skalicka-Woźniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gaweł-Bęben, K.; Antosiewicz, B. Characterization and biological evaluation of propolis from Poland. Molecules 2017, 22, 1159. [Google Scholar] [CrossRef] [PubMed]
- Falcão, S.I.; Vale, N.; Gomes, P.; Domingues, M.R.; Freire, C.; Cardoso, S.M.; Vilas-Boas, M. Phenolic profiling of Portuguese propolis by LC–MS spectrometry: Uncommon propolis rich in flavonoid glycosides. Phytochem. Anal. 2013, 24, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharmacol. Pharm. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef]
- De Groot, A.C.; Popova, M.P.; Bankova, V.S. An Update on the Constituents of Poplar-Type Propolis; Acdegroot Publishing: Wapserveen, The Netherlands, 2014. [Google Scholar]
- Saleh, K.; Zhang, T.; Fearnley, J.; Watson, D.G. A comparison of the constituents of propolis from different regions of the United Kingdom by liquid chromatography-high resolution mass spectrometry using a metabolomics approach. Curr. Metabolom. 2015, 3, 42. [Google Scholar] [CrossRef]
- Kardar, M.; Zhang, T.; Coxon, G.; Watson, D.; Fearnley, J.; Seidel, V. Characterisation of triterpenes and new phenolic lipids in Cameroonian propolis. Phytochemistry 2014, 106, 156–163. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Iwashina, T. The structure and distribution of the flavonoids in plants. J. Plant Res. 2000, 113, 287. [Google Scholar] [CrossRef]
- Mahamat, A.A.; Nyemb, J.N.; Gade, I.S.; Ngenge, A.T.; Talla, E.; Céline, H.; Sophie, L.; Mbafor, J.T. A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts. Open Chem. 2020, 18, 239–243. [Google Scholar] [CrossRef]
- Talla, E.; Tamfu, A.N.; Gade, I.S.; Yanda, L.; Mbafor, J.T.; Laurent, S.; Elst, L.V.; Popova, M.; Bankova, V. New mono-ether of glycerol and triterpenes with DPPH radical scavenging activity from Cameroonian propolis. Nat. Prod. Res. 2017, 31, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Tamfu, A.N.; Sawalda, M.; Fotsing, M.T.; Kouipou, R.M.T.; Talla, E.; Chi, G.F.; Epanda, J.J.E.; Mbafor, J.T.; Baig, T.A.; Jabeen, A. A new isoflavonol and other constituents from Cameroonian propolis and evaluation of their anti-inflammatory, antifungal and antioxidant potential. Saudi J. Biol. Sci. 2020, 27, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Siheri, W.; Ebiloma, G.U.; Igoli, J.O.; Gray, A.I.; Biddau, M.; Akrachalanont, P.; Alenezi, S.; Alwashih, M.A.; Edrada-Ebel, R.; Muller, S. Isolation of a novel flavanonol and an alkylresorcinol with highly potent anti-trypanosomal activity from Libyan propolis. Molecules 2019, 24, 1041. [Google Scholar] [CrossRef]
- Segueni, N.; Zellagui, A.; Moussaoui, F.; Lahouel, M.; Rhouati, S. Flavonoids from Algerian propolis. Arab. J. Chem. 2016, 9, S425–S428. [Google Scholar] [CrossRef]
- Sokeng, S.D.; Talla, E.; Sakava, P.; Fokam Tagne, M.A.; Henoumont, C.; Sophie, L.; Mbafor, J.T.; Tchuenguem Fohouo, F.-N. Anti-inflammatory and analgesic effect of arachic acid ethyl ester isolated from propolis. BioMed Res. Int. 2020, 2020, 8797284. [Google Scholar] [CrossRef]
- Ccana-Ccapatinta, G.V.; Mejía, J.A.A.; Tanimoto, M.H.; Groppo, M.; de Carvalho, J.C.A.S.; Bastos, J.K. Dalbergia ecastaphyllum (L.) Taub. and Symphonia globulifera Lf: The botanical sources of isoflavonoids and benzophenones in Brazilian red propolis. Molecules 2020, 25, 2060. [Google Scholar] [CrossRef]
- Nina, N.; Lima, B.; Feresin, G.E.; Giménez, A.; Salamanca Capusiri, E.; Schmeda-Hirschmann, G. Antibacterial and leishmanicidal activity of Bolivian propolis. Lett. Appl. Microbiol. 2016, 62, 290–296. [Google Scholar] [CrossRef]
- Nina, N.; Quispe, C.; Jiménez-Aspee, F.; Theoduloz, C.; Giménez, A.; Schmeda-Hirschmann, G. Chemical profiling and antioxidant activity of Bolivian propolis. J. Sci. Food Agric. 2016, 96, 2142–2153. [Google Scholar] [CrossRef]
- Mitsui, T.; Hotta, S.; Tazawa, S.; Arai, Y.; Kato, K.; Ichihara, K. Chemical constituents of Brazilian propolis from the state of Bahia and their growth inhibitory activities against cancer cells. Biosci. Biotechnol. Biochem. 2018, 82, 417–421. [Google Scholar] [CrossRef]
- Cuesta-Rubio, O.; Fernández, M.C.; Hernández, I.M.; Jaramillo, C.G.J.; González, V.H.; Porto, R.M.D.O.; Delange, D.M.; Fidalgo, L.M.; Piccinelli, A.L.; Campone, L. Chemical profile and anti-leishmanial activity of three Ecuadorian propolis samples from Quito, Guayaquil and Cotacachi regions. Fitoterapia 2017, 120, 177–183. [Google Scholar] [CrossRef]
- Nina, N.; Quispe, C.; Jiménez-Aspee, F.; Theoduloz, C.; Feresín, G.E.; Lima, B.; Leiva, E.; Schmeda-Hirschmann, G. Antibacterial activity, antioxidant effect and chemical composition of propolis from the Región del Maule, Central Chile. Molecules 2015, 20, 18144–18167. [Google Scholar] [CrossRef] [PubMed]
- Sanpa, S.; Popova, M.; Bankova, V.; Tunkasiri, T.; Eitssayeam, S.; Chantawannakul, P. Antibacterial compounds from propolis of Tetragonula laeviceps and Tetrigona melanoleuca (Hymenoptera: Apidae) from Thailand. PLoS ONE 2015, 10, e0126886. [Google Scholar] [CrossRef]
- Zhao, L.; Yu, M.; Sun, M.; Xue, X.; Wang, T.; Cao, W.; Sun, L. Rapid determination of major compounds in the ethanol extract of geopropolis from Malaysian stingless bees, Heterotrigona itama, by UHPLC-Q-TOF/MS and NMR. Molecules 2017, 22, 1935. [Google Scholar] [CrossRef]
- Ismail, T.N.N.T.; Sulaiman, S.A.; Ponnuraj, K.T.; Man, C.N.; Hassan, N.B. Chemical constituents of Malaysian Apis mellifera propolis. Sains Malays. 2018, 47, 117–122. [Google Scholar] [CrossRef]
- Ishizu, E.; Honda, S.; Ohta, T.; Vongsak, B.; Kumazawa, S. Component analysis and antiangiogenic activity of Thailand stingless bee propolis. Makara J. Technol. 2019, 23, 78–83. [Google Scholar] [CrossRef]
- Georgieva, K.; Trusheva, B.; Uzunova, V.; Stoyanova, T.; Valcheva, V.; Popova, M.; Tzoneva, R.; Bankova, V. New cycloartane triterpenes from bioactive extract of propolis from Pitcairn Island. Fitoterapia 2018, 128, 233–241. [Google Scholar] [CrossRef]
- Aminimoghadamfarouj, N.; Nematollahi, A. Propolis diterpenes as a remarkable bio-source for drug discovery development: A review. Int. J. Mol. Sci. 2017, 18, 1290. [Google Scholar] [CrossRef]
- Trusheva, B.; Stancheva, K.; Gajbhiye, N.; Dimitrova, R.; Popova, M.; Saraf, R.; Bankova, V. Two new prenylated stilbenes with an irregular sesquiterpenyl side chain from propolis from Fiji Islands. Rec. Nat. Prod. 2016, 10, 465. [Google Scholar]
- Duke, C.C.; Tran, V.H.; Duke, R.K.; Abu-Mellal, A.; Plunkett, G.T.; King, D.I.; Hamid, K.; Wilson, K.L.; Barrett, R.L.; Bruhl, J.J. A sedge plant as the source of Kangaroo Island propolis rich in prenylated p-coumarate ester and stilbenes. Phytochemistry 2017, 134, 87–97. [Google Scholar] [CrossRef]
- Nishimura, E.; Murakami, S.; Suzuki, K.; Amano, K.; Tanaka, R.; Shinada, T. Structure determination of monomeric phloroglucinol derivatives with a cinnamoyl group isolated from propolis of the stingless bee, Tetragonula carbonaria. Asian J. Org. Chem. 2016, 5, 855–859. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Spivak, M. Propolis and bee health: The natural history and significance of resin use by honey bees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef]
- Nicodemo, D.; De Jong, D.; Couto, R.; Malheiros, B. Honey bee lines selected for high propolis production also have superior hygienic behavior and increased honey and pollen stores. Genet. Mol. Res. 2013, 12, 6931–6938. [Google Scholar] [CrossRef]
- Borba, R.S.; Klyczek, K.K.; Mogen, K.L.; Spivak, M. Seasonal benefits of a natural propolis envelope to honey bee immunity and colony health. J. Exp. Biol. 2015, 218, 3689–3699. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Borba, R.S.; Wilson, M.; Spivak, M. Propolis counteracts some threats to honey bee health. Insects 2017, 8, 46. [Google Scholar] [CrossRef]
- Wilson, M.B.; Pawlus, A.D.; Brinkman, D.; Gardner, G.; Hegeman, A.D.; Spivak, M.; Cohen, J.D. 3-Acyl dihydroflavonols from poplar resins collected by honey bees are active against the bee pathogens Paenibacillus larvae and Ascosphaera apis. Phytochemistry 2017, 138, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Nicodemo, D.; Malheiros, E.B.; De Jong, D.; Couto, R.H.N. Increased brood viability and longer lifespan of honeybees selected for propolis production. Apidologie 2014, 45, 269–275. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.D.; Spivak, M. Increased resin collection after parasite challenge: A case of self-medication in honey bees? PLoS ONE 2012, 7, e34601. [Google Scholar] [CrossRef]
- Borba, R.S.; Spivak, M. Propolis envelope in Apis mellifera colonies supports honey bees against the pathogen, Paenibacillus larvae. Sci. Rep. 2017, 7, 11429. [Google Scholar] [CrossRef] [PubMed]
- Saelao, P.; Borba, R.S.; Ricigliano, V.; Spivak, M.; Simone-Finstrom, M. Honeybee microbiome is stabilized in the presence of propolis. Biol. Lett. 2020, 16, 20200003. [Google Scholar] [CrossRef]
- Antúnez, K.; Harriet, J.; Gende, L.; Maggi, M.; Eguaras, M.; Zunino, P. Efficacy of natural propolis extract in the control of American Foulbrood. Vet. Microbiol. 2008, 131, 324–331. [Google Scholar] [CrossRef]
- Bastos, E.M.A.; Simone, M.; Jorge, D.M.; Soares, A.E.E.; Spivak, M. In vitro study of the antimicrobial activity of Brazilian propolis against Paenibacillus larvae. J. Invertebr. Pathol. 2008, 97, 273–281. [Google Scholar] [CrossRef]
- Bilikova, K.; Popova, M.; Trusheva, B.; Bankova, V. New anti-Paenibacillus larvae substances purified from propolis. Apidologie 2013, 44, 278–285. [Google Scholar] [CrossRef]
- Popova, M.; Antonova, D.; Bankova, V. Chemical composition of propolis and American foulbrood: Is there any relationship? Bulg. Chem. Commun. 2017, 49, 171–175. [Google Scholar]
- Damiani, N.; Fernández, N.J.; Maldonado, L.M.; Álvarez, A.R.; Eguaras, M.J.; Marcangeli, J.A. Bioactivity of propolis from different geographical origins on Varroa destructor (Acari: Varroidae). Parasitol. Res. 2010, 107, 31–37. [Google Scholar] [CrossRef]
- Garedew, A.; Lamprecht, I.; Schmolz, E.; Schricker, B. The varroacidal action of propolis: A laboratory assay. Apidologie 2002, 33, 41–50. [Google Scholar] [CrossRef]
- Pusceddu, M.; Floris, I.; Mura, A.; Theodorou, P.; Cirotto, G.; Piluzza, G.; Bullitta, S.; Angioni, A.; Satta, A. The effects of raw propolis on Varroa-infested honey bee (Apis mellifera) workers. Parasitol. Res. 2018, 117, 3527–3535. [Google Scholar] [CrossRef]
- Drescher, N.; Klein, A.-M.; Neumann, P.; Yañez, O.; Leonhardt, S.D. Inside honeybee hives: Impact of natural propolis on the ectoparasitic mite Varroa destructor and viruses. Insects 2017, 8, 15. [Google Scholar] [CrossRef]
- Pusceddu, M.; Piluzza, G.; Theodorou, P.; Buffa, F.; Ruiu, L.; Bullitta, S.; Floris, I.; Satta, A. Resin foraging dynamics in Varroa destructor-infested hives: A case of medication of kin? Insect Sci. 2019, 26, 297–310. [Google Scholar] [CrossRef]
- Popova, M.; Reyes, M.; Le Conte, Y.; Bankova, V. Propolis chemical composition and honeybee resistance against Varroa destructor. Nat. Prod. Res. 2014, 28, 788–794. [Google Scholar] [CrossRef]
- Mura, A.; Pusceddu, M.; Theodorou, P.; Angioni, A.; Floris, I.; Paxton, R.J.; Satta, A. Propolis consumption reduces Nosema ceranae infection of European honey bees (Apis mellifera). Insects 2020, 11, 124. [Google Scholar] [CrossRef]
- Regan, T.; Barnett, M.W.; Laetsch, D.R.; Bush, S.J.; Wragg, D.; Budge, G.E.; Highet, F.; Dainat, B.; de Miranda, J.R.; Watson, M. Characterisation of the British honey bee metagenome. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ravoet, J.; Schwarz, R.S.; Descamps, T.; Yañez, O.; Tozkar, C.O.; Martin-Hernandez, R.; Bartolomé, C.; De Smet, L.; Higes, M.; Wenseleers, T. Differential diagnosis of the honey bee trypanosomatids Crithidia mellificae and Lotmaria passim. J. Invertebr. Pathol. 2015, 130, 21–27. [Google Scholar] [CrossRef]
- Castelli, L.; Branchiccela, B.; Invernizzi, C.; Tomasco, I.; Basualdo, M.; Rodriguez, M.; Zunino, P.; Antúnez, K. Detection of Lotmaria passim in Africanized and European honey bees from Uruguay, Argentina and Chile. J. Invertebr. Pathol. 2019, 160, 95–97. [Google Scholar] [CrossRef]
- Schwarz, R.S.; Bauchan, G.R.; Murphy, C.A.; Ravoet, J.; de Graaf, D.C.; Evans, J.D. Characterization of two species of Trypanosomatidae from the honey bee Apis mellifera: Crithidia mellificae Langridge and McGhee, and Lotmaria passim n. gen., n. sp. J. Eukaryot. Microbiol. 2015, 62, 567–583. [Google Scholar] [CrossRef]
- Gómez-Moracho, T.; Buendía-Abad, M.; Benito, M.; García-Palencia, P.; Barrios, L.; Bartolomé, C.; Maside, X.; Meana, A.; Jiménez-Antón, M.D.; Olías-Molero, A.I. Experimental evidence of harmful effects of Crithidia mellificae and Lotmaria passim on honey bees. Int. J. Parasitol. 2020, in press. [Google Scholar]
- Ruiz-González, M.X.; Brown, M.J. Honey bee and bumblebee trypanosomatids: Specificity and potential for transmission. Ecol. Entomol. 2006, 31, 616–622. [Google Scholar] [CrossRef]
- Runckel, C.; DeRisi, J.; Flenniken, M.L. A draft genome of the honey bee trypanosomatid parasite Crithidia mellificae. PLoS ONE 2014, 9, e95057. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; de Castro, S. Propolis extracts are effective against Trypanosoma cruzi and have an impact on its interaction with host cells. J. Ethnopharmacol. 1994, 43, 149–155. [Google Scholar] [CrossRef]
- de Castro, S.; Higashi, K. Effect of different formulations of propolis on mice infected with Trypanosoma cruzi. J. Ethnopharmacol. 1995, 46, 55–58. [Google Scholar] [CrossRef]
- Marcucci, M.C.; Ferreres, F.; Garcıa-Viguera, C.; Bankova, V.; de Castro, S.; Dantas, A.; Valente, P.; Paulino, N. Phenolic compounds from Brazilian propolis with pharmacological activities. J. Ethnopharmacol. 2001, 74, 105–112. [Google Scholar] [CrossRef]
- Dantas, A.P.; Salomão, K.; Barbosa, H.S.; de Castro, S.L. The effect of Bulgarian propolis against Trypanosoma cruzi and during its interaction with host cells. Mem. Inst. Oswaldo Cruz 2006, 101, 207–211. [Google Scholar] [CrossRef]
- Dantas, A.P.; Olivieri, B.P.; Gomes, F.H.; de Castro, S.L. Treatment of Trypanosoma cruzi-infected mice with propolis promotes changes in the immune response. J. Ethnopharmacol. 2006, 103, 187–193. [Google Scholar] [CrossRef]
- Salomão, K.; De Souza, E.; Barbosa, H.; de Castro, S. Propolis and derivatives of megazol: In vitro and in vivo activity on Trypanosoma cruzi, mechanism of action and selectivity. Int. J. Infect. Dis. 2010, 14, e441–e442. [Google Scholar] [CrossRef][Green Version]
- Almutairi, S.; Eapen, B.; Chundi, S.M.; Akhalil, A.; Siheri, W.; Clements, C.; Fearnley, J.; Watson, D.G.; Edrada-Ebel, R. New anti-trypanosomal active prenylated compounds from African propolis. Phytochem. Lett. 2014, 10, 35–39. [Google Scholar] [CrossRef]
- Almutairi, S.; Edrada-Ebel, R.; Fearnley, J.; Igoli, J.O.; Alotaibi, W.; Clements, C.J.; Gray, A.I.; Watson, D.G. Isolation of diterpenes and flavonoids from a new type of propolis from Saudi Arabia. Phytochem. Lett. 2014, 10, 160–163. [Google Scholar] [CrossRef]
- Siheri, W.; Igoli, J.O.; Gray, A.I.; Nasciemento, T.G.; Zhang, T.; Fearnley, J.; Clements, C.J.; Carter, K.C.; Carruthers, J.; Edrada-Ebel, R. The isolation of antiprotozoal compounds from Libyan propolis. Phytother. Res. 2014, 28, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.; Ebiloma, G.U.; Williams, R.; Alenezi, S.; Donachie, A.-M.; Guillaume, S.; Igoli, J.O.; Fearnley, J.; De Koning, H.P.; Watson, D.G. European propolis is highly active against trypanosomatids including Crithidia fasciculata. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Rakotomanga, M.; Blanc, S.; Gaudin, K.; Chaminade, P.; Loiseau, P. Miltefosine affects lipid metabolism in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 2007, 51, 1425–1430. [Google Scholar] [CrossRef]
- Lee, J.S.; Cho, Y.S.; Park, E.J.; Kim, J.; Oh, W.K.; Lee, H.S.; Ahn, J.S. Phospholipase Cγ1 inhibitory principles from the sarcotestas of Ginkgo biloba. J. Nat. Prod. 1998, 61, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Kozubek, A.; Tyman, J.H. Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity. Chem. Rev. 1999, 99, 1–26. [Google Scholar] [CrossRef]
- Baker, N.; Glover, L.; Munday, J.C.; Andrés, D.A.; Barrett, M.P.; De Koning, H.P.; Horn, D. Aquaglyceroporin 2 controls susceptibility to melarsoprol and pentamidine in African trypanosomes. Proc. Natl. Acad. Sci. USA 2012, 109, 10996–11001. [Google Scholar] [CrossRef]
- Munday, J.C.; Eze, A.A.; Baker, N.; Glover, L.; Clucas, C.; Aguinaga Andrés, D.; Natto, M.J.; Teka, I.A.; McDonald, J.; Lee, R.S.; et al. Trypanosoma brucei aquaglyceroporin 2 is a high-affinity transporter for pentamidine and melaminophenyl arsenic drugs and the main genetic determinant of resistance to these drugs. J. Antimicrob. Chemother. 2014, 69, 651–663. [Google Scholar] [CrossRef]
- Munday, J.C.; Settimo, L.; De Koning, H.P. Transport proteins determine drug sensitivity and resistance in a protozoan parasite, Trypanosoma brucei. Front. Pharmacol. 2015, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.H.; Munday, J.C.; Campagnaro, G.; Gurvič, D.; Svensson, F.; Okpara, C.E.; Kumar, A.; Quintana, J.F.; Martin Abril, M.E.; Milic, P.; et al. Positively selected modifications in the pore of TbAQP2 allow pentamidine to enter Trypanosoma brucei. eLife 2020, 9, e56416. [Google Scholar] [CrossRef]
- Matovu, E.; Stewart, M.L.; Geiser, F.; Brun, R.; Mäser, P.; Wallace, L.J.; Burchmore, R.J.; Enyaru, J.C.; Barrett, M.P.; Kaminsky, R. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot. Cell 2003, 2, 1003–1008. [Google Scholar] [CrossRef]
- Bridges, D.J.; Gould, M.K.; Nerima, B.; Mäser, P.; Burchmore, R.J.; De Koning, H.P. Loss of the high-affinity pentamidine transporter is responsible for high levels of cross-resistance between arsenical and diamidine drugs in African trypanosomes. Mol. Pharmacol. 2007, 71, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.P. The drugs of sleeping sickness: Their mechanisms of action and resistance, and a brief history. Trop. Med. Infect. Dis. 2020, 5, 14. [Google Scholar] [CrossRef]
- Giordani, F.; Morrison, L.J.; Rowan, T.G.; De Koning, H.P.; Barrett, M.P. The animal trypanosomiases and their chemotherapy: A review. Parasitology 2016, 143, 1862–1889. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, S.S.; Natto, M.J.; Igoli, J.O.; Gray, A.I.; Fearnley, F.; Fearnley, H.; De Koning, H.P.; Watson, D.G. Novel flavanones with anti-trypanosomal activity isolated from Zambian and Tanzanian propolis samples. Int. J. Parasitol. Drugs Drug Resist. 2020. [Google Scholar] [CrossRef]
- Aregawi, W.G.; Agga, G.E.; Abdi, R.D.; Büscher, P. Systematic review and meta-analysis on the global distribution, host range, and prevalence of Trypanosoma evansi. Parasit. Vectors 2019, 12, 67. [Google Scholar] [CrossRef]
- Rassi, A., Jr.; Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Dantas Silva, R.P.; Machado, B.A.S.; de Abreu Barreto, G.; Costa, S.S.; Andrade, L.N.; Amaral, R.G.; Carvalho, A.A.; Padilha, F.F.; Barbosa, J.D.V.; Umsza-Guez, M.A. Antioxidant, antimicrobial, antiparasitic, and cytotoxic properties of various Brazilian propolis extracts. PLoS ONE 2017, 12, e0172585. [Google Scholar] [CrossRef]
- Salomao, K.; de Souza, E.M.; Henriques-Pons, A.; Barbosa, H.S.; de Castro, S.L. Brazilian green propolis: Effects in vitro and in vivo on Trypanosoma cruzi. Evid. Based Complement. Alternat. Med. 2011, 2011, 185918. [Google Scholar] [CrossRef] [PubMed]
- Nweze, N.E.; Okoro, H.O.; Al Robaian, M.; Omar, R.M.; Tor-Anyiin, T.A.; Watson, D.G.; Igoli, J.O. Effects of Nigerian red propolis in rats infected with Trypanosoma brucei brucei. Comp. Clin. Pathol. 2017, 26, 1129–1133. [Google Scholar] [CrossRef]
- Figueiredo, R.; Rosa, D.; Gomes, Y.; Nakasawa, M.; Soares, M. Reservosome: An endocytic compartment in epimastigote forms of the protozoan Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae). Correlation between endocytosis of nutrients and cell differentiation. Parasitology 2004, 129, 431. [Google Scholar] [CrossRef] [PubMed]
- Salomão, K.; Dantas, A.P.; Borba, C.M.; Campos, L.; Machado, D.; Aquino Neto, F.; de Castro, S. Chemical composition and microbicidal activity of extracts from Brazilian and Bulgarian propolis. Lett. Appl. Microbiol. 2004, 38, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Prytzyk, E.; Dantas, A.P.; Salomão, K.; Pereira, A.S.; Bankova, V.S.; de Castro, S.L.; Neto, F.R.A. Flavonoids and trypanocidal activity of Bulgarian propolis. J. Ethnopharmacol. 2003, 88, 189–193. [Google Scholar] [CrossRef]
- Santana, L.C.; Carneiro, S.M.P.; Caland-Neto, L.B.; Arcanjo, D.D.; Moita-Neto, J.M.; Citó, A.M.; Carvalho, F.A.A. Brazilian brown propolis elicits antileishmanial effect against promastigote and amastigote forms of Leishmania amazonensis. Nat. Prod. Res. 2014, 28, 340–343. [Google Scholar] [CrossRef]
- Monzote, L.; Cuesta-Rubio, O.; Campo Fernandez, M.; Márquez Hernandez, I.; Fraga, J.; Pérez, K.; Kerstens, M.; Maes, L.; Cos, P. In vitro antimicrobial assessment of Cuban propolis extracts. Mem. Inst. Oswaldo Cruz 2012, 107, 978–984. [Google Scholar] [CrossRef]
- Devequi-Nunes, D.; Machado, B.A.S.; de Abreu Barreto, G.; Rebouças Silva, J.; da Silva, D.F.; da Rocha, J.L.C.; Brandão, H.N.; Borges, V.M.; Umsza-Guez, M.A. Chemical characterization and biological activity of six different extracts of propolis through conventional methods and supercritical extraction. PLoS ONE 2018, 13, e0207676. [Google Scholar] [CrossRef]
- Ayres, D.C.; Marcucci, M.C.; Giorgio, S. Effects of Brazilian propolis on Leishmania amazonensis. Mem. Inst. Oswaldo Cruz 2007, 102, 215–220. [Google Scholar] [CrossRef]
- Trusheva, B.; Popova, M.; Bankova, V.; Simova, S.; Marcucci, M.C.; Miorin, P.L.; da Rocha Pasin, F.; Tsvetkova, I. Bioactive constituents of Brazilian red propolis. Evid. Based Complement. Alternat. Med. 2006, 3, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, P.; Shaddel, M.; Yakhchali, M.; Raoufi, N.; Shamsi, H.; Dastgheib, M. Antileishmanial effects of propolis against Leishmania major in vitro and in vivo. Ann. Milit. Health Sci. Res. 2020, 18, e100630. [Google Scholar] [CrossRef]
- Ferreira, F.M.; Castro, R.A.; Batista, M.A.; Rossi, F.M.; Silveira-Lemos, D.; Frézard, F.; Moura, S.A.; Rezende, S.A. Association of water extract of green propolis and liposomal meglumine antimoniate in the treatment of experimental visceral leishmaniasis. Parasitol. Res. 2014, 113, 533–543. [Google Scholar] [CrossRef]
- da Silva, S.S.; Mizokami, S.S.; Fanti, J.R.; Miranda, M.M.; Kawakami, N.Y.; Teixeira, F.H.; Araújo, E.J.; Panis, C.; Watanabe, M.A.; Sforcin, J.M. Propolis reduces Leishmania amazonensis-induced inflammation in the liver of BALB/c mice. Parasitol. Res. 2016, 115, 1557–1566. [Google Scholar] [CrossRef]
- Ayres, D.C.; Fedele, T.A.; Marcucci, M.C.; Giorgio, S. Potential utility of hyperbaric oxygen therapy and propolis in enhancing the leishmanicidal activity of glucantime. Rev. Inst. Med. Trop. Sao Paulo 2011, 53, 329–334. [Google Scholar] [CrossRef]
- Jihene, A.; Rym, E.; Ines, K.J.; Majdi, H.; Olfa, T.; Abderrabba, M. Antileishmanial potential of propolis essential oil and its synergistic combination with Amphotericin B. Nat. Prod. Commun. 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Essid, R.; Rahali, F.Z.; Msaada, K.; Sghair, I.; Hammami, M.; Bouratbine, A.; Aoun, K.; Limam, F. Antileishmanial and cytotoxic potential of essential oils from medicinal plants in Northern Tunisia. Ind. Crops Prod. 2015, 77, 795–802. [Google Scholar] [CrossRef]
- Orsatti, C.; Missima, F.; Pagliarone, A.; Bachiega, T.F.; Búfalo, M.; Araújo, J., Jr.; Sforcin, J. Propolis immunomodulatory action in vivo on Toll-like receptors 2 and 4 expression and on pro-inflammatory cytokines production in mice. Phytother. Res. 2010, 24, 1141–1146. [Google Scholar] [CrossRef]
- Rebouças-Silva, J.; Celes, F.S.; Lima, J.B.; Barud, H.S.; de Oliveira, C.I.; Berretta, A.A.; Borges, V.M. Parasite killing of Leishmania (V) braziliensis by standardized propolis extracts. Evid. Based Complement. Alternat. Med. 2017, 2017, 6067172. [Google Scholar] [CrossRef]
- Ribeiro, T.G.; Chávez-Fumagalli, M.A.; Valadares, D.G.; França, J.R.; Rodrigues, L.B.; Duarte, M.C.; Lage, P.S.; Andrade, P.H.; Lage, D.P.; Arruda, L.V. Novel targeting using nanoparticles: An approach to the development of an effective anti-leishmanial drug-delivery system. Int. J. Nanomed. 2014, 9, 877. [Google Scholar]
- Ahmad, A.; Syed, F.; Shah, A.; Khan, Z.; Tahir, K.; Khan, A.U.; Yuan, Q. Silver and gold nanoparticles from Sargentodoxa cuneata: Synthesis, characterization and antileishmanial activity. RSC Adv. 2015, 5, 73793–73806. [Google Scholar] [CrossRef]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid–polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef]
- Do Nascimento, T.G.; Da Silva, P.F.; Azevedo, L.F.; Da Rocha, L.G.; de Moraes Porto, I.C.C.; Moura, T.F.A.L.; Basílio-Júnior, I.D.; Grillo, L.A.M.; Dornelas, C.B.; da Silva Fonseca, E.J. Polymeric Nanoparticles of Brazilian red propolis extract: Preparation, characterization, antioxidant and leishmanicidal activity. Nanoscale Res. Lett. 2016, 11, 301. [Google Scholar] [CrossRef]
- Wilson, M.B.; Spivak, M.; Hegeman, A.D.; Rendahl, A.; Cohen, J.D. Metabolomics reveals the origins of antimicrobial plant resins collected by honey bees. PLoS ONE 2013, 8, e77512. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. The phytochemistry of the honeybee. Phytochemistry 2018, 155, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ravoet, J.; Maharramov, J.; Meeus, I.; De Smet, L.; Wenseleers, T.; Smagghe, G.; De Graaf, D.C. Comprehensive bee pathogen screening in Belgium reveals Crithidia mellificae as a new contributory factor to winter mortality. PLoS ONE 2013, 8, e72443. [Google Scholar] [CrossRef]
- Alenezi, S.; Manal, N.; Natto, J.; De Konıng, H.P.; Igoli, J.; Watson, D. Chemical profiling of Papua New Guinea propolis and assay its antiprotozoal activity. J. Apither. Nat. 2018, 1, 35. [Google Scholar]
- Al Rofaidi, M.; Alotaibi, A.; Alqarni, A.; Alghamdi, A.; Fearnley, J.; Watson, D.G. A preliminary study of the absorption and metabolism of temperate propolis by human subjects. J. Food Nutr. Metab. 2020, 3, 2–6. [Google Scholar]
- De Koning, H.P. Drug resistance in protozoan parasites. Emerg. Topics Life Sci. 2017, 1, 627–632. [Google Scholar]
- Beekmann, K.; Actis-Goretta, L.; van Bladeren, P.J.; Dionisi, F.; Destaillats, F.; Rietjens, I.M. A state-of-the-art overview of the effect of metabolic conjugation on the biological activity of flavonoids. Food Funct. 2012, 3, 1008–1018. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.-M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodríguez, G.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.; Chen, C.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Zanatta, L.; Alberton, E.H.; Bonorino Figueiredo, M.S.R.; Folador, P.; Damazio, R.G.; Pizzolatti, M.G.; Barreto Silva, F.R.M. Flavonoids: Prospective drug candidates. Mini Rev. Med. Chem. 2008, 8, 1429–1440. [Google Scholar] [CrossRef]
- Silberberg, M.; Morand, C.; Mathevon, T.; Besson, C.; Manach, C.; Scalbert, A.; Remesy, C. The bioavailability of polyphenols is highly governed by the capacity of the intestine and of the liver to secrete conjugated metabolites. Eur. J. Nutr. 2006, 45, 88–96. [Google Scholar] [CrossRef] [PubMed]

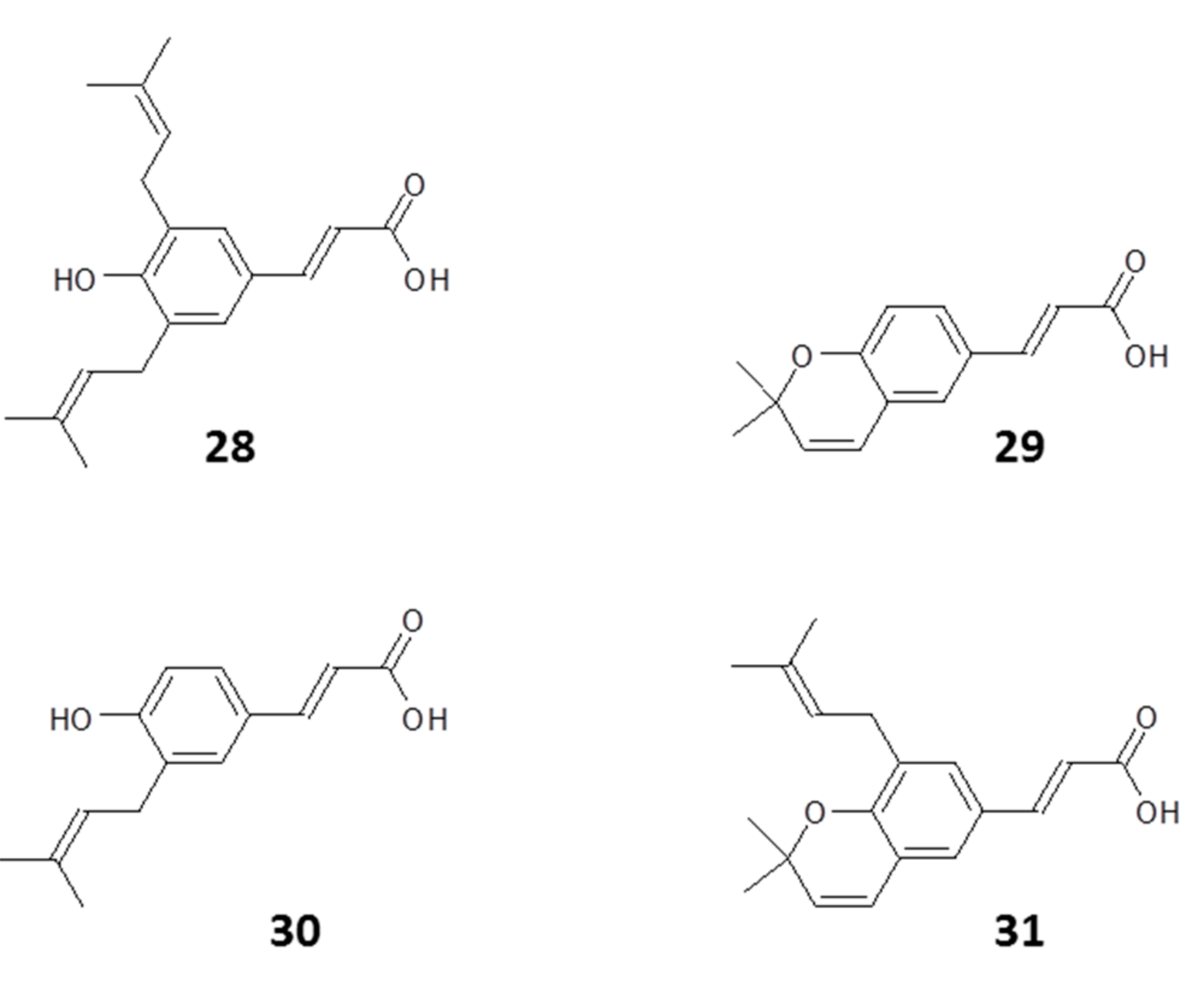
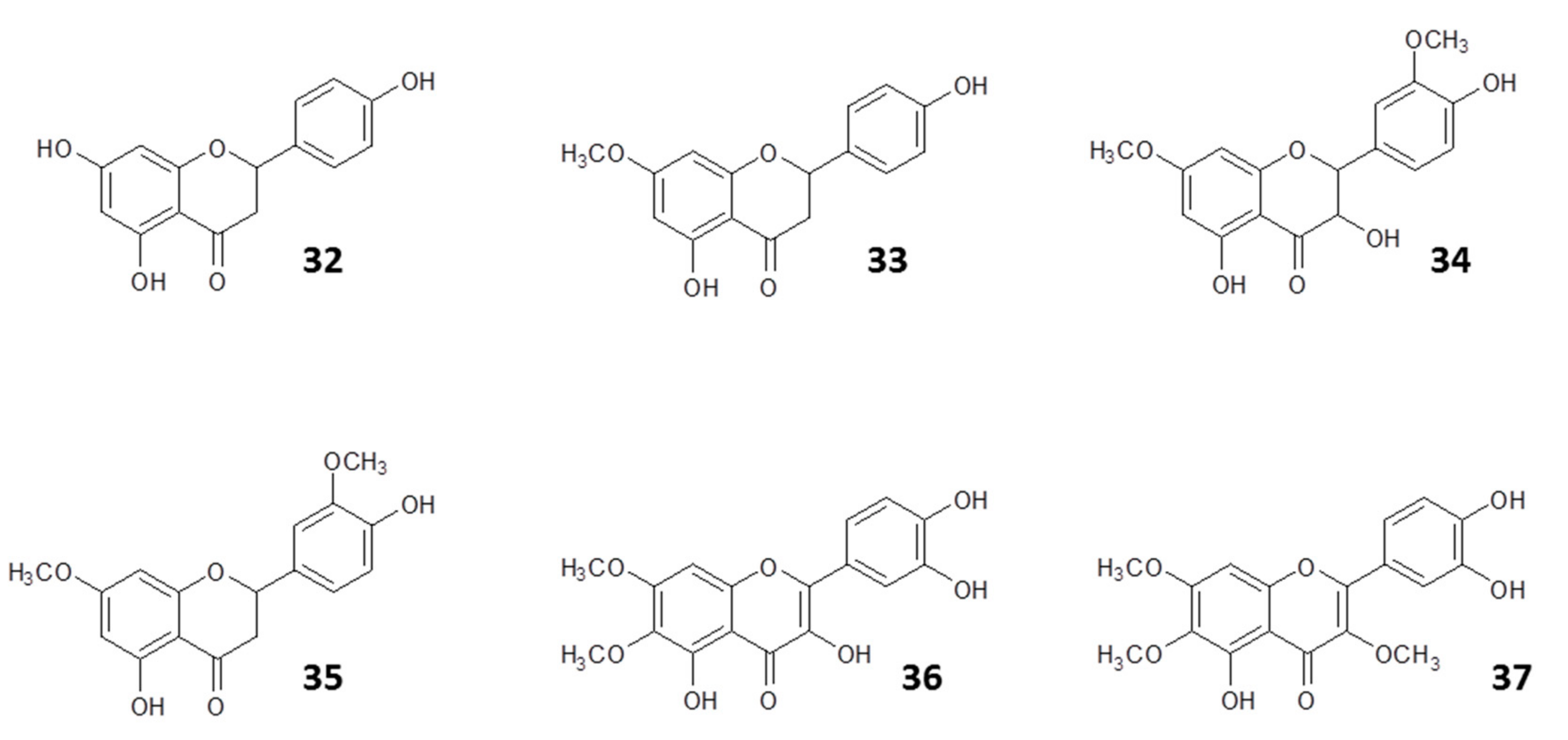
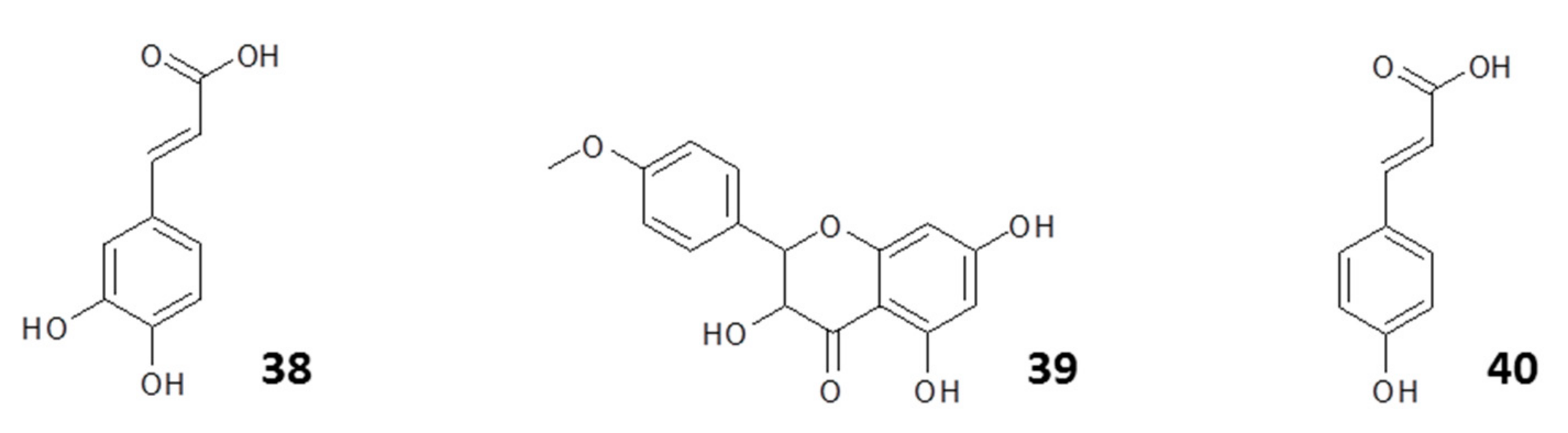
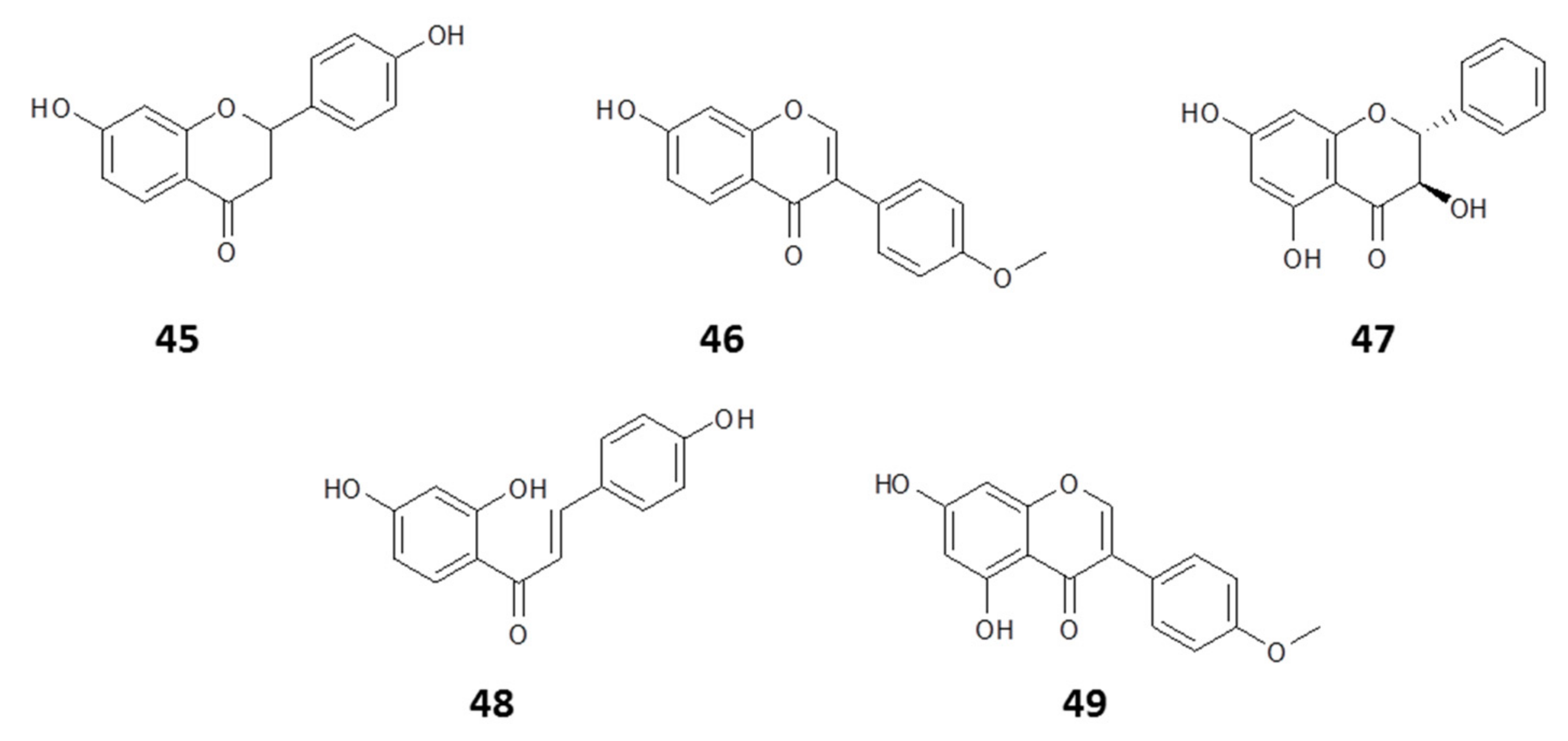
| Name | Class of Compound | Country | Reference |
|---|---|---|---|
| Lupenone | Triterpenoid | Cameroon | [55] |
| α-Amyrin (16) | Triterpenoid | Cameroon/Nigeria | [15,55] |
| β-Amyrin | Triterpenoid | Cameroon | [55] |
| Methyl-3β,27-dihydroxycycloart-24-en-26-oate | Triterpenoid | Cameroon | [56] |
| Oleanolic acid | Triterpenoid | Cameroon | [57] |
| β-Amyrin acetate | Triterpenoid | Cameroon | [57] |
| Lupeol | Triterpenoid | Cameroon | [57] |
| Betulinic acid | Triterpenoid | Cameroon | [57] |
| Lupeol acetate | Triterpenoid | Cameroon | [57] |
| Cycloartanol | Cycloartane triterpene | Libya | [58] |
| Mangiferolic acid (4) | Cycloartane triterpene | Libya/Nigeria | [15,58] |
| Mangiferonic acid (5) | Cycloartane triterpene | Libya/Nigeria | [15,58] |
| Ambolic acid | Cycloartane triterpene | Libya | [58] |
| 27-Hydroxymangiferonic acid (6) | Cycloartane triterpene | Libya | [58] |
| Ambonic acid (15) | Cycloartane triterpene | Nigeria | [15] |
| 13-Epitorulosol | Diterpene | Libya | [58] |
| Acetylisocupressic acid (7) | Diterpene | Libya | [58] |
| Agathadiol (8) | Diterpene | Libya | [58] |
| Isocupressic acid (9) | Diterpene | Libya | [58] |
| Isoagatholal (10) | Diterpene | Libya | [58] |
| 2-Hydroxy-8-prenylbiochanin A | Flavonoid | Cameroon | [57] |
| Taxifolin-3-acetyl-4′methyl ether (11) | Flavanoid | Libya | [58] |
| 3,8-dihydroxy-9-methoxy-pterocarpan | Flavonoid | Nigeria | [39] |
| Astrapterocarpan | Flavonoid | Nigeria | [39] |
| Vesticarpan | Flavonoid | Nigeria | [39] |
| Vestitol (21) | Flavonoid | Nigeria | [15,39] |
| Broussonin B | Flavonoid | Nigeria | [39] |
| Calycosin | Flavonoid | Nigeria | [15] |
| Liquiritigenin (45) | Flavonoid | Nigeria | [15] |
| Pinocembrin | Flavonoid | Nigeria | [15] |
| Isosativan, (2′-hydroxy-7,4′-dimethoxyisoflavan) | Flavonoid | Nigeria | [37] |
| Medicarpin | Flavanoid | Nigeria | [39] |
| Pectolinarigenin | Flavonoid | Algeria | [59] |
| 6,7-Dihydroxy-7,4′-dimethoxyflavone (Ladanein) | Flavonoid | Algeria | [59] |
| 8-Prenylnaringenin (20) | Prenylated flavonoid | Nigeria | [15] |
| 6-Prenylnaringenin | Prenylated flavonoid | Nigeria | [15] |
| Propolin D | Prenylated flavonoid | Nigeria | [15] |
| Macarangin | Prenylated flavonoid | Nigeria | [15] |
| Gerontoxanthone H (12) | Xanthone | Nigeria | [15] |
| 6-Deoxy-γ-mangostin (13) | Xanthone | Nigeria | [15] |
| 1,7-Dihydro-3-O-(3-methylbut-2-enyl)-8(3-methylbut-2-enyl) xanthone (14) | Xanthone | Nigeria | [15] |
| Demethylpiperitol (1) | Lignan | Libya | [59] |
| 5′-Methoxypiperitol (2) | Lignan | Libya | [59] |
| Riverinol | Benzofuran | Nigeria | [15] |
| Triacontyl ρ-coumarate | Coumarin | Cameroon | [57] |
| Arachic/arachidic acid ethyl ester (PEN4) | Alkylphenol | Cameroon | [60] |
| Cardol | Alkylresorcinol | Libya/Cameroon | [55,58] |
| 1′-O-Eicosanyl glycerol | Acylglycerol | Cameroon | [56] |
| Oleic acid | Fatty acid | Nigeria | [37] |
| Propyl stearate | Fatty acid ester | Nigeria | [37] |
| Hexatriacontanoic acid | Fatty acid | Cameroon | [55] |
| 2′,3′-Dihydroxypropyltetraeicosanoate | Fatty acid | Cameroon | [57] |
| Name | Class of Compound | Country | Reference |
|---|---|---|---|
| β-Amyrin | Triterpenoid | Brazil | [61] |
| Glutinol | Triterpenoid | Brazil | [61] |
| Cycloart-24-en-3β-ol | Triterpenoid | Bolivia | [62,63] |
| Cycloart-24-en-3β,26-diol | Triterpenoid | Bolivia | [62,63] |
| 24(E)-Cycloart-24-en-26-ol-3-one | Cycloartane triterpene | Bolivia | [62,63] |
| Cycloart-24-en-3-one | Cycloartane triterpene | Bolivia | [62,63] |
| Lupeol | Pentacyclic triterpene | Bolivia | [62,63] |
| Cycloartenone | Cycloartane triterpene | Bolivia | [62,63] |
| Liquiritigenin (45) | Flavonoid | Brazil | [61] |
| Isoliquiritigenin (48) | Flavonoid | Brazil | [61] |
| Formononetin (46) | Flavonoid | Brazil | [61] |
| Vestitol (21) | Flavonoid | Brazil | [61] |
| Neovestitol | Flavonoid | Brazil | [61] |
| Medicarpin | Flavonoid | Brazil | [61] |
| 7-O-Neovestitol | Flavonoid | Brazil | [61] |
| 3-O-Methylquercetin | Flavonoid | Brazil | [64] |
| 3,6,4′-Trimethoxychrysin | Flavonoid | Brazil | [64] |
| 3,6-Dimethoxyapigenin | Flavonoid | Brazil | [64] |
| 6-Methoxykaempferol | Flavonoid | Brazil | [64] |
| 6-Methoxyapigenin | Flavonoid | Brazil | [64] |
| 5,7,4′-Trihydroxyflavanone (Naringenin) | Flavonoid | Ecuador | [65] |
| 5,4′-Dihydroxy-7-methoxyflavanone (Sakuranetin) | Flavonoid | Ecuador | [65] |
| 3,5,4′-Trihydroxy-7,3′-dimethoxyflavanone | Flavonoid | Ecuador | [65] |
| 5,4′-Dihydroxy-7,3′-dimethoxyflavanon | Flavonoid | Ecuador | [65] |
| 3,5,3′,4′-Tetrahydroxy-6,7-dimethoxy flavone (Eupatolitin) | Flavonoid | Ecuador | [65] |
| 3,5,4′-Trihydroxy-7,3′-dimethoxy flavone (Rhamnazin) | Flavonoid | Ecuador | [65] |
| Pinocembrin | Flavonoid | Chile | [66] |
| Chrysin | Flavonoid | Chile | [66] |
| Kaempferol 3-methyl ether | Flavonoid | Bolivia | [62,63] |
| Kaempferol 7-O-methyl ether | Flavonoid | Bolivia | [62,63] |
| 2-Phenoxychromone | Benzopyran derivative | Brazil | [64] |
| Cinnamic acid | Phenyl propanoid | Bolivia | [62,63] |
| 3-Prenyl-p-coumaric acid (Drupanin) | Coumarin | Bolivia | [62,63] |
| Benzyl benzoate | Benzyl ester | Bolivia | [62,63] |
| Guttiferone E | Polyprenylated benzophenone | Brazil | [61] |
| Oblongifolin B | Polyprenylated benzophenone | Brazil | [61] |
| (E)-3-Hydroxy-1,7-diphenylhept-1-ene-5-acetate | Diarylheptanoid | Chile | [66] |
| (E)-5-Hydroxy-1,7-diphenylhept-1-ene-3-acetate | Diarylheptanoid | Chile | [66] |
| Name | Class of Compound | Country | Reference |
|---|---|---|---|
| Mangiferolic acid | Cycloartane triterpenoid | Indonesia | [28] |
| Cycloartenol | Cycloartane triterpenoid | Indonesia | [28] |
| Mangferonic acid (5) | Cycloartane triterpenoid | Indonesia | [28] |
| Ambonic acid (15) | Cycloartane triterpenoid | Indonesia | [28] |
| Ambolic acid | Cycloartane triterpenoid | Indonesia | [28] |
| 3-O-Acetyl ursolic acid | Triterpenoid | Thailand | [67] |
| Ocotillone I | Triterpenoid | Thailand | [67] |
| Ocotillone II | Triterpenoid | Thailand | [67] |
| Ursolic aldehyde | Triterpenoid | Thailand | [67] |
| Oleanolic aldehyde | Triterpenoid | Thailand | [67] |
| 20-Hydroxy-24-dammaren-3-one | Triterpenoid | Malaysia | [68] |
| Dipterocarpol | Triterpenoid | Thailand | [67] |
| Cabralealactone | Triterpenoid | Thailand | [67] |
| Isocabralealactone | Triterpenoid | Thailand | [67] |
| β-Panasinsene | Sesquiterpene | Malaysia | [69] |
| α-Mangostin | Prenylated xanthone | Thailand | [70] |
| γ-Mangostin | Prenylated xanthone | Thailand | [70] |
| Cochinchinone T | Prenylated xanthone | Thailand | [70] |
| β-Mangostin | Prenylated xanthone | Thailand | [70] |
| Gartanin | Prenylated xanthone | Thailand | [70] |
| 8-Deoxygartanin | Prenylated xanthone | Thailand | [70] |
| 9-Hydroxycalabaxanthone | Prenylated xanthone | Thailand | [70] |
| Mangostanol | Prenylated xanthone | Thailand | [70] |
| Mangostanin | Xanthone | Thailand | [67] |
| Garcinone B | Xanthone | Thailand | [67] |
| Methylpinoresinol | Lignan | Thailand | [67] |
| Name | Class of Compound | Country | Reference |
|---|---|---|---|
| 3-Oxo-cycloart-24E-en-21,26-diol-21,26-diacetate | Triterpenoid | Pitcairn Island | [71] |
| 3-Oxo-cycloart-24E-en-21,26-diol | Triterpenoid | Pitcairn Island | [71] |
| 3-Oxo-cycloart-24E-en-21,26-diol-21-acetate | Triterpenoid | Pitcairn Island | [71] |
| 3-Oxo-cycloart-24E-en-21,26-diol-26-acetate | Triterpenoid | Pitcairn Island | [71] |
| 3-Oxo-cycloart-24-en-26-al | Triterpenoid | Pitcairn Island | [71] |
| 7,8,18-Trihydroxyserrulat-14-ene | Diterpene | Australia | [72] |
| 5,18-Epoxyserrulat-14-en-7,8-dione | Diterpene | Australia | [72] |
| (18RS)-5,18-Epoxyserrulat-14-en-8,18-diol | Diterpene | Australia | [72] |
| Abietinal | Diterpene | Pitcairn Island | [71] |
| Glyasperin | Flavonoid | Fiji Islands | [73] |
| (E)-4-(3-Methyl-2-buten-1-yl)-3,4′,5-trihydroxy-3′-methoxystilbene | Stilbene | Kangaroo Island | [74] |
| (E)-2-(3-Methyl-2-buten-1-yl)-3,4′,5-trihydroxystilbene (2-prenylresveratrol) | Stilbene | Kangaroo Island | [74] |
| (E)-2,4-Bis(3-methyl-2-buten-1-yl)-3,3′,4′,5-tetrahydroxystilbene | Stilbene | Kangaroo Island | [74] |
| (E)-2-(3-Methyl-2-buten-1-yl)-3-(3-methyl-2-butenyloxy)-3′,4′,5-trihydroxystilbene | Stilbene | Kangaroo Island | [74] |
| (E)-2,6-Bis(3-methyl-2-buten-1-yl)-3,3′,5,5′-tetrahydroxystilbene | Stilbene | Kangaroo Island | [74] |
| (E)-2,6-Bis-(3-methyl-2-buten-1-yl)-3,4′,5-trihydroxy-3′-methoxystilbene | Stilbene | Kangaroo Island | [74] |
| Tetragocarbone A | Phenol | Australia | [75] |
| Tetragocarbone B | Phenol | Australia | [75] |
| Solomonin B | Stilbene | Fiji Islands | [73] |
| Solomonin C | Stilbene | Fiji Islands | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebiloma, G.U.; Ichoron, N.; Siheri, W.; Watson, D.G.; Igoli, J.O.; De Koning, H.P. The Strong Anti-Kinetoplastid Properties of Bee Propolis: Composition and Identification of the Active Agents and Their Biochemical Targets. Molecules 2020, 25, 5155. https://doi.org/10.3390/molecules25215155
Ebiloma GU, Ichoron N, Siheri W, Watson DG, Igoli JO, De Koning HP. The Strong Anti-Kinetoplastid Properties of Bee Propolis: Composition and Identification of the Active Agents and Their Biochemical Targets. Molecules. 2020; 25(21):5155. https://doi.org/10.3390/molecules25215155
Chicago/Turabian StyleEbiloma, Godwin U., Nahandoo Ichoron, Weam Siheri, David G. Watson, John O. Igoli, and Harry P. De Koning. 2020. "The Strong Anti-Kinetoplastid Properties of Bee Propolis: Composition and Identification of the Active Agents and Their Biochemical Targets" Molecules 25, no. 21: 5155. https://doi.org/10.3390/molecules25215155
APA StyleEbiloma, G. U., Ichoron, N., Siheri, W., Watson, D. G., Igoli, J. O., & De Koning, H. P. (2020). The Strong Anti-Kinetoplastid Properties of Bee Propolis: Composition and Identification of the Active Agents and Their Biochemical Targets. Molecules, 25(21), 5155. https://doi.org/10.3390/molecules25215155








